Abstract
Aim:
Oxidative stress (OS) is largely thought to be a central mechanism responsible for liver damage, inflammation and fibrosis in nonalcoholic steatohepatitis (NASH). Our aim was to investigate whether suppression of OS in the liver via redox nanoparticles (RNPs) reduces liver damage in a mouse model of NASH.
Materials & methods:
RNPs were prepared by self-assembly of redox polymers possessing antioxidant nitroxide radicals and were orally administered by daily gavage for 4 weeks.
Results:
The redox polymer was delivered to the liver after disintegration of nanoparticle in the stomach. RNP treatment in NASH mice via gavage led to a reduction of liver OS, improvement of fibrosis, and significant reduction of inflammation.
Conclusion:
These findings uncover RNP as a novel potential NASH therapy.
Keywords: : anti-inflammatory polymer drug, oral antioxidants, redox nanoparticles
Nonalcoholic fatty liver disease (NAFLD) has become the most common form of chronic liver disease in both children and adults in the world, especially affecting within the American Population where up to 30% are affected. NAFLD is tightly associated with obesity and encompasses a wide spectrum of conditions associated with the overaccumulation of fat in the liver, ranging from hepatic steatosis to nonalcoholic steatohepatitis (NASH) and cirrhosis [1]. Hepatic steatosis is characterized by the isolated accumulation of lipids in the liver and is generally thought to follow a relatively benign, nonprogressive clinical course. NASH is a serious condition, with about 5–25% of patients progressing to fibrosis and cirrhosis with its associated complications of portal hypertension, liver failure and hepatocellular carcinoma [2]. Although the pathophysiology of NAFLD is complex and involves a close interaction between host genetics and various environmental factors, growing evidence supports a central role for oxidative stress (OS) caused by an increase in reactive oxygen species (ROS) in the progression of disease inflammation and fibrosis [3,4].
Due to the importance of OS in NASH development, antioxidant interventions, mainly related to vitamin E (RRR-α-tocopherol) supplementation, have been tested in both adults and children with mixed results [5,6]. The nitroxide compounds, such as 4-hydroxy-2,2,6,6-tetramethylpiperidine-1-oxyl (TEMPOL), a membrane-permeable radical scavenger, can catalytically react with ROS and are anticipated as new antioxidant therapies for several diseases [7]. However, these compounds have not been able to advance to the clinical setting, due in part to problems with their short half-life and several observed side effects [8]. One of the most important issues for low-molecular-weight (LMW) antioxidants such as TEMPOL is that excessive uptake in healthy cells may disrupt normal redox reactions triggering mitochondrial dysfunction and apoptosis [9,10]. It has also been reported that the administration of 2,2,6,6-tetramethylpiperidine-1-oxyl (TEMPO) derivatives, such as TEMPOL, in animals may induce a dramatic reduction in blood pressure [11]. As a result, the therapeutic effects of LMW ROS scavengers remain limited. To overcome these obstacles, a new strategy must be required. Heckman et al. reported that cerium oxide nanoparticles work as antioxidants against a free radical mediated autoimmune degenerative disease in the brain [12]. It is an interesting approach, however, cerium cation erodes from the nanoparticle, which causes strong toxicity even in the normal tissues and cells [13]. In order to prevent strong adverse effects to normal organs, tissues and cells, we must prevent disturbance of normal redox reaction in healthy parts. In addition, oral administration is preferable for chronic diseases even when using nanomedicine. Our strategy is to employ low-molecular weight polymers, which tend to uptake into the blood stream via oral administration and avoid internalization in healthy cells. Our developed materials are referred to as redox polymers, which consist of poly(ethylene glycol)-b-poly[4-(2,2,6,6-tetramethylpiperidine-1-oxyl)aminomethylstyrene] (MeO-PEG-b-PMNT) (10 kDa) [8]. This redox polymer possesses antioxidant nitroxide radicals in the hydrophobic segments via covalent linkages and forms a polymeric micelle under physiological conditions, which confines the nitroxide radicals in its core and is 40 nm in diameter (referred to as the redox nanoparticles [RNPs]).
Since the RNPs are not internalized in healthy cells due to the intact cell membrane, almost no cellular dysfunction is observed in healthy cells [9]. Because RNPs possess amine linkages in the core, they disintegrate under acidic conditions due to the protonation of amino groups. Thus, RNP has been confirmed to disintegrate into individual polymers within the stomach and be absorbed as dissociated polymer into the blood stream across the intestinal epithelium [14]. For the treatment of chronic liver diseases, such as NASH, oral medications are preferred owing to the convenience and noninvasiveness to patient. Most nanomedicines are not used as orally administered drugs for systemic diseases because nanoparticles between the sizes of 10 and 100 nm are not absorbed via the gastrointestinal tract. In contrast, since redox polymers are absorbed in the blood after oral administration of RNPs, it is potentially an ideal oral medication for chronic diseases. Nanoparticle formulation allows patients to drink easily owing to its low viscosity. Considering the long-term treatment of chronic diseases, this advantage helps patients to take medicine.
Thus far, we have confirmed a therapeutic effect of our redox polymer (PEG-b-PMNT) on the senescence-accelerated prone mouse model (SAMP8) by scavenging ROS in the brain after oral administration of RNPs [14].
In this study, we investigated whether redox polymers travel into the injured NASH liver by oral administration of RNPs and improve liver injury via OS suppression in a human pathophysiologically relevant murine model of fibrotic NASH. Our results demonstrated that when given orally, redox polymers reached the liver after the disintegration of RNP, and this led to a reduction of liver inflammation and fibrosis, at least in part through OS suppression in liver tissue. This is the first report that oral administration of RNP improves liver fibrosis and reduces inflammation associated with experimental NASH. These findings uncover orally administered RNP as a potentially novel anti-inflammatory and antifibrotic therapy for NASH.
