Abstract
Tissue and organ transplants between genetically distinct individuals are always or nearly always rejected. The universality and speed of transplant rejection distinguishes this immune response from all others. Although this distinction is incompletely understood, some efforts to shed light on transplant rejection have revealed broader insights, including a relationship between activation of complement in grafted tissues, the metabolism of heparan sulfate proteoglycan and the nature of immune and inflammatory responses that ensue. Complement activation on cell surfaces, especially on endothelial cell surfaces, causes the shedding heparan sulfate, an acidic saccharide, from the cell surface and neighboring extracellular matrix. Solubilized in this way, heparan sulfate can activate leukocytes via toll like receptor-4, triggering inflammatory responses and activating dendritic cells, which migrate to regional lymphoid organs where they spark and to some extent govern cellular immune responses. In this way local ischemia, tissue injury and infection, exert systemic impact on immunity. Whether or in what circumstances this series of events explains the distinct characteristics of the immune response to transplants is still unclear but the events offer insight into the inception of immunity under the sub-optimal conditions accompanying infection and mechanisms by which infection and tissue injury engender systemic inflammation.
Keywords: Complement, Endothelial cells, Heparan sulfate, Antigen presenting cell, Dendritic cell, Toll-like receptor, Systemic inflammatory response syndrome, Sepsis, Accommodation
8.1 The Thinking Process
In June 1999, Tom and Ray Magliozzi delivered the commencement address at the Massachusetts Institute of Technology (MIT). The Magliozzi brothers were alumni of MIT and had a popular radio program, Car Talk, in which they entertained audiences with humorous stories and with advice on automobile repair and human nature. According to one report of the address [1], the brothers proposed a theory that happiness is an inverse function of phylogeny and declared their mantra to be: “non impediti ratione cogitatonis” or “unencumbered by the thought process.” One unifying conclusion was that rational thinking is inimical to happiness but another conclusion, more pertinent for the fields of immunology and transplantation, might be that dumb luck often solves the most important problems; but, just as often, rational thinking prevents us from seeing that the problems are solved. Below we describe and reinterpret some conclusions we drew from fortuitous observations made as we investigated immunity and transplantation. We make no attempt to discuss the broader literature on these subjects since we cannot know which observations of others were truly fortuitous and because we believe the evolution of thinking and not the thinking process per se has more lasting value than the details.
8.2 The Immune Response to Transplantation
Three decades ago we began to explore the immune response to transplantation. The question that seemed most urgent at that time (and still today) was why transplantation evokes immunity that is universal, rapid and powerful. Conventional immune reactions, typified by initial exposure to Mycobacterium bovis, attenuated and optimized in dosage as Bacillus Calmette–Guérin (BCG) vaccine, are detected in approximately 50 % of those first exposed approximately 4–6 weeks after exposure and detection required re-administration of antigen in the form of a skin test. In contrast, immune responses to transplantation occur in nearly 100 % of recipients, can be detected within a few days and in the absence of immunosuppression destroy the grafted tissue or organ [2–5]. Our original approach to understanding what might distinguish the immune response to transplantation was to explore the numbers of leukocytes of varying phenotypes that populated rejecting grafts [6] and delayed type hypersensitivity reactions [7]. The phenotype of leukocytes in DTH differed somewhat from the phenotype of leukocytes in rejection but the kinetics and other characteristics differed more [7]. Thus, this thought process brought an end to what had been a productive line of research and led to research aimed linking phenotype with functions.
The functions of the phenotypic markers initially studied, CD2, CD3, CD4, and CD8, BA-1, among others, were not then understood but since some markers were also expressed in development [8, 9], it seemed that understanding the processes governing the evolution of phenotypes in development would shed light on the function of the markers in mature tissue. It seemed further that changes in the phenotype and function of cells might be governed by glycosaminoglycans, the unique carbohydrate substitutions on proteoglycans, the metabolism of which had been found to drive cell-cell and cell-matrix interactions in development [10]. The lines of reasoning that brought us to investigate glycosaminoglycans and proteoglycans were entirely wrong, but the investigation nevertheless would bring some understanding of processes that can determine the fate of transplants
8.3 Proteoglycans in Ontogeny and Rejection of Kidneys
Proteoglycans consist of a core protein conjugated with glycosaminoglycan chains. Glycosaminoglycan chains are O-linked linear copolymers consisting of interdigitating hexuronic acid and hexosamine residues modified by N- and O-linked sulfate esters. The expression of a given core protein determines which glycosaminoglycan chain will be added to the core protein, where on or in the cell the proteoglycan will be situated and a few biological properties. However, it is the glycosaminoglycan chains that confer the predominant biological properties of proteoglycans we shall consider. Only a few of many outstanding reviews of the structure, biosynthesis and biological properties of proteoglycans are provided as references [11–13].
To understand the connection between the phenotype and function of cells, we explored the metabolism of proteoglycans in kidney organogenesis and the impact of perturbing that metabolism [14–16]. The kidney was selected for study because morphogenesis of that organ involves complex stereotypic cell-cell and cell-matrix interactions the disruption of which might cause dramatic and reproducible change in morphology and biochemistry. Disrupting chondroitin sulfate proteoglycan synthesis had clearly and also predictable changes. But, adding heparan sulfate had the greatest impact; it shut down development of branching structures without apparently impacting on maturation of epithelial element of glomeruli, which we found to be associated with degradation of heparan sulfate. We took these results to indicate that heparan sulfate controls the earliest events in nephron formation (induction of nephrogenic mesenchyme); but, it might also have reflected the inhibition of heparan sulfate depolymerization by heparanase or the elution of heparan sulfate binding peptides. We would later return to these possibilities in entirely different systems, ischemic tissues and rejecting organs [17–20]. However, we first turned our attention to the question of whether inflammation and immunity might change the metabolism of heparan sulfate proteoglycan.
