Abstract
Rationale: Treatments for patients with sepsis with intermediate lactate values (≥2 and <4 mmol/L) are poorly defined.
Objectives: To evaluate multicenter implementation of a treatment bundle (including timed intervals for antibiotics, repeat lactate testing, and intravenous fluids) for hemodynamically stable patients with sepsis and intermediate lactate values in the emergency department.
Methods: We evaluated patients in annual intervals before and after bundle implementation in March 2013. We evaluated bundle compliance and compared outcome measures across groups with multivariable logistic regression. Because of their perceived risk for iatrogenic fluid overload, we also evaluated patients with a history of heart failure and/or chronic kidney disease.
Measurements and Main Results: We identified 18,122 patients with sepsis and intermediate lactate values, including 36.1% treated after implementation. Full bundle compliance increased from 32.2% in 2011 to 44.9% after bundle implementation (P < 0.01). Hospital mortality was 8.8% in 2011, 9.3% in 2012, and 7.9% in 2013 (P = 0.02). Treatment after bundle implementation was associated with an adjusted hospital mortality odds ratio of 0.81 (95% confidence interval, 0.66–0.99; P = 0.04). Decreased hospital mortality was observed primarily in patients with a heart failure and/or kidney disease history (P < 0.01) compared with patients without this history (P > 0.40). This corresponded to notable changes in the volume of fluid resuscitation in patients with heart failure and/or kidney disease after implementation.
Conclusions: Multicenter implementation of a treatment bundle for patients with sepsis and intermediate lactate values improved bundle compliance and was associated with decreased hospital mortality. These decreases were mediated by improved mortality and increased fluid administration among patients with a history of heart failure and/or chronic kidney disease.
Keywords: hospital mortality, quality improvement, resuscitation, sepsis
At A Glance Commentary
Scientific Knowledge on the Subject
Patients with sepsis with intermediate lactate values contribute to substantial hospital mortality. However, corresponding treatments are poorly defined.
What This Study Adds to the Field
In this study, we evaluated the treatment and outcomes of more than 18,000 hemodynamically stable patients with intermediate lactate sepsis who presented through the emergency department in a multicenter contemporary setting after bundle implementation. Implementation was associated with improved bundle compliance and decreased mortality that was driven primarily by improved mortality in patients with a history of heart failure or chronic kidney disease.
Sepsis is the most expensive cause of hospitalization in the United States and plays a role in as many as one in two hospital deaths nationally (1, 2). Most prior studies of sepsis have been focused heavily on patients with the most severe disease (including those with septic shock and/or lactate values of 4 mmol/L or greater), who are treated primarily in intensive care units (ICUs) (3–8). In this population, the current standard of care is focused on early patient identification, prompt infection source control, and aggressive fluid administration with the addition of central venous catheter–based care based on clinical need (3, 5–8). Multiple observational studies suggest that implementation of this care within emergency department (ED)–based performance improvement programs has contributed to improved mortality (9–11).
In contrast, few studies have been focused on assessing optimal treatment strategies for patients with patients with less severe sepsis, including so-called intermediate lactate patients who have normal blood pressure and lactate values between 2 and 4 mmol/L (12–16). Such patients also face substantial hospital mortality and are often treated in less standardized, non-ICU hospital settings (12). Because patients with intermediate lactate values are also more common in hospitalized populations, they contribute to a number of overall hospital deaths nearly equivalent to that of patients with more severe sepsis (1). Despite this, the evidence base guiding treatment strategies in this population is poorly defined (15). However, prior work suggests that these patients may also benefit from similar ED-based early identification and care strategies.
As a result, in March 2013, the Kaiser Permanente Northern California (KPNC) integrated healthcare delivery system implemented a treatment strategy designed to improve care for hemodynamically stable patients with sepsis and intermediate lactate values in the ED. This quality improvement effort is centered on a treatment bundle focused on prompt antibiotic administration, aggressive fluid resuscitation, and early risk reassessment with repeat lactate testing. In this study, we compared treatment practices and outcomes before and after implementation of the intermediate lactate bundle quality improvement effort for patients with sepsis hospitalized at 21 community hospitals between March 2011 and February 2014.
Methods
This study was approved by the KPNC Institutional Review Board (CN-14-1815-H), which has jurisdiction over all study hospitals, with a waiver of informed consent.
Intermediate Lactate Bundle
In March 2013, KPNC implemented an intermediate lactate bundle quality improvement effort for eligible patients admitted to its 21 community-based hospitals. Bundle-eligible patients included those admitted through the ED with an initial lactate value greater than or equal to 2 mmol/L and less than 4 mmol/L who did not meet standard criteria for early goal-directed therapy (EGDT), including refractory hypotension, following an intravenous fluid bolus and/or an initial lactate value greater than or equal to 4 mmol/L. The bundle included three elements to be completed after the initial lactate test results were obtained (time 0), including (1) antibiotics administered within 3 hours, (2) repeat lactate testing within 1–4 hours of initial lactate testing, and (3) orders for 30 ml/kg (or at least 2 L) of intravenous fluid within 3 hours.
