Abstract
Contractile muscle fibers produce enormous intrinsic forces during contraction/relaxation waves. These forces are directly applied to their cytoplasmic organelles including mitochondria, sarcoplasmic reticulum, and multiple nuclei. Data from our analysis of Drosophila larval somatic muscle fibers suggest that an intricate network of organized microtubules (MT) intermingled with Spectrin-Repeat-Containing Proteins (SRCPs) are major structural elements that protect muscle organelles and maintain their structure and position during muscle contraction. Whereas the perinuclear MT network provides structural rigidity to the myonucleus, the SRCPs Nesprin and Spectraplakin form semiflexible filamentous biopolymer networks, providing nuclei with the elasticity required to resist the contractile cytoplasmic forces produced by the muscle. Spectrin repeats are domains found in numerous structural proteins, which are able to unfold under tension and are subject to mechanical stresses in the cell. This unique composite scaffold combines rigidity and resilience in order to neutralize the oscillating cellular forces occurring during muscle contraction/relaxation waves and thereby protect myonuclei. We suggest that the elastic properties of SRCPs are critical for nuclear protection and proper function in muscle fibers.
KEYWORDS: EB1, microtubules, muscles, Nesprin, semiflexible polymers, Spectraplakin, spectrin repeats
Myonuclear Strength and Stability: A Robust Organelle in a “Heavy Duty” Tissue
Muscle is an extraordinary and ancient tissue highly specialized for reiterative and directed force generation.1 It can be found in all eumetazoan, either as single contractile cells or as bundled myofibers like in the human body. Despite enormous diversity in morphology, organization, and function, all muscle cells face the same paradoxical challenge. Although contraction/relaxation waves generate tremendous periodical stresses, myocytes must maintain a robust intrinsic cytoarchitecture, including sarcomeric structure, organelle distribution and homogenous nuclear morphology. To date, the mechanisms associated with maintaining cytoplasmic order in contractile muscle fibers are not entirely clear.
Under a microscope, one hallmark of mature muscle architecture is the equal distribution of numerous flattened nuclei aligned along the muscle surface (Fig. 1A). Although this non-spherical, discoidal (plate-like) morphology and the peripheral position of myonuclei have long been described,2 their biological significance is unclear. Because aberrant nuclear morphology is characteristic of various muscle pathogenesis,3,4 it is assumed that these specific shape and distribution are physiologically important. Strikingly, the biological principles behind these attributes have remained uninvestigated. Herein, we focus on fully differentiated muscle fibers and try to lift the veil off myonuclear shape maintenance.
Figure 1.
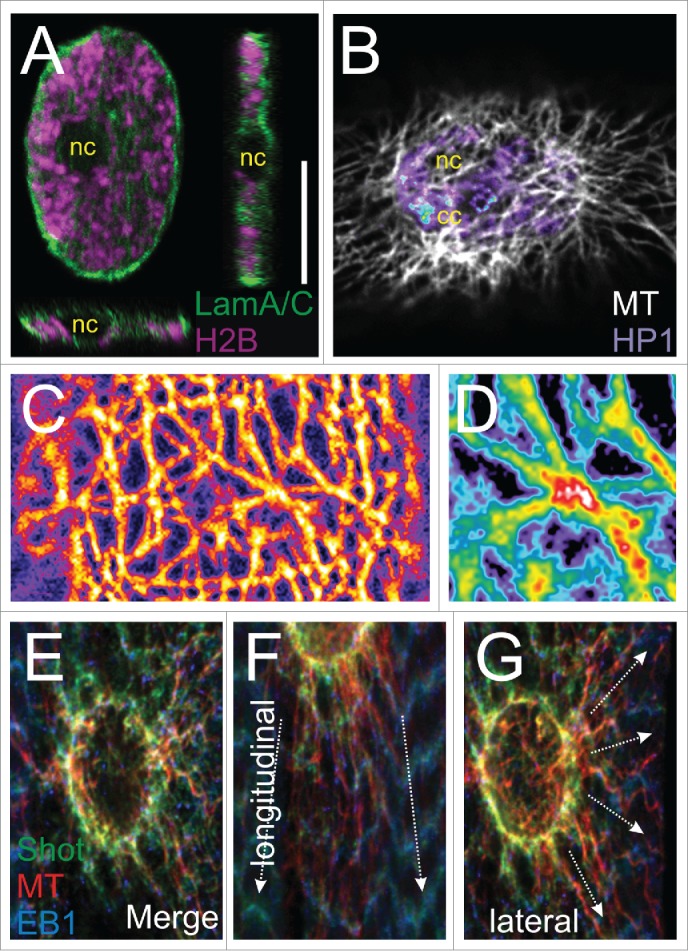
