Abstract
The prevalence of abnormal acid gastroesophageal reflux (GER) is higher in patients with idiopathic pulmonary fibrosis (IPF) than in matched control subjects. Several studies demonstrated that more than one-third of patients with IPF have abnormal esophageal acid exposures. In addition, many of these studies indicate that the majority of patients with IPF have silent reflux with no symptoms of GER. Findings of abnormal reflux persist in a large proportion of patients with IPF placed on antacid therapy such as proton pump inhibitors (PPIs). This seemingly paradoxical observation suggests that either patients with IPF are somehow resistant to PPI-based intervention or PPIs are inherently unable to suppress acid GER. By contrast, patients with IPF who undergo Nissen fundoplication surgery are effectively relieved from the complications of GER, and retrospective studies suggest improved lung function. Retrospective, anecdotal data suggest a beneficial role of PPIs in IPF including stabilization of lung function, reduction in episodes of acute exacerbation, and enhanced longevity. The recent evidence-based guidelines for treatment of IPF approved conditional recommendation of PPIs for all patients with IPF regardless of their GER status. Recently, we have reported that PPIs possess antiinflammatory and antifibrotic activities by directly suppressing proinflammatory cytokines, profibrotic proteins, and proliferation of lung fibroblasts. Our study provides an alternative explanation for the beneficial effect of PPIs in IPF. In this Perspective, we reviewed emerging progress on antifibrotic effect of PPIs using IPF as a disease model. In addition, we summarized surgical and pharmacological interventions for GER and their downstream effect on lung physiology.
Keywords: gastroesophageal reflux, antireflux surgery, proton pump inhibitors, lung inflammation, fibrosis
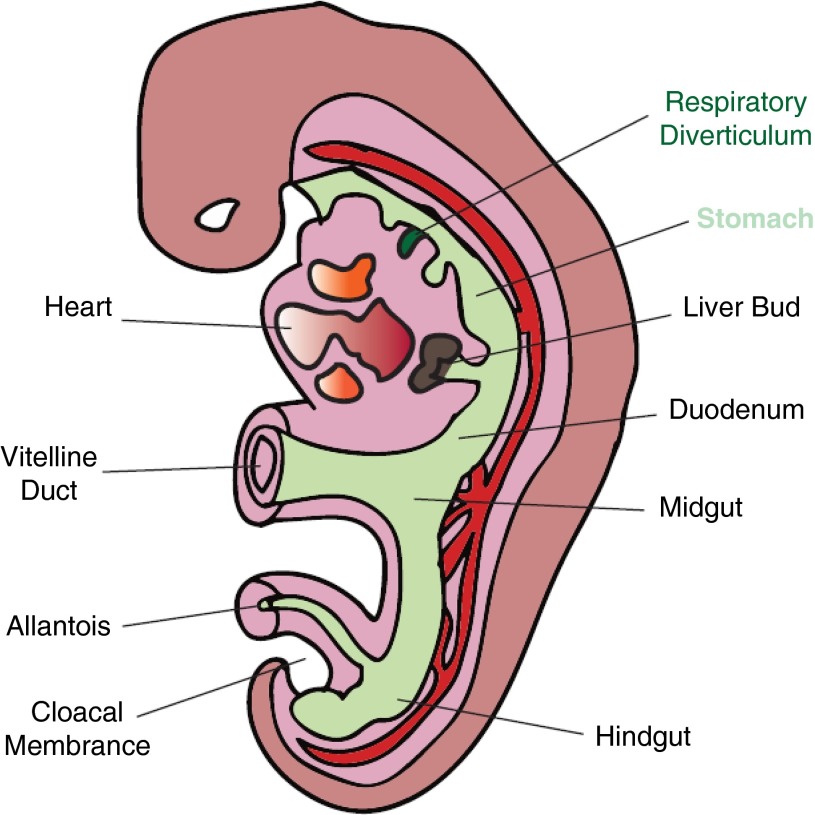
During the fourth week of gestation in mammalian embryogenesis, the foregut endoderm outpouches ventrally to create a respiratory diverticulum, a structure that forms the basis for lung development (Figure 1). Subsequently, the respiratory diverticulum differentiates, under the influence of the associated mesodermal structure, into tissues of the respiratory system including the larynx, trachea, and lungs (1). Further growth of the respiratory diverticulum forms trachea-esophageal ridges that eventually demarcate the respiratory system (trachea) from the upper part of the digestive system (esophagus). Despite this physical separation of respiratory tract tissues from the digestive system, the two structures may continue their interactions biologically. For example, gastric juice that is secreted by the digestive system may be able to reach the respiratory tract through reflux and/or microaspiration and influence biological processes in the airways.
Figure 1.
Outpouching of foregut endoderm and respiratory tract tissues during mammalian embryogenesis. The respiratory diverticula that form the lungs and the stomach are highlighted.
Gastroesophageal Reflux and IPF
Gastroesophageal reflux (GER) and GER disease (GERD) appear to disproportionately affect patients with idiopathic pulmonary fibrosis (IPF; 87–94%) (2, 3) compared with the general population (10–19%) (4) or patients with other advanced lung diseases, including cystic fibrosis (35–81%) (5), asthma (59–68%) (3, 6), chronic obstructive pulmonary disease (19–28%) (7, 8), and bronchiolitis obliterans syndrome (9). Although the exact mechanism for the higher GER prevalence in IPF is not clear, the relationship between GER and pulmonary fibrosis has been recognized for the past 40 years (10). In 1976, a prospective gastrointestinal series (barium swallow) study indicated that the incidence of hiatal hernia and gastric reflux was higher in patients with pulmonary fibrosis than age-matched control subjects (10).
A number of recent studies have used more precise techniques, including esophageal manometry and 24-hour esophageal pH monitoring probe, to evaluate the prevalence of abnormal acid GER in patients with well-defined IPF. Tobin and colleagues (2) prospectively evaluated 17 patients with IPF and found that 94% of these patients had abnormal esophageal acid exposure, and 75% were asymptomatic for classic GER symptoms such as heartburn and regurgitation. Raghu and colleagues (3) also monitored 65 patients with IPF using both esophageal manometry and 24-hour esophageal pH probe and reported that 87% of the patients had abnormal proximal and/or distal esophageal acid exposures. Similar to the study by Tobin and colleagues (2), a large proportion of patients with IPF (53%) in this study were asymptomatic, indicating that most patients with IPF have clinically occult acid GER.
Several other studies support the high prevalence of abnormal acid GER in IPF (11–14). In addition to abnormal intragastric pH, these studies also note that subsets of patients with IPF are more prone to episodes of acute exacerbation and rapid clinical deterioration, including in measures of lung function. Overall, these findings suggest interdependence among GER, hiatal hernia, microaspiration, and acute exacerbation events, and their possible influence on the disease process in IPF. Paradoxically, however, it is still unclear whether there is a causal relationship between IPF and GERD (i.e., whether GER increases risk of IPF or IPF increases risk of GER).
