Abstract
Although oral combination antiretroviral therapy effectively clears plasma HIV, patients on oral drugs exhibit much lower drug concentrations in lymph nodes than blood. This drug insufficiency is linked to residual HIV in cells of lymph nodes. While nanoformulations improve drug solubility, safety and delivery, most HIV nanoformulations are intended to extend plasma levels. A stable nanodrug combination that transports, delivers and accumulates in lymph nodes is needed to clear HIV in lymphoid tissues. This review discusses limitations of current oral combination antiretroviral therapy and advances in anti-HIV nanoformulations. A ‘systems approach’ has been proposed to overcome these limitations. This concept has been used to develop nanoformulations for overcoming drug insufficiency, extending cell and tissue exposure and clearing virus for treating HIV/AIDS.
Keywords: : combination antiretroviral therapy, cure for AIDS, HIV, long-acting, lymphatic drug insufficiency, nanoformulation, systems approach, targeted drug delivery
While there has been success in developing highly potent and specific compounds that suppress virus to undetectable levels in the blood of HIV-infected patients, it is well accepted that these treatments do not cure patients from HIV/AIDS. At opportune times, disruption of therapy due to noncompliance or medical conditions such as an inability to swallow oral tablets and capsules can readily result in viral rebound and progression to AIDS. Therefore, there is an urgent need to understand the source of residual virus; the underlying virologic, physiologic and pharmacologic mechanisms that allow viral persistence; and to develop targeted drug delivery strategies to eliminate residual virus in HIV patients.
Due to steady advancements in HIV research and drug discovery, HIV+ patients on current combination antiretroviral therapy (cART; also referred to as highly active ART or HAART) can effectively maintain undetectable plasma virus levels for years [1]. Long-term plasma viral suppression has improved life expectancy and quality and reduced the rate of disease progression in patients under medical care. Additionally, viral resistance to antiretroviral (ARV) drugs can be addressed with two to three drug combinations that target two or more drug targets within the same or different viral proteins that are pivotal for viral propagation. Should drug-resistant virus appear, clinicians typically switch to alternate drug combinations to suppress virus in plasma. In general, the drugs available for the treatment of HIV/AIDS are proven to be selective and effective with an acceptable safety profile to reduce plasma HIV levels to undetectable levels as long as patients are consistently on chronic oral cART.
Despite the availability of effective oral anti-HIV drugs to suppress virus, HIV persists in tissues within the body even in the absence of detectable plasma virus. This tissue persistence is a significant challenge and believed to be a key barrier to finding a cure for HIV. Even after years of effective plasma viral suppression to undetectable levels, discontinuation of cART results in a rapid viral rebound in patients’ plasma; viral RNA is often detectable within 2 weeks of discontinuation [2]. Rare exceptions to this rule have drawn significant research interest. For instance, genetic variation is thought to play a role in the ability of some individuals to retain high CD4+ T-cell counts despite HIV infection status (termed ‘long-term nonprogressors’), and a small subset is even able to suppress virus in the blood to below the level of detection without antiretroviral therapy (termed ‘elite controllers’). There have been rare cases of a ‘functional cure’ or ‘sterilizing cure’, such as the 2008 case of the Berlin stem cell transplant patient, who remains virus free after receiving stem cells with a mutation that inhibits HIV cell entry [3]. However, despite these rare successes, the vast majority of individuals are unable to control HIV viral replication without daily cART, and the necessity of adherence to therapy is a constant challenge. In addition, due to the high frequency (two- to four-times daily) of oral cART dosing schedules, patient populations at risk for noncompliance such as those unable to swallow, IV drug abusers and recreational drug users become at risk of disease progression.
Even in the USA with advanced medical care and a high living standard, 2011 CDC data showed that only 40% of the 1.2 million HIV-infected people were engaged in medical care (treatment), and only 30% had achieved viral suppression [4]. The reasons for this ‘leaky cascade of care’ are multifold. Individuals that are unemployed, homeless or living in poverty may not have the ability to access or afford regular medical care. Mental health problems can make adherence to daily therapy difficult. Adolescents may lack the mental and emotional maturity to take their medication consistently. Users of recreational injectable drugs are not only at higher risk of transmitting HIV but also may miss doses due to their drug use. In order to engage these individuals in medical care and adequate treatment, new drug delivery methods or dosage forms are needed to provide long-acting therapy and enhance drug exposure in tissues, targeting the residual virus that is pivotal to viral rebound.
Long-acting therapy, referred to as sustained release or extended release (XR) oral dosage forms, has had an extensive history and proven record of contribution to improving patient compliance and effective drug use. In fact, formulation research and development has been a major driver and contributor to growth in the life-cycle (patent life) extension of innovator drugs for large pharmaceutical companies. For chronic treatments, converting multiple daily doses to once-a-day dosing with an XR dosage form has been reported to improve patient compliance [5]. Also, the application of nanoformulations or pharmaceuticals of small size is a proven and important area of pharmaceutical research and development. Pharmaceuticals of small size include nanocrystal, colloids, nanoparticles, lipid vesicles or liposomes, biopolymers, or protein and protein aggregates. When these drug-carrier particles or complexes are small and in the range of 10–1000 nm, they are often referred to as nanoparticles or nanomedicine, and the process or method to produce the dosage form is generally known as nanoformulation of drugs. These nanoformulations are intended to improve pharmaceutical properties and drug response by improving drug solubility, stability, biodistribution, pharmacokinetics, safety and efficacy. Some examples of nanoformulations in clinical use are shown in Table 1 [6–11].
Table 1. . Examples of commercially available nanoformulations.
| Drug | Indication | Particle diameter (nm) | Drug delivery system | Ref. |
|---|---|---|---|---|
| Doxorubicin (Doxil) |
Kaposi's sarcoma |
50–100 |
Liposome |
[6,7] |
| Amphotericin B (Ambisome) |
Fungal infections |
50–100 |
Liposome |
[8] |
| Sirolimus (Rapamune) |
Transplant rejection |
20–1000 |
Nanocrystal |
[9] |
| Aprepitant (Emend) |
Antiemetic |
20–1000 |
Nanocrystal |
[9] |
| Fenofibrate (Tricor) |
High cholesterol |
20–1000 |
Nanocrystal |
[9] |
| Albumin-Paclitaxel (Abraxane) |
Breast cancer |
≈130 |
Protein–drug conjugate |
[10] |
| Gamma Globulin (IVIG) | Autoimmune disease | 5 | Immunoglobulin | [11] |
On a laboratory scale, many novel nanoformulations are reported to improve antiretroviral therapy. Drug delivery platforms that include emulsomes [12], nanoemulsions [13] and solid lipid nanocapsules [14] have shown improved targeting of HIV drugs to the liver, brain and testes, respectively. Unfortunately, the liver is not the major site of HIV replication. In the case of emulsomes, retention of zidovudine in liver tissue with a cationic carrier was shown to be higher relative to a neutral carrier, indicating an ionization-dependent localization of particles. For the nanoemulsion, incorporation of saquinavir (a protease inhibitor with highly variable bioavailability) into a lipid emulsion allowed for improved absorption and higher concentrations in the CNS. Solid lipid nanocapsules were shown to prevent P-gp efflux of indinavir and improve the concentration of drug in the testes and brain. These examples highlight the profound impact that size, morphology and composition can have on the distribution of the active pharmaceutical ingredient. With an understanding of physiochemical properties, hydrophobic/hydrophilic interactions and distributive processes in the body, researchers have demonstrated the potential of nanosize drug formulations in laboratory and early conceptual studies.
Traditional sustained drug release platforms generally act by way of a drug depot at the site of injection or within the gastrointestinal (GI) tract, with slow release of native drug from the dosage form or drug-carrier complex. The slow and sustained release of drug from a reservoir deposited in a local site provides effective plasma drug levels in the blood. With technological advances and a fuller understanding of mechanisms of drug release, transport, distribution, metabolism and elimination over time (sometimes referred to as pharmacokinetics, drug transport and metabolism, or PKDTM), the characteristic physiochemical profile of an active pharmaceutical ingredient can be used to construct nanoformulations capable of increasing drug exposure in the target tissues and cells. In other words, nanoformulations should and could be used not only as an XR platform but also to transport and localize drug preferentially in tissues laden with a high density of a drug-target (e.g., HIV-infected cells or HIV proteins). In this way, nanoformulations not only provide prolonged drug levels in blood but also prolong the time spent within target tissues where virus persists. This is important because extended exposure in the blood alone may not translate to extended drug exposure in tissue, resulting in subtherapeutic effects at sites of interest.
Compared to other areas of therapeutics, interest in developing long-acting HIV drug therapy (once-a-day or lower dosing frequency) is a relatively new. With the availability of highly potent HIV drugs and well-defined tissue and cellular targets, recent innovations in nanoformulation design have offered new strategies to enhance drug exposure over time and increase drug penetration into tissues. It should be reminded that overall tissue exposure requires both achieving therapeutic target drug concentrations in the target tissue and maintaining those concentrations for sufficient time. This is especially true for HIV, where the current goal is to reduce and eliminate residual virus in tissues such as lymph nodes and to some extent in the brain. There are a number of excellent reviews on the use of HIV drug formulations for prevention, as well as nanoformulations intended for suppressing HIV in the brain and CNS. Please refer to [15–20] for additional details.
In the following, we will discuss recent advances in anti-HIV nanoformulation research intended for treating AIDS patients with emphasis on the rationale, progress and potential impact in overcoming the drug insufficiency shown to exist in lymphoid tissues and cells. We will first briefly review HIV infection and current treatment strategies, limitations and challenges of oral dosage forms. We will also highlight the link between limited tissue exposure and the lack of clearance of HIV in tissues, followed by discussion of the systems approach to targeting drugs to tissues and cells – from concept to implementation and validation in primate HIV models. This review will conclude with discussion on future directions in nanomedicine to enhance drug exposure in HIV-infected tissues and cells, as well as patient compliance/acceptance in pursuit of a cure for HIV.
HIV infection & treatment
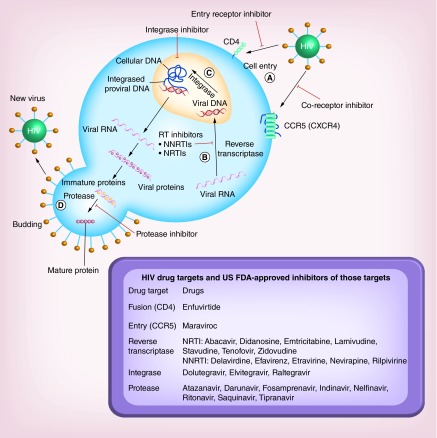
HIV infection was first described in the early 1980s when scientists isolated a retrovirus capable of horizontal transmission in humans with symptoms that often preceded AIDS [21]. Shortly thereafter, a concerted effort between clinicians and scientists allowed elucidation of HIV virus as a causative agent in the development of AIDS [22]. With significant investments of NIH funding for HIV research and tireless effort of HIV researchers, almost all proteins that regulate the HIV life cycle are now identified, and their structure and functional details are characterized, allowing their validation as HIV drug targets. As a result, proteins pivotal to HIV replication are now known in the context of the HIV life cycle, which is presented schematically in Figure 1.