The myonucleus and the surrounding microtubules in Drosophila larval muscle. (A) Orthogonal views of a muscle nucleus from the third instar larva. The nuclear lamina was labeled in green and the histone associated with chromatin in magenta. The dark round spot in the center is the nucleolus (nc). Under specific physiological and cellular conditions, the geometry of a myonucleus can vary, but in general they are flat rather than spherical. (B) A nucleus on the muscle surface embedded in a microtubule meshwork (labeled in white). The chromatin is labeled with HP1a in an intensity-dependent manner. The chromocenter (cc), abundant with pericentric heterochromatin, is indicated by the blue spot. (C) A microtubule scaffold on the nuclear surface. (D) An enlargement showing a possible crossover of microtubules. (E) The perinuclear ring structure consists of microtubules associated with the plus-end binding proteins Shot and EB1. (F, G) The ring is anchored to the sarcomeres by longitudinal (F) and lateral (G) MTs.
The nucleus is considered to be the largest and the stiffest organelle in most mammalian cells and can greatly influence cell mechanics.5 Its shape is greatly influenced by the nucleoskeleton, a relatively stiff frame of a network of lamin-based intermediate filaments that associates with the inner nuclear membrane. Because muscles change their shape dramatically during contraction and relaxation, we suggest that the combination of a flattened shape and peripheral position helps to minimize the impact of rapid oscillation between axial tension and lateral compression during muscle function. Furthermore, the well-defined and homogenous oval and flattened nuclear shape indicates anisotropic forces applied to the muscle nuclei, which are possibly counteracted by a shape-forming scaffold and robust nuclear anchorage to the cytoskeleton. In addition to nuclear shape, such a mechanism would maintain equal nuclear positioning along the entire muscle fiber. Indeed, centralized nuclear aggregates with aberrant shape are seen in diverse mutants lacking the mechanical linkage between the cytoskeleton on one side and the chromatin on the other side of the nuclear envelope.6 Emerging evidence indicates that changes in the transmission of external mechanical stimuli to the nuclear interior induced by modulating components of the Linker of Nucleoskeleton and Cytoskeleton (LINC) complex8,9 may affect genome stability, suggesting that LINC proteins couple chromatin and gene expression with mechanosensitive signals. Recently, it has been shown that a stable covalent disulfide bond that forms between a cysteine residue in SUN1/2 and a cysteine in the KASH domain of Nesprins (nuclear envelope spectrin repeat proteins) in the perinuclear space assists in load bearing and force transmission to the nucleus.7
Composite Biopolymer Scaffolds Shape Muscle Nucleus: Spectrin-Repeat-Containing Proteins (SRPCs) alongside Microtubules (MT)
Similarly to immobile or migrating adherent cells plated on matrigel or micropattern, muscle fibers generate elastic stresses through actomyosin contraction. Nevertheless, they differ from cultured cells in that they are permanently anchored through tendons to bones, thus producing polarized cyclic stresses counteracted by these rigid structures. In response to physiological challenges, muscles use cytoskeletal components and other filamentous biopolymers of distinct physical properties to balance between stability and flexibility. High stability is achieved by reinforcement with ordered arrays of long, parallel stiffeners such as MTs, which may