Abstract
The synthesis of novel phosphatidylcholines with geranic and citronellic acids in sn-1 and sn-2 positions is described. The structured phospholipids were obtained in high yields (59–87%) and evaluated in vitro for their cytotoxic activity against several cancer cell lines of different origin: MV4-11, A-549, MCF-7, LOVO, LOVO/DX, HepG2 and also towards non-cancer cell line BALB/3T3 (normal mice fibroblasts). The phosphatidylcholines modified with monoterpene acid showed a significantly higher antiproliferative activity than free monoterpene acids. The highest activity was observed for the terpene-phospholipids containing the isoprenoid acids in sn-1 position of phosphatidylcholine and palmitic acid in sn-2.
Introduction
Isoprenoids are one of the most widely occurring group of natural compounds. Many of them exhibit wide range of biological activities. Among documented useful properties of isoprenoids the following activities need to be mentioned: fungicidal, antibacterial, antiviral and anti-inflammatory [1–4]. The results of many studies show that they inhibit proliferation of cancer cell lines, causing inhibition of cell division and transition of cells into the phase of programmed cell death [5,6]. The administered doses, even in high concentrations do not cause such an effect in the case of healthy cells [7].
In recent years there has been also a rapid progress in the field of bioengineering and phospholipid technology [8,9]. Many new discoveries in this area completely changed the idea that phospholipids are just the integral components of biomembrane ensuring their integrity and functionality and energetic and supplementary material [10,11]. It is currently known that phospholipids are also involved in many metabolic and neurological reactions [12,13]. They regulate fundamental biological processes and play an important role in the transmission of information as intracellular mediators [14,15]. For the description of phospholipids-protein interactions, the mechanisms of their action in membrane as signaling molecules and relation between their structure and activity the key point is preparation of a great number of phospholipid derivatives. Especially important in this area is development of new synthetic methods for the preparation of biologically active phospholipids (PLs) with a defined structure and high purity.
Keeping in view of the properties of isoprenoids and phospholipids the production of novel hybrid molecules with the combination of these two biologically important products could result in bioactive analogues—terpene-phospholipids, which could be characterized by enhanced biological activities. It has been reported that phospholipid modifications of drugs is the method of modulation their polarity, to prevent degradation and enhance the biological activity of molecules attached to the PLs [16–20].
Materials and Methods
General Procedures
Geranic acid (mixture of cis/trans isomers; 23:67) (1a) and racemic citronellic acid (1b) were purchased from Sigma–Aldrich Chemical Co. Phospholipase A2 (Lecitase 10L; 10,000 LEU/mL) was a gift from Novozymes. The enantiomerically pure GPC was purchased from Bachem and converted to the cadmium chloride complex (GPC × CdCl2) using the method described elsewhere. All of the solvents used in liquid chromatography were of HPLC grade (LiChrosolv Reagents) and were purchased from Merck.
Analytical TLC was performed on silica gel 60, F254 plates (Merck) with mixture of chloroform: methanol: water (65:25:4; v/v/v) as developing system. Products were detected by spraying the plates with a solution of 10 g of Ce(SO4)2 and 20 g of phosphomolybdic acid in 1 L of 10% H2SO4 followed by heating or 0.05% primuline solution (8: 2; acetone/H2O; v/v) followed by UV (365 nm). Column chromatography was performed on silica gel (Kieselgel 60, 230–400 mesh (Merck)). 1H, 13C, and 31P NMR spectra were recorded using a Bruker Advance II 600 MHz spectrometer. Chemical shifts are given in ppm downfield from tetramethylsilane (TMS) as the internal standard. In 31P NMR spectra, chemical shifts were referenced to 85% H3PO4 as a standard. Coupling constant (J) values are given in hertz. Elemental analyses were performed on Waters ESI-Q-TOF Premier XE spectrometer using electron spray ionization (ESI) technique.
The purity of synthesized products were monitored by HPLC using an Ultimate 3000 DIONEX chromatograph equipped with a DGP-3600A dual-pump fluid control module, a TCC-3200 thermostatted column compartment, and a WPS-3000 autosampler. A Corona charged aerosol detector (CAD) from ESA Biosciences was used. The acquisition range 100 pA, digital filter set to none, the N2 pressure was 0.24 MPa. The system was controlled and data acquisition was carried out using the Chromeleon 6.80 software (Dionex Corporation). HPLC analysis was carried out using a Betasil DIOL 5-μm column (Thermo, 150 × 2.1 mm).
The column temperature was maintained at 30°C. The gradient had a constant flow rate 1.5 mL min-1 with solvent A = 1% HCOOH, 0.1% triethylamine (TEA) in water, B = hexane, and C = propan-2-ol. Gradient time: 3/40/57 (%A/%B/%C), at 4 min 10/40/50, at 9 min 10/40/50, at 9.1 min 3/40/57 and at 19 min 43/40/57. The total time of analysis was 19 min. The injection volume was 15 μL in all of the experiments and the cooling temperature for the samples was 20°C.
Preparation of Cadmium Chloride Complex of GPC (GPC × CdCl2)
The solution of sn-glycero-3-phosphocholine (GPC) (10 g, 38.7 mmol) in methanol (65 mL) was added to a solution of cadmium chloride (CdCl2 × 21/2H2O) (8.88 g, 38.7 mmol) in cold water (30 mL). A heavy white precipitate appeared and was stored at 0°C for 4 h. The precipitate (GPC × CdCl2) was next filtered, lyophilized and then dried in an Abderhalden’s drying pistol over P2O5 at the boiling temperature of acetone to give 15.65 g of white powder in 91% yield.
General procedure for the 1,2-diacylation of GPC × CdCl2 (3a, 3b)
The cadmium complex of GPC has been acetylated with isoprenoid acids in positions sn-1 and sn-2. Geranic acid (1a) or citronellic acid (1b) (0.92 mmol) was dried by repeated evaporation with an anhydrous CH2Cl2 and then dissolved in anhydrous CH2Cl2 (1 mL). To the 100 mg (0.23 mmol) of GPC × CdCl2 complex added 56 mg (0.46 mmol) of DMAP and the solution of 200 mg of DCC (0.97 mmol) in 6 mL of the anhydrous CH2Cl2. The reaction flask was flushed with dry N2 and next tightly sealed. The mixture was stirred at room temperature under protection from light for 72 h. After this time (TLC) the formed during the reaction course precipitate was removed using a Schott funnel. The solution was mixed with ion-exchange resin (DOWEX 50W X8, H+ form) for 30 min to dislodge the cadmium chloride and DMAP. DOWEX was filtered off and the solvent was evaporated under vacuum. The crude PC was purified by silica gel column chromatography (65: 25: 4, CHCl3/CH3OH/H2O, (v/v/v)). Fractions containing products (Rf 0.3) were collected and evaporated under vacuum (45°C) to afford 1,2-diisoprenoyl-sn-glycero-3-phosphocholine 3 as a mixture of two stereoisomers.
