Abstract
Background:
This study explores the use of hydrophilic poly(ethylene glycol)-conjugated poly(lactic-co-glycolic acid) nanoparticles (PLGA-PEG-NPs) as delivery system to improve the antitumor effect of antiobesity drug orlistat for triple-negative breast cancer (TNBC) therapy by improving its bioavailability.
Materials & methods:
PLGA-PEG-NPs were synthesized by emulsion-diffusion-evaporation method, and the experiments were conducted in vitro in MDA-MB-231 and SKBr3 TNBC and normal breast fibroblast cells.
Results:
Delivery of orlistat via PLGA-PEG-NPs reduced its IC50 compared with free orlistat. Combined treatment of orlistat-loaded NPs and doxorubicin or antisense-miR-21-loaded NPs significantly enhanced apoptotic effect compared with independent doxorubicin, anti-miR-21-loaded NPs, orlistat-loaded NPs or free orlistat treatments.
Conclusion:
We demonstrate that orlistat in combination with antisense-miR-21 or current chemotherapy holds great promise as a novel and versatile treatment agent for TNBC.
Keywords: : anti-miR, cancer therapy, orlistat, PLGA nanoparticles, TNBC
Systemically injected chemotherapy drugs have several drawbacks, including premature drug degradation and clearance, poor bioavailability, low water solubility and cytotoxic effects on healthy cells [1]. Thus, it is critical to develop strategies for delivering drugs specifically to the tumor site, in order to maintain drug efficacy and avoid adverse side effects. Several studies have explored the use of nanoparticles (NPs) for this purpose [2,3]. Biodegradable polymeric NPs address many of the aforementioned issues, as they provide a medium for sustained drug release, have reduced toxicity (since they are metabolized by the body), tend to gather in tumor tissues by the enhanced permeability and retention effect, are modifiable with targeting moieties to increase site-specific delivery and can carry a wide range of chemically diverse drugs [4,5]. In the past few years, the area of nanomedicine has shown significant progress, and various NP drug delivery systems are already in different stages of clinical development [6].
Poly(ethylene glycol)-conjugated poly(lactic-co-glycolic acid) (PLGA-PEG) NPs are particularly effective drug nanocarriers. PLGA is a US FDA-approved biocompatible polymer that gets hydrolyzed and broken down into nontoxic lactic acid and glycolic acid monomers, which can then be metabolized by the body [4]. On its own, due to its hydrophobicity, PLGA may often be targeted by macrophages that are part of the reticuloendothelial system, but with the attachment of the hydrophilic PEG, it largely escapes immune detection [5].
Breast cancer is the leading cause of cancer-associated deaths in women around the world. It constitutes nearly a quarter of all female cancers, and affected over 1.7 million women in 2012 [6]. The heterogeneity of breast cancers makes diagnosis and treatment difficult, but these challenges are even more acute in triple-negative breast cancer (TNBC), which is negative for the expression of all three receptors, estrogen, progesterone and HER2/neu, and accounts for approximately 15% of all breast cancers [7].
Current neoadjuvant and adjuvant treatment options for TNBC include various cytotoxic drug combination therapies consisting of cyclophosphamide, anthracyclines (daunorubicin, doxorubicin), taxanes (paclitaxel, docetaxel), and platinums (cisplatin, carboplatin), all of which induce cell death through various mechanisms [8,9]. Unfortunately, many of these drugs also cause serious cardio-, gastro-, neuro- and nephrotoxicity, and while TNBC tumors are initially sensitive to these treatments, often they develop resistance. The aggressive nature of TNBC, combined with lack of treatment choices and the high prevalence of the disease, especially among younger women [10], means that the search continues for more targeted and effective treatments.
Drug development is a time-consuming, cost-intensive high-effort process; often, even a very promising drug candidate does not ultimately result in a therapy approved for clinical use. Because of this, drug repurposing or the identification of new applications for an existing drug, is a worthwhile venture, since US FDA-approved drugs have already passed through preliminary safety reviews. Orlistat (Xenical), also known as tetrahydrolipstatin, is an FDA-approved drug used for bodyweight reduction in diabetic patients, and is now available over-the-counter as a weight-loss drug [11]. However, several studies have also demonstrated its antitumor effect [12]. Orlistat inhibits the lipogenic activity of fatty acid synthase (FAS), which is an oncogenic antigen-519 that is upregulated in >50% of breast cancers and is associated with poor prognosis. Even though the signal transduction pathways regulating FAS expression in normal and cancer cells share several downstream pathways, the upstream mechanism controlling FAS expression in cancer cells is different from that in normal tissues; hence tumor-associated FAS expression is insensitive to nutritional signals. This process makes it more tumor specific, sparing liver and adipose tissues, which are normally involved in fatty acid synthesis [13]. Chemoresistance is a major obstacle in the treatment of cancer, particularly TNBC, and occurs when a cell alters the expression of regulatory proteins that are involved in the pathway targeted by a chemotherapeutic agent [14]. This, in turn, interferes with drug action and inhibits the procession of the apoptotic pathway. A valuable strategy for dealing with this problem is chemosensitization, or the use of a pretreatment strategy that sensitizes tumor cells to chemotherapy.
miRNAs (miRs) are a class of small, non-coding endogenous RNAs ranging in size from 18 to 24 bp that are involved in post-transcriptional regulation of gene expression as part of the RNAi pathway. They function by binding to miRs, and depending on the degree of complementarity, either induce miR cleavage or repress translation. Dysregulation of miRs has been implicated in various cancers. Antagomirs or anti-miRs or antisense-miRs are small chemically modified antisense oligonucleotides that can block endogenous miRs activity by irreversibly binding to the target miR. Specific miRs can be silenced by anti-miRs complementary to the mature miR sequences. miR-21, a miR with antiapoptotic activity and other oncogenic properties, has been found to be significantly upregulated in TNBC, and are associated with tumor proliferation and drug resistance. Anti-miR-21 has been reported to impair tumor cell growth, induce apoptosis and reduce the migration and invasion of cancer cells expressing miR-21 at high levels. In our previous studies, which tested the effect of PLGA-b-PEG NPs coloaded with anti-miR-21 and anti-miR-10b on TNBC cell lines, we reported significant apoptotic induction and reduction in cell proliferation in vitro, as well as substantial tumor growth reduction and metastatic inhibition in mice [15].