Materials & methods
Preparation of RNP & rhodamine-RNP
Methoxy-poly(ethylene glycol)-b-poly(4-chloromethylstyrene) (MeO-PEG-b-PCMS) was synthesized by the radical telomerization of 4-chloromethylstyrene using methoxy-poly(ethylene glycol)-sulphanyl (MeO-PEG-SH; Mn = 5000; NOF corporation, Tokyo, Japan) as a telogen. Unit number of PCMS segment was 16. To obtain MeO-PEG-b-PMNT, chloromethyl groups on the PCMS segment of the block copolymer were reacted with 4-amino-TEMPO via amination in dimethylsulfoxide. Based on molecular weight of PEG-b-PMNT and electron spin resonance (ESR) signal intensity of nitroxide radicals covalently conjugated to PMNT segment, the conjugation efficiency of 4-amino-TEMPO was 83%. RNP was prepared from MeO-PEG-b-PMNT by the dialysis method. MeO-PEG-b-PMNT was dissolved in N,N-dimethylformamide (DMF), and the polymer solution was transferred into a membrane tube (Spectra/Por, molecular-weight cutoff size: 3500; Spectrum Laboratories, Inc., CA, USA) and then dialyzed for 24 h against 2 l of water, which was changed after 2, 5, 8 and 20 h. Dynamic light scattering measurements were carried out to determine the diameter of the obtained RNP following dialysis using a Zetasizer Nano ZS (Malvern Instruments, Ltd, Malvern, UK). Control nanoparticles (cNPs) were prepared by MeO-PEG-b-PCMS via dialysis method, similar to preparation of RNPs.
The in vivo localization of polymer (MeO-PEG-b-PMNT) was determined using fluorescent rhodamine-labeled RNPs. Rhodamine was introduced to MeO-PEG-b-PMNT via a thiourethane bond between MeO-PEG-b-PMNT possessing reduced TEMPO moieties and rhodamine B isothiocyanate (Sigma-Aldrich, MO, USA) in the presence of sodium hydride. Briefly, after 30 μmol (300 mg) of the MeO-PEG-b-PMNT were weighed into a 100-ml flask, a CHCl3 solution (2 ml) of phenylhydrazine (300 μmol, 33 mg) was added to the flask and stirred for 10 min at room temperature. The reacted polymer was recovered by precipitation into 10 ml of diethyl ether, followed by filtration to obtain polymers possessing reduced TEMPO moieties. The obtained polymers (200 mg) were dissolved in anhydride DMF (1 ml) and added to sodium hydride (150 μmol, 5.4 mg) and rhodamine B isothiocyanate (90 μmol, 48 mg) in anhydride DMF solution (1 ml) and stirred for 10 h at room temperature, followed by dialysis with methanol to remove free rhodamine B isothiocyanate. Rhodamine-RNP was prepared from rhodamine-labeled MeO-PEG-b-PMNT by the dialysis method.
Preparation of 125I-labeled RNP
A solution of Na125I in 10 mM phosphate-buffered saline (PBS) (100 μl, 74 MBq/ml, PerkinElmer, Inc., USA) was added to a solution of RNP (2ml, 50 mg/ml). A solution of chloramine T in 10 mM PBS (40 μl, 600 mM, Sigma-Aldrich) was added to the reaction mixture, which was incubated at room temperature for 10 min. After incubation, the unreacted 125I and other chemicals were removed by three rounds of centrifugation (3200 rpm) using a membrane ultrafiltration (Vivaspin, MWCO: 5000, GE Healthcare, USA). To confirm the purification, the gel filtration chromatography was conducted on a PD-10 column (GE Healthcare, USA) using saline as the eluent. The radioactivity of each fraction was measured using a γ-counter (ARC-380; Aloka, Japan).
Pharmacokinetics of MeO-PEG-b-PMNT by 125I-labeled RNP
Radioisotope experiments for evaluation of biodistribution of redox polymer were performed at University of Tsukuba according to the Guide for the Care and Use of Laboratory Animals at the University of Tsukuba. Male ICR mice (bodyweight, 30 g; Charles River, Yokohama, Japan) were used for radioisotope experiment. The mice were fasted 1 day before the experiment and then 0.5 ml of 125I-labeled RNPs (20 mg/ml) was orally administered to ICR mice. The mice were subsequently sacrificed at 0.25, 0.5, 1, 2, 4, 8, 12 and 24 h after oral administration. Their radioactivities of isolated liver and kidney were measured by a γ-counter (ARC-380; Aloka, Japan). The percentage of radioactivity in each organ was determined based on the initial total radioactivity.
Animal studies
The use and care of the animals was reviewed and approved by the Institutional Animal Care and Use Committee at the University of California, San Diego. Male C57BL/6 mice were purchased from Harlan Laboratories (CA, USA) and were aged between 6 and 8 weeks at the beginning of this study. Mice were fed a choline deficient amino acid defined (CDAA; Dyets, Inc., PA, USA) diet for 20 weeks to induce NASH. During the last 4 weeks of the feeding course, mice fed with the CDAA diet received daily administration of the RNPs, Control NPs, or Buffer (water) via gavage (300 mg/kg/day) (n = 4 each group). The dose of nitroxide radicals in the RNPs was 42.5 mg/kg/day.
Detection of MeO-PEG-b-PMNT
For distribution of MeO-PEG-b-PMNT, mice fed with the CDAA diet for 20 weeks received rhodamine-RNPs or RNPs (300 or 600 mg/kg) via gavage and had their plasma and liver collected at 30–60 min postadministration. The total amount of drug (nitroxide radicals + hydroxyamines) in blood and homogenized liver were estimated from X-band ESR measurements of the sample after addition of (K3Fe[CN]6) (see Supplementary Material for more details).
Liver sample preparation
All mice were sacrificed at the termination of treatment – 20 weeks of CDAA diet. A piece of liver tissue was fixed in 10% formalin for 24 h and embedded in paraffin, quickly frozen in OCT (Sakura Finetek, CA, USA), or incubated with RNAlater Solution (Life technologies/Fisher, CA, USA) for RNA extraction. The rest of the liver tissue was quickly frozen in liquid nitrogen and stored at -80°C.
