Abstract
Rationale: Pulmonary sarcoidosis is classically defined by T-helper (Th) cell type 1 inflammation (e.g., IFN-γ production by CD4+ effector T cells). Recently, IL-17A–secreting cells have been found in lung lavage, invoking Th17 immunity in sarcoidosis. Studies also identified IL-17A–secreting cells that expressed IFN-γ, but their abundance as a percentage of total CD4+ cells was either low or undetermined.
Objectives: Based on evidence that Th17 cells can be polarized to Th17.1 cells to produce only IFN-γ, our goal was to determine whether Th17.1 cells are a prominent source of IFN-γ in sarcoidosis.
Methods: We developed a single-cell approach to define and isolate major Th-cell subsets using combinations of chemokine receptors and fluorescence-activated cell sorting. We subsequently confirmed the accuracy of subset enrichment by measuring cytokine production.
Measurements and Main Results: Discrimination between Th17 and Th17.1 cells revealed very high percentages of Th17.1 cells in lung lavage in sarcoidosis compared with controls in two separate cohorts. No differences in Th17 or Th1 lavage cells were found compared with controls. Lung lavage Th17.1-cell percentages were also higher than Th1-cell percentages, and approximately 60% of Th17.1-enriched cells produced only IFN-γ.
Conclusions: Combined use of surface markers and functional assays to study CD4+ T cells in sarcoidosis revealed a marked expansion of Th17.1 cells that only produce IFN-γ. These results suggest that Th17.1 cells could be misclassified as Th1 cells and may be the predominant producer of IFN-γ in pulmonary sarcoidosis, challenging the Th1 paradigm of pathogenesis.
Keywords: lymphocyte, chemokine receptor, inflammation
At a Glance Commentary
Scientific Knowledge on the Subject
The idea that T-helper (Th) type 1 cells play a key role in sarcoidosis is largely based on experimental observations of increased numbers of CD4+ effector T cells in sarcoidosis bronchoalveolar lavage fluid that produce IFN-γ. Th17 cells have also been invoked in sarcoidosis immunity and can be polarized into a Th1-like phenotype, whereby they can produce IFN-γ and exhibit pathogenic characteristics. Prior studies have not measured the prevalence of Th17 cells that produce only IFN-γ and, therefore, these cells could have been misclassified as Th1 cells based on the immunophenotyping methods previously used.
What This Study Adds to the Field
Applying robust single-cell phenotyping methods to define Th subsets using more discerning criteria on paired blood and lung samples from patients with sarcoidosis, this study found that Th17.1 cells (and not Th1 cells) were the major producers of IFN-γ in sarcoidosis lung lavage. Validation of these findings in a second cohort challenges prior assumptions of a Th1 paradigm in disease pathogenesis and may provide new directions for clinical studies.
Sarcoidosis is a granulomatous disease in which the inflammation is thought to be driven in part by activated T-helper (Th) type 1 effector T cells (1–4). The idea that Th1 cells play a key role in sarcoidosis is largely based on experimental observations of increased numbers of CD4+ effector T cells, which produce IFN-γ, in sarcoidosis bronchoalveolar lavage (BAL) fluid (3, 5–7). After the recent discovery of a new class of Th cells, Th17 cells, several investigators have used a variety of flow cytometry gating strategies to identify IL-17A–producing cells in BAL fluid from patients with sarcoidosis (8–13). Several of these studies also identified IL-17A–secreting cells that expressed IFN-γ, but their abundance as a percentage of total CD4+ cells was either low or undetermined (9, 11, 12).
Recent studies in Crohn’s disease identified a subset of IFN-γ–producing Th17 cells, called Th17.1 cells. This subset expressed a specific pattern of chemokine receptors and exhibited functional attributes indicating that they were proinflammatory and resistant to corticosteroids (14). Therefore, identification of these cells in sarcoidosis would have important implications for treatment approaches.
Th17.1 cells are thought to be derived from classically polarized Th17 cells. Many studies show that Th17 cells are plastic and can be polarized to differentiate into a Th1-like phenotype in which they produce significant IFN-γ (15–19). In vitro experiments have shown that cytokines prevalent in sarcoidosis, IFN-γ and IL-12, promote this transformation (18). The nomenclature for this “Th1-polarized Th17 subset” is not uniform, and these cells have been referred to as Th17/Th1 (20, 21), Th1/17 (22), and Th17.1 cells to capture their transformed state (14). We refer to this Th17 subset as Th17.1 to be consistent with prior studies that used chemokine receptor expression as part of their definition for these cells (14, 23). Because the majority of Th17.1 cells produce only IFN-γ, we hypothesized that Th17.1 cells have largely been misclassified as Th1 cells because measurement of cytokine production has been the usual method for defining Th1 and Th17 cells. For example, production of IL-17A has been used to define “Th17” cells (8–13), and therefore the proportions of Th17 cells that produced only IFN-γ would be completely missed.
To address whether Th17.1 cells could be a predominant source of IFN-γ in pulmonary sarcoidosis, we used definitions for Th cells based on the latest immunology (14), which consisted of a combination of three chemokine receptors, CCR4, CCR6, and CXCR3. We first applied single-cell sorting techniques using chemokine receptor expression to isolate cells from paired blood and lung samples from sarcoidosis and controls. We then confirmed appropriate cytokine secretion in the sorted and enriched populations of Th-cell subsets. These techniques allowed for a high degree of cell separation in which to study Th subsets (and subsets within subsets) and make new observations in sarcoidosis, such as finding that IFN-γ–producing Th17.1 cells are the predominant effector cell in sarcoidosis BAL in two separate cohorts.
Methods
Subjects
Participants in the U.S. cohort underwent written informed consent and the study was approved by the University of California, San Francisco Committee on Human Research. Sarcoidosis diagnosis was based on consistent clinical features, absence of alternative diagnoses, and biopsy of the lung or mediastinal lymph nodes showing noncaseating granulomas according to accepted criteria (24). Exclusion criteria included a smoking history, cancer, chronic infections, autoimmune diseases, other pulmonary diseases, or organ transplant. Subjects underwent chest X-ray, high-resolution chest computed tomography (CT) scan, BAL, and blood collection. Noncontrast axial images (1.25 mm) were obtained supine during full inspiration for a 10-second breath hold. Imaging protocol was defined by the National Institutes of Health (NIH) study (NCT01831739). Organ involvement was determined as described previously (25). Healthy control data were obtained from a concurrent study (NCT01484691) to measure the same immunological parameters.
The validation cohort, referred to as the Erasmus MC cohort, consisted of European patients newly diagnosed with pulmonary sarcoidosis using the same diagnostic and exclusionary criteria (24). In addition, patients could not be taking immunomodulatory medication in the 3 months before enrollment; however, a smoking history was accepted. The control group consisted of individuals who underwent bronchoscopy for community-acquired pneumonia or chronic obstructive pulmonary disease. The Medical Ethics Committee of the Erasmus MC (Rotterdam, the Netherlands) approved this study.
BAL and Peripheral Blood Mononuclear Cells
The bronchoscopy protocol with BAL was developed by the NIH study, Genomic Research in Alpha-1 Antitrypsin Deficiency and Sarcoidosis (NCT01831739). Cells were resuspended in 0.1% bovine serum albumin plus 2 mM ethylenediaminetetraacetic acid in phosphate-buffered saline (PBS) and immediately processed for flow cytometry. Peripheral blood mononuclear cells (PBMCs) were isolated as described previously (26).
