ABSTRACT
Despite the search for new therapeutic strategies for gastric cancer (GC), there is much evidence of progression due to resistance to chemotherapy. Multidrug resistance (MDR) is the ability of cancer cells to survive after exposure to chemotherapeutic agents. The involvement of miRNAs in the development of MDR has been well described but miRNAs able to modulate the sensitivity to chemotherapy by regulating hypoxia signaling pathways have not yet been fully addressed in GC. Our aim was to analyze miR-20b, miR-27a and miR-181a expression with respect to (epirubicin/oxaliplatin/capecitabine (EOX)) chemotherapy regimen in a set of GC patients, in order to investigate whether miRNAs deregulation may influence GC MDR also via hypoxia signaling modulation. Cancer biopsy were obtained from 21 untreated HER2 negative advanced GC patients, retrospectively analyzed. All patients received a first-line chemotherapy (EOX) regimen. MirWalk database was used to identify miR-27a, miR-181a and miR-20b target genes. The expression of miRNAs and of HIPK2, HIF1A and MDR1 genes were detected by real-time PCR. HIPK2 localization was assessed by immunohistochemistry. Our data showed the down-regulation of miR-20b, miR-27a, miR-181a concomitantly to higher levels of MDR1, HIF1A and HIPK2 genes in GC patients with a progressive disease respect to those with a disease control rate. Moreover, immunohistochemistry assay highlighted a higher cytoplasmic HIPK2 staining, suggesting a different role for it. We showed that aberrant expression of miR-20b, miR27a and miR-181a was associated with chemotherapeutic response in GC through HIF1A, MDR1 and HIPK2 genes modulation, suggesting a possible novel therapeutic strategy.
KEYWORDS: Gastric cancer, hypoxia signaling pathways, MDR, miR-27a, miR-181a, miR-20b
Abbreviations
- cHIPK2
cytoplasm HIPK2
- DCR
disease control rate
- EOX
epirubicin/oxaliplatin/capecitabine
- GC
gastric cancer
- MDR
multidrug resistance
- nHIPK2
nuclear HIPK2
- PD
progression disease
Introduction
Gastric cancer (GC) is the sixth leading cause of cancer in Europe and the second leading cause of mortality worldwide.1 65% of patients with GC present an advanced or metastatic stage at diagnosis.2 In this setting, Her2-neu positive GC patients receive first line therapy containing Trastuzumab associated with chemotherapy, achieving a median overall survival of 13.8 months. Conversely, Her2-neu negative patients who can be treated with chemotherapy alone have a shorter survival.3 Currently for this latter group of patients the gold standard includes treatment with the triplet formed by epirubicin, oxaliplatin, and capecitabine (EOX).4,5 Despite the search for new therapeutic strategies with targeted therapy in GC (future oncology), there is much evidence of progression under 12 months of diagnosis due to resistance to chemotherapy, the greatest obstacle to treatment success.6
Multidrug resistance (MDR) is the ability of cancer cells to survive after exposure to different chemotherapeutic agents and constitutes the most important obstacle for the effectiveness of cancer treatment. Recently, the role of the hypoxic tumor microenvironment in chemotherapy failure has been increasingly considered. Emerging evidence has described the role of hypoxia inducible factor-1 (HIF-1a) as a transcriptional regulator of P-glycoprotein (P-gp) expression.7,8 P-gp is a product of the MDR1 gene and is known to play a role in the efflux of drugs from cancer cells.9 Among the mechanisms underlying MDR, the over-expression of P-gp seems to be the major form of resistance to chemotherapy. In the last years, increasing evidence has highlighted the contributions of homeodomain-interacting protein kinase-2 (HIPK2) in counteracting hypoxia-induced cancer chemoresistance. Besides its role as a regulator of p53 apoptotic function, HIPK2 exerts antitumor activity by modulating the transcription activity of several transcription factors, including HIF-1α. Inhibition of HIF-1α activity by HIPK2 reduces the expression of MDR1 and stimulates drug-induced apoptosis in p53-dependent and -independent ways.10-12 Recently, microRNAs (miRNAs), small non coding RNAs that act as post-transcriptional repressors of gene expression, have been suggested to play essential roles in modulating chemotherapeutic tumor response.13-17 We selected some miRNAs that recent literature and bioinformatics tools have highlighted to be involved in MDR1, HIF1A and HIPK2 gene modulation. Several studies have suggested the ability of miR-27a to modulate MDR1/P-gp expression 18-20 and the association of miR-181a deregulation with the modulation of HIPK2.21 Moreover, emerging evidence documented miR-20b as a direct regulator of HIF1α expression. 22 Given that hypoxia is one of the major factors that promotes MDR, we investigated whether miRNA deregulation may influence gastric cancer MDR by regulating the expression of the MDR1 gene also via hypoxia signaling modulation. For this purpose, we analyzed the expression of miR-20b, miR-27a and miR-181a with respect to a first-line chemotherapy (epirubicin/oxaliplatin/capecitabine (EOX)) regimen in a set of GC patients, in order to investigate their eventual predictive role.