In this study, we utilized PLGA-PEG NPs as drug nanocarriers for efficient delivery of the hydrophobic antiobesity drug orlistat as an anticancer agent. We evaluated the antiproliferative and apoptotic action of the developed drug-loaded NPs in vitro on MDA-MB-231 (triple-negative), SKBr3 (HER2 positive, ER [estrogen receptor] and PR [progesterone receptor] negative) and MCF10A (noncancerous) cell lines, and showed that the delivery of orlistat via PLGA-PEG NPs significantly increases its bioavailability. In addition, given the drug-resistant properties of TNBC, we tested the use of the drug in combination with codelivered chemosensitizer antisense miR-21. In a parallel vein, we explored the potential of orlistat use as a chemosensitizer itself, and used it in combination with doxorubicin to evaluate whether combined treatment with orlistat-loaded NPs enhanced the therapeutic effect of this chemotherapeutic agent. Finally, in order to decode its mechanism of action, we tested the effect of this drug on PARP and caspase proteins levels and cleavage.
Materials & methods
Materials
All chemical reagents used in this study were of analytical grade or above. NH2-PEG-COOH (MW 3400) was purchased from JenKem Technology (TX, USA). Acid terminated Poly(D,L-lactide-co-glycolide) (50/50) (PLGA, inherent viscosity 0.16–0.24 dL/g, MW 7000–17000), N-hydroxysuccinimide (NHS), 1-ethyl-3-(3 [dimethylamino]propyl)carbodiimide (EDC) and diisopropylethylamine (DIPEA), MTT (Thiazolyl Blue Tetrazolium Bromide) reagent were obtained from Sigma-Aldrich (MO, USA). Orlistat ([−]-tetrahydrolipstatin) was purchased from Sigma (MO, USA). Antisense-miR-21 were custom synthesized by PAN Facility at Stanford, at purity above 90%. MDA-MB-231, SKBr3 and MCF10A cells were purchased from ATCC (MO, USA). Cell culture medium, culture flasks, fetal bovine serum (FBS), penicillin, streptomycin, other supplements and acrylamide gel were from Invitrogen (CA, USA).
Methods
Synthesis, preparation, characterization & optimization
Synthesis of PLGA-b-PEG copolymer
To a solution of PLGA-COOH (250 mg, 0.02 mmol) in dry dichloromethane (CH2Cl2), EDC (40 mg, 0.2 mmol) in dry CH2Cl2 was added and then NHS (24 mg, 0.2 mmol) in dry CH2Cl2 was added and stirred at room temperature (RT) for 4 h. The resulted PLGA-NHS was precipitated using mixture of ice-cold methanol (MeOH)/diethylether (Et2O) (1:1). The precipitate was centrifuged at 5000 rpm for 5 min, the supernatant was decanted and the pellet was further washed twice with ice-cold MeOH/Et2O (1:1). The pellet was dried under vacuum for 2 h. The PLGA-NHS pellet was dissolved in dry chloroform and treated with heterobifunctional NH2-PEG-COOH (70 mg, 0.02 mmol) followed by addition of diisopropylethylamine (36 µl, 0.2 mmol). The reaction mixture was stirred at RT for 24 h. The resulting PLGA-b-PEG copolymer was precipitated by addition of cold MeOH/Et2O (1:1), washed twice with cold MeOH/Et2O (1:1), dried under vacuum and characterized by 1H-NMR (yield: 79%).
Synthesis & characterization of orlistat-loaded PLGA-PEG-block copolymer nanoparticles by emulsion-diffusion-evaporation method
A premixed solution of PLGA-b-PEG (10 mg) and orlistat (1 mg) in ethyl acetate was added drop wise to 2% poly vinyl alcohol (PVA) (w/v) with slight stirring. The resulting mixture was sonicated at 60% amplitude for 1 min at 0°C using sonic dismembrator to yield the first emulsion. The first emulsion was diffused by addition of ultrapure water (5 ml) and stirred at RT for 4 h to evaporate the ethyl acetate and harden the NPs. The resulting NPs were filtered using 0.45 µM syringe filter (Puradisc 25 AS, PES membrane, Whatman), and washed using centrifugal filters (100 kDA MWCO, EMD-Millipore, MA, USA). The concentrated NPs were further washed with ultrapure water three-times to remove nonencapsulated orlistat. The NPs were diluted to known volume, lyophilized with 10% sucrose and the resulting NPs powder was stored at -20°C. The prepared NPs size was evaluated using dynamic light scattering and transmission electron microscopy (TEM). The amount of orlistat present in NPs was calculated using HPLC.
In vitro drug release study
In vitro drug release study of orlistat-loaded PLGA-b-PEG NPs was conducted at pH 7.0 and pH 5.5 conditions in a dialysis process. Orlistat-loaded NP (1 ml) was loaded in dialysis cassettes (Slide-A-Lyzer™ Cassettes, mw cut-off 3.5 kilo Dalton), and the dialysis cassettes were allowed to float in a beaker containing 200 ml of ultrapure water (pH 7.0) and acidified water (pH 5.5), then the dialysis was performed at 37°C in an incubator. At each time point, 40 ml of samples was removed from the outside solution and was substituted with same volume of fresh water of corresponding pH. After each time points, the collected released orlistat samples were concentrated by lyophilization. Then all the freeze-dried samples were redissolved in methanol and were analyzed using UV absorbance at 205 nm to determine orlistat concentration in the released fraction at each condition.
Transmission electron microscopy
Transmission electron microscopy images were obtained using an FEI TITAN 80–300 kV ETEM (environmental transmission electron microscope) at Stanford's Nanocharacterization Laboratory by operating at 80 kV using negatively stained NPs. In brief, for negative staining, approximately 5 µl of PLGA-b-PEG NPs was mixed with equal volume of 1% phosphotungstic acid (1% PTA in water, pH 7.5). The solution was incubated for 3 min at RT before being plated on a carbon film-coated copper grid. The grid was left for 3 min at RT, excess solution was drained off, the sample was air dried and observed under TEM. Size analysis was performed using IMAGEJ software.