Flow Cytometry and Sorting
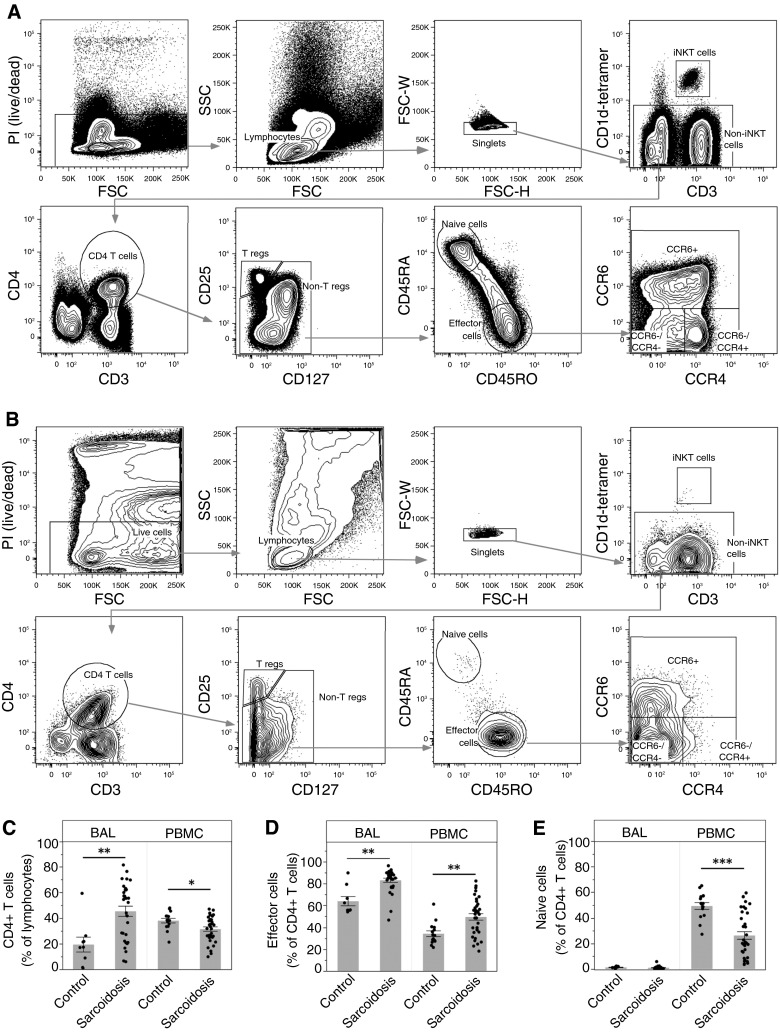
For surface staining, BAL cells and PBMCs were incubated with fluorescent antibodies (CD3 [BD Horizon, San Jose, CA], CCR4 [BD Pharmingen, San Jose, CA], CD127, CD4, CD25, and CCR6 [eBioscience, San Diego, CA], and CD45RO, CD45RA, and CXCR3 [Biolegend, San Diego, CA]), and CD1d-tetramer-PBS57 [NIH Tetramer Facility, Atlanta, GA]) for 30 minutes at 4°C using methods recommended by the manufacturers. Viability was measured using propidium iodide (BD Biosciences, San Jose, CA). We used the Aria II fluorescence-activated cell sorter (FACS) to isolate CCR4- and CCR6-positive and -negative cells (BD Biosciences). FACS sorting was performed using a number of gating strategies: we gated on live cells, followed by lymphocytes, singlets, noninvariant natural killer T cells, CD4+ T cells, nonregulatory T cells, and effector cells (Figures 1A and 1B). CCR6+, CCR4+CCR6−, and CCR4−CCR6− effector cells were sorted into separate chambers to analyze cytokine function. Figures 1A and 1B show the three-gate sorting strategy. After discovery of the high prevalence of IFN-γ–producing CCR6+ cells, a fourth gate was added to sort effector T cells into four chambers based on chemokine expression: CCR4+CCR6+, CCR4+CCR6−, CCR4−CCR6+, and CCR4−CCR6−. CXCR3 was used in the FACS-sorting panel and functional panel to allow more stringent gating of Th17, Th17.1, Th1, and Th2 populations as defined in Table 1 and Figure E1 in the online supplement, and as previously described (14). Samples from the European cohort were analyzed similarly (see the online supplement).
Figure 1.
Flow cytometry sorting strategy and comparative analysis of CD4+ T-cell subsets. Peripheral blood mononuclear cells (PBMCs) and bronchoalveolar lavage (BAL) cells were stained and analyzed by an LSRII Fortessa cytometer (BD Biosciences). (A and B) Representative sample is shown for blood (A) and BAL cells (B) from a patient with sarcoidosis. Lymphocytes were analyzed using the following series of subgates: live cells, lymphocytes, singlets, non–invariant natural killer (iNKT), CD4 T cells (CD4+CD3+), nonregulatory T cells (CD4+CD127+CD25− T cells), memory effector cells (CD45RA−/CD45RO+ CD4 T cells), and three subpopulations of memory effector cells using CCR4 and CCR6. We excluded iNKT cells (CD1d tetramer-PBS57), regulatory T cells (CD4+CD3+CD127−CD25+), and naive T cells (CD4+CD3+CD45RA+CD45RO−) from the CD4+ population. (C) CD4+ T cells as a percentage of lymphocytes are significantly increased in the lungs of patients with sarcoidosis (**P = 0.0019), and decreased in the blood (*P = 0.012). (D) Effector cells as a percentage of CD4+ T cells are significantly increased in both the lungs (**P = 0.0015) and the blood (**P = 0.0004) of patients with sarcoidosis. (E) There are essentially no naive cells in the lungs, and a significant decrease of naive cells as a percentage of CD4+ T cells in the blood of patients with sarcoidosis (***P < 0.0001). Data are expressed as mean (histogram bars) ± SEM. Overlaid dots represent individual patient values. BAL, n = 32 subjects with sarcoidosis and 9 control subjects; PBMC, n = 35 subjects with sarcoidosis and 18 control subjects. FSC = forward scatter; PI = propidium iodide; SSC = side scatter; T regs = T regulatory cells.
Table 1.
T Effector Cell Subset Phenotypes
| CCR4−/CXCR3+ | CCR4+/CXCR3− | |
|---|---|---|
| CCR6− | Th1 | Th2 |
| CCR6+ | Th17.1 | Th17 |
Definition of abbreviation: Th = T helper.
Cell Stimulation and Cytokine Analysis
Freshly isolated cells were stimulated at 37°C for 4 hours in 10 nM phorbol 12-myristate 13-acetate, 1 μM ionomycin, and 5 μg/ml brefeldin A. Antibody staining against CXCR3 (eBioscience), and live/dead Amcyan stain (Invitrogen, Grand Island, NY) was performed for 30 minutes at 4°C. Cells were fixed in 2% paraformaldehyde and permeabilized (BD Biosciences) for intracellular cytokine staining of IL-17A, IL-4, and IFN-γ (eBioscience). Additional details are provided in the online supplement.
Statistical Analysis
FlowJo 9 (FlowJo, Ashland, OR), JMP 10 (SAS Institute, Cary, NC), and GraphPad Prism 6 (GraphPad Software, San Diego, CA) software were used. Normality was measured using Shapiro-Wilk test. Two group comparisons were made using an unpaired Student’s t test or rank sum test as appropriate. Chi-square analysis was used to assess sex, race, and ethnicity differences, and Student’s t test for age. A P value less than 0.05 was considered significant.
Results
Patient Characteristics
A total of 35 patients with sarcoidosis and 18 healthy control subjects were enrolled in the U.S. cohort. Patients with sarcoidosis had pulmonary disease (Table 2) and met criteria for chronic disease (lack of disease resolution by 2 yr after diagnosis [27]). There were no significant differences in sex between groups; however, the control population was younger, on average. Both groups consisted of more white than African American subjects or those of other races. Six patients with sarcoidosis were on immunosuppression for progressive pulmonary disease at enrollment. Three patients were taking 20 mg or less of prednisone daily; two patients were taking 15 mg of methotrexate weekly, and one of these was also taking infliximab. One patient was taking 50 mg of azathioprine daily. We found no significant differences between T-cell profiles of these six patients compared with those not taking immunosuppression. Fewer subjects were stage I compared with stages II/III and IV. Fibrosis was confirmed in stage IV patients by CT scanning. There was a trend in decreasing lung function with increasing stage (Table 3). Average BAL fluid return was 114 ml (60–169 ml) with 77% (69–81%) alveolar macrophages, 22% (17–30%) lymphocytes, and 1% (0–3%) neutrophils.