As a result, two long-standing hypotheses regarding the cause-effect relationship remain unresolved:
-
(1)
Chronic microaspiration causes recurrent injury to the bronchiolar and alveolar epithelium and drives the disease process in susceptible individuals to manifest IPF.
-
(2)
Decreased lung compliance of the fibrotic lung in patients with IPF causes increased swings in intrathoracic pressure and leads to dysfunctional lower esophageal sphincter, GERD, and microaspiration that perpetuate and/or accelerate the IPF disease process.
The Contribution of Gastric Juice to Lung Injury
The constituents of gastric juice include several acidic and nonacidic mixtures, such as bile acids, salts, pepsin, endotoxin, and food particles. A number of cell biological and preclinical studies have demonstrated that crude gastric juice or its differential components are associated with harmful effects, including stimulation of immune response, increased cell membrane permeability, airway inflammation, and lung remodeling (15–17). In particular, studies have shown that alveolar/bronchial epithelial cells are exquisitely sensitive to acid-mediated (e.g., bile acids) and nonacid-mediated (e.g., pepsin, bile salts) triggers (16, 18). For example, Perng and colleagues (16) cultured primary human alveolar epithelial cells (HAECs) and exposed them to a major component of bile acids, chenodeoxycholic acid (CD), before assessing the gene and protein expression of transforming growth factor-β (TGF-β). Intriguingly, brief (24 h) treatment of HAECs with CD increased TGF-β gene expression by 2.5-fold and stimulated release of the fibrogenic cytokine by 170-fold in comparison to unstimulated controls. In addition, incubation of primary human lung fibroblasts with conditioned media harvested from CD-stimulated HAECs nearly doubled their proliferation capacity, suggesting direct and biological involvement of a gastric juice component in fibrogenesis.
Subsequent mechanistic studies indicate that bile acids induce the expression of TGF-β and its downstream target, connective tissue growth factor (CTGF), via activation of p38 mitogen-activated protein kinase (19). CTGF is known to enhance fibroblast proliferation and collagen synthesis and has been reported to be substantially up-regulated (by more than tenfold) in bronchoalveolar lavage cells isolated from patients with IPF (20). Additional preclinical studies have shown that acid aspiration induces proinflammatory cytokines and promotes recruitment of inflammatory cells (e.g., neutrophils and alveolar macrophages) into the alveolar interstitial space (21–23). These studies implicate the involvement of bile acid aspiration in lung scarring at least in part due to stimulation of fibroblast proliferation and activation of TGF-β along with its downstream targets, including CTGF.
Furthermore, the role of nonacidic components of gastric juice in the pathobiology of lung injury has been discussed. For example, Johnston and colleagues (24) performed electron microscopy study and demonstrated that airway epithelial cells uptake pepsin by receptor-mediated endocytosis. Once internalized, pepsin is known to induce cytotoxicity, inflammatory response (e.g., up-regulation of IL-6 and IL-8), and airway remodeling (18). In addition, endotoxins are often found in gastric juice likely due to lysis of bacteria in the stomach (25). There is substantial evidence that endotoxins are capable of activating innate immune response including the nuclear factor-κB pathway and altering cellular signaling to aberrantly influence the structure and function of the lungs (26–28).
Clinically, several studies propose the involvement of gastric refluxate in lung remodeling and acute exacerbation events in IPF. However, there are no convincing data on the direct involvement of gastric aspiration in lung remodeling or acute exacerbations. In addition, possible correlation between the severity of GERD, including size of hiatal hernia and volume of gastric aspiration, with the frequency of exacerbation or overall lung structure and function is still obscure (29, 30). Previously, a study of 65 well-characterized patients with IPF did not find a linear correlation between the severity of acid GER and impairment in lung function, including diffusing capacity of the lung for carbon monoxide (DlCO) and FVC (3).
Surgical and Pharmacological Interventions for GER
As described above, the association of GERD with several types of chronic lung disease, including IPF, has been subjectively and objectively explored. In addition, the occurrence and contribution of reflux and gastric aspiration to the pathogenesis of lung remodeling, including acute exacerbation events, have been conceptually discussed. In fact, several studies have indicated gastric aspiration by detecting pepsin in airway samples obtained from patients with respiratory diseases (15, 31–33). Recently, Lee and colleagues (30) reported the presence of pepsin in bronchoalveolar lavage samples obtained from the lower respiratory tract of patients with IPF during episodes of acute exacerbation. This finding not only suggests possible involvement of GER and microaspiration in the IPF disease process but also infers the contribution of this digestive enzyme to lung remodeling.
As described above, several studies have demonstrated that pepsin regulates the physiology of airway epithelial cells (18, 32, 34), including cell survival and generation of inflammatory cytokines (18). Given the known enzymatic action of pepsin and injurious effects of other nonacidic components of gastric juice on lung parenchyma, the potential implications of gastric reflux in the pathobiology of pulmonary fibrosis are evident. Thus, it is important to consider medical and nonmedical measures to mitigate and/or modulate potentially deleterious effects associated with nonacid components of GER, particularly when reflux symptoms are suppressed with antacid therapy.
Altogether, these strategies motivate the need to prospectively assess the application of comprehensive antireflux measures, including pharmacological agents, conservative approaches, and surgical procedures, on the pathogenesis of IPF. Medically, for example, the use of gastroprokinetic agents such as domperidone and metoclopramide may be useful in improving gastrointestinal motility and broadly regulating reflux. Conservative, nonpharmaceutical interventions including lifestyle modifications to decrease abdominal girth (tailored to the individual); changes in social, behavioral, and sleeping habits that include cessation of cigarette smoking; and avoiding or restricting food and beverages known to increase acid production, provoke GER, and/or relax esophageal sphincter may help to minimize risks for GER and microaspiration.
Surgical therapeutic interventions include repair of hiatal hernia and Nissen fundoplication to treat GERD by suppressing GER (35). This strategy effectively suppresses the reflux of both acidic and nonacidic gastric material from the stomach, and thus the risks of silent or overt aspiration are minimized (36). The feasibility of this surgical procedure to improve lung function has been evaluated (37–39). In a retrospective study, decreased oxygen supplementation needs during exercise and increased distance covered during 6-minute-walk tests were observed in patients with IPF subjected to Nissen fundoplication (40). It is hoped that the ongoing prospective and randomized phase 2 clinical study WRAP-IPF (Weighing Risks and Benefits of Laparoscopic Anti-Reflux Surgery in Patients with Idiopathic Pulmonary Fibrosis) will determine the safety and efficacy of laparoscopic antireflux surgery (Nissen fundoplication) in patients with IPF with abnormal acid GER (41).