Liver histology & immunostaining
Paraffin-embedded liver sections were stained for hematoxylin and eosin (H&E). Steatosis and inflammation were scored by an experienced pathologist (BGP) on the basis of the nonalcoholic fatty liver disease (NAFLD) activity score (Design and validation of a histological scoring system for nonalcoholic fatty liver disease) [15]. Liver fibrosis was assessed by Sirius Red staining with liver sections incubated for 2 h at room temperature with Fast Green FCF (Fisher Scientific, PA, USA) and Direct Red (Sigma-Aldrich) in saturated picric acid (Sigma-Aldrich). Immunohistochemistry staining for myeloperoxidase (Myeloperoxidase Ab-1, Thermo Scientific, MA, USA) and Ly6C (Abcam, MA, USA) were performed in paraffin embedded liver sections according to the manufacturer's instruction. All pictures were taken by NanoZoomer 2.0HT Slide Scanning System (Hamamatsu, Japan) and quantitated on Image J software. Frozen liver sections were stained for Oil Red O using Oil red O (Sigma-Aldrich) in 60% 2-propanol (Sigma-Aldrich). Frozen liver sections (10 μm) were fixed with cold-acetone for 2 min, washed with PBS and incubated with 20 μM dihydroethidium (DHE; Life technologies/Fisher) for 30 min at 37°C. Oil Red O or DHE staining was observed using immunofluorescence microscopy (Olympus, USA) and quantitated on Image J software.
Gene expression
Total RNA was isolated from liver tissue using Trizol Reagents (Life technologies) following RNeasy Tissue Mini kit (Qiagen, CA, USA) and reverse transcribed by iScript cDNA Synthesis kit (Bio-Rad, CA, USA) according to the manufacture's instructions. Real-time PCR quantification was performed with two, forward and reverse, primers at final concentration of 200 nmol and KAPA SYBR FAST qPCR master mix (KAPA biosystems, MA, USA) in CFX96 Touch Real-Time PCR Detection System (Bio-Rad) according to the manufacture's instructions. The PCR primers used to amplify each gene are listed in Supplementary Table 1. Ct values were automatically obtained on CFX96 Touch Real-Time PCR Detection System and mean values were normalized to β2 microglobulin for mRNA.
Immunoblot analysis
80 µg whole-liver lysate was resolved by a gradient gel, transferred to nitrocellulose membrane, and blotted with primary antibody (see Supplementary Material for more details).
Statistical analysis
All data are expressed as mean ± SEM unless otherwise noted. Data were analyzed using t-test among the groups of interest (chow vs Buffer, Buffer vs RNP and cNP vs RNP). The data were transformed when necessary to achieve homogeneity of variance. Differences were considered to be significant at p ≤ 0.05.
Results
Redox polymers derived from RNPs accumulate in NASH liver after disintegration in the stomach & do not alter mouse bodyweight
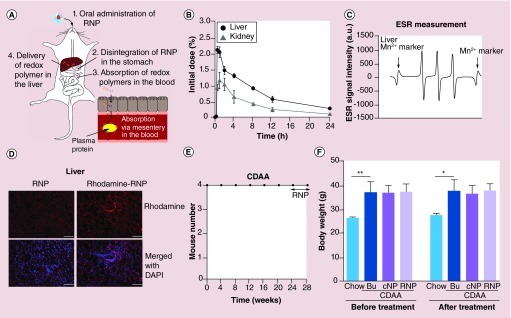
RNPs are polymeric micelles composed of self-assembling amphiphilic block copolymers containing a hydrophilic poly(ethylene glycol) (PEG) segment and hydrophobic poly(4-methylstyrene) segment possessing nitroxide radicals via amine linkage (Supplementary Figure 1A). RNPs, or control NPs (cNP, which has no TEMPO moiety.), are intact nanoparticles of 36–37 nm in diameter as assessed via dynamic light scattering (Supplementary Figure 1B). However, in more acidic environments (<pH 7.0) RNPs disintegrate into MeO-PEG-b-PMNT (redox polymer), which are solubilized and, therefore, made available for intestinal uptake [16,17]. Our previous observations led us to expect the redox polymers to be delivered into the liver across the intestinal epithelium after RNP disintegration in the stomach (Figure 1A). We checked the biodistribution of redox polymers administered via gavage (oral) in both wild-type (WT) and NASH mice. In WT mice, we revealed that 5–7 ID% of the redox polymers, which disintegrate in the stomach, appear in the bloodstream across the intestinal epithelium [14]. Half-life of redox polymers in the blood was ca. 4 h. Their bioavailability was 18.8% (Supplementary Figure 2). Once redox polymers were in the bloodstream, they were delivered to liver, as well as the kidney, but most of them were cleared from the mouse body within 24 h via renal excretion due to the low-molecular weight of redox polymers (less than 20 kDa) (Figure 1B). To investigate whether the redox polymers are delivered more efficiently into the livers of a murine model of NASH, RNPs were administered orally via gavage to mice fed a choline-deficient l-amino acid defined (CDAA) diet for 20 weeks. The presence of redox polymer (MeO-PEG-b-PMNT) in liver homogenates and blood samples was measured by ESR. After 30 min postadministration of RNPs, the ESR signal was detected in the liver (Figure 1C), as well as in the blood (Supplementary Figure 3A). Detected ESR signal was a clear triplet signal corresponding to the ESR signal of redox polymer [16]. Additionally, to validate these results we used a second approach where rhodamine-conjugated RNPs were administered orally via gavage and the distribution of redox polymers was explored by assessing fluorescent rhodamine signal in the liver section. As can clearly be seen in Figure 1D, the fluorescent signal based on rhodamine conjugating in the polymers was detected more strongly in the liver compared with that of the control. Rhodamine signal was also detected slightly in the intestine (Supplementary Figure 3B). After demonstrating that orally administered RNPs resulted in the accumulation of RNP derived redox polymers in the liver, we next examined the side effects. Mice were fed a CDAA diet for 16 weeks (20 weeks total) and were then treated with RNPs, control NPs (300 mg/kg/day), or control (buffer) via gavage while still on the diet for 4 additional weeks (n = 4). All NASH mice survived the RNP treatment (Figure 1E), as well as other treatments (data not shown). Furthermore, there were no changes in total bodyweight during the gavage treatment in any of the groups (Figure 1F).