Cell culture & treatment conditions
In vitro cytotoxic effect of free orlistat & orlistat-loaded nanoparticles in MDA-MB-231, SKBr3 & MCF10A cells
MCF10a cells were cultured in MEBM (mammary epithelial basal medium) medium, while SKBr3 and MDA-MB-231 cells were cultured in DMEM high-glucose medium supplemented with 10% FBS and 1% penicillin and streptomycin. The cells were maintained at 37°C with 5% CO2 in a humid atmosphere. All three cell lines were plated in 96-well plates with 5000 cells/well in the corresponding media. The next day, cells were treated with various concentrations of free orlistat and orlistat-loaded NP, ranging from 0.625 to 20 μM orlistat-equivalent, and incubated at 37°C with 5% CO2, in 2% FBS containing media. After appropriate incubation time (24, 48 and 72 h), the media was carefully removed and 50 μl of phenol red free DMEM with 2% FBS containing 0.5 mg/ml of MTT reagent (Sigma, MO, USA) was added. The cells were incubated further for 2 h and the violet formazan crystals formed in the cells were dissolved in 100 μl of DMSO by incubating at 37°C for 30 min in dark. The soluble MTT-formazan derivative absorption was optically measured by TECAN-Safire UV-Vis spectrophotometer at 540 nm. The relative cell viability (%) compared with control cells was calculated as follows:
 |
In vitro combined treatment of MDA-MB-231 cells with doxorubicin & orlistat-loaded nanoparticles
MDA-MB-231 cells were plated in 6-well plates at 1.5 × 105 cells/well in regular DMEM with 10% FBS. Cells were treated with varying concentrations (ranging from 0 to 3 μg/ml) of doxorubicin alone, doxorubicin in combination with 1.25 μM free orlistat or doxorubicin in combination with 1.25 μM orlistat-equivalent of orlistat-loaded PLGA-PEG NPs. After 48 h of incubation at 37°C with 5% CO2, both dead and live cells were trypsinized, collected and fixed in 70% ice cold ethanol. The samples were washed once in PBS and stained with 0.5 ml of PBS (phosphate-buffered saline) containing 2.5 μg/ml propidium iodide, 100 μg/ml RNAse A, and 0.1% TritonX-100. After 15 min of incubation at RT in the dark, cells were subjected to FACS analysis (BD FACS-Aria™ III), and generated data was analyzed by FlowJo 8.8.6 software for the quantification of live and dead cells.
In vitro combined treatment of MDA-MB-231 cells with anti-miR-21-loaded nanoparticles & orlistat-loaded nanoparticles
MDA-MB-231 cells were plated in 24-well plates with 7.5 × 104 cells/well in regular DMEM with 10% FBS. Cells were pretreated with either 10 pmol anti-miR-21-loaded NPs or 10 pmol control NPs. The next day, each set was treated with varying concentrations ranging from 0.625 to 20 μM of either free orlistat or orlistat-loaded NPs in DMEM with 2% FBS. After 48 h of incubation at 37°C with 5% CO2, the media was carefully removed and 200 μl of phenol red free DMEM with 2% FBS containing 0.5 mg/ml of MTT reagent was added. The cells were incubated further for 2 h and the violet formazan crystals formed in the cells were dissolved in 200 μl of DMSO by incubating at 37°C for 30 min in dark. The soluble MTT-formazan derivative was optically measured for its absorption by TECAN-Safire UV-Vis spectrophotometer at 540 nm. The relative cell viability (%) compared with control cells was calculated as follows:
 |
Immunoblot analysis for Caspase-3, Bax, Bcl2, p53 & p21 levels to elucidate the mechanism of apoptotic induction by orlistat & anti-miR-21 delivered by PLGA-PEG nanoparticles in MDA-MB-231 cells
MDA-MB-231 cells treated with different concentrations of orlistat-loaded PLGA-PEG NPs (1.25, 2.5 and 10 μM orlistat equivalent) in the presence or absence of 10 pmol anti-miR-21 equivalent of PLGA-PEG NPs were grown for 24 h and assessed for the expression of Caspase-3, Bax, Bcl2, p53, p21 and tubulin levels by immunoblot analysis. Cells treated with PLGA-PEG NP without orlistat and with 10 pmol anti-miR-21 served as controls. To obtain total cell lysate, after treatment, cells were collected and resuspended in 100 μl of lysis buffer (50 mM Tris, pH 7.5, 150 mM NaCl, 1% Nonidet P-40, 5 mM EDTA, protease inhibitors [Roche, CA, USA]) and lysed by keeping on ice for 30 min with intermittent mixing. 30 μg of total protein from the cell lysates were resolved in 4–12% gradient polyacrylamide gel by electrophoresis, and electroblotted onto a nitrocellulose membrane (Schleicher & Schuell, NH, USA) of 0.2 µm pore size, with subsequent exposure of the membrane to rabbit anti-Caspase-3 antibody (1:1000 dilution, Cell Signaling, MA, USA), rabbit anti-p53 antibody (1:1000 dilution, Cell Signaling), rabbit anti-p21 antibody (1:1000 dilution, Abcam MA), rabbit anti-Bcl2 antibody (1:2000 dilution, Cell Signaling) and rabbit anti-Bax antibody (1:2000 dilution, Cell Signaling) for 12 h in TBS (tris-buffered saline)/0.05% Tween 20 and 3% dry milk. The membrane was washed and probed with anti-rabbit or anti-mouse HRP-conjugated antibody (1:10,000 dilution, Sigma-Aldrich). The signal was detected using ECL system (Thermo Scientific, CA, USA). The membrane was stripped and probed for antitubulin (1:1000 dilution, Sigma-Aldrich, MI, USA) as loading control.
