ABSTRACT
Telomerase activation is one of the key mechanisms that allow cells to bypass replicative senescence. Telomerase activity is primarily regulated at the level of transcription of its catalytic unit- hTERT. Prostate cancer (PCa), akin to other cancers, is characterized by high telomerase activity. Existing data suggest that hTERT expression and telomerase activity are positively regulated by androgenic stimuli in androgen-dependent prostate cancer (ADPC) cells. A part of the present study reaffirmed this by demonstrating a decline in the hTERT expression and telomerase activity on “loss of AR” in ADPC cells. The study further addressed 2 unresolved queries, i) whether AR-mediated signaling is of any relevance to hTERT expression in castration-resistant prostate cancer (CRPC) and ii) whether this signaling involves EGR1. Our data suggest that AR-mediated signaling negatively regulates hTERT expression in CRPC cells. Incidental support for the possibility of EGR1 being a regulator of hTERT expression in PCa was provided by i) immunolocalization of hTERT and EGR1 proteins in the same cell type (secretory epithelium) of PCa and BPH tissues; ii) significantly (p< 0.001) higher levels of both these proteins in CRPC (PC3 and DU145), compared with ADPC (LNCaP) cells. A direct evidence for the role of EGR1 in hTERT expression was evident by a significant (p<0.0001) decrease in the hTERT transcript levels in the EGR1-silenced CRPC cells. Further, “gain of AR” led to a significant reduction in the levels of hTERT and EGR1 in CRPC cells. However, restoration of EGR1 levels prevented the decline in the hTERT transcript levels in these cells. Taken together, our data indicate that AR regulates the expression of EGR1, which in turn acts as a positive regulator of hTERT expression in CRPC cells. Thus, AR exerts an inhibitory effect on hTERT expression and telomerase activity by modulating EGR1 levels in CRPC cells.
KEYWORDS: Androgen receptor, EGR-1, hTERT, prostate cancer, telomerase
Abbreviations
- hTERT
human Telomerase Enzyme Reverse Transcriptase
- EGR1
Early Growth Response1
- AR
Androgen Receptor
- ADPC
Androgen- Dependent Prostate Cancer
- CRPC
Castration- Resistant Prostate Cancer
- ADT
Androgen Deprivation Therapy
- hTR
human Telomerase RNA
- PCa
Prostate Cancer
- BPH
Benign Prostate Hyperplasia
- FGF
Fibroblast Growth Factor
- WNT
Wingless and Integrase
- qRT-PCR
Quantitative Reverse Transcription-Polymerase Chain Reaction
- DHT
Dihydrotestosterone
- ARE
Androgen Response Element
- BPH
Benign Prostatic Hyperplasia
- HRP
Horseradish Peroxidase
- DPX
Distyrene Plasticizer and Xylene
- FITC
Fluorescein isothiocyanate
- GAPDH
Glyceraldehyde-3-Phosphate Dehydrogenase
- SO
Scrambled Oligos
- RQ
Relative Quantity
- CT
Threshold Cycle
- SDS PAGE
Sodium Dodecyl Sulfate -Polyacrylamide Gel
- PVDF
Polyvinylidenedifluoride
- ECL
Enhanced Chemi Luminescence
- IQTL
ImageQuant TL
- BSA
Bovine Serum Albumin
- TRAP
Telomeric Repeat Amplification Protocol
Introduction
Androgen signaling plays a critical role in the initiation and progression of prostate cancer. ADT thus remains the mainstay of the therapy for patients with locally advanced and metastatic prostate cancer. However, the benefits of ADT are short-lived, and almost all prostate cancers eventually acquire more aggressive castration-resistant phenotype.1 Aggressiveness of prostate tumors has been correlated with telomerase activity.2 Telomerase, a multimeric enzyme, consists of the catalytic subunit- hTERT,3 hTR4 and other associated proteins.5 Telomerase activity is regulated at several levels such as hTERT transcription, alternative splicing, phosphorylation, chaperone-mediated folding, assembly and nuclear transport of various telomerse subunits.6 Of these, transcriptional control of hTERT expression is reported to be of crucial significance in the regulation of telomerase activity.
Androgenic stimuli evoke contrasting effects on telomerase activity in normal and cancerous cells of the prostate. Androgens exert an inhibitory effect on telomerase activity in normal prostate and a stimulatory effect on hTERT expression in ADPC cells.7,8 Androgen deprivation led to an abrogation of hTERT expression and telomerase activity in ADPC cells.9 Further, a decline in hTERT immunostaining was observed in prostate cancer biopsies from patients who were on ADT.10 Collectively, these investigations are suggestive of a stimulatory effect of androgenic stimuli on hTERT expression in ADPC cells. However, the role of AR-mediated signaling in hTERT expression or telomerase activity in CRPC remains to be deciphered. Interestingly, Methylseleninic acid (MSA), an anti-cancer drug that targets AR, led to a reduction in the hTERT levels in an ADPC cell line, but not in a CRPC cell line.11 This was despite the fact that AR-mediated signaling remains functional in CRPC cells. A recent study suggests that AR drives a distinct transcriptional program in CRPC.12 In the wake of these observations, it is imperative to investigate whether in CRPC cells also, AR regulates the expression of hTERT and thereby modulates telomerase activity.