Bundle implementation occurred within the context of a mature sepsis performance improvement program at KPNC that was initiated in 2008. Before implementation, hospital sepsis champions, quality improvement staff, and operational leadership were educated about the bundle at a regional sepsis summit meeting. These educational meetings were initially instituted in 2010 to facilitate improvements in the care of patients with sepsis using EGDT. Subsequent meetings addressed quality and performance improvement as well as novel sepsis-related initiatives. Similarly, after implementation, intermediate lactate bundle performance metrics were added to an existing electronic sepsis scorecard that was already being distributed to all hospitals for monthly performance review. Sepsis scorecards included monthly performance metrics at the hospital and regional levels for a wide variety of sepsis-related initiatives. As part of earlier efforts to improve KPNC sepsis-related care, lactate testing triggered by meeting systemic inflammatory response syndrome criteria and/or on the basis of blood cultures became standardized practice. Across KPNC, the use of lactate testing in the ED had already increased from an annual average of 4,886 tests between 2005 and 2007 to 42,718 tests between 2009 and 2011.
Subjects
To evaluate the impact of the intermediate lactate bundle, we conducted a retrospective study of bundle-eligible patients with sepsis aged 18 years or older admitted for nonobstetric overnight hospitalizations divided into yearly intervals including March 2011 to February 2012 (labeled “Prior” or 2011), March 2012 to February 2013 (labeled “Pre” or 2012), and March 2013 to February 2014 (labeled “Post” or 2013). We compared patient characteristics, treatments, and outcomes across these intervals. Our cohort included patients with (1) a diagnosis of “present on admission” sepsis (based on International Classification of Diseases, Ninth Revision [ICD-9], diagnostic codes 038, 995.91, and 995.92), (2) an initial lactate value greater than or equal to 2 mmol/L and less than 4 mmol/L, and (3) antibiotics that were administered in the ED and also within 12 hours of ED arrival. From among this initial population, we excluded patients who met EGDT eligibility criteria during their ED stay on the basis of manually validated data prospectively collected by local quality improvement staff using regional standards and Internet-based data tools (12).
Patient and Hospitalization Data
We linked patients with sepsis with corresponding KPNC databases using methods detailed in prior studies (17, 18). We quantified comorbid disease burden using the Comorbidity Points Score, version 2 (COPS2) (19). We quantified acute severity of illness using the Laboratory Acute Physiology Score, version 2 (LAPS2), which incorporates 15 laboratory and 6 vital sign values preceding inpatient admission into a single score that independently predicts mortality (19). We determined predicted hospital mortality on the basis of a previously developed automated hospital risk prediction model that demonstrated good discrimination in this population (c-statistic, 0.78) (17). We determined whether patients were admitted to an ICU on the basis of bed history records and grouped them within direct ICU (from ED to ICU without other transfer) or ever ICU (ICU at any time during hospitalization) categories. We grouped patients’ resuscitation care order status at hospital admission as full code or not full code (17). Because our clinicians reported concerns about the administration of aggressive fluid therapy in patients with a higher potential for iatrogenic fluid overload, we also specifically assessed whether patients had a prior history of heart failure (ICD-9 code 428) or chronic kidney disease (ICD-9 code 585). We ascertained hospital mortality from inpatient records and 30-day mortality from a combination of records, KPNC membership tables, and state and national death record files.
Bundle Elements Data
We determined compliance with bundle elements on the basis of previously established methods for analyzing electronic medical record data (12). For antibiotic measures, we captured all enteral and intravenous antibiotics and calculated the elapsed time from ED entry and time 0 to administration time. For lactate testing, we denoted the first lactate test result as the index lactate value and evaluated elapsed time to subsequent lactate tests after time 0, including those within the 1- to 4-hour window. We calculated the percentage of patients in whom repeat lactate values within 12 hours demonstrated a 10% reduction. For fluid administration, we determined the amount of fluid ordered and given from the medication administration record within relevant intervals based on methods detailed in prior studies. We also quantified weight-based fluid doses by dividing total fluid volume recorded by each patient’s most recent prehospital weight. Among patients without weight measurements, we divided total fluid volume by 70 for men and 60 for women. We defined patients as achieving full bundle compliance if they received all three bundle elements within allotted time intervals.
Statistical Analysis
Continuous data are presented as mean ± SD or median (interquartile range). Categorical data are presented as number (percentage). We compared characteristics between the yearly interval groups with analysis of variance, χ2 tests, or Kruskal–Wallis tests. We then used logistic regression to compare changes in mortality over time, first in unadjusted analyses and then adjusting for patient age, sex, LAPS2 score, COPS2 score, predicted hospital mortality, first resuscitation care order, need for direct ICU admission, index lactate value, history of heart failure, history of chronic kidney disease, month of the study period, and hospital as a random effect.
To account for potential secular changes in overall hospital practice and mortality occurring over the study period, we conducted a difference-in-difference regression including high-risk patients hospitalized through the ED (not including those identified in our primary cohort of interest). To identify high-risk inpatients, we selected patients presenting within the highest quartile of predicted mortality at hospital admission (predicted mortality ≥3.75%) and evaluated the significance of the interaction P value between intermediate lactate sepsis cohort membership and time period as well as the association between time period and mortality in high-risk inpatients. Finally, we stratified our intermediate lactate sepsis sample on the basis of the presence of heart failure and/or kidney disease and evaluated differences in bundle achievement and mortality. We conducted analyses using STATA/SE version 11.2 software (StataCorp, College Station, TX) and considered a P value less than 0.05 to be significant.
