Abstract
A fixed-dose inhaled corticosteroid/long-acting β2-agonist (ICS/LABA) combination of extrafine beclometasone dipropionate and formoterol fumarate (BDP/FF) has been recently approved for use in chronic obstructive pulmonary disease (COPD). Small airway inflammation and remodelling are cardinal features of COPD; therefore, the ability of this extrafine formulation to reach the small, as well as the large, airways is likely to be therapeutically important by enabling treatment of inflammatory processes in the whole bronchial tree. The clinical development of extrafine BDP/FF has demonstrated significant benefits over extrafine FF in terms of lung function improvement and reduction of the exacerbation rate, thus supporting the beneficial effect of an ICS combined to a LABA in COPD patients. Head-to-head comparison studies versus other ICS/LABA combinations have shown that the extrafine formulation enables the clinical benefits to be achieved with a lower dose of ICS. Extrafine BDP/FF showed lung function and dyspnoea improvements comparable to other ICS/LABAs, and a significantly faster onset of action was observed when compared with a salmeterol-containing fixed-dose combination. This review summarises the clinical evidence supporting the efficacy of extrafine BDP/FF in COPD and confirming that extrafine BDP/FF achieves the type of health benefit expected from such a targeted ICS/LABA combination in COPD.
Introduction
Chronic obstructive pulmonary disease (COPD) is a condition characterised by poorly reversible airflow limitation that is generally progressive and causes serious disability. Exacerbations and co-morbidities contribute to the overall severity in individual patients.
The Global initiative for Obstructive Lung Disease recommends that the assessment of disease severity should be multidimensional, taking into account symptoms, degree of airflow limitation and risk of exacerbations. This multidimensional severity assessment divides patients into four categories (A, B, C and D).1 The mainstays of drug therapy for stable COPD patients are bronchodilators, and in patients at risk of exacerbations fixed combinations of inhaled corticosteroids (ICS) and long-acting β2-agonist (LABA) are recommended as a first-choice treatment.1
Several studies have shown that a LABA combined with an ICS is more effective than the individual components alone for improving lung function and symptoms, reducing exacerbation rates and improving general health status.2–7 β-agonists and corticosteroids have different pharmacological targets and some synergistic interactions have been documented:
ICS increase the β2-adrenoreceptor density in the airways, which can counterbalance receptor downregulation and therefore prevent the development of LABA tolerance;8–10
LABAs favour the nuclear translocation of glucocorticoid receptors, therefore enhancing their mechanism of action.11
The clinical benefits of ICS/LABA combination treatment were demonstrated, among other studies, in the TOwards a Revolution in COPD Health (TORCH) study, a 3-year randomised trial of over 6,000 patients with moderate to severe COPD that compared inhaled fluticasone propionate (FP) and salmeterol (S) alone or in combination, with placebo.12 There was a 17.5% lower risk of death (absolute risk reduction of 2.6%) in the combination therapy group compared with placebo, which just missed statistical significance (95% confidence interval (CI), 0.681 to 1.002; P=0.052). ICS/LABA therapy reduced moderate/severe exacerbations to a greater extent compared with placebo and monocomponents. In addition, ICS/LABA treatment was associated with a slower rate of lung function deterioration compared with placebo.13 This benefit on the rate of lung function decline has also been observed in the SUMMIT trial, investigating the effects of fluticasone furoate/vilanterol.14
A fixed-dose combination of the ICS beclometasone dipropionate (BDP) and LABA formoterol fumarate (FF) has been recently approved in Europe for the treatment of COPD. This fixed-dose combination is characterised by an extrafine (i.e., mean mass aerodynamic diameter (MMAD) <2.0 μm) formulation of both active components and has a nominal dose of BDP 100 μg and FF 6 μg per actuation.15 The extrafine formulation enables efficient lung deposition, allowing a reduction to about half the equivalent dose of a conventional BDP aerosol and minimising systemic exposure. Small airway inflammation and narrowing are cardinal features of COPD,16 and the ability of this extrafine formulation to reach the small airways is therefore likely to be therapeutically important.
BDP/FF 100/6 μg is licensed for use in COPD using a pressurised metred dose inhaler (pMDI) or a dry powder inhaler (DPI). Other ICS/LABAs licensed for COPD are DPIs. Many patients prefer to use pMDIs, and the option to use this extrafine formulation using a pMDI with or without a spacer therefore offers a potentially valuable treatment option for COPD patients.17
This review critically evaluates the effects of extrafine BDP/FF compared with other COPD pharmacotherapies, and discusses the evidence for the potential advantages of using an extrafine combination inhaler in COPD. Two types of clinical trials involving BDP/FF were selected for inclusion in this review: (1) trials evaluating the deposition of extrafine BDP/FF in the airways of COPD patients and (2) phase 3 clinical trials in COPD patients conducted according to the regulations of the European Medical Agency (EMA) to investigate the efficacy and safety of extrafine BDP/FF in COPD patients. The focus of this article is a critical review of the strengths and limitations of three clinical trials (phase 3) of extrafine BDP/FF in COPD patients, and the relevance of the results for the treatment of COPD patients in primary care.
Lung deposition of extrafine BDP/FF
The ability of extrafine BDP/FF to achieve central and peripheral lung deposition was investigated in an open, single-dose, parallel-group study involving 10 healthy volunteers and 8 patients with stable COPD (mean forced expiratory volume in the first second (FEV1) 112 and 44% of predicted, respectively).18 Patients inhaled four actuations of radiolabelled 99mTc BDP/FF and subsequent gamma camera imaging measured activity in the entire lung and extra-thoracic region, as well as the amount of exhaled activity. Lung deposition was remarkably consistent in the two groups, and it was 34% and 33% of the nominal dose in healthy volunteers and COPD patients, respectively, with a homogeneous deposition in both large and small airways regardless of the pathophysiological condition. The amount of drug exhaled was small and ranged from 2.8 to 3.4% of the nominal dose, thus confirming that only a minimal amount of extrafine particles are exhaled.
DeBacker et al. used multi-slice computed tomography (CT) scans and Computational Fluid Dynamics to evaluate the effects of the extrafine BDP/FF formulation on airway geometry in COPD patients.19 The administration of extrafine BDP/FF led to a significant improvement in airway geometry at 4–6 h, which was greater in the lower airways compared with the upper airways. After 6 months of treatment, the hyperinflation at the lobar level at total lung capacity was significantly reduced compared with baseline values. These changes were associated with a reduction in hyperinflation measured by functional residual capacity (FRC) and improved FEV1. These findings indicate the efficacy of extrafine BDP/FF in improving lung physiology in the small airways.