Results
Analysis of mir-27a, miR-181a and miR-20b and their target genes HIF1A, MDR1 and HIPK2
The miRNA target analysis tool miRWalk 23 was used to screen validated targets of miR-27a, miR-181a and miR-20b: HIF1A was found to be a validated target of miR-20b, MDR1 a validated target of miR-27a and HIPK2a gene regulated by miR-181a and miR-27a. To confirm the targeting of HIF1A, MDR1 and HIPK2 by miR-27a, miR-181a and miR-20b, a correlation analysis was carried out. A negative correlation was found between each gene HIF1A, MDR1 and HIPK2 and the correlated miRNA (HIF1A/miR-20b: Spearman r = −0.21; P = 0.34; MDR1/miR-27a: Spearman r = −0.5; P = 0.01; HIPK2/miR-181a: Spearman r = −0.4; P = 0.06; HIPK2/miR-27a: Spearman r = −0.66, P = 0.0009) (Figs. 1A–D).
Figure 1.
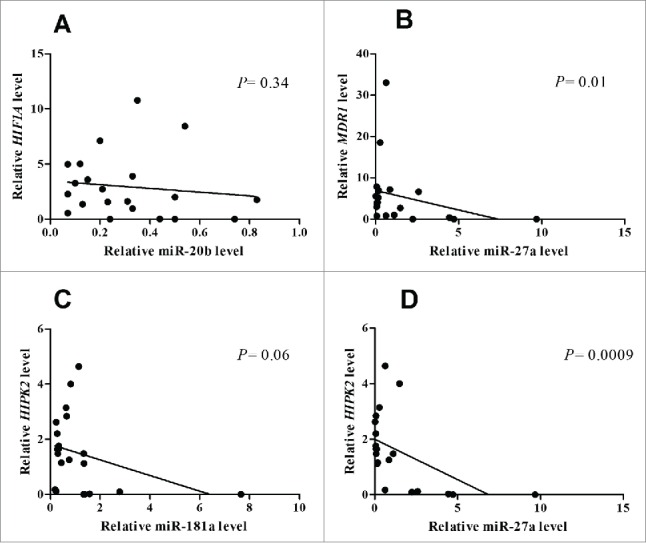
Correlation analysis betweenHIF1A and miR-20bexpression (A) MDR1 and miR-27a levels (B) and HIPK2 and miR-181a (C) and HIPK2 and miR-27a (D) in GC patients.
MiRNAs and target genes expression with regards to clinico-pathological characteristics
The clinico-pathological characteristics of all advanced GC patients are summarized in Table 2. The expression level of mir-27a, miR-181a and miR-20b was evaluated with regards to different clinico-pathological features in order to highlight their eventual prognostic role in GC patients. Lower median expression levels of all 3 miRNAs resulted associated with wider nodal involvement, older age (>63 years) and male gender as reported in Table 2.
Table 2.
Correlation between miR-27a, miR-181a, miR20b and relative target genes expression with clinicopathologic characteristics in GC patients.
| miR-27a median level | miR-181a median level | miR-20b median level | MDR-1 median level | HIF1A median level | HIPK2 median level | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age | ||||||||||||
| ≤63 (n = 11) | 1.11 | P = 0.13 | 1.14 | P = 0.2 | 0.33 | P = 0.41 | 3.02 | P = 0.91 | 1.99 | P = 0.75 | 1.25 | P = 0.77 |
| >63 (n = 10) | 0.13 | 0.39 | 0.22 | 3.6 | 2.16 | 1.56 | ||||||
| T | ||||||||||||
| II-III (n = 15) | 0.62 | P = 0.87 | 0.66 | P = 0.53 | 0.24 | P = 0.45 | 3.94 | P = 0.81 | 2.27 | P = 0.61 | 1.25 | P = 0.34 |
| IV (n = 6) | 0.69 | 0.72 | 0.27 | 1.83 | 1.68 | 1.56 | ||||||
| Lymph nodes status | ||||||||||||
| N0-1-2 (n = 8) | 1.17 | P = 0.36 | 0.79 | P = 0.46 | 0.38 | P = 0.27 | 0.80 | P = 0.05 | 1.48 | P = 0.32 | 0.71 | P = 0.58 |
| N3 (n = 13) | 0.18 | 0.44 | 0.21 | 5.22 | 2.72 | 1.48 | ||||||
| Gender | ||||||||||||
| F (n = 10) | 1.3 | P = 0.34 | 1.08 | P = 0.5 | 0.32 | P = 0.37 | 1.83 | P = 0.06 | 1.68 | P = 0.54 | 1.3 | P = 0.39 |
| M (n = 11) | 0.27 | 0.63 | 0.23 | 5.54 | 2.72 | 1.48 | ||||||
The expression of the 3 miRNA target genes HIF1A, MDR1 and HIPK2 was studied. Interestingly, an inverse trend of gene expression was highlighted compared to that of mir-27a, miR-181a and miR-20b. Higher median expression levels of HIF1A, MDR1 and HIPK2 were found in gastric N3 tumors and in males, while no difference was found in gene expression with respect to tumor size (T) or GC patient age.
