To the Editor:
The diagnosis of pulmonary tuberculosis in HIV-infected individuals is particularly challenging as HIV-induced alterations of the immune system lead to reduced cavitation, limiting the sensitivity of sputum-based assays (1). Thus, alternate markers are needed to distinguish between latent tuberculosis infection (LTBI) and active tuberculosis (aTB) in this high-risk group. Several attributes of Mycobacterium tuberculosis (Mtb)-specific CD4+ T cells have been shown to efficiently delineate LTBI and aTB in HIV-uninfected individuals, including their polyfunctional or memory profiles (2–4). Moreover, Adekambi and colleagues recently demonstrated that the activation profile of Mtb-specific CD4+ T cells accurately discriminates between LTBI and aTB (5). As chronic HIV infection is characterized by persistent systemic immune activation (6), it is plausible that these blood-based markers may not be relevant for HIV-infected individuals.
We, therefore, compared the potential of the activation and polyfunctional profiles of Mtb-specific CD4+ T cells to distinguish between LTBI and aTB in HIV-uninfected and HIV-infected individuals. We analyzed 76 participants divided in four groups according to their TB and HIV status: LTBI/HIV− (n = 17; median age, 22 yr; 47% female), aTB/HIV− (n = 17; median age, 27 yr; 29% female), LTBI/HIV+ (n = 21; median age, 29 yr; 67% female; median CD4 count, 316 cells/mm3; interquartile range [IQR], 231–543 cells/mm3), and aTB/HIV+ (n = 21; median age, 35 yr; 57% female; CD4 count, 250 cells/mm3; IQR, 155–295 cells/mm3). LTBI was defined as tuberculin skin test positive, IFN-γ release assay positive, sputum culture negative, and normal chest X-ray. aTB was diagnosed on the basis of symptoms suggestive of tuberculosis and Mtb-positive smear and/or sputum culture, as previously described (7). All HIV-infected participants were antiretroviral therapy–naive. The University of Cape Town ethics committee approved the study, and written consent was obtained from participants. Cryopreserved peripheral blood mononuclear cells were stimulated for 16 hours with early secretory antigenic target-6 (ESAT-6)/culture filtrate protein-10 (CFP-10) peptide pool, and intracellular staining, using a live/dead marker and antibodies toward CD3, CD4, CD8, human leukocyte antigen-DR (HLA-DR), Ki67, CD38, IFN-γ, tumor necrosis factor (TNF)-α, and IL-2, was performed. Positive ESAT-6/CFP-10 responses (defined as twice the background) were detectable in 16 subjects in the LTBI/HIV− and LTBI/HIV+ groups, and in 15 and 18 individuals in the aTB/HIV− and aTB/HIV+ groups, respectively. No significant differences were observed in the overall magnitude of IFN-γ+ responses between the four groups (data not shown).
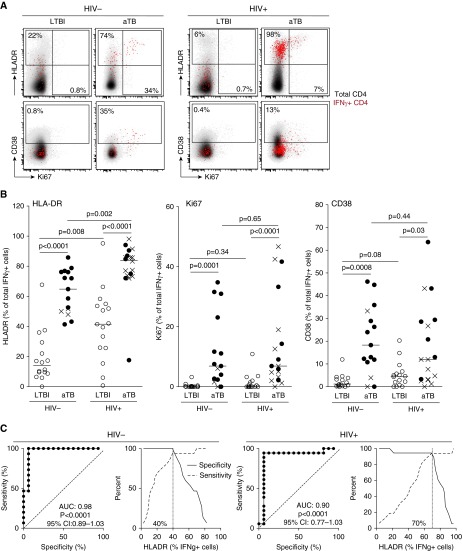
We first compared the activation profile of IFN-γ+ Mtb-specific CD4+ T cells between the four groups (Figure 1A). As previously shown (5), in HIV-uninfected persons, HLA-DR, Ki67, and CD38 expression on IFN-γ+ Mtb-specific CD4+ T cells was significantly higher in aTB participants than in those with LTBI (Figure 1B). Interestingly, although HLA-DR expression on Mtb-specific CD4+ T cells in the LTBI/HIV+ group (median, 41.7%; IQR, 25.7–54.6%) was significantly higher than in the LTBI/HIV− group (median, 13.7%; IQR, 8.9–27.5%), HLA-DR expression on these cells was significantly further increased in HIV-infected individuals with aTB (median, 84%; IQR, 