1',2'-di(E)-geranoyl)-sn-glycero-3'-phosphocholine + 1',2'-di(Z)-geranoyl)-sn-glycero-3'-phosphocholine (3a)
Colourless greasy solid (82% yield, 99% purity (according to HPLC)); 1H NMR (600 MHz, CDCl3/CD3OD 2:1 (v/v)), δ: 1.29 (two s, 12H, CH3-9sn-1 (A), CH3-9sn-1 (B), CH3-9sn-2 (A), CH3-9sn-2 (B)), 1.36 (s, 12H, CH3-8sn-1 (A), CH3-8sn-1 (B), CH3-10sn-2 (A), CH3-10sn-2 (B)), 1.81–1.85 (m, 8H, CH2-4sn-1 (A), CH2-4sn-1 (B), CH2-5sn-2 (A), CH2-5sn-2 (B)), 2.91 (s, 18H, -N(CH3)3 (A), N(CH3)3 (B)), 3.32–3.35 (m, 4H, CH2-β (A), CH2-β (B)), 3.70–3.77 (m, 4H, CH2-3' (A), CH2-3' (B)), 3.91 (dd, J = 12.0, 6.6 Hz, 2H, one of CH2-1' (A), one of CH2-1' (B)), 3.96–4.01 (m, 4H, CH2-α (A), CH2-α (B), 4.06 (m, 2H, one of CH2-1' (A), one of CH2-1' (B), 4.80 (m, 2H, H-6sn-1 (B), H-6sn-2 (B)), 4.84 (m, 2H, H-6sn-1 (A), H-6sn-2 (A)), 4.94 (m, 2H, H-2' (A), H-2' (B)), 5.35 (s, 4H, H-2sn-1 (A), H-2sn-1 (B), H-2sn-2 (A), H-2sn-2 (B)); 13C NMR (151 MHz, CDCl3/CD3OD 2:1 (v/v)) δ: 15.39, 15.44, 15.46, 15.49, 24.70, 24.74, 24.88 (C-8sn-1 (A), C-8sn-1 (B), C-8sn-2 (A), C-8sn-2 (B), C-10sn-1 (A), C-10sn-1 (B), C-10sn-2 (A), C-10sn-2 (B)), 16.88, 16.89, 16.93, 16.95 (C-9sn-1 (A), C-9sn-1 (B), C-9sn-2 (A), C-9sn-2 (B)), 25.58, 25.59, 26.26, 26.28 (C-5sn-1 (A), C-5sn-1 (B), C-5sn-2 (A), C-5sn-2 (B)), 40.52, 40.54, 40.55, 40.57 (C-4sn-1 (A), C-4sn-1 (B), C-4sn-2 (A), C-4sn-2 (B)), 53.65 (t, J = 3.7 Hz, -N(CH3)3 (A), -N(CH3)3 (B)), 58.59 (d, J = 4.8 Hz, C-α (A), C-α (B)), 61.73 (C-1' (A), C-1' (B)), 63.24 (d, J = 4.8 Hz, C-3' (A), C-3' (B)), 66.10 (m, C-β (A), C-β (B)), 70.28 (d, J = 8.4 Hz, C-2' (A), C-2' (B)), 114.49, 114.60, 115.00, 115.10 (C-2sn-1 (A), C-2sn-1 (B), C-2sn-2 (A), C-2sn-2 (B)), 121.85, 121.88, 122.35, 122.37 (C-6sn-1 (A), C-6sn-1 (B), C-6sn-2 (A), C-6sn-2 (B)), 128.11, 128.15 (C-7sn-1 (A), C-7sn-1 (B), C-7sn-2 (A), C-7sn-2 (B)), 131.44, 132.13 (C-3sn-1 (A), C-3sn-1 (B), C-3sn-2 (A), C-3sn-2 (B)), 161.05, 161.19, 165.69, 165.78 (C-1sn-1 (A), C-1sn-1 (B), C-1sn-2 (A), C-1sn-2 (B)); 31P NMR (243 MHz, CDCl3/CD3OD 2:1 (v/v)) δ: -0.63; HRMS (ESI): m/z calcd. for C28H48NO8P [M + H]+ 558.3196; found 558.3217
(2'R)-1',2'-di[(3R)-3,7-dimethylocta-6-enyl]-sn-glycero-3'-phosphocholine + (2'R)-1',2'-di[(3S)-3,7-dimethylocta-6-enyl]-sn-glycero-3'-phosphocholine (3b)
Colourless greasy solid (86% yield, 99% purity (according to HPLC)); 1H NMR (600 MHz, CDCl3/CD3OD 2:1 (v/v)), δ: 0.70, 0.71 (two d, J = 7.8 Hz, 12H, CH3-9sn-1 (A), CH3-9sn-1 (B), CH3-9sn-2 (A), CH3-9sn-2 (B)), 0.99 (m, 4H, one of CH2-4sn-1 (A), one of CH2-4sn-1 (B), one of CH2-4sn-2 (A), one of CH2-4sn-2 (B)), 1.12 (m, 4H, one of CH2-4sn-1 (A), one of CH2-4sn-1 (B), one of CH2-4sn-2 (A), one of CH2-4sn-2 (B)), 1.36, 1.44 (two s, 24H, CH3-8sn-1 (A), CH3-8sn-1 (B), CH3-10sn-1 (A), CH3-10sn-1 (B), CH3-8sn-2 (A), CH3-8sn-2 (B), CH3-10sn-2 (A), CH3-10sn-2 (B)), 1.66–1.81 (m, 12H, CH2-5sn-1 (A), CH2-5sn-1 (B), CH2-5sn-2 (A), CH2-5sn-2 (B), H-3sn-1 (A), H-3sn-1 (B), H-3sn-2 (A), H-3sn-2 (B)), 1.86–1.95 (m, 4H, one of CH2-2sn-1 (A), one of CH2-2sn-1 (B), one of CH2-2sn-2 (A), one of CH2-2sn-2 (B)), 2.07–2.14 (m, 4H, one of CH2-2sn-1 (A), one of CH2-2sn-1 (B), one of CH2-2sn-2 (A), one of CH2-2sn-2 (B)), 2.99 (s, 18H, -N(CH3)3 (A), N(CH3)3 (B)), 3.38–3.39 (m, 4H, CH2-β (A), CH2-β (B)), 3.77 (m, 4H, CH2-3' (A), CH2-3' (B)), 3.92 (m, 2H, one of CH2-1' (A), one of CH2-1' (B)), 4.03 (m, 4H, CH2-α (A), CH2-α (B)), 4.19 (m, 2H, one of CH2-1' (A), one of CH2-1' (B)), 4.84 (t, J = 7.2 Hz, 4H, H-6sn-1 (A), H-6sn-1 (B), H-6sn-2 (A), H-6sn-2 (B)), 5.00 (m, 2H, H-2' (A), H-2' (B)); 13C NMR (151 MHz, CDCl3/CD3OD 2:1 (v/v)) δ: 16.91, 24.96 (C-8sn-1 (A), C-8sn-1 (B), C-8sn-2 (A), C-8sn-2 (B), C-10sn-1 (A), C-10sn-1 (B), C-10sn-2 (A), C-10sn-2 (B)), 18.77, 18.78. 18.85 (C-9sn-1 (A), C-9sn-1 (B), C-9sn-2 (A), C-9sn-2 (B)), 24.88 (C-5sn-1 (A), C-5sn-1 (B), C-5sn-2 (A), C-5sn-2 (B)), 29.49, 29.53, 29.56 (C-3sn-1 (A), C-3sn-1 (B), C-3sn-2 (A), C-3sn-2 (B)), 36.20, 36.23 (C-4sn-1 (A), C-4sn-1 (B), C-4sn-2 (A), C-4sn-2 (B)), 40.99, 41.15, 41.16 (C-2sn-1 (A), C-2sn-1 (B), C-2sn-2 (A), C-2sn-2 (B)), 53.58 (t, J = 3.5 Hz, -N(CH3)3 (A), -N(CH3)3 (A)), 58.75 (d, J = 4.8 Hz, C-α (A), C-α (B)), 62.21 (C-1' (A), C-1' (B)), 63.27 (d, J = 4.7 Hz, C-3' (A), C-3' (B)), 65.93 (m, C-β (A), C-β (B)), 69.89, (d, J = 7.7 Hz, C-2' (A)), 69.91 (d, J = 8.3 Hz, C-2' (B)), 123.64 (C-6sn-1 (A), C-6sn-1 (B), C-6sn-2 (A), C-6sn-2 (B)), 131.09 (C-7sn-1 (A), C-7sn-1 (B), C-7sn-2 (A), C-7sn-2 (B)), 172.55, 172.56, 172.97 (C-1sn-1 (A), C-1sn-1 (B), C-1sn-2 (A), C-1sn-2 (B)); 31P NMR (243 MHz, CDCl3/CD3OD 2:1 (v/v)) δ: -0.79; HRMS (ESI): m/z calcd. for C28H52NO8P [M + H]+ 562.3509; found 562.3530
General procedure for 1,2-dipalmitoyl-sn-glycero-3-phosphocholine enzymatic hydrolysis
Hydrolysis of 1,2-dipalmitoyl-sn-glycero-3-phosphocholine (5) was carried out according to the method described by Morgado et al. [21]. The detailed data were described earlier [22]. 1-Palmitoyl-2-hydroxy-sn-glycero-3-phosphocholine (6) was obtained in 99% yield and its physical and spectroscopic data were consistent with those found in literature [23].
General procedure for 1-palmitoyl-2-isoprenoyl-sn-glycero-3-phosphocholine (7a, 7b) synthesis
Terpene acids (1a or 1b, 0.604 mmol), solution of DMAP (74 mg, 0.6 mmol) in 3 mL of CH2Cl2 and finally a solution of DCC (267 mg, 1.3 mmol) in 5 mL of CH2Cl2 were added to the solution of 1-palmitoyl-2-hydroxy-sn-3-glycero-phosphocholine (6) (150 mg, 0.302 mmol) dissolved in 4 mL of anhydrous CH2Cl2. The reaction was carried out for 72 h under a nitrogen atmosphere in the dark at 40°C. The product was extracted according to the method described for compound 3a and 3b. 1-Palmitoyl-2-isoprenoyl-sn-glycero-3-phosphocholine (7) was obtained as the mixture of two stereoisomers.