Results
Cohort Characteristics
Over the study period, we identified a total of 18,122 patients with sepsis and intermediate lactate values. Among the cohort, 36.1% were hospitalized after bundle implementation (Table 1). The patients’ mean age was 71 ± 16 years, and 51.5% (n = 9,337) of the cohort was male. Between 2011 and 2013, acute severity of illness, based on LAPS2 scores, decreased modestly while comorbid disease burden, based on COPS2 scores, increased modestly. Overall, predicted mortality was 9.5 ± 11.3% in 2011 compared with 8.9 ± 10.4% in 2013 (P < 0.01). Index lactate values also decreased slightly, from 2.7 ± 0.6 mmol/L to 2.6 ± 0.6 mmol/L (P < 0.01), over the same intervals.
Table 1.
Baseline Characteristics and Outcomes before and after Implementation of the Intermediate Lactate Bundle
| Prior (2011) | Prebundle (2012) | Postbundle (2013) | P Value | |
|---|---|---|---|---|
| Number | 5,636 | 5,942 | 6,544 | |
| Age, yr | 71 ± 16 | 71 ± 16 | 71 ± 16 | 0.99 |
| Male sex | 2,914 (51.7) | 3,093 (52.1) | 3,330 (50.9) | 0.41 |
| Body mass index | 28 ± 8 | 28 ± 8 | 28 ± 8 | 0.87 |
| LAPS2 (severity) | 108 ± 35 | 107 ± 35 | 106 ± 34 | <0.01 |
| COPS2 (comorbidity) | 55 ± 49 | 61 ± 53 | 66 ± 56 | <0.01 |
| Predicted mortality, % | 9.5 ± 11.3 | 9.3 ± 11.1 | 8.9 ± 10.4 | 0.02 |
| History of heart failure | 1,407 (25.0) | 1,449 (24.4) | 1,558 (23.8) | 0.33 |
| History of chronic kidney disease | 1,782 (31.6) | 2,070 (34.8) | 2,433 (37.2) | <0.01 |
| Index lactate value, mmol/L | 2.7 ± 0.6 | 2.6 ± 0.6 | 2.6 ± 0.6 | 0.05 |
| Direct ICU admission | 1,214 (22.1) | 1,112 (18.7) | 1,154 (17.6) | <0.01 |
| Admitted to medical service | 5,210 (92.4) | 5,480 (92.2) | 6,007 (91.8) | 0.40 |
| Full code at admission | 4,096 (72.7) | 4,387 (73.8) | 4,831 (73.8) | 0.16 |
| ED length of stay, h | 5.5 (4.3–7.3) | 5.4 (4.1–7.2) | 5.2 (4.0–7.0) | <0.01 |
| Hospital length of stay, d | 4.0 (2.6–6.7) | 3.9 (2.5–6.6) | 3.8 (2.4–6.3) | <0.01 |
| Ever in the ICU | 1,738 (30.8) | 1,643 (27.7) | 1,763 (26.9) | <0.01 |
| Hospital mortality | 498 (8.8) | 552 (9.3) | 517 (7.9) | 0.02 |
| Living, discharge to home | 3,823 (74.4) | 3,979 (73.8) | 4,564 (75.7) | 0.13 |
| 30-d mortality | 772 (13.7) | 838 (14.1) | 821 (12.6) | 0.03 |
| Readmission within 30 d | 1,090 (19.3) | 1,175 (19.8) | 1,296 (19.8) | 0.78 |
Definition of abbreviations: COPS2 = Comorbidity Points Score, version 2; ED = emergency department; ICU = intensive care unit; LAPS2 = Laboratory Acute Physiology Score, version 2.
Values are mean ± SD, median (interquartile range), or number (percentage).
Bundle Compliance
The fraction of patients receiving all elements of the bundle increased from 32.2% in 2011 to 44.9% in the postimplementation phase (P < 0.01) (Table 2 and Figure 1). Increases in bundle achievement were driven by increases in lactate goal (49.7–63.1%; P < 0.01) and fluid goal (59.6–67.1%; P < 0.01) attainment. Antibiotic goal attainment was unchanged between periods (P = 0.16); however, early antibiotics were already achieved at high rates before implementation (>95%). All secondary metrics for the lactate and fluid bundle elements differed after implementation, including a decreased mean time to index and repeat lactate values as well as an increased mean volume of fluid administered in the ED and within the first 24 hours after presentation (P < 0.01 for all).
Table 2.
Completion of Intermediate Lactate Bundle Elements, by Period
| Prior (2011) | Prebundle (2012) | Postbundle (2013) | P Value | |
|---|---|---|---|---|
| All bundle goals met, n (%) | 1,813 (32.2) | 2,006 (33.8) | 2,938 (44.9) | <0.01 |
| Antibiotic goals met, n (%) | 5,391 (95.7) | 5,656 (95.2) | 6,275 (95.9) | 0.16 |
| Mean ± SD time to antibiotics after lactate, h | 0.7 ± 1.1 | 0.8 ± 1.1 | 0.7 ± 1.1 | 0.01 |
| Mean ± SD time to antibiotics after ED entry, h | 2.4 ± 1.6 | 2.4 ± 1.5 | 2.3 ± 1.5 | 0.04 |
| Lactate goals met, n (%) | 2,800 (49.7) | 3,015 (50.7) | 4,132 (63.1) | <0.01 |
| Mean ± SD time to first lactate, h | 2.0 ± 1.4 | 1.9 ± 2.1 | 1.9 ± 1.5 | <0.01 |
| Mean ± SD time to second lactate from first lactate, h | 4.5 ± 2.6 | 4.4 ± 2.7 | 3.8 ± 2.5 | <0.01 |
| >10% lactate reduction within 12 h, n (%) | 4,518 (80.2) | 4,732 (79.6) | 5,391 (82.4) | <0.01 |
| Fluid goals met, n (%) | 3,359 (59.6) | 3,571 (60.1) | 4,391 (67.1) | <0.01 |
| Mean ± SD MAR fluid total within 3 h of lactate, L | 1.8 ± 1.3 | 1.8 ± 1.2 | 1.9 ± 1.2 | <0.01 |
| Mean ± SD MAR fluid total within 3 h, ml/kg | 24.3 ± 18.6 | 24.6 ± 18.0 | 26.3 ± 17.8 | <0.01 |
| Mean ± SD MAR fluid total over 24 h, L | 2.6 ± 1.7 | 2.6 ± 1.7 | 2.7 ± 1.7 | <0.01 |
Definition of abbreviations: ED = emergency department; MAR = medication administration record.
