Abstract
Since its discovery almost three decades ago, HIV-1 has grown into the most aggressive pandemic of modern time. Following the implementation of combination antiretroviral therapy, the pathological outcome of HIV infection has substantially improved. However, combination antiretroviral therapy is limited by several factors including, long-term toxicity, serious side effects and complex dosing regimens, and so on. In this regard, researchers have directed their attention toward enhancing current treatment strategies and/or developing alternative HIV-1 therapeutics. In recent years, this attention has fixated on nanomedicine-based anti-HIV therapeutics (HIV-1 nanotherapeutics). In the present study, we have reviewed several HIV-1 nanotherapeutics that have shown success at the preclinical level and/or Phase I/II clinical trials. We also discuss the possible benefits of these nanomedicine-based approaches and their future outlook.
Keywords: : AIDS, cART, HIV, nanomedicine, nanoparticles
HIV was identified in 1983 as the retrovirus responsible for the onset of AIDS [1]. In a relatively short period of time, HIV/AIDS transformed into a global pandemic that resulted in the death of millions of people. The Joint United Nations Program on HIV/AIDS (UNAIDS) 2014 report calculated that approximately 35 million people are living with HIV worldwide. In 2013, 3.2 million children under the age of 15 years were living with HIV globally – 91% of whom are located in sub-Saharan Africa [2]. Another 2014 UNAIDS report highlighted inferior access to HIV prevention, treatment, care and support, as the reason for this disparity. The report concluded that focusing on populations who are underserved and at higher risk of HIV will be the key to end the AIDS epidemic [3].
It has also become increasingly important to understand how HIV treatment fails over time in patients. As the molecular mechanisms for treatment failure are still not fully understood, patients who begin showing resistance to one regimen of drugs will be asked to change to a different regimen. Long-term adherence to these treatment regimens can be difficult due to their high expense and adverse side effects. At the same time, current treatments do not guarantee that infected patients will not progress to AIDS [3].
Combination antiretroviral therapy (cART) has significantly improved the morbidity and mortality of HIV-infected patients [4]. However, cART cannot eradicate the virus from reservoir organs, such as, the CNS, lymphoid tissues, testis, and so on [4]. Moreover, pharmacology issues such as drug–drug interaction, acute and long-term toxicity have presented an immense challenge toward controlling the progression and spread of HIV [4].
Limitations of conventional cART in treating HIV-1
Presently, no cure for HIV has been discovered nor has a viable therapeutic vaccine been developed. Prevention measures (e.g., condom use, needle exchange programs, peripartum antiretroviral prophylaxis) play an important role in circumventing HIV numbers but, once the virus is transmitted, the infection is irreversible. The current method of combating HIV infection is through cART, which involves the combined administration of three or more different types of drugs. cART has had wide success in improving patients’ lives, but not without serious adverse side effects. The potential risk of developing adverse side effects varies from drug to drug. The long-term use of protease inhibitors has been linked with hyperlipidemia, body fat redistribution and diabetes mellitus [5]. While nucleoside analogous have been associated with lactic acidosis, which can ultimately lead to liver failure and death. Moreover, almost all antiretrovirals have been associated with increased risk of transaminitis and hepatotoxicity [6]. In addition, cART is a life-long treatment that often consists of patients taking a variety of drugs daily. These impractical regimens have led to unfavorable results in treatment as well as the enhanced development of drug-resistance when patients fail to take the drugs as prescribed. This ‘lack of adherence’ to drug regimens has resulted in patients having ineffective drug levels in their body, which allows the virus to mutate and replicate further [4].
In addition to the problem of daily doses required by cART, researchers are also working toward creating therapeutics that is effective in combating the disease mechanism [7]. One of the issues with halting the disease mechanism with cART is the lack of a targeted approach to decrease viral reservoirs (where the replication of the virus may result in consistent activation of the immune system) [8]. cART may be leaving behind HIV-replicating cells in these reservoirs, which would normally be eliminated by the immune system, but since cART reduces the effectiveness of memory CD4 T cells designated to attack the virus, patients who have been treated with cART over a long periods of time are susceptible to HIV (replication) in the viral reservoirs [8]. Different research approaches are attempting to completely eliminate HIV within the viral reservoirs through nontoxic and low-dosage therapeutics, thereby obviating the need for a lifetime of daily drug administration [4].
HIV-1 nanotherapeutics
The limitations of cART may be addressed in the coming years by nanotechnological applications in pharmacology. The serious side effects associated with cART may be ameliorated through nanomedicine-based enhancements to current treatment. The use of modified nanocarriers and/or nanosuspensions will allow for the emergence of novel targeted drug delivery and controlled drug release methods. These exciting nanomedical applications toward current treatment may reduce the potentially toxic fluctuations in drugs levels caused by uneven tissue distribution and bioavailability. Nanomedicine has also driven the development of new prevention and treatment approaches to HIV-1 infection, including vaccine delivery, gene therapy, immunotherapy and preventive microbicides. Most nanomedical applications toward HIV are preclinical or have failed at the clinical stage. However, clinical trials investigating the controlled and sustained release of cART drugs via nanoformulations have shown immense promise (Table 1) [7].
Table 1. . Selected examples of antiretroviral compounds and nanocarriers in development for HIV-1 nanotherapeutics.
| Antiretroviral compound(s) | Type | Nanocarriers | Development stage | Refs. |
|---|---|---|---|---|
| TMC278-LA (long-acting) |
Non-nucleoside reverse-transcriptase inhibitor |
Nanosuspension with poloxamer 338 and d-a-tocopheryl polyethylene glycol 1000 succinate |
Phase II clinical trials |
[9] |
| GSK1265744 |
Intregrase inhibitor |
Nanosuspension with polysorbate 20 and polyethylene glycol 3350 |
Phase II clinical trials |
[10] |
| Enfuvirtide |
Fusion inhibitor |
Iron oxide nanoparticles coated with amphiphilic polymer |
In vitro |
[11] |
| DermaVir Patch | HIV antigen-coding DNA plasmid | Polyethyleimine mannose nanoparticles | Phase II clinical trials | [12] |
Common nanomaterials used in HIV-1 nanotherapeutics
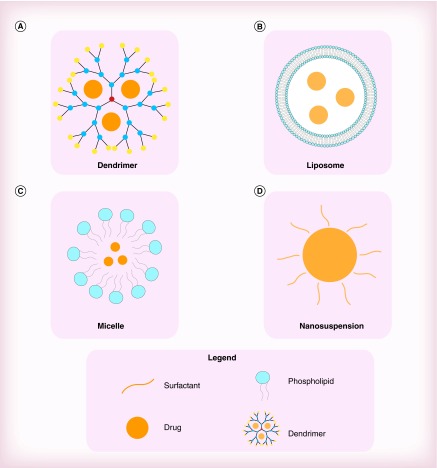
Nanomaterials are defined by their very small size – featuring at least one dimension measuring less than 100 nm. Materials on this scale possess unique properties, such as high reactivity and large surface volumes. Nanomaterials have provided researchers with novel approaches to advance treatment and prevention strategies for HIV-1. There is an extensive range of nanomaterials used in biomedicine. Some of the most prevailing nanomaterials that have been investigated for their use in HIV-1 infection are dendrimers, liposomes, micelles and nanosuspensions (Figure 1) [8].