Table 2.
Demographics and Clinical Characteristics
| Control | Sarcoidosis | P Value | |
|---|---|---|---|
| n | 18 | 35 | |
| Female | 9 (50) | 16 (46) | 0.78 |
| Age, median (range), yr | 30 (25–54) | 53 (32–69) | <0.01 |
| Immunosuppression | — | 6 (17) | — |
| Stage, I/II–III/IV | — | 5/17/13 | — |
| Lung/heart/LN/eye/skin | 35/2/4/1/2 | ||
| Race | |||
| African American | 3 (17) | 3 (9) | 0.39 |
| White | 11 (61) | 31 (89) | 0.02 |
| Other | 4 (22) | 1 (3) | 0.03 |
| Ethnicity | |||
| Hispanic | 1 (6) | 2 (6) | 0.98 |
Definition of abbreviation: LN = lymph node.
Data are shown as n (%) unless otherwise stated.
Table 3.
Clinical Characteristics by Radiographic Stage of Disease
| Stage I |
Stage II/III |
Stage IV |
|
|---|---|---|---|
| (n = 5) | (n = 17) | (n = 13) | |
| Age, median (range), yr | 53 (37–71) | 53 (34–68) | 58 (34–71) |
| Female, n (%) | 3 (60) | 8 (47) | 5 (38) |
| Race, n | |||
| White | 5 | 14 | 12 |
| African American | 0 | 3 | 0 |
| Other | 0 | 0 | 1 |
| Current immunosuppression use, n | 0 | 3 | 3 |
| FEV1, % predicted, mean ± SD | 101 ± 24 | 98 ± 14 | 91 ± 18 |
| FVC, % predicted, mean ± SD | 102 ± 21 | 100 ± 10 | 97 ± 14 |
| DlCO, % predicted mean, ± SD | 80 ± 22 | 80 ± 11 | 75 ± 9 |
Definition of abbreviation: DlCO = diffusing capacity of the lung for carbon monoxide.
Total n = 35.
A second validation cohort from Europe, the Erasmus MC cohort, consisted of 30 subjects with sarcoidosis and 12 disease control subjects, 8 of whom underwent bronchoscopy for community-acquired pneumonia, and 4 patients with chronic obstructive pulmonary disease. The control group showed a trend toward older age (Table 4). There were no statistically significant differences in sex or age between groups. The majority of the patients were white. One patient had Lofgren syndrome. Patients with sarcoidosis were newly diagnosed and not on immunosuppressive treatment. The lymphocyte percentages were consistent with alveolitis (Table 4).
Table 4.
Demographics and Clinical Characteristics for the Erasmus MC Cohort
| Control | Sarcoidosis | P Value | |
|---|---|---|---|
| n | 12 | 30 | |
| Female | 5 (42) | 12 (40) | 0.92 |
| Age, median (range), yr | 57 (18–80) | 43 (24–70) | 0.07 |
| Immunosuppression* | — | 0 | |
| Stage, I/II–III/IV/CT | — | 11/16/0/3† | — |
| Lung/heart/LN/eye/skin | 30/0/0/9/2 | ||
| Smoking, never/former/current/unknown | — | 16/8/4/2 | — |
| Race | |||
| African | — | 2 (7) | — |
| White | — | 19 (63) | — |
| Asian | — | 4 (13) | — |
| Unknown | — | 5 (17) | — |
| Ethnicity | |||
| Hispanic | — | 1 (3) | — |
| Lymphocyte BALF | |||
| Median (range), % | — | 27 (3–68) | — |
| Average (SD), % | — | 32 (21) | — |
| CD4/CD8 ratio BALF | |||
| Median (range) | — | 4.4 (0.5–14.34) | — |
| Average (SD) | — | 5.2 (3.4) | — |
Definition of abbreviations: BALF = bronchoalveolar lavage fluid; CT = computed tomography; LN = lymph node.
Data are shown as n (%) unless otherwise stated.
Patients were newly diagnosed and not on immunosuppressive treatment.
Three subjects were found to have mediastinal and hilar lymph node involvement by CT scan.
CD4+ T-Cell Analysis and Enrichment of Th Subsets Using Chemokine Receptor Expression
Sarcoidosis is characterized by abnormal increases in IFN-γ–producing BAL CD4+ T cells (2, 3, 28). Our goal was to use chemokine receptor expression to define and distinguish among Th17 subsets to understand their full capacity to produce IFN-γ and how numerous they were (14, 23). We used single-cell analysis and FACS sorting to isolate these Th subset populations (Figures 1A and 1B). We use the terminology “enriched” subsets to acknowledge that these populations cannot be 100% pure, but we show, using cytokine measurements, that the purities are very high.
As expected, we found that CD4+ T cells (as a percentage of lymphocytes) were markedly increased in BAL from patients with sarcoidosis compared with healthy control subjects (Figure 1C). In contrast, we found that CD4+ T cells were decreased in the blood in sarcoidosis compared with control, consistent with the idea of CD4+ T cells homing to the lungs from the blood (2). Decreased circulating CD4+ T-cell numbers have been associated with more severe forms of sarcoidosis, including decreased pulmonary function (29). In support of this idea, we also found that patients with sarcoidosis with fibrosis on chest CT scan had significantly lower numbers of CD4+ T cells in the blood compared with those without fibrosis (Figure E2). As anticipated, there were essentially no naive cells (CD3+CD4+CD1d−CD25loCD45RA+CD45RO−) in the BAL fluid in any participant (Figure 1E); in the blood, naive cells as a percentage of CD4+ T cells were significantly decreased in sarcoidosis compared with control, as has been recently observed in a separate cohort (30). Effector memory cells (CD3+CD4+CD1d−CD25loCD45RA−CD45RO+) as a percentage of CD4+ T cells were significantly increased in sarcoidosis BAL (average, ∼83%) compared with controls (Figure 1D). This expansion of effector memory cells is consistent with the idea of antigen-driven stimulation, as has been suggested by prior findings of oligoclonal expansion of T cells expressing specific αβ T-cell receptor genes in the lung and blood in sarcoidosis (31, 32).
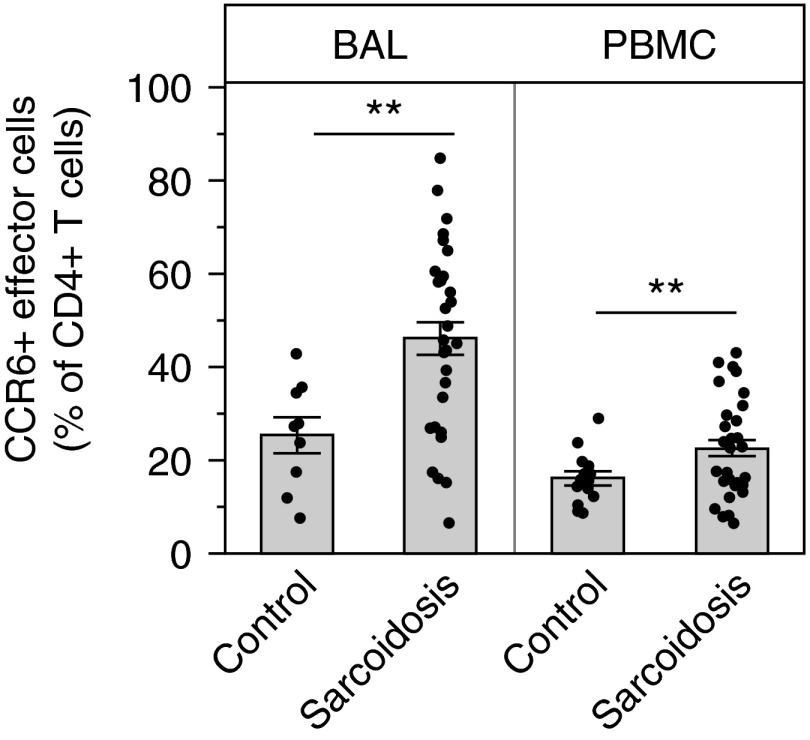
Within the population of effector memory cells, we found that, on average, approximately 50% of the CD4+ T effector cells in the lung expressed CCR6 (Figure 2). Almost one-third of subjects with sarcoidosis had over approximately 60% CCR6+ effector cells in the BAL. Although CCR6 is expressed on other cell types, it is a marker for Th17 cells, and this finding supports the hypothesis that large numbers of Th17 cells (or their subsets) are present in sarcoidosis BAL (33). In contrast, we did not find an increase in the percentage of CCR4+ effector cells in the lungs of patients with sarcoidosis compared with control subjects (P = 0.25).