73.7–87.9%) (Figure 1B). Additional analyses showed that in LTBI/HIV+ individuals, HLA-DR expression on Mtb-specific CD4+ T cells mirrors HLA-DR expression in the whole CD4 compartment (P = 0.02; r = 0.56), but this association was not apparent in aTB/HIV+ individuals (data not shown). Unlike HLA-DR, Ki67 and CD38 expression levels were comparable between HIV-uninfected and HIV-infected individuals with LTBI. In HIV-infected persons with aTB, Ki67 expression on IFN-γ+ Mtb-specific CD4+ T cells was significantly higher (P < 0.0001) than in LTBI, whereas the up-regulation of CD38 was more modest between these two groups (P = 0.03). Of note, in the aTB/HIV+ group, the expression of CD38 was significantly higher in individuals with a positive smear when compared with smear-negative participants (P = 0.01; data not shown), suggesting that CD38 expression could reflect bacterial load. To assess the accuracy of these markers to discriminate between LTBI and aTB status, receiver operating characteristic curves and crossover plots were performed. Figure 1C shows the data for HLA-DR; area under the curve (AUC) and P values reflect that HLA-DR expression on IFN-γ+ Mtb-specific CD4+ T cells distinguishes LTBI and aTB in both the HIV− and HIV+ groups (AUC = 0.98 [P < .0001]; and AUC = 0.9 [P < 0.0001], respectively). However, the optimum cutoff values discriminating LTBI from aTB were distinct for HIV-uninfected (40%) and HIV-infected (70%) individuals. In our experimental setting, the expression of Ki67 and CD38 was less robust to differentiate TB status in HIV-uninfected (AUC = 0.896 [P = 0.00017], cutoff = 1.4%; AUC = 0.858 [P = 0.0007], cutoff = 4%, respectively) and HIV-infected (AUC = 0.89 [P = 0.0002], cutoff = 2.4%; AUC = 0.72 [P = 0.026], cutoff = 5%, respectively) individuals (data not shown). Our data were comparable to those of Adekambi and colleagues (5) despite disparity in the cutoff value for these markers, which could be explained by flow-cytometry technical differences.
Figure 1.
Comparison of the activation profile of IFN-γ+ early secretory antigenic target-6/culture filtrate protein-10–specific CD4+ T cells between HIV-uninfected and HIV-infected individuals with latent tuberculosis infection (LTBI) or active tuberculosis (aTB). (A) Representative overlay plots of human leukocyte antigen-DR (HLA-DR), CD38, and Ki-67 expression in total CD4+ T cells (gray) and IFN-γ+ Mycobacterium tuberculosis (Mtb)-specific CD4+ T cells (red). (B) Expression of HLA-DR, Ki67, and CD38 on IFN-γ+ Mtb-specific CD4+ T cells in LTBI/HIV− (n = 16), aTB/HIV− (n = 15), LTBI /HIV+ (n = 16), and aTB/HIV+ (n = 18) participants. Open circles depict LTBI individuals, solid circles represent smear-positive patients with aTB, and crosses correspond to smear-negative and culture-positive individuals with aTB. Horizontal lines indicate the median. Statistical comparisons were performed using a nonparametric Mann-Whitney U test. (C) Receiver operating characteristic curves and specificity/sensitivity crossover plots for HLA-DR expression level in IFN-γ+ Mtb-specific CD4+ T cells to discriminate between LTBI or aTB in HIV-uninfected and HIV-infected individuals. The area under the curve (AUC), P value, and confidence intervals (CIs) are shown. The diagonal dashed line depicts an AUC of 0.5, representing a random test. The dashed vertical line on the crossover plots represents the optimal threshold to distinguish LTBI and aTB individuals.