Figure 1. . Comparison between four common nanomaterials used in HIV-1 nanotherapeutics.
(A) Dendrimers: increases the uptake of efavirenz into macrophages. (B) Micelles: increases the bioavailability of efavirenz. (C) Liposomes: increases the transdermal delivery of indinavir. (D) Nanosuspensions: increases the circulation time of several antiretrovirals.
Dendrimers consist of repetitively branched molecules that radiate from a central core. They are quite unique in their overall uniformity, narrow molecular weight distribution and dynamic internal cavities. Dendrimers also possess a multifunctional terminal surface – primarily composed of amine functional groups. Dendrimers can be used as nanocarriers by conjugating/absorbing drugs unto the dendrimer surface or through drug encapsulation by its internal cavities. Their low manufacturing costs and easy scalability also make dendrimers a highly attractive option. In one in vitro study, poly(propyleneimine) dendrimers were shown to improve the uptake of efavirenz by circulating macrophages [13].
Micelles are lipid molecules that form spherical aggregates in aqueous solutions. The water-soluble exterior of a micelle forms a kind of shell that allows drugs to be encapsulated in its hydrophobic core. These spherical structures are highly versatile as their size and block copolymer chemistries can be fine-tuned for a wide range of biomedical applications [14]. Polymeric micelles have been shown to increase the aqueous solubility and oral bioavailability of efavirenz [15].
Liposomes are spherical structures that are made up of one or more phospholipid bilayer membranes surrounding an aqueous core. When the phospholipids bilayer membranes of liposomes are hydrated they form closed structures that can be used as vesicles to transport aqueous and lipid drugs. Liposomes offer many advantages as drug carriers owing to their nontoxic, non-immunogenic, biocompatible and biodegradable nature [8]. The transdermal delivery of Indinavir – a protease inhibitor – was significantly enhanced by use of ethanolic liposomes [16].
Nanosuspensions are colloidal dispersions of nanosized drug particles that are typically stabilized by a surfactant in order to avoid agglomeration. Drug bioavailability can be significantly improved through nanosuspension formulation, especially in drugs with poor solubility and/or permeability. Stabilizers that are commonly used in nanosuspensions are cellulosics, poloxamer, polysorbates and lecithin. Injectable nanosuspensions are preferred for drugs that are degraded or not absorbed in GI tract (GIT), and/or have shown limited improvement in bioavailability when stabilized by surfactants [17]. Nanosuspensions of several antiretrovirals have resulted in sustained release properties that may allow for lower dosing frequency and improved adherence [4].
Controlled release of cART
The applications of nanomedicine toward HIV may tackle the problem of low adherence and subsequent treatment failure by implementing a drug therapy that is not required to be taken daily. Nanomedicine may apply controlled release delivery systems to anti-HIV drugs so that their half-lives may be prolonged, thus keeping the drug molecules in circulation longer [4]. If the drugs are effective in patients for a longer period of time, then they would not have to be taken as frequently, which could make it much easier for patients to remain on their recommended treatment regimens.
Recently, injectable long acting nanoformulations of several cART drugs have been developed and tested by many researchers in order to observe their potential for improving the dosing regimen from current daily dosing to weekly or monthly dosing. This approach may dramatically improve the quality of life of patients by avoiding side effects and related toxicity. Long-acting injectable nanoformulations of rilpivirine and GSK1265744 (cabotegravir) have progressed to the latter stages of the development pipeline. GSK1265744 is an HIV-1 integrase strand transfer inhibitor and analogue of dolutegravir. In a Phase II clinical trial, GSK1265744 was detected in plasma at 48 weeks [18]. Complementary pharmacologic properties, resistance profiles, metabolic pathways, lack of drug interactions and low daily oral doses, offer the potential for combination use. Mean absorption-limited apparent terminal-Phase half-life ranged from 21 to 50 days as compared with approximately 40 h following single-dose oral administration. This longer apparent half-life following injection is a result of low solubility of the drug nanoparticles and inherent low tissue perfusion, prompting a slow absorption rate of drug from the injectable formulation [19]. A similar kind of long-acting parenteral formulation of antiretrovirals could facilitate maintenance and prophylactic treatment of HIV using the poorly water soluble non-nucleoside reverse transcriptase inhibitor (NNRTI) rilpivirine (TMC278). Baert et al. have developed such a formulation, which has been shown to significantly increase the biological half-life of TMC278 via injectable nanosuspension [20]. TMC278 free base is a stable crystalline polymorphic drug substance with very low water solubility (<0.1 mg/ml). Further preclinical investigation of TMC278-LA (200 nmol/l injectable nanosuspension) in animal models confirmed sustained plasma concentrations and dose-proportional release lasting more than 2 months in rats and more than 6 months in dogs. Absolute bioavailability approached 100%, indicating complete release from the injection site [21]. A subsequent Phase I clinical trial of TMC278-LA (300 mg/ml nanosuspension) evaluated with single gluteal intramuscular or abdominal subcutaneous injections in 51 HIV-negative adult patients established that rilpivirine can slowly release from the injection site into plasma with drug concentrations of more than 10 ng/ml for 12–26 weeks [22].
A previous report from our group indicated that weekly administered injections of long-acting nanoformulated cART drugs in a mouse model led to similar concentrations as those effective in humans and resulted in limited toxicity. The CD4 T-cell levels of nanodrug treated humanized mice were significantly higher than those of conventionally treated (standard drug) mice [23]. Furthermore, a weekly injection of P-188 nanoformulation with cART drugs atazanavir and ritonavir showed serum and tissue concentrations up to 270-fold higher than unformulated drugs throughout 8 weeks of study in mice. Importantly, nanoformulated drugs were localized in nonlysosomal compartments in mouse liver macrophages, creating intracellular depot sites. Similar observations were also made in representative rhesus macaque studies using the same formulations. These nanoformulations also did not indicate any immediate liver and renal toxicity to mice and macaques, respectively [24].
Despite the natural worries about patient acceptability issues regarding invasive administration of drugs, the development of the first injectable long acting antiretroviral formulation has created a significant interest among patient populations. A recent study found that nearly 73% of patients were interested in using injectable nanoformulated antiretroviral therapy depending on the dosing schedule [25]. Monthly administration seems feasible contrasting with poor acceptance of weekly injections. In this regard, formulations of rilpivirine and GSK1265744 have been developed with the potential for once monthly intramuscular administration. Moreover, physiologically based pharmacokinetic modeling suggests that dolutegravir, efavirenz, emtricitabine, raltegravir, rilpivirine and tenofovir are potential candidate for once monthly dosing with an optimized clinical condition [26].