Figure 1. . Schematic representation of the life cycle of HIV beginning with the entry of a viral particle into a healthy cell via membrane proteins such as CD4 or CCR5/CXCR4 and the therapeutic agents approved by the US FDA for use in the treatment of HIV.
After entry into the cell (A), viral RNA is converted into viral DNA by reverse transcriptase (B). Viral DNA is then incorporated into the human genome by integrase (C). Ultimately, expression of viral proteins result in a budding process (D). Newly budded viral particles must undergo cleavage by protease enzymes to mature into infectious viruses.
The pathogenesis of HIV begins with a single infectious virus entering the host CD4+ T lymphocyte, while other cells such as macrophages and mononuclear phagocytes harbor HIV, CD4+ T cells are the major host of viral replication and pathogenesis. In addition to CD4, other host cell receptors such as CCR5 and CXCR4 also serve as coreceptors of HIV envelope protein that mediate viral entry into host cells. Upon entry, as a retrovirus, HIV RNA is transcribed to DNA by HIV reverse transcriptase, which allows HIV DNA to be integrated into host DNA for producing HIV progeny [23]. The viral protein integrase is responsible for incorporating the viral DNA into the human genome. As HIV reverse transcriptase converts viral RNA into viral DNA with a high degree of error, it may introduce genetic variations. In patients on antiretroviral therapy, these genetic variations are responsible for the high rate of drug resistance development [24]. Once integrated into the host genome, HIV can enter its replication cycle through transcription/translation of the HIV viral sequence to produce viral progeny. Viral protein and newly synthesized RNA are transported to the cell plasma membrane and initiate a ‘budding’ process, resulting in new viral particles. These viral particles then require the HIV protease to cleave whole viral proteins into viable, infectious virus for initiating subsequent host cell infection [25].
As the mechanisms and proteins essential for HIV replication are characterized, most of these viral proteins have been used as drug targets for inhibiting HIV replication. Potent inhibitors of viral entry, replication, integration and maturation have been developed as therapeutic agents. These drugs are generally referred to as entry (CCR5/CD4), reverse transcriptase, integrase and protease inhibitors, respectively. Reverse transcriptase inhibitors have been elucidated in detail, which revealed two distinct (nucleoside and non-nucleoside analogs) classes of molecules that inhibit enzyme activity through unique mechanisms. In combination, these two reverse transcriptase inhibitors greatly reduce the risk of inducing drug-resistant virus. In the relatively short span (˜30 years after the discovery of HIV), there are 26 or more drugs available for the treatment of HIV in the USA. Clinically, therapeutic (cART) regimens often target multiple checkpoints in viral replication such as the combination of protease inhibitors with reverse transcriptase inhibitors [26,27]. The development of these agents for cART has been effective at suppressing plasma viral load and preventing drug resistance [28,29].
Residual virus & oral ARV drug exposure in tissues
Lymphoid tissue drug exposure is expected to be low for orally administered cART
Despite the success of oral cART and its ability to reduce the mortality of HIV patients and increase their life expectancy, treatment of HIV is a lifetime commitment [30–34]. While patients on oral cART achieve undetectable virus levels in blood, virus rebounds readily if cART is stopped [35–37]. Residual virus isolated from the lymph nodes of patients without detectable virus in their blood is still responsive to cART [38,39]. Therefore, instead of drug resistance, limited access of orally administered drug to sites of viral persistence in the lymphatic system is likely the cause of the inability to clear residual virus [40,41]. While it is possible that HIV sequences integrated into host DNA may produce latent virus that is later reactivated by some cellular or immunological trigger, it is also likely that insufficient oral drug penetration into lymphoid tissue leads to insufficient tissue drug exposure. Additionally, rapid re-equilibration of the small fraction of penetrated drug back into the blood can exacerbate this problem. The resulting insufficient drug exposure in lymph nodes and other lymphoid tissues could lead to rapid viral rebound soon after stoppage of oral cART.
Physiologic mechanisms of oral versus subcutaneous drug administration relating to overall drug exposure in lymphoid tissues
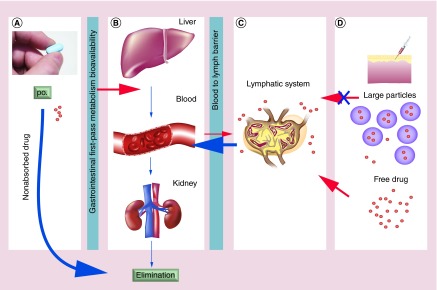
Lymphatic drug insufficiency of oral HIV therapies can be explained from a physiologic and anatomic perspective (Figure 2). First, orally administered HIV drug encounters several barriers and sites of loss in its sequential movement from the GI tract to the systemic circulation. Dissolution, a prerequisite to movement across the gut wall, is the first step (Figure 2A). Incomplete dissolution, slow penetration of the GI membranes, metabolism and decomposition in the gut lumen can all lead to poor plasma HIV drug bioavailability or overall drug exposure in plasma. Second, blood supply draining the gut goes through the liver before reaching the systemic circulation. The liver is the major organ for drug metabolism, excretion and clearance. Limited drug absorption via the gut [42] and early biotransformation and elimination by the liver and kidney lead to only a fraction of orally administered drug entering the systemic circulation (Figure 2B). Third, once absorbed systemically, drug in the blood exists in either protein bound or free soluble form (typically measured as plasma drug concentrations). The drug in free soluble form will be delivered by the blood to most tissues, including organs of elimination such as the liver and kidney. Thus, an even smaller fraction of HIV drug in plasma that presents in free soluble form will be available to penetrate the blood–lymphatic barriers to reach lymphoid tissues (Figure 2C) [43]. Due to nonspecific drug distribution as well as the restriction of biological barriers, lymphoid drug exposure after oral dosing is likely very limited. Fourth, soluble free drug that is found in lymphoid tissue can readily diffuse back into the blood with little resistance, which also likely contributes to lymphoid drug insufficiency by shortening the residence time of drug in the lymphatic system (Figure 2D).
Figure 2. . The distribution of drug varies depending on particle size and route of administration.
(A) Represents issues in bioavailability including solubility in gastrointestinal fluids and absorption through the gastrointestinal and first-pass metabolism. (B) Shows the possible accumulation of drug in off-target tissues as well as clearance of drug through the liver and kidneys. Peripheral accumulation of drug can result in toxicities and adverse reactions. (C) Shows the effect of particle size on drug retention in the lymph. Free drug that absorbs into the lymph is subject to rapid reabsorption into the blood. (D) Shows the effect of particle size on the drug's ability to move in the interstitial space and migrate to the lymph nodes. Free drugs and smaller nanoparticles can readily move into the lymph while larger particles (>1000 nm) stay at the site of injection.
po.: Per os.
Subcutaneous administration of HIV drug in solution is expected to improve drug bioavailability in blood by overcoming problems of oral drug absorption and first-pass metabolism. The free soluble drug molecule in the subcutaneous space can gain equal and direct access to both lymphatic and blood circulatory systems through capillary vessels in the subcutaneous space. The fraction of drug drained and found in the lymphatic system could distribute into the nodes without being subjected to first-passage metabolism before appearing in the blood. However, the drug found in lymphoid tissues can still readily diffuse out of the vessels into tissue and return to the blood via blood capillaries or the interstitial space in the body. Therefore, the subcutaneously administered HIV drug in solution could bypass the need of drug penetration from blood to lymph but would not provide duration of drug retention in the lymphatic system to improve the overall lymphoid tissue drug exposure.
On the other hand, drug formulated in large particles such as microspheres may improve drug retention in the subcutaneous space by inhibiting the drug backflow from lymph nodes (Figure 2D). However, the hindrance of significantly larger particle size also makes it difficult for those large particles to enter the lymphatic system. Thus, only drug molecules released (slowly) from the particles at the injection site are available to lymph, leading to apparent sustained but low levels of drug in the blood and lymph; overall, this process provides some improvement over oral drug dosages but still carries significant limitations to improve drug exposure in lymphoid tissues. Therefore, subcutaneous administration of free drug or a large particle formulation is unlikely to significantly improve tissue and cell drug exposure and address HIV drug insufficiency. As a result, strategies are urgently needed to increase lymphoid drug exposure that not only improve drug distribution but also enhance the retention time of drug in the lymphatic system.
HIV drug insufficiency in lymphoid tissues after oral dosing: hypothesis & data validation
While logical, the hypothesis we proposed in 2003 about lymphoid tissue drug insufficiency in HIV patients on oral drug therapy needed validation. To determine whether HIV drug levels in lymphoid tissue are lower than in blood, we have investigated and compared drug concentrations in cells in lymph nodes and the equivalent cells in blood from patients on oral cART. These results were published in 2003 [44,45]. We found that in patients on chronic oral cART therapy (with steady-state drug levels), intracellular indinavir levels in lymph node cells were about 66% lower than the same set of cells in blood (Table 2 [44,46]). Based on these data, we proposed that drug insufficiency in lymphoid tissues after oral dosing is one of the factors likely to contribute to residual virus. We also highlighted the need to address this issue for improved effectiveness of HIV therapy. Recently, our initial finding about lymphatic HIV drug insufficiency in treated patients was confirmed and extended to multiple classes of HIV drugs [47]. In a prospective study, Fletcher et al. reported that multiple drug concentrations in lymph nodes were as much as 99% lower than those in blood for the same patient (Table 3 [47]). Low lymphatic drug levels in patients also paralleled the residual virus and viral replication detected in tissues [47]. Thus, the association between suboptimal concentrations of ARV drugs in lymphoid tissues and viral persistence is established. While a number of oral ARV combination drug therapies can reduce detectable viral load in blood to nearly undetectable levels in HIV-infected patients, they are unable to maximally suppress or clear the virus in lymphoid tissue compartments due to the limited drug exposure [48].
Table 2. . Indinavir concentrations in peripheral blood mononuclear cells relative to concentrations in plasma and lymph node mononuclear cells in HIV-1-infected humans receiving oral therapy.† .
| PBMC (ng/ml)‡ | PBMC/plasma ratio | LNMC/PBMC ratio | |
|---|---|---|---|
| Patient A |
860 |
1.40 |
0.35 |
| Patient B |
940 |
NA |
0.28 |
| Patient C | 265 | NA | 0.23 |
†Drug concentrations were determined in patients who had received 800 mg indinavir t.i.d. (3×/day) orally for at least 1 month as part of the combination therapy. PBMCs and LNMCs were isolated from blood and lymph node tissue, respectively, as described in ref. [44].
‡Intracellular drug concentrations were calculated assuming an intracellular volume of 4 × 10–9 ml per cell [46].