Extensive efforts have been made to elucidate the molecular mechanisms underlying the transition of ADPC to CRPC.13 In addition to modifications in AR signaling, several growth and survival pathways such as RAS/MAPK, Wnt/β-catenin, FGF have been implicated in the evolution of CRPC. EGR1, a nuclear transcription factor, has also been shown to be more highly expressed in CRPC clinical specimens than in ADPC samples.14 EGR1, known for its role in cell proliferation and apoptosis, was earlier considered a tumor suppressor. However, recent evidences indicate that EGR1 promotes the progression of prostate cancer.15 Yang et al (2006) also demonstrated that EGR1 facilitates the androgen-independent growth of prostate carcinoma cells in vitro and in vivo.16
We focused on EGR1 as a potential regulator of hTERT expression. Some reports implicate EGR1 as a hTERT regulator in cervical and placental cells.17,18 A consensus EGR1 binding motif is present between −273 and −281 nucleotides in the hTERT promoter region.17 However, the mode of hTERT regulation by EGR1 appears to be cell-type specific. EGR1 represses the endogenous hTERT expression in cervical cells and cancer tissue cells.17 In contrast, EGR1 activates the transcription of hTERT in placental cells.18 However, there are no reports that allude to EGR1 being a regulator of hTERT or a target of AR in prostate cancer cells.
The present study explored whether androgen receptor regulates the expression of hTERT and EGR1 in CRPC cells. The study also explored the possibility of hTERT regulation by EGR1 in CRPC cells.
Results
Subcellular localization of hTERT and EGR1 in BPH and PCa tissues
hTERT and EGR1 proteins were predominantly localized in the nuclei of the basal epithelial cells of BPH glands (Figs. 1a,b). In the PCa tissues, these proteins were intensely localized in the nuclear as well as cytoplasmic compartments of the secretory epithelial cells. Thus, both hTERT and EGR1 proteins showed a similar pattern of subcellular immunolocalization in PCa and BPH tissues.
Figure 1.
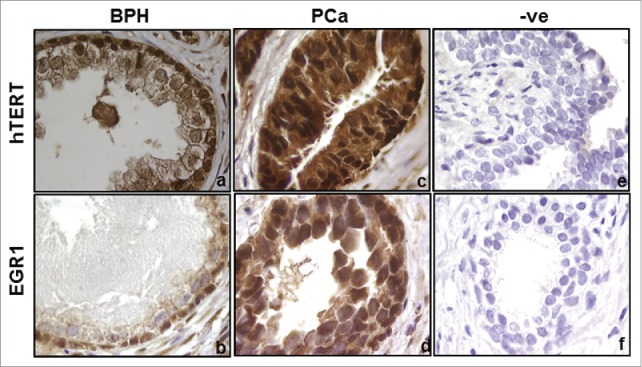
Immunolocalization of hTERT (a,c) and EGR1 (b,d) proteins in the prostate tissues of BPH and PCa patients. Panels e and f represent the sections where primary antibodies were replaced with IgGs. Magnification- 100X.
Basal levels of hTERT and EGR1 in PCa cell lines
qRT-PCR studies showed significantly (p<0.01) higher levels of EGR1 (Fig. 2A) and hTERT (Fig. 2B) transcripts in castration-resistant PC3 and DU145 cells, compared with androgen-dependent LNCaP cells.
Figure 2.
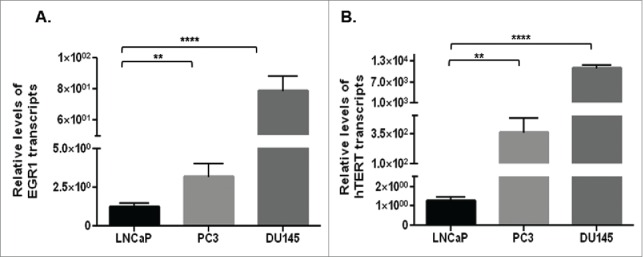
Basal levels of EGR1 (A) and hTERT (B) transcripts in castration-resistant (PC3 and DU145) cells and androgen-dependent (LNCaP) cells, as assessed by qRT-PCR. ** p value < 0.01, ****p value < 0.0001.
Role of androgenic stimuli on hTERT and EGR1 expression in androgen-dependent PCa Cells
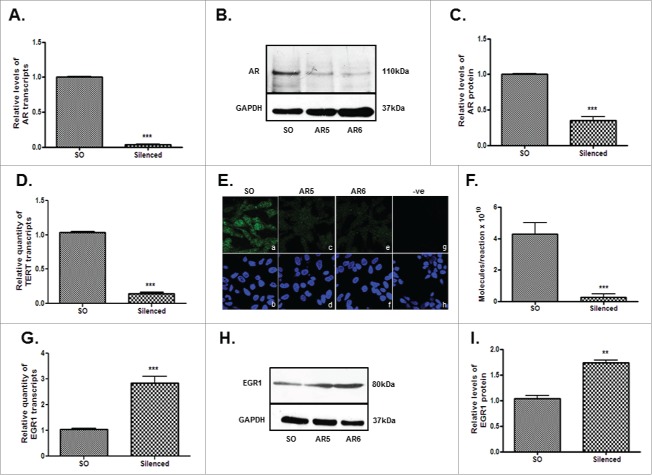
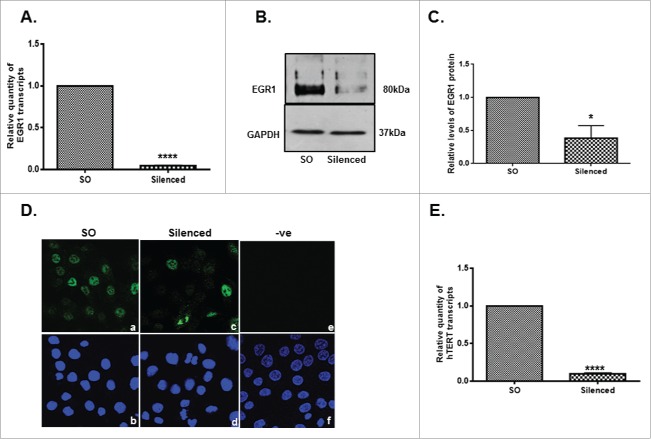
Stimulation of LNCaP cells with 5α-DHT led to a dose-dependent (0.1–10 nM) decrease (p<0.0001) in the levels of EGR1 and a significant increase (p<0.001) in the levels of AR transcripts (Fig. 3). Further AR silencing (Figs. 4A-C) led to a significant decrease in the levels of hTERT transcript (Fig. 4D), protein (Fig. 4E) and telomerase activity (Fig. 4F). Interestingly, a significant increase (p<0.001) was observed in the levels of EGR1 after AR silencing in LNCaP cells (Figs. 4G–I). Collectively, these observations indicate that EGR1 expression is down-regulated by androgenic stimuli in androgen-dependent PCa cells.