be further stabilized locally by posttranslational modification and by binding to microtubule-associated proteins (MAPs). Flexibility is attained by SRCPs (Nesprin/MSP300 and Spectraplakin/Shot), which are elastic molecules presumably due to spectrin repeats. Because these networks are interdependent and associate with the nuclear membrane, the interplay between the 2 systems will determine the ultimate physical forces applied to the muscle nuclei. Interestingly, the interaction between SRCPs and MTs seems to be quite ancient. Recently, the spectrin-like protein EzrA was shown to regulate polymer bundling of the tubulin homolog FtsZ in bacterial cell division.8
Microtubules Play a Predominant Role in Muscle Cell Architecture
During myogenesis, microtubules perform versatile roles and undergo extensive rearrangement. Interestingly, whereas the myoblast exhibits a conspicuous microtubule organizing center (MTOC), mature myofibers no longer possess it. Instead, a circular perinuclear microtubule organizing complex is formed,9,10 presumably facilitating the reorganization of the MT perinuclear network. How the MTOC is inactivated and rearranged into a perinuclear ring remains to be elucidated. In mature muscle, MTs are abundant in the periphery of the muscle and especially around each of the nuclei (Fig. 1B). The perinuclear MTs can be divided into 2 parts. The first forms a cage-like structure of a high-density meshwork (Fig. 1C) and may be responsible for nuclear shaping and mechanical protection. The fact that mechanical stability and rigidity of networks depends on their connectivity, raises the question if these MTs are cross-linked and bundled (Fig. 1D). The other part is circular (Fig. 1E) and radial-anisotropic MTs, which are either polarized in the direction of contraction (Fig. 1F) or in the lateral direction (Fig. 1G). Apparently, this part anchors the nucleus in the correct position but may also transduce forces from the sarcomeres at long distance.
Nesprins are Required for Tensional Homeostasis and Force Transmission
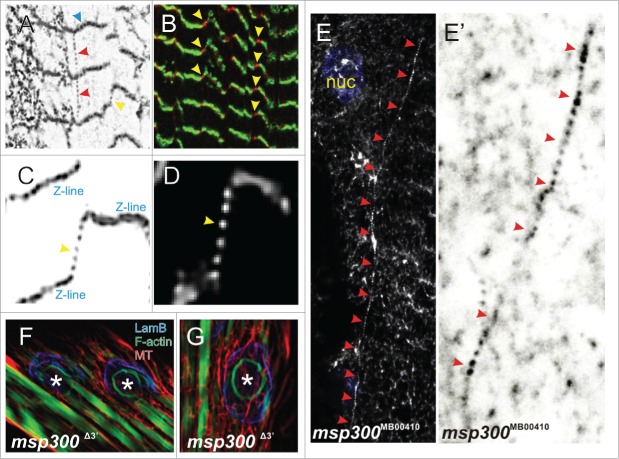
The equilibration of variable mechanical forces applied to the muscle nuclei during contraction/relaxation waves necessitates an elastic network of Drosophila Nesprin MSP300 (muscle-specific protein 300 kDa).11 High resolution microscopy unveils an organization of a flexible polymer network (Fig. 2A). The small puncta, connecting either neighboring Z-lines (red arrowheads) or massive lines (blue arrowheads), are both regions of multiple spectrin repeats, representing the epitope recognized by the anti-MSP300 antibody. These structures, which seem to be cross-linked, connect separate myofibrils with each other (Figs. 2A-D, yellow arrowheads). This observation suggests that Nesprin/MSP300 filaments redistribute variable local cytoplasmic stresses and reestablish global tensional homeostasis within the muscle by force-induced unfolding. Consistently, upon application of external force to the muscles by stretching the larvae, the distance between these dots increases. Our interpretation is that the dotted lines may represent a single Nesprin fiber formed by head-to-tail oligomerization, whereas the thick line overlapping with the Z-lines is probably parallel-bundled fibers.
Figure 2.