It has also been observed in several clinical trials that lower pill burden is associated with better virological suppression and overall adherence to treatment. In an attempt to capitalize on this finding, many clinical trials have been designed to evaluate the short- and long-term effects of injectable nanoformulations of anti-HIV drugs versus a once daily oral tablet [26,27]. In one example, the injectable nanodrug, TMC278, was suggested for one (1200 mg) injection at 8-week intervals rather than a 25 mg oral capsule once daily [26]. In a separate ongoing study the injectable HIV medicine, GSK1265744, was tested for its safety, pharmacokinetics and therapeutic efficacy as an injectable and oral medication once or twice a week [10,28]. Likewise, a similar kind of long-acting injectable drugs, TMC278 and GSK1265744, were also tested for their safety, tolerability and pharmacokinetics of single versus three consecutive doses in humans by Tibotec Pharmaceuticals and GlaxoSmithKline [9,29–30]. A single tablet containing co-formulated darunavir, cobicistat, emtricitabine and tenofovir alafenamide fumarate is currently in development. If approved by US FDA, this will be a first line tablet regimen [31]. A co-formulation of tenofovir alafenamide fumarate with elvitegravir, cobicistat (adrug enhancer) and emtricitabine (FTC) is also in development, which is also a potential candidate for single tablet regimens [32–37]. All the above studies have strongly indicated that once daily, single tablet regimes offer several advantages over combination therapy. Indeed, replacing complicated drug cocktails with a single daily pill increased treatment adherence, lowered healthcare costs and improved overall quality of life [37,38]. With these improved drug formulations, it is possible that there will be long-term reduction of neuronal and renal toxicity which itself improves the chances of longer survival of infected patients. Moreover, one pill a day regimens have achieved 95% treatment adherence and a 23% reduction in hospitalization, which resulted in a 17% reduction in the healthcare costs associated with HIV [38].
Nanomedical approaches for improving the delivery of cART drugs
Complete eradication of HIV via cART is hampered by the continued presence of HIV in anatomical and cellular reservoir sites. The CNS acts as a vital reservoir site to the persistence of HIV infection following cART [39]. The chronic presence of HIV in the CNS has also been associated with a range of neurocognitive disorders [40]. Although current cART drugs can suppress HIV in peripheral blood, they have limited access to the CNS due to efflux exclusion at the blood–brain barrier (BBB). Therefore, the CNS acts as a safe haven for HIV which allows the virus to evolve and enhance its fitness.
Nanomedical approaches to cART may allow therapeutic doses of cART drugs to be delivered into the CNS. One nanomedical drug delivery approach is to absorb or conjugate cART drugs unto nanocarriers. Some of the materials that have been investigated as nanocarriers include liposomes, dendrimers, micelles. These nanocarriers may allow cART drugs to penetrate the BBB and be administered into the CNS [41]. More recently, iron oxide nanoparticles coated with an amphiphilic polymer were found to increase the translocation of enfuvirtide across the BBB [11].
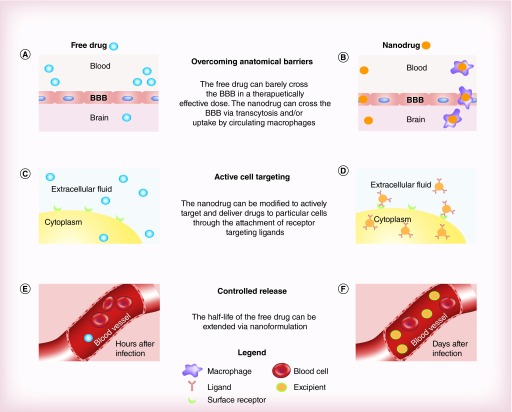
The transfer of hydrophobic/hydrophilic drugs into specific tissues may also be improved due to the small size of the nanoparticles used as delivery systems [4]. Nanomedicine has exhibited improved delivery of poorly water-soluble drugs, the ability to target specific cells or tissues for drug delivery, and intracellular delivery of macromolecules. Nanocarriers can be engineered to deliver drugs to specific cells by modifying their surface chemistries. One commonly employed method is the conjugation of ligands to the nanocarrier surface. These factors could be used to improve treatment of HIV-1 infection [42]. In addition, these drugs may be significantly less toxic once nanoformulated [43,44]. This is due to less fluctuant systemic levels of antiretroviral drugs as yielded by sustained release from nanoparticles. An overall difference between unformulated and nanoformulated anti-HIV drug delivery has been illustrated in Figure 2 indicating hurdles of conventional drug delivery compared with nanodrug delivery.
Figure 2. . The advantage of nanoformulated drug vs free drug in several applications.
(A) The free drugs are mostly prevented from reaching the CNS; (B) whereas the nanodrugs are able to reach the CNS in a therapeutically effective dose. (C) The free drugs are distributed uniformly with low levels being delivered to target cells. (D) The intercellular delivery of the free drugs is enhanced via a nanoformulation that contains receptor targeting ligands. (E) Most of the free drugs have been metabolized and eliminated within few hours after administration. (F) The nanodrug remains at therapeutically effective dose even days after injection.
BBB: Blood–brain barrier.
HIV nanoformulated vaccine
While the controlled release of cART drugs via nanoformulations has shown the most success in clinical trials, another promising application of nanomedicine toward HIV is the DermaVir patch (Genetic Immunity, Budapest, Hungary). The DermaVir patch is a therapeutic vaccine that has entered Phase II clinical trials. The formulation of the patch involves mannosylated poly(ethyleneimine), glucose and an HIV antigen-coding DNA plasmid, which are formulated into nanoparticles. The patch delivers these nanoparticles to epidermal cells, which then engulf the nanoparticles and evolve to produce an immune response. The DermaVir patch is one of the first nanomedical treatments for HIV to reach clinical trials and is intended to be used as adjuvant to conventional cART. It has been shown to induce long-term responses in memory T cells and reduce HIV load in viral reservoirs. Phase I clinical trials indicated that the patch was safe and tolerable by patients [45]. No therapeutic vaccine has yet been made available because of the complicated nature of the disease mechanism and its interaction with the immune system; the fact that the immune escape has not been eliminated by T cells is due to the high genetic variability of the virus and the inability to stimulate widely reactive neutralizing antibodies [4,7]. However, efforts are underway in order to tackle these and other limiting issues in HIV vaccine development [45].
Nanomedicine & prevention of HIV sexual transmission
As advances continue to be made in the field of nanomedicine, approaches to eliminating HIV-1 in patients globally may sway from mainly treating the virus via drug therapeutics to preventative measures. One possible method of prevention is the use of topical microbicides, defined as products containing one or more anti-HIV compounds and intended to be locally administered around the time of sexual intercourse (coitus-dependent microbicides) or otherwise allow sustained drug levels for prolonged periods irrespective of the time of use (coitus-independent microbicides) [46]. Microbicides have been tested in an attempt to prevent transmission of HIV-1 through vaginal and/or rectal routes.