Figure 3.
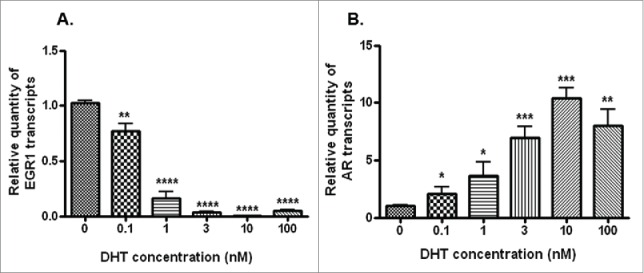
Transcript levels of EGR1 (A) and AR transcripts (B) in LNCaP cells stimulated with different concentration (0.1–100 nM) of DHT, as assessed by qRT-PCR. * p value <0.05, ** p value<0.01, ***p value< 0.001, ****p value< 0.0001.
Figure 4.
Effect of AR silencing on hTERT and EGR-1 expression in androgen-dependent LNCaP cells. AR5 and AR6 siRNAs target 2 different regions of AR. Panels A-C display the levels of AR transcripts (A) and protein (B, C) in the AR silenced cells. Panels D-F demonstrate the levels of hTERT transcripts (D), protein (E) and telomerase activity (F) in AR-silenced cells. Panel E shows detection of immunoreactive hTERT in the SO-transfected (a), AR5-silenced (c) and AR6-silenced (e) cells. Panel g shows cells stained only with secondary antibody. Lower panels - b, d, f and h are the counterstained (DAPI) images of the cells shown in panels a, c, e and h, respectively. Panels G-I show the effect of AR silencing on the levels of EGR1 transcripts (G) and protein (H, I). Panels C and I represent densitometric analysis of the immunoreactive bands (AR or EGR1) in the SO and AR (AR6) siRNA transfected cells. ** p value < 0.01, *** p value < 0.001, **** p value < 0.0001.
Effect of “AR gain” on hTERT expression and telomerase activity in castration-resistant PCa cells
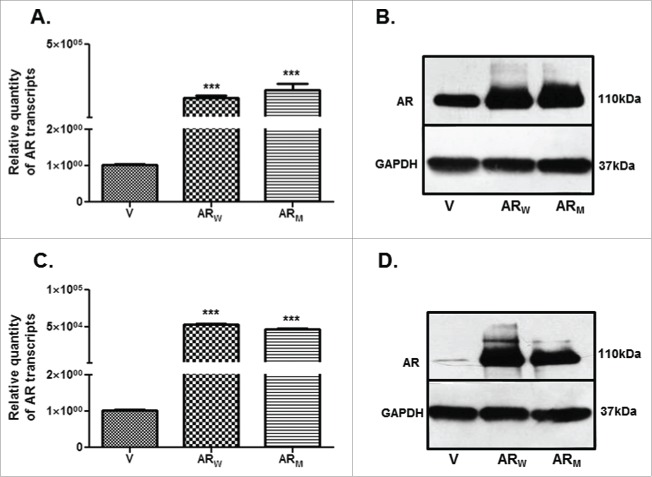
PC3 and DU145 cells were transfected with the respective constructs to express human wild type (ARW) or mutant AR (ARM-T877A mutation in the hormone binding domain of AR) protein. Significantly (p < 0.001) higher expression of AR was evident at transcript (Figs. 5A,C) and (Figs. 5B,D) levels in these cells. PC3 and DU145 cells expressing high levels of AR are hereafter referred to as PC3-AR and DU-AR respectively.
Figure 5.
Relative levels of AR transcript (A,C) and protein (B,D) in PC3 (A,B) and DU145 (C,D) cells, transfected either with the wild type (ARW) or mutant (ARM) cDNA constructs, compared with the respective parental cells transfected with the empty vector (V) construct. *** p value < 0.001.
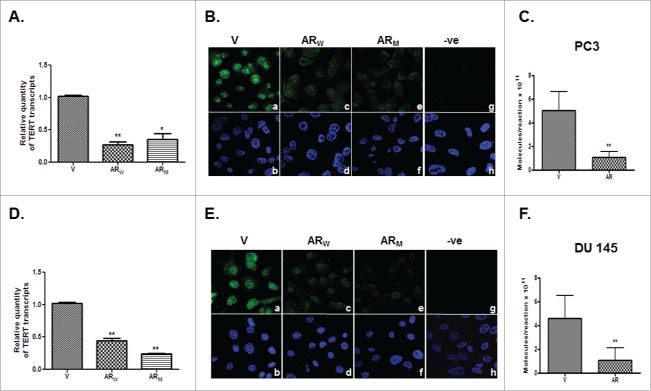
The levels of hTERT transcript (Figs. 6A,D) and protein (Figs. 6B,E) were found to be significantly (p<0.01) lower in the PC3-AR (Figs. 6A,B) and DU-AR (Figs. 6D,E) cells than in the respective parental cells. This decrease in hTERT expression was observed, irrespective of the fact whether the cells expressed wild type or mutant AR.