Results & discussion
Loading orlistat in PLGA-PEG-NP
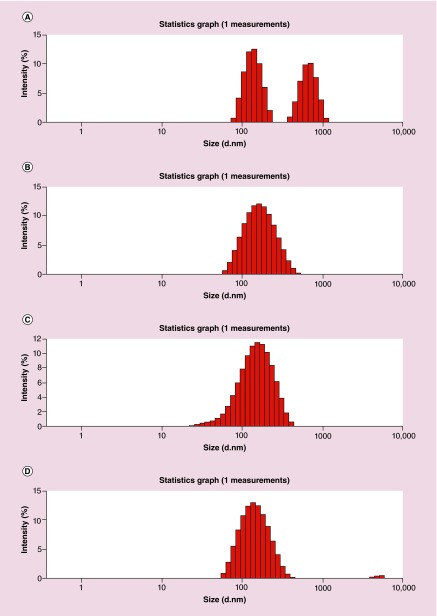
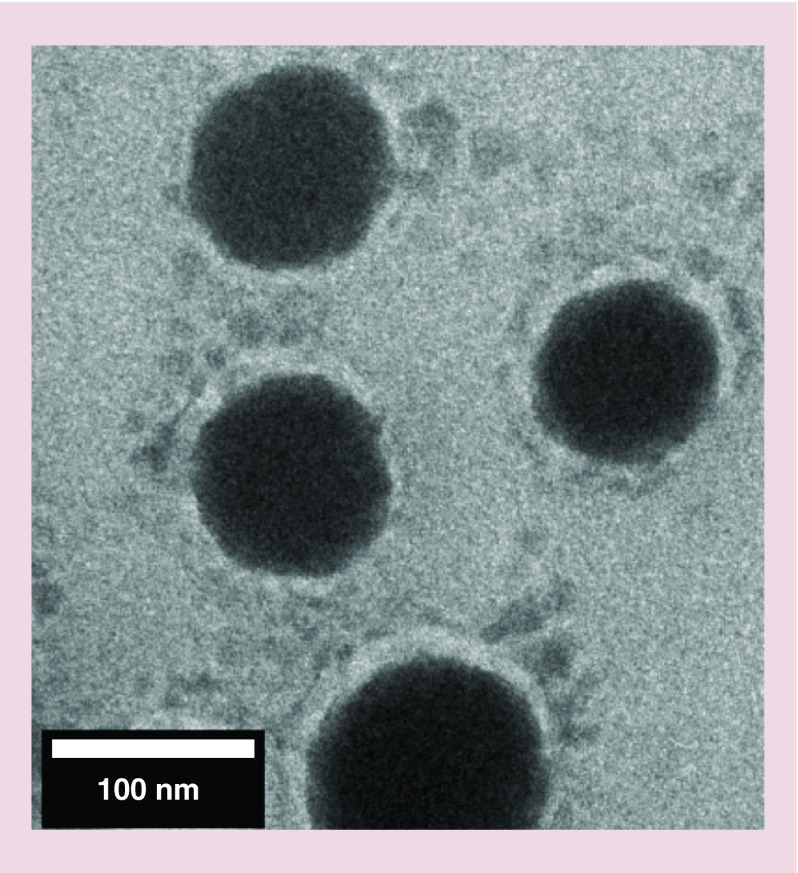
PLGA-b-PEG copolymer was synthesized from the conjugation of acid terminated PLGA-COOH and heterobifunctional amino-PEG-carboxylic acid (H2N-PEG-CO2H) as shown previously by us [15,16]. Orlistat is practically water insoluble, so we formulated PLGA-b-PEG NPs loaded with orlistat by using an emulsion-diffusion-evaporation (EDE) method with polyvinyl alcohol (PVA) as an emulsifier to stabilize the NPs. However, since no previous study has attempted to load orlistat in PLGA-PEG, we also tested other methods such as nanoprecipitation with different concentration of PVA (1 and 2%), and Tween 80 (1%) in combination with Span 80 (3%) as a mixture for NP stabilization. Dynamic light scattering of the NPs prepared by different methods showed hydrodynamic size ranges from 130 to 360 nm before filtration, while the PDI was higher for the particles prepared by nanoprecipitation compared with EDE method (Table 1). Significant percentage of self-aggregated orlistat of average particle sizes of around 400 nm was found in NPs prepared by nanoprecipitation method (Figure 1). Hence, we used the particles prepared by optimized EDE method that did not result in any orlistat self-aggregation, for further evaluation of orlistat encapsulation and loading efficiency using HPLC. The results showed orlistat encapsulation efficiency of 72 ± 4% with the loading efficiency of 7.1% (Table 1). We used PLGA-PEG-loaded anti-miR-21 prepared by water-in-oil-in-water (w/o/w) double emulsion method optimized by our previous work for the study [15,16]. Transmission electron micrograph of orlistat-loaded PLGA-PEG-NPs showed clearly dispersed particles of around 150 nm (Figure 2).
Table 1. . Optimization studies for orlistat encapsulation in PLGA-b-PEG nanoparticles.
| Entry | Sample ID | Formulation | Mean size (nm)† before and after filtration through 0.45 µm filter | PDI‡ before and after filtration through 0.45 µm filter | ζ potential before and after filtration through 0.45 µm filter | Orlistat EE and loading efficiency (%) | Orlistat (concentration)§ |
|---|---|---|---|---|---|---|---|
| 1 |
NP-231 |
Nanoprecipitation 2% PVA (2 ml) DCM (1 ml) |
352.5 (149.4) |
0.466 (0.169) |
-32.7 (1.8) |
ND§ |
ND§ |
| 2 |
NP-232 |
Nanoprecipitation 1% PVA (5 ml) DCM (1 ml) |
272.7 (159.3) |
0.496 (0.170) |
-26.0 (-16.0) |
ND§ |
ND§ |
| 3 |
NP-233 |
Emulsion-diffusion-evaporation 2% PVA (2 ml) EA (1 ml) Water (5 ml) |
138.9 (138.3) |
0.201 (0.181) |
-20.2 (-25.3) |
72.1 (7.21)¶ |
240.3 µg/ml¶ |
| 4 | NP-234 | Nanoprecipitation 1% Tween 80 (5 ml) 3% Span 80 (1 ml) |
248.7 (172.8) | 0.524 (0.428) | -12.2 (-29.4) | ND§ | ND§ |
†Average of three dynamic light scattering experiments.
‡PDI (average of three dynamic light scattering readings).
§Not determined.
¶Determined by HPLC.
DCM: Dichloromethane; EA: Ethyl acetate; EE: Encapsulation efficiency; ND: Not determined; PDI: Polydispersity index; PLGA: Poly(lactic-co-glycolic acid); PVA: Polyvinyl alcohol.
Figure 1. . Dynamic light scattering images of orlistat-loaded PLGA-b-PEG nanoparticles.
(A) Mixture of PLGA-b-PEG nanopartices and self-aggregated orlistat particles observed in nanoparticles prepared by nanoprecipitation method. (B) PLGA-b-PEG NPs (A) after removal of orlistat self-aggregates through 0.45 μm filter. (C) Orlistat-loaded PLGA-b-PEG nanoparticles prepared using emulsion–diffusion–evaporation method. (D) PLGA-b-PEG nanoparticles in (C) after filtration through 0.45 μm filter.
PLGA: Poly(lactic-co-glycolic acid).
Figure 2. . Transmission electron microscopic image of orlistat-loaded PLGA-b-PEG nanoparticles, which are stained with 1% phosphotungstic acid.
PLGA: Poly(lactic-co-glycolic acid).
In vitro drug release studies
We have conducted the in vitro drug release studies of orlistat-loaded NPs (Supplementary Figure 1). These NPs showed initial burst release effect with 32 ± 2% and 36 ± 2.5% orlistat release after 24 h at pH 7.0 and pH 5.5, respectively. Afterward, orlistat release was in a sustained manner with the release of 17 ± 3% in every 24 h at both pH. We observed 68 ± 3% to 77 ± 4% of orlistat released after 72 h, and almost 90% of the orlistat was released in 96 h. There was no significant variation observed between pH 5.5 and pH 7.0.