8.4 Metabolism of Heparan Sulfate Proteoglycan in Inflammation and Immunity
Having found that metabolism of heparan sulfate and chondroitin sulfate proteoglycans are linked to organogenesis of kidney [14, 21], we wondered whether metabolism of these also changes in inflammation and immunity. The query was focused on endothelial cells, which we considered the principal target of cell-mediated and humoral immunity [17, 22, 23]. Mature endothelial cells that we used produced far more heparan sulfate than chondroitin sulfate and hence we direct our work at the metabolism of heparan sulfate proteoglycan. But, there was another, far better reason for focusing on endothelial cell heparan sulfate proteoglycan. Heparan sulfate proteoglycan on cell surfaces and extracellular matrices exerted or at least supported all of the key physiologic functions of endothelial cells that inflammation, immunity and disease disrupt (Fig. 8.1). Heparan sulfate maintains the integrity of the endothelial lining, providing a key barrier to diffusion of proteins and migration of cells. Heparan sulfate maintains the fluidity of blood by tethering and activating antithrombin III and tissue factor pathway inhibitor. Heparan sulfate also regulates activation of complement, in part by its action of antithrombin III and in part by tethering factors H and properdin and helps to limit oxidant injury by tethering superoxide dismutase. Heparan sulfate also potentially regulates inflammation and immunity by attaching chemokines and many cytokines to endothelial surfaces. Therefore, we reasoned that if one had to name a molecule the metabolism of which would transform the biology of tissues and organs, one could find no better candidate to name than heparan sulfate.
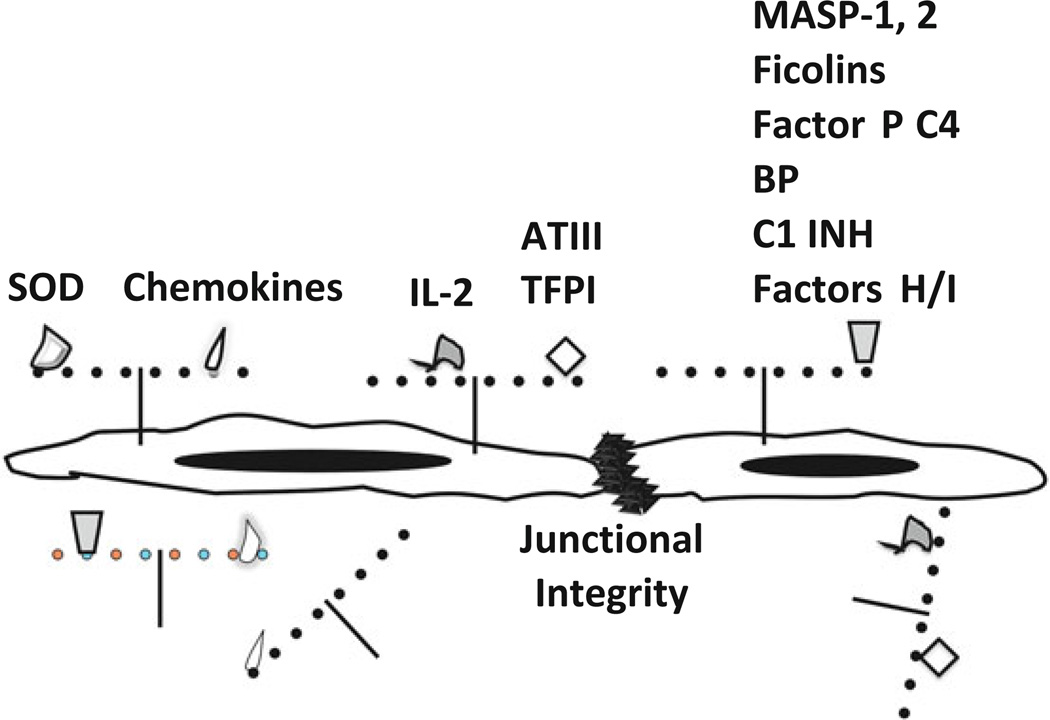
Fig. 8.1.
Heparan sulfate proteoglycan and the functions of endothelium. Heparan sulfate proteoglycans, consisting of a core protein conjugated with heparan sulfate glycosaminoglycan chains (strings of pearls) contribute to many function of normal blood vessels. These functions include (a) providing negative cell surface-charge that regulates complement, coagulation and cellular interactions; (b) maintaining the junctional integrity of the endothelial barrier to efflux of cells, solutes and plasma; (c) tethering and in some cases activating proteins that regulate oxidants such as superoxide dismutase (SOD); adherence, migration and activation of leukocytes, stem cells (chemokines) and lymphocytes (IL-2); coagulation [antithrombin III (ATIII) and tissue factor pathway inhibitor (TFPI)] and complement [MBL associated serine protease (MASP-1) and (MASP-2), ficolins, Factor P, C4 binding protein (C4 BP), C1 inhibitor (C1 INH), factor H and factor I]. Shedding of heparan sulfate deprives endothelial cells of these functions leading to cellular injury, extravasation of blood cells and plasma from blood vessels, activation of complement, coagulation and hemostasis and inflammation
This reasoning led us to investigate whether and how inflammation modifies heparan sulfate metabolism in endothelial cells. In this one case, perhaps owing to dumb luck, we were apparently non impediti ratione cogitatonis. Thus, activation of complement on endothelial cells, as it might occur in ischemia-reperfusion injury or graft rejection, caused the quantitative shedding of heparan sulfate from the cells [22]. Interaction of neutrophils [24] and activated T cells also caused shedding of heparan sulfate [25]. Shedding of heparan sulfate caused by complement occurred within a few minutes, shedding caused by neutrophils proceeded over 20–60 min and was less complete, shedding caused by activated T cells took place over about 1 h and the loss represented <50 % of heparan sulfate. It thus seemed that acute inflammation and immunity might, as an early manifestation, disrupt the barrier, anticoagulant, and anti-inflammatory functions of blood vessels and in this way set the stage for the dramatic changes in endothelial cell physiology and activation that would be seen over the ensuing hours [26–30]. It seemed also that the burgeoning interest in endothelial cell biology could not be explored in full unless changes in heparan sulfate proteoglycan were taken into account.