LNMC: Lymph node mononuclear cell; NA: Not available; PBMC: Peripheral blood mononuclear cell; t.i.d.: Three times a day.
Data taken from [44].
Table 3. . Drug concentrations in lymph nodes compared with blood for different anti-HIV drugs.† .
| HIV drug | Viral RNA in lymph node (over 6 months) | Drug present in LN vs PBMC (%)‡ |
|---|---|---|
| TDF |
+ |
20 |
| FTC |
+ |
35 |
| ATV |
+ |
0 |
| DRV |
+ |
0 |
| EFV | + | 5 |
†12 HIV+ patients were administered combination antiretroviral therapy for 6 months with LN and blood samples taken monthly for drug and virological analyses.
‡Median drug concentrations over the 6-month study period were determined in LNs and blood, and the ratio of ([drug]LN/[drug]blood) was taken. Data are expressed as a percentage.
ATV: Atazanavir; DRV: Darunavir; EFV: Efavirenz; FTC: Emtricitabine; LN: Lymph node; TDF: Tenofovir disoproxil fumarate.
Current status of HIV drug nanoformulations
Various forms of nanoformulations including liposomes, polymeric nanoparticles (NPs), solid lipid nanoparticles (SLNs), dendrimers and micelles are reported to enhance the effective delivery of ARV drugs for HIV prevention and therapy (Table 4) [44,49–74]. Among them, liposome formulations have been extensively used for delivery of anti-HIV drugs to enhance solubility of insoluble drugs with high log P, protect drugs from enzymatic degradation, improve intracellular uptake and encapsulate both hydrophobic as well as hydrophilic drugs [44,49–61]. While many reports are in a preformulation or formulation characterization stage, some have progressed to in vivo small-animal studies. Jin et al. [54] investigated the pharmacokinetics and tissue distribution of zidovudine in rats following intravenous administration of zidovudine myristate (a prodrug of zidovudine)-loaded liposomes. The prodrug resulted in greater entrapment efficiency and a longer plasma half-life in rats. Other studies showed that PEG-coated liposomes have benefits as long circulation carriers for lymphatic tissue delivery after intravenous administration and were less cytotoxicity in mice [57,58]. In addition to conventional liposomal delivery system, Gagne et al. developed an indinavir-loaded targeted liposome (immunoliposome), which showed 21- to 126-fold enhanced drug accumulation in the liver, spleen and lymph nodes when compared with free indinavir [59]. Garg et al. used mannose and galactose as targeting moieties on liposomes, which were intended to improve uptake by macrophages with galactose receptors. Stavudine loaded into mannosylated and galactosylated liposomes exhibited greater cellular uptake by cells of the mononuclear phagocytic system. Unfortunately, this strategy also enhanced accumulation in other organs of the mononuclear phagocytic system such as the liver, spleen and lungs in Sprague–Dawley rats (compared with free drug solution and control liposomes) [60,61]. Development and characterization of HIV drugs in polymeric nanoparticles, micelles, dendrimers and SLNs, and the results of some of the representative formulations are summarized briefly in Table 4 [62–74].
Table 4. . Brief summary of selected nanoformulations of HIV drugs and in vitro/animal experimental model.
| Nanosystem | Anti-HIV drug | Summary | Study model | Ref. |
|---|---|---|---|---|
| Conventional liposomes | TFV | TFV-loaded cationic proliposomes improved the permeation of TFV | In vitro study: Caco-2 cell model[51] NA [52] | [51,52] |
| Nevirapine | Nevirapine showed better encapsulation and quick release from liposomes in PBS and DMEM | In vitro release kinetics study | [53] | |
| Indinavir | Lipid–drug association enhanced HIV-1 protease inhibitor indinavir localization in lymphoid tissues | In vivo study: protease macaque model | [44] | |
| AZT | AZT myristate prodrug liposomes showed higher release of AZT in plasma, RES and brain; plasma concentration of drug after intravenous administration of drug-loaded liposomes was twofold higher compared with plain drug | In vivo study: the pharmacokinetic profiles and tissue distribution of AZT in rat model | [54] | |
| 2′,3′-Dideoxyinosine (ddI) | Liposome encapsulation of ddI enhanced drug accumulation in the reticuloendothelial system | In vitro study: murine monocyte – macrophage RAW 264.7 cells and human premonocytoid U937 cells | [55] | |
| In vivo study: female SD rats model | ||||
| |
Zalcitabine (ddC) |
Drug-loaded liposomes were taken up rapidly by mouse Mac cell line and compared with the free drug |
In vitro study: Macrophage RAW 264.7 cells |
[56] |
| PEGylated liposomes | Saquinavir | PEGylated liposomes loaded with saquinavir were less cytotoxicity | In vitro study: Jurkat T-cells | [57] |
| AZT | PEGylated elastic liposomal formulation for lymphatic targeting of AZT. Biodistribution study indicated 27-fold higher accumulation of drug in lymphoid tissues after transdermal application compared with free drug | In vitro study: lymphoid cells (MT-2 cell line) | [58] | |
| |
|
|
In vivo study: rat model |
|
| Targeted liposomes | Indinavir | Immunoliposomes were very efficient in delivering high concentrations of indinavir to lymphoid tissues | In vitro study: PM1 cells and Sup-T1 cells; in vivo study: female C3H mice model | [59] |
| Stavudine | Mannosylated liposomes maintained a significant level of stavudine in the liver, spleen and lungs | In vitro study: MT-2 cell line | [60,61] | |
| In vivo study: intravenous injection in SD rats[60] | ||||
| In vitro study: the frozen MT2 cell line | ||||
| |
|
|
In vivo study: female New Zealand rabbits model [61] |
|
| Polymeric nanoparticles | TFV | pH-sensitive nanoparticles for intravaginal delivery of anti-HIV/AIDS microbicide TFV and TFV disoproxil fumarate | In vitro study: Vaginal/endocervical cell lines | [62] |
| Saquinavir | Poly(ethylene oxide)-modified poly(epsilon-caprolactone) provides a versatile platform for encapsulation of saquinavir and intracellular delivery in Mo/Mac cells | In vitro study: THP-1 human Mo/Mac cell line | [63] | |
| Lamivudine | In vitro blood–brain barrier permeability of drug increased up to ten–18-fold with polymeric nanoparticles compared with conventional formulation | In vitro study: bovine brain-microvascular endothelial cells; brain-microvascular endothelial cells | [64] | |
| Nevirapine | Poly(lactide-co-glycolide) nanoparticles were grafted with transferrin enhanced the permeability of nanoparticles in human brain-microvascular endothelial cells | In vitro study: human brain-microvascular endothelial cells | [65] | |
| Didanosine | Nanoparticles successfully transported didanosine to Macs in vitro and may control HIV infection effectively at an early stage | In vitro study: macrophage cells from mice (Swiss albino, female 22 ± 2 g) | [66] | |
| |
Zidovudine |
Polylactic acid nanoparticles were more efficiently phagocytosed than polylactic acid/PEG (polyethylene glycol) blends |
NA |
[67] |
| Polymeric micelles | EFV | Intranasal administration of EFV loaded micelles for targeting brain | In vitro study: male 3-month-old Wistar rats | [68] |
| |
Saquinavir |
Combination of PEI/γ-PGA/PLGA NPs increased the permeability of drug across the blood–brain barrier in vitro |
In vitro study: human brain-microvascular endothelial cells |
[69] |
| Polydendrimer |
EFV |
Poly(propyleneimine) dendrimer-based nanocontainers for targeting of EFV to Mo/Mac cells |
In vitro study: human Mo/Mac cells |
[70] |
| Dendrimers |
NA |
Carbosilane dendrimer as a propitious molecule may against HIV replication |
In vitro study: peripheral blood mononuclear cells and MT2 cells |
[71] |
| SLNs | EFV | EFV-loaded SLNs exhibited 5.32-fold increase in peak plasma concentration (Cmax) and 10.98-fold increase in AUC in comparison to EFV suspension | In vivo study: pharmacokinetic studies in albino rats model | [72] |
| |
Nevirapine |
SLNs entraped more nevirapine than nanostructured lipid carriers, HAS-grafted SLN increased the viability of human brain-microvascular endothelial cells about 10% |
In vitro study: human brain-microvascular endothelial cells |
[73] |
| Nanofibers | TFV | Electrospun cellulose acetate phthalate nanofibers have been reported to have intrinsic antimicrobial activity, efficiently neutralize HIV in vitro | In vitro study: human vaginal epithelial cells | [74] |
AUC: Area under the curve; AZT: Zidovudine; EFV: Efavirenz; Mac: Macrophage; Mo: Monocyte; SD: Sprague–Dawley; SLN: Solid lipid nanoparticle; TFV: Tenofovir.
To varying degrees, nanotechnology-based drug delivery systems could potentially enhance uptake of anti-HIV drugs into HIV host and infected cells in vitro and improve the pharmacokinetics, pharmacodynamics and biodistribution of ARV agents in various rodent models. It should be noted that most of these reports, if not all, are in early stage and use only a single agent formulation, which is no longer acceptable clinical practice for HIV therapy. Nevertheless, these reports demonstrate potential of nanotechnology to modify tissue distribution and extend the plasma half-life of HIV drugs [63]. When an anti-HIV drug is encapsulated in a nanosystem, its absorption, metabolism and excretion is not exclusively governed by drug properties; rather, the nanosystem's physical–chemical properties, particularly surface-exposed molecules and electric charge, and its size, could significantly modify the resident time and metabolic and elimination rates [75,76]. To our knowledge, most current nanomedicine platforms for HIV treatment focus on drug delivery in the blood and on improving pharmacokinetic profiles. Minimal research effort has been directed at developing drug delivery systems that would target other major sites of residual HIV, for example, lymphoid tissues in the mucosa such as gut-associated lymphoid tissue (GALT) and in the peripheral and visceral nodes throughout the lymphatic system [50].
To reduce off-target effects and improve on-target drug distribution into tissues and cells that mediate or are linked to a clinical syndrome, an innovative nanoformulation must be stable both in vitro and in vivo for sufficient duration and exhibit physiochemical properties that allow distribution and localization of drug particles within the sites of interest (e.g., lymphoid tissues and nodes), while minimizing peripheral toxicities (e.g., liver and kidney). Consideration of the systems context and practical prospects for clinical translation is also essential, as many of what were considered highly effective but complex formulations have proven to be impractical to scale up and/or unstable in vitro or in vivo for clinical development. Furthermore, the majority of work done to date in the field of nanocarrier ARV drug delivery systems involves the use of single ARV agents. As mentioned, single-agent ART is no longer clinically relevant. Given that clinical use of combinations of drugs is more efficacious than HIV monotherapy, the understanding of HIV pathophysiology and the fact that the residual virus target is linked to drug insufficiency could be leveraged to develop novel cART nanodrug formulations with high relevant and increasing their potential clinical impact.