Analysis of mir-27a, miR-181a and miR-20b and treatment response
To understand if miR-27a, miR-181a and miR-20b can regulate response to chemotherapy, patients were divided into those with progression disease (PD) (n = 15) and those with disease control rate (DCR) (n = 6). The expression of mir-27a, miR-181a and miR-20b and of their target genes was measured with respect to the response.
The median levels of the 3 miRNAs were lower in GC patients with PD compared to those with DCR (miR-27a: 0.18 vs 2.04; P = 0.07; miR-181a: 0.63 vs 1.08; P = 0.72 and miR-20b: 0.21 vs 0.4; P = 0.21) (Fig. 2A). Also considering median expression level as cutoff, all 3 miRNAs resulted more frequently overexpressed in GC patients with DCR compared to those with PD (miR-27a: 83.3% vs 40%; P = 0.14; miR-181a: 66.7% vs 46.7%;P = 0.63 and miR-20b: 83.3% vs 40%; P = 0.14). Probably the low number of the enrolled cases prevented reaching a statistical significance (Fig. 2B).
Figure 2.

Median levels ofmiR-27a, miR-181a and miR-20b in GC patients with PD vs those with DCR (A). Percentage of miR-27a, miR-181a and miR-20b overexpression in GC patients with PD vs those with DCR (B). Median levels of HIF1A, MDR1 and HIPK2 in GC patients with PD vs those with DCR (C). Percentage of HIF1A, MDR1 and HIPK2 overexpression in GC patients with PD vs those with DCR (D).
We investigated the expression of the 3 genes in the treatment response GC subgroups. The median expression level of the 3 genes resulted higher in GC patients with PD compared to those with DCR (Fig. 2C). However, when considering the frequency of HIF1A, MDR1 and HIPK2 overexpression, a higher percentage of HIF1A and MDR1 overexpression was observed in advanced GC with PD compared to those with DCR (HIF1A: 60% vs 33.3%; P = 0.36; MDR1: 66.7% vs 16.7%; P = 0.06). On the contrary, there was no difference in the frequency of HIPK2 gene overexpression in the 2 GC subgroups (Fig. 2D). The role of the 3 genes in PD patients was stressed by the correlation among gene expression: a positive and significant correlation was observed between HIF1A and HIPK2 (Spearman r = 0.97; P< 0.0001), between HIF1A and MDR1 (Spearman r = 0.63; P = 0.01) and finally between HIPK2 and MDR1 (Spearman r = 0.69; P = 0.003) (Figs. 3A-3B). Conversely, a significant relationship between HIF1A and MDR1 (Spearman r = 0.94; P = 0.01) was found in GC patients with DCR.
Figure 3.
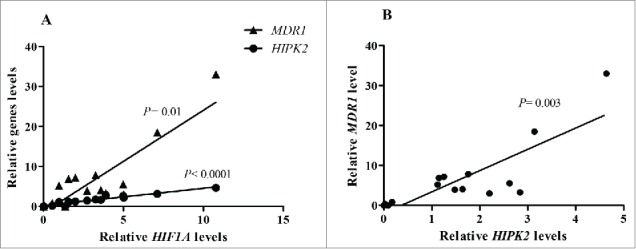
Correlation analysis between HIF1A and HIPK2 levels (A) HIF1A and MDR1 levels (A) and HIPK2 and MDR1 (B) in GC patients with PD.
HIPK2 subcellular localization was explored by immunohistochemistry assay. In detail, cytoplasmic HIPK2 (cHIPK2) (25%, range 0–60%) was much more expressed than nuclear HIPK2 (nHIPK2) (3,7%, range 0–8%) (P = 0,0009) (Fig. 4).
Figure 4.
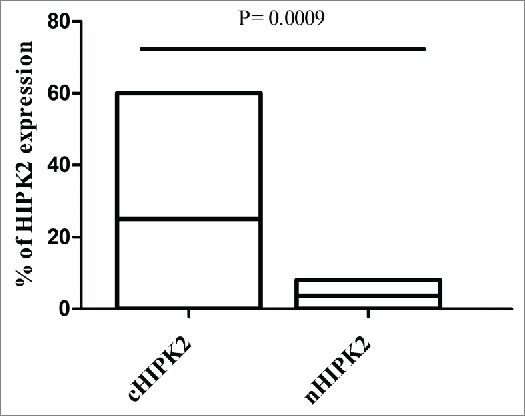
Percentage of HIPK2 protein expression in cytoplasm and nucleus of GC patients. Bars in the histogram represent percentage range and median value of HIPK2 protein expression.