Figure 2.
Significant increases in CCR6+ effector cells, consistent with the T-helper 17 lineage, in sarcoidosis. Bronchoalveolar lavage (BAL) cells and peripheral blood mononuclear cells (PBMCs) were stained and analyzed by flow cytometry as described in Methods. As a percentage of CD4+ T cells, CCR6+ effector cells are significantly increased in both the lungs (**P = 0.0006) and the blood (**P = 0.0051) of patients with sarcoidosis compared with control subjects. Data are shown as mean (histogram bars) ± SEM. Overlaid dots represent individual patient values. BAL, n = 32 subjects with sarcoidosis and 9 control subjects; PBMC, n = 35 subjects with sarcoidosis and 18 control subjects.
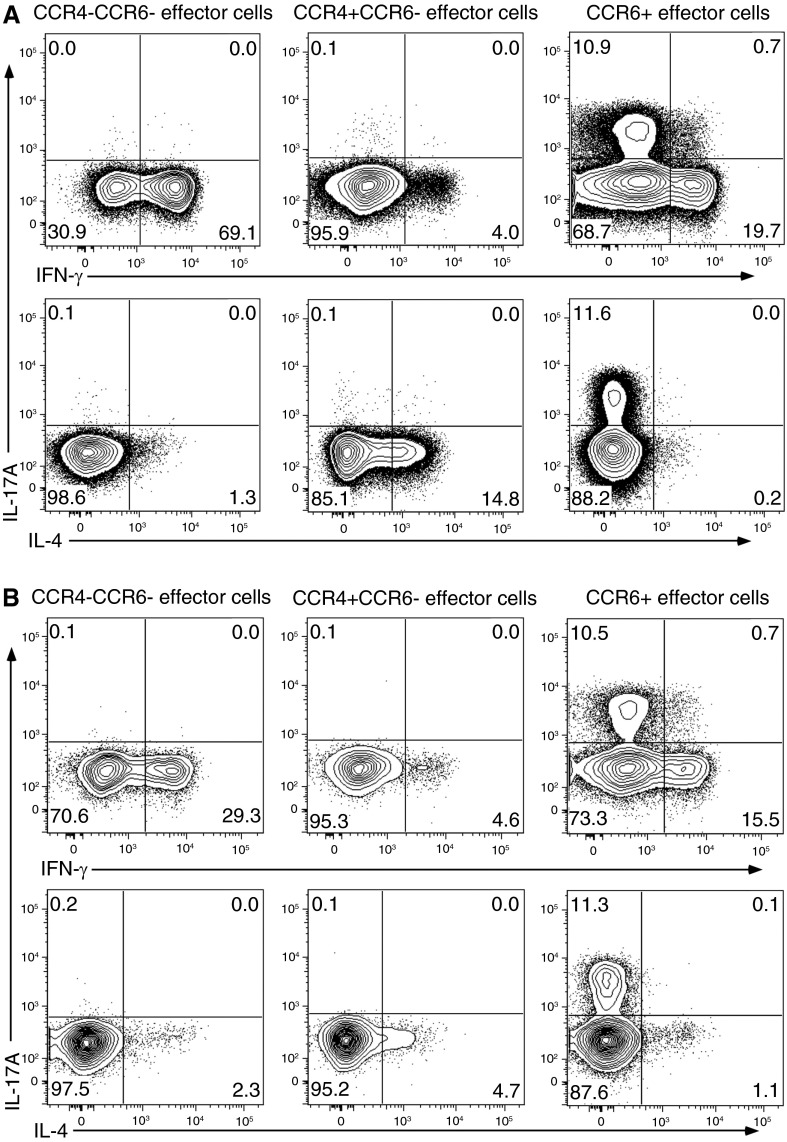
Functional Analysis of Th-Cell CCR4/CCR6 Subset Enrichments
We next assessed the accuracy of the Th subset enrichment strategy by stimulating the FACS-sorted CCR4 and CCR6 subsets and measuring production of IL-4, IL-17A, and IFN-γ. The CCR4−CCR6− population produced IFN-γ and virtually no IL-4 or IL-17A, consistent with Th1 characteristics (Figure 3). The CCR4+CCR6− effector cells produced IL-4 with little to no production of IL-17A or IFN-γ, supporting Th2 characteristics. Finally, the CCR6+ effector cells produced IL-17A as expected, but, in addition, a significant percentage also produced IFN-γ, and a very small population showed polyfunctional capacity to produce both IL-17A and IFN-γ. These data demonstrate the presence of at least three functionally diverse Th17 cells in sarcoidosis: a large percentage of CCR6+ cells that only produced IFN-γ, a smaller percentage of cells that produce only IL-17A, and a very small percentage of cells able to produce both IFN-γ and IL-17A.
Figure 3.
Isolation of discrete T-helper (Th)-cell populations from peripheral blood mononuclear cells (PBMCs) using FACS sorting confirmed accurate separation and revealed that CCR6+ cells demonstrated different patterns of cytokine secretion. FACS-sorted cells were stimulated and stained with fluorescent antibodies, as described in Methods. Shown is a representative sarcoidosis (A) and healthy control (B) sample of FACS-sorted PBMC effector cell subsets demonstrating predicted patterns of cytokine secretion for Th1 (CCR6−CCR4−) and Th2 (CCR4+CCR6−) cells. FACS-sorted CCR6+ effector cells show increased frequencies of cells expressing IFN-γ or IL-17 and a low frequency of cells expressing both cytokines. FACS = fluorescence-activated cell sorter.
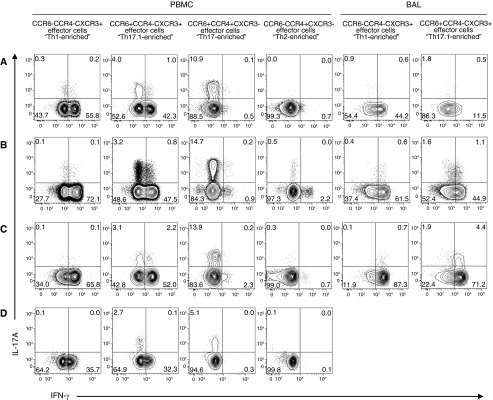
Discrimination of Th-Cell Subsets Using CXCR3, CCR6, and CCR4
Given the large subpopulation of IFN-γ–producing CCR6+ cells we analyzed the expression of CXCR3, a chemokine receptor known to be expressed on Th1 and Th17.1 cells (14, 34). Adding CXCR3 to our analysis allowed for a more stringent gating strategy to determine the percentages of Th1, Th17, and Th17.1 subsets (Table 1 and Figure E1), as well as to measure cytokine production. Functional analysis of both BAL and PBMC confirmed IFN-γ production with no significant IL-17A production by the sorted Th1-enriched (CCR6−CCR4−CXCR3+) population (Figure 4). In comparison, we found marked production of IFN-γ by Th17.1-enriched cells (CCR6+CCR4−CXCR3+), but only a small percentage of Th17.1 cells were capable of IL-17A expression (Figure 4, row “Th17.1-enriched”). In contrast to Th17.1 cells, the Th17-enriched cells (CCR6+CCR4+CXCR3−) showed essentially no production of IFN-γ and an increased capacity to produce IL-17A compared with Th17.1 (Figure 4, row “Th17-enriched”). Figure E3 compiles the functional data for PBMC samples from all subjects with sarcoidosis and demonstrates the striking differences in cytokine production between the Th17 subsets. Thus, these data show that we have identified three discernible populations of Th cells, namely, Th1, Th17, and Th17.1, in human sarcoidosis, and, specifically, that a large population of Th17.1 cells produce only IFN-γ.