Figure 6.
Relative levels of hTERT transcripts (A,D) and protein (B,E) in PC3-AR (A,B) and DU-AR (D,E) cells transfected with either wild type (ARW) or mutant (ARM) AR, compared with the respective parental cells transfected with the empty vector (V) construct. Panels B and E show immunofluorescent detection of hTERT in PC3 (B) and DU145 (E) cells transfected with empty vector (a) or AR cDNA constructs (c, e). Panels g in B and E show cells stained only with secondary antibody. Lower panels - b, d, f and h are the DAPI stained images of the cells shown in panels a, c, e and g, respectively. Magnification-63X. *p value < 0.05, **p value < 0.01, ***p value< 0.001.
A significant (p<0.01) decrease was also observed in the telomerase activity in PC3-AR (Figs. 6C) and DU-AR (Figs. 6F) cells, compared with the respective parental cells. Thus, AR elicits an inhibitory effect on hTERT expression and telomerase activity in CRPC cells. This is in contrast to a stimulatory effect of AR on hTERT expression in ADPC cells.
Effect of “AR gain” on EGR1 expression in castration-resistant PCa cells
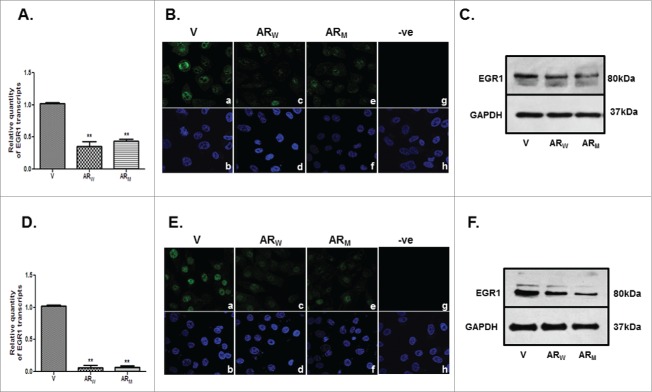
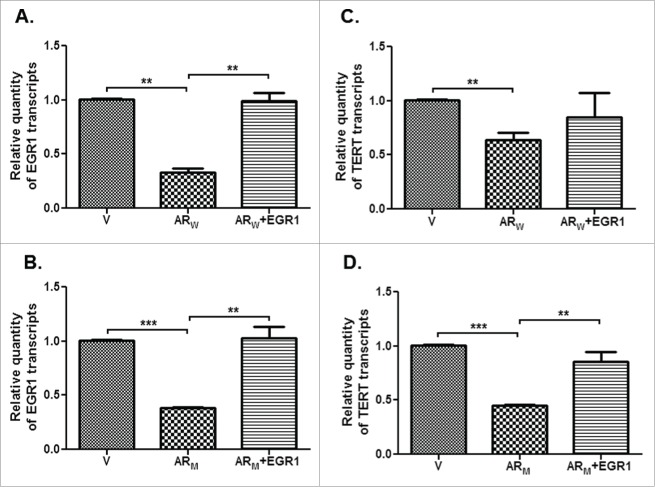
A significant (p<0.001) decline was observed in the levels of EGR1 transcript and protein in PC3-AR (Figs. 7A-C) and DU-AR (Figs. 7D-F) cells, compared with the respective parental cells. Again these effects were independent of the genotype of the AR (wild type versus mutant) constructs.
Figure 7.
Relative levels of EGR1 transcript and protein in PC3 (A,B,C) and DU145 (D,E,F) cells, transfected with AR cDNA (ARW/ARM) or empty vector (V) constructs; as assessed by qRT-PCR (A,D), immunofluorescence (B,E) and immunoblotting (C,F). B and E represent immunofluorescent detection of EGR1 in PC3 (B) and DU145 (E) cells transfected with empty vector (a) or AR cDNA constructs (c, e). Panels g in B and E show cells stained only with secondary antibody. Lower panels -b, d, f and h are the DAPI stained images of the cells shown in panels a, c, e and g, respectively. Magnification-63X. ** p value < 0.01.
A significant (p<0.0001 ) reduction was observed in the levels of hTERT transcript (Fig. 8E) in the EGR1- silenced DU145 cells, compared to the SO-transfected cells (Figs. 8A-D). Taken together, these observations are suggestive of an inhibitory effect of AR on hTERT and EGR1 expression in CRPC cells.
Figure 8.
EGR1 silencing and its effect on hTERT expression in DU145 cells. qRT-PCR (A) and immunoblotting (B) were done to assess the efficiency of EGR1 silencing . Panel C represents mean ratios of the intensities of EGR1 to that of GAPDH bands in the cells transfected with either scrambled oligos (SO) or EGR1 siRNA (silenced). Panel D represents immunofluorescent detection of EGR1 protein in SO (a) and EGR1 (c) siRNA transfected cells. Panel e shows the cells stained only with secondary antibody. Lower panels -b, d and f are the DAPI stained images of the cells shown in panels a, c and e, respectively. Magnification-63X. Panel E shows relative levels of hTERT transcripts in cells transfected with SO and EGR1 siRNA. * p value <0.05, **** p value< 0.0001.
Further, hTERT transcript levels were found to be restored in DU-AR cells transfected with the EGR1 cDNA construct (Figs. 9C,D). Thus, a decline in the levels of EGR1 contributes to a reduction in the hTERT levels in AR-expressing CRPC cells. This observation suggests that AR exerts an inhibitory effect on hTERT expression and this effect is mediated via EGR1 in CRPC cells.
Figure 9.
Relative levels of EGR1 (A,B) or hTERT (C,D) transcripts in DU145 cells transfected with either vector alone (V) or AR (ARW or ARM) alone or with EGR1 construct. ** p value < 0.01, *** p value< 0.001.