Figure 1. . Redox polymers derived from redox nanoparticles accumulated in the nonalcoholic steatohepatitis liver after disintegration of redox nanoparticles in the stomach.
(A) Scheme of redox polymer delivery from oral administration of RNP to liver. (B) Biodistribution of redox polymer in liver and kidney. (C) ESR measurement in liver. RNP (300 mg/kg) were administered to mouse for measurement of ESR in liver. (D) Fluorescence microscopy of liver section administered with 600 mg/kg rhodamine-RNP or RNP (no rhodamine) as a negative control. Scale bar: 100 μm. (E) Survival curve in mice fed with a CDAA diet administered with RNP and (F) bodyweight at beginning or termination of treatment in mice fed with a chow diet, fed with a CDAA diet administered with control (buffer), cNP, or RNP. Values are mean ±SEM.
*p < 0.05; **p < 0.01.
Bu: Buffer; CDAA: Choline deficient amino acid defined; cNP: Control nanoparticle; ESR: Electron spin resonance; RNP: Redox nanoparticle; SEM: Standard error of the mean.
RNP treatment reduces ROS activity in the liver independent of steatosis & changes in liver weight
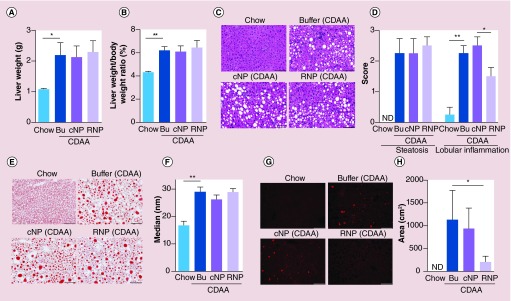
We next investigated the effectiveness of RNP treatment in reducing liver OS. There were no changes in liver weight or liver/bodyweight ratio during the gavage treatment in any of the groups (Figure 2A & B).
Figure 2. . Redox nanoparticles do not improve steatosis, but reduces reactive oxygen species activity in the liver.
(A) Liver weight or (B) liver/bodyweight ratio in mice fed with a chow diet or fed with a CDAA diet administered with control (buffer), cNP, or RNP. Values are mean ± SEM. **p < 0.01; *p < 0.05. (C) Haematoxylin-eosin staining of liver sections in mice fed with a chow diet or fed with a CDAA diet administered with control, cNP, or RNP. Scale bar: 100 μm. (D) Bar graph shows liver histological score for degree of steatosis and lobular inflammation based on NAFLD activity score (see ‘Materials & methods’ section for detail) on H&E staining. Values are mean ± SEM. **p < 0.01; *p < 0.05. (E) Oil Red O staining of liver sections in mice fed with a chow diet or fed with a CDAA diet administered with control, cNP, or RNP. Scale bar: 100 μm. (F) Bar graph shows quantification of positive area of Oil Red O. Values are mean ± SEM. **p < 0.01. (G) DHE staining of liver sections in mice fed with a chow diet, fed with a CDAA diet administered with control, cNP, or RNP. Scale bar: 100 μm. (H) Bar graph shows quantification of positive area of DHE. Values are mean ±SEM. *p < 0.05.
Bu: Buffer; CDAA: Choline deficient amino acid defined; cNP: Control nanoparticle; DHE: Dihydroethidium; H&E: Hematoxylin and eosin; NAFLD: Nonalcoholic fatty liver disease; ND: Not detected; RNP: Redox nanoparticle; SEM: Standard error of the mean.
After the termination of treatment, liver sections were stained with H&E and Oil Red O. As expected, mice fed with the CDAA diet for 20 weeks showed significant lipid accumulation in the liver compared with mice fed with the control diet (Figure 2C–F). RNP treated CDAA fed mice showed no significant differences in severity of steatosis (Figure 2C–F). However, liver inflammation score was significantly dropped in RNP treated CDAA fed mice (Figure 2D). Notably, a significant effect was observed in measurements of OS in the RNP treated animals as determined by DHE staining in the liver sections (Figure 2G & H). Indeed, OS was increased in the NASH mouse model fed with a CDAA diet compared with mice fed with a chow diet (Figure 2G & H). RNP treatment led to a significant reduction in liver OS compared with control NPs (cNPs) or control (buffer) as assessed by DHE staining (Figure 2G & H). These results indicate that redox polymers derived from RNP reduced the ROS activity in the NASH liver and these effects were independent of lipid accumulation in the liver.