Although PPIs may decrease/suppress the acidity of the gastric juice, the GER is not suppressed, and thus the refluxate containing the nonacid components of the gastric contents could still be aspirated into the lungs (42). Besides, PPIs may not effectively decrease the intragastric pH in all patients. Indeed, a study by Raghu and colleagues (3) documented that 63% of the patients with IPF treated with standard doses of PPI persisted in having an abnormal acid esophageal pH test. Because the vast majority of patients with IPF with abnormal acid GER are asymptomatic for GER/GERD, one cannot assume that the acidity of gastric juice is effectively suppressed by PPIs in these patients. Several other studies have reported the referral of patients with GERD to undergo antireflux surgery after repeated failure of medical therapy to suppress acid GER or treat GERD (40). Thus, gastric aspirations may contain both acidic and nonacidic components despite PPI therapy. In addition, PPIs are unlikely to modulate volume of gastric secretion or biochemistry of nonacidic contents of gastric juice, including pepsin and bile salts (32).
PPIs and Their Emerging Antiinflammatory–Antifibrotic Role
Since their approval for the treatment of gastric acidity in the late 1980s, PPIs have been widely used to manage patients with abnormal gastrointestinal conditions, including GERD, dyspepsia, Zollinger-Ellison syndrome, Barrett esophagus and Helicobacter pylori infection–induced gastric disorder. However, emerging data indicate that the utility of PPIs extends beyond the gastrointestinal system into the regulation of immune, vascular endothelial, and airway epithelial biology (43). Several molecular and cell biological studies have demonstrated that PPIs favorably regulate the oxidant–antioxidant system by scavenging reactive oxygen species, preventing depletion of detoxifying enzymes such as glutathione, and inducing the expression and activity of antioxidant stress proteins including heme oxygenase 1 and superoxide dismutase (44).
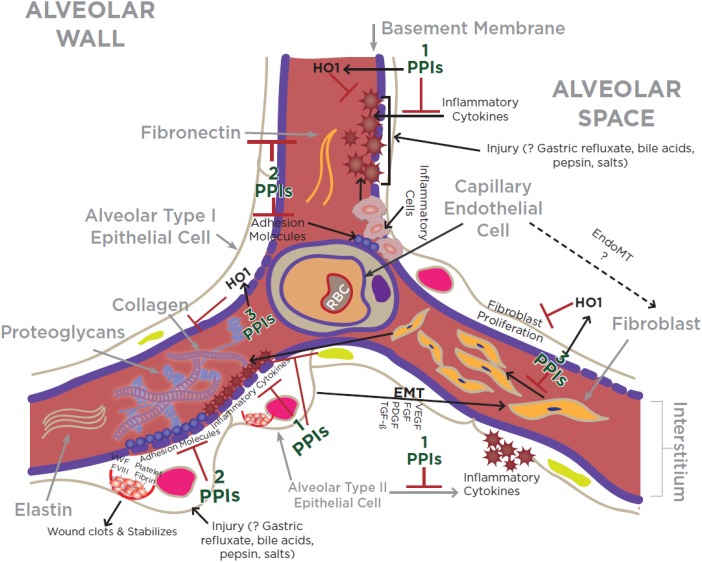
In addition, PPIs have been shown to suppress key inflammatory molecules in nongastric cells, including vascular endothelial and tracheal epithelial cells. Some of the PPI-regulated molecules include members of the integrin superfamily CD11b (integrin αMβ2) and CD18 (integrin β2), adhesion molecules (intercellular adhesion molecule-1 and vascular cell adhesion molecule-1), and proinflammatory cytokines (tumor necrosis factor-α, IL-1β, IL-6, and IL-8) (45, 46) (Figure 2). Moreover, PPIs are reported to directly inhibit the migration and interaction of inflammatory cells with vascular endothelial cells (45) (Figure 2). These effects of PPIs, however, were not reproduced with histamine H2-receptor antagonists (H2RA) such as ranitidine and famotidine (45).
Figure 2.
Schematic illustration of the key cellular and molecular events associated with an injured alveolar wall in a genetically predisposed person manifesting pulmonary fibrosis. Note that proton pump inhibitors (PPIs) suppress key events in lung inflammation and fibrosis including (1) release of proinflammatory molecules from injured epithelial cells (43, 47); (2) expression of adhesion molecules and adherence of inflammatory cells into vascular wall (45–47); and (3) proliferation of fibroblasts including extracellular matrix deposition (47). The ? indicates “unclear.” EMT = epithelial-to-mesenchymal transition; EndoMT = endothelial-to-mesenchymal transition; FGF = fibroblast growth factor; FVIII = factor VIII; HO1 = heme oxygenase 1; PDGF = platelet-derived growth factor; RBC = red blood cell; TGF-β = transforming growth factor-β; VEGF = vascular endothelial growth factor; VWF = Von Willenbrand factor.
Our recent mechanistic study demonstrated that an archetype PPI, esomeprazole, possesses a pleiotropic salutary effect in regulating processes involved in the development and progression of lung inflammation and fibrosis (47). We demonstrated this effect of esomeprazole using in vitro studies of primary lung fibroblasts, endothelial and epithelial cells exposed to bleomycin or ionizing radiation. More specifically, we showed that esomeprazole suppressed the transcriptional expression of (Figure 2): (1) inflammatory cytokines (e.g., tumor necrosis factor-α, IL-1β, and IL-6); (2) adhesion molecules (vascular cell adhesion molecule-1 and intercellular adhesion molecule-1), fibronectin (e.g., FN1), and matrix metalloproteinase enzymes (e.g., matrix metalloproteinase-7). (3) We also found that esomeprazole strongly (by up to 70-fold) up-regulated the cytoprotective enzyme heme oxygenase 1 and favorably regulated markers of oxidative/nitrosative stress. Our cell proliferation study also demonstrated that esomeprazole dose-dependently inhibited the proliferation of lung fibroblasts and reduced TGF-β–induced soluble collagen release from IPF-derived lung fibroblasts.