Study NCT00476099
The study design, primary aims and key inclusion criteria for the three clinical development studies (NCT00476099, FORWARD and FUTURE) are summarised in Table 1. NCT00476099 was a 1-year, double-blind, double-dummy, randomised, multinational, multicentre, 3-arm parallel-group trial.20 The primary aims were to test the non-inferiority of extrafine BDP/FF 100/6 μg versus budesonide/formoterol fumarate (BUD/FF) 200/6 μg in terms of pulmonary function (FEV1) and the superiority of extrafine BDP/FF versus FF in terms of exacerbation rate in COPD patients with severe airflow limitation.
Table 1. Main characteristics of COPD studies with BDP/FF.
| Name of the study | Treatments | Study duration | Rand. patients | Primary end points | Key inclusion criteria |
|---|---|---|---|---|---|
| NCT0047609920 | BDP/FF (200/12 μg) BUD/FF (400/12 μg) FF (12 μg) BID | 48 weeks | 718 | Change from baseline to 48 weeks in pre-dose FEV1 and mean rate of COPD exacerbations. | Age ⩾40 years ⩾20 pack-years PB FEV1 30%÷50% of pred. and FEV1/FVC<0.7 One severe exacerbation in the previous year Change in FEV1 <12% of pred. after salbutamol Free from COPD exacerbations for 12 weeks before randomisation |
| FORWARD28 | BDP/FF (200/12 μg) FF (12 μg) BID | 48 weeks | 1199 | Change from baseline to 12 weeks in pre-dose morning FEV1 and mean rate of COPD exacerbations | Age >40 years ⩾10 pack-years PB FEV1 30%÷50% of pred. and FEV1/FVC<0.7 Documented history of at least one exacerbation in the previous year |
| FUTURE37 | BDP/FF (200/12 μg) FP/S (500/50 μg) BID | 12 weeks | 419 | TDI score at week 12, and AUC0-30min at randomisation | Age ⩾ 40 years ⩾10 pack-years PB FEV1 30%÷60% of pred. and FEV1/FVC<0.7 BDI⩽10 An increase in FEV1 ⩾ 5% from baseline following administration of salbutamol |
Abbreviations: AUC, area under the curve; BDI, baseline dyspnoea index; BID, bis in die; BDP, beclometasone; BUD, budesonide; FF, formoterol fumarate; FP, fluticasone; FVC, forced vital capacity; PB, postbronchodilator; pred., predicted; S, salmeterol, TDI, transitional dyspnoea index.
The non-inferiority of extrafine BDP/FF to BUD/FF was demonstrated; the difference between the adjusted mean FEV1 of the extrafine BDP/FF and BUD/FF group was −0.002 l, and the lower limit of the two-sided 95% CI for the difference between groups was −0.052 l. In addition, extrafine BDP/FF led to a statistically significant improvement in FEV1 in comparison with FF (difference 0.051 l; 95% CI 0.001 to 0.102; P=0.046).
The exacerbation rate in this study was much lower (less than half) than the rates usually observed in COPD studies that have specifically studied the effects of ICS on exacerbations (Table 2), which made it difficult to draw firm conclusions on this outcome. The total number of COPD exacerbations over the 48-week treatment period were similar across all three treatment groups (~0.4 per patient per year).
Table 2. Demographic and other baseline characteristics of NCT00476099 and FORWARD studies.
|
NCT00476099
|
FORWARD
|
||||
|---|---|---|---|---|---|
| BDP/FF (N=232) | BUD/FF (N=238) | FF (N=233) | BDP/FF (N=595) | FF (N=591) | |
| Male, n (%) | 184 (79.3) | 194 (81.5) | 189 (81.1) | 408 (68.6) | 410 (69.4) |
| Female, n (%) | 48 (20.7) | 44 (18.5) | 44 (18.9) | 187 (31.4) | 181 (30.6) |
| Age, years, mean (s.d.) | 63.0 (9.0) | 64.1 (9.1) | 63.7 (8.8) | 64.6 (8.6) | 63.9 (8.6) |
| BMI, kg/m2, mean (s.d.) | 26.8 (5.3) | 26.6 (5.0) | 26.7 (4.7) | 26.5 (5.4) | 26.5 (5.3) |
| FEV1, L, mean (s.d.) | 1.22 (0.3) | 1.23 (0.3) | 1.22 (0.3) | 1.15 (0.3) | 1.16 (0.3) |
| FEV1, % pred. mean (s.d.) | 41.9 (5.8) | 42.3 (6.0) | 42.5 (5.9) | 41.86 (6.0) | 41.61 (6.0) |
| FVC, L, mean (s.d.) | 2.46 (0.6) | 2.47 (0.6) | 2.46 (0.6) | 2.46 (0.7) | 2.52 (0.7) |
| FEV1/FVC, mean (s.d.) | 0.51 (0.1) | 0.51 (0.1) | 0.51 (0.1) | 0.48 (0.1) | 0.48 (0.1) |
| Disease duration, years, mean (s.d.) | 9.4 (7.0) | 9.9 (7.8) | 9.8 (6.7) | 7.9 (5.9) | 7.5 (5.7) |
| Exacerbations/patient, mean (s.d.) | 1.7 (1.0) | 1.7 (1.0) | 1.8 (1.0) | 1.5 (0.9) | 1.4 (0.9) |
| Current smokers, n (%) | 90 (38.8) | 86 (36.1) | 87 (37.3) | 231 (38.8) | 237 (40.1) |
| Ex-smokers, n (%) | 142 (61.2) | 152 (63.9) | 146 (62.7) | 364 (61.2) | 354 (59.9) |
| Pack-years, mean (s.d.) | 37.3 (14.1) | 37.8 (14.6) | 39.7 (19.1) | 43.1 (23.5) | 42.7 (22.9) |
| Tiotropium users at randomisation, n (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 318 (53.4) | 298 (50.4) |
| SGRQ total, mean (s.d.) | 51.0 (15.4) | 49.7 (15.8) | 50.6 (16.2) | 47.3 (17.9) | 48.0 (17.2) |
Abbreviations: BMI, body mass index; FVC, forced vital capacity; SGRQ, St George's Respiratory Questionnaire.
At the end of the study, the mean morning pre-dose forced vital capacity (FVC) was significantly higher than baseline in the extrafine BDP/FF group (0.09 l; P=0.005) but not in the BUD/FF group (0.05 l; P=0.15) and the FF group (0.02 l; P=0.58), although there was no statistical difference between groups (P=0.39). These significant changes in FVC are likely to reflect changes in lung volumes related to improvements in distal airway function.