Extranuclear MSP300 distribution and organization in muscle cells. (A) MSP300 is localized to the sarcomeric Z-lines (blue arrowhead) and is also seen between the Z-lines (red arrowhead) or between the staggered Z-lines (yellow arrowhead). Note that MSP300 is also able to form a network, suggesting a cross-linking activity. (B) The Z-lines were labeled in green. MSP300 (red) was found between staggered Z-lines and therefore interconnects adjacent myofibrils. (C) High resolution details of MSP300 organization (grayscale inverted) on and between staggered Z-lines. (D) Enlarged part of (C) without inversion. (E, E') In the isoform-specific mutation Msp300MB00410, the assembly patterns of MSP300 fibers are perturbed. Grayscale-inverted enlargement of (E) is shown in (E'). (F,G) In a-mutant of Msp300, an F-actin ring can occasionally be observed.
Strikingly, in isoform-specific mutants, for example in Msp300MB00410, the stereotypical assembly pattern described above is impaired, as MSP300 molecules are either disorganized (Fig. 2E) or assembled as abnormally long fibers (red arrowheads in Figures 2E and E'). We postulate that different Nesprin isoforms serve as molecular building bricks for modular self-assembly of the Nesprin/MSP300 network. We predict a stoichiometric and hierarchical organization of Nesprin, depending on cell type, developmental stage and physiological conditions.
In vertebrates, Nesprin-coding genes generate a large palette of transcripts possessing varied number of spectrin repeats. Moreover, many of the gene products lack either a nuclear envelope anchoring KASH-domain, or a cytoskeleton-associating domain, or both. This indicates that they are highly versatile components, rather than a plain linear linker between nuclear membrane and cytoskeletal elements.12-14 It remains unclear if invertebrates like Drosophila possess intranuclear Nesprin isoforms, too, and if so, how they enter the nuclear envelope and whether they directly interact with the inner nuclear membrane (INM) and chromatin.
Traditionally, Nesprins have been illustrated in conceptual models of diverse literatures as simple and diminutive linkers between cytoskeleton and outer nuclear membrane (ONM). However, the diversity of isoforms of varying lengths suggests a more complex organization. Homology modeling based on spectrin structure suggests that even as a monomer, the Nesprin molecule will reach a gigantic size. A single Spectrin Repeat (SR) contains 99-114 amino acids and has an approximate length of 5 nm (Figs. 3 A and B). As contiguous SRCPs, a Spectrin tetramer is about 180-190 nm long, which is confirmed by super-resolution microscopy.15,16 Moreover, the physiological resting length of spectrin can undergo a 3-fold extension, which can be explained by a compact supercoil model.17 Excluding the linker regions between SRs, a giant isoform of Nesprin-1 possesses 74 SRs and MSP300 at least 78 SRs 18(our unpublished data). Thus, their static length should be more than 370 nm and the de facto total length under tension can be much longer.
Figure 3.
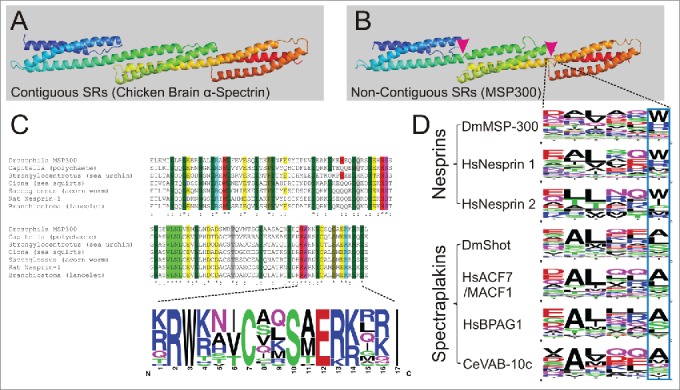
Highly conserved non-canonical motifs in spectrin-repeat-containing proteins. (A) Ribbon diagram of the chicken brain α-spectrin fragment (1u4q) exemplifies 3 contiguous SRs. (B) Homology modeling of MSP300 isoform D (5140-5460) based on 1u4q exemplifies 3 non-contiguous SRs. The magenta arrowheads indicate the position of the linkers. (C) A highly conserved but non-canonical SR is found in nesprins of different animal groups. (D) The hexapeptide linker regions between 2 consecutive SRs are conserved in nesprins and spectraplakins. The blue frame demonstrates the typical tryptophan (W) / histidine (H) residue variants at the -1 of helix C.