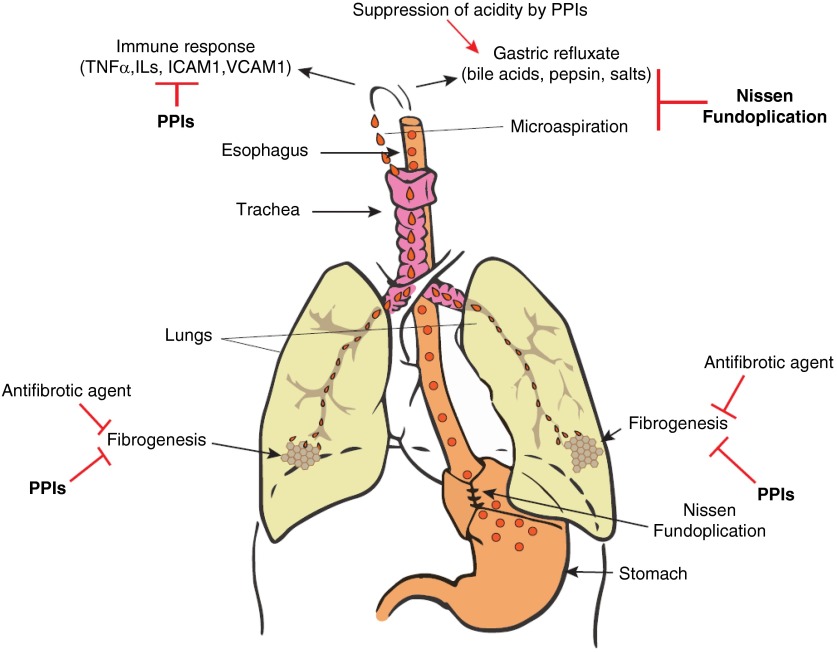
Our preclinical in vivo study revealed that oral esomeprazole mitigates inflammatory and fibrotic responses (about 50% reduction of each parameter) in a rodent model of bleomycin-induced lung injury (47). Overall, the reported data from in vitro and in vivo studies demonstrating the beneficial role of PPIs on processes involved in lung inflammation and fibrogenesis suggest that PPIs, in contrast to antireflux surgery, may exert favorable effects in measures of lung function and survival in IPF through biological regulation of inflammatory and fibrotic processes rather than suppression of gastric reflux or microaspiration (Figure 3). In addition, it is possible that PPI-mediated changes in intragastric pH may contribute to reduced insult from gastric droplets in the event of microaspiration and/or gastric reflux (Figure 3).
Figure 3.
In a genetically predisposed individual, pulmonary fibrosis/idiopathic pulmonary fibrosis may develop as a result of chronic microaspiration and reflux of gastric contents to the respiratory system. Potential preventative and/or therapeutic mechanism of surgical (e.g., Nissen fundoplication) or pharmacological (e.g., antifibrotic agent or proton pump inhibitor [PPI]) agents is shown. ICAM = intercellular adhesion molecule; TNF = tumor necrosis factor; VCAM = vascular cell adhesion molecule.
Treatment of IPF with PPIs?
The newly approved antifibrotic agents—pirfenidone and nintedanib—appear to be promising in the modulation of fibrogenesis and decreasing the rate of progression of pulmonary fibrosis as assessed by decline in FVC measurements. Meanwhile, there are several reported studies that support the beneficial role of antacid therapy (i.e., PPIs or H2RA) in general and PPIs in particular in the management of IPF (Table 1). For example, a case series study by Raghu and colleagues (48) reported that empirical treatment of patients with IPF with PPIs was associated with stabilized or improved lung function and significantly fewer incidences of hospitalization due to respiratory problems, including acute exacerbations. Noth and colleagues (29) also demonstrated that patients with IPF with hiatal hernia taking antacid medications (of whom 94% were on PPIs) had significantly greater DlCO and improved composite physiologic index scores than matched control subjects. In addition, two studies by Lee and colleagues (49, 50) described the beneficial effect of antacid therapy on transplant-free survival and on measures of lung function, including slower decline in FVC and reduction in acute exacerbation events.
Table 1.
The Use of Antacid Therapy and Clinical Outcome in Patients with Well-defined Idiopathic Pulmonary Fibrosis
| Study | No. of Patients with IPF |
Outcome | |
|---|---|---|---|
| PPI/H2RA (% on PPI) | Control | ||
| Raghu et al., 2006 (48) | 4 (100)* | NA | Stabilized or improved lung function |
| No hospitalization for respiratory problems | |||
| PFT deterioration with poor PPI adherence† | |||
| No episode of acute exacerbations | |||
| Lee et al., 2011 (49) | 96 (87)‡ | 107 | Prolonged median survival time: PPI/H2RA (1,967 d) vs. control subjects (896 d) |
| Lower radiologic fibrosis score: PPI/H2RA group (14%) vs. control subjects (19%) | |||
| Lee et al., 2013 (50) | 124 (91) | 118 | Lower loss in lung function (FVC) in PPI/H2RA |
| Fewer acute exacerbations: none on PPI/H2RA vs. 9 events in control subjects | |||
| Ghebremariam et al., 2015 (47) | 130 (100) | 85 | Prolonged transplant-free survival: PPI group (1,241 d) vs. control subjects (730 d) |
Definition of abbreviations: H2RA = antacid histamine H2-receptor antagonist; IPF = idiopathic pulmonary fibrosis; NA = not applicable; PFT = pulmonary function test; PPI = proton pump inhibitor.
Of the number of patients with IPF on antacid therapy, the percentage on PPIs is shown in the second column. H2RA is an alternate antacid.
One patient was on PPI and had antireflux surgery.
PFT was measured by change in FVC and diffusing capacity of the lung for carbon monoxide.
Two percent of the patients were on both PPI and H2RA.
Notably, a large proportion (>85%) of the patients with IPF in the cohorts described above were placed on PPIs compared with H2RA (Table 1). Interestingly, one of the studies (50) conducted differential analysis and showed that the significance of clinical benefit (e.g., estimated change in FVC) of antacid therapy continued to uphold even after exclusion of patients taking H2RA, indicating major contribution of the PPIs to the study outcome. Recently, we reported that patients with IPF on PPIs had significantly prolonged lung transplant–free survival (47). Unlike many of the studies above, our patients on “antacid therapy” in this study were exclusively on PPIs.
It is apparent that the recent conditional recommendation of antacid treatment for IPF was based on such reports (51). The updated 2015 evidence-based guideline for treatment of IPF conditionally recommended the use of antacids for the treatment of IPF but also surfaced the low quality of supporting evidence on which the recommendation was based. The accompanying note from the editor points out that some of the conflicted panel members of the IPF committee that updated the evidence-based guidelines for treatment of IPF in 2015 expressed concerns with the favorable recommendation.
At the 2015 European Respiratory Society meeting, a presentation of post hoc analyses of the INPULSIS clinical trials that determined the safety and efficacy of nintedanib in patients with IPF suggests that patients treated with PPI at baseline may actually do worse (52). However, the presented data were results of a post hoc analysis comparing patients with IPF taking concomitant antacid medication (PPIs or H2RA) versus those who were not. As analyzed, the pooled data from the two INPULSIS trials showed that there was a greater decline in FVC in the patients receiving antacid medication at baseline and continued on placebo-for-nintedanib compared with the patients who received antacid medication at baseline and continued on nintedanib (52).