For quality of life, the St George's Respiratory Questionnaire showed significant improvements in all treatment groups, with no difference between treatments. The use of rescue medication at the end of the study was significantly reduced from baseline by extrafine BDP/FF (−0.27 puffs; P<0.001) and BUD/FF (−0.24 puffs; P=0.013) but not by FF (−0.04 puffs, P>0.05).
The mean changes in the distance covered during the six-minute walking test (6MWT) were 41.1 metres in the extrafine BDP/FF group, 35.4 metres in the BUD/FF group and 35.2 metres in the FF group (P<0.001 in all groups). No statistically significant differences were found in the comparisons between groups. However, only extrafine BDP/FF provided an improvement of walking ability above the threshold of 37 metres, which is thought to be a clinically significant change (the minimal clinically important difference; MCID).21,22 The authors suggest that the ability to walk further after treatment with BDP/FF may be linked to the improvement in air trapping.20
NCT00476099: strengths
NCT00476099 was performed in patients likely to be suitable candidates for ICS/LABA treatment—i.e., severe airflow obstruction plus a history of ⩾1 exacerbation in the previous year. This was the first long-term study of extrafine BDP/FF in COPD patients, which succeeded in one of its co-primary aims, to demonstrate a similar effect on lung function compared wth BUD/FF, which is widely used in clinical practice.
NCT00476099: Limitations
The major limitation of this study was the very low exacerbation rate observed, despite the study being conducted in severe COPD patients with a history of ⩾1 exacerbation in the previous year. This reduced the ability of the study to measure the impact of ICS/LABA compared with LABA on this outcome measure. Possible reasons for the low exacerbation rate observed include the following:
Patients were required to be free from COPD exacerbations for 12 weeks before randomisation. This may have inadvertently biased the recruitment towards more stable patients who were less likely to exacerbate thereafter, as the best predictor of exacerbation risk is the previous history of frequent exacerbations and exacerbations tend to cluster;23,24
Many patients tend to self-medicate to manage their symptom worsening.25,26 Many exacerbations after randomisation might not have been reported by patients, leading to under-estimation of the exacerbation frequency;
The major risks for COPD exacerbations are community-based viral infections, such as influenza, and upper respiratory tract infections. These vary between seasons, between different years and between countries. In the period of the study conduct, the burden of respiratory tract infection/influenza over Europe was low.27
It should also be noted that the sample size per treatment arm was lower than in many other COPD clinical trials that have investigated the effects of inhaled treatments on exacerbations as a primary end point.
Forward study
The primary aims of the FORWARD study were to compare extrafine BDP/FF 100/6 μg with extrafine FF 6 μg with two co-primary efficacy end points: COPD exacerbation rate over 1 year and change in pre-dose morning FEV1 from baseline (randomisation visit) to Week 12.28 The FORWARD study was designed specifically to fill the gap in evidence for the effect of extrafine BDP/FF on exacerbations by enhancing the capture of exacerbation events. Specifically, (i) patients were recruited in three ‘winter waves’ in two consecutive years across the globe (November to April for the Northern hemisphere, and April to September for the Southern Hemisphere) in order to capture the winter exacerbation peak. The validity of this approach is confirmed by the post hoc analysis of the TORCH study, in which a major proportion of exacerbations fell in the winter months of both Northern and Southern hemispheres;29 (ii) as previous exacerbation history is the most important risk factor for future exacerbations,30 documented evidence of an exacerbation in the last year (e.g., medical letter, hospital records) was required rather than just relying on a patient verbal report as in NCT00476099; (iii) the wash-out period free from exacerbations was limited to 6 weeks (4 weeks before screening plus 2 weeks for the run-in period), rather than 12 weeks as NCT00476099 (8 weeks before screening plus 4 weeks for the run-in period); (iv) the EXAcerbations of Chronic Pulmonary Disease Tool (EXACT)- Patient-Reported Outcome (PRO) questionnaire was used to measure symptoms. This 14-item questionnaire31 was recorded daily using a digital platform. The daily electronic transmission of symptom data allowed the investigators to monitor for deteriorations in health. This instrument was used also as a trigger for physicians to contact patients for evaluation of whether changes in symptoms were due to an exacerbation.
The use of tiotropium as a concomitant medication was permitted, except for a 72-h wash-out period before each clinic visit. This meant that this study was conducted in a more ‘real-life’ setting with a severe COPD population, as a large proportion (50–70%) of severe COPD patients in Western Europe use tiotropium.32
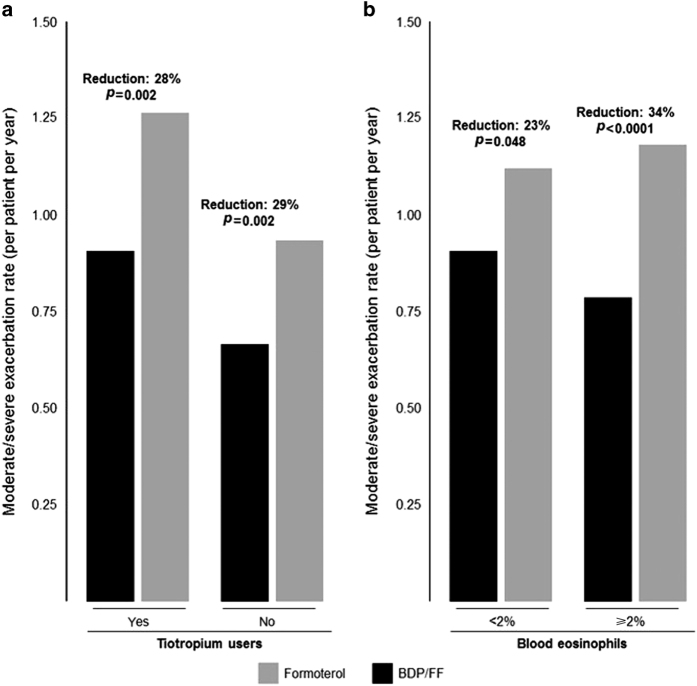
Extrafine BDP/FF was superior to extrafine FF in terms of the annual COPD exacerbation rate; there was a 28% reduction of moderate-to-severe exacerbations with extrafine BDP/FF compared with FF, P<0.001. Moreover, a subgroup analysis, stratifying patients by tiotropium use at randomisation, showed that extrafine BDP/FF was superior to FF for reduction of exacerbations both in tiotropium users and in non-tiotropium users (Figure 1a).
Figure 1.