Discussion
MDR, defined as a lack of sensitivity of cancer cells to different drugs, remains the major clinical obstacle to the effectiveness of chemotherapy. Recently, miRNAs have been suggested to play a key role in the development of MDR.24 Through regulating gene expression, miRNAs can impact on different pathways associated with sensitivity or resistance to chemotherapeutic drugs in various tumors.13-17 Several miRNAs, such as miR-106a,25 miR-508-5p26 and miRNA-19a/b27 have been reported to play a role in the development of MDR in GC. However, miRNAs able to modulate the sensitivity to chemotherapy by regulating genes involved in the hypoxia signaling pathways have not yet been fully addressed in GC. In the last years, the influence of hypoxia on chemotherapeutic effects has been well described,28 thus becoming one of the main focuses of research in cancer treatment. In this study, we selected miR-27a, miR-181a and miR-20b because of literature and bioinformatics tools shown to be involved in MDR1, HIF1A and HIPK2 genes modulation. With regard to treatment response, higher median values of HIF1A gene expression were observed in the PD subgroup. Interestingly, in line with the literature which reported the ability of miR-20b to target the HIF1A transcript,22 lower expression levels of miR-20b were found in the GC subgroup showing elevated amounts of HIF1A mRNA. Moreover, the negative correlation observed between miR-20b and HIF1A suggested a direct regulation of this miRNA on HIF1A. With regards to the impact of hypoxia on the MDR, mounting evidence has recently described the influence of increasing HIF-1α levels on the expression and transport function of P-gp.7,8,29 P-gp is the product of the MDR1 gene able to regulate the bioavailability of drugs and its elevated levels represent the most highly frequent feature related to the MDR phenotype.30 In addition to higher levels of HIF1A mRNA, patients with PD also showed a high expression of the MDR1 gene, suggesting a close association between the 2 genes also confirmed by their positive correlation. Besides a transcriptional regulation of the MDR1 gene by HIF1α, we also supposed an epigenetic modulation of this gene. For this purpose, we explored the expression of miR-27a that both the literature and bioinformatics tools highlighted to target MDR1. In line with Feng et al's study31 in which miR-27a was inversely correlated with MDR1 and transfection of exogenous miR-27a down-regulated MDR1, our results showed a significant and negative correlation between miR-27a and MDR1 transcript. When miRNA expression was evaluated with respect to chemotherapy response, lower values of miR-27a and a higher expression of the MDR1 gene were observed in patients with a PD. Both a direct and an indirect regulation on MDR1 by miR-27a was described,32 although a downregulation of miR-27a with a decreased expression of P-gp was reported in GC.33 Interestingly, bioinformatics software (mirWalk)23 indicated HIPK2 to be a validated target of miR-27a as well as of miR-181a. Recent studies have demonstrated the capacity of miR-27a to modulate MDR1/P-glycoprotein expression by targeting HIPK2 in human ovarian cancer cells,18 highlighting a new player in hypoxia-mediated chemoresistance. HIPK2 is a tumor suppressor gene involved in the activation of p53 pro-apoptotic function, but it also acts as a co-repressor for many transcription factors, such as HIF1α.11 Inhibition of HIF-1α activity by HIPK2 reduces MDR1 gene expression and restrains HIF-1-induced tumor chemoresistance.12 Despite the extensive knowledge on HIPK2 function in many tumors,34 there are no reports about the role of HIPK2 in GC. Unexpectedly, in contrast to its tumor suppressor role, elevated levels of HIPK2 mRNA were observed in PD patients showing a lower expression of both miR-27a and miR-181a. A recent study reported a down-regulation of miR-181a with a subsequent modulation of HIPK2 after cisplatin treatment in neck squamous cell carcinoma,21 but in our study the role of HIPK2 in hypoxia-mediated MDR became less clear. Recently it has been reported that HIPK2 is not exclusively localized in the cell nucleus in which it exerts an pro-apoptotic role, but it can also be distributed into the cytoplasm35,36 where it was found associated to High-Mobility Group A1 (HMGA1) overexpression leading to inhibition of p53-mediated apoptosis.37 A dual role depending on subcellular distribution has been recently described for HIPK2,38 but it is still unclear whether altered HIPK2 localization could differently affect hypoxia-mediated chemoresistance. In order to verify the sublocalization of HIPK2 in patients of our cohort, an immunohistochemistry assay was performed. Interestingly, the immunohistochemistry assay revealed higher cytoplasm HIPK2 than nuclear dislocation in both the DCR and PD subgroups. No data are available on the link between cytoplasmic HIPK2 and HIF1α or P-gp, but we speculated that the cytoplasm dislocation of HIPK2 could be a mechanism of tumors cells to contrast HIPK2 function on hypoxia-mediated MDR. Our results showed a significant and positive correlation of HIPK2 with both HIF1A and MDR1 genes only within PD in the GC subset, suggesting a close and diverse association of HIPK2 to hypoxia in non-responder GC patients.
In this study, we explored miRNA-mediated MDR by affecting hypoxia signaling in GC. We showed that aberrant expression of miR-20b, miR27a and miR-181a was associated with chemotherapeutic response in GC through HIF1A, MDR1 and HIPK2 gene modulation, suggesting a possible novel strategy for the reversal of the hypoxia effect on MDR in GC. These results merit confirmation in larger, prospective clinical series to better understand the correlation between miRNA and MDR associated genes.