1'-palmitoyl-2'-(E)-geranoyl-sn-glycero-3'-phosphocholine + 1'-palmitoyl-2'-(Z)-geranoyl-sn-glycero-3'-phosphocholine (7a)
Colourless greasy solid (59% yield, 98% purity (according to HPLC)); 1H NMR (600 MHz, CDCl3/CD3OD 2:1 (v/v)) δ: 0.60 (t, J = 6.6 Hz, 6H, CH3(CH2)14C(O) (A), CH3(CH2)14C(O) (B)), 1.98–1.02 (m, 48H, CH3(CH2)12CH2CH2C(O) (A), CH3(CH2)12CH2CH2C(O) (B)), 1.32 (m, 4H, CH3(CH2)13CH2CH2C(O) (A), CH3(CH2)13CH2CH2C(O) (B)), 1.35 (s, 6H, CH3-9 (A), CH3-9 (B)), 1.41 (s, 12H, CH3-8 (A), CH3-8 (B), CH3-10 (A), CH3-10 (B)), 1.85–1.91 (m, 8H, CH2-4 (A), CH2-4 (B), CH2-5 (A), CH2-5 (B)), 2.03 (m, 4H, CH3(CH2)13CH2C(O) (A), CH3(CH2)13CH2C(O) (B)), 2.94 (s, 18H, -N(CH3)3 (A), -N(CH3)3 (B)), 3.31–3.35 (m, 4H, CH2-β (A), CH2-β (B)), 3.72 (m, 4H, CH2-3' (A), CH2-3' (B)), 3.92 (m, 2H, one of CH2-1' (A), one of CH2-1' (B), 3.96–4.00 (m, 4H, CH2-α (A), CH2-α (B)), 4.14 (m, 2H, one of CH2-1' (A), one of CH2-1' (B)), 4.80, 4.85 (two t, J = 6.6 Hz, 2H, H-6 (A), H-6 (B)), 4.96–5.05 (two m, 2H, H-2' (A), H-2' (B)), 5.39 (s, 2H, H-2 (A), H-2 (B)); 13C NMR (151 MHz, CDCl3/CD3OD 2:1 (v/v)) δ: 13.37 (CH3CH2(CH2)11CH2CH2C(O) (A), CH3CH2(CH2)11CH2CH2C(O) (B)), 16.94, 16.95 (C-9 (A), C-9 (B)), 22.15 (CH3CH2(CH2)11CH2CH2C(O) (A), CH3CH2(CH2)11CH2CH2C(O) (B)), 24.33, 24.34 (CH3CH2(CH2)11CH2CH2C(O) (A), CH3CH2(CH2)11CH2CH2C(O) (B)), 24.90 (C-8 (A), C-10 (A), C-8 (B), C-10 (B)), 25.53, 25.55 (C-5(A), C-5 (B)), 28.63, 28.77, 28.84, 28.98, 29.11, 29.13, 29.16 (CH3CH2(CH2)11CH2CH2C(O) (A), CH3CH2(CH2)11CH2CH2C(O) (B), 33.54 (CH3CH2(CH2)11CH2CH2C(O) (A), CH3CH2(CH2)11CH2CH2C(O) (B)), 40.53, 40.56 (C-4 (A), C-4 (B)), 53.56 (m, -N(CH3)3 (A), -N(CH3)3 (B)), 58.59 (d, J = 4.7 Hz, C-α (A), C-α (B)), 62.20 (C-1' (A), C-1' (B)), 63.06, 63.11 (two d, J = 5.2 Hz, C-3' (A), C-3' (B)), 65.95–65.99 (two m, C-β (A), C-β (B)), 70.24, 70.31 (two d, J = 7.8 Hz, C-2' (A), C-2' (B)), 114.93, 114.41 (C-2 (A), C-2 (B)), 121.78, 121.80 (C-6 (A), C-6 (B)), 126.89, 127.14 (C-7 (A), C-7 (B)), 127.70, 128.12 (C-3 (A), C-3 (B)), 161.45, 165.68 (C-1 (A), C-1 (B)), 173.57, 173.68 (CH3CH2(CH2)11CH2CH2C(O) (A), CH3CH2(CH2)11CH2CH2C(O) (B)); 31P NMR (243 MHz, CDCl3/CD3OD 2:1 (v/v)) δ: 0.64; HRMS (ESI): m/z calcd. for C34H64NO8P [M + H]+ 646.4448; found 646.4451
(2'R)-1'-palmitoyl-2'-[(3R)-3,7-dimethylocta-6-enyl]-sn-glycero-3'-phosphocholine + (2'R)-1'-palmitoyl-2'-[(3S)-3,7-dimethylocta-6-enyl]-sn-glycero-3'-phosphocholine (7b)
Colourless greasy solid (62% yield, 99% purity (according to HPLC)); 1H NMR (600 MHz, CDCl3/CD3OD 2:1 (v/v)), δ: 0.64 (t, J = 6.6 Hz, 6H, CH3(CH2)14C(O) (A), CH3(CH2)14C(O) (B)), 0.72 (d, J = 7.2 Hz, 6H, CH3-9 (A), CH3-9 (B)), 0.96–1.05 (m, 50H, CH3(CH2)12CH2CH2C(O) (A), CH3(CH2)12CH2CH2C(O) (B), one of CH2-4 (A), one of CH2-4 (B)), 1.12 (m, 2H, one of CH2-4 (A), one of CH2-4 (B)), 1.31–1.39 (m, 4H, CH3(CH2)13CH2CH2C(O) (A), CH3(CH2)13CH2CH2C(O) (B)), 1.36, 1.44 (two s, 12H, CH3-8 (A), CH3-8 (B), CH3-10 (A), CH3-10 (B)), 1.65–1.81 (m, 6H, CH2-5 (A), CH2-5 (B), H-3 (A), H-3 (B)), 1.87–1.93 (two dd, J = 15.0, 8.4 Hz, 2H, one of CH2-2 (A), one of CH2-2 (B)), 2.07 (t, J = 7.8 Hz, 4H, CH3(CH2)13CH2C(O) (A), CH3(CH2)13CH2C(O) (B)), 2.09–2.15 (two dd, J = 15.0, 6.0 Hz, 1H, one of CH2-2 (A), one of CH2-2 (B)), 2.99 (s, 18H, -N(CH3)3 (A), -N(CH3)3 (B)), 3.39 (m, 4H, CH2-β (A), CH2-β (B)), 3.77 (m, 4H, CH2-3' (A), CH2-3’ (B), 3.92 (m, 2H, one of CH2-1' (A), one of CH2-1' (B)), 4.03 (m, 4H, CH2-α (A), CH2-α (B)), 4.17 (m, 2H, one of CH2-1' (A), one of CH2-1' (B)), 4.84 (t, J = 6.6 Hz, 2H, H-6 (A), H-6 (B)), 5.00 (m, 2H, H-2' (A), H-2' (B)); 13C NMR (151 MHz, CDCl3/CD3OD 2:1 (v/v)) δ: 13.44 (CH3(CH2)14C(O) (A), CH3(CH2)14C(O) (B)), 16.97, 25.02 (C-8 (A), C-8 (B), C-10 (A), C-10 (B)), 18.93 (C-9 (A), C-9 (B)), 22.20 (CH3CH2(CH2)13C(O) (A), CH3CH2(CH2)13C(O) (B)), 24.45 (CH3(CH2)12CH2CH2C(O) (A), (CH3(CH2)12CH2CH2C(O) (B)), 24.94 (C-5 (A), C-5 (B)), 28.67, 28.87, 28.89, 29.06, 29.19, 29.21, 29.23, 31.47 (CH3CH2(CH2)11CH2CH2C(O) (A), CH3CH2(CH2)11CH2CH2C(O) (B)), 29.55, 29.56 (C-3 (A), C-3 (B)), 33.78 (CH3(CH2)13CH2C(O) (A), CH3(CH2)13CH2C(O) (B)), 36.28 (C-4 (A), C-4 (B)), 41.06 (C-2 (A), C-2 (B)), 53.66 (t, J = 3.6 Hz, -N(CH3)3 (A), -N(CH3)3 (B)), 58.79 (d, J = 4.9 Hz, C-α (A), C-α (B)), 62.12, 62.14 (C-1' (A), C-1' (B)), 63.39 (d, J = 5.1 Hz, C-3' (A), C-3' (B)), 65.00 (m, C-β (A), C-β (B)), 69.95 (d, J = 7.8 Hz, C-2' (A), C-2' (B)), 123.68 (C-6 (A), C-6 (B)), 131.13 (C-7 (A), C-7 (B)), 172.95 (C-1 (A), C-1 (B)), 173.19 (CH3(CH2)13CH2C(O) (A), CH3(CH2)13CH2C(O) (B); 31P NMR (243 MHz, CDCl3/CD3OD 2:1 (v/v)) δ: -0.83; HRMS (ESI): m/z calcd. for C34H66NO8P [M + H]+ 648.4604; found 648.4615
General procedure the synthesis of 1-isoprenoyl-2-hydroxy-sn-glycero-3-phosphocholine (9a, 9b)
The synthesis of 1-isoprenoyl-2-hydroxy-sn-glycero-3-phosphocholine (9a, 9b) was carried out according to the procedure described by Niezgoda et al. [24]. GPC (300 mg, 1.16 mmol) and dibutyltin oxide (DBTO) (290 mg, 1.17 mmol) were suspended in 10 mL of anhydrous propan-2-ol and refluxed for 1 h. The reaction was cooled to an ambient temperature and TEA (2.4 mmol) was added followed by terpene chloride (281 mg, 2.4 mmol). After 1 h of the reaction, the mixture was filtreted using diatomaceous earth (Celite® 545) and washed CH2Cl2. The organic solvent was evaporated under vacuum (45°C), after which the crude residue was purified on a silica gel column (65: 25: 4 CHCl3/CH3OH/H2O, (v/v/v)). Product-containing fractions of Rf 0.1 were collected and solvent evaporated under vacuum (45°C) to gave 1-isoprenoyl-2-hydroxy-sn-glycero-3-phosphocholine (9a, 9b). The chemical structure was confirmed by 1H and 13C NMR spectra.