COPD exacerbations during the FORWARD study in patients stratified by different baseline characteristics. (a) Use of tiotropium before study entry; data from Wedzicha et al. 28 (b) Percentage count of baseline blood eosinophils; data on file. BDP/FF, beclometasone dipropionate/formoterol.
Extrafine BDP/FF was superior to FF also for the change in pre-dose morning FEV1 from baseline to Week 12, confirming the results obtained in NCT00476099. In the ITT population, the difference in the adjusted mean change between the two groups (0.069 l; P<0.001) in favour of extrafine BDP/FF was evident irrespective of tiotropium use. There was an improvement in the St George's Respiratory Questionnaire total score in the BDP/FF group compared with FF, with an adjusted mean difference of 2.78 units (95%: −4.51, −1.05; P=0.002).
FORWARD: strengths
The sample size of FORWARD was greater than NCT00476099, thus increasing the statistical power to measure a difference between treatments on exacerbations. The key baseline characteristics of NCT00476099 and FORWARD patient populations are presented in Table 2; the populations are very similar, including the retrospectively reported exacerbation history in the previous year. However, the design of FORWARD increased the prospective capture of these events compared with NCT00476099 (~1.1 versus 0.4, respectively, for the LABA treatment arm).
The superiority of extrafine BDP/FF versus extrafine FF in terms of exacerbation reduction in tiotropium users is a novel aspect of FORWARD, as all previous ICS/LABA clinical trials have required the discontinuation of LAMA treatment. This subset analysis of tiotropium users provides useful information on the value of triple therapy (ICS+LABA+LAMA) versus dual bronchodilator therapy (LABA+LAMA) on exacerbations. There are little published data on this particular issue.
FORWARD: limitations
FORWARD succeeded in its primary aim of demonstrating a greater effect of extrafine BDP/FF compared with FF on exacerbation reduction in severe COPD patients. The patients recruited were required to have a history of ⩾1 exacerbation in the previous year. Global initiative for Obstructive Lung Disease recommends that ICS/LABA treatments can be used in patients with ⩾2 exacerbations in the previous year. To date, no ICS/LABA clinical trials have used this inclusion criteria, so the evidence from clinical trials such as FORWARD comes from a population different from that stated by Global initiative for Obstructive Lung Disease.
FORWARD studied COPD patients with FEV1 <50% predicted. Some other ICS/LABA clinical trials have used an FEV1 cutoff up to 70% predicted,6 and have demonstrated an effect on exacerbations. One could assume that extrafine BDP/FF would also be effective in patients with FEV1 50–70% and a history of exacerbations, but FORWARD did not specifically investigate this issue.
The FORWARD study provided the data that EU regulators required in order to grant a license for extrafine BDP/FF in COPD patients. There is debate concerning how closely the effects observed in clinical trials will predict the benefits in real-life.33 For example, the inclusion criteria of COPD clinical trials often exclude patients with more severe and/or symptomatic disease who are unable to withdraw inhaled medication; in FORWARD, ICS withdrawal was required during the run-in. Furthermore, patients with significant co-morbidities such as cardiovascular disease are often excluded. Clinical trials closely monitor adherence to medication, whereas in real-life this is often a significant problem. There is a need for real-life studies that overcome these issues, so that we can better understand the effects of ICS/LABA combination therapies, such as extrafine BDP/FF, in COPD patients.
FORWARD: blood eosinophils and exacerbation rates
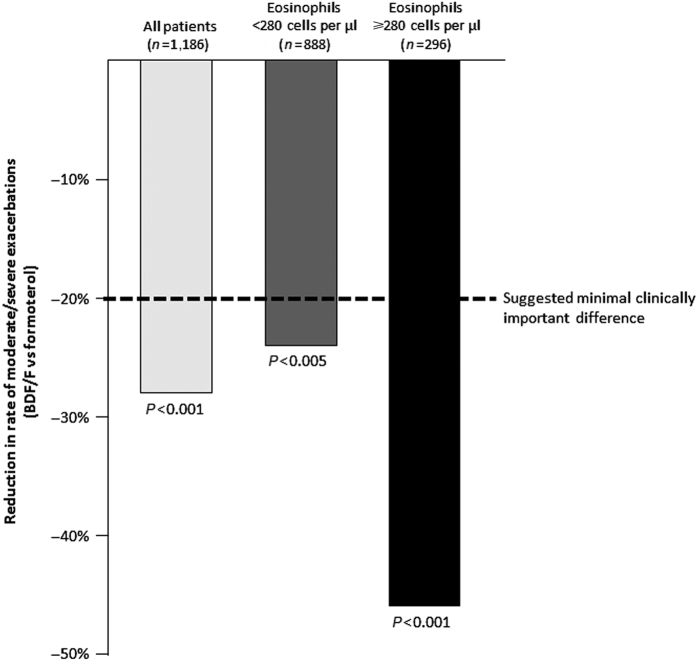
Blood eosinophil count is a potential biomarker of response to ICS therapy in COPD patients.34 A post hoc analysis of the FORWARD study stratified patients into quartiles using the baseline blood eosinophil counts.35 The reduction in exacerbation rate with BDP/FF in comparison with FF alone ranged from 22% in patients in the lowest quartile to 46% in patients in the highest quartile. Overall, there was a comparable exacerbation rate across the ranges of eosinophil counts in patients receiving BDP/FF, whereas an increasing pattern of exacerbation rate with increasing eosinophil counts was observed in patients treated with FF alone. This suggests an increasing treatment effect size for BDP/FF in patients with higher blood eosinophil counts. A blood eosinophil count ⩾2% has been suggested as a threshold to classify patients as having ‘eosinophilic COPD’; 37% of COPD patients had eosinophil counts persistently ⩾2% over 3 years in the ECLIPSE study.36 Subdividing the patients in FORWARD using this threshold, Figure 1b shows a significant benefit of BDP/FF over FF in both groups, but with a larger effect size in patients with eosinophils ⩾2% (34% versus 23% reduction). Similarly, using a cutoff level of 280 cells per μl (the upper quartile in FORWARD), there is a greater benefit (46% reduction of exacerbations) in patients with higher eosinophils (>280 cells per μl), but still a significant effect (24%) in patients with eosinophil counts below this threshold (Figure 2). This was a post hoc analysis, and thus it was not statistically powered to define the effect sizes in the quartiles. Nevertheless, the ICS effect appears to increase with higher eosinophil counts, possibly because of exacerbations that are not effectively treated with bronchodilators. These results from a post hoc analysis should be confirmed in prospective clinical trials, to confirm the observation and provide further guidance on the blood eosinophil cutoff level(s) that could be used in clinical practice.
Figure 2.