P values are based on χ2 tests or analysis of variance.
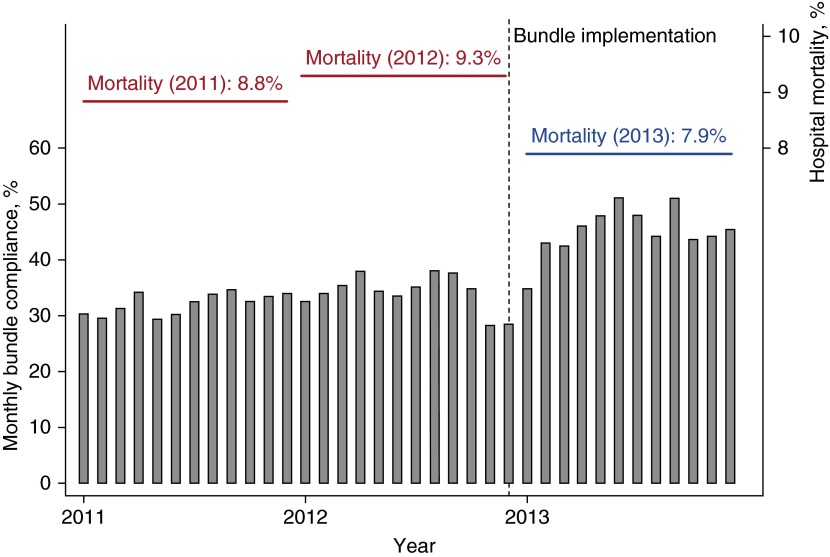
Figure 1.
Monthly full bundle compliance before and after implementation and hospital mortality rates by implementation period. The year markers along the x-axis indicate March of that year.
Outcomes
Hospital mortality was 8.8% in 2011, 9.3% in 2012, and 7.9% in 2013 (P = 0.02) (Table 1). Thirty-day mortality also decreased after implementation (P = 0.03). In unadjusted analysis, treatment in the year after bundle implementation was associated with odds ratios of 0.86 (95% CI, 0.77–0.97; P < 0.01) for hospital mortality and 0.89 (95% CI, 0.81–0.97; P = 0.01) for 30-day mortality (Table 3). In fully adjusted regression, treatment after bundle implementation was associated with an odds ratio of 0.79 (95% CI, 0.65–0.96; P = 0.02). In the fully adjusted model, the odds ratio of 30-day mortality was 0.84 (95% CI, 0.71–0.99).
Table 3.
Analysis of Hospital Mortality before and after Implementation
| Variable | Unadjusted | Multivariable |
|---|---|---|
| Period | ||
| Preimplementation | Reference | Reference |
| Postimplementation | 0.86 (0.77–0.96) | 0.79 (0.65–0.96) |
| P value | <0.01 | 0.02 |
| Age, per decade | — | 1.16 (1.11–1.22) |
| Male sex | — | 1.01 (0.91–1.13) |
| LAPS2 score, per 20 points | — | 1.10 (1.08–1.12) |
| COPS2 score, per 20 points | — | 1.01 (1.00–1.01) |
| Predicted mortality, per 10% | 1.14 (1.05–1.25) | |
| Direct ICU transfer | — | 1.60 (1.38–1.80) |
| Full code at admission | — | 0.71 (0.62–0.82) |
| History of heart failure | — | 1.28 (1.14–1.46) |
| History of kidney disease | — | 0.76 (0.68–0.86) |
| Index lactate value, by 1 mmol/L | — | 1.16 (1.05–1.28) |
| Month of study period | — | 1.00 (1.00–1.00) |
Definition of abbreviations: COPS2 = Comorbidity Points Score, version 2; ICU = intensive care unit; LAPS2 = Laboratory Acute Physiology Score, version 2.
Data are shown as odds ratios and 95% confidence intervals derived from univariable and multivariable logistic regression analyses.
The odds of hospital mortality were identical among high-risk inpatients in the year after bundle implementation compared with before implementation (see Table E2 in the online supplement) (odds ratio, 1.00; 95% CI, 0.92–1.09; P = 0.94). The interaction P value in the difference-in-difference regression was significant (P = 0.03), indicating that the implementation period had a differential impact on mortality among the sepsis cohort of interest compared with general high-risk inpatients.