1'-(E)-geranoyl-2'-hydroxy-sn-glycero-3'-phosphocholine + 1'-(Z)-geranoyl-2'-hydroxy-sn-glycero-3'-phosphocholine) (9a)
Colourless greasy solid (75% yield, 99% purity (according to HPLC)); 1H NMR (600 MHz, CDCl3/CD3OD 2:1 (v/v)), δ: 1.38 (s, 6H, CH3-9 (A), CH3-9 (B)), 1.45 (s, 24H, CH3-8 (A), CH3-8 (B), CH3-10 (A), CH3-10 (B)), 1.89–1.84 (m, 8H, CH2-4 (A), CH2-4 (B), CH2-5 (A), CH2-5 (B)), 2.99 (s, 18H, -N(CH3)3 (A), -N(CH3)3 (B)), 3.37–3.39 (m, 4H, CH2-β (A), CH2-β (B)), 3.65 (m, 4H, one of CH2-3' (A), one of CH2-3' (B)), 3.71–3.78 (two m, 4H, H-2' (A), H-2' (B), one of CH2-3' (A), one of CH2-3' (B)), 3.87–3.96 (two m, 4H, CH2-1' (A), CH2-1' (B)), 4.02–4.05 (m, 4H, CH2-α (A), CH2-α (B)), 4.86 (m, 2H, H-6 (A), H-6 (B)), 5.48 (s, 2H, H-2 (A), H-2 (B)); 13C NMR (151 MHz, CDCl3/CD3OD 2:1 (v/v)) δ: 17.00, 18.24 (C-9 (A), C-9 (B)), 24.97 (C-8 (A), C-8 (B), C-10 (A), C-10 (B)), 25.58 (C-5 (A), C-5 (B)), 40.56, 41.19 (C-4 (A), C-4 (B)), 53.63 (t, J = 3.8 Hz, -N(CH3)3 (A), -N(CH3)3 (B)), 58.63 (d, J = 4.9 Hz, C-α (A), C-α (B)), 64.88 (C-1' (A), C-1' (B)), 66.03 (m, C-β (A), C-β (B)), 66.41 (d, J = 5.6 Hz, C-3' (A)), 66.58 (d, J = 5.7 Hz, C-3' (B)), 68.24 (d, J = 6.6 Hz, C-2' (A)), 68.41 (d, J = 6.8 Hz, C-2' (B)), 113.22, 114.58 (C-2 (A), C-2 (B)), 122.41, 123.12 (C-6 (A), C-6 (B)), 131.51, 132.13 (C-7 (A), C-7 (B)), 141.68 (C-3 (A), C-3 (B)), 160.88, 166.63 (C-1 (A), C-1 (B)); 31P NMR (243 MHz, CDCl3/CD3OD 2:1 (v/v)) δ: 0.02; HRMS (ESI): m/z calcd. for C18H34NO7P [M + H]+ 408.2151; found 408.2175
(2'R)-1'-[(3R)-3,7-dimethylocta-6-enyl]-2'-hydroxy-sn-glycero-3'-phosphocholine + (2'R)-1'-[(3S)-3,7-dimethylocta-6-enyl]-2'-hydroxy-sn-glycero-3'-phosphocholine (9b)
Colourless greasy solid (87% yield, 98% purity (according to HPLC)); 1H NMR (600 MHz, CDCl3/CD3OD 2:1 (v/v)), δ: 0.72 (d, J = 6.6 Hz, 6H, CH3-9 (A), CH3-9 (B)), 0.96–1.15 (two m, 4H, CH2-4 (A), CH2-4 (B)), 1.37, 1.45 (two s, 12H, CH3-8 (A), CH3-8 (B), CH3-10 (A), CH3-10 (B)), 1.70–1.82 (m, 6H, CH2-5 (A), CH2-5 (B), H-3 (A), H-3 (B)), 1.93 (dd, J = 15.0, 8.4 Hz, 2H, one of CH2-2 (A), one of CH2-2 (B)), 2.14 (dd, J = 15.0, 5.4 Hz, 2H, one of CH2-2 (A), one of CH2-2 (B)), 2.99 (s, 18H, -N(CH3)3 (A), -N(CH3)3 (B)), 3.37–3.39 (m, 4H, CH2-β (A), CH2-β (B)), 3.62–3.73 (two m, 4H, CH2-3' (A), CH2-3' (B)), 3.74–3.77 (m, 2H, H-2' (A), H-2' (B)), 3.87–3.95 (two m, 4H, CH2-1' (A), CH2-1' (B)), 4.02–4.04 (m, 4H, CH2-α (A), CH2-α (B)), 4.85 (t, J = 7.2 Hz, 2H, H-6 (A), H-6 (B)); 13C NMR (151 MHz, CDCl3/CD3OD 2:1 (v/v)) δ: 16.92, 24.98 (C-8 (A), C-8 (B) C-10 (A), C-10 (B)), 18.86 (C-9 (A), C-9 (B)), 24.91 (C-5 (A), C-5 (B)), 29.53 (C-3 (A), C-3 (B)), 36.30 (C-4 (A), C-4 (B)), 41.09 (C-2 (A), C-2 (B)), 53.61 (t, J = 3.8 Hz, -N(CH3)3 (A), -N(CH3)3 (B), 58.62 (d, J = 5.1 Hz, C-α (A), C-α (B)), 64.49 (C-1' (A), C-1' (B)), 66.01 (m, C-β (A), C-β (B)), 66.47 (d, J = 5.6 Hz, C-3' (A), C-3' (B)), 68.30 (d, J = 6.5 Hz, C-2' (A), C-2' (B)), 123.72 (C-6 (A), C-6 (B)), 131.08 (C-7 (A), C-7 (B)), 173.39 (C-1 (A), C-1 (B)); 31P NMR (243 MHz, CDCl3/CD3OD 2:1 (v/v)) δ: -0.03; HRMS (ESI): m/z calcd. for C18H36NO7P [M + H]+ 410.23075; found 410.2311
General procedure for the synthesis of 1-isoprenoyl-2-palmitoyl-sn-glycero-3-phosphocholine (10a, 10b)
Compounds 10a and 10b were prepared from 1-isoprenoyl-2-hydroxy-sn-glycero-3-phosphocholines (9a, 9b) (0.14 mmol) by adding palmitic acid (0.56 mmol) dissolved in 2 mL of anhydrous CH2Cl2, DMAP (0.28 mmol) and finally a solution of DCC (0.58 mmol) in 3 mL of CH2Cl2. The reaction was carried out for 72 h under a nitrogen atmosphere in the dark at 40°C. The product was extracted according to the method described for compounds 3a, 3b and pure 1-isoprenoyl-2-palmitoyl-sn-glycero-3-phosphocholine (10) was obtained as the mixture of two stereoisomers.
1'-(E)-geranoyl-2'-palmitoyl-sn-glycero-3'-phosphocholine + 1'-(Z)-geranoyl-2'-palmitoyl-sn-glycero-3'-phosphocholine) (10a)
Colourless greasy solid (67% yield, 99% purity (according to HPLC)); 1H NMR (600 MHz, CDCl3/CD3OD 2:1 (v/v)) δ: 0.63 (t, J = 6.6 Hz, 6H, CH3(CH2)14C(O) (A), CH3(CH2)14C(O) (B)), 1.03–1.04 (m, 48H, CH3(CH2)12CH2CH2C(O)), 1.35 (m, 4H, CH3(CH2)13CH2CH2C(O) (A), CH3(CH2)13CH2CH2C(O) (B)), 1.36 (s, 6H, CH3-9 (A), CH3-9 (B)), 1.43 (s, 12H, CH3-8, CH3-10), 1.86–1.92 (two m, 8H, CH2-4 (A), CH2-4 (B), CH2-5 (A), CH2-5 (B)), 2.07 (t, J = 6.6 Hz, 4H, CH3(CH2)13CH2C(O) (A), CH3(CH2)13CH2C(O) (B)), 2.98 (s, 18H, -N(CH3)3 (A), -N(CH3)3 (B),), 3.36–3.39 (m, 4H, CH2-β (A), CH2-β (B)), 3.77 (m, 4H, CH2-3' (A), CH2-3' (B)), 3.94 (m, 2H, one of CH2-1' (A), one of CH2-1' (B)), 4.00–4.05 (m, 4H, CH2-α (A), CH2-α (B)), 4.18 (m, 2H, one of CH2-1' (A), one of CH2-1' (B)), 4.83 (m, 2H, H-6 (A). H-6 (B)), 4.96–5.03 (m, 2H, H-2' (A), H-2' (B)), 5.41 (s, 2H, H-2 (A), H-2 (B)); 13C NMR (151 MHz, CDCl3/CD3OD 2:1 (v/v)) δ: 13.38 (CH3CH2(CH2)11CH2CH2C(O) (A), CH3CH2(CH2)11CH2CH2C(O) (B)), 16.95, 16.97 (C-9 (A), C-9 (B)), 22.16 (CH3CH2(CH2)11CH2CH2C(O) (A), CH3CH2(CH2)11CH2CH2C(O) (B)), 24.38, 24.43 (CH3CH2(CH2)11CH2CH2C(O) (A), CH3CH2(CH2)11CH2CH2C(O) (B)), 24.92 (C-8 (A), C-8 (B), C-10 (A), C-10 (B)), 25.55 (C-5 (A), C-5 (B)), 28.59, 28.63, 28.82, 28.85, 29.01, 29.14, 29.16, 29.18 (CH3CH2(CH2)11CH2CH2C(O) (A), CH3CH2(CH2)11CH2CH2C(O) (B)), 33.70, 33.76 (CH3(CH2)13CH2C(O) (A), CH3(CH2)13CH2C(O) (B)), 40.54, 41.13 (C-4 (A), C-4 (B)), 53.57 (t, J = 3.2 Hz, -N(CH3)3 (A), -N(CH3)3 (A)), 58.69 (d, J = 4.7 Hz, C-α (A), C-α (B)), 62.50 (C-1' (A), C-1' (B)), 63.17 (d, J = 5.1 Hz, C-3' (A)), 63.37 (d, J = 4.8 Hz, C-3' (B)), 65.94 (m, C-β (A), C-β (B)), 69.85 (d, J = 7.8 Hz, C-2' (A)), 70.08 (d, J = 8.0 Hz, C-2' (B)), 113.31, 114.32 (C-2 (A), C-2 (B)), 122.32, 123.03 (C-6 (A), C-6 (B)), 131.48, 132.09 (C-7 (A), C-7 (B)), 141.46 (C-3 (A), C-3 (B)), 161.13, 166.16 (C-1 (A), C-1 (B)), 173.13, 173.23 (CH3CH2(CH2)11CH2CH2C(O) (A), CH3CH2(CH2)11CH2CH2C(O) (B)); 31P NMR (243 MHz, CDCl3/CD3OD 2:1 (v/v)) δ: 0.83; HRMS (ESI): m/z calcd. for C34H64NO8P [M + H]+ 646.4448; found 646.4474