Comparison of reduction in the rate of moderate/severe exacerbations with BDP/FF versus formoterol in COPD patients. Adapted from FORWARD study: all patients (Wedzicha et al. 28) and data pooling from post hoc analysis on baseline blood eosinophil count (Siddiqui et al. 35). BDP/FF, beclometasone dipropionate/formoterol. 20% is the suggested minimal clinically important difference for exacerbations.47
Future study
This was a 12-week multicentre, multinational, randomised, double-blind, double-dummy, 2-arm parallel-group design comparing extrafine BDP/FF 100/6 μg (n=211) with FP/S (Seretide, Accuhaler GlaxoSmithKline, Middlesex, UK, 500/50 μg; n=208) in patients with moderate to severe COPD.37 The primary objectives of the study were to demonstrate the superiority of extrafine BDP/FF versus FP/S, in terms of pulmonary function (FEV1 standardised area under the curve between time 0 and 30 min (AUC0-30min)) after drug inhalation on the morning of Day 1, and the equivalence between treatments in terms of the Transition Dyspnoea Index (TDI) score at week 12. St George's Respiratory Questionnaire was also assessed at baseline and at week 12.
The mean (±s.d.) FEV1 AUC0-30min was greater in the extrafine BDP/FF group (0.17±0.13 l) compared with the FP/S group (0.09±0.10 l) on day 1; the adjusted mean difference between groups was 0.073 l (95% CI: 0.050 to 0.095, (P<0.001). This difference between treatments was also demonstrated after dose at week 12. In patients with FEV1% predicted<50%, similar results were observed.
The mean (±s.d.) TDI score was 1.47±2.64 with extrafine BDP/FF and 1.31±2.86 with FP/S; the adjusted mean difference between groups was 0.165, with the 95% CI for the difference (−0.387 to 0.718) lying entirely within the pre-defined±1 equivalence margins (P=0.56 between groups). A TDI score ⩾1 (the MCID21) was observed in 93 patients (44.1%) in the BDP/FF group and in 89 (43.0%) in the FP/S group (P=0.92 between groups).
Secondary efficacy end point measurements showed no difference between treatments, including the pre-dose FEV1. Both treatments improved the quality of life (St George's Respiratory Questionnaire), but only extrafine BDP/FF provided an improvement greater than the 4-unit MCID.
FUTURE: strengths
This is the first head-to-head study comparing extrafine BDP/FF with one of the most commonly used drugs for COPD patients (FP/S), evaluating different ICS dosages in fixed combination therapies. ICS can cause side effects such as osteoporosis and pneumonia.38 FUTURE demonstrated that a lower ICS dose in extrafine BDP/FF compared with high-dose FP/S resulted in equivalent improvements in dyspnoea and trough FEV1. This may be important when considering the potential of ICS to cause long-term side effects.
A potential advantage of extrafine BDP/FF over FP/S is the significantly greater effect immediately after dosing in the morning that is present at the first dose and also persists during long-term treatment. This is likely to be due to the well-known faster onset of action of formoterol compared with salmeterol.39 Early-morning symptoms are common for many COPD patients,40 and an ICS/LABA with a faster onset of bronchodilation may be very useful for such patients.
FUTURE: limitations
FUTURE provided a comparison of extrafine BDP/FF and FP/S over 12 weeks. The duration of the study was short for measuring exacerbations, and the population recruited was not enriched for a history of exacerbations. The similar effect of the extrafine BDP/FF and FP/S on other outcome measurements suggests that exacerbation reduction would also be similar, but this conclusion cannot definitively be drawn.
Safety
NCT00476099 and the FORWARD study were long-term studies that provided relevant information on adverse events (AEs). In the NCT00476099 study, the incidence of AEs were similar between treatments: 42.8% of patients in the extrafine BDP/FF group, 40.9% in the BUD/FF group and 44.1% in the FF group, with AEs leading to study discontinuation reported in only 9 (3.8%), 6 (2.5%) and 5 (2.1%) patients, respectively. A similar pattern in AEs leading to discontinuation was observed in FORWARD: 26 (4.3%) and 28 (4.7%) for BDP/FF and FF, respectively.
Pneumonia has been identified as a risk for COPD patients treated with ICS/LABA combinations.41 The overall incidence of such events has generally been reported as low (<10%; Table 3) and should be interpreted in the overall context of such risks against the benefits of reducing exacerbations and hospitalisation for life-threatening events. It should also be noted that the increase risk of occurrence of pneumonia with ICS-LABAs compared with the corresponding monotherapy is not associated with increased mortality.42 In the NCT00476099 study, pneumonia was reported in only five patients (2.1%) in the extrafine BDP/FF group, seven (2.9%) patients in the BUD/FF group and in one (0.4%) patient in the FF group. In the FORWARD study, pneumonia was reported by 23 patients (3.8%) in the extrafine BDP/FF group and 11 patients (1.8%) in the FF group. Overall, the rate of pneumonia observed in the two long-term BDP/FF studies was very low; it is generally in line with those of a meta-analysis on long-term use of ICS and risk of pneumonia in COPD.43
Table 3. Percentage of patients with pneumonia in recent studies investigating ICS/LABA combinations versus LABA monotherapy in COPD patients.
| Study reference | Drugs and total daily dose (μg) | Study duration |
% of patients with pneumonia
|
|
|---|---|---|---|---|
| ICS/LABA | LABA | |||
| Calverley et al. 12 | FP/S 1000/100 versus S 100 | 3 years | 19.6 | 13.3 |
| Kardos et al. 45 | FP/S 1000/100 versus S 100 | 44 weeks | 4.5 | 1.4 |
| Calverley et al. 3 | BUD/FF 640/18 versus FF 18 | 12 months | 3.1 | 2.7 |
| Ferguson et al. 4 | FP/S 500/100 versus S 100 | 12 months | 7.0 | 4.0 |
| Rennard et al. 7 | BUD/FF 320/9 versus FF 9 | 12 months | 3.0 | 3.4 |
| Rennard et al. 7 | BUD/FF 160/9 versus FF 9 | 12 months | 3.0 | 3.4 |
| Anzueto et al. 46 | FP/S 500/100 versus S 100 | 52 weeks | 6.6 | 2.5 |
| Carlverley et al. 20 | BDP/FF 400/24 versus FF 24 | 48 weeks | 2.1 | 0.4 |
| Carlverley et al. 20 | BUD/FF 800/24 versus FF 24 | 48 weeks | 2.9 | 0.4 |
| Sharafkhaneh et al. 44 | BUD/FF 320/9 versus FF 9 | 12 months | 6.4 | 2.7 |
| Sharafkhaneh et al. 44 | BUD/FF 160/9 versus FF 9 | 12 months | 4.7 | 2.7 |
| Dransfield et al. 6 | FF/Vil 100/25 versus Vil 25 | 52 weeks | 6.3 | 3.3 |
| Wedzicha et al. 28 | BDP/FF 400/24 versus FF 24 | 48 weeks | 3.8 | 1.8 |
Studies >6 months duration were included.