Figure 4.
T-helper (Th) 17.1 cells showed coproduction of IFN-γ and IL-17A to a small extent but significant production of IFN-γ, which was not found for Th17 cells. Shown are representative dot plot images displaying IFN-γ versus IL-17A production from FACS-sorted effector cell subsets from subjects with sarcoidosis (A–C) and a healthy control subject (D). After FACS sorting by surface markers, the memory effector cell subsets were stimulated, fixed, permeabilized, stained intracellularly for cytokines, and analyzed by flow cytometry. These methods revealed marked differences in IL-17 and IFN-γ cytokine production between Th17- and Th17.1-enriched cells. In the bronchoalveolar lavage (BAL) samples, too few events fell within the CCR4+ gate to quantify CCR6+CCR4+CXCR3− “Th17-enriched” cells and CCR6−CCR4+CXCR3− “Th2-enriched.” Functional stimulation assays were not available from healthy control BAL samples owing to a paucity of available cells. FACS = fluorescence-activated cell sorter.
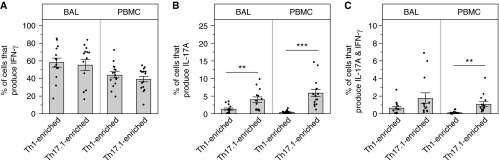
We next compiled the functional data from the sarcoidosis cohort to compare cytokine production between BAL and PBMC and Th1 and Th17.1 cells. We found that a remarkably high percentage (average, ∼60%) of Th17.1-enriched cells from BAL produced IFN-γ (Figure 5A). Importantly, the frequency of Th17.1 cells producing IFN-γ was just as high as that measured from Th1-enriched cells. The percentage of IFN-γ–producing Th1 and Th17.1 cells were also similar in the PBMC compartment (Figure 5A). Figure 5B shows that the frequency of Th17.1 cells producing IL-17 was modest (∼5% of total Th17.1 sorted cells in both BAL and PBMC) as predicted from the individual FACS plots shown in Figure 4. Finally, the frequency of Th17.1 cells capable of coproduction of IL-17A and IFN-γ was even lower (∼2% or lower; Figure 5C). Taken together, these data show a much greater capacity for Th17.1 cells to produce IFN-γ alone compared with IL-17A or both cytokines together, supporting the hypothesis that Th17.1 cells expressing only IFN-γ could have been misclassified as Th1 cells.
Figure 5.
T-helper (Th) 17.1-enriched cells were equipotent in their ability to produce IFN-γ compared with Th1-enriched cells. Bronchoalveolar lavage (BAL) and peripheral blood mononuclear cells (PBMCs) cells were FACS sorted and subsequently stimulated and stained for intracellular cytokines, as described in Methods. (A–C) Functional data collected from flow cytometry on patients with sarcoidosis were compiled and displayed. (A) Percentage of cells producing IFN-γ only. (B) Percentage of cells producing IL-17A only (BAL, **P = 0.0056; PBMC, ***P < 0.0001). (C) Percentage of polyfunctional cells producing both IL-17A and IFN-γ (PBMC, **P = 0.0024). Data are expressed as mean (histogram bars) ± SEM. Overlaid dots represent individual patient values. Sarcoidosis BAL, n = 14, and PBMC, n = 15. FACS = fluorescence-activated cell sorter.
Th17.1, but Not Th1 or Th17, Cells Are Increased in Sarcoidosis
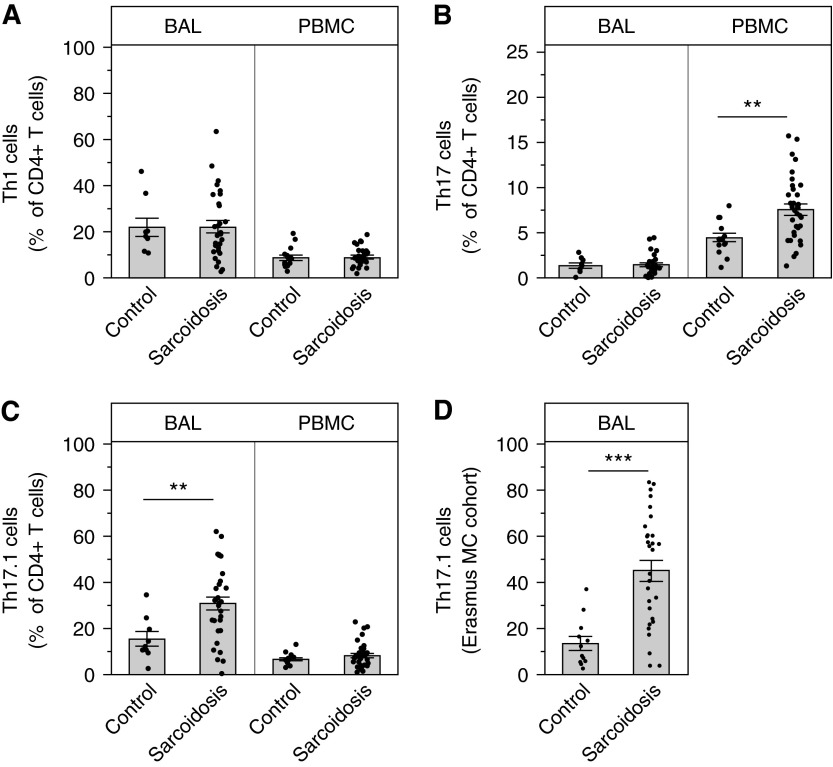
Sarcoidosis is characterized as a Th1-mediated disease. However, we found no significant differences in the percentage of Th1 cells in both BAL and blood in patients with sarcoidosis compared with control subjects (Figure 6A). Furthermore, the size of the Th1 population in BAL was low in both groups (∼20% of CD4+ T cells in BAL; Figure 6A). Consistent with our hypothesis, we found, on average, that approximately 30% of the CD4+ T cells in sarcoidosis BAL were Th17.1 cells, which was significantly increased compared with control subjects and not found for Th17 cells, respectively (Figures 6B and 6C). Th17.1 cells accounted for up to 60% of all the BAL CD4+ T cells in some subjects. In sarcoidosis blood, we found the opposite pattern: Th17 cells were increased, but Th17.1 cells were not. Because the U.S. control group was younger than the patients with sarcoidosis (Table 2), we also studied a second cohort of European control subjects and newly diagnosed patients with sarcoidosis that were better matched for age (Table 4). Figure 6D displays the marked expansion of Th17.1 cells in patients with sarcoidosis compared with the disease control subjects, which validates the U.S. cohort findings.
Figure 6.