RNP treatment reverses NASH fibrosis through the inhibition of markers of hepatic stellate cell activation
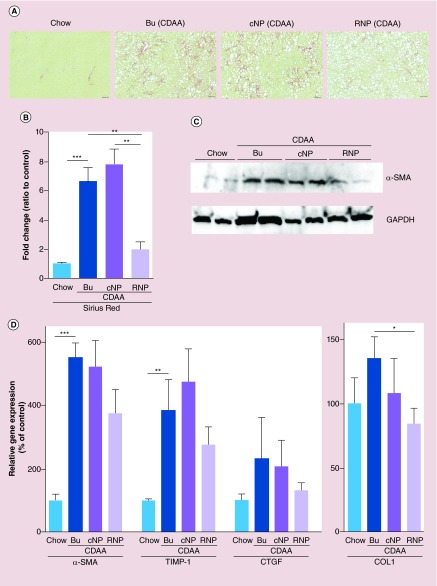
The reduction, and/or the reversal, of fibrosis is crucial to stop the progression of NASH into the more serious forms of this disease, namely cirrhosis. To investigate whether RNP treatment reverses fibrosis we started the treatment after 16 weeks of CDAA feeding, a time point that has been shown to be associated with significant fibrotic changes. Changes in liver fibrosis were assessed via Sirius red staining, α-SMA abundance via immunoblotting, the expression of various fibrogenic genes, as well as a key marker of activated hepatic stellate cells, via qPCR. As expected, liver fibrosis was extensive in the untreated mice fed the CDAA diet (Figure 3A–D). Importantly, fibrosis induced by CDAA feeding was reversed in the RNP-treated group as compared with control NPs, or control (buffer), as determined by a significant reduction in the area of Sirius red stain assessed by digital image morphometric quantitation (Figure 3A & B). Corresponding to the changes observed in Sirius red staining, the abundance of α-SMA in the whole liver was also decreased in the NASH mouse model treated with RNPs (Figure 3C). Although the mRNA expression of various fibrogenic genes was also reduced, statistical significance was only achieved for Collagen 1-alpha (Figure 3D). Taken together, these results suggest that oral RNP therapy impacted the progression of fibrosis induced by CDAA feeding.
Figure 3. . Redox nanoparticles treatment reversed nonalcoholic steatohepatitis fibrosis through reduction of hepatic stellate cell activation.
(A) Sirius-red staining of liver sections in mice fed with a chow diet, fed with a CDAA diet administered with control (Bu), cNP or RNP. Scale bar: 100 μm. (B) Bar graph shows quantification of positive area of Sirius-red as shown by percentage compared with mice fed with a chow diet. Values are mean ± SEM. ***p < 0.001; **p < 0.01. (C) Gene expression of α-SMA in whole liver by immunoblotting. (D) Gene expression of fibrogenic genes by qPCR. All gene expression levels were normalized to housekeeping control, β2 microglobulin, and shown relative to the expression levels of mice fed with a chow diet. Values are mean ± SEM.
***p < 0.001; **p < 0.01; *p < 0.05.
Bu: Buffer; CDAA: Choline deficient amino acid defined; cNP: Control nanoparticle; qPCR: Quantitative PCR; RNP: Redox nanoparticle; SEM: Standard error of the mean.
RNP treatment reduces liver inflammation
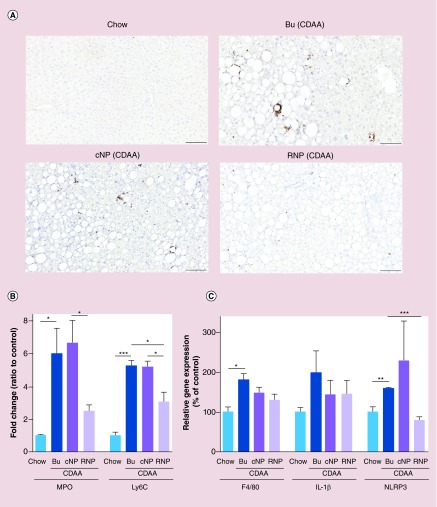
Since we observed the reduction in liver OS following improvement of liver histology by RNP treatment, we further assessed liver inflammation (neutrophil and monocyte infiltration) via immunohistochemistry against myeloperoxidase (MPO) and Ly6C, respectively. In addition we measured the expression of F4/80 – a marker of infiltrated macrophages – assessed the expression of some proinflammatory cytokines via qPCR. In the NASH mouse model, infiltration of neutrophils and monocytes was significantly increased (Figure 4A & B), as well as the number of macrophages and the expression of proinflammatory cytokines (Figure 4C). The number of neutrophils (MPO positive cells) in the NASH mouse model samples treated with RNPs was reduced compared with control NPs or control (buffer) treated mice (Figure 4A & B). In addition, the number of infiltrated monocytes (Ly6C positive cells) in mice treated with RNPs was significantly decreased compared with control treatments (Figure 4B). The mRNA expression of F4/80, a marker of mature macrophages, as well as the expression of proinflammatory genes, IL-1β and NLRP3, although not statistically significant, was reduced in RNP treated animals (Figure 4C). These results revealed that RNPs suppressed liver inflammation and reduced the infiltration of neutrophils, monocytes and macrophages through a mechanism associated with the reduction of ROS activity.
Figure 4. . Hepatocyte inflammation was reduced by redox nanoparticle treatment.
(A) Immunohistochemical staining specific for MPO (neutrophils) of liver sections in mice fed with a chow diet, fed with a CDAA diet administered with control (Bu), cNP or RNP. Scale bar: 100 μm. (B) Bar graph shows quantification of MPO- or Ly6C-positive cells. Values are mean ± SEM. ***p < 0.001; *p < 0.05. (C) Gene expression of inflammatory genes by qPCR. All gene expression levels were normalized to housekeeping control, β2 microglobulin, and shown relative to the expression levels of mice fed with a chow diet. Values are mean ± SEM.
***p < 0.001; **p < 0.01; *p < 0.05.
Bu: Buffer; CDAA: Choline deficient amino acid defined; cNP: Control nanoparticle; MPO: Myeloperoxidase; qPCR: Quantitative PCR; RNP: Redox nanoparticle; SEM: Standard error of the mean.
Discussion
In this study we demonstrated that RNP treatment in a diet-induced NASH mouse model led to the reversal of hepatic fibrosis and a decrease in liver inflammation and infiltration of neutrophils and monocytes through a mechanism associated with the reduction of ROS. ROS are chemically reactive molecules containing oxygen and represent one of the key factors that may trigger liver damage and further lead to the development of NASH [3]. Therefore, reducing ROS in the liver presents an important therapeutic approach for treating or reversing NASH.