What seemed most interesting then and now, from a practical perspective, was not the loss of heparan sulfate per se, as important as that might be, but rather the mechanism of the loss (which one might wish to prevent therapeutically). Both proteases and an endoglycosidase, heparanase (endo-β-glucuronidase), were known to degrade heparan sulfate proteoglycans associated with normal and malignant cells (although an impact on blood vessels and blood vessel functions in inflammation and immunity had not been described). Proteases might cause release of nearly full-sized proteoglycans; heparanase might release small fragments of individual glycosaminoglycan chains; both enzymes, acting together would release partly degraded fragments of proteoglycans and glycosaminoglycans. Thus, the size of heparan sulfate proteoglycans and fragments thereof could provide clues to the enzyme activities responsible for the shedding of the molecules. Determining the size of shed molecules was especially important for discovering how complement had acted because the sera used as a source of complement might contain abundant amounts of platelet heparanase [31], the activity of which would confound probing this subject. Using endothelial cells in which heparan sulfate had been biosynthetically labeled we traced the fate and size of the label, [35S]sulfate, after exposure of the cells to complement, neutrophils and activated (and resting) T cells. In each of these setting the preponderance of heparan sulfate initially released from the labeled cells was found in nearly full-size proteoglycans [32]. Thus, the earliest step, especially after complement was activated [20, 33], involved the action of proteases, the inhibition of which would preserve endothelial cell heparan sulfate, at least under the conditions used in our experiments (Fig. 8.2).
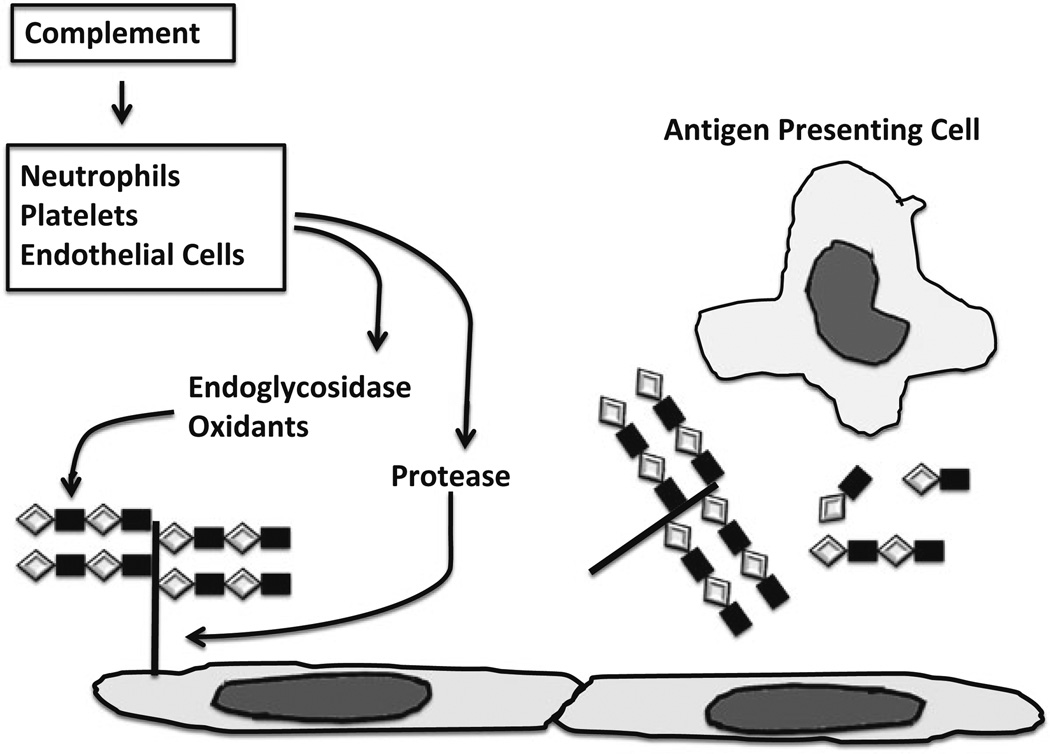
Fig. 8.2.
Complement activation and the inciting of inflammation and immunity. Complement activation on endothelial cells incites inflammation and immunity via several mechanisms. Illustrated in the figure is one mechanism involving degradation of heparan sulfate proteoglycan. Complement activation causes neutrophils, platelets and endothelial cells to secrete proteases and heparanase, an endoglycosidese that specifically cleaves heparan sulfate. Heparan sulfate proteoglycans and partially degraded heparan sulfate chains so released activate inflammatory cells and dendritic cells (antigen presenting cells) which, via secretion of cytokines, such as IL-1, increased antigen processing and presentation and co-stimulation and migration to regional lymph nodes, activate naïve T cells
That is not to say that heparanase and/or oxidants are not important in the overall sequence of events. Activated T cells had been found to produce heparanase and use it to penetrate matrices and inhibition of heparanase appears to halt migration of T cells [34, 35]. Likewise, oxidants produced by inflammatory cells and endothelial cells might also cleave heparan sulfate [36]. However, degradation by heparanase and oxidants is much slower and requires not only prior activation of immune inflammatory cells but persistence of those cells in the vicinity of the proteoglycans to be degraded. Thus, inflammation or immunity involving complement probably does not depend on heparanase and oxidants, at least at the inception.
8.5 Heparan Sulfate and the Immune Response
If shedding of heparan sulfate was an early event in tissue injury, infection or transplantation, could it impact in some way on the activation of T cells? Heparin, which is structurally similar to but more sulfated than heparan sulfate had been shown previously to inhibit autoimmune disease and allograft rejection [37, 38]. But, heparin was commonly used as an anticoagulant in transplant recipients and seemed to have no appreciable impact on rejection in that setting. Besides, rejection of a graft would be preceded by T cell differentiation and expansion and migration and we were interested in understanding the potential impact of heparan sulfate shed at the earliest time, when cells of the immune system would either be ignited to respond or held in check (tolerance).