T-helper (Th) 17.1 cells from sarcoidosis bronchoalveolar lavage (BAL) fluid were significantly increased in two separate sarcoidosis cohorts and present in higher percentages compared with Th1 cells. BAL cells and peripheral blood mononuclear cells (PBMCs) were stained, FACS sorted, and the acquired data were analyzed to include all three chemokine receptors to define Th1 and Th17.1 cells, as described in Methods, Table 1, and Figure E1. (A) There were no significant differences in the percentages of Th1 cells (CCR6−CCR4−CXCR3+ effector cells) in the blood or lungs in subjects with sarcoidosis compared with healthy control subjects. (B) The percentages of Th17 cells (defined CCR6+CCR4+CXCR3− effector cells) are significantly increased in the blood of patients with sarcoidosis (**P = 0.0002), but not in the lungs, compared with healthy control subjects. (C) The percentages of Th17.1 cells (defined CCR6+CCR4−CXCR3+) were significantly increased in the lungs of patients with sarcoidosis (**P = 0.0016), but not in the blood. (D) The percentages of Th17.1 cells were markedly increased in the BAL of patients with sarcoidosis from the Erasmus MC cohort compared with similarly aged disease control subjects (***P ≤ 0.0001). Data are expressed as mean (histogram bars) ± SEM. Overlaid dots represent individual patient values. U.S. cohort: BAL, n = 32 subjects with sarcoidosis and 9 control subjects; PBMC, n = 35 subjects with sarcoidosis and 18 control. Erasmus MC cohort: BAL, n = 30 subjects with sarcoidosis and 12 control subjects. FACS = fluorescence-activated cell sorter.
Discussion
Sarcoidosis is a classic Th1-mediated disease (35–37). Given the evidence for plasticity of Th17 cells toward a Th1 phenotype (14, 17–20, 22, 23, 34, 38–40), we speculated that the intense IFN-γ inflammation in the lungs of patients with sarcoidosis could transform Th17 cells into cells with characteristics of Th1 cells. These cells have been referred to as Th17.1, and large subpopulations of them have been shown to produce only IFN-γ. Using methods that are highly accurate in discriminating subsets of Th cells (14), we identified two separate Th17 subsets in sarcoidosis, namely, Th17 and Th17.1. These subsets express different chemokine receptors and demonstrate different cytokine effector functions. Importantly, Th17.1 cells have shown pathogenic characteristics, such as up-regulation of proinflammatory cytokines and corticosteroid resistance (14), demonstrating the importance of identifying this Th subset in sarcoidosis. We found that Th17.1 cells were markedly expanded in sarcoidosis BAL, and were present in even higher frequencies than classical Th1 cells. Furthermore, sarcoidosis Th17.1-enriched cells were equipotent in their ability to produce IFN-γ compared with Th1 cells. We also analyzed Th17.1 cells from a separate European cohort, which validated the original observations. Taken together, these findings stimulate questions regarding the established view that Th1 cells are the major contributor in sarcoidal inflammation. This study also demonstrates the importance of detailed characterization of human T cells, because Th17.1 cells, which are skewed to produce only IFN-γ, have likely been misclassified as Th1 cells due to their cytokine profiles. Ultimately, functional studies of CD4+ T cells in sarcoidosis, without regard to more detailed distinction of the type of Th subset, may be misleading.
Direct comparison of the frequencies of Th17 and Th17.1 effector cell subsets between the blood and BAL compartment identified significant elevations of Th17-enriched cells in the blood, but not the BAL, of subjects with sarcoidosis compared with control subjects. Conversely, Th17.1-enriched cells were significantly increased in the BAL, but not in the blood, when compared with healthy control subjects. This observation could relate to plasticity of Th17-cell subsets in vivo if they are transiting from blood to the inflamed lung (36, 41, 42). Interesting prior research supports our results by finding elevated expression of CCR6 on CD4+ T cells in sarcoidosis, but this study did not use immunophenotyping assays that would allow for identification of the full population of Th17.1 cells or identify the marked differences in cytokine production of Th17.1 cells compared with Th17 cells (43). Other studies focused on CD4+ cells that secreted IL-17 or IFN-γ without incorporation of multiple phenotypic markers and FACS sorting, resulting in the possible misclassification of Th17.1 cells as Th1 cells (9, 11, 12). By highlighting the heterogeneity of IFN-γ–producing cells in this chronic human disease, our results suggest the more general conclusion that lymphocytes in vivo, especially in chronic diseases, may be more heterogeneous than those observed in controlled experimental models.
Studies such as that by Ramesh and colleagues (14) provide insight into the complicated functional role of Th17-cell subsets. The authors asked why patients with Crohn’s disease receiving secukinumab, a fully humanized anti–IL-17A monoclonal antibody, developed exacerbations of their disease (44), even though IL-17A is thought to be an important contributor of gut inflammation. Using human blood and gut tissue, they showed that Th17.1 cells (which the authors defined as CCR6+CXCR3hiCCR4loCCR10−CD161+MDR1+) expressed a proinflammatory transcriptional signature that mimicked disease-inducing Th17 subsets in mice and also predicted increased responsiveness to IL-23, which is thought to be a regulator of pathogenic Th17 function (14). In contrast, a study examining T cells expressing CCR6+CXCR3+CCR4− (i.e., Th17.1 cells) found that their circulating numbers were increased in patients with latent tuberculosis infection compared with control subjects (45). The authors performed RNA sequencing of these cells and found lineage-specific signatures of both Th1 and Th17 cells, which would be expected, but also differential expression of genes associated with susceptibility to tuberculosis, and enhanced T-cell activation and cell survival (45). Thus, the functional complexity of Th17 subsets appears to relate to the local inflammatory signals and, although considered pathogenic in one inflammatory disease, they may be necessary for eradication of the infection in another. Further studies are needed to understand the role of Th17-cell subsets, their cellular products, and the regulators of Th17 pathology (e.g., IL-23) in sarcoidosis to make sense of results from existing studies (46) and facilitate the development of more effective therapies.
Our study has some potential limitations. We found that Th17.1-cell numbers were markedly increased in sarcoidosis BAL as compared with that from two control groups, whereas Th1-cell numbers were not. However, each control group has a potential limitation. Our first control group was comprised of healthy control subjects, but they were somewhat younger, on average, than those in our sarcoidosis group. Our second control group was comprised of control subjects with disease rather than healthy control subjects. Nonetheless, we did not find any expansion of Th17.1 cells in BAL in either of these control groups, and we believe the consistency of our findings across these two control groups is supportive of our general conclusions.
In summary, we isolated CD4+ T effector cells by their chemokine receptors to understand their distribution and functional attributes in the BAL and blood of sarcoidosis to uncover significantly elevated frequencies of BAL Th17.1 cells. It is likely that these cells have been misclassified as Th1 cells in sarcoidosis. These new findings raise questions about prior assumptions of disease pathogenesis and may provide new directions for clinical studies and treatment targets. We also need to learn more about their presence in granulomatous tissues and what role(s) they play in disease progression.
Acknowledgments
Acknowledgment
The authors thank the subjects, nurses, and physicians that participated in the study. They thank Suresh Garudadri, Michael Li, and Elijah Darnell for assistance with bronchoscopy sample collection, and Marthe Paats, Menno van der Eerden, and Ingrid Bergen for providing control bronchoalveolar lavage samples.
Footnotes
Supported by National Institutes of Health grants U01HL112696 (L.L.K.), P01HL107202 (P.G.W.), HL107202, and HL109102 (K.M.A.); a Scholar award from the Leukemia and Lymphoma Society (K.M.A.); and Framework Program 7 Marie Curie Career Integration Grant of the European Union (M.K.).