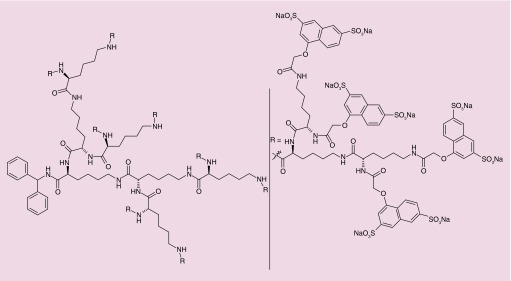
Among different options for product design, nanotechnology-based approaches have been proposed over recent years and a few products are in various stages of preclinical and even clinical development. The most advanced nano-microbicide product in the development pipeline is Vivagel® (Starpharma, Melbourne, Australia). This carbomer gel contains a dendrimer (SPL7013) with inherent but unspecific antiviral activity [47]. SPL7013 is a fourth generation dendrimer comprising naphthalene disulfonate-terminated polylisine branches attached to a central benzhydrylamine amide group (Figure 3). The outer sulfonate groups are responsible for interacting with viral gp120, which has been shown to inactivate HIV at submicromolar concentrations in vitro [48]. Animal in vivo data on safety and efficacy [49,50] provided support for progressing into human testing. Phase I clinical trials have been conducted and results were generally favorable concerning the safety of Vivagel [51,52]. However, advance into subsequent clinical stages seems to be halted. Other dendrimers are being actively developed, with promising anti-HIV activity being demonstrated in vitro [53]. In particular, carbosilane dendrimers have been proposed by the group of Muñoz-Fernández and tested in vivo. Results obtained using mouse models showed that different carbosilane dendrimers were safe [54,55] and one in particular (G2-S16, a ∼8 nm, second generation, silica core and 16 sulfonate end-groups dendrimer) was able to significantly reduce HIV transmission upon vaginal challenge (84% of animals protected) [56].
Figure 3. . Chemical structure of SPL7013.
Reproduced with permission from [47] © American Chemical Society (2005).
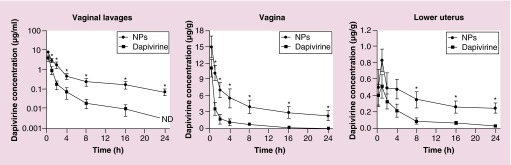
Current nanotechnology approaches for the development of microbicides seem to be shifting toward the delivery of potent antiretroviral drugs rather than the use of nanostructures with inherent activity [42]. Several molecules of various nature have been incorporated into different nanocarriers, in particular polymeric nanoparticles, and tested for their potential as microbicide systems (Table 2). As an example, 150–200 nm carriers based on polycaprolactone have been shown useful in modulating the in vitro activity, toxicity and cell targeting ability of the NNRTI dapivirine [57]. In particular, the formulation steps of nanoparticles were shown critical in achieving systems with suitable colloidal stability [58] and ability to be transported across a simulated vaginal fluid [59]. Furthermore, optimized poly(ethylene oxide)-modified polycaprolactone nanoparticles were shown to be safe and provided favorable pharmacokinetics after vaginal delivery in a mouse model. Higher and prolonged levels of dapivirine at the lower genital tract were observed as compared with the drug in suspension (Figure 4) [46]. These results suggest that the use of nanoparticles may potentially increase the protection window of dapivirine against vaginal HIV transmission.
Table 2. . Selected examples of antiretroviral compounds and nanocarriers used for developing vaginal microbicides.
| Antiretroviral compound(s) | Nanocarriers | Development stage | Ref. |
|---|---|---|---|
| Tenofovir |
Solid lipid nanoparticles |
In vitro |
[60] |
| Tenofovir |
Poly(lactate-co-glycolate)/methacrylic acid copolymer nanoparticles |
In vitro |
[61] |
| Tenofovir |
Chitosan-based nanoparticles |
In vitro |
[62] |
| Dapivirine |
Polycaprolactone nanoparticles |
In vivo |
Reviewed in [63] |
| Dapivirine |
Poly(lactate-co-glycolate) nanoparticles |
In vitro |
[64] |
| Raltegravir and efavirenz |
Poly(lactate-co-glycolate) nanoparticles |
In vitro |
[65] |
| Rilpivirine |
Poly(lactate-co-glycolate) nanoparticles |
In vivo |
[66] |
| MC1220 |
Lecithin/cholesterol-based liposomes |
In vivo |
[67] |
| PSC-RANTES |
Poly(lactate-co-glycolate) nanoparticles |
In vitro |
[68] |
| Maraviroc, zidovudine, tenofovir, etravirine, other compounds with pharmacological activity (e.g., contraceptives, anti-HSV drugs), either alone or in combination | Polymeric nanofibers | In vivo | Reviewed in [69] |
Figure 4. . Dapivirine levels in vaginal lavages and genital tissues (vagina and lower half of the uterine horns) following vaginal administration of dapivirine-loaded poly(ethylene oxide)-modified polycaprolactone nanoparticles or the drug suspension in phosphate-buffered saline (dapivirine).
Note the different scales and units in y-axes, including the log-scale for vaginal lavages. Individual points represent mean values and vertical bars the standard error of the mean (n = 5). *p < 0.05 when comparing nanoparticles with free dapivirine at the same time point.
ND: Not detected; NP: Nanoparticle.
Reproduced with permission from [70] © Springer Science and Business Media (2014).
In another recent work, Kovarova et al. [66] showed the ability of poly(lactate-co-glycolate) nanoparticles loaded with the NNRTI rilpivirine to protect HIV-susceptible BLT mice from vaginal transmission. Nanoparticles (around 60–70 nm) were incorporated in a Pluronic®-based thermosensitive gel in order to facilitate vaginal retention and provide adequate mucosal distribution upon administration as assessed by fluorescent confocal microscopy. Most important, mice administered with the gel 1.5 h prior to vaginal viral challenge were completely protected from infection; however, protection decreased to 50% when the gel was administered 24 h before HIV challenge. Despite these interesting results, no control data were obtained for a gel containing rilpivirine in suspension, thus limiting the interpretation as to the real importance of incorporating the drug into nanoparticles.
Another interesting approach for obtaining new microbicide formulations has been the use of polymeric fibers, namely those presenting cross-sectional diameter in the nanometer range, to incorporate different antiretroviral drugs (Table 2). Developments in microbicide nanofibers have been mostly fueled by the work of Woodrow and collaborators, namely by producing various electrospun systems using materials such as poly(ethylene oxide), poly(lactate-co-glycolate) and poly(vinyl alcohol) [71]. Among other advantages, fibers allow incorporating high amounts of different active molecules, either alone or in combination, while drug release can be easily modulated in order to meet specific needs (i.e., coitally-dependent or -independent microbicides) [72,73]. At the same time, obtention of readily usable vaginal dosage forms upon electrospinning of nanofibers and their manufacturing scale-up seems to be feasible [74].