Discussion
Androgen receptor mediated signaling plays a major role in the pathogenesis of prostate cancer. Therefore, the majority of PCa therapies are directed toward either reducing the levels of circulating androgens or blocking the AR-mediated signaling pathways.19 However, blocking these events is not a very effective strategy. Patients on these therapies eventually develop castration-resistant metastatic prostate cancers which often result in mortality. This necessitates conducting detailed investigations to determine whether the molecular cascades involved in AR signaling are similar in androgen-sensitive and CRPC cells. Knowledge gained through such investigations may help identify novel therapeutic targets for CRPC.
The present study demonstrated a decrease in the hTERT levels and telomerase activity in the AR-silenced ADPC cells. This supports a previous observation indicating positive regulation of hTERT expression by androgenic stimuli in ADPC cells. However, contrasting effects were observed in CRPC cells in the present study. “Gain of AR” led to a significant decrease in the hTERT expression and telomerase activity, irrespective of the fact whether the cells expressed wild type or mutant AR. These observations are in contrast to a previous study indicating an inhibition of hTERT expression by the wild-type AR and loss of this ability by the mutant AR.20 However, a careful review of their data indicated that the wild type and mutant ARs differ only in their efficiencies as the repressors of hTERT expression. Mutant AR did not completely lack the ability to inhibit hTERT expression. Further, the trans-repression of hTERT promoter by AR was found to be agonist-dependent.20 Interestingly, Moehren et al reported a decrease in the hTERT promoter activity in androgen-stimulated LNCaP cells. 20 This observation is also in contrast to several existing reports and the present study indicating an androgen-induced upregulation of hTERT expression and telomerase activity in ADPC cells. Our data suggest that both wild type and mutant ARs have the ability to repress the hTERT expression in CRPC cells. Further, our qRT-PCR data showed significantly higher levels of hTERT transcripts in CRPC- PC3 and DU145 cells, compared with ADPC-LNCaP cells. It may be added here that all 3 cell lines harbor mutations in the hormone binding domain of AR. While LNCaP cells harbor DNA mutations, DU145 and PC3 cells acquire these mutations due to RNA editing.21 Thus, the differential effects of AR on telomerase activity in ADPC and CRPC cells cannot be attributed solely to AR mutations. Instead, differential effects on the telomerase activity may signify a divergent transcriptional program of AR in ADPC and CRPC cells.
To our knowledge, the present study is the first report indicating EGR1 as one of the target genes of AR in prostate cancer cells. In-silico search through −1 to −5000 nucleotides of human EGR1 gene (accession no NM_001964) indicated the absence of AREs in the scanned regions. This suggested that EGR1 may not be a direct transcriptional target of AR. Recently it has been demonstrated that EGR1 expression in prostate cancer cells is mediated by an ERK signaling cascade.22 Interestingly ERK1/2 pathway was found to be deregulated in the AR-expressing CRPC cells in the present study (data not shown). It is likely that “AR gain” deregulates ERK1/2 pathway, that in turn contributes to a decrease in the EGR1 expression. In CRPC cells also, AR acts as a negative regulator of EGR1 expression. 5α-DHT stimulated a decrease whereas AR knock-down caused an increase in the EGR1 levels in ADPC cells. The relevance of an increase in the expression of EGR1 following AR silencing in ADPC cells is a subject of speculation. We hypothesize that the regulation of hTERT expression by AR is EGR1-independent in ADPC cells. It is reported that EGR1 mediates translocation of AR to nucleus and enables prostate cancer cells to grow in low androgen concentrations.23 The present study adds another dimension to the existing data by demonstrating that AR regulates the expression of EGR1 in PCa cells. This raises a possibility of the existence of a bidirectional cascade between EGR1 and AR.
In brief, the present study demonstrates that AR acts as a positive regulator of hTERT expression in ADPC cells and as a negative regulator in CRPC cells. The study also demonstrates that EGR1 positively regulates the expression of hTERT in prostate cancer cells. Thus, hTERT expression is regulated by AR in a cell context-dependent manner whereas regulation of EGR1 expression by AR appears to be cell context-independent.
We observed a decrease in the expression of hTERT on “AR gain” in CRPC cells. Therefore it may be surmised from the present study that complete neutralization of AR functions may not be effective as an anti-cancer therapy. There exist evidences to support this corollary. The downregulation of AR expression, often associated with long-term androgen deprivation, has been shown to contribute to recurrent prostate tumor growth.24 Zhu and Kyprianou also demonstrated that AR maintenance is essential for the regulation of PCa metastasis.25 Further, intermittent, rather than continuous, ADT has been found beneficial to patients with locally advanced metastatic prostate tumors.26,27 Our study indirectly reasserts that maintenance of the AR levels, to a certain extent, may be essential for an effective treatment of prostate cancer.
Materials and methods
Antibodies
Monoclonal antibody against human AR (M3562), mouse secondary antibodies conjugated to HRP (P0161), rabbit secondary antibodies conjugated to HRP (P0448), mouse secondary antibodies conjugated to FITC (F0232), rabbit secondary antibodies conjugated to FITC (F0205) were procured from Dako. Antibody against human EGR1 (sc-189) was procured from Santacruz Biotechnology Inc. GAPDH (CB 1001) and hTERT (582000) antibodies were procured from Calbiochem. Alexa fluor-488 (A31628) and biotin (PK4001) conjugated anti-rabbit secondary antibodies were procured from Life Technologies and Vector Laboratories, respectively. Mouse (PP542-K) and rabbit (PP64-K) IgGs were procured from Millipore.
Human prostate biopsies collection
BPH tissues (n = 5) and PCa biopsies (n = 5) were obtained using transurethral resection and core needle biopsy respectively. The protocol for prostate tissue samples was approved by the NIRRH Ethics Committee for Clinical Studies (Project no.176/2010).