Author Contributions: J.R., C.E.B., and L.L.K. conceived of the project; J.R., C.E.B., B.S.B., L.J.S., K.M.A., S.A.S., M.E.H., P.G.W., N.R.B., L.C., C.P.N., B.J.A., R.W.H., B.v.d.B., M.K., and L.L.K. generated data for the project; J.R., C.E.B., L.J.S., K.M.A., L.C., M.K., and L.L.K. analyzed data for the project; J.R., C.E.B., and L.L.K. wrote the manuscript; J.R., C.E.B., B.S.B., L.J.S., K.M.A., S.A.S., M.E.H., P.G.W., N.R.B., L.C., C.P.N., B.J.A., R.W.H., B.v.d.B., M.K., and L.L.K. reviewed and edited the manuscript, gave final approval for submission, and were accountable for all aspects of the work in terms of ensuring accuracy of the data.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.201507-1499OC on December 9, 2015
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Chen ES, Moller DR. Sarcoidosis—scientific progress and clinical challenges. Nat Rev Rheumatol. 2011;7:457–467. doi: 10.1038/nrrheum.2011.93. [DOI] [PubMed] [Google Scholar]
- 2.Hunninghake GW, Crystal RG. Pulmonary sarcoidosis: a disorder mediated by excess helper T-lymphocyte activity at sites of disease activity. N Engl J Med. 1981;305:429–434. doi: 10.1056/NEJM198108203050804. [DOI] [PubMed] [Google Scholar]
- 3.Robinson BW, McLemore TL, Crystal RG. Gamma interferon is spontaneously released by alveolar macrophages and lung T lymphocytes in patients with pulmonary sarcoidosis. J Clin Invest. 1985;75:1488–1495. doi: 10.1172/JCI111852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Agostini C, Meneghin A, Semenzato G. T-lymphocytes and cytokines in sarcoidosis. Curr Opin Pulm Med. 2002;8:435–440. doi: 10.1097/00063198-200209000-00016. [DOI] [PubMed] [Google Scholar]
- 5.Prasse A, Georges CG, Biller H, Hamm H, Matthys H, Luttmann W, Virchow JC., Jr Th1 cytokine pattern in sarcoidosis is expressed by bronchoalveolar CD4+ and CD8+ T cells. Clin Exp Immunol. 2000;122:241–248. doi: 10.1046/j.1365-2249.2000.01365.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Möllers M, Aries SP, Drömann D, Mascher B, Braun J, Dalhoff K. Intracellular cytokine repertoire in different T cell subsets from patients with sarcoidosis. Thorax. 2001;56:487–493. doi: 10.1136/thorax.56.6.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wahlström J, Katchar K, Wigzell H, Olerup O, Eklund A, Grunewald J. Analysis of intracellular cytokines in CD4+ and CD8+ lung and blood T cells in sarcoidosis. Am J Respir Crit Care Med. 2001;163:115–121. doi: 10.1164/ajrccm.163.1.9906071. [DOI] [PubMed] [Google Scholar]
- 8.Huang H, Lu Z, Jiang C, Liu J, Wang Y, Xu Z. Imbalance between Th17 and regulatory T-Cells in sarcoidosis. Int J Mol Sci. 2013;14:21463–21473. doi: 10.3390/ijms141121463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Richmond BW, Ploetze K, Isom J, Chambers-Harris I, Braun NA, Taylor T, Abraham S, Mageto Y, Culver DA, Oswald-Richter KA, et al. Sarcoidosis Th17 cells are ESAT-6 antigen specific but demonstrate reduced IFN-γ expression. J Clin Immunol. 2013;33:446–455. doi: 10.1007/s10875-012-9817-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ostadkarampour M, Eklund A, Moller D, Glader P, Olgart Höglund C, Lindén A, Grunewald J, Wahlström J. Higher levels of interleukin IL-17 and antigen-specific IL-17 responses in pulmonary sarcoidosis patients with Löfgren’s syndrome. Clin Exp Immunol. 2014;178:342–352. doi: 10.1111/cei.12403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tondell A, Moen T, Borset M, Salvesen O, Ro AD, Sue-Chu M. Bronchoalveolar lavage fluid IFN-γ+ Th17 cells and regulatory T cells in pulmonary sarcoidosis. Mediators Inflamm. 2014;2014:438070. doi: 10.1155/2014/438070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ten Berge B, Paats MS, Bergen IM, van den Blink B, Hoogsteden HC, Lambrecht BN, Hendriks RW, Kleinjan A. Increased IL-17A expression in granulomas and in circulating memory T cells in sarcoidosis. Rheumatology (Oxford) 2012;51:37–46. doi: 10.1093/rheumatology/ker316. [DOI] [PubMed] [Google Scholar]
- 13.Facco M, Cabrelle A, Teramo A, Olivieri V, Gnoato M, Teolato S, Ave E, Gattazzo C, Fadini GP, Calabrese F, et al. Sarcoidosis is a Th1/Th17 multisystem disorder. Thorax. 2011;66:144–150. doi: 10.1136/thx.2010.140319. [DOI] [PubMed] [Google Scholar]
- 14.Ramesh R, Kozhaya L, McKevitt K, Djuretic IM, Carlson TJ, Quintero MA, McCauley JL, Abreu MT, Unutmaz D, Sundrud MS. Pro-inflammatory human Th17 cells selectively express P-glycoprotein and are refractory to glucocorticoids. J Exp Med. 2014;211:89–104. doi: 10.1084/jem.20130301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ahern PP, Schiering C, Buonocore S, McGeachy MJ, Cua DJ, Maloy KJ, Powrie F. Interleukin-23 drives intestinal inflammation through direct activity on T cells. Immunity. 2010;33:279–288. doi: 10.1016/j.immuni.2010.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hirota K, Duarte JH, Veldhoen M, Hornsby E, Li Y, Cua DJ, Ahlfors H, Wilhelm C, Tolaini M, Menzel U, et al. Fate mapping of IL-17–producing T cells in inflammatory responses. Nat Immunol. 2011;12:255–263. doi: 10.1038/ni.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee YK, Turner H, Maynard CL, Oliver JR, Chen D, Elson CO, Weaver CT. Late developmental plasticity in the T helper 17 lineage. Immunity. 2009;30:92–107. doi: 10.1016/j.immuni.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lexberg MH, Taubner A, Albrecht I, Lepenies I, Richter A, Kamradt T, Radbruch A, Chang HD. IFN-γ and IL-12 synergize to convert in vivo generated Th17 into Th1/Th17 cells. Eur J Immunol. 2010;40:3017–3027. doi: 10.1002/eji.201040539. [DOI] [PubMed] [Google Scholar]
- 19.Sundrud MS, Trivigno C. Identity crisis of Th17 cells: many forms, many functions, many questions. Semin Immunol. 2013;25:263–272. doi: 10.1016/j.smim.2013.10.021. [DOI] [PubMed] [Google Scholar]
- 20.Annunziato F, Cosmi L, Santarlasci V, Maggi L, Liotta F, Mazzinghi B, Parente E, Filì L, Ferri S, Frosali F, et al. Phenotypic and functional features of human Th17 cells. J Exp Med. 2007;204:1849–1861. doi: 10.1084/jem.20070663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maggi L, Santarlasci V, Capone M, Rossi MC, Querci V, Mazzoni A, Cimaz R, De Palma R, Liotta F, Maggi E, et al. Distinctive features of classic and nonclassic (Th17 derived) human Th1 cells. Eur J Immunol. 2012;42:3180–3188. doi: 10.1002/eji.201242648. [DOI] [PubMed] [Google Scholar]
- 22.Duhen T, Campbell DJ. IL-1β promotes the differentiation of polyfunctional human CCR6+CXCR3+ Th1/17 cells that are specific for pathogenic and commensal microbes. J Immunol. 2014;193:120–129. doi: 10.4049/jimmunol.1302734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nistala K, Adams S, Cambrook H, Ursu S, Olivito B, de Jager W, Evans JG, Cimaz R, Bajaj-Elliott M, Wedderburn LR. Th17 plasticity in human autoimmune arthritis is driven by the inflammatory environment. Proc Natl Acad Sci USA. 2010;107:14751–14756. doi: 10.1073/pnas.1003852107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Statement on sarcoidosis. Joint Statement of the American Thoracic Society (ATS), the European Respiratory Society (ERS) and the World Association of Sarcoidosis and Other Granulomatous Disorders (WASOG) adopted by the ATS Board of Directors and by the ERS Executive Committee, February 1999. Am J Respir Crit Care Med. 1999;160:736–755. doi: 10.1164/ajrccm.160.2.ats4-99. [DOI] [PubMed] [Google Scholar]