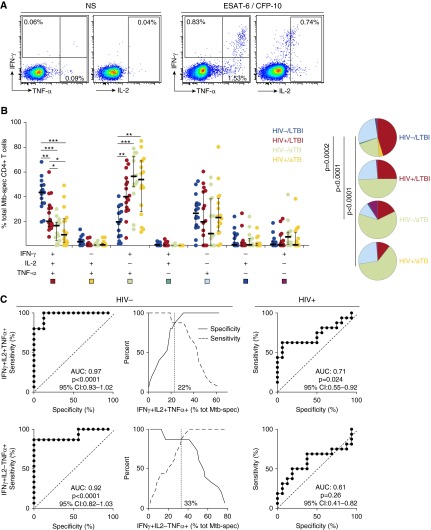
The polyfunctional profile of Mtb-specific CD4+ T cells has also been shown to discriminate between LTBI and aTB in HIV-uninfected individuals (2, 3), but conflicting data exist for HIV-infected persons (8–10). Thus, we compared the profile of ESAT-6/CFP-10-specific CD4+ T cells on the basis of their capacity to secrete IFN-γ, TNF-α, and/or IL-2, between the four groups (Figure 2A). HIV-uninfected individuals with LTBI were characterized by a predominant proportion of IFN-γ+IL-2+TNF-α+ cells (median, 44%; IQR, 35–49%), a subset that was significantly lower in individuals with HIV (median, 20%; IQR, 15–32%), aTB (median, 16%; IQR, 4–19%), or both (median, 9%; IQR, 2.6–22%) (Figure 2B). In participants with HIV and/or aTB, IFN-γ+IL-2−TNF-α+ cells counterweighed the reduction of triple-positive cells. Of note, unlike previously reported (2), no differences in the proportion of TNF-α single-positive Mtb-specific CD4+ T cells were observed; these differences could arise from significant disparities in the age, ethnicity, and TB diagnosis in the study cohorts. Receiver operating characteristic curve analyses (Figure 2C) show that the proportion of IFN-γ+IL-2+TNF-α+ or IFN-γ+IL-2−TNF-α+ Mtb-specific CD4+ T cells allowed the distinction between LTBI and aTB in HIV-uninfected individuals (AUC = 0.97 [P < 0.0001]; AUC = 0.92 [P < 0.0001], respectively), but not in HIV-infected persons.
Figure 2.
Comparison of the polyfunctional profile of early secretory antigenic target-6 (ESAT-6)/culture filtrate protein-10 (CFP-10)–specific CD4+ T cells between HIV-uninfected and HIV-infected individuals with latent tuberculosis infection (LTBI) or active tuberculosis (aTB). (A) Representative dot plots of IFN-γ, tumor necrosis factor (TNF)-α, and IL-2 production in response to ESAT-6/CFP-10 peptide pool in one LTBI/HIV− individual. NS = no stimulation. Numbers represent the frequencies of cytokine-producing cells expressed as a percentage of the total CD4+ T-cell population. (B) Proportion of Mycobacterium tuberculosis (Mtb)-specific CD4+ T cells producing any possible combinations of IFN-γ, TNF-α, or IL-2. Horizontal bars represent the median values and interquartile range. Statistical analysis was performed using Mann-Whitney test; significant differences are indicated by asterisks (***P < 0.001, **P < 0.01, *P < 0.05). Each slice of the pie corresponds to a distinct combination of cytokine. A key to colors used in the pie charts is shown at the bottom of the graph. (C) Receiver operating characteristic curves and specificity/sensitivity crossover plots for the proportion of IFN-γ+ IL-2+ TNF-α+ (top), and IFN-γ+ IL-2−TNF-α+ (bottom) Mtb-specific CD4+ T cells to discriminate between LTBI or aTB in HIV-uninfected and HIV-infected individuals. The diagonal dashed line depicts an area under the curve (AUC) of 0.5, representing a random test. The dashed vertical line on the crossover plots represents the optimal threshold to distinguish LTBI and aTB individuals. CI = confidence interval.
In summary, these data show that HLA-DR expression on IFN-γ+ Mtb-specific CD4+ T cells represents a robust marker to distinguish between LTBI and aTB in both HIV-uninfected and antiretroviral therapy–naive HIV-infected individuals. This suggests that despite HIV-induced systemic immune activation, active bacterial replication promotes further up-regulation of HLA-DR on Mtb-specific CD4+ T cells. On the contrary, the polyfunctional profile of Mtb-specific CD4+ T cells associated with TB status solely in HIV-uninfected individuals, suggesting HIV infection may alter the secretion potential and/or localization of Mtb-specific CD4+ T cells even in the absence of bacterial replication. One main limitation of such assays, requiring cell stimulation to identify Mtb-specific CD4+ T cells, is that the analysis is restricted to individuals with detectable Mtb responses. Inclusion of additional immunodominant Mtb antigens could improve the “coverage” of Mtb responders. Further experiments will be needed to confirm these data in a larger study including HIV-infected participants receiving antiretroviral treatment. Nevertheless, this study confirms that HLA-DR expression could represent an important alternate tool to assess TB status in HIV-uninfected individuals and expand this finding to HIV-infected subjects.