Sectioning of tissue blocks and immunohistochemical localization
Paraffin embedded prostate tissue sections (5 µm) were deparaffinized in xylene and then rehydrated using descending grades of methanol. The sections were quenched for their endogenous peroxidase activity and then processed for antigen retrieval and nuclear permeabilization.28 After blocking, the sections were incubated at 4°C overnight with primary antibody (EGR1 at 2 µg/ml; hTERT at 1:200 dilution) or respective rabbit/mouse IgGs at same concentration and then with respective biotinylated secondary antibodies. Sections were then incubated with avidin-biotin-horseradish peroxidase complex (Vector Laboratories). Immunoprecipitates were detected using 1.0 mg/ml diaminobenzidene (Sigma-Aldrich). Further, immunostained sections were counterstained using haemotoxylin and mounted in DPX. Image analysis software Aperio Image scope version v11.2.0.780 (Aperio, Vista, CA, USA) was used to determine the intensities of the immunoprecipitates. Integrated optical density (IOD) values were obtained for randomly selected 10 areas in each section.
Cell line maintenance
Androgen-dependent prostate carcinoma cell line LNCaP-FGC (CRL-1740) was obtained from American Type Culture Collection (ATCC). Castration-resistant or androgen-independent prostate carcinoma cell lines- PC3 (CRL-1435) and DU145 (ATCC-HTB-81) were obtained from National Center for Cell Sciences. These cell lines were propagated in phenol red-free RPMI-1640 media (R8755, Sigma-Aldrich), supplemented with 2 mM L-glutamine, 10% heat-inactivated fetal bovine serum (10082–147, Invitrogen), pen-strep (50unit/ml penicillin and 50 µg/ml streptomycin, 15070–063, Invitrogen) at 37°C in a humidified atmosphere of 5% CO2 in air.
siRNA transfections
Cells were transfected with specific siRNAs targeting AR or EGR1 (Ambion Silencer Select validated siRNAs) transcripts or with respective SO siRNAs (4390843, Ambion) as per the manufacturer's instructions. For immunofluorescence studies, transfections were carried out on cells grown on coverslips. After transfection, cells were suspended in RLT lysis buffer (74004, Qiagen) or fixed in 3.7% paraformaldehyde.
cDNA transfections
Cells were transfected with the wild type AR cDNA construct (pCMV5.AR), a kind gift from Dr. Donald Tindall (Mayo Clinic, Rochester, MN, USA) or with the empty vector construct (without the AR insert). Mutant AR cDNA construct (pCMV.ARM –with T877A mutation in the ligand binding domain) and wild type EGR1 cDNA (pCMV5.EGR1) constructs29 were kindly gifted by Prof. Shiv Srivastava (Center for Prostate Disease Research, Bethseda, MD, USA) and Dr Gerald Thiel (Institute for Genetics, University of Cologne, Germany) respectively. Cells (1×105 cells/well) were transfected with a complex of cDNA construct (0.8 µg) and Lipofectamine PLUS (15338100, Invitrogen) prepared in OPTI-MEM. For co-transfection studies, DU145 cells were transfected with AR cDNA (wild type or mutant) constructs for 30 hrs and then with the EGR1 cDNA construct for additional 18 hrs. After 48 hrs, cells were harvested in RLT buffer or protein lysis buffer.
RNA isolation, cDNA synthesis and qRT-PCR
As per the manufacturer's instructions, total RNA (1 µg) was converted to cDNA using High Prime cDNA synthesis kit (4368814, Applied Biosystems). Specific TaqMan primer/probes (4331182, Applied Biosystems) were used to amplify the transcripts encoded by the gene of interest (AR/EGR-1/hTERT) or endogenous control (18S rRNA) gene using 7900 HT Real Time PCR system (Applied Biosystems). RQ of transcripts was calculated by ΔΔ CT method using the formula: RQ= 2-ΔΔCt, where Δ CT is calculated by subtracting CT of the endogenous control from CT of the target gene and ΔΔ CT by subtracting Δ CT of control from Δ CT of test. Values were expressed as RQ ±S .E.M.
Immunoblotting
Protein extracts (10 µg) electrophoresed in 10% SDS-PAGE was blotted to PVDF membranes (IPVH 00010, Millipore). After blocking, the membranes were probed with primary antibody {AR antibody (3 µg/ml) or EGR1 (0.2 µg/ml)} for 16 hrs at 4°C, then with respective HRP conjugated secondary antibodies. Immunoreactive bands on the membranes were detected using advanced ECL detection kit (Amersham, RPN2135). Densities of the bands of interest were measured using IQTL software (GE Biosciences). After detection, membranes were stripped off the bound antibodies and reprobed with GAPDH antibody (1 µg/ml).
Immunofluorescence
Cells fixed on coverslips were permeabilized using 0.1% Triton-X 100. After blocking with 1% BSA, cells were incubated with respective primary antibody EGR1 (2 µg/ml) or hTERT (1:200 dilution)} for 16 hrs at 4°C and then with respective FITC or Alexa Fluor conjugated secondary antibodies. Immunofluorescence was detected using confocal laser microscopy (Carl Zeiss).