There are a variety of antioxidants, such as vitamin C (ascorbic acid), glutathione, lipoic acid, uric acid, carotenes, vitamin E (α-tocopherol), and coenzyme Q (ubiquinol), available as natural substances. Due to the importance of OS in NASH development, antioxidant interventions have been postulated as an attractive therapeutic approach. Up to date, vitamin E has been the one most studied including large randomized placebo controlled trials in both adults and children with mixed results [5,6]. While in adult patients vitamin E therapy demonstrated improvement in steatohepatitis [5], it failed to show a reduction of serum ALT that was the primary endpoint in the pediatric study [6]. In both studies vitamin E had no effect on liver fibrosis [18]. Internalization of vitamin E in healthy cells may result in unwanted effects on normal redox reactions, which prohibited to increase doses up to effective level. Because many antioxidant compounds show similar issues (viz, they scavenge important intracellular ROS in healthy cells) antioxidant strategies using low-molecular weight drugs may fail to achieve the protective effects expected in conditions with heightened OS. Selective scavenging of excessively generated ROS in inflamed areas (outside of cells), and protecting the normal redox reactions inside healthy cells, is an attractive alternative strategy. TEMPOL, a synthetic antioxidant, is the most extensively studied nitroxide shown to be effective in detoxifying ROS in both cell culture and animal studies [11]. Indeed, Sepodes et al. reported that TEMPOL reduced acute liver injury in rat models of hepatic ischemia [19]. However, TEMPOL administration leads to a significant lowering of blood pressure, thus limiting its potential clinical utility [11]. TEMPOL is also internalized in normal cells nonspecifically [9–11]. Therefore, we developed a novel way to deliver TEMPO to the site of injury (high ROS production) with minimal exposure to the blood stream, thus reducing TEMPO's side effects. We developed RNPs, which are composed of redox polymers able to disintegrate under low pH conditions [20]. Covalent conjugation of the TEMPO moiety to the polymer used is one of the most important considerations to avoid possible adverse effects. Notably, RNPs do not induce a decrease in blood pressure [17]. We have previously demonstrated that RNP treatment protected against tissue damage in acute brain, as well as kidney, injury mouse models and improved chronic disease symptoms in colitis or small intestinal inflammation mouse models [21–23]. This study is the first to show that RNPs may treat or reverse NASH, and the associated liver fibrosis, in a human pathophysiologically-relevant mouse model that mimics several of the key features of human NASH. We detected a strong ESR signal, indicating the presence of redox polymers, both in the blood and liver. The ESR spectra of RNPs can provide information on morphological changes in vivo. Although RNPs show a broad singlet ESR signal, the dissociated polymers show a triplet signal [17]. In Figure 1C & Supplementary Figure 2A, ESR signals in the blood and liver were triplet signals, indicating that redox polymers are present at these sites, but not RNP (because of disintegration in stomach and internalized in blood stream by molecularly dissolved redox polymer state). We also detected a rhodamine signal, which was conjugated to redox polymers, in the liver. In previous paper, we demonstrated that pH-insensitive RNPs, which consist of poly(ethylene glycol)-b-poly[4-(2,2,6,6-tetramethylpiperidine-1-oxyl)oxymethylstyrene], accumulate in the mucosa of intestine and do not pass through bloodstream across the intestinal epithelium because pH-insensitive RNPs do not disintegrate even in the harsh environment of gastrointestinal tract [24]. When rhodamine-labeled pH-insensitive RNPs were orally administered, strong fluorescent signal was detected in the mucosa of intestine. This difference of fluorescent image in the intestine suggests that PEG-b-PMNT does not form polymeric micelles in the intestine. Taken together, our results reveal that orally administered RNPs are disintegrated to redox polymers in the stomach, and redox polymers are absorbed in the blood stream through the intestines and circulate for ca. 24 h, and ultimately reach the liver.
In this study we observed that RNP therapy administered to a diet-induced NASH mouse model led to a significant decrease in ROS and inflammation in the liver, especially notable was the reduction in the number of infiltrated proinflammatory macrophages and neutrophils. In addition, we observed a reduction in the expression of proinflammatory markers, in particular NLRP3-inflammasome and IL-1β, two key components of sterile inflammation that have been linked to liver injury and fibrosis in the context of NASH [25,26]. The reduction of liver ROS and inflammasome activity induced by RNP therapy was further associated with a decrease in inflammatory activity in the livers of these mice. In addition, we observed a modest reversal of liver fibrosis with 4-week oral RNP treatment initiated at the 16-week timepoint of CDAA diet, which is associated with severe liver damage. Based on these results, future studies that provide a longer timecourse of RNP treatment, as well as testing higher doses of TEMPO, are warranted and may result in a more robust antifibrotic effect.
Conclusion
Liver OS is the key factor in progressive liver disease. This study suggests that novel antioxidant RNPs, which are prepared by self-assembling amphiphilic block copolymers composed of a hydrophilic poly(ethylene glycol) segment and hydrophobic poly(4-methylstyrene) segment possessing nitroxide radical moieties via amine linkage, reduced liver OS promoting improvement of liver fibrosis and inflammation. RNPs are disintegrated under low pH, such as stomach, and redox polymers are delivered to the liver via the intestine. Contrary to the low-molecular weight antioxidants, RNPs and disintegrated polymers could not internalize into healthy cells, which maintained normal redox reaction in healthy cells. This RNP treatment modality will be a novel anti-oxidant delivery system to treat NASH by oral administration.