As documented above, most advances in the field of nanotechnology-based microbicides have so far been made with the purpose of preventing vaginal HIV transmission, while little work has been conducted concerning the rectal route. Apart from increasing interest in using similar principles for developing rectal products, preliminary in vitro and ex vivo data suggest that this approach may be advantageous for protecting both male and female individuals engaged in anal intercourse [75–77].
Conclusion & future perspective
In the coming years, nanomedicine may fundamentally transform approaches toward the treatment and prevention of HIV/AIDS. Although many novel HIV-1 nanotherapeutic strategies have been proposed, only a small minority have reached clinical trials, most of which, aim to improve the safety and efficacy of conventional cART. Presently, there are 28 antiretroviral drugs that are recommended as first-line regimens in antiretroviral therapy. In addition, two more drug regimens are currently being tested for standard care against HIV-1 infection [78]. While conventional cART regimes are known to significantly reduce viral load, their high toxicity, and associated side effects limit the adherence of patients to long-term treatment. In order to tackle this problem, researchers have focused on developing new drug delivery methods that allow for active targeting of therapeutic sites and/or controlled release of antiretrovirals. Several long-acting nanoformulated drugs have demonstrated considerable success in Phase I/II clinical trials. For instance, combination therapy with long-acting nanoformulations of GSK1265744 and TMC278-LA has achieved adequate and sustained drug concentrations in blood. Furthermore, novel preventive strategies that block HIV-1 transmission and have also entered the development pipeline. There are several important considerations with respect to basic and clinical research that should be highlighted regarding the development of these future therapeutic strategies to combat HIV-1 infection. The full potential of nanomedicine toward HIV-1 will likely take some time to be realized as high levels of toxicity, poor scalability and adverse reactions, including: undesirable interactions with plasma proteins in systemic circulation and toxicity through the accumulation of nanomaterials in major end organs have undermined progress [42].
In time, a more detailed understanding of the safety, efficacy and pharmacodynamics of these HIV-1 nanotherapeutics will be assessed. Furthermore, viral reservoir reduction, renal clearance and the side effects of HIV-1 nanotherapeutics on major end organs such as, liver, kidney, heart, lungs and brain, will likely be deciding factors with respect to the continuation of research in this direction. The present review has also mentioned the enthusiasm among patients toward accepting these novel therapies. Promising data indicate that advances in nanomedicine toward HIV-1 may substantially reduce the healthcare costs of patients. It is also important to understand whether these HIV-1 nanotherapeutics can be delivered to patients in the developing world – where HIV is most prevalent – in a cost effective manner. There are many exciting nanomedical applications toward HIV on the horizon that may significantly improve the lives of HIV-1-infected patients [79].
Executive summary.
Challenges associated with the treatment of HIV/AIDS
HIV/AIDS has triggered a devastating global epidemic with over 35 million people currently infected with the virus.
Progress toward curing HIV/AIDS has been limited. Current combination antiretroviral therapy (cART) regimens are expensive, induce adverse side effects and have inconvenient dosing regimens, all of which leads to suboptimal adherence to cART.
Full adherence to cART cannot completely eliminate the virus in infected patients. The virus lays dormant in cellular and anatomical reservoirs and it may come back to blood circulation after cART is stopped due to viral reactivation.
Applications of nanomedicine toward the treatment & prevention of HIV/AIDS
Nanomedicine may significantly change HIV treatment by improving the health outcomes of patients by lowering the cost of cART drugs, decreasing antiretroviral drug exposure and contributing new methods of drug delivery toward elimination of HIV/AIDS.
Nanomedical applications toward HIV that have shown initial success in clinical trials include the controlled and sustained release of cART drugs, vaccine delivery, preventive microbicides and drug delivery.
Controlled release of cART drugs via nanosuspension
Currently, the controlled and sustained release of cART drugs via long acting nanoformulations shows considerable promise in reducing toxicity and improving adherence to cART.
Experimental nanoformulated cART drugs such as long-acting GSK1265744 and TMC278-LA may allow patients to move from daily dosing to weekly or monthly dosing.
Patients have expressed enthusiasm for long-acting nanoformulations of CART drugs.
Nanomedical approaches for improving the delivery of cART drugs
HIV-1 nanotherapeutic drug delivery may dramatically improve the safety and efficacy of cART.
HIV-1 nanotherapeutic drug delivery systems can be used to overcome anatomical and cellular barriers to HIV-1 eradication, such as the blood–brain barrier, and delivery antiretrovirals.
Recently engineered iron oxide nanoparticles were utilized to increases the translocation of enfuvirtide across the blood–brain barrier.
Nanoformulated HIV-1 vaccine
The DermaVir patch is only nano formulated HIV-1 vaccine to reach advance stage clinical trials.
The DermaVir patch as shown to be safe and tolerable in both preclinical and Phase I clinical trial.
Nanomedicine & prevention of HIV-1 sexual transmission
Nanomedicine-based topical microbicides are being developed at the preclinical and clinical level to prevent the sexual transmission of HIV-1.
Vivagel is a vaginally delivered nanomicrobicide that is presently the most advanced product of its kind in the development pipeline.
New microbicide formulations consisting of various antiretrovirals and nanocarriers have also been developed in recent years that have shown success at the preclinical level.
Footnotes
Financial & competing interests disclosure
This work was supported by NIH grants (1R01DA037838-01, 1R01DA027049, 1R21MH101025 & R01DA034547-01), Herbert Wertheim College of Medicine developmental grant, Fundação para a Ciência e a Tecnologia, Portugal (FCT) grant (VIH/SAU/0021/2011), FCT/MEC through National Funds and co-financed by FEDER via the PT2020 Partnership Agreement under the 4293 Unit I&D. José das Neves also acknowledges FCT for financial support (grant SFRH/BPD/92934/2013). The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
- 1.Gallo RC, Montagnier L. The discovery of HIV as the cause of AIDS. N. Engl. J. Med. 2003;349(24):2283–2285. doi: 10.1056/NEJMp038194. [DOI] [PubMed] [Google Scholar]
- 2.Joint United Nations Programme on HIV/AIDS (UNAIDS) Fast-track: ending the AIDS epidemic by 2030. Geneva: UNAIDS. 2014 www.unaids.org/sites/default/files/media_asset/JC2686_WAD2014report_en.pdf [Google Scholar]
- 3.Joint United Nations Programme on HIV/AIDS (UNAIDS) The gap report. Geneva: UNAIDS. 2014 www.unaids.org/sites/default/files/en/media/unaids/contentassets/documents/unaidspublication/2014/UNAIDS_Gap_report_en.pdf [PubMed] [Google Scholar]
- 4.Mamo T, Moseman EA, Kolishetti N, et al. Emerging nanotechnology approaches for HIV/AIDS treatment and prevention. Nanomedicine (Lond.) 2010;5(2):269–285. doi: 10.2217/nnm.10.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schooley RT. Longer-term immunologic effects and side effects of successful antiretroviral therapy. Clin. Infect. Dis. 1999;29(1):12–18. doi: 10.1086/520139. [DOI] [PubMed] [Google Scholar]
- 6.Nolan D, Reiss P, Mallal S. Adverse effects of antiretroviral therapy for HIV infection: a review of selected topics. Expert Opin. Drug Safety. 2005;4(2):201–218. doi: 10.1517/14740338.4.2.201. [DOI] [PubMed] [Google Scholar]
- 7.Lisziewicz J, Toke ER. Nanomedicine applications towards the cure of HIV. Nanomedicine (Lond.) 2013;9(1):28–38. doi: 10.1016/j.nano.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 8.Fletcher CV, Staskus K, Wietgrefe SW, et al. Persistent HIV-1 replication is associated with lower antiretroviral drug concentrations in lymphatic tissues. Proc. Natl Acad. Sci. USA. 2014;111(6):2307–2312. doi: 10.1073/pnas.1318249111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.https://clinicaltrials.gov/ct2/show/NCT02165202 ClinicalTrials Database: NCT02165202.