Systems approach to target HIV drugs & overcome lymphatic drug insufficiency
The systems approach concept
Lymphoid tissue is now a well-established target tissue in HIV patients on cART with low or no detectable HIV in their blood [44,47,77–78]. While it is clear that drug insufficiency in lymphoid cells parallels residual virus in lymphoid tissues [44,47], it is less-well appreciated that the use of either subcutaneous dosing of drug or traditional sustained-release formulations to gain access to the lymphatic system may not address the much needed enhancement of HIV drug exposure in lymphoid tissues. As mentioned, subcutaneous drug administration can provide first passage of drug into the lymph and lymph nodes; however, free drug in solution or released from a sustained-release dosage form will readily diffuse out of the lymphoid tissue and redistribute into the blood. Therefore, only very limited short-acting and transient gains will be realized. This short-term gain is unlikely to improve lymphoid tissue drug exposure and overcome drug insufficiency. A more concerted and integrated approach is needed to enhance drug resident time and improve the drug concentration in lymphoid tissue.
Therefore, beginning in 2003, we began to integrate knowledge in HIV pathogenesis with other advancements in bioanalytical markers and assessment tools to develop a novel drug delivery platform that efficiently tackles barriers to HIV eradication. For example, the rebound of viral load and the precipitate decline of the HIV target CD4+ T cells are now validated to be pathological/surrogate markers of HIV progression to AIDS. In addition, advances in bioanalytical capabilities using a number of tools such as chromatographic mass spectrometry have allowed probing of tissue drug exposure with sensitivity and specificity necessary to evaluate the detailed time course and effects of nanodrug combination formulations in small tissue and cell samples. With these advances and converging knowledge about the virology, cellular immunology and pathology of disease progression in HIV/AIDS along with the ability to isolate residual virus in lymphoid tissues, we have proposed and implemented an integrated approach called the systems approach to nanomedicine, also referred to as the systems approach to targeted drug delivery (Figure 3).
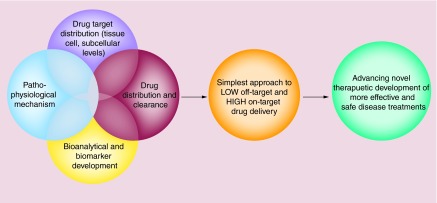
Figure 3. . Schematic representation of the systems approach concept.
It involves integration of the detailed understanding of four core areas of biomedical knowledge: first, drug target distribution and localization in the body at molecular, intra-/extra-cellular and tissue/organ levels; second, drug biodistribution and clearance of drug in the body over time; third, quantitative or bioanalytical markers that could be measured in blood or other samples for predicting drug levels and disease outcome and fourth, physiological and pathological consequences of the aberration of drug targets that reflect disease symptoms. This is in addition to selecting the right drug(s) with potent efficacy and high safety. The integration of these four areas of knowledge is the basis for the development of the simplest approach of a drug delivery platform that leads to low off-target and high on-target effects. Finally, the ultimate outcome of a successful systems approach is a novel therapeutic that is more effective and safe at treating a disease.
In brief, the systems approach to targeted drug delivery is an integrated drug delivery and targeting platform designed to address lymphoid tissue drug insufficiency and HIV persistence. It is a holistic approach for drug development that includes careful consideration of: first, the detailed pathobiological and pathophysiological context of a disease and hence drug targets and their biological distribution; second, drug distribution (at molecular, intra-/extracellular, tissue, organ and blood levels), elimination and action; and third, validated measures or surrogate biomarkers of disease outcomes coupled with bioanalytical capabilities. It integrates molecular, cellular and physiological knowledge, and an understanding of pharmacology (the study of drug action) and pharmacokinetics (how the body handles drug) of drug–target interactions. The time course of drug concentrations and the extent of drug exposure at target sites in the body relative to the blood are carefully contrasted and considered. Moreover, an intricate understanding and definition of key-limiting factors that impede total elimination of a disease is considered in this approach to design and build a delivery system that can best deliver drug to molecular, intra-/extracellular and tissue targets, and realize maximal overlap between the distribution of drug molecules (and exposure) and that of drug targets. The overall goal of the systems approach is to reduce off-target effects and metabolic conversion and to improve on-target drug distribution into the precise disease targets that mediate or are linked to a clinical HIV/AIDS syndrome. For practicality and sustainability, a minimalist approach is preferred to arrive at the simplest tissue and cell localization strategy – this should enable a cost-effective and scalable drug delivery system with the fewest preparation steps needed to ensure a stable pharmaceutical dosage formulation specifically targeted to well-defined sites of action.
Application of the systems approach to enhance tissue & cell drug exposure in lymph nodes: the tissue of drug insufficiency in HIV infection
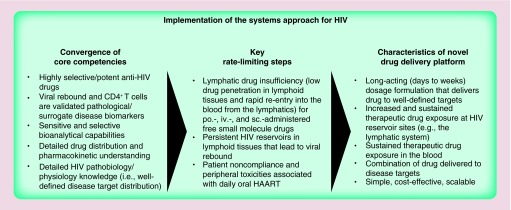
HIV is a prototypical case study on the implementation of the systems approach because the components required to implement the systems approach are mostly in place (Figure 4). HIV target proteins, as well as their respective inhibitors (drugs), are well characterized and have been validated to modify virus levels and rate of HIV progression to AIDS. This detailed knowledge forms the basis of the molecular targets that are validated by having drugs approved for human use with selective, inhibitory efficacy against HIV, but minimal cross-activity against human host protein counterparts. In addition, in the case of residual virus and virus-infected cells in lymphoid tissues with limited drug exposure, the tissue distribution of HIV drug–target (enzymes) is also defined to be cells of lymph nodes and associated tissues. While viremia is an important factor, precipitate decline in CD4+ T cells is among the best clinical hallmarks and surrogate biomarkers of HIV progression to AIDS [79]. Having a validated surrogate biomarker that represents disease outcome to assist in early decisions on drug development is invaluable to the design and implementation of the systems approach is designed to preferentially deliver to and improve drug exposure in lymphoid tissues. As mentioned above, the tissues that HIV infects are also well understood, which primarily include the blood, the lymphatic system and CNS. A key consideration is if the extent of drug exposure in target tissues is sufficient for therapeutic efficacy to take place. Thus, a detailed understanding of HIV drug uptake, distribution (including the influence of drug transporter proteins, such as P-gp), metabolism and elimination in tissues and cells within the body over time is needed. Inconsistent drug penetration and insufficient drug exposure in tissues other than highly perfused organs such as the liver, kidneys and lungs can pose a unique set of challenges [43]. As discussed above, only a very small fraction of orally administered drug gains access to the lymphatic tissues. This poor drug exposure in lymph nodes may be a key underlying mechanism of the low but detectable and persistent virus in lymph nodes. Finally, as mentioned above, bioanalytical capabilities exist that use chromatographic mass spectrometry and target tissue and cell isolation to detect drug molecules inside cells with high sensitivity and selectivity. Highly sensitive assays capable of detecting a single virus copy of HIV's ribonucleotide sequences have been developed to prove that cART reduces the detectable viral load in blood to nearly undetectable levels in HIV patients. Thus, in summary, the following six components have coalesced for HIV to make the systems approach applicable to novel anti-HIV therapeutic development: first, pathobiology/physiology of disease mechanisms; second, definition and characteristics of disease targets at molecular, intra-/extracellular and tissue levels; third, selective and effective drug molecules; fourth, validated surrogate biomarker(s) that represents disease outcome; fifth, drug and drug–target (residual viral-infected cells) distribution (including influence of drug transporter proteins) and pharmacokinetics in cells, tissue and blood within the body and sixth, sensitive and selective bioanalytical capabilities.
Figure 4. . Schematic representation of implementation of the systems approach for HIV nanoformulations.
Following the systems approach principle, implementation from concept to practice could be divided into three general components: first, convergence of core competencies; second, identifying key rate-limiting steps and third, definition of the therapeutic product profile for the nanoformulation.
HAART: Highly active antiretroviral therapy; iv.: Intravenously; po.: Per os; sc.: Subcutaneously.
Lymphatic drug insufficiency and persistent HIV in the lymphatic system have been identified as the drug delivery target and a limiting step in viral eradication in HIV patients on oral cART [80,81]. The key to overcoming drug insufficiency and residual HIV is improving drug exposure in lymphatic tissue above and beyond what is currently achievable [82]. In other words, drug delivered to the lymphatic system must accumulate and be retained/trapped in the tissues (i.e., lymph nodes) and cells for extended periods of time, so it can carry out its therapeutic action at its target (i.e., HIV proteins essential for replication).
After integrating the systems knowledge and clearly defined target of lymphoid tissues and cells, we then look for characteristics of drug and drug particle properties that could gain not only a first-pass advantage but also accumulate in the lymph nodes for an extended time. Following the early discovery that ink particles of 40–80 nm (not soluble fountain pen ink) are trapped in the interstitial space within the lymph nodes [83,84], we reasoned that having nanodrug particles of 50–100 nm that drain from the subcutaneous space into lymph vessel could allow accumulation of drug molecules as they flow throughout the lymphatic system (the resulting drug–lipid nanoformulation will be discussed below) [44].
For clinical translation, the simplest tissue and cell localization strategy is favored to provide a cost-effective and scalable pharmaceutical preparation that accelerates product development. To address lymphatic drug insufficiency and suppress viral rebound, we systematically developed and evaluated anti-HIV drug particles that distribute throughout the lymphatic system after subcutaneous administration and avoid metabolic liabilities that occur with chronic oral dosing. In initial proof-of-principle nonhuman primate studies, we used the protease inhibitor indinavir in lipid particles in which the lipid stabilizes water-insoluble (hydrophobic) indinavir in 50–90 nm lipid–drug particles at neutral pH (7–8), and then readily releases drug from particles at pH 4–5. After subcutaneous administration, indinavir concentrations were greatly enhanced in lymph nodes throughout the lymphatic system (e.g., inguinal, mesenteric, submandibular and other peripheral and visceral nodes) compared with free indinavir given orally. Moreover, the natural course of HIV progression was disrupted by CD4+ T-cell decline being reversed, and both viremia and residual viral levels in lymph nodes were reduced [44,45]. Therefore, these studies demonstrated that lipid-bound drug targets the system of interest (the lymphatics) and retains pharmacologic activity.
These proof-of-principle studies with indinavir–lipid nanoparticles demonstrated the potential of the systems approach to develop a method to target HIV drug to sites of viral persistence, overcome lymphatic drug insufficiency and enhance viral suppression. However, since monotherapy is no longer an accepted HIV treatment paradigm due to the high risk of drug resistance, a combination of at least three drugs targeting at least two viral proteins is required to sufficiently combat HIV. Thus, to move our drug delivery system forward in a clinically relevant direction, we pursued a combination anti-HIV drug nanoparticle formulation to increase clinical efficacy and reduce the risk of harboring drug-resistant HIV.