Abbreviations: COPD, chronic obstructive pulmonary disease; ICS/LABA, inhaled corticosteroid/long-acting β2-agonist.
NCT00476099 and FORWARD; comparison with other ICS/LABA studies
For COPD patients with a history of exacerbations, international guidelines recommend the use of an ICS/LABA combination based on evidence showing significant improvement of lung function, symptoms and health status, and significant reduction of exacerbations.1 The clinical trial programme for extrafine BDP/FF has shown that this combination has a greater effect than the FF component alone on lung function in NCT00476099 and FORWARD. The improvements in pre-dose FEV1 at the end of NCT00476099 and FUTURE (0.077 and 0.07 l, respectively) were similar to the effects reported in clinical trials of other ICS/LABA combinations, as shown in Table 4;2–7,20,28,44 for most studies, the effect was between 50 and 90 ml. BDP/FF caused a 28% reduction of exacerbations compared with FF in FORWARD; again, this is similar to the effect sizes reported on other ICS/LABA studies, which generally varies between 25 and 30%3–7,12,28,44–46 (listed in Table 5). There is no direct comparison of the effect of BDP/FF with other ICS/LABA combinations on exacerbation rate reduction, but the indirect comparison with the results in Table 5 indicates that BDP/FF has similar effects to the ICS/LABA combinations FP/S and BUD/FF that are commonly used in clinical practice.
Table 4. Pre-dose FEV1 improvements in studies of ICS/LABA combinations in COPD.
| Clinical studies | Pre-dose FEV 1 at baselinea (ml) | Pre-dose FEV 1 versus baselineb (ml) | Pre-dose FEV 1 versus LABAb (ml) | Timepoint considered for FEV 1 comparison |
|---|---|---|---|---|
| Calverley2 ,c | 1,266 | 120 | 73 | 52 weeks |
| Ferguson4 ,c | 950 | 102 | 74 | 52 weeks |
| Szafranski5 ,d | 960 | 80 | 80 | 12 months |
| Calverley3 ,d | 980 | N/A | 50 | 12 months |
| Wedzicha28 ,e | 1,052 | 70 | 65 | 48 weeks |
| Calverley20 ,e | 1,140 | 77 | 50 | 48 weeks |
| Rennard7 ,d | 1,020 | 120 | 90 | 12 months |
| Sharafkhaneh44 ,d | 1,000 | 70 | 30 | 12 months |
| Dransfield6 ,f | N/A | N/A | 40 | 52 weeks |
Studies >6 months duration were included. Studies with pre-dose FEV1 presented were included.
Abbreviations: LABA, long-acting β2-agonist; N/A, not available.
FEV1 before first dose (baseline).
Change in FEV1 caused by ICS/LABA.
Studies conducted with: fluticasone propionate/salmeterol.
Budesonide/formoterol fumarate.
Beclometasone dipropionate/formoterol fumarate.
Fluticasone furoate/vilanterol.
Table 5. COPD exacerbation annual rate in recent studies of ICS/LABA combinations.
| Study reference | Drugs and total daily dose (μg) | Study duration |
Annual rate of moderate/severe exacerbations
|
||
|---|---|---|---|---|---|
| ICS/LABA | LABA | Reduction ICS/LABA versus LABA (%) | |||
| Calverley et al. 3 | BUD/FF 800/24 versus FF 24 | 12 months | 1.38 | 1.85 | 25.5a |
| Calverley et al. 12 | FP/S 1000/100 versus S 100 | 3 years | 0.85 | 0.97 | 12a |
| Szafranski et al. 5 | BUD/FF 800/24 versus FF 24 | 12 months | 1.42 | 1.84 | 23a |
| Kardos et al. 45 | FP/S 1000/100 versus S 100 | 44 weeks | 0.92 | 1.4 | 35a |
| Ferguson et al. 4 | FP/S 500/100 versus S 100 | 12 months | 1.06 | 1.53 | 30.5a |
| Rennard et al. 7 | BUD/FF 400/12 versus FF 12 | 12 months | n/a | n/a | 25a |
| Rennard et al. 7 | BUD/FF 200/12 versus FF 12 | 12 months | n/a | n/a | 29a |
| Anzueto et al. 46 | FP/S 500/100 versus S 100 | 52 weeks | 1.10 | 1.59 | 30.4a |
| Sharafkhaneh et al. 44 | BUD/FF 400/12 versus FF 12 | 12 months | 0.70 | 1.07 | 34.6a |
| Sharafkhaneh et al. 44 | BUD/FF 200/12 versus FF 12 | 12 months | 0.79 | 1.07 | 25.9a |
| Dransfield et al. 6 | FF/Vil 100/25 versus Vil 25 | 52 weeks | 0.81 | 1.11 | 30b |
| Wedzicha et al. 28 | BDP/FF 400/24 versus FF 24 | 48 weeks | 0.80 | 1.12 | 28.1a |
Studies >6 months were included.
NCT00476099 excluded from this summary, because of a low exacerbation rate.20
Abbreviations: BDP/FF, beclometasone dipropionate/formoterol fumarate; BUD/FF, budesonide/formoterol fumarate; FP/S, fluticasone propionate/salmeterol; FF/Vil, fluticasone furoate/vilanterol.
P value ICS/LABA versus LABA alone <0.05.
P value not available.
Clinical trial data for extrafine BDP/FF; relevance to primary care
Clinical trials of ICS/LABA combinations have generally been conducted using criteria that exclude many patients who would receive these medicines in a primary-care setting in real-life, as already discussed. There is a need for real-life studies that evaluate the effectiveness of ICS/LABA combinations, including extrafine BDP/FF. Real-life studies also allow the impact of patient compliance of treatment effectiveness to be assessed. In the absence of such studies, we are left with the information from randomised controlled trials to make treatment decisions.
ICS/LABA combinations are frequently prescribed in both primary and secondary care. FORWARD recruited from both primary and secondary care clinics, and thus the results support the use of extrafine BDP/FF in both these clinical settings.
ICS/LABA combinations including FP/S and BUD/FF have historically been widely used to treat COPD patients in primary care. The direct comparison of extrafine BDP/FF with FP/S in the FUTURE study, and the indirect comparisons presented in Tables 4 and 5, indicate similar effects of extrafine BDP/FF compared with other ICS/LABA combination inhalers. In this situation, the decision of a health-care professional regarding which inhaler to use will be influenced by factors other than efficacy, such as cost and inhaler device preference.