- 10.https://clinicaltrials.gov/ct2/show/NCT02076178 ClinicalTrials Database: NCT02076178.
- 11.Fiandra L, Colombo M, Mazzucchelli S, et al. Nanoformulation of antiretroviral drugs enhances their penetration across the blood brain barrier in mice. Nanomedicine (Lond.) 2015;11(6):1387–1397. doi: 10.1016/j.nano.2015.03.009. [DOI] [PubMed] [Google Scholar]
- 12.Rodriguez B, Asmuth DM, Matining RM, et al. Safety, tolerability, and immunogenicity of repeated doses of dermavir, a candidate therapeutic HIV vaccine, in HIV-infected patients receiving combination antiretroviral therapy: results of the ACTG 5176 trial. J. Acquir. Immune Defic. Syndr. 2013;64(4):351–359. doi: 10.1097/QAI.0b013e3182a99590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dutta T, Agashe HB, Garg M, Balakrishnan P, Kabra M, Jain NK. Poly (propyleneimine) dendrimer based nanocontainers for targeting of efavirenz to human monocytes/macrophages in vitro . J. Drug Target. 2007;15(1):89–98. doi: 10.1080/10611860600965914. [DOI] [PubMed] [Google Scholar]
- 14.Parboosing R, Maguire GE, Govender P, Kruger HG. Nanotechnology and the treatment of HIV infection. Viruses. 2012;4(4):488–520. doi: 10.3390/v4040488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chiappetta DA, Hocht C, Taira C, Sosnik A. Efavirenz-loaded polymeric micelles for pediatric anti-HIV pharmacotherapy with significantly higher oral bioavailability [corrected] Nanomedicine (Lond.) 2010;5(1):11–23. doi: 10.2217/nnm.09.90. [DOI] [PubMed] [Google Scholar]
- 16.Dubey V, Mishra D, Nahar M, Jain V, Jain NK. Enhanced transdermal delivery of an anti-HIV agent via ethanolic liposomes. Nanomedicine (Lond.) 2010;6(4):590–596. doi: 10.1016/j.nano.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 17.Arunkumar N, Deecaraman M, Rani C. Nanosuspension technology and its applications in drug delivery. Asian J. Pharm. 2009;3(3):168. [Google Scholar]
- 18.https://clinicaltrials.gov/ct2/show/NCT02178800 ClinicalTrials Database: NCT02178800.
- 19.Spreen WR, Margolis DA, Pottage JC., Jr Long-acting injectable antiretrovirals for HIV treatment and prevention. Curr. Opin. HIV AIDS. 2013;8(6):565–571. doi: 10.1097/COH.0000000000000002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baert L, van'T Klooster G, Dries W, et al. Development of a long-acting injectable formulation with nanoparticles of rilpivirine (TMC278) for HIV treatment. Eur. J. Pharm. Biopharm. 2009;72(3):502–508. doi: 10.1016/j.ejpb.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 21.Doblecki-Lewis S, Cohen S, Liu A. Clinical treatment options infectious diseases: update on PrEP implementation, adherence, and advances in delivery. Curr. Treat. Options Infect. Dis. 2015;7(2):101–112. doi: 10.1007/s40506-015-0046-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Verloes R, van't Klooster G, Baert L, et al. 17th International AIDS Conference. Mexico City, Mexico: 3–8 August 2008. TMC278 long acting - a parenteral nanosuspension formulation that provides sustained clinically relevant plasma concentrations in HIV-negative volunteers. [Google Scholar]
- 23.Roy U, Mcmillan J, Alnouti Y, et al. Pharmacodynamic and antiretroviral activities of combination nanoformulated antiretrovirals in HIV-1-infected human peripheral blood lymphocyte-reconstituted mice. J. Infect. Dis. 2012;206(10):1577–1588. doi: 10.1093/infdis/jis395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gautam N, Roy U, Balkundi S, et al. Preclinical pharmacokinetics and tissue distribution of long-acting nanoformulated antiretroviral therapy. Antimicrob. Agents Chemother. 2013;57(7):3110–3120. doi: 10.1128/AAC.00267-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Williams J, Sayles HR, Meza JL, et al. Long-acting parenteral nanoformulated antiretroviral therapy: interest and attitudes of HIV-infected patients. Nanomedicine (Lond.) 2013;8(11):1807–1813. doi: 10.2217/nnm.12.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rajoli RK, Back DJ, Rannard S, et al. Physiologically based pharmacokinetic modelling to inform development of intramuscular long-acting nanoformulations for HIV. Clin. Pharmacokinet. 2015;54(6):639–650. doi: 10.1007/s40262-014-0227-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Truong WR, Schafer JJ, Short WR. Once-daily, single-tablet regimens for the treatment of HIV-1 infection. PT. 2015;40(1):44–55. [PMC free article] [PubMed] [Google Scholar]
- 28.https://clinicaltrials.gov/ct2/show/NCT02120352 ClinicalTrials Database: NCT02120352.
- 29.https://clinicaltrials.gov/ct2/show/NCT01031589 ClinicalTrials Database: NCT01031589.
- 30.https://clinicaltrials.gov/ct2/show/NCT01593046 ClinicalTrials Database: NCT01593046.
- 31.https://clinicaltrials.gov/ct2/show/NCT01565850 ClinicalTrials Database: NCT01565850.