Application of the systems approach to design nanoformulation with multiple HIV drugs
Patient compliance remains a significant hurdle in HIV treatment strategies. Several key demographics, including those suffering from economic hardship, mental disability or injectable drug use, have increased rates of treatment interruption and cessation due to lack of access to continuous care and difficulties complying with oral dosing frequencies of twice or even three-times daily administration. In the case of nanoformulations for parenteral administration, daily dosing would be even more impractical. Drug delivery strategies that achieve prolonged drug exposure after a single injectable dose could allow access to therapy for many underserved at-risk demographics. A survey conducted by the University of Nebraska and Johns Hopkins University indicates a high degree of patient interest in weekly or less frequent injectable anti-HIV therapy, especially among young people and among users of recreational drugs [85]. Thus, applying the systems approach to develop long-acting nanoformulations capable of targeting drug combinations to the lymphatic system after subcutaneous delivery may not only overcome lymphatic drug insufficiency that currently enables viral persistence but also address current concerns of patient compliance.
Targeted long-acting combination antiretroviral therapy intended for simultaneous delivery of multiple anti-HIV drugs
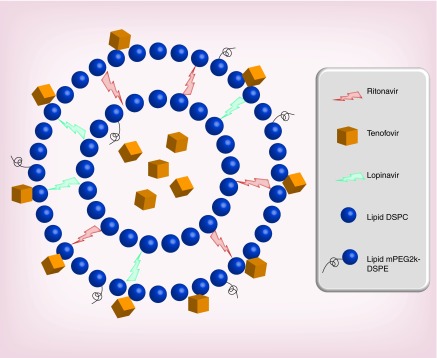
The targeted long-acting combination antiretroviral therapy (TLC-ART) formulation composed of drug–lipid nanocomplexes that carries multiple ARV drugs in a single nanoformulation. It is intended to enhance exposure of drug in lymphoid tissues after subcutaneous administration [41]. The overall goal is to enhance and prolong drug exposure in the lymph node and lymphoid tissue targets (to overcome tissue drug insufficiency) and to extend the duration of plasma drug concentrations. We have chosen two protease inhibitors, lopinavir (LPV) and ritonavir (RTV), and a nucleoside reverse transcriptase inhibitor (PMPA) as a test combination for TLC-ART (Figure 5). The lipophilic protease inhibitors lopinavir and ritonavir naturally associate with the lipid excipient in TLC-ART, resulting in a high degree of lipid–drug binding. A significant fraction of hydrophilic tenofovir (PMPA) can also be simultaneously and stably associated.
Figure 5. . Schematic representation of a lipid nanoparticle with combined antiretroviral drugs.
The lipophilic protease inhibitors (ritonavir and lopinavir) are shown to be integrated into the lipid compartment of nanoparticles. Tenofovir is shown to be associated within the aqueous compartment and on the hydrophilic surface due to its hydrophilicity. This formulation enables the loading of multiple drugs with variable solubility/absorption properties into the same lipid nanoparticle composed of DSPC (1,2-distearoyl-sn-glycero-3-phosphocholine) and mPEG2000DSPE (1,2-distearoyl- sn-glycero-3-phosphoethanolamine-N-methoxy-polyethylene glycol-2000).
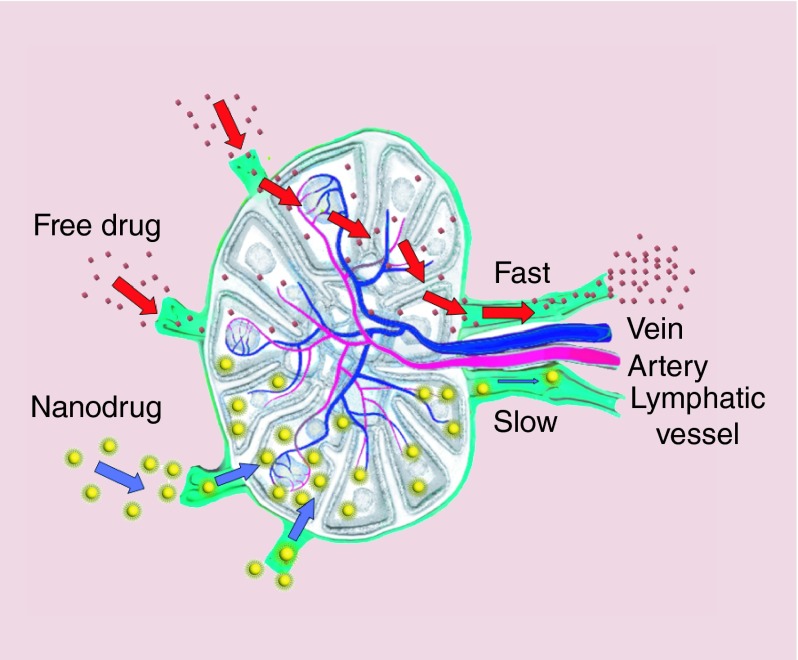
While traditional sustained-release drug delivery systems (a depot or biodegradable implant at the injection site) slowly release native drug over time, TLC-ART nanodrug combination formulation has proven to gain preferential lymphatic access from the subcutaneous injection site, enhance drug penetration and retain within cells. In addition, the nanodrug combination formulation prolonged circulation time of the drugs in the blood (Figure 6) [86]. With a particle size of 50–70 nm, the TLC-ART formulation is ideally sized for lymphatic uptake, distribution and retention within lymph nodes [87]. This capability has been demonstrated in nonhuman primates, which is one of the most appropriate models for anti-HIV nanotherapeutics intended to enhance drug exposure in tissue and cells.
Figure 6. . Schematic representation of differences in lymphatic uptake, distribution and retention of lipid nanoparticles in the lymph nodes in comparison to free drug.
Translation of ARV drug combination nanoparticles intended to enhance intracellular drug exposure in lymphoid tissues & cells
Following the systems approach, verification of systemic targeting to lymphoid tissues and cells by in vivo evaluation can, and often should, be conducted. These studies should be done as a priority and prior to the addition of targeting moieties to a nanoformulation for cell-specific localization, such as a CD4-selective peptides and antibodies or aptamers. Inclusion of these cell-selective moieties not only increases complexity and cost but also the challenges in scaling from laboratory testing to large-animal testing, which is essential for clinical translation and development. While we have developed capabilities to target HIV drug nanoformulations to CD4+ T cells [88], which is the HIV host, we deliberately did not proceed with clinical translation to find the simplest solution for addressing lymphoid tissue drug insufficiency. No CD4 targeting is necessary to improve drug exposure in cells of lymph nodes [89].
Evaluations of anti-HIV therapies in nonhuman primates are the most appropriate model for multiple physiological and virological reasons. Rodents have very loose connective tissue in the subcutaneous space, resulting in wide lateral expansion of administered compounds, potentially impacting drug absorption and distribution [90]. While swines have tight attachments in the subcutaneous space that mimic that in humans, they also have inverted lymph node architecture that may impact trafficking through the lymphatic system [91]. Not only do the larger size and comparable lymphatic physiology make nonhuman primates a preferable model for subcutaneously administered compounds, but they are also an ideal model for HIV research due to their ability to develop AIDS [92]. Importantly, SIV-infected macaques (a nonhuman primate) treated with antiretroviral therapy demonstrate the same plasma viral suppression and rebound viremia seen in humans [93].
When administered to macaques, drug–lipid nanoparticles containing lopinavir, ritonavir and tenofovir resulted in enhanced and extended drug concentrations of all three drugs in plasma and peripheral blood mononuclear cells with drugs still detectable 7 days after administration [87]. Total drug exposure (plasma AUC from 0 to 168 h) was enhanced sevenfold or more compared with free drug in suspension. This was particularly remarkable for hydrophilic tenofovir which, with only 12% lipid-association, not only demonstrated increased total drug exposure but also showed a dramatic shift toward delayed drug exposure with 51% of exposure occurring after 24 h. By contrast, administration of free drug resulted in only 0.1% of tenofovir exposure occurring after 24 h. Triple-drug particles also enhanced intracellular drug concentrations within peripheral lymph nodes, supporting our previous findings with monodrug particles containing indinavir, which enhanced drug concentrations in peripheral and visceral lymph nodes throughout the body [44]. Importantly, no significant adverse effects were observed.
Few other anti-HIV nanoformulations have been evaluated in primates, likely indicative of the challenges involved in translating from small-scale nanoparticle development to the larger scale aseptic production needed for in vivo studies. Liposomes containing antithrombin III, a serpin with anti-HIV activity, were administered subcutaneously to SIV-infected macaques and resulted in a decrease in plasma viral load [92]. However, tissue drug concentration was not evaluated, and the clinical relevance of antithrombin III has yet to be demonstrated. Polymer-coated crystalline forms of RTV and atazanavir (ATV), prepared separately and mixed at the time of administration, resulted in sustained drug release in plasma, but this was accomplished via a slow-release drug depot in the subcutaneous space, and lymphatic tissue concentrations were not evaluated [94]. This is in contrast to a long-circulating nanoformulation that is sized for direct lymphatic uptake. Although possible, mixing of two or more single-drug nanoformulations could circumvent the need to produce a nanoformuation containing multiple HIV drugs. However, the mixing approach may lead to heterogeneous intracellular drug concentrations for each drug, which could pose drug resistance concerns. Uneven peak and trough intracellular concentrations could be minimized with a fixed ratio of drug combinations in a single nanoformulation such as that proposed in the TLC-ART strategy. As presented above, the TLC-ART formulation with a three HIV drug combination allows for simultaneous delivery of multiple complimentary drugs to target tissues and cells. Taken together, by combining our understanding of lymphatic physiology, drug pharmacokinetics and clinical need, we have developed lipid–drug complexes containing multiple ARV drugs that provide enhanced lymphatic delivery and prolonged drug concentrations in blood and cells. With a target dosing frequency of once-weekly or less, TLC-ART has the potential to address challenges of lymphatic drug insufficiency and patient noncompliance inherent to current oral cART. While incorporation of CD4-binding peptides in the TLC-ART nanoformulation provided selective delivery of drug to CD4+ T cells [88], such a strategy will add cost to final product and is therefore reserved as a backup strategy.
Conclusion & future perspective
Nanomedicine has provided new tools and rationales in the development and search for new and more powerful strategies for HIV/AIDS treatment. The rational design and manipulation of diverse nanosystems, including liposomes, dendrimers, NPs and SLNs, has provided new candidates against HIV with improved solubility, stability and pharmacokinetic and adherence properties. However, most current nanomedicine platforms for HIV treatment focus on drug delivery in the blood and on improving pharmacokinetic profiles. Nano- or microsustained drug release formulations could maintain plasma drug concentrations for weeks and months, especially those implanted or injected in the muscular or subcutaneous space. While accumulated data indicate that most of the sustained-release formulations prolong the plasma drug exposure, these formulations do not fully address lymphoid tissue drug insufficiency. In this review, we discussed a systems approach to targeted drug delivery to residual HIV-infected cells and tissues. The systems approach leverages and integrates the pathology, virology and well-defined tissues and cells of drug insufficiency and residual virus to develop long-acting nanoformulations capable of improving local drug concentrations and retention at these sites of interest. As a result, drug exposure in off-target tissues such as the liver and kidney will likely be reduced. By doing so, this approach could contribute significantly to reducing adverse systemic toxicities while improving efficacy for existing and new products. Through the integration of pathophysiological understanding with physiochemical interactions as well as the characteristics of drug pharmacokinetic properties and bioanalytical capabilities, this approach has the potential to produce multiple drugs in a single nanoformulation and to accelerate development of breakthrough therapeutics in a wide range of diseases including cancer. It also could accelerate the development of systems for bacterial, fungal and mycobacterial diseases.