With the exception of the N-terminal tandem calponin homology (CH) domains and the C-terminal KASH domain in Nesprins or EF-hand-GAS2 in spectraplakins, interspecies sequence conservation of SRs is relatively low. This is probably because SRs are merely structural elements and, therefore, under low selection pressure during evolution. Nevertheless, in Nesprins we recognized a highly conserved SR with clear consensus (Fig. 3C). Surprisingly, this highly conserved sequence is non-canonical and may harbor functionally indispensable structures or residues for oligomerization. Its helix C, which was thought to contain a bipartite NLS-consensus,19 can be found in both mammalian Nesprin-1 and 2, but also in homologs from 22 different species including mollusc, polychaete, arthropods, echinoderm, hemichordate, cephalochordate but also lower vertebrates (Fig. 3C). Thus, this SR sequence might be very informative for understanding the biology of Nesprins.
In contrast to spectrins, which are stringently organized in contiguous tandem repeats (Fig. 3A), both Nesprins and Spectraplakins possess a hexapeptide linker (Fig. 3D). Interestingly, sequences of these linkers are even more conserved than the SRs themselves. This suggests either that they are not only connective elements but also serve other functions, for instance to provide additional flexibility or to form curvatures, or, in the case of Nesprins, that the last position is either an aromatic tryptophan or positively charged and protonable histidine. We suggest that this is an important and unique feature of Nesprins in general, which might be correlated to their cellular functions. For example, filamentous molecules of that size must be well organized and folded before the assembly into even longer structures. The stoichiometry of the possible MSP300 oligomer needs to be deciphered in order to understand the control of the hierarchical self-assembly process in vivo.
A Newly Discovered Ancient role for Spectraplakin in Mechanical Coupling between Nucleus and Cytoskeleton
Several independent pieces of evidence have revealed the role of Spectraplakin in organizing MTs in myocytes. Similar to our observation, depletion of microtubule-actin cross-linking factor 1 (MACF1), the mammalian homolog of short stop (Shot), in neonatal rat cardiomyocytes perturbs microtubule organization. Proper MT organization is essential for ventricle adaptation to hemodynamic overload. Under aortic pressure overload, MACF1 expression in heart was increased, indicating a specific correlation between cardiovascular malfunction and mechanical responses.20 Shot, the only member of the plakin family in the fly genome, was originally identified as a provider of mechanical linkage between the cortical actin and MTs.21,22 Similarly in C. elegans, the intracellular role of spectraplakins in nuclear anchoring and movement 23 seems to be more ancient. Interestingly, in a recent S2-cell based screen, nesprins and Shot were also identified as factors involved in nuclear morphology,24 possibly indicating a more general role in protecting nuclei.
Genes encoding primitive but complete predecessors of spectraplakin can also be found in animals without tissue organization, such as the placozoan Trichoplax adhaerens or the sponge Amphimedon queenslandica (our unpublished data). Noteworthy, vertebrate genomes contain a few genes encoding different plakins, although their functions partially overlap. Conversely, invertebrate genome usually contains only one plakin gene; but, this gene expresses a large number of splice variants, both with and without plectin repeats. As most arthropods, like Drosophila, lack cytoplasmic intermediate filaments, the yet uncharacterized cellular roles of the plectin repeats-containing isoforms are very interesting.
Last but not least, Drosophila proteomic studies have revealed that both MSP300 and Shot undergo multiple phosphorylation,25,26 suggesting a potential role for phosphorylation in regulating mechanical stability. Also, a general mechanism for force-induced conformational change by exposing cryptic sites has been identified in lamins 27,28 and emerin.29
“Faster, Higher, Stronger:” Breaking Bottlenecks in Muscle Nuclear Biology
Recently, the main bottleneck in the research of muscle nuclear biology is being eliminated by rapid development and integration of new techniques. With few exceptions,30-32 in vivo myocyte studies have so far been conducted mainly in fixed condition. Due to contractility, investigating aspects of muscle cell biology, such as cytoskeleton dynamics, turnover and rearrangement, under physiological conditions requires a combination of high-speed and high-resolution image acquisition. Advancement in visualization technology has opened new experimental opportunities. Another technical barrier that is being overcome is the ability to directly measure myonuclear-specific biomechanics. Under forces, changes in curvature, length and orientation of myonuclei, as well as their displacement over time, are highly informative for a dynamic stress field analysis.