Subpopulations
After stratifying patients by their prior history of either heart failure or kidney disease, patients with a prior history of either condition demonstrated statistically significant improvements in hospital and 30-day mortality in the postimplementation period (P < 0.01 for all) (Table 4). In contrast, when we evaluated only patients with no prior history of heart failure or kidney disease, we found that bundle implementation was not associated with a reduction in mortality rates (P ≥ 0.40 for all). The rates of compliance with antibiotic timing and lactate reassessment were similar between these patient groups before and after implementation.
Table 4.
Hospital Mortality in Heart Failure and Chronic Kidney Disease Subgroups
| Mortality (%) |
|||||
|---|---|---|---|---|---|
| n | Prior (2011) | Prebundle (2012) | Postbundle (2013) | P Value | |
| All patients | 18,122 | ||||
| Hospital | 8.8 | 9.3 | 7.9 | 0.02 | |
| 30 d | 13.7 | 14.1 | 12.6 | 0.03 | |
| History of heart failure | 4,144 | ||||
| Hospital | 13.0 | 14.8 | 11.6 | 0.03 | |
| 30 d | 18.8 | 20.7 | 17.8 | 0.13 | |
| History of kidney disease | 6,285 | ||||
| Hospital | 9.7 | 11.5 | 7.5 | <0.01 | |
| 30 d | 15.9 | 17.7 | 13.3 | <0.01 | |
| Heart failure or kidney disease | 8,322 | ||||
| Hospital | 10.7 | 12.5 | 8.7 | <0.01 | |
| 30 d | 16.8 | 18.3 | 14.5 | <0.01 | |
| No heart failure or kidney disease | 9,800 | ||||
| Hospital | 7.4 | 6.5 | 7.2 | 0.40 | |
| 30 d | 11.3 | 10.5 | 10.8 | 0.60 | |
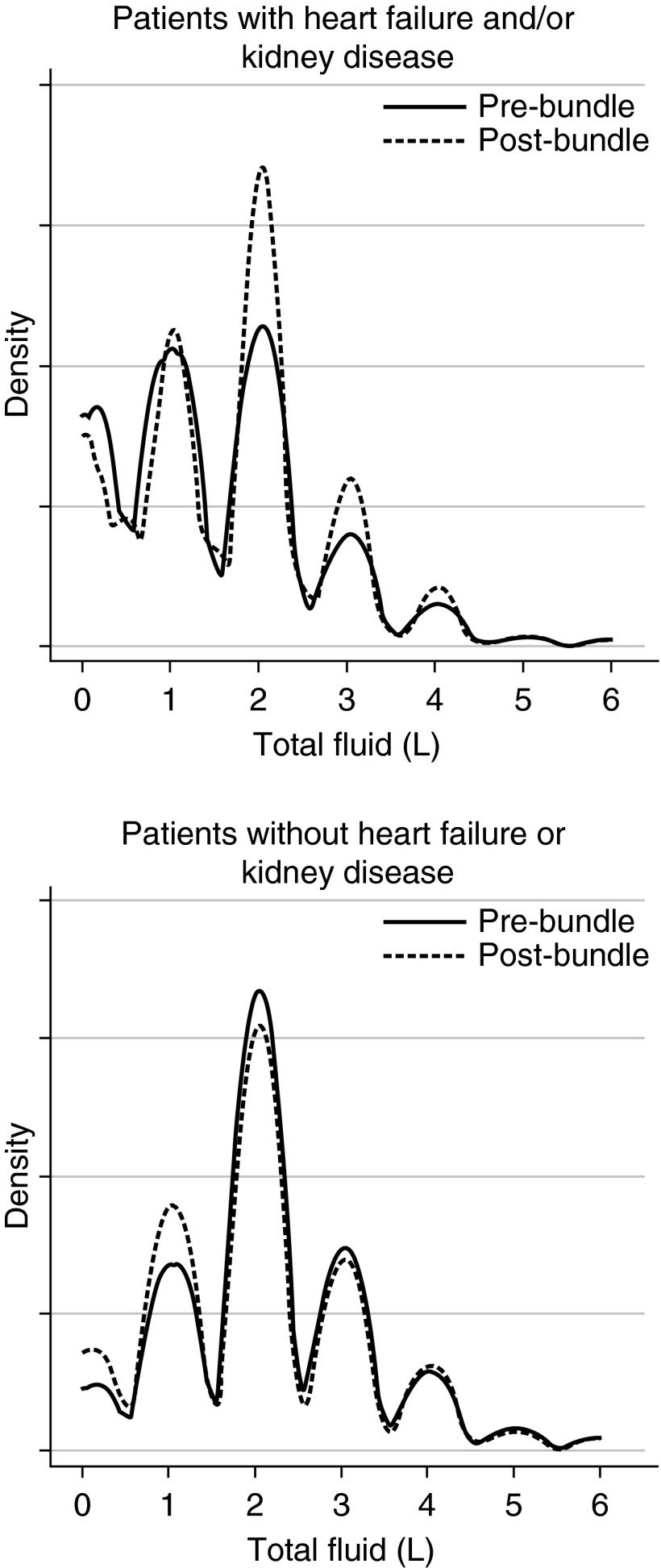
However, compliance with fluid administration targets differed substantially between patients with and without a history of heart failure or kidney disease (Figure E1). For example, even before implementation, 69.9% of patients without heart failure or kidney disease history already met the bundle fluid targets. Among patients with heart failure and/or kidney disease, bundle implementation was associated with substantial increases in total fluid administration (Figure 2), with an increase in mean fluid totals from 1.4 to 1.7 L (P < 0.01). At the same time, implementation was not associated with increased hospital length of stay or the need for immediate or late transfer to the ICU.