The initial testing of the impact of heparan sulfate on T cell activation was conducted in mixed leukocyte cultures, which, in mouse, are prepared by mixing splenocytes from different strains, or cultures of splenocytes and mitogens of various types [39]. In these systems, we observed that heparan sulfate amplified T cell proliferative responses and development of effector functions, especially under suboptimal conditions we believed would model the condition in which T cell activation normally occurs [39, 40].
However, the most interesting finding was not that heparan sulfate stimulated T cells; indeed, we observed that heparan sulfate had no appreciable direct impact on T cells. Rather, the most interesting finding was that heparan sulfate modified the function of antigen presenting cells (APC), the leukocytes, such as dendritic cells, that actually take up and present antigen to T cells. This finding made more sense than any impact heparan sulfate might have on T cells because it is the APC in transplants or infected tissues that take up antigen at sites of tissue injury, and hence the site of heparan sulfate shedding, and carry the antigen to lymphoid organs where naïve T cells reside.
A further observation was also of much interest. If the presence of heparan sulfate was limited to the first day of a 5-day mixed leukocyte culture, it had the most profoundly stimulatory impact, while the presence of heparan sulfate only during the last several days of a 5-day mixed culture had a profoundly inhibitory impact on the proliferative response. The early impact of heparan sulfate thus seemed to model rather well a circumstance in which an APC takes up antigen and becomes activated and then migrates to a microenvironment, the lymphoid tissue, which lacks a surfeit of shed of heparan sulfate.
We also found that the apparently disparate actions of heparan sulfate on APC were owed, at least in part, to secretion of IL-1 and IL-6 early after stimulation and PGE2 later and were associated with activation and nuclear translocation of NFκB [41, 42]. We also found that the panoply of changes in cellular functions was relatively specific for heparan sulfate. Heparin, which has structural similarity to heparan sulfate but contains twice as many sulfate esters and is mainly confined to cellular granules, had far less stimulatory effect than heparan sulfate (and since heparin has some sequences modified like heparan sulfate, the heparin sequences might well have had no impact). Chondroitin sulfate, which has similar charge density but differ disaccharide units but is expressed outside cells, caused little change except at the highest concentrations used. And, only heparan sulfate incited production of PGE2 [42]. In its action on APC, heparan sulfate seemed to trigger a number of signaling intermediates in APC, the constellation of which could not be ascribed to any one receptor or cell surface perturbation and we concluded that heparan sulfate probably delivered signals via two or more discrete surface events, but the net effect would enhance T cell activation. The production of PGE2 on the other hand seemed unrelated to the overall propensity of heparan sulfate to promote immune response to sub-optimal stimuli (which we figured then as now represent the condition when immunity to foreign organisms and toxins most needs stimulation). Instead, the production of PGE2 days after APC were activated might limit the expansion of T cells responding to antigen or avoid ongoing activation of antigen specific responses.
8.6 Orchestrating T Cell Responses In Vivo
Studying T cells in mixed cultures of splenocytes has revealed much of what is known about the specificity of alloimmune responses. However, neither alloimmune nor conventional immune responses arise by a mixing of splenocytes. Rather, they arise when a small number of dendritic cells, take up antigen and receive activating signals such as lipopolysaccharide (LPS). Activated dendritic cells migrate from tissues to regional lymph nodes where the dendritic cells are brought together with a large number naïve T cells in lymphoid tissues. Dendritic cells are sometimes referred to as “professional antigen presenting cells” because unlike the various cells that might be used to probe T cell specificity and biochemical processes of antigen presentation, dendritic cells have the unique abilities to engulf antigen in various forms, migrate from the source of antigen to key positions in lymphoid organs, and present antigen and the key accessory signals needed to activate naïve T cells or in the absence of the accessory signals to generate energy [43–45]. Hence, to know whether and how heparan sulfate might actually impact on T cell activation (or suppression), it would be necessary to probe these events using dendritic cells.
As a first step, we asked whether heparan sulfate changes the differentiation and function of dendritic cells of the mouse [46]. Immature bone marrow derived dendritic cells were incubated with small amounts of heparan sulfate or with control substances (including heparan sulfate that had been depolymerized by treatment with HNO2, to assure absence of contaminating substances) and then “activation” was evaluated by assaying expression of proteins typically found on mature dendritic cells. Immature dendritic cells expressed MHC class II at intermediate levels and low levels of CD40, CD54, and CD86 at low levels. Dendritic cells exposed to heparan sulfate expressed high levels of these proteins. Exposure to heparan sulfate also caused functional changes in the dendritic cells—(a) the cells secreted appreciable amounts of TNF, IL-1β and IL-6; (b) the uptake and processing of antigen ceased while the MHC class II molecules became “fixed” at the surface; and (c) the number of dendritic cells needed to evoke an alloimmune response decreased by an order of magnitude. Thus, immature dendritic cells exposed to heparan sulfate behaved like mature, activated dendritic cells poised to induce cellular immune responses [44, 47]. In contrast, the cells kept under control conditions continued to appear and behaved as immature dendritic cells, which induce immunological tolerance in some systems [48–53].
It seemed as though heparan sulfate acted initially as an agonist to cause dendritic cells to mature and in this way to promote cellular immunity but that the promoting of cellular immunity was circumscribed and indeed ultimately suppressed by the later production of PGE2. Thus action of heparan sulfate on antigen presenting cells could explain both the expansion and also the eventual contraction of a cellular immune response to foreign antigen [20]. The suppression of T cell proliferation caused by PGE2 clearly differed from the condition of energy generated by immature dendritic cells.