BDP/FF is licensed for use in COPD patients with both a pMDI and a DPI. Other ICS/LABA combinations are licensed for COPD using DPI devices. The pMDI option that extrafine BDP/FF provides is likely to be useful practically for many COPD patients in primary care.17
Conclusions
Extrafine BDP/FF is the only ICS/LABA approved for use in COPD patients as both a pMDI and a DPI. This allows health-care professionals to select the most appropriate inhaler based on specific patient needs. The extrafine formulation enables drug delivery from both inhalers to both the large and small airways, and allows the clinical benefits to be achieved with a lower ICS dose compared with larger-particle ICS/LABA combinations. The clinical studies performed show a benefit of extrafine BDP/FF over FF in terms of lung function and the risk of exacerbations that is comparable to the effect sizes observed for other ICS/LABA combinations. Overall, the clinical development of extrafine BDP/FF demonstrates that this extrafine formulation achieves the type of health benefits expected from such a targeted ICS/LABA combination.
Acknowledgments
FUNDING
The authors declare that no funding was received.
Footnotes
D.S. has received sponsorship to attend and speak at international meetings, consultancy fees, honoraria for lecturing or attending advisory boards and research grants from various pharmaceutical companies including Almirall, AstraZeneca, Boehringer Ingelheim, Chiesi, Genentech, GlaxoSmithKline, Glenmark, Johnson and Johnson, Merck, NAPP, Novartis, Pfizer, Skypharma, Takeda, Teva, Therevance and Verona. M.C. has received honoraria for lecturing from Chiesi Farmaceutici. M.S. and S.P. are employees of Chiesi Farmaceutici. A.P. has received grants, personal fees and non-financial support from AstraZeneca, Chiesi Farmaceutici, GlaxoSmithKline, Boehringer Ingelheim, Merck Sharp & Dohme, Menarini, Novartis, Zambon, TEVA, Pfizer, Takeda and Mundipharma.
References
- Global Strategy for the Diagnosis, Management and Prevention of COPD. Global Initiative for Chronic Obstructive Lung Disease (GOLD). Available at http://www.goldcopd.org/ (2015).
- Calverley, P. et al. Combined salmeterol and fluticasone in the treatment of chronic obstructive pulmonary disease: a randomised controlled trial. Lancet 361, 449–456 (2003). [DOI] [PubMed] [Google Scholar]
- Calverley, P. et al. Maintenance therapy with budesonide and formoterol in chronic obstructive pulmonary disease. Eur. Respir. J. 22, 912–929 (2003). [DOI] [PubMed] [Google Scholar]
- Ferguson, G. T. et al. Effect of fluticasone propionate/salmeterol (250/50 microg) or salmeterol (50 microg) on COPD exacerbations. Respir. Med. 102, 1099–1108 (2008). [DOI] [PubMed] [Google Scholar]
- Szafranski, W. et al. Efficacy and safety of budesonide-formoterol in the management of chronic obstructive pulmonary disease. Eur. Respir. J. 21, 74–81 (2003). [DOI] [PubMed] [Google Scholar]
- Dransfield, M. T. et al. Once-daily inhaled fluticasone furoate and vilanterol versus vilanterol only for prevention of exacerbations of COPD: two replicate double-blind, parallel-group, randomised controlled trials. Lancet Respir. Med. 1, 210–223 (2013). [DOI] [PubMed] [Google Scholar]
- Rennard, S. I. et al. Efficacy and tolerability of budesonide/formoterol in one hydrofluoroalkane pressurized metered-dose inhaler in patients with chronic obstructive pulmonary disease: results from a 1-year randomized controlled clinical trial. Drugs 69, 549–565 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulet, L. P. et al. Tolerance to the protective effects of salmeterol on methacholine-induced bronchoconstriction: influence of inhaled corticosteroids. Eur. Respir. J. 11, 1091–1097 (1998). [DOI] [PubMed] [Google Scholar]
- Tan, K. S. , McFarlane, L. C. & Lipworth, B. J. Concomitant administration of low-dose prednisolone protects against in vivo beta-2-adrenoceptor subsensitivity induced by regular formoterol. Chest 113, 34–41 (1998). [DOI] [PubMed] [Google Scholar]
- Aziz, I. & Lipworth, B. J. A bolus of inhaled budesonide rapidly reverses airway subsensitivity and b2-adrenoceptor down-regulation after regular inhaled formoterol. Chest 115, 623–628 (1999). [DOI] [PubMed] [Google Scholar]
- Haque, R. et al. Inhaled long-acting β2 agonists enhance glucocorticoid receptor nuclear translocation and efficacy in sputum macrophages in COPD. J. Allergy Clin. Immunol. 132, 1166–1173 (2013). [DOI] [PubMed] [Google Scholar]
- Calverley, P. et al. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N. Engl. J. Med. 356, 775–789 (2007). [DOI] [PubMed] [Google Scholar]
- Celli, B. et al. Effect of pharmacotherapy on rate of decline of lung function in chronic obstructive pulmonary disease: results from the TORCH study. Am. J. Respir. Crit. Care Med. 178, 332–338 (2008). [DOI] [PubMed] [Google Scholar]
- Vestbo, J. et al. Fluticasone furoate and vilanterol and survival in chronic obstructive pulmonary disease with heightened cardiovascular risk (SUMMIT): a double-blind randomised controlled trial. Lancet. 387, 1817–1826 (2016). [DOI] [PubMed] [Google Scholar]
- Dhillon, S. & Keating, G. M. Beclometasone dipropionate/formoterol: in an HFA-propelled pressurised metered-dose inhaler. Drugs 66, 1475–1483 (2006). [DOI] [PubMed] [Google Scholar]
- Hogg, J. C. et al. The nature of small-airway obstruction in chronic obstructive pulmonary disease. N. Engl. J. Med. 350, 2645–2653 (2004). [DOI] [PubMed] [Google Scholar]
- Aggarwal, B. & Gogtay, J. Use of pressurized metered dose inhalers in patients with chronic obstructive pulmonary disease: review of evidence. Expert Rev. Respir. Med. 8, 349–356 (2014). [DOI] [PubMed] [Google Scholar]
- De Backer, W. et al. Lung deposition of BDP/formoterol HFA pMDI in healthy volunteers, asthmatic, and COPD patients. J. Aerosol. Med. Pulm. Drug Deliv. 23, 137–148 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Backer, J. et al. The effects of extrafine beclometasone/formoterol (bdp/f) on lung function, dyspnea, hyperinflation, and airway geometry in copd patients: novel insight using functional respiratory imaging. J. Aerosol. Med. Pulm. Drug. Deliv. 28, 88–99 (2014). [DOI] [PubMed] [Google Scholar]
- Calverley, P. M. et al. Beclomethasone/formoterol in the management of COPD: a randomised controlled trial. Respir. Med. 104, 1858–1868 (2010). [DOI] [PubMed] [Google Scholar]
- Cazzola, M. et al. ATS/ERS Task Force Outcomes for COPD pharmacological trials: from lung function to biomarkers. Eur. Respir. J. 31, 416–468 (2008). [DOI] [PubMed] [Google Scholar]
- Donohue, J. F. Minimal clinically important differences in COPD lung function. COPD 2, 111–124 (2005). [DOI] [PubMed] [Google Scholar]
- Miravitlles, M. et al. Effect of exacerbations on quality of life in patients with chronic obstructive pulmonary disease: a 2-year follow up study. Thorax 59, 387–395 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurst, J. R. et al. Temporal clustering of exacerbations in chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 179, 369–374 (2009). [DOI] [PubMed] [Google Scholar]
- Langsetmo, L. , Platt, R. W. , Ernst, P. & Bourbeau, J. Under-reporting exacerbation of chronic obstructive pulmonary disease in a long-term cohort. Am. J. Respir Crit. Care Med. 177, 396–401 (2008). [DOI] [PubMed] [Google Scholar]
- Vijayasaretha, K. & Stockley, R. A. Reported and unreported exacerbations of COPD: analysis by diary card. Chest 133, 34–41 (2008). [DOI] [PubMed] [Google Scholar]
- European centre for disease prevention and control. Available at http://ecdc.europa.eu/en/publications/surveillance_reports/influenza.