Orlistat as a repurposing anticancer drug for triple negative breast cancer therapy
The antiobesity drug orlistat works by inhibiting gastric and pancreatic lipases that normally cleave triglycerides in the intestine [17]. Having been tested in several clinical trials, it is now available in several countries over-the-counter as a weight-loss drug, and is usually prescribed at doses of 60 or 120 mg. Recently, it was found that orlistat also inhibits the function of fatty acid synthase, a multienzyme protein involved in fatty acid synthesis that is overexpressed in many cancers [18]. For this reason, several studies have explored its antitumorigenic effect on prostate, endometrial, breast, ovarian and lung cancers [19–23]. Previously, we showed that orlistat has a concentration-dependent cytotoxic effect on TNBC cells [24]. However, as a hydrophobic drug, orlistat has a very low bioavailability (<1%), which leads to two major problems [25]. First, since most of the drug gets cleared through gastric excretion, it has a very poor therapeutic efficiency, and must, therefore, be administered in high doses to garner any noticeable effect. Second, gastric absorption during the clearance of orlistat in humans causes severe gastrointestinal side effects, which are exacerbated by the high dose requirement. In the aforementioned study, it was hypothesized that by increasing the bioavailability of the drug, it would be possible to reduce the required orlistat dosage and its gastric excretion, and consequently reduce the severity of side effects, thereby improving the drug's efficacy for both antiobesity and anticancer applications. We showed that loading orlistat into folate receptor targeted HEA-b-EHA micellar NPs enhanced the solubility of the hydrophobic orlistat, and that the level of apoptosis induced in TNBC cells by orlistat-loaded micellar NPs was even higher than that induced by free orlistat [24].
PLGA-PEG NPs offer certain advantages over micellar NPs, as they are easier to synthesize, more stable, have been US FDA-approved for drug delivery in humans, and are more conducive to changes in concentration. Therefore, in this study, we built upon our previous work and began by comparing the cytotoxic effect of orlistat-loaded PLGA-PEG NPs in MDA-MB-231 (TNBC) and SKBr3 cells (used as a reference control) to that of free orlistat. MCF10A (noncancerous breast fibroblast cells) was used as a negative control. We also tested anti-miR-21-mediated improvement in the therapeutic effect of orlistat, and orlistat as a sensitizer for improving current chemotherapeutic drug (doxorubicin) for TNBC treatment.
In-vitro cytotoxic effect of free orlistat & orlistat-loaded PLGA-PEG nanoparticles in MDA-MB-231, SKBr3 & MCF10A cells
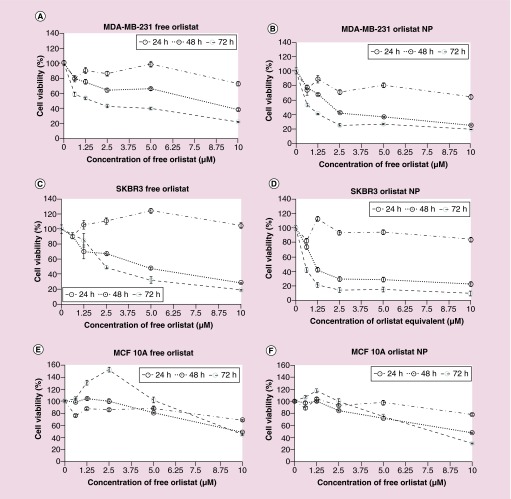
MDA-MB-231, SKBr3 and MCF10A cells were treated with 0–10 μM of free orlistat or orlistat-loaded PLGA-PEG-NPs in complete medium with 2% FBS. After the appropriate incubation periods (24, 48 and 72 h), an MTT assay was carried out to assess cell proliferation/cytotoxicity. The results showed a time- and dose-dependent decrease in cell viability in both MDA-MB-231 (Figure 3A & Supplementary Figures 2A & 3) and SKBr3 (Figure 3B & Supplementary Figures 2B & 4) cells treated with both free orlistat and orlistat-loaded PLGA-NPs. With the control values normalized to 100%, cell viability of MDA-MB-231 cells decreased to 72.3 ± 2.3%, 37.7 ± 1.2% and 21.8 ± 1.1%, respectively at 24, 48 and 72 h post treatment with 10 μM of free orlistat. The IC50 decreased from 7.85 μM at 48 h to 1.60 μM at 72 h. Similarly, the cell viability of SKBr3 cells decreased to 28.7 ± 1.3% and 18.9 ± 0.6%, respectively, at 48 and 72 h post treatment with with 10 μM of free orlistat. The IC50 decreased from 4.70 μM at 48 h to 2.50 μM at 72 h. In contrast, MDA-MB-231 and SKBr3 cells treated with orlistat-loaded PLGA-PEG-NPs showed significantly greater decreases in cell viability compared with free orlistat, at all concentrations of orlistat used for treatment and at all time points studied (24, 48 and 72 h) (Figure 3C & D).
Figure 3. . Cell viability of MDA-MB-231, SKBr3 and MCF10A cells after treated with various concentrations of free orlistat or orlistat nanoparticles ranging from 0 to 10 μM, assessed by MTT assay at 24, 48 and 72 h.
(A) MDA-MB-231 cells treated with free orlistat. (B) MDA-MB-231 cells treated with orlistat NPs. (C) SKBr3 cells treated with free orlistat, (D) SKBr3 cells treated with orlistat NPs. (E) MCF10A cells treated with free orlistat and (F) MCF10A cells treated with orlistat NPs.
NP: Nanoparticle.
On the other hand, in MCF10A, cell viability decreased to 48.6 ± 0.69% at 48 h and to 44.6 ± 1.35% at 72 h, at 10 μM free orlistat. Interestingly, the IC50 values were much higher for MCF10A (9.85 μM at 48 h), and decreased slightly to 9.54 μM at 72 h post treatment. Similarly, MCF10A cells treated with orlistat-loaded PLGA-PEG NPs, the IC50 values decreased from 9.49 μM at 48 h to 7.79 μM at 72 h (Figure 3E & F & Supplementary Figure 5). In summary, at all concentrations at 48 and 72 h post treatment, in both free orlistat and orlistat NP-treated cells, cell viability was significantly lower in MDA-MB-231 cells than in MCF10A cells (p < 0.0001). This demonstrates the selective nature of orlistat in inducing cell death in cancer cells.