Although there are benefits and advantages of applying the systems approach to novel nanoformulations, there are features and challenges that must be overcome in the future to successfully translate ongoing studies to the clinical setting. These include the biocompatibility of excipients (biopolymers, lipids, novel materials, etc.), safety, stability and their robust scale-up procedure for large-scale preparations. The integration of virological information, drug target and drug distribution, elimination and kinetics has allowed for the development of new ways of clearing residual virus through maintaining therapeutic drug concentrations in the hard-to-reach tissues. With the goal of clinical translation in mind, an improved nonformulation that combines multiple ARV drugs that target multiple HIV proteins should be selected to overcome tissue drug insufficiency. Other nanomedicine platforms should also be explored to develop combination therapies for safe and effective long-acting HIV therapy to improve patient compliance. Future clinical trials and in vivo research studies that address these challenges may prevent the spread of the HIV/AIDS epidemic worldwide and may help find a cure for those who are infected.
Executive summary.
Challenges of HIV/AIDS treatment
Current HIV drug combinations are given orally, which reduce virus to undetectable levels. However, they are unable to completely eradicate the virus.
HIV persists in cells and tissues such as lymph nodes and other privileged sites, and rebound upon cessation of drug therapy.
The tissue persistence is a significant challenge and believed to be a key barrier to finding a cure for HIV.
As pills are taken daily, or more frequent, patient adherence to dosing schedule is also a key challenge.
Lymphatic drug insufficiency: hypothesis & data validation
Orally given HIV drugs are found in cell of lymph node at 1/3 that of blood counterpart.
Lymphatic drug insufficiency of oral HIV therapies could be rationally explained from a physiologic and anatomic context, and has been verified directly with experimental evidence.
Lymphatic drug insufficiency and viral rebound from persistent and residual HIV in the lymphatic system in HIV patients on oral highly active antiretroviral therapy have been identified and validated independently as rate-limiting steps in viral eradication.
Nanotechnology-based drug delivery for HIV/AIDS treatment
Various nanocarriers such as liposomes, polymeric nanoparticles, solid lipid nanoparticles, dendrimers and micelles have been reported with varying degree of success to enhance and overcome lymphatic drug insufficiency.
Subcutaneous administration of nanoformulation could help improve the drug bioavailability in the lymphatic system.
The systems approach concept
The systems approach to targeted drug delivery integrates knowledge of drug target at tissue and cell levels and engineering insights to develop appropriate nanoformulation to improve drug exposure at target.
The overall goal is to reduce off-target effects and improve on-target drug distribution into the precise tissue/cell targets that mediate a clinical response.
Application of the systems approach has proven to accelerate nanoformulation design that enhances tissue and cell drug exposure in lymph nodes, the tissue of drug insufficiency in HIV infections.
Targeted long-acting combination antiretroviral therapy for simultaneous delivery of multiple anti-HIV drugs
Unlike most nanodrug formulations carrying a single drug, targeted long-acting combination antiretroviral therapy formulations carry multiple antiretroviral drugs in a single drug–lipid nanoformulation.
Targeted long-acting combination antiretroviral therapy nanoformulations have been shown to enhance intracellular drug exposure in lymphoid tissues and cells; it is currently a part of clinical translation effort.
Footnotes
Financial & competing interests disclosure
This work is supported in part by NIH grants numbers AI-077390, AI-077390-S1, AI-077390-S2, AI-077390-S3, UM1 AI-120176 and 1UL1-RR025014. RJY Ho is also supported by the Milo Gibaldi endowment. This work is also supported in part by the National Natural Science Foundation of China (Nos. 81472767 and 81201709). J Shao now holds an appointment at Fuzhou University, PR China. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as: •• of considerable interest
- 1.Sarmati L, D'Ettorre G, Parisi SG, Andreoni M. HIV replication at low copy number and its correlation with the HIV reservoir: a clinical perspective. Curr. HIV Res. 2015;13(3):250–257. doi: 10.2174/1570162X13666150407142539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Joos B, Fischer M, Kuster H, et al. HIV rebounds from latently infected cells, rather than from continuing low-level replication. Proc. Natl Acad. Sci. USA. 2008;105(43):16725–16730. doi: 10.1073/pnas.0804192105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Allers K, Hutter G, Hofmann J, et al. Evidence for the cure of HIV infection by CCR5Delta32/Delta32 stem cell transplantation. Blood. 2011;117(10):2791–2799. doi: 10.1182/blood-2010-09-309591. [DOI] [PubMed] [Google Scholar]
- 4.Bradley H, Hall HI, Wolitski RJ, et al. Vital signs: HIV diagnosis, care, and treatment among persons living with HIV – United States, 2011. MMWR Morb. Mortal Wkly Rep. 2014;63(47):1113–1117. [PMC free article] [PubMed] [Google Scholar]
- 5.Levy J, Cobas RA, Gomes MB. Assessment of efficacy and tolerability of once-daily extended release metformin in patients with Type 2 diabetes mellitus. Diabetol. Metab. Syndr. 2010;2:16. doi: 10.1186/1758-5996-2-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chauhan VP, Stylianopoulos T, Martin JD, et al. Normalization of tumour blood vessels improves the delivery of nanomedicines in a size-dependent manner. Nat. Nanotechnol. 2012;7(6):383–388. doi: 10.1038/nnano.2012.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ho RJY, Gibaldi M. Biotechnology and Biopharmaceuticals: Transforming Proteins and Genes into Drugs. Wiley-Blackwell; NJ, USA: 2013. [Google Scholar]
- 8.Boswell GW, Buell D, Bekersky I. AmBisome (liposomal amphotericin B): a comparative review. J. Clin. Pharmacol. 1998;38(7):583–592. doi: 10.1002/j.1552-4604.1998.tb04464.x. [DOI] [PubMed] [Google Scholar]
- 9.Junghanns JU, Muller RH. Nanocrystal technology, drug delivery and clinical applications. Int. J. Nanomedicine. 2008;3(3):295–309. doi: 10.2147/ijn.s595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miele E, Spinelli GP, Miele E, Tomao F, Tomao S. Albumin-bound formulation of paclitaxel (Abraxane ABI-007) in the treatment of breast cancer. Int. J. Nanomedicine. 2009;4:99–105. doi: 10.2147/ijn.s3061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ballow M. Clinical experience with Flebogamma 5% DIF: a new generation of intravenous immunoglobulins in patients with primary immunodeficiency disease. Clin. Exp. Immunol. 2009;157(Suppl. 1):22–25. doi: 10.1111/j.1365-2249.2009.03951.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vyas SP, Subhedar R, Jain S. Development and characterization of emulsomes for sustained and targeted delivery of an antiviral agent to liver. J. Pharm. Pharmacol. 2006;58(3):321–326. doi: 10.1211/jpp.58.3.0005. [DOI] [PubMed] [Google Scholar]
- 13.Vyas TK, Shahiwala A, Amiji MM. Improved oral bioavailability and brain transport of Saquinavir upon administration in novel nanoemulsion formulations. Int. J. Pharm. 2008;347(1–2):93–101. doi: 10.1016/j.ijpharm.2007.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pereira de Oliveira M, Garcion E, Venisse N, Benoit JP, Couet W, Olivier JC. Tissue distribution of indinavir administered as solid lipid nanocapsule formulation in MDR1a (+/+) and MDR1a (-/-) CF-1 mice. Pharm. Res. 2005;22(11):1898–1905. doi: 10.1007/s11095-005-7147-6. [DOI] [PubMed] [Google Scholar]
- 15.Nowacek A, Gendelman HE. NanoART, neuroAIDS and CNS drug delivery. Nanomedicine (Lond.) 2009;4(5):557–574. doi: 10.2217/nnm.09.38. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• This review article focuses on how nanotechnology can serve to improve the delivery of antiretroviral medicines, termed nanoART, across the blood–brain barrier and affect the biodistribution and clinical benefit for HIV-1 disease.
- 16.Liner KJ, 2nd, Ro MJ, Robertson KR. HIV, antiretroviral therapies, and the brain. Curr. HIV/AIDS Rep. 2010;7(2):85–91. doi: 10.1007/s11904-010-0042-8. [DOI] [PubMed] [Google Scholar]
- 17.Gannon P, Khan MZ, Kolson DL. Current understanding of HIV-associated neurocognitive disorders pathogenesis. Curr. Opin. Neurol. 2011;24(3):275–283. doi: 10.1097/WCO.0b013e32834695fb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khalil NM, Carraro E, Cotica LF, Mainardes RM. Potential of polymeric nanoparticles in AIDS treatment and prevention. Expert Opin. Drug Deliv. 2011;8(1):95–112. doi: 10.1517/17425247.2011.543673. [DOI] [PubMed] [Google Scholar]
- 19.Mamo T, Moseman EA, Kolishetti N, et al. Emerging nanotechnology approaches for HIV/AIDS treatment and prevention. Nanomedicine (Lond.) 2010;5(2):269–285. doi: 10.2217/nnm.10.1. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Another review provides effective treatment and prevention for HIV/AIDS by nanotechnology-based advancing ART, gene therapy, immunotherapy, vaccinology and microbicides.