Future perspective
Recently, development of nanotechnology-based drug delivery systems has made rapid progress and a part of them has already passed into the stage of practical use. However, most of their targets are cancer therapy via intravenous administration. On the other hand, since chronic diseases such as NASH and arteriosclerosis basically require long-term treatment, oral medication is much preferable, but it was difficult to deliver nanosized materials systemically via oral administration. We have already confirmed that extremely high dispersion-stable nanoparticles accumulate in the intestinal mucosa but not internalized in the blood stream via mesentery by our pH-insensitive RNPs [22,24]. Here, we have succeeded to deliver polymer nanomedicine to the blood circulation from oral route by controlling molecular weight and pH-sensitive self-assembling characters, resulting in therapeutic effects on NASH. One of the important points of our material design is covalent linkage of nitroxide radicals to low-molecular weight polymer for selectively suppressing oxidative stress which aggravates chronic inflammation without disturbance of normal redox reaction in the body. Since absorbed redox polymers are delivered to the entire body, treatment with oral administered RNPs might be applicable to not only treatment of liver diseases but also other chronic systemic diseases such as autoimmune disorders, diabetes, arteriosclerosis, and so on. In fact, most recently, we have confirmed that orally administered RNPs almost completely revived the cognition in 17-week-old SAMP8 mice and published it [14]. We believe that the oral nanomedicines using synthetic polymer-drug conjugate will attract great attentions as new concepts. We will progress the development for practical use of oral nanomedicies using RNP.
Executive summary.
Liver delivery of redox polymer after oral administration of redox nanoparticle
Oral administration of redox nanoparticles (RNPs) results in the disintegration of redox nanoparticles in the stomach, absorption of redox polymer into the blood and delivery of redox polymer to liver resulting in liver enrichment.
Long-term toxicity of redox polymer
After oral administration of RNPs, most of redox polymers are eliminated within 24 h from whole body.
Administration of RNPs orally for 4 weeks did not have any impact on bodyweight or survival in mice fed the control or nonalcoholic steatohepatitis diet.
Therapeutic effects of orally administered RNP on nonalcoholic steatohepatitis model mice
RNP treatment significantly reduces ROS activity in the liver independent of lipid buildup.
Liver inflammation and, to a lesser extent, liver fibrosis are recovered by RNP treatment.
RNP treatment via oral administration is a potentially novel antioxidant delivery system to treat nonalcoholic steatohepatitis.
Supplementary Material
Acknowledgements
The authors thank Yuki Ozaki and Pennapa Chonpathompikunlert (Graduate School of Pure and Applied Sciences, University of Tsukuba) for their technical help in the biodistribution experiment using 125I-labeled RNP. The authors thank the UCSD Neuroscience Core, especially Jennifer Santini for microscopy assistance.
Footnotes
Author contributions
A Eguchi: all experiments in NASH mouse model; T Yoshitomi: RNP preparation, and measurements of ESR and distribution in wild-type mice by 125I-labeled RNP.
Financial & competing interests disclosure
This study was presented in part during an oral ePOSTER presentation of the Annual Meeting of the European Association for the Study of the Liver (ESAL), held in Amsterdam, The Netherlands, April 2013 and in one of the Poster of Distinction of Falk symposium 191, held in London, October 2013. This work was supported by NIH grants U01AA022489 and DK082451 to AE Feldstein, supported by a Grant-in-Aid for Scientific Research S (no. 25220203) and the World Premier International Research Center Initiative (WPI Initiative) on Materials Nanoarchitronics to Y Nagasaki, and supported by a Grant-in-Aid for challenging Exploratory Research (no. 24659014) from the Ministry of Education, Culture, Sports, Science, and Technology, Japan to T Yoshitomi. UCSD Neuroscience Core for microscopy is supported by a grant P30 CA23100. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Ethical conduct of research
The authors state that they have obtained appropriate institutional review board approval or have followed the principles outlined in the Declaration of Helsinki for all human or animal experimental investigations. In addition, for investigations involving human subjects, informed consent has been obtained from the participants involved.
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- 1.Wree A, Broderick L, Canbay A, Hoffman HM, Feldstein AE. From NAFLD to NASH to cirrhosis-new insights into disease mechanisms. Nat. Rev. Gastroenterol. Hepatol. 2013;10(11):627–636. doi: 10.1038/nrgastro.2013.149. [DOI] [PubMed] [Google Scholar]; • Review article of inflamassome in liver disease.
- 2.Angulo P. Nonalcoholic fatty liver disease. N. Engl. J. Med. 2002;346(16):1221–1231. doi: 10.1056/NEJMra011775. [DOI] [PubMed] [Google Scholar]
- 3.Schuppan D, Schattenberg JM. Non-alcoholic steatohepatitis: Pathogenesis and novel therapeutic approaches. J. Gastroenterol. Hepatol. 2013;28(Suppl. 1):68–76. doi: 10.1111/jgh.12212. [DOI] [PubMed] [Google Scholar]
- 4.Koek GH, Liedorp PR, Bast A. The role of oxidative stress in non-alcoholic steatohepatitis. Clin. Chim. Acta. 2011;412(15–16):1297–1305. doi: 10.1016/j.cca.2011.04.013. [DOI] [PubMed] [Google Scholar]
- 5.Sanyal AJ, Chalasani N, Kowdley KV, et al. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N. Engl. J. Med. 2010;362(18):1675–1685. doi: 10.1056/NEJMoa0907929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lavine JE, Schwimmer JB, Van Natta ML, et al. Effect of vitamin E or metformin for treatment of nonalcoholic fatty liver disease in children and adolescents: the TONIC randomized controlled trial. JAMA. 2011;305(16):1659–1668. doi: 10.1001/jama.2011.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yuksel BC, Serdar SE, Tuncel A, et al. Effect of tempol, a membrane-permeable radical scavenger, on mesenteric blood flow and organ injury in a murine cecal ligation and puncture model of septic shock. Eur. Surg. Res. 2009;43(2):219–227. doi: 10.1159/000225984. [DOI] [PubMed] [Google Scholar]
- 8.Nagasaki Y. Nitroxide radicals and nanoparticles: a partnership for nanomedicine radical delivery. Ther. Deliv. 2012;3(2):165–179. doi: 10.4155/tde.11.153. [DOI] [PubMed] [Google Scholar]; •• Review article of redox nanoparticles (RNPs).