Although the above findings cast uncertainty on the possible treatment benefits of antacid treatment for patients with IPF, it is assumed that patients were taking the antacids throughout the study period because of an abnormal acid GER that prompted the patients to have been on PPI at the time of enrollment into the INPULSIS study. However, the data gathered during the clinical trials did not capture the objective assessment of GER in the patients. Because PPIs do not stop the actual GER, and it is known that about 60% of patients treated with PPIs persist to have abnormal acid GER (3), it is possible that the persistent acid GER and/or the nonacid components present in the refluxate of the abnormal GER may have been the reason for deleterious outcomes of patients taking antacids at the time of enrollment.
In light of these findings and that the 2015 conditional recommendation of antacid treatment for IPF was based on low-quality retrospective evidence, there is a pressing need for a well-defined, randomized, controlled clinical study to prospectively evaluate the safety and therapeutic efficacy of PPIs/antacids and antireflux surgery for IPF. Such studies should determine the safety and efficacy of PPIs to treat IPF as “stand-alone” or “add-on” to current and/or other new antifibrotic strategies. Concurrent mechanistic studies may also be used to understand the mechanism(s) by which PPIs may regulate lung function to benefit patients with IPF.
Because PPIs are generically available as over-the-counter medication, the potential for the drug to be cost effective, if found to be efficacious for the treatment of IPF, is evident. However, it is important to recognize that there are several nonfatal side effects associated with intermittent and/or prolonged use of PPIs, including rash, fatigue, diarrhea, headache, acid rebound after discontinuation, increase in nonacid reflux events, risk of osteoporosis, and increased risk of community-acquired pneumonia (53). In addition, recent bioinformatics studies indicate that chronic use of PPIs may increase the risk of dementia (54) and major adverse cardiovascular events (55, 56). Another possible adverse effect of PPIs is on the homeostasis of the gastric microbiome and risk of microbial infection. This concern was partially alleviated by a recent metaanalysis of an observational study that found no statistically significant increase in the risk of hospitalization for community-acquired pneumonia among PPI users (57).
In addition to the effect of PPIs on the gastric microbiome, contribution of the microbiome to reflux disorders and to IPF disease progression has been recently evaluated. For example, comparison of the esophageal microbiome among patients with reflux disorders such as reflux esophagitis and Barrett esophagus to that of the microbiome in normal subjects revealed the transformation of the esophageal microbiome to gram-negative bacteria in the disease setting (58–60). In addition, studies have found association between increased abundance of specific bacterial sequences or bacterial loads and IPF disease progression, including the rate of lung volume decline (61–63).
All of the aforementioned clinical anecdotes and bioinformatic inferences call for the need to determine the safety and efficacy of long-term treatment with PPIs.
Conclusions
A number of observational studies have documented a strong association between GERD and IPF. However, several questions need to be addressed, particularly in relation to the role of GER and/or microaspiration in lung injury, remodeling, and progression to IPF. In addition, the interdependence or mutual exclusivity between abnormal acid and nonacid GER and IPF in any given number of patients needs to be carefully examined. In light of this, particular attention should be given to patients presenting with symmetrical or asymmetrical disease that may selectively affect progression of fibrotic changes on one side of the lungs over the other (64).
The evidence reviewed here suggests that antacid therapy (e.g., PPIs) may play a beneficial role in IPF despite their inability in controlling the gastric reflux per se or microaspiration. The alternate and biologically plausible mechanism(s) that may underlie the beneficial effect of PPIs in IPF may include down-regulation of fibroinflammatory molecules, up-regulation of cytoprotective mechanisms, inhibition of fibroblast proliferation, and suppression of gastric acidity. These emerging frontiers of PPI use for extraintestinal applications deserve further investigation. More specifically, the anecdotal and possibly reflux-independent benefit of PPIs in IPF needs to be prospectively examined in controlled clinical trials that enroll patients with IPF with normal and abnormal GER, symptomatic and asymptomatic GERD, acidic and nonacidic reflux, and symmetrical and asymmetrical disease and compared with other conservative and surgical measures that are deployed to minimize risks for GER and microaspiration.
Footnotes
Supported by National Institutes of Health NHLBI-funded project exploring treatment of IPF with laparoscopic antireflux surgery grant 5 UM1HL119089 (G.R.); the Stanford School of Medicine Dean’s fellowship grant number 1049528-149-KAVFB, Stanford SPARK Translational Research Program, the Tobacco-Related Disease Research Program of the University of California grant 20FT-0090, intramural funding from the Houston Methodist Research Institute (Y.T.G.); NHLBI grant K01HL118683-04 (Y.T.G.); and intramural funding from Baylor College of Medicine, Department of Radiation Oncology (Y.T.G.).
Author Contributions: G.R. and Y.T.G. conceived the idea and discussed and organized the topics and outline. Y.T.G. drafted the first version of the manuscript. Both authors reviewed, revised, and finalized the manuscript and accepted the submitted version.
Originally Published in Press as DOI: 10.1164/rccm.201512-2316PP on April 25, 2016
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Duke University; Lung and diaphragm development. 2015 [accessed 2015 Nov 20]. Available from: https://web.duke.edu/anatomy/embryology/respiratory/lungDiaphragm.html.