Figure 2.
Kernel density plot showing the distribution of fluids administered, based on medication administration records, to patients stratified by their history of heart failure or kidney disease before and after bundle implementation. The top panel shows patients with a history of heart failure and/or kidney disease, and the bottom panel shows patients without such history. The solid lines represent estimated densities before bundle implementation, and the dashed lines represent estimated densities after bundle implementation.
Discussion
In this report, we describe changes in practices and outcomes following a multicenter implementation of a process-oriented bundle for hemodynamically stable patients with sepsis and intermediate lactate values in the ED. Compared with patients in the 2 years preceding implementation, patients treated in the year after implementation achieved a modest increase in the attainment of all bundle metrics. This increase occurred in concert with an increased frequency of repeat lactate testing and with larger volumes of fluid administration. Over the same period, mortality rates fell substantially, such that patients in the early implementation period had a 19% reduction in their odds of hospital death, as well as reduced odds of death at 30 days with borderline significance. Importantly, these improvements appeared to be driven primarily by increased fluid administration and decreased mortality in patients with a history of heart failure and/or chronic kidney disease.
Sepsis has been called a hidden public health disaster because of its deleterious impact on short- and long-term patient health (20, 21). Prior studies have been focused primarily on improving treatment for the most severely ill patients with sepsis—those with shock and/or lactate values greater than or equal to 4 mmol/L—a group whose hospital mortality rates exceed 20–30% (4–8). To our knowledge, this is the first study to describe multicenter implementation of a therapeutic approach for patients with less severe sepsis and intermediate lactate values. Such patients have lower mortality rates than those with septic shock; however, their hospital and 30-day mortality rates were substantially higher than those of the general hospital population (12, 15). In single-center studies, others have reported that ED patients with infection and intermediate lactate values had hospital mortality rates between 9% and 10% (14, 16). Mikkelsen and others also reported a substantially increased risk of 28-day mortality among patients with nonshock sepsis with intermediate lactate values (13). Also, while they have lower mortality rates than septic shock patients, patients with sepsis and intermediate lactate values contribute to a nearly equivalent number of overall hospital deaths (1).
Despite this, there are no broadly implemented treatment guidelines for patients with sepsis and intermediate lactate values (15). Because most of these patients are treated outside critical care settings, they are also less likely to receive standardized care approaches (12, 14). As a result, we sought to implement a standardized treatment strategy that incorporated the principles of effective sepsis care defined in prior studies. First, we adapted the Surviving Sepsis Campaign’s 3-hour bundle focused on process-oriented elements, including prompt administration of antibiotics, early reassessment of mortality risk with repeat lactate testing, and aggressive fluid administration and volume resuscitation (3). Simultaneously, we implemented an improvement process that incorporated multidisciplinary team communication and education, standardized data collection and measurement, and low latency feedback to facilitate continuous performance improvement (22).
Our results demonstrate, for the first time to our knowledge, that quality improvement efforts in the patients with intermediate lactate values successfully improved bundle compliance and were also associated with improvements in mortality. These findings are concordant with many prior studies that emphasized the importance of improving sepsis care through coordinated quality improvement initiatives (3, 9–11, 23). At the same time, we were surprised to discover that these improvements appeared to be mediated by decreased mortality in the sepsis population with a history of underlying heart failure and/or kidney disease. In the process of bundle design and implementation, many of our clinicians raised concerns that increasing fluid administration in patients at high risk for fluid overload could result in iatrogenic complications, including increases in the need for positive pressure ventilation or intensive care and in overall length of stay.
To assess whether bundle implementation could result in harm to patients, we prospectively evaluated our balancing measures (length of stay, mortality, and the need for immediate or delayed critical care) at 6 months and 1 year after implementation in this subpopulation. We did not identify an increase in adverse events following implementation, despite increases in the volume of early fluid administration. Few prior studies have specifically addressed fluid resuscitation targets in patients with conditions commonly associated with fluid overload and in those for whom clinicians tend to be cautious about overly aggressive fluid administration. Unlike patients with septic shock, for example, who are frequently cared for in critical care units with close monitoring of respiratory status, the vast majority of patients with intermediate lactate values are treated in medical-surgical wards with only intermittent monitoring (12). Our findings suggest that these subpopulations deserve further consideration and study, efforts that are likely to offer important guidance to clinicians making decisions about fluid resuscitation and triage from the ED.
Recent evidence derived from large, multicenter randomized controlled trials in patients with septic shock or lactate values greater than or equal to 4 mmol/L demonstrate that mandatory central line–based treatment strategies do not improve outcomes compared with standard approaches incorporating early and aggressive sepsis care (5, 6). As a result, we expect that the prior discontinuities in practice that resulted from arbitrary lactate value cutoffs are likely to become less relevant for designing treatment approaches. In light of this new evidence, we have initiated a more uniform approach to sepsis care, regardless of whether patients’ index lactate values are in the intermediate or higher range. Nonetheless, the use of lactate values for screening as well as initial and dynamic risk prognostication will continue to be a cornerstone of our treatment approach (12, 13, 16, 24, 25).