- 9.Shimizu M, Yoshitomi T, Nagasaki Y. The behavior of ROS-scavenging nanoparticles in blood. J. Clin. Biochem. Nutr. 2014;54(3):166–173. doi: 10.3164/jcbn.13-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Monti E, Supino R, Colleoni M, Costa B, Ravizza R, Gariboldi MB. Nitroxide TEMPOL impairs mitochondrial function and induces apoptosis in HL60 cells. J. Cell. Biochem. 2001;82(2):271–276. doi: 10.1002/jcb.1160. [DOI] [PubMed] [Google Scholar]
- 11.Wilcox CS, Pearlman A. Chemistry and antihypertensive effects of tempol and other nitroxides. Pharm. Rev. 2008;60(4):418–469. doi: 10.1124/pr.108.000240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heckman KL, Decoteau W, Estevez A, et al. Custom cerium oxide nanoparticles protect against a free radical mediated autoimmune degenerative disease in the brain. ACS Nano. 2013;7(12):10582–10596. doi: 10.1021/nn403743b. [DOI] [PubMed] [Google Scholar]
- 13.Park EJ, Choi J, Park YK, Park K. Oxidative stress induced by cerium oxide nanoparticles in cultured BEAS-2B cells. Toxicology. 2008;245(1–2):90–100. doi: 10.1016/j.tox.2007.12.022. [DOI] [PubMed] [Google Scholar]
- 14.Chonpathompikunlert P, Yoshitomi T, Vong LB, Imaizumi N, Ozaki Y, Nagasaki Y. Recovery of cognitive dysfunction via orally administered redox-polymer nanotherapeutics in SAMP8 mice. PLoS ONE. 2015;10(5):e0126013. doi: 10.1371/journal.pone.0126013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kleiner DE, Brunt EM, Van Natta M, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41(6):1313–1321. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 16.Yoshitomi T, Suzuki R, Mamiya T, Matsui H, Hirayama A, Nagasaki Y. pH-sensitive radical-containing-nanoparticle (RNP) for the L-band-EPR imaging of low pH circumstances. Bioconjug. Chem. 2009;20(9):1792–1798. doi: 10.1021/bc900214f. [DOI] [PubMed] [Google Scholar]
- 17.Yoshitomi T, Hirayama A, Nagasaki Y. The ROS scavenging and renal protective effects of pH-responsive nitroxide radical-containing nanoparticles. Biomaterials. 2011;32(31):8021–8028. doi: 10.1016/j.biomaterials.2011.07.014. [DOI] [PubMed] [Google Scholar]; • Therapeutic effect of intravenously administered RNPs on renal ischemia–reperfusion injury is described in this paper.
- 18.Pacana T, Sanyal AJ. Vitamin E and nonalcoholic fatty liver disease. Curr. Opin. Clin. Nutr. Metabol. 2012;15(6):641–648. doi: 10.1097/MCO.0b013e328357f747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Violi F, Cangemi R. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N. Engl. J. Med. 2010;363(12):1185–1186. doi: 10.1056/NEJMc1006581. author reply 1186. [DOI] [PubMed] [Google Scholar]
- 20.Yoshitomi T, Miyamoto D, Nagasaki Y. Design of core – shell-type nanoparticles carrying stable radicals in the core. Biomacromolecules. 2009;10(3):596–601. doi: 10.1021/bm801278n. [DOI] [PubMed] [Google Scholar]
- 21.Marushima A, Suzuki K, Nagasaki Y, et al. Newly synthesized radical-containing nanoparticles enhance neuroprotection after cerebral ischemia-reperfusion injury. Neurosurgery. 2011;68(5):1418–1425. doi: 10.1227/NEU.0b013e31820c02d9. discussion 1425– 1416. [DOI] [PubMed] [Google Scholar]; •• Therapeutic effect of intravenously administered RNPs on cerebral ischemia–reperfusion injury is described in this paper.
- 22.Vong LB, Tomita T, Yoshitomi T, Matsui H, Nagasaki Y. An orally administered redox nanoparticle that accumulates in the colonic mucosa and reduces colitis in mice. Gastroenterology. 2012;143(4):1027–1036. doi: 10.1053/j.gastro.2012.06.043. e1023. [DOI] [PubMed] [Google Scholar]; • Therapeutic effect of pH-insensitive RNPs on colitis is described in this paper.
- 23.Chonpathompikunlert P, Fan CH, Ozaki Y, Yoshitomi T, Yeh CK, Nagasaki Y. Redox nanoparticle treatment protects against neurological deficit in focused ultrasound-induced intracerebral hemorrhage. Nanomedicine. 2012;7(7):1029–1043. doi: 10.2217/nnm.12.2. [DOI] [PubMed] [Google Scholar]; •• Therapeutic effect of intravenously administered RNPs on intracerebral hemorrhage is described in this paper.
- 24.Sha S, Vong LB, Chonpathompikunlert P, Yoshitomi T, Matsui H, Nagasaki Y. Suppression of NSAID-induced small intestinal inflammation by orally administered redox nanoparticles. Biomaterials. 2013;34(33):8393–8400. doi: 10.1016/j.biomaterials.2013.06.032. [DOI] [PubMed] [Google Scholar]; • Therapeutic effect of pH-insensitive RNPs on small intestinal inflammation is described in this paper.
- 25.Wree A, Eguchi A, Mcgeough MD, et al. NLRP3 inflammasome activation results in hepatocyte pyroptosis, liver inflammation, and fibrosis in mice. Hepatology. 2014;59(3):898–910. doi: 10.1002/hep.26592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wree A, Mcgeough MD, Pena CA, et al. NLRP3 inflammasome activation is required for fibrosis development in NAFLD. J. Mol. Med. 2014;92(10):1069–1082. doi: 10.1007/s00109-014-1170-1. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Involvement of NLRP3 inflammasome in the non-alcoholic steatohepatitis progression is describe in this paper.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.