- 2.Tobin RW, Pope CE, II, Pellegrini CA, Emond MJ, Sillery J, Raghu G. Increased prevalence of gastroesophageal reflux in patients with idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 1998;158:1804–1808. doi: 10.1164/ajrccm.158.6.9804105. [DOI] [PubMed] [Google Scholar]
- 3.Raghu G, Freudenberger TD, Yang S, Curtis JR, Spada C, Hayes J, Sillery JK, Pope CE, II, Pellegrini CA. High prevalence of abnormal acid gastro-oesophageal reflux in idiopathic pulmonary fibrosis. Eur Respir J. 2006;27:136–142. doi: 10.1183/09031936.06.00037005. [DOI] [PubMed] [Google Scholar]
- 4.Dent J, El-Serag HB, Wallander MA, Johansson S. Epidemiology of gastro-oesophageal reflux disease: a systematic review. Gut. 2005;54:710–717. doi: 10.1136/gut.2004.051821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Robinson NB, DiMango E. Prevalence of gastroesophageal reflux in cystic fibrosis and implications for lung disease. Ann Am Thorac Soc. 2014;11:964–968. doi: 10.1513/AnnalsATS.201401-044FR. [DOI] [PubMed] [Google Scholar]
- 6.Havemann BD, Henderson CA, El-Serag HB. The association between gastro-oesophageal reflux disease and asthma: a systematic review. Gut. 2007;56:1654–1664. doi: 10.1136/gut.2007.122465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mokhlesi B, Morris AL, Huang CF, Curcio AJ, Barrett TA, Kamp DW. Increased prevalence of gastroesophageal reflux symptoms in patients with COPD. Chest. 2001;119:1043–1048. doi: 10.1378/chest.119.4.1043. [DOI] [PubMed] [Google Scholar]
- 8.Kim J, Lee JH, Kim Y, Kim K, Oh YM, Yoo KH, Rhee CK, Yoon HK, Kim YS, Park YB, et al. Association between chronic obstructive pulmonary disease and gastroesophageal reflux disease: a national cross-sectional cohort study. BMC Pulm Med. 2013;13:51. doi: 10.1186/1471-2466-13-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.King BJ, Iyer H, Leidi AA, Carby MR. Gastroesophageal reflux in bronchiolitis obliterans syndrome: a new perspective. J Heart Lung Transplant. 2009;28:870–875. doi: 10.1016/j.healun.2009.05.040. [DOI] [PubMed] [Google Scholar]
- 10.Mays EE, Dubois JJ, Hamilton GB. Pulmonary fibrosis associated with tracheobronchial aspiration: a study of the frequency of hiatal hernia and gastroesophageal reflux in interstitial pulmonary fibrosis of obscure etiology. Chest. 1976;69:512–515. doi: 10.1378/chest.69.4.512. [DOI] [PubMed] [Google Scholar]
- 11.Patti MG, Tedesco P, Golden J, Hays S, Hoopes C, Meneghetti A, Damani T, Way LW. Idiopathic pulmonary fibrosis: how often is it really idiopathic? J Gastrointest Surg. 2005;9:1053–1056, discussion 1056–1058. doi: 10.1016/j.gassur.2005.06.027. [DOI] [PubMed] [Google Scholar]
- 12.Salvioli B, Belmonte G, Stanghellini V, Baldi E, Fasano L, Pacilli AM, De Giorgio R, Barbara G, Bini L, Cogliandro R, et al. Gastro-oesophageal reflux and interstitial lung disease. Dig Liver Dis. 2006;38:879–884. doi: 10.1016/j.dld.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 13.Sweet MP, Patti MG, Leard LE, Golden JA, Hays SR, Hoopes C, Theodore PR. Gastroesophageal reflux in patients with idiopathic pulmonary fibrosis referred for lung transplantation. J Thorac Cardiovasc Surg. 2007;133:1078–1084. doi: 10.1016/j.jtcvs.2006.09.085. [DOI] [PubMed] [Google Scholar]
- 14.Gao F, Hobson AR, Shang ZM, Pei YX, Gao Y, Wang JX, Huang WN. The prevalence of gastro-esophageal reflux disease and esophageal dysmotility in Chinese patients with idiopathic pulmonary fibrosis. BMC Gastroenterol. 2015;15:26. doi: 10.1186/s12876-015-0253-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blondeau K, Mertens V, Vanaudenaerde BA, Verleden GM, Van Raemdonck DE, Sifrim D, Dupont LJ. Gastro-oesophageal reflux and gastric aspiration in lung transplant patients with or without chronic rejection. Eur Respir J. 2008;31:707–713. doi: 10.1183/09031936.00064807. [DOI] [PubMed] [Google Scholar]
- 16.Perng DW, Chang KT, Su KC, Wu YC, Wu MT, Hsu WH, Tsai CM, Lee YC. Exposure of airway epithelium to bile acids associated with gastroesophageal reflux symptoms: a relation to transforming growth factor-beta1 production and fibroblast proliferation. Chest. 2007;132:1548–1556. doi: 10.1378/chest.07-1373. [DOI] [PubMed] [Google Scholar]
- 17.D’Ovidio F, Mura M, Tsang M, Waddell TK, Hutcheon MA, Singer LG, Hadjiliadis D, Chaparro C, Gutierrez C, Pierre A, et al. Bile acid aspiration and the development of bronchiolitis obliterans after lung transplantation. J Thorac Cardiovasc Surg. 2005;129:1144–1152. doi: 10.1016/j.jtcvs.2004.10.035. [DOI] [PubMed] [Google Scholar]
- 18.Bathoorn E, Daly P, Gaiser B, Sternad K, Poland C, Macnee W, Drost EM. Cytotoxicity and induction of inflammation by pepsin in acid in bronchial epithelial cells. Int J Inflam. 2011:2011–569416. doi: 10.4061/2011/569416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Perng DW, Wu YC, Tsai CC, Su KC, Liu LY, Hsu WH, Lee YC. Bile acids induce CCN2 production through p38 MAP kinase activation in human bronchial epithelial cells: a factor contributing to airway fibrosis. Respirology. 2008;13:983–989. doi: 10.1111/j.1440-1843.2008.01402.x. [DOI] [PubMed] [Google Scholar]
- 20.Allen JT, Knight RA, Bloor CA, Spiteri MA. Enhanced insulin-like growth factor binding protein-related protein 2 (Connective tissue growth factor) expression in patients with idiopathic pulmonary fibrosis and pulmonary sarcoidosis. Am J Respir Cell Mol Biol. 1999;21:693–700. doi: 10.1165/ajrcmb.21.6.3719. [DOI] [PubMed] [Google Scholar]