(2'R)-2'-palmitoyl-1'-[(3R)-3,7-dimethylocta-6-enyl]-sn-glycero-3'-phosphocholine + (2'R)-2'-palmitoyl-1'-[(3S)-3,7-dimethylocta-6-enyl]-sn-glycero-3'-phosphocholine (10b)
Colourless greasy solid (70% yield, 99% purity (according to HPLC)); 1H NMR (600 MHz, CDCl3/CD3OD 2:1 (v/v)) δ: 0.64 (t, J = 6.6 Hz, 6H, CH3(CH2)14C(O) (A), CH3(CH2)14C(O) (B)), 0.70 (d, J = 6.6 Hz, 6H, CH3-9 (A), CH3-9 (B)), 0.90–1.34 (m, 50H, CH3(CH2)12CH2CH2C(O) (A), CH3(CH2)12CH2CH2C(O) (B), one of CH2-4 (A), one of CH2-4 (B)), 1.13 (m, 2H, one of CH2-4 (A), one of CH2-4 (B)), 1.35–1.40 (m, 4H, CH3(CH2)13CH2CH2C(O) (A), CH3(CH2)13CH2CH2C(O) (B)), 1.36, 1.44 (two s, 12H, CH3-8 (A), CH3-8 (B), CH3-10 (A), CH3-10 (B)), 1.68–1.78 (m, 6H, CH2-5 (A), CH2-5 (B), H-3 (A), H-3 (B)), 1.87–1.92 (m, 2H, one of CH2-2 (A), one of CH2-2 (B)), 2.06–2.12 (m, 2H, one of CH2-2 (A), one of CH2-2 (B)), 2.09 (t, J = 6.6 Hz, 4H, CH3(CH2)13CH2C(O) (A), CH3(CH2)13CH2C(O) (B)), 2.99 (s, 18H, -N(CH3)3 (A), -N(CH3)3 (B)), 3.39–3.40 (m, 4H, CH2-β (A), CH2-β (B)), 3.78 (m, 4H, CH2-3' (A), CH2-3’ (B), 3.93 (m, 2H, one of CH2-1' (A), one of CH2-1' (B)), 4.01–4.04 (m, 4H, CH2-α (A), CH2-α (B)), 4.20 (m, 2H, one of CH2-1' (A), one of CH2-1' (B)), 4.84 (t, J = 6.0 Hz, 2H, H-6 (A), H-6 (B)), 4.99 (m, 2H, H-2' (A), H-2' (B)); 13C NMR (151 MHz, CDCl3/CD3OD 2:1 (v/v)) δ: 13.61 (CH3(CH2)14C(O) (A), CH3(CH2)14C(O) (B)), 17.14, 25.19 (C-8 (A), C-8 (B), C-10 (A), C-10 (B)), 18.94, 18.97 (C-9 (A), C-9 (B)), 22.31 (CH3CH2(CH2)13C(O) (A), CH3CH2(CH2)13C(O) (B)), 24.49 (CH3(CH2)12CH2CH2C(O) (A), (CH3(CH2)12CH2CH2C(O) (B)), 25.03 (C-5 (A), C-5 (B)), 28.78, 28.94, 28.99, 29.14, 29.27, 29.29, 29.30, 29.32, 31.57 (CH3CH2(CH2)11CH2CH2C(O) (A), CH3CH2(CH2)11CH2CH2C(O) (B)), 29.69, 29.71 (C-3 (A), C-3 (B)), 33.72 (CH3(CH2)13CH2C(O) (A), CH3(CH2)13CH2C(O) (B)), 36.34, 36.36 (C-4 (A), C-4 (B)), 41.30 (C-2 (A), C-2 (B)), 53.81 (t, J = 3.5 Hz, -N(CH3)3 (A), -N(CH3)3 (B)), 58.86 (d, J = 4.8 Hz, C-α (A), C-α (B)), 62.41, 62.43 (C-1' (A), C-1' (B)), 63.42 (d, J = 4.8 Hz, C-3' (A), C-3' (B)), 66.09 (m, C-β (A), C-β (B)), 69.95 (d, J = 7.8 Hz, C-2' (A)), 69.96 (d, J = 7.7 Hz, C-2' (B)), 123.76 (C-6 (A), C-6 (B)), 131.27 (C-7 (A), C-7 (B)), 172.66, 172.68 (C-1 (A), C-1 (B)), 173.71 (CH3(CH2)13CH2C(O) (A), CH3(CH2)13CH2C(O) (B)); 31P NMR (243 MHz, CDCl3/CD3OD 2:1 (v/v)) δ: -0.88; HRMS (ESI): m/z calcd. for C34H66NO8P [M + H]+ 648.4604; found 648.4630
Biological studies
Cell cultures
Established in vitro, human cancer cell lines: MV4-11 (human biphenotypic B myelomonocytic leukaemia), A-549 (non-small cell lung cancer), MCF-7 (breast cancer), LoVo (colon cancer), LoVo/DX (colon cancer drug resistant), HepG2 (liver cancer) and BALB/3T3 (normal mice fibroblasts) were used.
All lines were obtained from American Type Culture Collection (Rockville, Maryland, U.S.A.). The cell line is being maintained in the Institute of Immunology and Experimental Therapy, Wroclaw, Poland.
The leukaemia cell line was cultured in RPMI 1640 medium (IIET, Wroclaw, Poland) with supplemented with 2 mM L-glutamine adjusted to contain 1.0 mM sodium pyruvate, 10% fetal bovine serum (all from Sigma-Aldrich Chemie GmbH, Steinheim, Germany). A549, LoVo and LoVo/DX cells were cultured in the mixture of RPMI 1640+Opti-MEM (1:1) (both from Gibco, Scotland, UK), MCF-7 cells in Eagle medium (IIET, Wroclaw, Poland), HepG2 in DMEM medium (Gibco, Scotland, UK), BALB/3T3 in DMEM medium (IIET, Wroclaw, Poland) supplemented with 2 mM L-glutamine and 1.0 mM sodium pyruvate, 10% fetal bovine serum (all from Sigma-Aldrich, Germany). The MCF-7 cell culture was supplemented with 0.8 mg/L of insulin (Sigma-Aldrich Chemie GmbH, Steinheim, Germany) and the LoVo/DX cell culture was supplemented with 0.1 μg/mL of doxorubicin (Accord). All culture media were supplemented with 100 units/mL penicillin, and 100 μg/mL streptomycin (both from Polfa Tarchomin S.A., Warsaw, Poland). All cell lines were grown at 37°C with 5% CO2 humidified atmosphere.
Compounds
Prior to usage, the compounds were dissolved in DMSO or in mixture of 99.8% ethanol and DMSO (1:1) to the concentration of 25 or 50 mM, and subsequently diluted in culture medium to reach the required concentrations (ranging from 5 to 625 μM).
Antiproliferative assay in vitro
24 hours before addition of the tested compounds, the cells were plated in 96-well plates at density of 1×104 cells per well. An assay was performed after 72 hours exposure to varying concentrations of the tested agents.
Cytotoxic test MTT was applied for the cytotoxicity screening against leukaemia cells growing in suspension culture and were carried out according to the method described before [25]. Cytotoxic test SRB was applied against A549, MCF-7, LoVo, LoVo/DX, BALB/3T3 and HepG2 cells and were carried out according to the method described before [25].
The results were calculated as an IC50 (inhibitory concentration 50)–the concentration of tested agent, which inhibits proliferation of 50% of the cell population. IC50 values were calculated for each experiment separately and mean values ± SD is presented in the Table 1. Each compound in each concentration was tested in triplicate in a single experiment, which was repeated 3–5 times.