8.7 What About Complement, PGE2 and Control of the Immune Response?
Our finding that heparan sulfate causes APC to produce PGE2 and our thinking that PGE2 might help circumscribe cellular immune responses was eclipsed by another observation. We discovered that IL-2 can be tethered to cells by heparan sulfate and that IL-2 so tethered can induce apoptosis of newly activated T cells, which express receptors for IL-2 [54]. In fact, the tethered form of IL-2 and not IL-2 in solution appeared to account for the impact of IL-2 in the generation and control of immune responses to model antigens delivered in vivo [55]. We imagined that interaction or lack thereof between complement and endothelial or parenchymal cells might govern immune responses—promoting responses when complement causes heparan sulfate to be shed and suppressing response when complement does not (Fig. 8.3) [56].
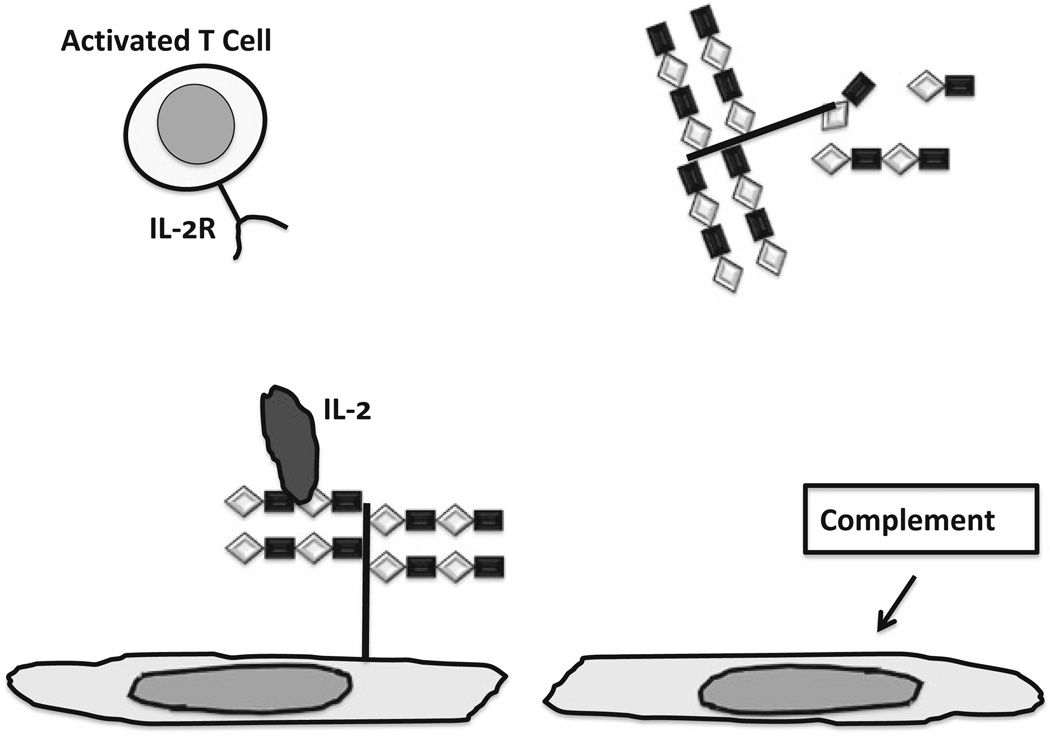
Fig. 8.3.
Impact of heparan sulfate proteoglycan and shedding of heparan sulfate proteoglycan and glycosaminoglycan on control of cellular immunity. Left : Intact heparan sulfate chains bind IL-2, which can cause activated T cells to undergo apoptosis, contributing to immunological tolerance. Right : Loss of heparan sulfate caused by activation of complement deprives endothelium of IL-2, allowing activated to cells to attach, transmigrate and exert effector functions
Although we did not forget entirely about PGE2 [20], our thinking about complement, heparan sulfate and IL-2, we did not pursue an apparent disparity concerning the involvement of complement and heparan sulfate in activation versus suppression of cellular immunity. If activation of complement causes heparan sulfate to be shed, and by now we had compelling evidence for that in vivo, and if the shed heparan sulfate activated APC which migrated to lymph nodes to activate T cells, then the production of PGE2 would subvert the expansion and function of effector T cells before they arrived in infected or transplanted tissues. We should have considered how PGE2 might impact of T cell activation in the microenvironment of lymph nodes. For, in the microenvironment of lymph nodes, PGE2 might conceivably promote foreign antigen-specific immunity and hinder auto-antigenspecific immunity, at least as we imagine these potentially occur. Decades ago, PGE2 was shown to profoundly suppress random migration of helper T cells [57]. The investigation of PGE2 focused on the impact on T cells of known specificity and function. In a lymph node, the migrating dendritic cells encounter T cells of diverse specificities and activation of those recognizing peptide-MHC complexes depends on the duration of specific interaction with TCR. Since, as mentioned above, the activated dendritic cells have peptide-MHC complexes relatively fixed, the duration of TCR engagement with the complexes will depend on the number of MHC bearing a given peptide and the propensity of T cells to migrate away. In this setting, as opposed to the conditions in a mixed leukocyte culture, PGE2 would favor the full activation of T cells. Further, if PGE2 or other factors failed to slow the separation of newly activated T cells from activated dendritic cells, then T cells bearing TCR that recognize self peptide-MHC complexes might gain access to the activated dendritic cells, leading to autoimmunity.
8.8 How Heparan Sulfate Activates Dendritic Cells
While we clearly missed the opportunity to explore what might be a pivotal involvement of heparan sulfate metabolism in sculpting, via PGE2, the T cell response to antigen, we did not forget our earlier question about how heparan sulfate might interact with leukocytes in the first place.
We had found, as mentioned above, that heparan sulfate triggers many signaling pathways in APC [41], the constellation of which seemed incompatible with utilization of a single type of receptor. However, since the stimulated cells produced cytokines, it was possible some of the pathways were activated by an autocrine loop. Indeed, we had found that complement activates endothelial cells through such an autocrine loop—the membrane attack complex triggers transcriptional activation and secretion of IL-1α which acts on the endothelial cell to evoke the broad range of changes [26, 27, 58]. However, the pathways induced by heparan sulfate involved activation of protein kinase cascades and NFκB [41]. These pathways happened to be the same pathways utilized by LPS and for that reason we used LPS as a positive control when we first tested how heparan sulfate if at all would activate dendritic cells [46]. LPS proved an excellent control because it evoked responses quite similar to heparan sulfate.