- 20.Date AA, Destache CJ. A review of nanotechnological approaches for the prophylaxis of HIV/AIDS. Biomaterials. 2013;34(26):6202–6228. doi: 10.1016/j.biomaterials.2013.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barre-Sinoussi F, Chermann JC, Rey F, et al. Isolation of a T-lymphotropic retrovirus from a patient at risk for acquired immune deficiency syndrome (AIDS) Science. 1983;220(4599):868–871. doi: 10.1126/science.6189183. [DOI] [PubMed] [Google Scholar]
- 22.Levy JA, Hoffman AD, Kramer SM, Landis JA, Shimabukuro JM, Oshiro LS. Isolation of lymphocytopathic retroviruses from San Francisco patients with AIDS. Science. 1984;225(4664):840–842. doi: 10.1126/science.6206563. [DOI] [PubMed] [Google Scholar]
- 23.Keele BF, Giorgi EE, Salazar-Gonzalez JF, et al. Identification and characterization of transmitted and early founder virus envelopes in primary HIV-1 infection. Proc. Natl Acad. Sci. USA. 2008;105(21):7552–7557. doi: 10.1073/pnas.0802203105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Iyidogan P, Anderson KS. Current perspectives on HIV-1 antiretroviral drug resistance. Viruses. 2014;6(10):4095–4139. doi: 10.3390/v6104095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Freed EO. HIV-1 replication. Somat. Cell. Mol. Genet. 2001;26(1–6):13–33. doi: 10.1023/a:1021070512287. [DOI] [PubMed] [Google Scholar]
- 26.Moore RD, Chaisson RE. Natural history of HIV infection in the era of combination antiretroviral therapy. AIDS. 1999;13(14):1933–1942. doi: 10.1097/00002030-199910010-00017. [DOI] [PubMed] [Google Scholar]
- 27.Gallant JE, Gerondelis PZ, Wainberg MA, et al. Nucleoside and nucleotide analogue reverse transcriptase inhibitors: a clinical review of antiretroviral resistance. Antivir. Ther. 2003;8(6):489–506. [PubMed] [Google Scholar]
- 28.Broder S. The development of antiretroviral therapy and its impact on the HIV-1/AIDS pandemic. Antiviral Res. 2010;85(1):1–18. doi: 10.1016/j.antiviral.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gunthard HF, Aberg JA, Eron JJ, et al. Antiretroviral treatment of adult HIV infection: 2014 recommendations of the International Antiviral Society-USA Panel. JAMA. 2014;312(4):410–425. doi: 10.1001/jama.2014.8722. [DOI] [PubMed] [Google Scholar]
- 30.Saag MS, Kilby JM. HIV-1 and HAART: a time to cure, a time to kill. Nat. Med. 1999;5(6):609–611. doi: 10.1038/9452. [DOI] [PubMed] [Google Scholar]
- 31.Blankson JN, Persaud D, Siliciano RF. The challenge of viral reservoirs in HIV-1 infection. Annu. Rev. Med. 2002;53:557–593. doi: 10.1146/annurev.med.53.082901.104024. [DOI] [PubMed] [Google Scholar]
- 32.Mills EJ, Nachega JB, Bangsberg DR, et al. Adherence to HAART: a systematic review of developed and developing nation patient-reported barriers and facilitators. PLoS Med. 2006;3(11):e438. doi: 10.1371/journal.pmed.0030438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Varatharajan L, Thomas SA. The transport of anti-HIV drugs across blood-CNS interfaces: summary of current knowledge and recommendations for further research. Antiviral Res. 2009;82(2):A99–A109. doi: 10.1016/j.antiviral.2008.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wong JK, Gunthard HF, Havlir DV, et al. Reduction of HIV-1 in blood and lymph nodes following potent antiretroviral therapy and the virologic correlates of treatment failure. Proc. Natl Acad. Sci. USA. 1997;94(23):12574–12579. doi: 10.1073/pnas.94.23.12574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lafeuillade A, Chollet L, Hittinger G, Profizi N, Costes O, Poggi C. Residual human immunodeficiency virus type 1 RNA in lymphoid tissue of patients with sustained plasma RNA of <200 copies/ml. J. Infect. Dis. 1998;177(1):235–238. doi: 10.1086/517362. [DOI] [PubMed] [Google Scholar]
- 36.Harrigan PR, Whaley M, Montaner JS. Rate of HIV-1 RNA rebound upon stopping antiretroviral therapy. AIDS. 1999;13(8):F59–F62. doi: 10.1097/00002030-199905280-00001. [DOI] [PubMed] [Google Scholar]
- 37.Siliciano JD, Kajdas J, Finzi D, et al. Long-term follow-up studies confirm the stability of the latent reservoir for HIV-1 in resting CD4+ T cells. Nat. Med. 2003;9(6):727–728. doi: 10.1038/nm880. [DOI] [PubMed] [Google Scholar]
- 38.Cavert W, Notermans DW, Staskus K, et al. Kinetics of response in lymphoid tissues to antiretroviral therapy of HIV-1 infection. Science. 1997;276(5314):960–964. doi: 10.1126/science.276.5314.960. [DOI] [PubMed] [Google Scholar]
- 39.Freeling JP, Ho RJ. Anti-HIV drug particles may overcome lymphatic drug insufficiency and associated HIV persistence. Proc. Natl Acad. Sci. USA. 2014;111(25):E2512–E2513. doi: 10.1073/pnas.1406554111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wong JK, Hezareh M, Gunthard HF, et al. Recovery of replication-competent HIV despite prolonged suppression of plasma viremia. Science. 1997;278(5341):1291–1295. doi: 10.1126/science.278.5341.1291. [DOI] [PubMed] [Google Scholar]; •• This paper reported HIV-1 provirus persistence in the lymphocytes cells of patients with complete and sustained suppression of plasma viremia, providing evidence for viral latency rather than drug failure.
- 41.Freeling JP, Koehn J, Shu C, Sun J, Ho RJ. Long-acting three-drug combination anti-HIV nanoparticles enhance drug exposure in primate plasma and cells within lymph nodes and blood. AIDS. 2014;28(17):2625–2627. doi: 10.1097/QAD.0000000000000421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang Y, Benet LZ. The gut as a barrier to drug absorption: combined role of cytochrome P450 3A and P-glycoprotein. Clin. Pharmacokinet. 2001;40(3):159–168. doi: 10.2165/00003088-200140030-00002. [DOI] [PubMed] [Google Scholar]
- 43.Trainor GL. The importance of plasma protein binding in drug discovery. Expert Opin. Drug Discov. 2007;2(1):51–64. doi: 10.1517/17460441.2.1.51. [DOI] [PubMed] [Google Scholar]
- 44.Kinman L, Brodie SJ, Tsai CC, et al. Lipid-drug association enhanced HIV-1 protease inhibitor indinavir localization in lymphoid tissues and viral load reduction: a proof of concept study in HIV-2287-infected macaques. J. Acquir. Immune Defic. Syndr. 2003;34(4):387–397. doi: 10.1097/00126334-200312010-00005. [DOI] [PubMed] [Google Scholar]; •• This paper reports that lipid association can greatly enhance delivery of the anti-HIV drug indinavir to lymph nodes at levels that cannot be achieved with soluble drug, provide significant virus load reduction and can potentially reverse CD4 T-cell depletion due to HIV infection.
- 45.Kinman L, Bui T, Larsen K, et al. Optimization of lipid-indinavir complexes for localization in lymphoid tissues of HIV-infected macaques. J. Acquir. Immune Defic. Syndr. 2006;42(2):155–161. doi: 10.1097/01.qai.0000214822.33905.87. [DOI] [PubMed] [Google Scholar]
- 46.Alberts B. Molecular Biology of the Cell. Garland Science; NY, USA: 2002. [Google Scholar]
- 47.Fletcher CV, Staskus K, Wietgrefe SW, et al. Persistent HIV-1 replication is associated with lower antiretroviral drug concentrations in lymphatic tissues. Proc. Natl Acad. Sci. USA. 2014;111(6):2307–2312. doi: 10.1073/pnas.1318249111. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• This article reports that HIV+ patients on oral ART exhibited lower drug concentrations in cells of numerous lymph nodes compared with those in blood.
- 48.Solas C, Lafeuillade A, Halfon P, Chadapaud S, Hittinger G, Lacarelle B. Discrepancies between protease inhibitor concentrations and viral load in reservoirs and sanctuary sites in human immunodeficiency virus-infected patients. Antimicrob. Agents Chemother. 2003;47(1):238–243. doi: 10.1128/AAC.47.1.238-243.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sagar V, Pilakka-Kanthikeel S, Pottathil R, Saxena SK, Nair M. Towards nanomedicines for neuroAIDS. Rev. Med. Virol. 2014;24(2):103–124. doi: 10.1002/rmv.1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Edagwa BJ, Zhou T, McMillan JM, Liu XM, Gendelman HE. Development of HIV reservoir targeted long acting nanoformulated antiretroviral therapies. Curr. Med. Chem. 2014;21(36):4186–4198. doi: 10.2174/0929867321666140826114135. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Excellent review on the development of nanotechnology-based anti-HIV drug delivery systems to target HIV reservoir sites using long-acting nanoformulated strategies.