- 21.Beck-Schimmer B, Rosenberger DS, Neff SB, Jamnicki M, Suter D, Fuhrer T, Schwendener R, Booy C, Reyes L, Pasch T, et al. Pulmonary aspiration: new therapeutic approaches in the experimental model. Anesthesiology. 2005;103:556–566. doi: 10.1097/00000542-200509000-00019. [DOI] [PubMed] [Google Scholar]
- 22.Folkesson HG, Matthay MA, Hébert CA, Broaddus VC. Acid aspiration-induced lung injury in rabbits is mediated by interleukin-8-dependent mechanisms. J Clin Invest. 1995;96:107–116. doi: 10.1172/JCI118009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kennedy TP, Johnson KJ, Kunkel RG, Ward PA, Knight PR, Finch JS. Acute acid aspiration lung injury in the rat: biphasic pathogenesis. Anesth Analg. 1989;69:87–92. [PubMed] [Google Scholar]
- 24.Johnston N, Wells CW, Blumin JH, Toohill RJ, Merati AL. Receptor-mediated uptake of pepsin by laryngeal epithelial cells. Ann Otol Rhinol Laryngol. 2007;116:934–938. doi: 10.1177/000348940711601211. [DOI] [PubMed] [Google Scholar]
- 25.Mertens V, Blondeau K, Vanaudenaerde B, Vos R, Farre R, Pauwels A, Verleden G, Van Raemdonck D, Dupont L, Sifrim D. Gastric juice from patients “on” acid suppressive therapy can still provoke a significant inflammatory reaction by human bronchial epithelial cells. J Clin Gastroenterol. 2010;44:e230–e235. doi: 10.1097/MCG.0b013e3181d47dc4. [DOI] [PubMed] [Google Scholar]
- 26.Brigham KL, Meyrick B. Endotoxin and lung injury. Am Rev Respir Dis. 1986;133:913–927. [PubMed] [Google Scholar]
- 27.Kao SJ, Su CF, Liu DD, Chen HI. Endotoxin-induced acute lung injury and organ dysfunction are attenuated by pentobarbital anaesthesia. Clin Exp Pharmacol Physiol. 2007;34:480–487. doi: 10.1111/j.1440-1681.2007.04598.x. [DOI] [PubMed] [Google Scholar]
- 28.Rojas M, Woods CR, Mora AL, Xu J, Brigham KL. Endotoxin-induced lung injury in mice: structural, functional, and biochemical responses. Am J Physiol Lung Cell Mol Physiol. 2005;288:L333–L341. doi: 10.1152/ajplung.00334.2004. [DOI] [PubMed] [Google Scholar]
- 29.Noth I, Zangan SM, Soares RV, Forsythe A, Demchuk C, Takahashi SM, Patel SB, Strek ME, Krishnan JA, Patti MG, et al. Prevalence of hiatal hernia by blinded multidetector CT in patients with idiopathic pulmonary fibrosis. Eur Respir J. 2012;39:344–351. doi: 10.1183/09031936.00099910. [DOI] [PubMed] [Google Scholar]
- 30.Lee JS, Song JW, Wolters PJ, Elicker BM, King TE, Jr, Kim DS, Collard HR. Bronchoalveolar lavage pepsin in acute exacerbation of idiopathic pulmonary fibrosis. Eur Respir J. 2012;39:352–358. doi: 10.1183/09031936.00050911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Farhath S, He Z, Nakhla T, Saslow J, Soundar S, Camacho J, Stahl G, Shaffer S, Mehta DI, Aghai ZH. Pepsin, a marker of gastric contents, is increased in tracheal aspirates from preterm infants who develop bronchopulmonary dysplasia. Pediatrics. 2008;121:e253–e259. doi: 10.1542/peds.2007-0056. [DOI] [PubMed] [Google Scholar]
- 32.Bardhan KD, Strugala V, Dettmar PW. Reflux revisited: advancing the role of pepsin. Int J Otolaryngol. 2012;2012:646901. doi: 10.1155/2012/646901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Farhath S, Aghai ZH, Nakhla T, Saslow J, He Z, Soundar S, Mehta DI. Pepsin, a reliable marker of gastric aspiration, is frequently detected in tracheal aspirates from premature ventilated neonates: relationship with feeding and methylxanthine therapy. J Pediatr Gastroenterol Nutr. 2006;43:336–341. doi: 10.1097/01.mpg.0000232015.56155.03. [DOI] [PubMed] [Google Scholar]
- 34.Rosen R, Johnston N, Hart K, Khatwa U, Nurko S. The presence of pepsin in the lung and its relationship to pathologic gastro-esophageal reflux. Neurogastroenterol Motil. 2012;24:129–133, e84–e85. doi: 10.1111/j.1365-2982.2011.01826.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nissen R. Gastropexy and “fundoplication” in surgical treatment of hiatal hernia. Am J Dig Dis. 1961;6:954–961. doi: 10.1007/BF02231426. [DOI] [PubMed] [Google Scholar]
- 36.Dallemagne B, Weerts J, Markiewicz S, Dewandre JM, Wahlen C, Monami B, Jehaes C. Clinical results of laparoscopic fundoplication at ten years after surgery. Surg Endosc. 2006;20:159–165. doi: 10.1007/s00464-005-0174-x. [DOI] [PubMed] [Google Scholar]
- 37.Patti MG, Arcerito M, Tamburini A, Diener U, Feo CV, Safadi B, Fisichella P, Way LW. Effect of laparoscopic fundoplication on gastroesophageal reflux disease-induced respiratory symptoms. J Gastrointest Surg. 2000;4:143–149. doi: 10.1016/s1091-255x(00)80050-5. [DOI] [PubMed] [Google Scholar]
- 38.Allen CJ, Anvari M. Does laparoscopic fundoplication provide long-term control of gastroesophageal reflux related cough? Surg Endosc. 2004;18:633–637. doi: 10.1007/s00464-003-8821-6. [DOI] [PubMed] [Google Scholar]
- 39.Hoppo T, Jarido V, Pennathur A, Morrell M, Crespo M, Shigemura N, Bermudez C, Hunter JG, Toyoda Y, Pilewski J, et al. Antireflux surgery preserves lung function in patients with gastroesophageal reflux disease and end-stage lung disease before and after lung transplantation. Arch Surg. 2011;146:1041–1047. doi: 10.1001/archsurg.2011.216. [DOI] [PubMed] [Google Scholar]
- 40.Linden PA, Gilbert RJ, Yeap BY, Boyle K, Deykin A, Jaklitsch MT, Sugarbaker DJ, Bueno R. Laparoscopic fundoplication in patients with end-stage lung disease awaiting transplantation. J Thorac Cardiovasc Surg. 2006;131:438–446. doi: 10.1016/j.jtcvs.2005.10.014. [DOI] [PubMed] [Google Scholar]
- 41.ClinicalTrials.gov. Treatment of IPF with laparoscopic anti-reflux surgery (WRAP-IPF) Clinical Trials identifier: NCT01982968 [accessed 18 Nov 2015]. Available from: www.clinicaltrials.gov.