- 32.Sax P, Brar I, Elion R, et al. The 53rd Interscience Conference on Antimicrobial Agents and Chemotherapy. Denver, CO, USA: 10–13 September 2013. 48 week study of tenofovir alafenamide (TAF) vs. tenofovir disoproxil fumarate (TDF), each in a singletablet regimen (STR) with elvitegravir, cobicistat, and emtricitabine [E/C/F/TAF vs. E/C/F/TDF] for initial HIV treatment. Abstract IV-1464d. Presented at. [Google Scholar]
- 33.Liu Y, Kitrinos K, Babusis D, et al. The 53rd Interscience Conference on Antimicrobial Agents and Chemotherapy. Denver, CO, USA: 10–13 September 2013. Lack of tenofovir alafenamide (TAF) effect on primary osteoblasts in vitro at clinically relevant drug concentrations. Abstract H-664. Presented at. [Google Scholar]
- 34.Bristol-Myers Squibb and Gilead Sciences; Foster City, California, USA: 2013. Atripla (efavirenz/emtricitabine/tenofovir disoproxil fumarate) package insert. [Google Scholar]
- 35.Cada DJ, Torres S, Levien TL, Baker DE. Elvitegravir/Cobicistat/Emtricitabine/Tenofovir disoproxil fumarate tablets. Hosp. Pharm. 2013;48(1):48–56. doi: 10.1310/hpj4801-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nathan B, Bayley J, Waters L, Post FA. Cobicistat: a novel pharmacoenhancer for co-formulation with hiv protease and integrase inhibitors. Infect. Dis. Ther. 2013;2(2):111–122. doi: 10.1007/s40121-013-0013-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Juday T, Correll T, Anene A, Broder MS, Ortendahl J, Bentley T. Cost-effectiveness of the once-daily efavirenz/emtricitabine/tenofovir tablet compared with the once-daily elvitegravir/cobicistat/emtricitabine/tenofovir tablet as first-line antiretroviral therapy in HIV-infected adults in the US. Clinicoecon. Outcomes Res. 2013;5:437–445. doi: 10.2147/CEOR.S47486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cohen CJ, Meyers JL, Davis KL. Association between daily antiretroviral pill burden and treatment adherence, hospitalisation risk, and other healthcare utilisation and costs in a US medicaid population with HIV. BMJ Open. 2013;3(8) doi: 10.1136/bmjopen-2013-003028. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gomes MJ, Neves J, Sarmento B. Nanoparticle-based drug delivery to improve the efficacy of antiretroviral therapy in the central nervous system. Int. J. Nanomedicine. 2014;9:1757–1769. doi: 10.2147/IJN.S45886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gray F, Adle-Biassette H, Chretien F, Lorin de la Grandmaison G, Force G, Keohane C. Neuropathology and neurodegeneration in human immunodeficiency virus infection. Pathogenesis of HIV-induced lesions of the brain, correlations with HIV-associated disorders and modifications according to treatments. Clin. Neuropathol. 2001;20(4):146–55. [PubMed] [Google Scholar]
- 41.Sagar V, Pilakka-Kanthikeel S, Pottathil R, Saxena SK, Nair M. Towards nanomedicines for neuroAIDS. Rev. Med. Virol. 2014;24(2):103–124. doi: 10.1002/rmv.1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.das Neves J, Amiji MM, Bahia MF, Sarmento B. Nanotechnology-based systems for the treatment and prevention of HIV/AIDS. Adv. Drug Deliv. Rev. 2010;62(4–5):458–477. doi: 10.1016/j.addr.2009.11.017. [DOI] [PubMed] [Google Scholar]
- 43.Dash PK, Gendelman HE, Roy U, et al. Long-acting nanoformulated antiretroviral therapy elicits potent antiretroviral and neuroprotective responses in HIV-1-infected humanized mice. AIDS. 2012;26(17):2135–2144. doi: 10.1097/QAD.0b013e328357f5ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Narayanasamy P, Switzer BL, Britigan BE. Prolonged-acting, multi-targeting gallium nanoparticles potently inhibit growth of both HIV and mycobacteria in co-infected human macrophages. Sci. Rep. 2015;5:8824. doi: 10.1038/srep08824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Haynes BF. New approaches to HIV vaccine development. Curr. Opin. Immunol. 2015;35:39–47. doi: 10.1016/j.coi.2015.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rohan LC, Devlin B, Yang H. Microbicide dosage forms. Curr. Top. Microbiol. Immunol. 2014;383:27–54. doi: 10.1007/82_2013_357. [DOI] [PubMed] [Google Scholar]
- 47.Mccarthy TD, Karellas P, Henderson SA, et al. Dendrimers as drugs: discovery and preclinical and clinical development of dendrimer-based microbicides for HIV and STI prevention. Mol. Pharm. 2005;2(4):312–318. doi: 10.1021/mp050023q. [DOI] [PubMed] [Google Scholar]
- 48.Tyssen D, Henderson SA, Johnson A, et al. Structure activity relationship of dendrimer microbicides with dual action antiviral activity. PLoS ONE. 2010;5(8):e12309. doi: 10.1371/journal.pone.0012309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jiang YH, Emau P, Cairns JS, et al. SPL7013 gel as a topical microbicide for prevention of vaginal transmission of SHIV89.6P in macaques. AIDS Res. Hum. Retroviruses. 2005;21(3):207–213. doi: 10.1089/aid.2005.21.207. [DOI] [PubMed] [Google Scholar]
- 50.Patton DL, Cosgrove Sweeney YT, Mccarthy TD, Hillier SL. Preclinical safety and efficacy assessments of dendrimer-based (SPL7013) microbicide gel formulations in a nonhuman primate model. Antimicrob. Agents Chemother. 2006;50(5):1696–1700. doi: 10.1128/AAC.50.5.1696-1700.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mcgowan I, Gomez K, Bruder K, et al. Phase 1 randomized trial of the vaginal safety and acceptability of SPL7013 gel (VivaGel) in sexually active young women (MTN-004) AIDS. 2011;25(8):1057–1064. doi: 10.1097/QAD.0b013e328346bd3e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Moscicki AB, Kaul R, Ma Y, et al. Measurement of mucosal biomarkers in a Phase 1 trial of intravaginal 3% StarPharma LTD 7013 gel (VivaGel) to assess expanded safety. J. Acquir. Immune Defic. Syndr. 2012;59(2):134–140. doi: 10.1097/QAI.0b013e31823f2aeb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jiménez JL, Pion M, Mata FJDL, et al. Dendrimers as topical microbicides with activity against HIV. N. J. Chem. 2012;36(2):299–309. [Google Scholar]
- 54.Sepulveda-Crespo D, Gomez R, De La Mata FJ, Jimenez JL, Munoz-Fernandez MA. Polyanionic carbosilane dendrimer-conjugated antiviral drugs as efficient microbicides: recent trends and developments in HIV treatment/therapy. Nanomedicine (Lond.) 2015;11(6):1481–1498. doi: 10.1016/j.nano.2015.03.008. [DOI] [PubMed] [Google Scholar]