- Wedzicha, J. A. et al. Extrafine beclomethasone/formoterol in severe COPD patients with history of exacerbations. Respir. Med. 108, 1153–1162 (2014). [DOI] [PubMed] [Google Scholar]
- Jenkins, C. R. et al. Seasonality and determinants of moderate and severe COPD exacerbations in the TORCH study. Eur. Respir. J. 39, 38–45 (2012). [DOI] [PubMed] [Google Scholar]
- Hurst, J. R. et al. Susceptibility to exacerbation in chronic obstructive pulmonary disease. N. Engl. J. Med. 363, 1128–1138 (2010). [DOI] [PubMed] [Google Scholar]
- Leidy, N. K. et al. Measuring respiratory symptoms of COPD: performance of the EXACT- Respiratory Symptoms Tool (E-RS) in three clinical trials. Respir. Res. 15, 124 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neyt, M. , Devriese, S. , Thiry, N. & Van den Bruel, A. Tiotropium's cost-effectiveness for the treatment of COPD: a cost-utility analysis under real-world conditions. BMC Pulm. Med. 15, 10–47 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herland, K. , Akselsen, J. P. , Skjonsberg, O. H. & Bjermer, L. How representative are clinical study patients with asthma or COPD for a larger ‘real life’ population of patients with obstructive lung disease? Respir. Med. 99, 11–19 (2005). [DOI] [PubMed] [Google Scholar]
- Pascoe, S. , Locantore, N. , Dransfield, M. T. , Barnes, N. C. & Pavord, I. D. Blood eosinophil counts, exacerbations, and response to the addition of inhaled fluticasone furoate to vilanterol in patients with chronic obstructive pulmonary disease: a secondary analysis of data from two parallel randomised controlled trials. Lancet Respir. Med. 3, 435–442 (2015). [DOI] [PubMed] [Google Scholar]
- Siddiqui, S. et al. Blood Eosinophils: a biomarker of response to extrafine beclomethasone/formoterol in chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 192, 523–525 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh, D. et al. Eosinophilic inflammation in COPD: prevalence and clinical characteristics. Eur. Respir. J. 44, 1697–1700 (2014). [DOI] [PubMed] [Google Scholar]
- Singh, D. et al. Extrafine beclomethasone/formoterol compared to fluticasone/salmeterol combination therapy in COPD. BMC Pulm. Med. 12, 14 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matera, M. G. , Cardaci, V. , Cazzola, M. & Rogliani, P. Safety of inhaled corticosteroids for treating chronic pulmonary disease. Expert Opin. Drug. Saf. 14, 533–541 (2015). [DOI] [PubMed] [Google Scholar]
- Cazzola, M. et al. Effect of salmeterol and formoterol in patients with chronic obstructive pulmonary disease. Pulm. Pharmacol. 7, 103–107 (1994). [DOI] [PubMed] [Google Scholar]
- Stephenson, J. J. et al. Impact and factors associated with nighttime and early morning symptoms among patients with chronic obstructive pulmonary disease. Int. J. Chron. Obstruct. Pulm. Dis. 10, 577–586 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kew, K. M. & Seniukovich, A. Inhaled steroids and risk of pneumonia for chronic obstructive pulmonary disease. Cochrane Database Syst. Rev. 10, 3 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Festic, E. & Scanlon, P. D. Incident pneumonia and mortality in patients with chronic obstructive pulmonary disease. A double effect of inhaled corticosteroids? Am. J. Respir. Crit. Care Med. 15, 141–148 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh, S. , Amin, A. V. & Loke, Y. K. Long-term use of inhaled corticosteroids and the risk of pneumonia in chronic obstructive pulmonary disease: a meta-analysis. Arch. Intern. Med. 169, 219–229 (2009). [DOI] [PubMed] [Google Scholar]
- Sharafkhaneh, A. , Southard, J. G. , Goldman, M. , Uryniak, T. & Martin, U. J. Effect of budesonide/formoterol pMDI on COPD exacerbations: a double-blind, randomized study. Respir. Med. 106, 257–268 (2012). [DOI] [PubMed] [Google Scholar]
- Kardos, P. , Wencker, M. , Glaab, T. & Vogelmeier, C. Impact of salmeterol/fluticasone propionate versus salmeterol on exacerbations in severe chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 175, 144–149 (2007). [DOI] [PubMed] [Google Scholar]
- Anzueto, A. et al. Effect of fluticasone propionate/salmeterol (250/50) on COPD exacerbations and impact on patient outcomes. COPD 6, 320–329 (2009). [DOI] [PubMed] [Google Scholar]
- Calverley, P. M. Minimal clinically important difference—exacerbations of COPD. COPD 2, 143–148 (2005). [DOI] [PubMed] [Google Scholar]