Comparison of cytotoxic effect between free orlistat & orlistat-loaded PLGA-PEG-NPs in MDA-MB-231, SKBr3 & MCF10A cells
Comparing the results of the free orlistat treatment and the orlistat-loaded PLGA-PEG-NPs treatment in all three cell lines, we found that there was no significant difference in cell viability between untreated cells and those treated with control NPs at both 24 and 48 h time points. However, reduction in cell viability by orlistat-loaded PLGA-PEG-NPs was significantly greater than that induced by free orlistat in MDA-MB-231 and SKBr3 cells. In MDA-MB-231 cells (Figure 3A & B), the reduction in cell viability was statistically significant at concentrations ≥2.5 μM at 48 h (p < 0.001), and at all concentrations at 72 h (p < 0.01). In addition, the IC50 for free orlistat treatment (7.85 μM) decreased down to 2.15 μM for orlistat-loaded PLGA-PEG-NPs at 48 h post treatment. In SKBr3 cells (Figure 3C & D), this difference was statistically significant at concentrations ≥2.5 μM at 24 h (p < 0.05), and at all concentrations at 48 and 72 h (p < 0.05). In SKBr3, the IC50 for free orlistat treatment of 4.70 μM decreased to 1.10 μM for orlistat NP treatment at 48 h. This shows that delivery of orlistat via NPs greatly increases the bioavailability and efficacy of the drug in both MDA-MB-231 and SKBr3 cells.
Enhanced reduction of cell viability by doxorubicin in MDA-MB-231 cells (TNBC) through combined treatment with orlistat-loaded PLGA-PEG NPs
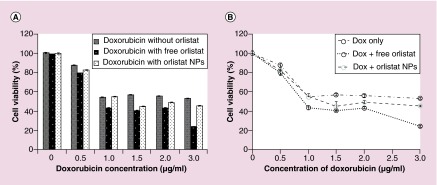
Doxorubicin is commonly used in the clinic for palliative chemotherapy for patients with advanced triple negative breast cancer. Unfortunately, the current doxorubicin dosage that is used for treatment often results in cardiotoxic and nephrotoxic side effects. Here, we hoped to see whether administering doxorubicin in conjunction with orlistat-loaded PLGA-PEG-NPs would enhance cell viability reduction and lower the doxorubicin doses needed for treatment. MDA-MB-231 cells were treated with either 0–3 μg/ml doxorubicin alone, in combination with 1.25 μM free orlistat, or in combination with 1.25 μM orlistat-equivalent of orlistat-loaded PLGA-PEG-NPs. Live and apoptotic cells were assessed 48 h post treatment by FACS analysis. We found that doxorubicin (3 μg/ml) on its own reduced cell viability by 47%, while doxorubicin in combination with free orlistat reduced cell viability by 75.2% (Figure 4A & B). Doxorubicin in combination with orlistat NPs reduced cell viability by 54.4%. Furthermore, the reported IC50 for doxorubicin in MDA-MB-231 cells is 3 μg/ml. This value was reduced to 0.75 μg/ml for doxorubicin when combined with free orlistat, and to 1.15 μg/ml when combined with orlistat-loaded PLGA-PEG-NPs. Since the half-life of doxorubicin in cell culture medium was estimated to be 24–30 h [26], orlistat-loaded PLGA-PEG-NPs tend to release orlistat over time when compared with the availability of free orlistat. Hence, we found slight increase in IC50 value for orlistat-loaded PLGA-PEG-NPs (1.15 μg/ml) compared with free orlistat-treated cells.
Figure 4. . Cell viability of MDA-MB-231 cells after treatment for 48 h with varying concentrations (0–3 μg/ml) of doxorubicin alone, in conjunction with 1.25 μM free orlistat and in conjunction with 1.25 μM orlistat-loaded PLGA-PEG nanoparticles as assessed by propidium iodide-staining-based FACS analysis.
Shown here is (A) the graph for cell viability for the range of concentrations of doxorubicin and (B) dose–response for each of the three conditions.
NP: Nanoparticle.
Antisense-miRNA-21-mediated reprogramming enhanced treatment effect of orlistat in MDA-MB-231 TNBC cells
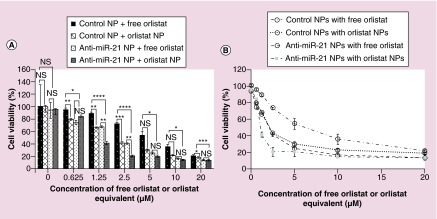
miR-21 is an oncomiR that is overexpressed in most cancers and contributes to drug resistance and cell proliferation. Silencing endogenous miR-21 function by antisense-miR-21 is a novel molecularly targeted anticancer therapy that reprograms the cancer cells and makes them sensitive to chemotherapies. In our previous study, we tested the effect of PLGA-b-PEG NPs coloaded with anti-miR-21 and anti-miR-10b on TNBC cell lines and found that they significantly reduced cell proliferation in vitro, invasion, metastasis and tumor growth in mice [15]. Thus, we hypothesized that pretreatment of MDA-MB-231 cells with anti-miR-21-loaded NPs can sensitize cancer cells to the treatment with orlistat NPs, and would substantially reduce cell viability compared with treatment with free orlistat. To test this hypothesis, cells were pretreated for 24 h with either control NPs or antisense-miR-21-loaded NPs followed by treatment with either free orlistat or orlistat-loaded PLGA-PEG-NPs. As expected, there was a significant reduction in cell viability at all concentrations of free orlistat or orlistat-NPs in cells pretreated with antisense-miR-21 NPs in comparison to free orlistat or orlistat NPs alone (Figure 5A & B). Furthermore, it was observed that the IC50 value in cells treated with free orlistat was reduced from 6.1 to 2.1 μM when pretreated with 10 pmol of antisense-miR-21 NPs. Similarly, the IC50 value in cells treated with orlistat NPs alone was reduced from 2.1 to 1.1 μM when pretreated with 10 pmol of antisense-miR-21 NP (Table 2 & Figure 5A & B). These results clearly show that orlistat-loaded NPs increase the bioavailability of the drug, since lower doses of the orlistat NP treatments were required to achieve the same level of cell viability inhibition, compared with the corresponding treatments with free orlistat. Additionally, we have shown that pretreating the cells with antisense-miR-21-loaded NPs works in conjunction with orlistat by significantly reducing cell viability, as well as the reduction of the IC50 value of orlistat.
Figure 5. . Cell viability of MDA-MB-231 cells measured after pretreatment with either control nanoparticles or antisense-miR-21-loaded nanoparticles, followed by treatment for 48 h with varying concentrations of either free orlistat or orlistat nanoparticles (0–20 μM orlistat equivalent).
Shown here are (A) the graph comparing cell viability for each of the four conditions at each concentration of orlistat and (B) dose–response for each of the four conditions.
*p < 0.05; **p < 0.01; ***p < 0.001.
NP: Nanoparticle; NS: Not significant.