Table 1. Antiproliferative activity of terpene-phospholipids against selected cell lines.
| Compounds | Acyl residue | Cell line /IC50 ±SD (μM) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| sn-1 | sn-2 | MV4-11 | A-549 | MCF-7 | LOVO | LOVO/DX | HepG2 | BALB/3T3 | ||
| 1a geranic acid | - | - | 310.77 ± 96.41 | n.a. | 447.09 ± 72.02 | 263.1 ± 32 | 492.3 ± 44.5 | n.a. | n.a. | |
| 1b citronellic acid | - | - | 254.49 ± 67.95 | n.a | 452.9 ± 96.0 | 249.3 ± 26.4 | 533.2 ± 36.7 | n.a. | n.a | |
| 3a | GA | GA | 96.66 ± 33.94* | 274.29 ± 9.42 | 236.33 ± 21.33* | 248.5 ± 9.3 | 278.8 ± 10.8* | 285.3 ± 5 | 273.16 ± 6.76 | |
| 3b | CA | CA | 130.08 ± 37.8^ | 203.1 ± 47.7 | 242.8 ± 45.1^ | 193.6 ± 22^ | 251.4 ± 23.4^ | 280.7 ± 1.8 | 283.7 ± 6.3 | |
| 7a | PA | GA | 60.89 ±10.86* | 72.1 ±14.4 | 104.66 ± 6.51* | 51.05±5.1* | 54.96±2.12* | 199.6±6.1 | 185.76 ± 15.2 | |
| 7b | PA | CA | 45.66 ± 7.63^ | 61.2 ± 6.0 | 155.0 ± 88.3^ | 53.9 ± 2.97^ | 57.1 ± 2.5^ | 103.4 ± 11.1 | 111.9 ± 29.3 | |
| 9a | GA | - | 323.5 ± 115.62 | n.a. | 522.96 ± 97.24 | 246.2 ± 9.9 | 526 ± 89.6 | n.a. | n.a. | |
| 9b | CA | - | 381.58 ± 24.49 | n.a | n.a | 286.7 ± 35.1 | 551.9 ± 18.5 | n.a. | n.a | |
| 10a | GA | PA | 38.21 ± 9.3* | 49.54 ± 1.88 | 52.45 ± 5.9* | 34.6 ± 1.6* | 51.9 ± 6.7* | 70.3 ± 8.6 | 65.8 ± 3.34 | |
| 10b | CA | PA | 57.06 ± 6.37^ | 60.2 ± 6.4 | 208.7 ± 25.7^ | 52.4 ± 3.6^ | 57 ± 1.8^ | 232.9 ± 14.7 | 92.9 ± 15.3 | |
| Cisplatin | - | - | 1.3 ± 0.47 | 8.6 ± 0.7 | 8.1 ± 0.03 | 2.56±0.35 | 3.17±0.2 | 2.38±0.64 | 4.2 ± 1.1 | |
| Doxorubicin | - | - | - | - | - | 0.117±0.012 | 6.53±0.93 | - | n.a. | |
n.a.—no activity in concentration of 5, 25, 125, 625 μM. IC50 –compound concentration leading to 50% inhibition of cell proliferation. Data are presented as mean ± SD of 3–5 independent experiments.
*—results within column which are significantly different in comparison to geranic acid, p < 0.05.
^—results within column which are significantly different in comparison to citronellic acid, p < 0.05.
Using the obtained IC50 values, the resistance indexes (RI) were calculated by dividing the IC50 values of the compounds tested against the cells of drug resistant cell LOVO/DX line by respective values obtained against the cells of drug sensitive LoVo line. According to Harker et al. [26] three categories of the cells could be distinguished: (a) the cells are drug-sensitive—if the ratio approaches 0–2; (b) the cells are moderately drug-resistant—if the ratio ranges from 2 to 10; (c) the cells are markedly drug-resistant—if the ratio is higher than 10.
Statistical analysis
Statistical analysis was performed using STATISTICA version 10 (StatSoft Inc., USA). Mann-Whitney U Test was used in the analysis and the results in Table 1 are given with statistical significance in comparison to geranic acid (*) or citronellic acid (^), p < 0.05.
Results and Discussion
One of the most widely studied phospholipids is phosphatidylcholine (PC). Yamamoto proposed the transphosphatidylation of 1,2-dioleoyl-sn-glycero-3-phosphocholine (DOPC) with terpene alcohols (geraniol, nerol, perillyl alcohol, myrtenol, farnesol, geranylgeraniol, phytol [19,20] as the method for joining two biologically active groups of molecules. In this way the novel carrier-linked prodrugs—phospholipids containing terpenes in the polar part of PC, which showed antiproliferative effects on human leukaemia cells (HL-60) and prostate cancer (PC-3) cells were prepared [19,20]. Promising results obtained by Yamamoto inspired us to create a novel strategy for the synthesis of phosphatidylcholine enriched in biologically active terpenes. In our aproach we decided to leave polar part of phosphatidylcholine (PC) untouched, because choline is biologically active compound, which is a substrate for the synthesis of the neurotransmitter acetylcholine and directly affects many vital functions such as breathing, heart rate, memory processes. It also protects the liver cells, prevents accumulation of fat in hepative cells, lowers cholesterol levels, reduces the risk of atherosclerosis and heart diseases by the reduction of homocysteine level [27,28]. Instead, we used two monoterpene carboxylic acids (geranic and citronellic) as acyl donors and introduced them into apolar part of PC in order to extend their application field and ensure their efficient transport in the human body. Geranic acid is known as a strong inhibitor of the melanine synthesis and the most potent isoprenoid with antifungal activity towards Fusarium graminearum and Colletotrichum graminicola [29,30]. Its structural 2,3-dihydroanalogue named citronellic acid occurs in many essential oils and as the carrier of specific odor exhibiting antimicrobial activity is often used in the cosmetic industry as valuable ingredient of various preparations [31]. The biological activities of phospholipids depend on the composition of fatty acids in the sn-1 and sn-2 positions. From that reason three groups of terpene-modified phosphatidylcholines were synthesized. First group contained the same residue of geranic acid (GA) (1a) or cytronellic acid (CA) (1b) in the sn-1 and sn-2 positions. In second variant, GA or CA was attached to the sn-2 position whereas in sn-1 position saturated fatty acid (palmitic acid) was introduced as it is observed in natural phospholipids. Third group of phospholipids possessed geranic or cytronellic acid residue in sn-1 position and palmitic acid in sn-2 position.
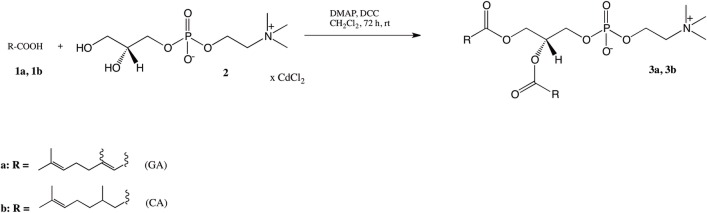
The first group of PC containing the same residue of geranic acid (1a) or citronellic acid (1b) in the sn-1 and sn-2 positions (3a, 3b) was obtained by known Steglich esterification method [32–34]. The cadmium complex of sn-glycero-3-phosphocholine (GPC×CdCl2) (2) was acylated with terpene acid (TA) 1a and 1b in the presence of 4-(N,N-dimethylamino)pyridine (DMAP) and a coupling agent N,N’-dicyclohexylcarbodiimide (DCC). All the reagents and solvent (dichloromethane) were freshly dried just before reaction to avoid the hydrolysis of DCC to dicyclohexylurea. In described condition using the molar ratio 1: 4: 2: 4.2 of GPC/TA/DMAP/DCC, 1,2-digeranoyl-sn-glycero-3-phosphocholine (3a) and 1,2-dicitroneloyl-sn-glycero-3-phosphocholine (3b) were obtained in 82% and 86% yields respectively after 72 hours of reaction (Fig 1).
Fig 1. Synthesis of 1,2-diacyl-sn-glycero-3-phosphocholine containing the GA and CA residues (3a, 3b).
The structures of products 3a and 3b were characterized based on comprehensive spectroscopic data interpretation. In the 1H NMR spectrum one can see signals from the incorporated isoprenoids. Signals from olefinic protons of terpene acids in the range δ 4.84–5.43 and protons from three methyl groups in δ 1.38–1.45 were observed. The carbon atoms of double bond systems present in terpenes acid residues gave signals in 13C NMR spectrum in the range 114.49–132.13 ppm for 3a and in the range 123.64–131.09 ppm for 3b. Signals in the 31P NMR spectrum at -0.63 ppm for 3a and -0.79 ppm for 3b proved that the phosphatidyl parts of molecules have been retained. Detailed assignments are given in the supplementary material (S1–S40 Figs).
The synthesis of asymmetrically substituted phospholipids containing terpene acids (TA) in the sn-2 position and palmitic acid in sn-1 position was started by acetylation of cadmium complex of GPC with palmitic acid (PA) (Fig 2). Then 1,2-dipalmitoyl-sn-3-glycero-phosphocholine was converted to the 2-lysophospholipid (6) by treatment with phospholipase A2. Subsequent reacetylation of 2-OH group of known 1-palmitoyl LPC by isoprenoid acids yielded the mixed diacyl phosphatidylcholines (7a, 7b) [35].
Fig 2. Synthesis of 1-palmitoyl-2-isoprenoyl-sn-glycero-3-phosphocholine (7a, 7b).
The time-course of esterification of 1-palmitoyl-2-hydroxy-sn-glycero-3-phosphocholine by isoprenoid acids proceeded very slowly (Fig 3). We prolonged the time of reaction but still the isolated yields of asymmetrically substitued phosphatidylocholines were lower than in the case of symmetrically substituted products. The progress of the reaction was monitored by HPLC and showed that even after 120 hours the content of structured PC (7a, 7b) in the reaction mixture was on the level of 58–84%. We decided to use then the higher molar excess of DCC and DMAP in relation of 1-palmitoyl-2-lysophospholipids and isoprenoid acids and increase temperature of reaction to 40°C which resulted the full conversion of substrate after 72 h of the reaction.
Fig 3. The time-course of esterification of 1-palmitoyl-2-hydroxy-sn-glycero-3-phosphocholine by isoprenoid acids.