- 55.Sepulveda-Crespo D, Sanchez-Rodriguez J, Serramia MJ, et al. Triple combination of carbosilane dendrimers, tenofovir and maraviroc as potential microbicide to prevent HIV-1 sexual transmission. Nanomedicine (Lond.) 2015;10(6):899–914. doi: 10.2217/nnm.14.79. [DOI] [PubMed] [Google Scholar]
- 56.Sepúlveda-Crespo D, Serramía MJ, Tager AM, et al. Prevention vaginally of HIV-1 transmission in humanized BLT mice and mode of antiviral action of polyanionic carbosilane dendrimer G2-S16. Nanomedicine (Lond.) 2015;11(6):1299–308. doi: 10.1016/j.nano.2015.04.013. [DOI] [PubMed] [Google Scholar]
- 57.Das Neves J, Michiels J, Ariën KK, et al. Polymeric nanoparticles affect the intracellular delivery, antiretroviral activity and cytotoxicity of the microbicide drug candidate dapivirine. Pharm. Res. 2012;29(6):1468–1484. doi: 10.1007/s11095-011-0622-3. [DOI] [PubMed] [Google Scholar]
- 58.das Neves J, Amiji M, Bahia MF, Sarmento B. Assessing the physical-chemical properties and stability of dapivirine-loaded polymeric nanoparticles. Int. J. Pharm. 2013;456(2):307–314. doi: 10.1016/j.ijpharm.2013.08.049. [DOI] [PubMed] [Google Scholar]
- 59.das Neves J, Rocha CM, Goncalves MP, et al. Interactions of microbicide nanoparticles with a simulated vaginal fluid. Mol. Pharm. 2012;9(11):3347–3356. doi: 10.1021/mp300408m. [DOI] [PubMed] [Google Scholar]
- 60.Alukda D, Sturgis T, Youan BB. Formulation of tenofovir-loaded functionalized solid lipid nanoparticles intended for HIV prevention. J. Pharm. Sci. 2011;100(8):3345–3356. doi: 10.1002/jps.22529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang T, Sturgis TF, Youan BB. pH-responsive nanoparticles releasing tenofovir intended for the prevention of HIV transmission. Eur. J. Pharm. Biopharm. 2011;79(3):526–536. doi: 10.1016/j.ejpb.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Meng J, Zhang T, Agrahari V, Ezoulin MJ, Youan BB. Comparative biophysical properties of tenofovir-loaded, thiolated and nonthiolated chitosan nanoparticles intended for HIV prevention. Nanomedicine (Lond.) 2014;9(11):1595–1612. doi: 10.2217/nnm.13.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.das Neves J, Nunes R, Machado A, Sarmento B. Polymer-based nanocarriers for vaginal drug delivery. Adv. Drug Deliv. Rev. 2014 doi: 10.1016/j.addr.2014.12.004. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 64.das Neves J, Sarmento B. Precise engineering of dapivirine-loaded nanoparticles for the development of anti-HIV vaginal microbicides. Acta Biomater. 2015;18:77–87. doi: 10.1016/j.actbio.2015.02.007. [DOI] [PubMed] [Google Scholar]
- 65.Date AA, Shibata A, Goede M, et al. Development and evaluation of a thermosensitive vaginal gel containing raltegravir+efavirenz loaded nanoparticles for HIV prophylaxis. Antiviral. Res. 2012;96(3):430–436. doi: 10.1016/j.antiviral.2012.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kovarova M, Council OD, Date AA, et al. Nanoformulations of rilpivirine for topical pericoital and systemic coitus-independent administration efficiently prevent HIV transmission. PLoS Pathog. 2015;11(8):e1005075. doi: 10.1371/journal.ppat.1005075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Caron M, Besson G, Etenna SL, et al. Protective properties of non-nucleoside reverse transcriptase inhibitor (MC1220) incorporated into liposome against intravaginal challenge of Rhesus macaques with RT-SHIV. Virology. 2010;405(1):225–233. doi: 10.1016/j.virol.2010.06.008. [DOI] [PubMed] [Google Scholar]
- 68.Ham AS, Cost MR, Sassi AB, Dezzutti CS, Rohan LC. Targeted delivery of PSC-RANTES for HIV-1 prevention using biodegradable nanoparticles. Pharm. Res. 2009;26(3):502–511. doi: 10.1007/s11095-008-9765-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ball C, Woodrow KA. Electrospun fibers for microbicide drug delivery. In: das Neves J, Sarmento B, editors. Drug Delivery and Development of Anti-HIV Microbicides. Pan Stanford; Singapore: 2014. pp. 459–507. [Google Scholar]
- 70.das Neves J, Araújo F, Andrade F, Amiji M, Bahia MF, Sarmento B. Biodistribution and pharmacokinetics of dapivirine-loaded nanoparticles after vaginal delivery in mice. Pharm. Res. 2014;31(7):1834–1845. doi: 10.1007/s11095-013-1287-x. [DOI] [PubMed] [Google Scholar]
- 71.Blakney AK, Ball C, Krogstad EA, Woodrow KA. Electrospun fibers for vaginal anti-HIV drug delivery. Antiviral. Res. 2013;100(Suppl.):S9–S16. doi: 10.1016/j.antiviral.2013.09.022. [DOI] [PubMed] [Google Scholar]
- 72.Ball C, Krogstad E, Chaowanachan T, Woodrow KA. Drug-eluting fibers for HIV-1 inhibition and contraception. PLoS ONE. 2012;7(11):e49792. doi: 10.1371/journal.pone.0049792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ball C, Woodrow KA. Electrospun solid dispersions of Maraviroc for rapid intravaginal preexposure prophylaxis of HIV. Antimicrob. Agents Chemother. 2014;58(8):4855–4865. doi: 10.1128/AAC.02564-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Krogstad EA, Woodrow KA. Manufacturing scale-up of electrospun poly(vinyl alcohol) fibers containing tenofovir for vaginal drug delivery. Int. J. Pharm. 2014;475(1–2):282–291. doi: 10.1016/j.ijpharm.2014.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nunes R, Sarmento B, das Neves J. Formulation and delivery of anti-HIV rectal microbicides: advances and challenges. J. Control. Release. 2014;194:278–294. doi: 10.1016/j.jconrel.2014.09.013. [DOI] [PubMed] [Google Scholar]
- 76.Sarmento B, das Neves J. Nanosystem formulations for rectal microbicides: a call for more research. Ther. Deliv. 2012;3(1):1–4. doi: 10.4155/tde.11.139. [DOI] [PubMed] [Google Scholar]
- 77.das Neves J, Araújo F, Andrade F, et al. In vitro and ex vivo evaluation of polymeric nanoparticles for vaginal and rectal delivery of the anti-HIV drug dapivirine. Mol. Pharm. 2013;10(7):2793–2807. doi: 10.1021/mp4002365. [DOI] [PubMed] [Google Scholar]
- 78.Parboosing R, Maguire GE, Govender P, Kruger HG. Nanotechnology and the treatment of HIV infection. Viruses. 2012;4(4):488–520. doi: 10.3390/v4040488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gulick R. HIV treatment 2020: what will it look like? J. Int. AIDS Soc. 2014;17(4 Suppl. 3):19528. doi: 10.7448/IAS.17.4.19528. [DOI] [PMC free article] [PubMed] [Google Scholar]