- 25.Judson MA, Costabel U, Drent M, Wells A, Maier L, Koth L, Shigemitsu H, Culver DA, Gelfand J, Valeyre D, et al. The WASOG Sarcoidosis Organ Assessment Instrument: an update of a previous clinical tool. Sarcoidosis Vasc Diffuse Lung Dis. 2014;31:19–27. [PubMed] [Google Scholar]
- 26.Snyder-Cappione JE, Nixon DF, Chi JC, Nguyen ML, Kirby CK, Milush JM, Koth LL. Invariant natural killer T (iNKT) cell exhaustion in sarcoidosis. Eur J Immunol. 2013;43:2194–2205. doi: 10.1002/eji.201243185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moller DR, Koth LL, Maier LA, Morris A, Drake W, Rossman M, Leader JK, Collman RG, Hamzeh N, Sweiss NJ, et al. GRADS Sarcoidosis Study Group *. Rationale and Design of the Genomic Research in Alpha-1 Antitrypsin Deficiency and Sarcoidosis (GRADS) study: sarcoidosis protocol. Ann Am Thorac Soc. 2015;12:1561–1571. doi: 10.1513/AnnalsATS.201503-172OT. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meyer KC, Kaminski MJ, Calhoun WJ, Auerbach R. Studies of bronchoalveolar lavage cells and fluids in pulmonary sarcoidosis. I. Enhanced capacity of bronchoalveolar lavage cells from patients with pulmonary sarcoidosis to induce angiogenesis in vivo. Am Rev Respir Dis. 1989;140:1446–1449. doi: 10.1164/ajrccm/140.5.1446. [DOI] [PubMed] [Google Scholar]
- 29.Sweiss NJ, Salloum R, Gandhi S, Alegre ML, Sawaqed R, Badaracco M, Pursell K, Pitrak D, Baughman RP, Moller DR, et al. Significant CD4, CD8, and CD19 lymphopenia in peripheral blood of sarcoidosis patients correlates with severe disease manifestations. PLoS One. 2010;5:e9088. doi: 10.1371/journal.pone.0009088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Braun NA, Celada LJ, Herazo-Maya JD, Abraham S, Shaginurova G, Sevin CM, Grutters J, Culver DA, Dworski R, Sheller J, et al. Blockade of the programmed death-1 pathway restores sarcoidosis CD4(+) T-cell proliferative capacity. Am J Respir Crit Care Med. 2014;190:560–571. doi: 10.1164/rccm.201401-0188OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moller DR. Involvement of T cells and alterations in T cell receptors in sarcoidosis. Semin Respir Infect. 1998;13:174–183. [PubMed] [Google Scholar]
- 32.Grunewald J, Janson CH, Eklund A, Ohrn M, Olerup O, Persson U, Wigzell H. Restricted V alpha 2.3 gene usage by CD4+ T lymphocytes in bronchoalveolar lavage fluid from sarcoidosis patients correlates with HLA-DR3. Eur J Immunol. 1992;22:129–135. doi: 10.1002/eji.1830220120. [DOI] [PubMed] [Google Scholar]
- 33.Sallusto F, Zielinski CE, Lanzavecchia A. Human Th17 subsets. Eur J Immunol. 2012;42:2215–2220. doi: 10.1002/eji.201242741. [DOI] [PubMed] [Google Scholar]
- 34.Zielinski CE, Mele F, Aschenbrenner D, Jarrossay D, Ronchi F, Gattorno M, Monticelli S, Lanzavecchia A, Sallusto F. Pathogen-induced human TH17 cells produce IFN-γ or IL-10 and are regulated by IL-1β. Nature. 2012;484:514–518. doi: 10.1038/nature10957. [DOI] [PubMed] [Google Scholar]
- 35.Bergeron A, Bonay M, Kambouchner M, Lecossier D, Riquet M, Soler P, Hance A, Tazi A. Cytokine patterns in tuberculous and sarcoid granulomas: correlations with histopathologic features of the granulomatous response. J Immunol. 1997;159:3034–3043. [PubMed] [Google Scholar]
- 36.Moller DR, Forman JD, Liu MC, Noble PW, Greenlee BM, Vyas P, Holden DA, Forrester JM, Lazarus A, Wysocka M, et al. Enhanced expression of IL-12 associated with Th1 cytokine profiles in active pulmonary sarcoidosis. J Immunol. 1996;156:4952–4960. [PubMed] [Google Scholar]
- 37.Walker C, Bauer W, Braun RK, Menz G, Braun P, Schwarz F, Hansel TT, Villiger B. Activated T cells and cytokines in bronchoalveolar lavages from patients with various lung diseases associated with eosinophilia. Am J Respir Crit Care Med. 1994;150:1038–1048. doi: 10.1164/ajrccm.150.4.7921434. [DOI] [PubMed] [Google Scholar]
- 38.Boniface K, Blumenschein WM, Brovont-Porth K, McGeachy MJ, Basham B, Desai B, Pierce R, McClanahan TK, Sadekova S, de Waal Malefyt R. Human Th17 cells comprise heterogeneous subsets including IFN-gamma–producing cells with distinct properties from the Th1 lineage. J Immunol. 2010;185:679–687. doi: 10.4049/jimmunol.1000366. [DOI] [PubMed] [Google Scholar]
- 39.Kebir H, Ifergan I, Alvarez JI, Bernard M, Poirier J, Arbour N, Duquette P, Prat A. Preferential recruitment of interferon-gamma–expressing TH17 cells in multiple sclerosis. Ann Neurol. 2009;66:390–402. doi: 10.1002/ana.21748. [DOI] [PubMed] [Google Scholar]
- 40.Annunziato F, Cosmi L, Liotta F, Maggi E, Romagnani S. Defining the human T helper 17 cell phenotype. Trends Immunol. 2012;33:505–512. doi: 10.1016/j.it.2012.05.004. [DOI] [PubMed] [Google Scholar]
- 41.Shigehara K, Shijubo N, Ohmichi M, Takahashi R, Kon S, Okamura H, Kurimoto M, Hiraga Y, Tatsuno T, Abe S, et al. IL-12 and IL-18 are increased and stimulate IFN-gamma production in sarcoid lungs. J Immunol. 2001;166:642–649. doi: 10.4049/jimmunol.166.1.642. [DOI] [PubMed] [Google Scholar]
- 42.Trinchieri G. Interleukin-12: a cytokine produced by antigen-presenting cells with immunoregulatory functions in the generation of T-helper cells type 1 and cytotoxic lymphocytes. Blood. 1994;84:4008–4027. [PubMed] [Google Scholar]
- 43.Facco M, Baesso I, Miorin M, Bortoli M, Cabrelle A, Boscaro E, Gurrieri C, Trentin L, Zambello R, Calabrese F, et al. Expression and role of CCR6/CCL20 chemokine axis in pulmonary sarcoidosis. J Leukoc Biol. 2007;82:946–955. doi: 10.1189/jlb.0307133. [DOI] [PubMed] [Google Scholar]
- 44.Hueber W, Sands BE, Lewitzky S, Vandemeulebroecke M, Reinisch W, Higgins PD, Wehkamp J, Feagan BG, Yao MD, Karczewski M, et al. Secukinumab in Crohn’s Disease Study Group. Secukinumab, a human anti–IL-17A monoclonal antibody, for moderate to severe Crohn’s disease: unexpected results of a randomised, double-blind placebo-controlled trial. Gut. 2012;61:1693–1700. doi: 10.1136/gutjnl-2011-301668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Arlehamn CL, Seumois G, Gerasimova A, Huang C, Fu Z, Yue X, Sette A, Vijayanand P, Peters B. Transcriptional profile of tuberculosis antigen–specific T cells reveals novel multifunctional features. J Immunol. 2014;193:2931–2940. doi: 10.4049/jimmunol.1401151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Judson MA, Baughman RP, Costabel U, Drent M, Gibson KF, Raghu G, Shigemitsu H, Barney JB, Culver DA, Hamzeh NY, et al. Safety and efficacy of ustekinumab or golimumab in patients with chronic sarcoidosis. Eur Respir J. 2014;44:1296–1307. doi: 10.1183/09031936.00000914. [DOI] [PubMed] [Google Scholar]