Table 2. . IC50 values for each of the three drugs used for the study.
| Treatment | IC50 |
|---|---|
| Control NPs with free orlistat |
6.1 μM |
| Control NPs with orlistat NPs |
2.1 μM |
| Anti-miR-21 NPs with free orlistat |
2.1 μM |
| Anti-miR-21 NPs with orlistat NPs | 1.1 μM |
NP: Nanoparticle.
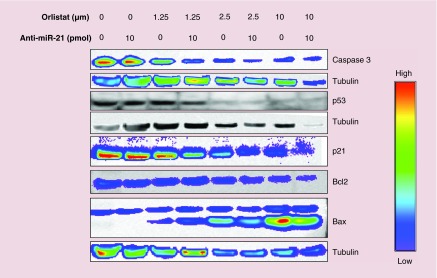
To determine the mechanism of action for PLGA-PEG NP-loaded orlistat and antisense-miR-21 coinduced apoptosis of MDA-MB-231 cells, we performed immunoblot analysis for Caspase-3, p53, p21, Bcl2 and Bax proteins. The results (Figure 6) showed an orlistat dose-dependent decrease in Caspase-3, p53, p21 and Bcl2 protein expression levels, and a dose-dependent increase in Bax protein expression levels. In both cases, there was an enhanced effect in samples that were cotreated with anti-miR-21.
Figure 6. . Immunoblot analysis of MDA-MB-231 cells treated with orlistat nanoparticle, antisense-miR-21 nanoparticle and orlisat nanoparticles after being pretreated with antisense-miR-21 nanoparticle for Caspase-3, p53, p21, Bcl2, Bax and tubulin proteins.
Conclusion & future perspective
In summary, we have successfully loaded the FDA-approved antiobesity drug orlistat into PLGA-PEG NPs, and demonstrated its utility in the treatment of triple negative breast cancer. We showed that orlistat NPs effectively increased the bioavailability of the hydrophobic drug in a number of different settings. Orlistat NPs increased apoptotic induction and reduced cell viability in both MDA-MB-231 (TNBC) and SkBr3 cells in vitro. Cotreatment of cells with orlistat NPs and the commonly used chemotherapy drug doxorubicin also enhanced the observed therapeutic effect, in comparison to doxorubicin alone or in combination with free orlistat, which suggests that lower concentrations of the chemotherapy drug would be required to achieve an improved effect if used in conjunction with orlistat. Last, we showed that cells treated with both antisense-miR-21 NPs and orlistat NPs showed a better therapeutic response than those treated with free orlistat alone, which suggests a possibility for a new and heretofore untested therapeutic strategy for treating triple-negative breast cancer. Interestingly, the observed system demonstrated synergistic, rather than additive effect, which would be highly beneficial when considering clinical translation option. Last, it must be noted that TNBC group of patients is highly heterogeneous, and different patients will therefore react to this strategy differently. We are in the process of establishing an experimental model that will allow us to culture primary cells from biopsied tissues and test their response to the potential treatment combinations in vitro before utilizing it as therapeutic means in the clinic. We are also in a process of establishing an in vivo mouse xenograft and orthotopic model that will enable us to test the effect of this potential therapeutic regiment for TNBC, with possible translation into the clinical setting.
Executive summary.
Objective
To explore the use of hydrophilic poly(ethylene glycol)-conjugated poly(lactic-co-glycolic acid) nanoparticles (PLGA-PEG-NPs) as a delivery system to improve the antitumor effect of the anti-obesity drug orlistat for triple-negative breast cancer therapy by improving its bioavailability.
Experimental setup
Orlistat-loaded PLGA-PEG-block copolymer NPs were synthesized and characterized by the emulsion-diffusion-evaporation method.
In vitro cytotoxic effect of free orlistat and orlistat-loaded NPs in MDA-MB231, SKBR3, and MCF10A cells was assessed.
In vitro combined treatment effect of MDA-MB231 cells with doxorubicin and orlistat-loaded NPs was performed.
In vitro combined treatment of MDA-MB231 cells with anti-miR-21-loaded NPs and orlistat-loaded NPs was performed.
Immunoblot analysis for Caspase 3, Bax, Bcl2, p53, and p21 levels was performed to elucidate the mechanism of apoptotic induction by orlistat and anti-miR-21 delivered by PLGA-PEG NPs in MDA-MB231 cells.
Results
Orlistat was successfully loaded in PLGA-PEG-NPs.
Reduction in cell viability by orlistat-loaded PLGA-PEG-NPs was significantly greater than that induced by free orlistat in MDA-MB231 and SKBR3 cells.
Enhanced reduction of cell viability by doxorubicin in MDA-MB-231 cells (triple-negative breast cancer) through combined treatment with orlistat-loaded PLGA-PEG NPs was observed.
Antisense-microRNA-21 mediated reprogramming enhanced treatment effect of orlistat in MDA-MB231 TNBC cells.
Conclusion
We have successfully loaded the US FDA-approved anti-obesity drug orlistat into PLGA-PEG NPs, and demonstrated its utility in the treatment of triple negative breast cancer.
Supplementary Material
Acknowledgements
The authors acknowledge the use of the SCi3 Core Facility and thank AL Koh, Stanford Nanocharacterization laboratory for her help with obtaining TEM images. The authors thank SS Gambhir, Chairman, Department of Radiology, Stanford University, for his constant support. The authors thank the department of Radiology and Canary Center for facility and support.
Footnotes
Authors’ contributions
R Paulmurugan conceived and designed the research. A Bhargava-Shah, K Foygel, R Devulapally and R Paulmurugan performed the experiments. A Bhargava-Shah, K Foygel and R Paulmurugan wrote the manuscript. All authors discussed the results and commented on the manuscript.
Financial & competing interests disclosure
This work was partially supported by the NIH (R01 CA161091 to R Paulmurugan). The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Ethical conduct of research
The authors state that they have obtained appropriate institutional review board approval or have followed the principles outlined in the Declaration of Helsinki for all human or animal experimental investigations. In addition, for investigations involving human subjects, informed consent has been obtained from the participants involved.