In summary, whereas we presented here novel aspects of the construction principles of myonuclear protection, numerous details of the assembly and architecture of this fundamental mechanism are still missing. For example factors controlling MT polarity around the myonucleus and their dynamic behavior are yet to be characterized. Furthermore, the architectural features, such as oligomerization patterns and in vivo elastic behavior of tandem SRs in large number should be further characterized by approaches such as nanoscale microscopy 15,16 and 2-dimensional infrared spectroscopy (2DIR). We believe and hope that the present data would encourage scientists of bioengineering background to further address the bioarchitecture of myonuclei in more quantitative approaches.
DISCLOSURE OF POTENTIAL CONFLICTS OF INTEREST
No potential conflicts of interest were disclosed.
Acknowledgments
We apologize to colleagues whose work could not be enumerated due to space constraint. We nevertheless thank them cordially for their valuable contributions to the enrichment of our knowledge.
Funding
This study was supported by grants from the Joint Lower Saxony - Israeli Research Project funding program by the Lower Saxony Ministry of Science and Culture (TV), and from the Minerva Foundation, grant number 711743 (TV).
REFERENCES
- 1.Steinmetz PR, Kraus JE, Larroux C, Hammel JU, Amon-Hassenzahl A, Houliston E, Worheide G, Nickel M, Degnan BM, Technau U. Independent evolution of striated muscles in cnidarians and bilaterians. Nature (2012); 487:231-4; PMID:22763458; http://dx.doi.org/ 10.1038/nature11180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Franke WW, Schinko W. Nuclear shape in muscle cells. J Cell Biol (1969); 42:326-31; PMID:4891914; http://dx.doi.org/ 10.1083/jcb.42.1.326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Folker ES, Baylies MK. Nuclear positioning in muscle development and disease. Front Physiol (2013); 4:363; PMID:24376424; http://dx.doi.org/ 10.3389/fphys.2013.00363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Isermann P, Lammerding J. Nuclear mechanics and mechanotransduction in health and disease. Curr Biol (2013); 23, R1113-1121; PMID:24355792; http://dx.doi.org/ 10.1016/j.cub.2013.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Caille N, Thoumine O, Tardy Y, Meister JJ. Contribution of the nucleus to the mechanical properties of endothelial cells. J Biomech (2002); 35:177-87; PMID:11784536; http://dx.doi.org/ 10.1016/S0021-9290(01)00201-9 [DOI] [PubMed] [Google Scholar]
- 6.Elhanany-Tamir H, Yu YV, Shnayder M, Jain A, Welte M, Volk T. Organelle positioning in muscles requires cooperation between two KASH proteins and microtubules. J Cell Biol (2012); 198:833-46; PMID:22927463; http://dx.doi.org/ 10.1083/jcb.201204102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jahed Z, Shams H, Mofrad MR. A Disulfide Bond Is Required for the Transmission of Forces through SUN-KASH Complexes. Biophys J (2015); 109:501-9; PMID:26244732; http://dx.doi.org/ 10.1016/j.bpj.2015.06.057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cleverley RM, Barrett JR, Basle A, Bui NK, Hewitt L, Solovyova A, Xu ZQ, Daniel RA, Dixon NE, Harry EJ, et al. . Structure and function of a spectrin-like regulator of bacterial cytokinesis. Nat Commun (2014); 5:5421; PMID:25403286; http://dx.doi.org/ 10.1038/ncomms6421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kronebusch PJ, Singer SJ. The microtubule-organizing complex and the Golgi apparatus are co-localized around the entire nuclear envelope of interphase cardiac myocytes. J Cell Sci (1987); 88 (Pt 1):25-34; PMID:3327863 [DOI] [PubMed] [Google Scholar]