In the late 1990s, toll-like receptor-4 (TLR-4) was reported to be the cellular protein through which LPS delivered signals to cells [59]. We immediately tested whether heparan sulfate might deliver signals through TLR-4. Using wild type mice and mice with mutations that encoded defective or absent TLR-4 or CD14 as sources of dendritic cells, we found that dendritic cells from the mutant strains of mice were inured to exposure to heparan sulfate while dendritic cells from wild type mice became activated as described above [60]. These results indicated quite clearly that heparan sulfate was utilizing TLR-4 in the same way as LPS, although how exactly either agonist utilized TLR-4 was not then clear. The results also suggested to us that since heparan sulfate proteoglycan undergoes degradation during the repair and remodeling of injured tissues, TLR might serve as monitors for the overall well being of tissues, and not just for infection [60, 61].
8.9 From Inflammation to the Immune Response to Transplantation?
Our investigation of heparan sulfate metabolism began with the question of whether processes such as ischemia and complement activation that damage endothelial cells could account in part for the unique characteristics of the cellular immune response to transplantation. Now, having found that transplantation (and complement activation) causes shedding of heparan sulfate and that the shed macromolecules activate dendritic cells, enabling T cell activation under suboptimal conditions, we were poised finally to test the overall model in transplantation. Defective signaling of all TLR, owing to deficiency of MyD88 in the transplant and in the recipient, had been reported to prevent development of rejection in tissues from male mice transplanted into female mice [62], a minor transplantation antigen mismatch. Using mice with aberrant or absent TLR4, we tested the concept both for minor and major (MHC) antigen mismatches and the results could not be more clear. Absence of TLR-4 function or protein had absolutely no impact on the kinetics of rejection. What did matter however was the genetic background of the strains of mice used. What explains such a result? Our earliest work showing that complement induces shedding of heparan sulfate from endothelial cells also showed that complement induces transcriptional activation of IL-1α, and that IL-1α acting as an autocrine agonist activates endothelial cells. Although we did not think about it at the time, the conditions that identified the seminal importance of IL-1α, including the replacing of medium bathing complement-treated cells and specifically blocking IL-1α, had proved that shed heparan sulfate was not essential for activation of endothelial cells. And, IL-1α was quite sufficient for activating macrophages and dendritic cells. Thus, if shedding of heparan sulfate and action of TLR-4 was important for ischemia and immune-mediated injury, and for the genesis of immunity in truly suboptimal conditions (when PGE2 is needed), it was not at all essential for the generation of alloimmunity, as the conditions in which transplantation immunity arises are far from suboptimal.
8.10 Whither Endogenous Agonists
Our work also led to an equally clear and less appealing conclusion. Immunologists impediti ratione cogitatonis were not at all ready to accept the possibility that something other than LPS or other exogenous (pathogen-derived) agonists could deliver signals through TLR. It took us nearly 4 years and layer upon layer of proof that heparan sulfate was not contaminated by bacterial products, to bring our findings to publication. During much of that time, TLR were said to be the receptors for PAMPS, pathogen associated molecular patterns [63, 64]. However, the idea that TLR could recognize endogenous agonists [61], apart from any contamination, eventually gained acceptance and the agonists came to be known as DAMPS, damage associated molecular patters.
But, the term “damage associated molecular patterns” may cloud more vital and universal functions for TLR and the metabolism of proteoglycans and some other macromolecules. As we knew from the work of others [10, 65] and confirmed [14, 15] at the outset of our work, development and possibly repair and regeneration, of tissues and organs depends absolutely on the degradation of proteoglycans (and perhaps other macromolecules from which ‘DAMP’ derive). Blocking degradation blocks development. This degradation then is not a reflection of ‘damage’ but is something essential for life in multicellular organisms [61]. Perhaps, then, developmental biologists might consider changing the acronym use to refer to the agonists of TLR or toll receptors, the invertebrate homologues of TLR, from DAMPS to “DAMPS,” the later referring, of course, to “development associated molecular patterns.”
With the more expansive definition of DAMPS in mind, we explored the potential impact of TLR on development and maturation of mice [66]. Mice deficient of TLR-4 or a co-receptor, CD14, looked very much like wild type mice and hence did not appear, at least to us, to have the gross development defects one might expect to see if TLR regulated the development of mice as toll regulates the development of insects. However, as wild type mice aged, they exhibited dramatic changes in weight, bone structure, and physique, becoming heavy, obese and osteoporotic and developing measurably weaker bones. In contrast, TLR-4-deficient or -defective mice and CD14-deficient mice remained lean and strong-boned and exhibited no osteoporosis as they aged. We thus referred to the phenotype of mice lacking TLR functions as the “Adonis phenotype.” Of particular interest, then, was the further observation that Adonis mice were no more active than wild type mice. Thus, despite the undoubted importance of exercise for overall health and well being, it did not explain the Adonis phenotype.
8.11 Heparan Sulfate in SIRS and Sepsis
Our interest in heparan sulfate as a potential agonist for TLR-4 led us to investigate an entirely different condition in which signaling by TLR generates biological changes. Besides their involvement in recruiting adaptive immune responses, TLR and particularly TLR-4 were best known as triggers for the sepsis syndrome and for the systemic inflammatory response syndrome or SIRS. SIRS was defined by the abrupt onset of fever, leukocytosis, shock and sometimes death in the absence of detectable infection; in the presence of infection, these findings would be called sepsis. SIRS occurs in such conditions as pancreatitis, multi-organ trauma, acute liver failure among others and the resemblance to sepsis is so close that investigators have asked repeatedly whether these conditions might cause by some means the entry of endotoxin into the system circulation. The answer has generally been no. Using the same strains of wild type and TLR-4 deficient or defective mice, we found that systemic administration of heparan sulfate had the same biological impact as LPS in wild type mice—it stimulated production of TNF and IL-6 and ultimately death—and like LPS, it had no appreciable effect on the mutant strains of mice [67]. Of particular note for those still skeptical about heparan sulfate was that a protein that specifically blocked the inflammatory impact of LPS did not impair the action of heparan sulfate.