The structures of obtained products 7a and 7b were established based on their spectral data. The 1-palmitoyl-2-geranoyl-sn-glycero-3-phosphocholine (7a) was synthesized earlier by Biodrowska in 27% yield but in our investigations using higher ratio of DCC and DMAP resulted in significantly higher yield (59%) [36]. The 1-pamitoyl-2-citroneloyl-sn-glycero-3-phosphocholine (7b) has not been reported in the literature and was obtained in 62% yield.
The synthesis of 1-isoprenoyl-2-palmitoyl-sn-glycero-3-phosphocholines is a task requiring regioselective incorporation of different acyl donors in two positions of the GPC backbone. The literature presents several useful methods for this type of mixed-chain phosphatidylcholines synthesis [29,24,37]. In all procedures the key step is production of 1-acyl-2-lyso-sn-glycero-3-phosphocholine. Initially, we applied chemoenzymatic strategy similar to that applied in the synthesis of compounds 7a and 7b. It was based on the hydrolysis of 1,2-diacyl-sn-glycero-3-phosphocholines (3a, 3b) obtained previously (Scheme 1) and subsequent reesterification of 1-acyl LPC with palmitic acid (PA) using DCC and DMAP. Unfortunately the yields of hydrolysis of terpene-PLs carried out with the phospholipase A2 (PLA2) were very low and we decided to use tin-mediated mono-functionalization of GPC described by D’Arrigo [35]. As it is presented in the Fig 4, in this process, GPC was first transformed into cyclic stannylene ketal by treatment with DBTO and then selectively acylated with isoprenoyl chlorides. Geranoyl and citroneloyl chlorides were obtained in situ using the procedure described by Mattson et al. [38]. We used the quantities of reagents suggested by D’Arrigo et al. [35], for the synthesis of 1-acyl-LPCs. Crude products were purified by a silica gel column chromatography to afford two new 1-isoprenoyl-2-hydroxy-sn-glycero-3-phosphocholines (9a, 9b) with 75% and 87% yields respectively. The last step was acylation of 9a and 9b with palmitic acid (PA) in the presence of DCC and DMAP, affording two new products 10a and 10b in 67 and 70% yields respectively. The structures of the final products were confirmed by their spectroscopic data.
Fig 4. Synthesis of 1-isoprenoyl-2-palmitoyl-sn-glycero-3-phosphocholine (10a, 10b).
All synthesized products (3a, 3b, 7a, 7b, 9a, 9b, 10a, 10b) were obtained as the mixture of diastereoisomers. In the case of phospholipids containing geranic acid (3a, 7a, 9a, 10a) it was the results of composition of cis/trans (23:67) isomers of starting isoprenoid. In the case of phosphatidylcholines modified with citronellic acid (3b, 7b, 9b, 10b) the formation of two diastereoisomers (50:50) was caused by introduction of racemic citronellic acid into optically pure sn-glycero-3-phosphocholine (GPC).
Biological studies
All terpene–modified PCs (1a, 1b, 3a, 3b, 7a, 7b, 9a, 9b, 10a, 10b) were tested for their antiproliferative activity towards human cancer cell lines, such as MV4-11 (human leukaemia), A549 (lung cancer), MCF-7 (breast cancer), HepG2 (liver cancer), LoVo (colon cancer) and also against doxorubicin-resistant colon cancer LoVo/DX (P-gp-dependent, MRP-, LRP-dependent multidrug resistance). The experiments of cytotoxicity were also performed towards BALB/3T3 (normal mice fibroblasts). The data for the in vitro anticancer activity (Table 1) were expressed as the IC50 –concentration of the compound (in μM) that inhibits proliferation of cells by 50% compared to the untreated control cells. The results were compared with the activity of free terpene acids (TA). Cisplatin and doxorubicin hydrochloride (for LoVo and LoVo/Dx cells) were used as a positive control.
The aim of this study was to determine whether a joining the terpenes with PC has impact on their biological activity. Increasing the lipophilicity of active compounds was expected to raise their cytotoxicity. We observed that almost all synthesized compounds were cytotoxic against tested cancer cell lines. The exception is compound 9a that is not active towards cells of A549 and HepG2 lines and 9b, which inhibits only the proliferation of leukaemia and colon cancer LoVo cells but in high concentration on the level 381.58 μM and 286.7 μM, respectively. Upon careful evaluation of data it can be concluded that cytotoxic activity of terpene-modified PCs is depended on the position of terpene moiety in the backbone of PC. Symmetrically substituted phospholipids (3a, 3b) containing terpene acids (TA) in the sn-1 and sn-2 position possessed the antiproliferative activity 2-3-fold higher than free acids. These compounds stronger than corresponding acids inhibit the proliferation of cancer cells. Free terpene acids (1a, 1b) did not showed any reduction of cells growth of A549, HepG2 even at 625 μM. The highest activity was observed for mixed-chain PC (7a, 7b, 10a, 10b). Their IC50 values were more than 9-fold lower than those determined for free terpene acids 1a, 1b towards leukaemia, LoVo, LOVO/DX and breast cell lines. For cells of lung cancer and HepG2 lines the activity was exhibited only after introduction of terpene acids into PC. Among eight tested terpene-PCs compound 10a showed the strongest antiproliferative effect towards all tested cell lines. Very high antiproliferative activity was demonstrated against leukaemia and colon cancer LoVo cells (IC50 38.21 and 34.6 μM, respectively). During the experiments we observed also that tested compounds exhibit activity against normal mice fibroblasts (BALB/3T3). However it is worth to notice that cytotoxic activity of compounds 7a, 7b, 10a and 10b against BALB/3T3 cells was in most cases lower in comparison to cytotoxicity exhibited against cancer cell lines, which is very promising in contest of potential application of tested compounds as the anticancer drugs. Generally, activity of terpene-modified PC increased in the following sequence: 1-isoprenoyl-LPC < diisoprenoyl-PC < monoisoprenoyl-PC.
We calculated also the resistance indexes (RI) by dividing the IC50 values of the compounds tested against the cells of drug resistant cell LoVo/DX by respective values obtained against the drug sensitive LoVo cell line (Table 2). All compounds were able to overcome the barrier of P-gp-dependent resistance with exception of two compounds: free terpene acids 1b and 9a, which revealed moderated ability to overcome drug resistance. Compounds 7a, 7b and 10b have the highest ability to overcome the barrier of resistance (RI = 1.08, 1.06 and 1.09).
Table 2. Resistance index (RI) values of terpene-phospholipids.
| Compounds | Acyl residue | ||
|---|---|---|---|
| sn-1 | sn-2 | RI | |
| 1a geranic acid | - | - | 1.87 |
| 1b citronellic acid | - | - | 2.14 |
| 3a | GA | GA | 1.12 |
| 3b | CA | CA | 1.3 |
| 7a | PA | GA | 1.08 |
| 7b | PA | CA | 1.06 |
| 9a | GA | - | 2.14 |
| 9b | CA | - | 1.93 |
| 10a | GA | PA | 1.5 |
| 10b | CA | PA | 1.09 |
| DOX | - | - | 55.81 |
DOX–doxorubicin
RI was calculated according to the formula RI = (IC50 estimated against resistant cell line)/(IC50 estimated against non-resistant cell line); values range: 0<RI<2-indicate that the tested compound is able to overcome drug resistance; 2<RI<10 –defines the moderate ability of the compound to overcome drug resistance; RI>10 –defines no influence on the drug resistance phenomenon.
In conclusion, all novel phospholipids containing the isoprenoid residue of geranic and citronellic acid in the non-polar part of phosphatidylcholine were synthesized in good yields (59–87%). It is the first report on the evaluation of anticancer activity of geranic and citronellic acid in the free form and after incorporation into PC. The results of cytotoxic studies confirmed that phospholipid modifications with geranic and citronellic acids enhanced their biological activity. It was indicated by the lower active doses of terpene-PCs comparison to doses of free terpene acids. The low activity observed for lysoderivatives may be a result of significantly increased polarity of those compounds comparing to 1,2-diacylphosphatidylcholines. We found that there is structure-activity relationship among the tested terpene-modified PCs but it is also important to determine how the structure of isoprenoid residue (the chain length, number of double bond etc.) influences on the activity of whole molecule. These studies are in progress. The aim of further work is also to elucidate the mechanism of the cytotoxicity terpene-modified PCs.
Supporting Information
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Acknowledgments
This project was financed by National Science Center of Poland no. 2013/09/D/NZ9/02457. Publication was supported by Wroclaw Centre of Biotechnology, programme The Leading National Research Centre (KNOW) for years 2014–2018 (http://know.wroc.pl).
Special thanks for Mr Paweł Dąbrowski (Wroclaw University of Technology) for NMR spectrum.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This project was financed by National Science Center of Poland no. 2013/09/D/NZ9/02457. Publication was supported by Wroclaw Centre of Biotechnology, programme The Leading National Research Centre (KNOW) for years 2014-2018 (http://know.wroc.pl).