References
- 1.Torchilin VP. Micellar nanocarriers: pharmaceutical perspectives. Pharm. Res. 2007;24(1):1–16. doi: 10.1007/s11095-006-9132-0. [DOI] [PubMed] [Google Scholar]
- 2.Cho K, Wang X, Nie S, Chen ZG, Shin DM. Therapeutic nanoparticles for drug delivery in cancer. Clin. Cancer Res. 2008;14(5):1310–1316. doi: 10.1158/1078-0432.CCR-07-1441. [DOI] [PubMed] [Google Scholar]
- 3.Haley B, Frenkel E. Nanoparticles for drug delivery in cancer treatment. Urol. Oncol. 2008;26(1):57–64. doi: 10.1016/j.urolonc.2007.03.015. [DOI] [PubMed] [Google Scholar]
- 4.Acharya S, Sahoo SK. PLGA nanoparticles containing various anticancer agents and tumour delivery by EPR effect. Adv. Drug Deliv. Rev. 2011;63(3):170–183. doi: 10.1016/j.addr.2010.10.008. [DOI] [PubMed] [Google Scholar]
- 5.Devulapally R, Paulmurugan R. Polymer nanoparticles for drug and small silencing RNA delivery to treat cancers of different phenotypes. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2014;6(1):40–60. doi: 10.1002/wnan.1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J. Clin. 2015;65(2):87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 7.Cascione L, Gasparini P, Lovat F, et al. Integrated microRNA and mRNA signatures associated with survival in triple negative breast cancer. PLoS ONE. 2013;8(2):e55910. doi: 10.1371/journal.pone.0055910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Joensuu H, Gligorov J. Adjuvant treatments for triple-negative breast cancers. Ann. Oncol. 2012;23(Suppl. 6):vi40–vi45. doi: 10.1093/annonc/mds194. [DOI] [PubMed] [Google Scholar]
- 9.Von Minckwitz G, Martin M. Neoadjuvant treatments for triple-negative breast cancer (TNBC) Ann. Oncol. 2012;23(Suppl. 6):vi35–vi39. doi: 10.1093/annonc/mds193. [DOI] [PubMed] [Google Scholar]
- 10.Boyle P. Triple-negative breast cancer: epidemiological considerations and recommendations. Ann. Oncol. 2012;23(Suppl. 6):vi7–vi12. doi: 10.1093/annonc/mds187. [DOI] [PubMed] [Google Scholar]
- 11.Ballinger A. Orlistat in the treatment of obesity. Expert Opin. Pharmacother. 2000;1(4):841–847. doi: 10.1517/14656566.1.4.841. [DOI] [PubMed] [Google Scholar]
- 12.Menendez JA, Vellon L, Lupu R. Antitumoral actions of the anti-obesity drug orlistat (Xenical™) in breast cancer cells: blockade of cell cycle progression, promotion of apoptotic cell death and PEA3-mediated transcriptional repression of Her2/neu (erbB-2) oncogene. Ann. Oncol. 2005;16(8):1253–1267. doi: 10.1093/annonc/mdi239. [DOI] [PubMed] [Google Scholar]
- 13.Menendez JA, Colomer R, Lupu R. Why does tumor-associated fatty acid synthase (oncogenic antigen-519) ignore dietary fatty acids? Med. Hypotheses. 2005;64(2):342–349. doi: 10.1016/j.mehy.2004.07.022. [DOI] [PubMed] [Google Scholar]
- 14.Shabbits JA, Hu Y, Mayer LD. Tumor chemosensitization strategies based on apoptosis manipulations. Mol. Cancer Ther. 2003;2(8):805–813. [PubMed] [Google Scholar]
- 15.Devulapally R, Sekar NM, Sekar TV, et al. Polymer nanoparticles-mediated codelivery of antimiR-10b and antimiR-21 for achieving triple negative breast cancer therapy. ACS Nano. 2015;9(3):2290–2302. doi: 10.1021/nn507465d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Devulapally R, Sekar TV, Paulmurugan R. Formulation of anti-miR-21 and 4-hydroxytamoxyfen co-loaded biodegradable polymer nanoparticles and its anti-proliferative effect on breast cancer cells. Mol. Pharm. 2015;12(6):2080–2092. doi: 10.1021/mp500852s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guerciolini R. Mode of action of orlistat. Int. J. Obes. Relat. Metab. Disord. 1997;21(Suppl. 3):S12–S23. [PubMed] [Google Scholar]
- 18.Kridel SJ, Axelrod F, Rozenkrantz N, Smith JW. Orlistat is a novel inhibitor of fatty acid synthase with antitumor activity. Cancer Res. 2004;64(6):2070–2075. doi: 10.1158/0008-5472.can-03-3645. [DOI] [PubMed] [Google Scholar]
- 19.Gansler TS, Hardman W, 3rd, Hunt DA, Schaffel S, Hennigar RA. Increased expression of fatty acid synthase (OA-519) in ovarian neoplasms predicts shorter survival. Hum. Pathol. 1997;28(6):686–692. doi: 10.1016/s0046-8177(97)90177-5. [DOI] [PubMed] [Google Scholar]
- 20.Nemoto T, Terashima S, Kogure M, et al. Overexpression of fatty acid synthase in oesophageal squamous cell dysplasia and carcinoma. Pathobiology. 2001;69(6):297–303. doi: 10.1159/000064636. [DOI] [PubMed] [Google Scholar]
- 21.Piyathilake CJ, Frost AR, Manne U, et al. The expression of fatty acid synthase (FASE) is an early event in the development and progression of squamous cell carcinoma of the lung. Hum. Pathol. 2000;31(9):1068–1073. doi: 10.1053/hupa.2000.9842. [DOI] [PubMed] [Google Scholar]
- 22.Pizer ES, Lax SF, Kuhajda FP, Pasternack GR, Kurman RJ. Fatty acid synthase expression in endometrial carcinoma: correlation with cell proliferation and hormone receptors. Cancer. 1998;83(3):528–537. [PubMed] [Google Scholar]
- 23.Rashid A, Pizer ES, Moga M, et al. Elevated expression of fatty acid synthase and fatty acid synthetic activity in colorectal neoplasia. Am. J. Pathol. 1997;150(1):201–208. [PMC free article] [PubMed] [Google Scholar]
- 24.Paulmurugan R, Bhethanabotla R, Mishra K, et al. Folate receptor targeted polymeric micellar nanocarriers for delivery of orlistat as a repurposed drug against triple negative breast cancer. Mol. Cancer Ther. 2015 doi: 10.1158/1535-7163.MCT-15-0579. pii: molcanther.0579.2015. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Drew BS, Dixon AF, Dixon JB. Obesity management: update on orlistat. Vasc. Health Risk Manag. 2007;3(6):817–821. [PMC free article] [PubMed] [Google Scholar]
- 26.Smith L, Watson MB, O'kane SL, Drew PJ, Lind MJ, Cawkwell L. The analysis of doxorubicin resistance in human breast cancer cells using antibody microarrays. Mol. Cancer Ther. 2006;5(8):2115–2120. doi: 10.1158/1535-7163.MCT-06-0190. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.