Our study should be interpreted in light of its limitations. First, this study was a retrospective study, making our findings vulnerable to commonly discussed biases and confounding. At the same time, our study also includes an extremely large and contemporary cohort of patients drawn from among a multicenter sample, which benefits from sophisticated risk adjustment methodology. Second, this study was conducted in the integrated healthcare delivery system of KPNC, which already had a mature sepsis-related quality improvement infrastructure. This allowed for relatively rapid dissemination of implementation measures and performance feedback, which may differ from the situation in other hospitals or systems (22, 26). Finally, the intermediate lactate bundle implemented in this study was adapted from Surviving Sepsis Campaign guidelines but has not been established in a randomized controlled trial as effective for this less severe sepsis population. Future randomized controlled studies should be undertaken to establish the efficacy of this approach, especially in subgroups at higher risk for iatrogenic volume overload.
In conclusion, multicenter implementation of a process-oriented bundle for patients with sepsis and intermediate lactate values successfully improved bundle compliance. Over the same period, there were marked interval decreases in hospital and 30-day mortality. These decreases appeared to be mediated primarily by increased fluid administration and improved survival among patients with underlying heart failure or chronic kidney disease. These findings should be evaluated in future randomized controlled trials.
Acknowledgments
Acknowledgment
The authors are deeply grateful for the contributions of hundreds of clinicians and support staff engaged in ongoing sepsis performance improvement work across Kaiser Permanente Northern California.
Footnotes
Supported by The Permanente Medical Group and Kaiser Foundation Hospitals; also supported by National Institute of General Medical Sciences grant K23GM112018 (V.X.L.).
Author Contributions: Study conception and design: V.X.L., J.W.M., M.S., G.J.E., and A.W.; acquisition, analysis, or interpretation of data: V.X.L., J.W.M., G.P.M., J.S., T.R., C.A., G.J.E., and A.W.; drafting and revision of the work as well as final approval of the submitted manuscript: all authors.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.201507-1489OC on December 22, 2015
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Liu V, Escobar GJ, Greene JD, Soule J, Whippy A, Angus DC, Iwashyna TJ. Hospital deaths in patients with sepsis from 2 independent cohorts. JAMA. 2014;312:90–92. doi: 10.1001/jama.2014.5804. [DOI] [PubMed] [Google Scholar]
- 2.Elixhauser A, Friedman B, Stranges E. Septicemia in U.S. hospitals, 2009. HCUP Statistical Brief #122. Rockville, MD: Agency for Healthcare Research and Quality; October 2011 [accessed 2015 Dec 31]. Available from: http://www.hcup-us.ahrq.gov/reports/statbriefs/sb122.pdf.
- 3.Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, Sevransky JE, Sprung CL, Douglas IS, Jaeschke R, et al. Surviving Sepsis Campaign Guidelines Committee including the Pediatric Subgroup. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med. 2013;41:580–637. doi: 10.1097/CCM.0b013e31827e83af. [DOI] [PubMed] [Google Scholar]
- 4.Rivers E, Nguyen B, Havstad S, Ressler J, Muzzin A, Knoblich B, Peterson E, Tomlanovich M Early Goal-Directed Therapy Collaborative Group. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345:1368–1377. doi: 10.1056/NEJMoa010307. [DOI] [PubMed] [Google Scholar]
- 5.Yealy DM, Kellum JA, Huang DT, Barnato AE, Weissfeld LA, Pike F, Terndrup T, Wang HE, Hou PC, LoVecchio F, et al. ProCESS Investigators. A randomized trial of protocol-based care for early septic shock. N Engl J Med. 2014;370:1683–1693. doi: 10.1056/NEJMoa1401602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peake SL, Delaney A, Bailey M, Bellomo R, Cameron PA, Cooper DJ, Higgins AM, Holdgate A, Howe BD, Webb SA, et al. ARISE Investigators; ANZICS Clinical Trials Group. Goal-directed resuscitation for patients with early septic shock. N Engl J Med. 2014;371:1496–1506. doi: 10.1056/NEJMoa1404380. [DOI] [PubMed] [Google Scholar]
- 7.Mouncey PR, Osborn TM, Power GS, Harrison DA, Sadique MZ, Grieve RD, Jahan R, Harvey SE, Bell D, Bion JF, et al. ProMISe Trial Investigators. Trial of early, goal-directed resuscitation for septic shock. N Engl J Med. 2015;372:1301–1311. doi: 10.1056/NEJMoa1500896. [DOI] [PubMed] [Google Scholar]
- 8.Peake SL, Delaney A, Bellomo R ARISE Investigators. Goal-directed resuscitation in septic shock. N Engl J Med. 2015;372:190–191. doi: 10.1056/NEJMc1413936. [DOI] [PubMed] [Google Scholar]