- 42.Raghu G, Meyer KC. Silent gastro-oesophageal reflux and microaspiration in IPF: mounting evidence for anti-reflux therapy? Eur Respir J. 2012;39:242–245. doi: 10.1183/09031936.00211311. [DOI] [PubMed] [Google Scholar]
- 43.Kedika RR, Souza RF, Spechler SJ. Potential anti-inflammatory effects of proton pump inhibitors: a review and discussion of the clinical implications. Dig Dis Sci. 2009;54:2312–2317. doi: 10.1007/s10620-009-0951-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Takagi T, Naito Y, Okada H, Ishii T, Mizushima K, Akagiri S, Adachi S, Handa O, Kokura S, Ichikawa H, et al. Lansoprazole, a proton pump inhibitor, mediates anti-inflammatory effect in gastric mucosal cells through the induction of heme oxygenase-1 via activation of NF-E2-related factor 2 and oxidation of kelch-like ECH-associating protein 1. J Pharmacol Exp Ther. 2009;331:255–264. doi: 10.1124/jpet.109.152702. [DOI] [PubMed] [Google Scholar]
- 45.Yoshida N, Yoshikawa T, Tanaka Y, Fujita N, Kassai K, Naito Y, Kondo M. A new mechanism for anti-inflammatory actions of proton pump inhibitors--inhibitory effects on neutrophil-endothelial cell interactions. Aliment Pharmacol Ther. 2000;14:74–81. doi: 10.1046/j.1365-2036.2000.014s1074.x. [DOI] [PubMed] [Google Scholar]
- 46.Sasaki T, Yamaya M, Yasuda H, Inoue D, Yamada M, Kubo H, Nishimura H, Sasaki H. The proton pump inhibitor lansoprazole inhibits rhinovirus infection in cultured human tracheal epithelial cells. Eur J Pharmacol. 2005;509:201–210. doi: 10.1016/j.ejphar.2004.12.042. [DOI] [PubMed] [Google Scholar]
- 47.Ghebremariam YT, Cooke JP, Gerhart W, Griego C, Brower JB, Doyle-Eisele M, Moeller BC, Zhou Q, Ho L, de Andrade J, et al. Pleiotropic effect of the proton pump inhibitor esomeprazole leading to suppression of lung inflammation and fibrosis. J Transl Med. 2015;13:249. doi: 10.1186/s12967-015-0614-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Raghu G, Yang ST, Spada C, Hayes J, Pellegrini CA. Sole treatment of acid gastroesophageal reflux in idiopathic pulmonary fibrosis: a case series. Chest. 2006;129:794–800. doi: 10.1378/chest.129.3.794. [DOI] [PubMed] [Google Scholar]
- 49.Lee JS, Ryu JH, Elicker BM, Lydell CP, Jones KD, Wolters PJ, King TE, Jr, Collard HR. Gastroesophageal reflux therapy is associated with longer survival in patients with idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2011;184:1390–1394. doi: 10.1164/rccm.201101-0138OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lee JS, Collard HR, Anstrom KJ, Martinez FJ, Noth I, Roberts RS, Yow E, Raghu G IPFnet Investigators. Anti-acid treatment and disease progression in idiopathic pulmonary fibrosis: an analysis of data from three randomised controlled trials. Lancet Respir Med. 2013;1:369–376. doi: 10.1016/S2213-2600(13)70105-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Raghu G, Rochwerg B, Zhang Y, Garcia CA, Azuma A, Behr J, Brozek JL, Collard HR, Cunningham W, Homma S, et al. American Thoracic Society; European Respiratory society; Japanese Respiratory Society; Latin American Thoracic Association. An official ATS/ERS/JRS/ALAT clinical practice guideline: treatment of idiopathic pulmonary fibrosis. An update of the 2011 clinical practice guideline. Am J Respir Crit Care Med. 2015;192:e3–e19. doi: 10.1164/rccm.201506-1063ST. [DOI] [PubMed] [Google Scholar]
- 52.Raghu G, Crestani B, Bailes Z, Schlenker-Herceg R, Costabel U. Effect of anti-acid medication on reduction in FVC decline with nintedanib. Presented at the European Respiratory Society Annual Meeting. September 26–30, 2015, Amsterdam, the Netherlands.
- 53.Gulmez SE, Holm A, Frederiksen H, Jensen TG, Pedersen C, Hallas J. Use of proton pump inhibitors and the risk of community-acquired pneumonia: a population-based case-control study. Arch Intern Med. 2007;167:950–955. doi: 10.1001/archinte.167.9.950. [DOI] [PubMed] [Google Scholar]
- 54.Gomm W, von Holt K, Thomé F, Broich K, Maier W, Fink A, Doblhammer G, Haenisch B. Association of proton pump inhibitors with risk of dementia: a pharmacoepidemiological claims data analysis. JAMA Neurol. 2016;73:410–416. doi: 10.1001/jamaneurol.2015.4791. [DOI] [PubMed] [Google Scholar]
- 55.Shih CJ, Chen YT, Ou SM, Li SY, Chen TJ, Wang SJ. Proton pump inhibitor use represents an independent risk factor for myocardial infarction. Int J Cardiol. 2014;177:292–297. doi: 10.1016/j.ijcard.2014.09.036. [DOI] [PubMed] [Google Scholar]
- 56.Shah NH, LePendu P, Bauer-Mehren A, Ghebremariam YT, Iyer SV, Marcus J, Nead KT, Cooke JP, Leeper NJ. Proton pump inhibitor usage and the risk of myocardial infarction in the general population. Plos One. 2015;10:e0124653. doi: 10.1371/journal.pone.0124653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Filion KB, Chateau D, Targownik LE, Gershon A, Durand M, Tamim H, Teare GF, Ravani P, Ernst P, Dormuth CR CNODES Investigators. Proton pump inhibitors and the risk of hospitalisation for community-acquired pneumonia: replicated cohort studies with meta-analysis. Gut. 2014;63:552–558. doi: 10.1136/gutjnl-2013-304738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pei Z, Yang L, Peek RM, Jr Levine SM, Pride DT, Blaser MJ. Bacterial biota in reflux esophagitis and Barrett’s esophagus. World J Gastroenterol. 2005;11:7277–7283. doi: 10.3748/wjg.v11.i46.7277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yang L, Lu X, Nossa CW, Francois F, Peek RM, Pei Z. Inflammation and intestinal metaplasia of the distal esophagus are associated with alterations in the microbiome. Gastroenterology. 2009;137:588–597. doi: 10.1053/j.gastro.2009.04.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Liu N, Ando T, Ishiguro K, Maeda O, Watanabe O, Funasaka K, Nakamura M, Miyahara R, Ohmiya N, Goto H. Characterization of bacterial biota in the distal esophagus of Japanese patients with reflux esophagitis and Barrett’s esophagus. BMC Infect Dis. 2013;13:130. doi: 10.1186/1471-2334-13-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Han MK, Zhou Y, Murray S, Tayob N, Noth I, Lama VN, Moore BB, White ES, Flaherty KR, Huffnagle GB, et al. COMET Investigators. Lung microbiome and disease progression in idiopathic pulmonary fibrosis: an analysis of the COMET study. Lancet Respir Med. 2014;2:548–556. doi: 10.1016/S2213-2600(14)70069-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Molyneaux PL, Cox MJ, Willis-Owen SA, Mallia P, Russell KE, Russell AM, Murphy E, Johnston SL, Schwartz DA, Wells AU, et al. The role of bacteria in the pathogenesis and progression of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2014;190:906–913. doi: 10.1164/rccm.201403-0541OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Molyneaux PL, Maher TM. Respiratory microbiome in IPF: cause, effect, or biomarker? Lancet Respir Med. 2014;2:511–513. doi: 10.1016/S2213-2600(14)70088-8. [DOI] [PubMed] [Google Scholar]
- 64.Tcherakian C, Cottin V, Brillet PY, Freynet O, Naggara N, Carton Z, Cordier JF, Brauner M, Valeyre D, Nunes H. Progression of idiopathic pulmonary fibrosis: lessons from asymmetrical disease. Thorax. 2011;66:226–231. doi: 10.1136/thx.2010.137190. [DOI] [PubMed] [Google Scholar]