Materials and methods
Patients and methods
Samples of cancer biopsy were obtained from 21 untreated HER2 negative advanced GC patients, retrospectively analyzed, from the tissue bank of the IRCCS, Istituto Tumori “Giovanni Paolo II” in Bari and of the Morgagni Pierantoni Hospital in Forlì (Italy). All patients received an intravenous bolus of epirubicin at a dose of 50 mg/m2 (day 1) in combination with oxaliplatin at a dose of 130 mg/m2 (day 1) and capecitabine at a twice-daily dose of 625 mg/m2 (days 1→21) (EOX) in a cycle scheduled every 3 weeks. According to the Response Evaluation Criteria in Solid Tumors (RECIST) definition 1.1, all patients were evaluated after 3 cycles of treatment.39 Information about clinical parameters such as sex, age, tumor size, lymph nodes status and response to chemotherapy were obtained from clinical records.
All patients were reviewed and the following data were collected (Table 1): gender (male versus female), age, tumor size, lymph nodes status and response to chemotherapy (CR: complete response; PR: partial response; ORR, objective response rate: CR + PR; SD: stable disease; DCR, disease control rate: ORR + SD, PD: progression disease).
Table 1.
Characteristics of GC patients.
| Responce to chemotherapy | n |
|---|---|
| PD patients | 15 |
| DCR patients | 6 |
| Age | |
| ≤63 | 11 |
| >63 | 10 |
| T | |
| II–III | 15 |
| IV | 6 |
| Lymph nodes | |
| N0-1-2 | 8 |
| N3 | 13 |
| Metastatic status | |
| M+ | 21 |
| M0 | 0 |
| Gender | |
| F | 10 |
| M | 11 |
This study was approved by the Ethics Committee of the IRCCS Istituto Tumori “Giovanni Paolo II,” Bari, Italy (prot. n. 312/2015). All patients signed an informed consent and all data have been processed with respect for privacy and anonymity.
RNA extraction and miRNA detection
MiRNA expression analysis was performed on the set of GC samples stratified into 15 patientswith PD and 6with DCR.
Total RNA was extracted from formalin-fixed, paraffin-embedded GC and matched adjacent non-cancerous tissues by the RNeasy FFPE Kit (QIAGEN) according to the manufacturer's protocol. Concentrations were estimated with the ND-8000 Spectrophotometer (NanoDrop Technologies). Briefly, for detection of miR- 27a, miR-181a and miR-20b expression levels, 10 ng of total RNA were reverse transcribed using the TaqMan® MicroRNA Reverse Transcription Kit using miRNA specific primers according to the manufacturer's protocol (Applied Biosystems). Real Time PCR analysis was performed on the ABI Prism 7000 Sequence Detection System (Applied Biosystems) using 3 μl of RT products in a reaction mixture containing TaqMan miRNA assay and the TaqMan Universal PCR Master Mix, according to the manufacturer's instructions (Applied Biosystems). All PCR reactions were performed in duplicate. Relative quantities of each miRNA were calculated using the ΔΔCt method after normalization with endogenous reference RNU 48.
HIF1A, MDR1 and HIPK2 expression
Total RNA was extracted from FFPE GC tissues, as described above. The concentration of the isolated RNA was measured by a NanoDrop 8000 Spectrophotometer v2 1.0 (Thermo Scientific). Total RNA was reverse transcribed to single-stranded cDNA using the High Capacity cDNA Reverse Transcription Kit (Applied Biosystem). Reaction was performed using 500 ng of total RNA in a total volume of 20 μl according to the manufacturer's protocol (Applied Biosystem). cDNA synthesis was performed at 25°C for 10 min, then the reaction was incubated at 37°C for 120min followed by 85°C for 5min.Quantitative real-time PCR was performed using 62.5ng of cDNA in a final volume of 20 μl according to the manufacturer's instructions (Applied Biosystems),on the ABI Prism 7000 Sequence Detection System(Applied Biosystems). The ID assays used were the following: human HIF1A (Hs00153153_m1), human MDR1 (Hs00184500_m1), human HIPK2 (Hs00179759_m1). RN18S1(Hs03928985_g1) was used as the endogenous reference. The samples underwent PCR analysis using the following cycling parameters: at 50°C for 2 min, at 95°C for 10 min, then at 95°C for 15 s and at 60°C for 1 min, for 45 cycles. Relative expression was calculated using the comparative Ct method. Each sample was tested in duplicate.
HIPK2 protein level and cellular dislocation were detected by immunohistochemistry.4-µm-thick slices were cut from formalin-fixed and paraffin-embedded histological blocks and these were immunohistochemically stained using standard immunoperoxidase techniques.40 The primary antibody was mouse monoclonal anti-HIPK2 (ab57328; Abcam; 1:300 dilution). Human gastric tissues were used as positive control and the primary antibody was omitted and replaced by PBS pH7.6 for negative control.
Statistical analysis
Data analysis was performed using the GraphPad Prism statistics software package (GraphPad Prism 5.01). Statistical significance was determined using the Mann–Whitney U-test. The two-tailed Fischer's exact test was used to compare both miRNAs and gene frequency of overexpression between the GC subgroups. The cases were divided into 2 groups with the expression above and below the median expression of miRNAs or genes.
The Spearman correlation coefficient was used to analyze the correlations between gene expression or between miRNAs and their targets. A P value ≤ 0.05 was considered statistically significant.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
The authors would like to thank Caroline Oakley for manuscript revision.