We next asked whether release of endogenous heparan sulfate would engender SIRS. Serine proteases cleave heparan sulfate core proteins near the transmembrane domain to generate proteoglycans of the same size as those released from endothelial cells and one such protease, elastase, is released in pancreatitis. Hence we administered elastase to wild type and mice with defective TLR-4 signaling [67]. Once again, the results were clear—SIRS occurred in wild type mice while no appreciably changes occurred mutant strains. The administration of elastase did cause release of heparan sulfate in both strains and especially in spleen where large numbers of inflammatory cells are found.
These studies led us to propose a working model for the events that lead to the systemic inflammation and death in SIRS and potentially in sepsis [68, 69]. Since mice and humans with defective receptors for LPS have substantially increased risk of death from sepsis the expression and function of TLR-4 is clearly adaptive, probably facilitating the local containment and walling off of infectious agents [20]. Yet, when containment and walling off fail, and TLR beyond the site of infection are stimulated, systemic manifestations ensue—this concept probably represents the consensus model. But, we are struck by the observations in multiple clinical trials that administration of antibodies or other agents that block LPS does not improve the pathophysiology or outcome of sepsis and neither does it make sepsis worse [70]. To some, this trial and the many other failed attempts to improve the outcome of sepsis by blocking LPS indicate that still better blocking agents are needed. To us, these observations suggest the possibility that despite 150 years of research on LPS, that substance might not actually cause the life threatening manifestations of sepsis in patients with infection. And, if that is so, then we should at least consider the possibility that it is endogenous agonists for TLR, such as heparan sulfate, at not exogenous substances, such as LPS or other PAMPS, that cause the pathophysiology of sepsis (and SIRS). This model would finally unify the pathogenesis of SIRS and sepsis and possibly encourage someone to invent an acronym more poetic than DAMPS.
8.12 Concluding Remarks
Today heparan sulfate is known to have more functions in endothelial cell biology, and graft rejection, and graft acceptance than editorial space would permit us to discuss. However, we would be remiss if we failed at least to mention that the presence of heparan sulfate in tissue and organ grafts probably plays a key part in protecting grafts from injury, as another glance at Figs. 8.1 and 8.3 might suggest, and in reestablishing cell and tissue function after ischemia and reperfusion. Because, in the absence of immunosuppression, immunity poses an absolute barrier to transplantation of foreign tissues and organs, the subject of the immune response to transplantation overshadows nearly every other biological consideration. However, recent work in developmental and ‘regenerative’ biology suggests that restoring the integrity of tissue architecture and facilitating engraftment will pose challenges at least as great as those posed by transplant immunity. And, to the extent that a tissue or organ can be made to resist injury from ischemia or immunity, the challenge of regeneration and engraftment will be more easily met. Toward that objective, we investigate with enthusiasm, and hopefully non impediti ratione cogitatonis, the condition of “accommodation,” which we discovered unexpectedly in transplants that apparently resisted immune and inflammatory injury that should have caused their destruction [23, 71–73]. Accommodation is now appreciated to occur not only in transplants, but also in tumors, infections and in cells exposed to environmental toxins [71, 74, 75]. Whether by way of happenstance or mechanism, we have observed de novo expression of heparan sulfate, previously shed from grafts, in accommodated organs [76].
Acknowledgments
We thank Charles A. C. Platt for insightful discussions about saccharides. The NIH supported the work summarized here.
Contributor Information
Jeffrey L. Platt, Email: plattjl@umich.edu, Transplantation Biology, Department of Surgery, University of Michigan, A520B Medical Sciences Research Building I, 1150W. Medical Center Drive, Ann Arbor, MI 48109-5656, USA; Department of Microbiology & Immunology, University of Michigan, Ann Arbor, MI, USA.
Lucile E. Wrenshall, Email: Lucile.wrenshall@wright.edu, Department of Surgery, Wright State University, Dayton, OH, USA; Department of Neuroscience, Cell Biology and Physiology, Wright State University, Dayton, OH, USA.
Geoffrey B. Johnson, Email: johnson.geoffrey@mayo.edu, Department of Radiology, Mayo Clinic, Rochester, MN, USA; Department of Immunology, Mayo Clinic, Rochester, MN, USA.
Marilia Cascalho, Email: marilia@umich.edu, Transplantation Biology, Department of Surgery, University of Michigan, A520B Medical Sciences Research Building I, 1150W. Medical Center Drive, Ann Arbor, MI 48109-5656, USA; Department of Microbiology & Immunology, University of Michigan, Ann Arbor, MI, USA.