- 9.Miller RR, III, Dong L, Nelson NC, Brown SM, Kuttler KG, Probst DR, Allen TL, Clemmer TP Intermountain Healthcare Intensive Medicine Clinical Program. Multicenter implementation of a severe sepsis and septic shock treatment bundle. Am J Respir Crit Care Med. 2013;188:77–82. doi: 10.1164/rccm.201212-2199OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ferrer R, Artigas A, Levy MM, Blanco J, González-Díaz G, Garnacho-Montero J, Ibáñez J, Palencia E, Quintana M, de la Torre-Prados MV Edusepsis Study Group. Improvement in process of care and outcome after a multicenter severe sepsis educational program in Spain. JAMA. 2008;299:2294–2303. doi: 10.1001/jama.299.19.2294. [DOI] [PubMed] [Google Scholar]
- 11.Levy MM, Dellinger RP, Townsend SR, Linde-Zwirble WT, Marshall JC, Bion J, Schorr C, Artigas A, Ramsay G, Beale R, et al. Surviving Sepsis Campaign. The Surviving Sepsis Campaign: results of an international guideline-based performance improvement program targeting severe sepsis. Crit Care Med. 2010;38:367–374. doi: 10.1097/CCM.0b013e3181cb0cdc. [DOI] [PubMed] [Google Scholar]
- 12.Liu V, Morehouse JW, Soule J, Whippy A, Escobar GJ. Fluid volume, lactate values, and mortality in sepsis patients with intermediate lactate values. Ann Am Thorac Soc. 2013;10:466–473. doi: 10.1513/AnnalsATS.201304-099OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mikkelsen ME, Miltiades AN, Gaieski DF, Goyal M, Fuchs BD, Shah CV, Bellamy SL, Christie JD. Serum lactate is associated with mortality in severe sepsis independent of organ failure and shock. Crit Care Med. 2009;37:1670–1677. doi: 10.1097/CCM.0b013e31819fcf68. [DOI] [PubMed] [Google Scholar]
- 14.Song YH, Shin TG, Kang MJ, Sim MS, Jo IJ, Song KJ, Jeong YK. Predicting factors associated with clinical deterioration of sepsis patients with intermediate levels of serum lactate. Shock. 2012;38:249–254. doi: 10.1097/SHK.0b013e3182613e33. [DOI] [PubMed] [Google Scholar]
- 15.Puskarich MA, Illich BM, Jones AE. Prognosis of emergency department patients with suspected infection and intermediate lactate levels: a systematic review. J Crit Care. 2014;29:334–339. doi: 10.1016/j.jcrc.2013.12.017. [DOI] [PubMed] [Google Scholar]
- 16.Shapiro NI, Howell MD, Talmor D, Nathanson LA, Lisbon A, Wolfe RE, Weiss JW. Serum lactate as a predictor of mortality in emergency department patients with infection. Ann Emerg Med. 2005;45:524–528. doi: 10.1016/j.annemergmed.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 17.Escobar GJ, Gardner MN, Greene JD, Draper D, Kipnis P. Risk-adjusting hospital mortality using a comprehensive electronic record in an integrated health care delivery system. Med Care. 2013;51:446–453. doi: 10.1097/MLR.0b013e3182881c8e. [DOI] [PubMed] [Google Scholar]
- 18.Escobar GJ, Greene JD, Scheirer P, Gardner MN, Draper D, Kipnis P. Risk-adjusting hospital inpatient mortality using automated inpatient, outpatient, and laboratory databases. Med Care. 2008;46:232–239. doi: 10.1097/MLR.0b013e3181589bb6. [DOI] [PubMed] [Google Scholar]
- 19.Escobar GJ, LaGuardia JC, Turk BJ, Ragins A, Kipnis P, Draper D. Early detection of impending physiologic deterioration among patients who are not in intensive care: development of predictive models using data from an automated electronic medical record. J Hosp Med. 2012;7:388–395. doi: 10.1002/jhm.1929. [DOI] [PubMed] [Google Scholar]
- 20.Angus DC. The lingering consequences of sepsis: a hidden public health disaster? JAMA. 2010;304:1833–1834. doi: 10.1001/jama.2010.1546. [DOI] [PubMed] [Google Scholar]
- 21.Iwashyna TJ, Ely EW, Smith DM, Langa KM. Long-term cognitive impairment and functional disability among survivors of severe sepsis. JAMA. 2010;304:1787–1794. doi: 10.1001/jama.2010.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Whippy A, Skeath M, Crawford B, Adams C, Marelich G, Alamshahi M, Borbon J. Kaiser Permanente’s performance improvement system, part 3: multisite improvements in care for patients with sepsis. Jt Comm J Qual Patient Saf. 2011;37:483–493. doi: 10.1016/s1553-7250(11)37061-4. [DOI] [PubMed] [Google Scholar]
- 23.Cooke CR, Iwashyna TJ. Sepsis mandates: improving inpatient care while advancing quality improvement. JAMA. 2014;312:1397–1398. doi: 10.1001/jama.2014.11350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jansen TC, van Bommel J, Schoonderbeek FJ, Sleeswijk Visser SJ, van der Klooster JM, Lima AP, Willemsen SP, Bakker J LACTATE study group. Early lactate-guided therapy in intensive care unit patients: a multicenter, open-label, randomized controlled trial. Am J Respir Crit Care Med. 2010;182:752–761. doi: 10.1164/rccm.200912-1918OC. [DOI] [PubMed] [Google Scholar]
- 25.Jones AE, Shapiro NI, Trzeciak S, Arnold RC, Claremont HA, Kline JA Emergency Medicine Shock Research Network (EMShockNet) Investigators. Lactate clearance vs central venous oxygen saturation as goals of early sepsis therapy: a randomized clinical trial. JAMA. 2010;303:739–746. doi: 10.1001/jama.2010.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu V, Greene JD, Baker JM, Skeath M, Whippy A, Escobar GJ. System-level changes in sepsis practices following implementation of a sepsis quality improvement program [abstract] Am J Respir Crit Care Med. 2015;191:A4003. [Google Scholar]