References
- 1.Ferro A, Peleteiro B, Malvezzi M, Bosetti C, Bertuccio P, Levi F, Negri E, La Vecchia C, Lunet N. Worldwide trends in gastric cancer mortality (1980–2011), with predictions to 2015, and incidence by subtype. Eur J Cancer 2014; 50:1330-44; PMID:24650579; http://dx.doi.org/ 10.1016/j.ejca.2014.01.029 [DOI] [PubMed] [Google Scholar]
- 2.Hundahl SA, Phillips JL, Menck HR. The National Cancer Data Base Report on poor survival of U.S. gastric carcinoma patients treated with gastrectomy: Fifth Edition American Joint Committee on Cancer staging, proximal disease, and the “different disease” hypothesis. Cancer 2000; 88:921-32; PMID:10679663; http://dx.doi.org/ [DOI] [PubMed] [Google Scholar]
- 3.Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, Lordick F, Ohtsu A, Omuro Y, Satoh T, et al.. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet 2010; 376:687-97; PMID:20728210; http://dx.doi.org/ 10.1016/S0140-6736(10)61121-X [DOI] [PubMed] [Google Scholar]
- 4.Cunningham D, Okines AF, Ashley S. Capecitabine and oxaliplatin for advanced esophagogastric cancer. N Engl J Med 2010; 362:858-9; PMID:20200397; http://dx.doi.org/ 10.1056/NEJMc0911925 [DOI] [PubMed] [Google Scholar]
- 5.Cunningham D, Starling N, Rao S, Iveson T, Nicolson M, Coxon F, Middleton G, Daniel F, Oates J, Norman AR. Capecitabine and oxaliplatin for advanced esophagogastric cancer. N Engl J Med 2008; 358:36-46; PMID:18172173; http://dx.doi.org/ 10.1056/NEJMoa073149 [DOI] [PubMed] [Google Scholar]
- 6.Huang S, Chen M, Shen Y, Shen W, Guo H, Gao Q, Zou X. Inhibition of activated Stat3 reverses drug resistance to chemotherapeutic agents in gastric cancer cells. Cancer Lett 2012; 315:198-205; PMID:22104727; http://dx.doi.org/ 10.1016/j.canlet.2011.10.011 [DOI] [PubMed] [Google Scholar]
- 7.Chen J, Ding Z, Peng Y, Pan F, Li J, Zou L, Zhang Y, Liang H. HIF-1alpha inhibition reverses multidrug resistance in colon cancer cells via downregulation of MDR1/P-glycoprotein. PLoS One 2014; 9:e98882; PMID: 24901645; http://dx.doi.org/12067980 10.1371/journal.pone.0098882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Comerford KM, Wallace TJ, Karhausen J, Louis NA, Montalto MC, Colgan SP. Hypoxia-inducible factor-1-dependent regulation of the multidrug resistance (MDR1) gene. Cancer Res 2002; 62:3387-94; PMID:12067980 [PubMed] [Google Scholar]
- 9.Breier A, Gibalova L, Seres M, Barancik M, Sulova Z. New insight into p-glycoprotein as a drug target. Anticancer Agents Med Chem 2013; 13:159-70; PMID:22931413; http://dx.doi.org/ 10.2174/187152013804487380 [DOI] [PubMed] [Google Scholar]
- 10.Nardinocchi L, Puca R, Sacchi A, Rechavi G, Givol D, D'Orazi G. Targeting hypoxia in cancer cells by restoring homeodomain interacting protein-kinase 2 and p53 activity and suppressing HIF-1alpha. PLoS One 2009; 4:e6819; PMID:19714248; http://dx.doi.org/ 10.1371/journal.pone.0006819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nardinocchi L, Puca R, Guidolin D, Belloni AS, Bossi G, Michiels C, Sacchi A, Onisto M, D'Orazi G. Transcriptional regulation of hypoxia-inducible factor 1alpha by HIPK2 suggests a novel mechanism to restrain tumor growth. Biochim Biophys Acta 2009; 1793:368-77; PMID:19046997; http://dx.doi.org/ 10.1016/j.bbamcr.2008.10.013 [DOI] [PubMed] [Google Scholar]
- 12.Nardinocchi L, Puca R, Sacchi A, D'Orazi G. Inhibition of HIF-1alpha activity by homeodomain-interacting protein kinase-2 correlates with sensitization of chemoresistant cells to undergo apoptosis. Mol Cancer 2009; 8:1; PMID:19128456; http://dx.doi.org/ 10.1186/1476-4598-8-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gisel A, Valvano M, El Idrissi IG, Nardulli P, Azzariti A, Carrieri A, Contino M, Colabufo NA. miRNAs for the detection of multidrug resistance: overview and perspectives. Molecules 2014; 19:5611-23; PMID:24786846; http://dx.doi.org/ 10.3390/molecules19055611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zheng T, Wang J, Chen X, Liu L. Role of microRNA in anticancer drug resistance. Int J Cancer 2010; 126:2-10; PMID:19634138; http://dx.doi.org/ 10.1002/ijc.24782 [DOI] [PubMed] [Google Scholar]