Telomere repeat amplification protocol assay
Telomerase activity in cell extracts was determined through their ability to synthesize telomeric repeats onto an oligonucleotide substrate (TSR) in vitro. Viable cells (105-104) were lysed in lysis buffer (provided with the Quantitative Telomerase Detection kit, Allied Biotech, MT3010) and centrifuged at 12,000 × g for 30 min at 4°C. Supernatants (test samples) were checked for their protein concentrations. Test (containing 1 µg protein) samples and standard samples were mixed with premix (provided in the kit). Standards were prepared by serial (1:5) dilutions (0.1, 0.02, 0.004, 0.0008, 0.00016 amoles/µl) of TSR oligonucleotide (Telomere primers, stock- 0.5amoles/µl) provided in the kit and 1 µl of each dilution was used for PCR amplification of the standards. The resultant extended products were amplified by highly sensitive SYBR Green fluorochrome based PCR using Step One Real Time PCR system (Applied Biosystems). Telomerase activity of the cells was measured by plotting the CT of the test samples against CT of known standard template (TSR) and expressed as molecules/reaction.
Statistical analysis
All experiments were performed at least thrice, and data expressed as ±S.D. Student's unpaired t-test was applied to determine the significance of a difference between control and experimental samples. A p- value of <0.05 was considered to be significant.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We appreciate the generosity of Prof D. Tindall (Mayo Clinic, USA) for providing human wild type AR cDNA clone. We are also extremely grateful to Prof. Shiv Srivastava (Center for Prostate Disease Research, Bethseda, MD, USA) for providing human mutant AR cDNA clone and also to Prof. Gerald Thiel (Institute for Genetics, University of Cologne, Germany) for human EGR1 cDNA clone. Dr. Nafisa Balasinor, Reshama Gaonkar, Shobha Potdar are thanked for their help in confocal microscopy. Ms. Jacob thanks DAE and ICMR for providing junior and senior research fellowships respectively.
Funding
Indian Council of Medical Research; Department of Atomic Energy (BRNS), Government of India.
References
- 1.Lonergan PE, Tindall DJ. Androgen receptor signaling in prostate cancer development and progression. J Carcinogenesis 2011; 10:20; PMID:21886458; http://dx.doi.org/ 10.4103/1477-3163.83937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Iczkowski KA, Pantazis CG, McGregor DH, Wu Y, Tawfik OW. Telomerase reverse transcriptase subunit immunoreactivity: a marker for high-grade prostate carcinoma. Cancer 2002; 95:2487-93; PMID:12467061; http://dx.doi.org/ 10.1002/cncr.10988 [DOI] [PubMed] [Google Scholar]
- 3.Nakamura TM, Morin GB, Chapman KB, Weinrich SL, Andrews WH, Lingner J, Harley CB, Cech TR. Telomerase catalytic subunit homologs from fission yeast and human. Science 1997; 277:955-9; PMID:9252327; http://dx.doi.org/ 10.1126/science.277.5328.955 [DOI] [PubMed] [Google Scholar]
- 4.Feng J, Funk WD, Wang SS, Weinrich SL, Avilion AAT, Chiu CP, Adams RR, Chang E, Allsopp RC, Yu J, et al.. The RNA component of human telomerase. Science 1995; 269:1236-41; PMID:7544491; http://dx.doi.org/ 10.1126/science.7544491 [DOI] [PubMed] [Google Scholar]
- 5.Harrington L, McPhail T, Mar V, Zhou W, Oulton R, Bass MB, Arruda I, Robinson MO. A mammalian telomerase-associated protein. Science 1997; 275:973-7; PMID:9020079; http://dx.doi.org/ 10.1126/science.275.5302.973 [DOI] [PubMed] [Google Scholar]
- 6.Wojtyla A, Gladych M, Rubis B. Human telomerase activity regulation. Mol Biol Rep 2011; 38:3339-49; PMID:21086176; http://dx.doi.org/ 10.1007/s11033-010-0439-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Meeker AK, Sommerfeld HJ, Coffey DS. Telomerase is activated in the prostate and seminal vesicles of the castrated rat. Endocrinology 1996; 137:5743-46; PMID:8940411 [DOI] [PubMed] [Google Scholar]
- 8.Guo C, Armbruster BN, Price DT, Counter CM. In vivo regulation of hTERT expression and telomerase activity by androgen. J Urol 2003; 170:615-8; PMID:12853842; http://dx.doi.org/ 10.1097/01.ju.0000074653.22766.c8 [DOI] [PubMed] [Google Scholar]
- 9.Suenaga M, Soda H, Oka M, Yamaguchi A, Nakatomi K, Shiozawa K, Kawabata S, Kasai T, Yamada Y, Kamihira S, et al.. Histone deacetylase inhibitors suppress telomerase reverse transcriptase mRNA expression in prostate cancer cells. Int J Cancer J Int du Cancer 2002; 97:621-5; PMID:11807787; http://dx.doi.org/ 10.1002/ijc.10082 [DOI] [PubMed] [Google Scholar]
- 10.Iczkowski KA, Huang W, Mazzucchelli R, Pantazis CG, Stevens GR, Montironi R. Androgen ablation therapy for prostate carcinoma suppresses the immunoreactive telomerase subunit hTERT. Cancer 2004; 100:294-9; PMID:14716763; http://dx.doi.org/ 10.1002/cncr.20002 [DOI] [PubMed] [Google Scholar]