- 51.Zidan AS, Spinks CB, Habib MJ, Khan MA. Formulation and transport properties of tenofovir loaded liposomes through Caco-2 cell model. J. Liposome Res. 2013;23(4):318–326. doi: 10.3109/08982104.2013.810645. [DOI] [PubMed] [Google Scholar]
- 52.Zidan AS, Spinks C, Fortunak J, Habib M, Khan MA. Near-infrared investigations of novel anti-HIV tenofovir liposomes. AAPS J. 2010;12(2):202–214. doi: 10.1208/s12248-010-9177-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ramana LN, Sethuraman S, Ranga U, Krishnan UM. Development of a liposomal nanodelivery system for nevirapine. J. Biomed. Sci. 2010;17:57. doi: 10.1186/1423-0127-17-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jin SX, Bi DZ, Wang J, Wang YZ, Hu HG, Deng YH. Pharmacokinetics and tissue distribution of zidovudine in rats following intravenous administration of zidovudine myristate loaded liposomes. Die Pharmazie. 2005;60(11):840–843. [PubMed] [Google Scholar]
- 55.Desormeaux A, Harvie P, Perron S, et al. Antiviral efficacy, intracellular uptake and pharmacokinetics of free and liposome-encapsulated 2′,3′-dideoxyinosine. AIDS. 1994;8(11):1545–1553. doi: 10.1097/00002030-199411000-00005. [DOI] [PubMed] [Google Scholar]
- 56.Makabi-Panzu B, Gourde P, Desormeaux A, Bergeron MG. Intracellular and serum stability of liposomal 2′,3′-dideoxycytidine. Effect of lipid composition. Cell. Mol. Biol. 1998;44(2):277–284. [PubMed] [Google Scholar]
- 57.Ramana LN, Sharma S, Sethuraman S, Ranga U, Krishnan UM. Investigation on the stability of saquinavir loaded liposomes: implication on stealth, release characteristics and cytotoxicity. Int. J. Pharm. 2012;431(1–2):120–129. doi: 10.1016/j.ijpharm.2012.04.054. [DOI] [PubMed] [Google Scholar]
- 58.Jain S, Tiwary AK, Jain NK. PEGylated elastic liposomal formulation for lymphatic targeting of zidovudine. Curr. Drug Deliv. 2008;5(4):275–281. doi: 10.2174/156720108785915078. [DOI] [PubMed] [Google Scholar]
- 59.Gagne JF, Desormeaux A, Perron S, Tremblay MJ, Bergeron MG. Targeted delivery of indinavir to HIV-1 primary reservoirs with immunoliposomes. Biochim. Biophys. Acta. 2002;1558(2):198–210. doi: 10.1016/s0005-2736(01)00432-1. [DOI] [PubMed] [Google Scholar]
- 60.Garg M, Asthana A, Agashe HB, Agrawal GP, Jain NK. Stavudine-loaded mannosylated liposomes: in-vitro anti-HIV-I activity, tissue distribution and pharmacokinetics. J. Pharm. Pharmacol. 2006;58(5):605–616. doi: 10.1211/jpp.58.5.0005. [DOI] [PubMed] [Google Scholar]
- 61.Garg M, Garg BR, Jain S, et al. Radiolabeling, pharmacoscintigraphic evaluation and antiretroviral efficacy of stavudine loaded 99mTc labeled galactosylated liposomes. Eur. J. Pharmaceut. Sci. 2008;33(3):271–281. doi: 10.1016/j.ejps.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 62.Zhang T, Sturgis TF, Youan BB. pH-responsive nanoparticles releasing tenofovir intended for the prevention of HIV transmission. Eur. J. Pharmaceut. Biopharmaceut. 2011;79(3):526–536. doi: 10.1016/j.ejpb.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shah LK, Amiji MM. Intracellular delivery of saquinavir in biodegradable polymeric nanoparticles for HIV/AIDS. Pharm. Res. 2006;23(11):2638–2645. doi: 10.1007/s11095-006-9101-7. [DOI] [PubMed] [Google Scholar]
- 64.Kuo YC, Chen HH. Effect of nanoparticulate polybutylcyanoacrylate and methylmethacrylate-sulfopropylmethacrylate on the permeability of zidovudine and lamivudine across the in vitro blood-brain barrier. Int. J. Pharm. 2006;327(1–2):160–169. doi: 10.1016/j.ijpharm.2006.07.044. [DOI] [PubMed] [Google Scholar]
- 65.Kuo YC, Lin PI, Wang CC. Targeting nevirapine delivery across human brain microvascular endothelial cells using transferrin-grafted poly(lactide-co-glycolide) nanoparticles. Nanomedicine (Lond.) 2011;6(6):1011–1026. doi: 10.2217/nnm.11.25. [DOI] [PubMed] [Google Scholar]
- 66.Pattnaik G, Sinha B, Mukherjee B, et al. Submicron-size biodegradable polymer-based didanosine particles for treating HIV at early stage: an in vitro study. J. Microencaps. 2012;29(7):666–676. doi: 10.3109/02652048.2012.680509. [DOI] [PubMed] [Google Scholar]
- 67.Mainardes RM, Gremiao MP. Nanoencapsulation and characterization of zidovudine on poly(L-lactide) and poly(L-lactide)-poly(ethylene glycol)-blend nanoparticles. J. Nanosci. Nanotechnol. 2012;12(11):8513–8521. doi: 10.1166/jnn.2012.6638. [DOI] [PubMed] [Google Scholar]
- 68.Chiappetta DA, Hocht C, Opezzo JA, Sosnik A. Intranasal administration of antiretroviral-loaded micelles for anatomical targeting to the brain in HIV. Nanomedicine (Lond.) 2013;8(2):223–237. doi: 10.2217/nnm.12.104. [DOI] [PubMed] [Google Scholar]
- 69.Kuo YC, Yu HW. Expression of ornithine decarboxylase during the transport of saquinavir across the blood–brain barrier using composite polymeric nanocarriers under an electromagnetic field. Colloids Surf. B Biointerfaces. 2011;88(2):627–634. doi: 10.1016/j.colsurfb.2011.07.053. [DOI] [PubMed] [Google Scholar]
- 70.Dutta T, Agashe HB, Garg M, Balakrishnan P, Kabra M, Jain NK. Poly(propyleneimine) dendrimer based nanocontainers for targeting of efavirenz to human monocytes/macrophages in vitro . J. Drug Target. 2007;15(1):89–98. doi: 10.1080/10611860600965914. [DOI] [PubMed] [Google Scholar]
- 71.Chonco L, Pion M, Vacas E, et al. Carbosilane dendrimer nanotechnology outlines of the broad HIV blocker profile. J. Control. Release. 2012;161(3):949–958. doi: 10.1016/j.jconrel.2012.04.050. [DOI] [PubMed] [Google Scholar]
- 72.Gaur PK, Mishra S, Bajpai M, Mishra A. Enhanced oral bioavailability of efavirenz by solid lipid nanoparticles: in vitro drug release and pharmacokinetics studies. BioMed. Res. Int. 2014;2014:363404. doi: 10.1155/2014/363404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kuo YC, Chung JF. Physicochemical properties of nevirapine-loaded solid lipid nanoparticles and nanostructured lipid carriers. Colloids Surf. B Biointerfaces. 2011;83(2):299–306. doi: 10.1016/j.colsurfb.2010.11.037. [DOI] [PubMed] [Google Scholar]
- 74.Huang C, Soenen SJ, van Gulck E, et al. Electrospun cellulose acetate phthalate fibers for semen induced anti-HIV vaginal drug delivery. Biomaterials. 2012;33(3):962–969. doi: 10.1016/j.biomaterials.2011.10.004. [DOI] [PubMed] [Google Scholar]
- 75.Li SD, Huang L. Pharmacokinetics and biodistribution of nanoparticles. Mol. Pharmaceut. 2008;5(4):496–504. doi: 10.1021/mp800049w. [DOI] [PubMed] [Google Scholar]
- 76.LaVan DA, Lynn DM, Langer R. Moving smaller in drug discovery and delivery. Nat. Rev. Drug Discov. 2002;1(1):77–84. doi: 10.1038/nrd707. [DOI] [PubMed] [Google Scholar]
- 77.Pantaleo G, Graziosi C, Butini L, et al. Lymphoid organs function as major reservoirs for human immunodeficiency virus. Proc. Natl Acad. Sci. USA. 1991;88(21):9838–9842. doi: 10.1073/pnas.88.21.9838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pantaleo G, Graziosi C, Demarest JF, et al. HIV infection is active and progressive in lymphoid tissue during the clinically latent stage of disease. Nature. 1993;362(6418):355–358. doi: 10.1038/362355a0. [DOI] [PubMed] [Google Scholar]
- 79.Liu Z, Cumberland WG, Hultin LE, Prince HE, Detels R, Giorgi JV. Elevated CD38 antigen expression on CD8+ T cells is a stronger marker for the risk of chronic HIV disease progression to AIDS and death in the Multicenter AIDS Cohort Study than CD4+ cell count, soluble immune activation markers, or combinations of HLA-DR and CD38 expression. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 1997;16(2):83–92. doi: 10.1097/00042560-199710010-00003. [DOI] [PubMed] [Google Scholar]
- 80.Schacker T, Little S, Connick E, et al. Rapid accumulation of human immunodeficiency virus (HIV) in lymphatic tissue reservoirs during acute and early HIV infection: implications for timing of antiretroviral therapy. J. Infect. Dis. 2000;181(1):354–357. doi: 10.1086/315178. [DOI] [PubMed] [Google Scholar]
- 81.Durand CM, Blankson JN, Siliciano RF. Developing strategies for HIV-1 eradication. Trends Immunol. 2012;33(11):554–562. doi: 10.1016/j.it.2012.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Notermans DW, Jurriaans S, de Wolf F, et al. Decrease of HIV-1 RNA levels in lymphoid tissue and peripheral blood during treatment with ritonavir, lamivudine and zidovudine. Ritonavir/3TC/ZDV Study Group. AIDS. 1998;12(2):167–173. doi: 10.1097/00002030-199802000-00006. [DOI] [PubMed] [Google Scholar]
- 83.Drinker CK, Field ME, Ward HK. The filtering capacity of lymph nodes. J. Exp. Med. 1934;59(4):393–405. doi: 10.1084/jem.59.4.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.van Hal SJ, Muthiah K, Matthews G, et al. Declining incidence of intestinal microsporidiosis and reduction in AIDS-related mortality following introduction of HAART in Sydney, Australia. Trans. R. Soc. Trop. Med. Hyg. 2007;101(11):1096–1100. doi: 10.1016/j.trstmh.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 85.Williams J, Sayles HR, Meza JL, et al. Long-acting parenteral nanoformulated antiretroviral therapy: interest and attitudes of HIV-infected patients. Nanomedicine (Lond.) 2013;8(11):1807–1813. doi: 10.2217/nnm.12.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Moghimi SM, Hunter AC, Murray JC. Long-circulating and target-specific nanoparticles: theory to practice. Pharmacol. Rev. 2001;53(2):283–318. [PubMed] [Google Scholar]
- 87.Freeling JP, Koehn J, Shu C, Sun J, Ho RJ. Anti-HIV drug-combination nanoparticles enhance plasma drug exposure duration as well as triple-drug combination levels in cells within lymph nodes and blood in primates. AIDS Res. Hum. Retroviruses. 2015;31(1):107–114. doi: 10.1089/aid.2014.0210. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• This article developed multidrug anti-HIV lipid nanoparticles to enhance intracellular drug concentrations in blood and lymph nodes. In primates, anti-HIV lipid nanoparticles produced over 50-fold higher intracellular concentrations of lopinavir and ritonavir in lymph nodes compared with free drug. Plasma and intracellular drug levels in blood were enhanced and sustained up to 7 days.
- 88.Endsley AN, Ho RJ. Design and characterization of novel peptide-coated lipid nanoparticles for targeting anti-HIV drug to CD4 expressing cells. AAPS J. 2012;14(2):225–235. doi: 10.1208/s12248-012-9329-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ryan GM, Kaminskas LM, Porter CJ. Nano-chemotherapeutics: maximising lymphatic drug exposure to improve the treatment of lymph-metastatic cancers. J. Control. Release. 2014;193:241–256. doi: 10.1016/j.jconrel.2014.04.051. [DOI] [PubMed] [Google Scholar]
- 90.Richter WF, Bhansali SG, Morris ME. Mechanistic determinants of biotherapeutics absorption following SC administration. AAPS J. 2012;14(3):559–570. doi: 10.1208/s12248-012-9367-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Fathallah AM, Balu-Iyer SV. Anatomical, physiological, and experimental factors affecting the bioavailability of SC-administered large biotherapeutics. J. Pharm. Sci. 2015;104(2):301–306. doi: 10.1002/jps.24277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ambrose Z, KewalRamani VN, Bieniasz PD, Hatziioannou T. HIV/AIDS: in search of an animal model. Trends Biotechnol. 2007;25(8):333–337. doi: 10.1016/j.tibtech.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 93.Horiike M, Iwami S, Kodama M, et al. Lymph nodes harbor viral reservoirs that cause rebound of plasma viremia in SIV-infected macaques upon cessation of combined antiretroviral therapy. Virology. 2012;423(2):107–118. doi: 10.1016/j.virol.2011.11.024. [DOI] [PubMed] [Google Scholar]
- 94.Gautam N, Roy U, Balkundi S, et al. Preclinical pharmacokinetics and tissue distribution of long-acting nanoformulated antiretroviral therapy. Antimicrob. Agents Chemother. 2013;57(7):3110–3120. doi: 10.1128/AAC.00267-13. [DOI] [PMC free article] [PubMed] [Google Scholar]