- 15.Kovalchuk O, Filkowski J, Meservy J, Ilnytskyy Y, Tryndyak VP, Chekhun VF, Pogribny IP. Involvement of microRNA-451 in resistance of the MCF-7 breast cancer cells to chemotherapeutic drug doxorubicin. Mol Cancer Ther 2008; 7:2152-9; PMID:18645025; http://dx.doi.org/ 10.1158/1535-7163.MCT-08-0021 [DOI] [PubMed] [Google Scholar]
- 16.Brunetti O, Russo A, Scarpa A, Santini D, Reni M, Bittoni A, Azzariti A, Aprile G, Delcuratolo S, Signorile M, et al.. Micro-RNA in pancreatic adenocarcinoma: predictive/prognostic biomarkers or therapeutic targets? Oncotarget 2015; 15:6(27):23323-41; PMID:26259238; http://dx.doi.org/26156293 10.18632/oncotarget.4492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pinto R, Strippoli S, De Summa S, Albano A, Azzariti A, Guida G, Popescu O, Lorusso V, Guida M, Tommasi S. MicroRNA expression in BRAF-mutated and wild-type metastatic melanoma and its correlation with response duration to BRAF inhibitors. Expert Opin Ther Targets 2015:1-9; PMID:26156293; http://dx.doi.org/ 10.1517/14728222.2015.1065818 [DOI] [PubMed] [Google Scholar]
- 18.Li Z, Hu S, Wang J, Cai J, Xiao L, Yu L, Wang Z. MiR-27a modulates MDR1/P-glycoprotein expression by targeting HIPK2 in human ovarian cancer cells. Gynecol Oncol 2010; 119:125-30; PMID:20624637; http://dx.doi.org/ 10.1016/j.ygyno.2010.06.004 [DOI] [PubMed] [Google Scholar]
- 19.Chen Z, Ma T, Huang C, Zhang L, Lv X, Xu T, Hu T, Li J. MiR-27a modulates the MDR1/P-glycoprotein expression by inhibiting FZD7/beta-catenin pathway in hepatocellular carcinoma cells. Cell Signal 2013; 25:2693-701; PMID:24018051; http://dx.doi.org/ 10.1016/j.cellsig.2013.08.032 [DOI] [PubMed] [Google Scholar]
- 20.Zhu H, Wu H, Liu X, Evans BR, Medina DJ, Liu CG, Yang JM. Role of MicroRNA miR-27a and miR-451 in the regulation of MDR1/P-glycoprotein expression in human cancer cells. Biochem Pharmacol 2008; 76:582-8; PMID:18619946; http://dx.doi.org/ 10.1016/j.bcp.2008.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang Y, Chuang A, Hao H, Talbot C, Sen T, Trink B, Sidransky D, Ratovitski E. Phospho-DeltaNp63alpha is a key regulator of the cisplatin-induced microRNAome in cancer cells. Cell Death Differ 2011; 18:1220-30; PMID:21274007; http://dx.doi.org/ 10.1038/cdd.2010.188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nallamshetty S, Chan SY, Loscalzo J. Hypoxia: a master regulator of microRNA biogenesis and activity. Free Radic Biol Med 2013; 64:20-30; PMID:23712003; http://dx.doi.org/ 10.1016/j.freeradbiomed.2013.05.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dweep H, Sticht C, Pandey P, Gretz N. miRWalk–database: prediction of possible miRNA binding sites by “walking” the genes of three genomes. J Biomed Inform 2011; 44:839-47; PMID:21605702; http://dx.doi.org/ 10.1016/j.jbi.2011.05.002 [DOI] [PubMed] [Google Scholar]
- 24.Xu K, Liang X, Shen K, Cui D, Zheng Y, Xu J, Fan Z, Qiu Y, Li Q, Ni L, et al.. miR-297 modulates multidrug resistance in human colorectal carcinoma by down-regulating MRP-2. Biochem J 2012; 446:291-300; PMID:22676135; http://dx.doi.org/ 10.1042/BJ20120386 [DOI] [PubMed] [Google Scholar]
- 25.Zhang Y, Lu Q, Cai X. MicroRNA-106a induces multidrug resistance in gastric cancer by targeting RUNX3. FEBS Lett 2013; 587:3069-75; PMID:23932924; http://dx.doi.org/ 10.1016/j.febslet.2013.06.058 [DOI] [PubMed] [Google Scholar]
- 26.Shang Y, Zhang Z, Liu Z, Feng B, Ren G, Li K, Zhou L, Sun Y, Li M, Zhou J, et al.. miR-508-5p regulates multidrug resistance of gastric cancer by targeting ABCB1 and ZNRD1. Oncogene 2014; 33:3267-76; PMID:23893241; http://dx.doi.org/ 10.1038/onc.2013.297 [DOI] [PubMed] [Google Scholar]
- 27.Wang F, Li T, Zhang B, Li H, Wu Q, Yang L, Nie Y, Wu K, Shi Y, Fan D. MicroRNA-19a/b regulates multidrug resistance in human gastric cancer cells by targeting PTEN. Biochem Biophys Res Commun 2013; 434:688-94; PMID:23603256; http://dx.doi.org/ 10.1016/j.bbrc.2013.04.010 [DOI] [PubMed] [Google Scholar]