Footnotes
C.R. is funded by the National Institutes of Health, Office of the Director (R21AI115977). K.A.W. is funded by the Medical Research Council UK. R.J.W. is supported by the Wellcome Trust (084323 and 104803), the Medical Research Council UK (U1175.02.002.00014.01), the European Union (FP7-Health-F3–2012–305578), South African National Research Foundation, and Medical Research Council South Africa Strategic Health Innovations Partnership.
Author Contributions: C.R. and K.A.W. designed the study; K.A.W. performed the experiments; C.R. and K.A.W. analyzed the data; T.O., H.P.G., and R.G. contributed to patient recruitment and diagnosis and sample collection and storage; C.R., K.A.W., and R.J.W. drafted the manuscript; all authors read, critically revised, and approved the final manuscript.
Author disclosures are available with the text of this letter at www.atsjournals.org.
References
- 1.Gardiner JL, Karp CL. Transformative tools for tackling tuberculosis. J Exp Med. 2015;212:1759–1769. doi: 10.1084/jem.20151468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Harari A, Rozot V, Bellutti Enders F, Perreau M, Stalder JM, Nicod LP, Cavassini M, Calandra T, Blanchet CL, Jaton K, et al. Dominant TNF-α+ Mycobacterium tuberculosis-specific CD4+ T cell responses discriminate between latent infection and active disease. Nat Med. 2011;17:372–376. doi: 10.1038/nm.2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lichtner M, Mascia C, Sauzullo I, Mengoni F, Vita S, Marocco R, Belvisi V, Russo G, Vullo V, Mastroianni CM. Multifunctional analysis of CD4+ T-cell response as immune-based model for tuberculosis detection. J Immunol Res. 2015;2015:217287. doi: 10.1155/2015/217287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Portevin D, Moukambi F, Clowes P, Bauer A, Chachage M, Ntinginya NE, Mfinanga E, Said K, Haraka F, Rachow A, et al. Assessment of the novel T-cell activation marker-tuberculosis assay for diagnosis of active tuberculosis in children: a prospective proof-of-concept study. Lancet Infect Dis. 2014;14:931–938. doi: 10.1016/S1473-3099(14)70884-9. [DOI] [PubMed] [Google Scholar]
- 5.Adekambi T, Ibegbu CC, Cagle S, Kalokhe AS, Wang YF, Hu Y, Day CL, Ray SM, Rengarajan J. Biomarkers on patient T cells diagnose active tuberculosis and monitor treatment response. J Clin Invest. 2015;125:3723. doi: 10.1172/JCI83279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Paiardini M, Müller-Trutwin M. HIV-associated chronic immune activation. Immunol Rev. 2013;254:78–101. doi: 10.1111/imr.12079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kaforou M, Wright VJ, Oni T, French N, Anderson ST, Bangani N, Banwell CM, Brent AJ, Crampin AC, Dockrell HM, et al. Detection of tuberculosis in HIV-infected and -uninfected African adults using whole blood RNA expression signatures: a case-control study. PLoS Med. 2013;10:e1001538. doi: 10.1371/journal.pmed.1001538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pollock KM, Whitworth HS, Montamat-Sicotte DJ, Grass L, Cooke GS, Kapembwa MS, Kon OM, Sampson RD, Taylor GP, Lalvani A. T-cell immunophenotyping distinguishes active from latent tuberculosis. J Infect Dis. 2013;208:952–968. doi: 10.1093/infdis/jit265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chiacchio T, Petruccioli E, Vanini V, Cuzzi G, Pinnetti C, Sampaolesi A, Antinori A, Girardi E, Goletti D. Polyfunctional T-cells and effector memory phenotype are associated with active TB in HIV-infected patients. J Infect. 2014;69:533–545. doi: 10.1016/j.jinf.2014.06.009. [DOI] [PubMed] [Google Scholar]
- 10.Canaday DH, Sridaran S, Van Epps P, Aung H, Burant CJ, Nsereko M, Mayanja-Kizza H, Betts MR, Toossi Z. CD4+ T cell polyfunctional profile in HIV-TB coinfection are similar between individuals with latent and active TB infection. Tuberculosis (Edinb) 2015;95:470–475. doi: 10.1016/j.tube.2014.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]