References
- 1.Fujita K, Kubo I. Multifunctional action of antifungal polygodial against Saccharomyces cerevisiae: involvement of pyrrole formation on cell surface in antifungal action. Bioorg Med Chem. 2005; 13(24): 6742–6747. [DOI] [PubMed] [Google Scholar]
- 2.Peng J, Franzblau SG, Zhang F, Hamann MT. Novel sesquiterpenes and a lactone from the Jamaican sponge Myrmekioderma styx. Tetrahedron Lett. 2002; 43: 9699–9702. [Google Scholar]
- 3.Singh SB, Felock P, Hazuda DJ. Chemical and enzymatic modifications of integric acid and HIV-1 integrase inhibitory activity. Bioorg Med Chem Lett. 2000; 10(3): 235–238. [DOI] [PubMed] [Google Scholar]
- 4.Hernandez V, Manez S, Recio MC, Giner RM, Rios JL. Anti-inflammatory profile of dehydrocostic acid, a novel sesquiterpene acid with a pharmacophoric conjugated diene. Eur J Pharma Sci. 2005; 26: 162–169. [DOI] [PubMed] [Google Scholar]
- 5.Burke YD, Stark MJ, Roach SL, Sen SE, Crowell PL. Inhibition of pancreatic cancer growth by the dietary isoprenoids farnesol and geraniol. Lipids. 1997; 32(2): 151–156. [DOI] [PubMed] [Google Scholar]
- 6.Adany I, Yazlovitskaya EM, Haug JS, Voziyan PA, Melnykovych G. Differences in sensitivity to farnesol toxicity between neoplastically- and non-neoplastically-derived cells in culture. Cancer Lett. 1994; 79: 175–179. [DOI] [PubMed] [Google Scholar]
- 7.Rioja A, Pizzey AR, Marson CM, Thomas NSB. Preferential induction of apoptosis of leukaemic cells by farnesol. FEBS Lett. 2000; 467: 291–295. [DOI] [PubMed] [Google Scholar]
- 8.Vance DE, Vance JE. Biochemistry of Lipids, Lipoproteins and Membranes, Elsevier; 2002. [Google Scholar]
- 9.Szuhaj BF, Nieuwenhuyzen WV, Nutrition and Biochemistry of Phospholipids, Illinois: AOCS Press; 2003. [Google Scholar]
- 10.Bezrukov SM. Functional consequences of lipid packing stress. Curr Opin Colloid In Sci. 2000; 5: 237–243. [Google Scholar]
- 11.White SH, Ladokhin AS, Jayasinghe S, Hirstova K. How membranes shape protein structure. J Biol Chem. 2001; 276: 32395–32398. [DOI] [PubMed] [Google Scholar]
- 12.Schlegel RA, Halleck MS, Williamson P. Nutrition and Biochemistry of Phospholipids Illinois: AOCS Press; 2003. [Google Scholar]
- 13.Lee TC. Biosynthesis and possible biological functions of plasmalogens. Biochim Biophys Acta. 1998; 1394: 129–145. [DOI] [PubMed] [Google Scholar]
- 14.Irvine RF, Schell MJ. Back in the water: the return of the inositol phosphates. Nat Rev Mol Cell Biol. 2001; 2(5): 327–338. [DOI] [PubMed] [Google Scholar]
- 15.Chrencik JE, Roth CB, Terakado M, Kurata H, Omi R, Kihara Y, I in. Crystal structure of antagonist bound human lysophosphatidic acid receptor 1. Cell. 2015; 161: 1633–1643. 10.1016/j.cell.2015.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lambert DM. Rationale and applications of lipids as prodrugs carriers. Eur J Pharm Sci. 2000; 11: S15–27. [DOI] [PubMed] [Google Scholar]
- 17.Hidaka N, Takami M, Suzuki YJ. Enzymatic phosphatidylation of thiamin, pantothenic acid and their derivatives. Nutr Sci Vitaminol. 2008; 54: 255–261. [DOI] [PubMed] [Google Scholar]
- 18.Takami M, Hidaka N, Miki S, Suzuki Y. Synthesis of novel phosphatidyldihydroxyacetone by phospholipase D. Biosci Biotechnol Biochem. 1994; 58: 1716–1717. [Google Scholar]
- 19.Yamamoto Y, Hokosawa M, Kurihara H, Miyashita K. Preparation of phosphatidylated terpenes via phospholipase D-mediated transphosphatidylation. J Am Oil Chem Soc. 2008; 85: 313–320. [Google Scholar]
- 20.Yamamoto Y, Masashi H, Kurihara H, Maoka T, Miyashita K. Synthesis of phosphatidylated-monoterpene alcohols catalyzed by phospholipase D and their antiproliferative effects on human cancer cells. Bioorg Med Chem Lett. 2008; 18: 4044–4046. 10.1016/j.bmcl.2008.05.113 [DOI] [PubMed] [Google Scholar]
- 21.Morgado MAP, Cabral JMS, Prazeres DMF. Hydrolysis of lecithin by phospholipase A2 in mixed reversed micelles of lecithin and sodium dioctyl sulphosuccinate. J Chem Technol Biot. 1995; 63: 181–189. [Google Scholar]
- 22.Niezgoda N, Gliszczyńska A, Gładkowski W, Kempińska K, Wietrzyk J, Wawrzeńczyk C. Phosphatidylcholine with cis-9,trans-11 and trans-10,cis-12 conjugated lin oleic acid isomers: synthesis and cytotoxic studiem. Aust J Chem. 2015; 68: 1065–1075. [Google Scholar]
- 23.Fasoli E, Arnone A, Caligiuri A, D’Arrigo P, de Ferra L, Servi S. Tin-mediated Synthesis of Lysophospholipids. Org Biomol Chem. 2006; 4: 2974–2978. [DOI] [PubMed] [Google Scholar]
- 24.Niezgoda N, Mituła P, Kempińska K, Wietrzyk J, Wawrzeńczyk C. Synthesis of Phosphatidylcholine with conjugated linoleic acid and studies on its cytotoxic activity. Aust J Chem. 2013, 66:354–361. [PubMed] [Google Scholar]
- 25.Gliszczyńska A, Gładkowski W, Świtalska M, Wietrzyk J, Szumny A, Gębarowska E, Wawrzeńczyk C. Dehalogenation activity of selected fungi towards δ-jodo-γ-lactone derived from trans, trans-farnesol. Chem Biodivers. 2016; 13: 477–482. 10.1002/cbdv.201500127 [DOI] [PubMed] [Google Scholar]
- 26.Harker WG, Slade DL, Drake FH, Parr RL. Mitoxantrone resistance in HL-60 leukemia cells: reduced nuclear topoisomerase II catalytic activity and drug-induced DNA cleavage in association with reduced expression of the topoisomerase II beta isoform. Biochem J. 1991; 30: 9953–9961. [DOI] [PubMed] [Google Scholar]
- 27.Innis SM, Davidson A, Geoge F, Melynk S, James SJ. Choline-related supplements improve abnormal plasma methioninehomocysteine metabolites and glutathione status in children with cystic fibrosis. Am J Clin Nutr. 2007; 85: 702–708. [DOI] [PubMed] [Google Scholar]
- 28.Wang T. Phospholipid Technology and Applications The Oily Press Lipid Library Series; 2008. [Google Scholar]
- 29.Masuda T, Odaka Y, Ogawa N, Nakamoto K, Kuninaga H. Identification of Geranic Acid, a Tyrosinase Inhibitor in Lemongrass (Cymbopogon citratus). J Agric Food Chem. 2008; 56: 597–601. [DOI] [PubMed] [Google Scholar]
- 30.Yang T, Stoopen G, Yalpani N, Vervoort J, de Vos R, Voster A, Verstappen FWA, Bouwmeester HJ, Jongsma MA. Metabolic engineering of geranic acid in maize to achieve fungal resistance is compromised by novel glycosylation patterns. Metab Eng. 2011; 13: 414–425. 10.1016/j.ymben.2011.01.011 [DOI] [PubMed] [Google Scholar]
- 31.Wuryatmo E, Klieber A, Scott ES. Inhibition of Citrus Postharvest Pathogens by Vapor of Citral and Related Compounds in Culture. J Agric Food Chem. 2003; 51: 2637–2640. [DOI] [PubMed] [Google Scholar]
- 32.Gupta CM, Radhakrishnan R, Khorana HG. Glycerophospholipid synthesis: improved general method and new analogs containing photoactivable groups. Proc Natl Acad Sci. USA 1977; 74: 4315–4319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Singh A. An efficient synthesis of phosphatidylcholines. J Lipid Res. 1990; 31: 1522–1525. [PubMed] [Google Scholar]
- 34.Ichihara K, Iwasaki H, Ueda K, Takizawa R, Naito H, Tomosugi M. Synthesis of phosphatidylcholine: an improved method without using the cadmium chloride complex of sn-glycero-3-phosphocholine. Chem Phys Lipids. 2005; 137: 94–99. [DOI] [PubMed] [Google Scholar]
- 35.D’Arrigo P, Fasoli E, Pedrocchi-Fantoni G, Rossi C, Saraceno C, Tessaro D, Servi S. A practical selective synthesis of mixed short/long chains glycerophosphocholines. Chem Phys Lipids. 2007; 147: 113–118. [DOI] [PubMed] [Google Scholar]
- 36.Biodrowska K, Draus A, Gliszczyńska A, Gładkowski W, Leśniak A, Wawrzeńczyk C. Synthesis of isoprenoid phospholipids. Przem Chem. 2012; 91: 692–696. [Google Scholar]
- 37.D’Arrigo P, Servi S. Synthesis of Lysophospholipids. Molecules. 2010; 15: 1354–1377. 10.3390/molecules15031354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mattson FH, Volpenhein RA. Synthesis and properties of glycerides. J Lipid Res. 1962; 3: 281–296. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.