- 28.Huang L, Ao Q, Zhang Q, Yang X, Xing H, Li F, Chen G, Zhou J, Wang S, Xu G, et al.. Hypoxia induced paclitaxel resistance in human ovarian cancers via hypoxia-inducible factor 1alpha. J Cancer Res Clin Oncol 2010; 136:447-56; PMID:19760195; http://dx.doi.org/ 10.1007/s00432-009-0675-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wartenberg M, Ling FC, Muschen M, Klein F, Acker H, Gassmann M, Petrat K, Putz V, Hescheler J, Sauer H. Regulation of the multidrug resistance transporter P-glycoprotein in multicellular tumor spheroids by hypoxia-inducible factor (HIF-1) and reactive oxygen species. FASEB J 2003; 17:503-5; PMID:12514119; http://dx.doi.org/ 10.1096/fj.02-0358fje [DOI] [PubMed] [Google Scholar]
- 30.He Q, Zhang G, Hou D, Leng A, Xu M, Peng J, Liu T. Overexpression of sorcin results in multidrug resistance in gastric cancer cells with up-regulation of P-gp. Oncol Rep 2011; 25:237-43; PMID:21109982; http://dx.doi.org/ 10.3892/or_00001066 [DOI] [PubMed] [Google Scholar]
- 31.Feng DD, Zhang H, Zhang P, Zheng YS, Zhang XJ, Han BW, Luo XQ, Xu L, Zhou H, Qu LH, et al.. Down-regulated miR-331-5p and miR-27a are associated with chemotherapy resistance and relapse in leukaemia. J Cell Mol Med 2011; 15:2164-75; PMID:21070600; http://dx.doi.org/ 10.1111/j.1582-4934.2010.01213.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Toscano-Garibay JD, Aquino-Jarquin G. Regulation exerted by miRNAs in the promoter and UTR sequences: MDR1/P-gp expression as a particular case. DNA Cell Biol 2012; 31:1358-64; PMID:22662865; http://dx.doi.org/ 10.1089/dna.2012.1703 [DOI] [PubMed] [Google Scholar]
- 33.Zhao X, Yang L, Hu J. Down-regulation of miR-27a might inhibit proliferation and drug resistance of gastric cancer cells. J Exp Clin Cancer Res 2011; 30:55; PMID:21569481; http://dx.doi.org/ 10.1186/1756-9966-30-55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Puca R, Nardinocchi L, Givol D, D'Orazi G. Regulation of p53 activity by HIPK2: molecular mechanisms and therapeutical implications in human cancer cells. Oncogene 2010; 29:4378-87; PMID:20514025; http://dx.doi.org/ 10.1038/onc.2010.183 [DOI] [PubMed] [Google Scholar]
- 35.Saul VV, de la Vega L, Milanovic M, Kruger M, Braun T, Fritz-Wolf K, Becker K, Schmitz ML. HIPK2 kinase activity depends on cis-autophosphorylation of its activation loop. J Mol Cell Biol 2013; 5:27-38; PMID:23000554; http://dx.doi.org/ 10.1093/jmcb/mjs053 [DOI] [PubMed] [Google Scholar]
- 36.Siepi F, Gatti V, Camerini S, Crescenzi M, Soddu S. HIPK2 catalytic activity and subcellular localization are regulated by activation-loop Y354 autophosphorylation. Biochim Biophys Acta 2013; 1833:1443-53; PMID:23485397; http://dx.doi.org/ 10.1016/j.bbamcr.2013.02.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pierantoni GM, Rinaldo C, Mottolese M, Di Benedetto A, Esposito F, Soddu S, Fusco A. High-mobility group A1 inhibits p53 by cytoplasmic relocalization of its proapoptotic activator HIPK2. J Clin Invest 2007; 117:693-702; PMID:17290307; http://dx.doi.org/ 10.1172/JCI29852 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 38.Polonio-Vallon T, Kirkpatrick J, Krijgsveld J, Hofmann TG. Src kinase modulates the apoptotic p53 pathway by altering HIPK2 localization. Cell Cycle 2014; 13:115-25; PMID:24196445; http://dx.doi.org/ 10.4161/cc.26857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, et al.. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009; 45:228-47; PMID:19097774; http://dx.doi.org/ 10.1016/j.ejca.2008.10.026 [DOI] [PubMed] [Google Scholar]
- 40.Mangia A, Caldarola L, Dell'Endice S, Scarpi E, Saragoni L, Monti M, Santini D, Brunetti O, Simone G, Silvestris N. The potential predictive role of nuclear NHERF1 expression in advanced gastric cancer patients treated with epirubicin/oxaliplatin/capecitabine first line chemotherapy. Cancer Biol Ther 2015; 16:1140-7; PMID:26126066; http://dx.doi.org/ 10.1080/15384047.2015.1056414 [DOI] [PMC free article] [PubMed] [Google Scholar]