- 11.Liu S, Qi Y, Ge Y, Duplessis T, Rowan BG, Ip C, Cheng H, Rennie PS, Horikawa I, Lustig AJ, et al.. Telomerase as an important target of androgen signaling blockade for prostate cancer treatment. Molecular cancer therapeutics 2010; 9:2016-25; PMID:20571066; http://dx.doi.org/ 10.1158/1535-7163.MCT-09-0924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang Q, Li W, Zhang Y, Yuan X, Xu K, Yu J, Chen Z, Beroukhim R, Wang H, Lupien M, et al.. Androgen receptor regulates a distinct transcription program in androgen-independent prostate cancer. Cell 2009; 138:245-56; PMID:19632176; http://dx.doi.org/ 10.1016/j.cell.2009.04.056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Karantanos T, Corn PG, Thompson TC. Prostate cancer progression after androgen deprivation therapy: mechanisms of castrate resistance and novel therapeutic approaches. Oncogene 2013; 32: 5501-11; PMID:23752182; http://dx.doi.org/ 10.1038/onc.2013.206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu B, Tang G, Xiao C, Wang L, Yang Q, Sun Y. Androgen deprivation therapy induces androgen receptor-dependent upregulation of Egr1 in prostate cancers. Int J Clin Exp Pathol 2014; 7: 2883-93; PMID:25031707 [PMC free article] [PubMed] [Google Scholar]
- 15.Gitenay D, Baron VT. Is EGR1 a potential target for prostate cancer therapy? Future Oncol 2009; 5: 993-03; PMID:19792968; http://dx.doi.org/ 10.2217/fon.09.67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang SZ, ELtoum IA, Abdulkadir SA. Enhanced EGR1 activity promotes the growth of prostate cancer cells in androgen-depleted environment. J Cell Biochem 2006; 97: 1292-99; PMID:16552752; http://dx.doi.org/ 10.1002/jcb.20736 [DOI] [PubMed] [Google Scholar]
- 17.Akutagawa O, Nishi H, Kyo S, Terauchi F, Yamazawa K, Higuma C, Inoue M, Isaka K. Early growth response-1 mediates downregulation of telomerase in cervical cancer. Cancer Sci 2008; 99:1401-6; PMID:18460021; http://dx.doi.org/ 10.1111/j.1349-7006.2008.00835.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Akutagawa O, Nishi H, Kyo S, Higuma C, Inoue M, Isaka K. Early growth response-1 mediates up-regulation of telomerase in placenta. Placenta 2007; 28:920-7; PMID:17485108; http://dx.doi.org/ 10.1016/j.placenta.2007.03.005 [DOI] [PubMed] [Google Scholar]
- 19.Ahmed A, Ali S, Sarkar FH. Advances in androgen receptor targeted therapy for prostate cancer. J Cell Physiol 2014; 229:271-6; PMID:24037862; http://dx.doi.org/ 10.1002/jcp.24456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moehren U, Papaioannou M, Reeb CA, Grasselli A, Nanni S, Asim M, Roell D, Prade I, Farsetti A, Baniahmad A. Wild-type but not mutant androgen receptor inhibits expression of the hTERT telomerase subunit: a novel role of AR mutation for prostate cancer development. FASEB J 2008; 22: 1258-67; PMID:17991730; http://dx.doi.org/ 10.1096/fj.07-9360com [DOI] [PubMed] [Google Scholar]
- 21.Martinez HD, Jasavala RJ, Hinkson I, Fitzgerald LD, Trimmer JS, Kung HJ, Wright ME. RNA editing of androgen receptor gene transcripts in prostate cancer cells. J Biol Chem 2008; 283: 29938-49; PMID:18708348; http://dx.doi.org/ 10.1074/jbc.M800534200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gregg J, Fraizer G. Transcriptional regulation of EGR1 by EGF and the ERK Signaling Pathway in Prostate Cancer Cells. Gen Cancer 2011; 9:900-9; PMID:NOT_FOUND; http://dx.doi.org/ 10.1177/1947601911431885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang SZ, Eltoum IA, Abdulkadir SA. Enhanced EGR1 activity promotes the growth of prostate cancer cells in an androgen-depleted environment. J Cell Biochem 2006; 97:1292-99; PMID:16552752; http://dx.doi.org/ 10.1002/jcb.20736 [DOI] [PubMed] [Google Scholar]
- 24.Liu T, Wu LY, Fulton MD, Johnson JM, Berkman CE. Prolonged androgen deprivation leads to downregulation of androgen receptor and prostate-specific membrane antigen in prostate cancer cells. Int J Oncol 2012; 41:2087-92; PMID:23041906; http://dx.doi.org/ 10.3892/ijo.2012.1649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhu ML, Kyprianou N. Role of androgens and the androgen receptor in epithelial-mesenchymal transition and invasion of prostate cancer cells. FASEB J 2010; 24:769-77; PMID:19901020; http://dx.doi.org/ 10.1096/fj.09-136994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boccon-Gibod L, Hammerer P, Madersbacher S, Mottet N, Prayer-Galetti T, Tunn U. The role of intermittent androgen deprivation in prostate cancer. BJU international 2007; 100:738-43; PMID:17662079; http://dx.doi.org/ 10.1111/j.1464-410X.2007.07053.x [DOI] [PubMed] [Google Scholar]
- 27.Suzuki H, Okihara K, Miyake H, Fujisawa M, Miyoshi S, Matsumoto T, Fujii M, Takihana Y, Usui T, Matsuda T, et al.. Alternative nonsteroidal antiandrogen therapy for advanced prostate cancer that relapsed after initial maximum androgen blockade. J Urol 2008; 180:921-7; PMID:18635218; http://dx.doi.org/ 10.1016/j.juro.2008.05.045 [DOI] [PubMed] [Google Scholar]
- 28.Jacob S, Nayak S, Fernandes G, Barai RS, Menon S, Chaudhari UK, Kholkute SD, Sachdeva G. Androgen receptor as a regulator of ZEB2 expression and its implications in epithelial-to-mesenchymal transition in prostate cancer. Endocrine-related cancer 2014; 21:473-86; PMID:24812058; http://dx.doi.org/ 10.1530/ERC-13-0514 [DOI] [PubMed] [Google Scholar]
- 29.Thiel G, Schoch S, Petersohn D. Regulation of synapsin 1 gene expression by the zinc-finger transcription factor zif268/egr-1. J Biol Chem 1994; 269:15294-301; PMID:8195167 [PubMed] [Google Scholar]