- 10.Zaal KJ, Reid E, Mousavi K, Zhang T, Mehta A, Bugnard E, Sartorelli V, Ralston E. Who needs microtubules? Myogenic reorganization of MTOC, Golgi complex and ER exit sites persists despite lack of normal microtubule tracks. PloS one (2011); 6:e29057; PMID:22216166; http://dx.doi.org/ 10.1371/journal.pone.0029057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang S, Reuveny A, Volk T. Nesprin provides elastic properties to muscle nuclei by cooperating with spectraplakin and EB1. J Cell Biol (2015); 209:529-38; PMID:26008743; http://dx.doi.org/ 10.1083/jcb.201408098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rajgor D, Shanahan CM. Nesprins: from the nuclear envelope and beyond. Expert Rev Mol Med (2013); 15:e5; PMID:23830188; http://dx.doi.org/ 10.1017/erm.2013.6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rajgor D, Mellad JA, Autore F, Zhang Q, Shanahan CM. Multiple novel nesprin-1 and nesprin-2 variants act as versatile tissue-specific intracellular scaffolds. PloS one (2012); 7:e40098; PMID:22768332; http://dx.doi.org/ 10.1371/journal.pone.0040098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Duong NT, Morris GE, Lam T, Zhang Q, Sewry CA, Shanahan CM, Holt I. Nesprins: tissue-specific expression of epsilon and other short isoforms. PloS one (2014); 9:e94380; PMID:24718612; http://dx.doi.org/ 10.1371/journal.pone.0094380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xu K, Zhong G, Zhuang X. Actin, spectrin, and associated proteins form a periodic cytoskeletal structure in axons. Science (2013); 339:452-6; PMID:23239625; http://dx.doi.org/ 10.1126/science.1232251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.D'Este E, Kamin D, Gottfert F, El-Hady A, Hell SW. STED nanoscopy reveals the ubiquity of subcortical cytoskeleton periodicity in living neurons. Cell Reports (2015); 10:1246-51; PMID:25732815; http://dx.doi.org/ 10.1016/j.celrep.2015.02.007 [DOI] [PubMed] [Google Scholar]
- 17.Brown JW, Bullitt E, Sriswasdi S, Harper S, Speicher DW, McKnight CJ. The physiological molecular shape of spectrin: a compact supercoil resembling a chinese finger trap PLoS Comput Biol (2015); 11:e1004302; PMID:26067675; http://dx.doi.org/ 10.1371/journal.pcbi.1004302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Autore F, Pfuhl M, Quan X, Williams A, Roberts RG, Shanahan CM, Fraternali F. Large-scale modelling of the divergent spectrin repeats in nesprins: giant modular proteins. PloS one (2013); 8:e63633; PMID:23671687; http://dx.doi.org/ 10.1371/journal.pone.0063633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang Q, Skepper JN, Yang F, Davies JD, Hegyi L, Roberts RG, Weissberg PL, Ellis JA, Shanahan CM. Nesprins: a novel family of spectrin-repeat-containing proteins that localize to the nuclear membrane in multiple tissues. J Cell Sci (2001); 114:4485-98; PMID:11792814 [DOI] [PubMed] [Google Scholar]
- 20.Fassett JT, Xu X, Kwak D, Wang H, Liu X, Hu X, Bache RJ, Chen Y. Microtubule Actin Cross-linking Factor 1 regulates cardiomyocyte microtubule distribution and adaptation to hemodynamic overload. PloS one (2013); 8:e73887; PMID:24086300; http://dx.doi.org/ 10.1371/journal.pone.0073887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Strumpf D, Volk T. Kakapo, a novel cytoskeletal-associated protein is essential for the restricted localization of the neuregulin-like factor, vein, at the muscle-tendon junction site. J Cell Biol (1998); 143:1259-70; PMID:9832554; http://dx.doi.org/ 10.1083/jcb.143.5.1259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gregory SL, Brown NH. kakapo, a gene required for adhesion between and within cell layers in Drosophila, encodes a large cytoskeletal linker protein related to plectin and dystrophin. J Cell Biol (1998); 143:1271-82; PMID:9832555; http://dx.doi.org/ 10.1083/jcb.143.5.1271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim HS, Murakami R, Quintin S, Mori M, Ohkura K, Tamai KK, Labouesse M, Sakamoto H, Nishiwaki K. VAB-10 spectraplakin acts in cell and nuclear migration in Caenorhabditis elegans. Development (2011); 138:4013-23; PMID:21831923; http://dx.doi.org/ 10.1242/dev.059568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ramdas NM, Shivashankar GV. Cytoskeletal control of nuclear morphology and chromatin organization. J Mol Biol (2015); 427:695-706; PMID:25281900; http://dx.doi.org/ 10.1016/j.jmb.2014.09.008 [DOI] [PubMed] [Google Scholar]
- 25.Bodenmiller B, Mueller LN, Pedrioli PG, Pflieger D, Junger MA, Eng JK, Aebersold R, Tao WA. An integrated chemical, mass spectrometric and computational strategy for (quantitative) phosphoproteomics: application to Drosophila melanogaster Kc167 cells. Molecular bioSystems (2007); 3:275-86; PMID:17372656; http://dx.doi.org/ 10.1039/b617545g [DOI] [PubMed] [Google Scholar]
- 26.Hilger M, Bonaldi T, Gnad F, Mann M. Systems-wide analysis of a phosphatase knock-down by quantitative proteomics and phosphoproteomics. Mol Cell Proteomics (2009); 8:1908-20; PMID:19429919; http://dx.doi.org/ 10.1074/mcp.M800559-MCP200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Swift J, Ivanovska IL, Buxboim A, Harada T, Dingal PC, Pinter J, Pajerowski JD, Spinler KR, Shin JW, Tewari M, et al.. Nuclear lamin-A scales with tissue stiffness and enhances matrix-directed differentiation. Science (2013); 341:1240104; PMID:23990565; http://dx.doi.org/ 10.1126/science.1240104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Buxboim A, Swift J, Irianto J, Spinler KR, Dingal PC, Athirasala A, Kao YR, Cho S, Harada T, Shin JW, et al. . Matrix elasticity regulates lamin-A,C phosphorylation and turnover with feedback to actomyosin. Curr Biol (2014); 24:1909-17; PMID:25127216; http://dx.doi.org/ 10.1016/j.cub.2014.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guilluy C, Osborne LD, Van Landeghem L, Sharek L, Superfine R, Garcia-Mata R, Burridge K. Isolated nuclei adapt to force and reveal a mechanotransduction pathway in the nucleus. Nat Cell Biol (2014); 16:376-81; PMID:24609268; http://dx.doi.org/ 10.1038/ncb2927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brosig M, Ferralli J, Gelman L, Chiquet M, Chiquet-Ehrismann R. Interfering with the connection between the nucleus and the cytoskeleton affects nuclear rotation, mechanotransduction and myogenesis. The international journal of biochemistry & cell biology (2010); 42:1717-28; PMID:20621196; http://dx.doi.org/ 10.1016/j.biocel.2010.07.001 [DOI] [PubMed] [Google Scholar]
- 31.Wilson MH, Holzbaur EL. Opposing microtubule motors drive robust nuclear dynamics in developing muscle cells. J Cell Sci (2012); 125:4158-69; PMID:22623723; http://dx.doi.org/ 10.1242/jcs.108688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oddoux S, Zaal KJ, Tate V, Kenea A, Nandkeolyar SA, Reid E, Liu W, Ralston E. Microtubules that form the stationary lattice of muscle fibers are dynamic and nucleated at Golgi elements. J Cell Biol (2013); 203:205-13; PMID:24145165; http://dx.doi.org/ 10.1083/jcb.201304063 [DOI] [PMC free article] [PubMed] [Google Scholar]