References
- 1.Dabek F. ‘Car Talk’ Brothers Address Graduates. The Tech. 1999;119:1–16. [Google Scholar]
- 2.Platt JL, Rubinstein P. Mechanisms and characteristics of allograft rejection. In: Sabiston DC Jr, Lyerly HK, editors. Textbook of surgery: the biological basis of modern surgical practice. 15th. Philadelphia: W.B.: Saunders; 1997. pp. 400–408. [Google Scholar]
- 3.Platt JL, Cascalho M. Transplantation immunology. In: Mulholland MW, Lillemoe KD, Doherty GM, Maier RV, Simeone DM, Upchurch GRJ, editors. Greenfield’s surgery: scientific principles and practice. 5th. Philadelphia: Lippincott Williams & Wilkins; 2011. pp. 497–514. [Google Scholar]
- 4.Platt JL, Cascalho M, West LJ. Lessons from cardiac transplantation in infancy. Pediatr Transplant. 2009;13:814–819. doi: 10.1111/j.1399-3046.2009.01143.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lynch RJ, Silva IA, Chen BJ, Punch JD, Cascalho M, Platt JL. Cryptic B cell response to renal transplantation. Am J Transplant. 2013;13(7):1713–1723. doi: 10.1111/ajt.12308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Platt JL, LeBien TW, Michael AF. Interstitial mononuclear cell populations in renal graft rejection: identification by monoclonal antibodies in tissue sections. J Exp Med. 1982;155:17–30. doi: 10.1084/jem.155.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Platt JL, Grant BW, Eddy AA, Michael AF. Immune cell populations in cutaneous delayed-type hypersensitivity. J Exp Med. 1983;158:1227–1242. doi: 10.1084/jem.158.4.1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Platt JL, LeBien TW, Michael AF. Stages of renal ontogenesis identified by monoclonal antibodies reactive with lymphohemopoietic differentiation antigens. J Exp Med. 1983;157:155–172. doi: 10.1084/jem.157.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Platt JL, Burke BA, Michael AF. Cellular antigens in nephroblastoma: identification with monoclonal antibodies which recognize hemopoietic cells. Clin Immunol Immunopathol. 1987;43:110–116. doi: 10.1016/0090-1229(87)90162-0. [DOI] [PubMed] [Google Scholar]
- 10.Bernfield M, Banerjee SD. The turnover of basal lamina glycosaminoglycan correlates with epithelial morphogenesis. Dev Biol. 1982;90(2):291–305. doi: 10.1016/0012-1606(82)90378-5. [DOI] [PubMed] [Google Scholar]
- 11.Iozzo RV. Matrix proteoglycans: from molecular design to cellular function. Annu Rev Biochem. 1998;67:609–652. doi: 10.1146/annurev.biochem.67.1.609. [DOI] [PubMed] [Google Scholar]
- 12.Sarrazin S, Lamanna WC, Esko JD. Heparan sulfate proteoglycans. Cold Spring Harb Perspect Biol. 2011;3(7) doi: 10.1101/cshperspect.a004952. a004952 (1–33) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mikami T, Kitagawa H. Biosynthesis and function of chondroitin sulfate. Biochim Biophys Acta. 2013;1830(10):4719–4733. doi: 10.1016/j.bbagen.2013.06.006. [DOI] [PubMed] [Google Scholar]
- 14.Platt JL, Brown DM, Granlund K, Oegema TR, Klein DJ. Proteoglycan metabolism associated with mouse metanephric development: morphologic and biochemical effects of b-D-xyloside. Dev Biol. 1987;123:293–306. doi: 10.1016/0012-1606(87)90388-5. [DOI] [PubMed] [Google Scholar]
- 15.Klein DJ, Brown DM, Moran A, Oegema TR, Platt JL. Chondroitin sulfate proteoglycan synthesis and reutilization of b-D-xyloside initiated chondroitin/dermatan sulfate glycosaminoglycans in fetal kidney branching morphogenesis. Dev Biol. 1989;133:515–528. doi: 10.1016/0012-1606(89)90054-7. [DOI] [PubMed] [Google Scholar]
- 16.Klein DJ, Brown DM, Oegema TR, Brenchely PE, Anderson JC, Dickinson MAJ, et al. Glomerular basement membrane proteoglycans are derived from a large precursor. J Cell Biol. 1988;106:963–970. doi: 10.1083/jcb.106.3.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ihrcke NS, Wrenshall LE, Lindman BJ, Platt JL. Role of heparan sulfate in immune system-blood vessel interactions. Immunol Today. 1993;14:500–505. doi: 10.1016/0167-5699(93)90265-M. [DOI] [PubMed] [Google Scholar]
- 18.Selvan RS, Ihrcke NS, Platt JL. Heparan sulfate in immune responses. Ann N Y Acad Sci. 1996;797:127–139. doi: 10.1111/j.1749-6632.1996.tb52955.x. [DOI] [PubMed] [Google Scholar]
- 19.Platt JL, Saadi S. The role of complement in transplantation. Mol Immunol. 1999;36:965–971. doi: 10.1016/s0161-5890(99)00119-4. [DOI] [PubMed] [Google Scholar]
- 20.Saadi S, Wrenshall LE, Platt JL. Regional manifestations and control of the immune system. FASEB J. 2002;16(8):849–856. doi: 10.1096/fj.01-0690hyp. [DOI] [PubMed] [Google Scholar]
- 21.Platt JL, Trescony P, Lindman BJ, Oegema TR. Heparin and heparan sulfate delimit nephron formation in fetal metanephric kidneys. Dev Biol. 1990;139:338–348. doi: 10.1016/0012-1606(90)90303-z. [DOI] [PubMed] [Google Scholar]
- 22.Platt JL, Vercellotti GM, Lindman BJ, Oegema TR, Jr, Bach FH, Dalmasso AP. Release of heparan sulfate from endothelial cells. Implications for pathogenesis of hyperacute rejection. J Exp Med. 1990;171(4):1363–1368. doi: 10.1084/jem.171.4.1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Platt JL, Vercellotti GM, Dalmasso AP, Matas AJ, Bolman RM, Najarian JS, et al. Transplantation of discordant xenografts: a review of progress. Immunol Today. 1990;11:450–456. doi: 10.1016/0167-5699(90)90174-8. [DOI] [PubMed] [Google Scholar]
- 24.Key NS, Platt JL, Vercellotti GM. Vascular endothelial cell proteoglycans are susceptible to cleavage by neutrophils. Arterioscler Thromb. 1992;12:836–842. doi: 10.1161/01.atv.12.7.836. [DOI] [PubMed] [Google Scholar]
- 25.Geller RL, Ihrcke NS, Platt JL. Release of endothelial cell-associated heparan sulfate proteoglycan by activated T cells. Transplantation. 1994;57:770–774. [PubMed] [Google Scholar]
- 26.Saadi S, Holzknecht RA, Patte CP, Stern DM, Platt JL. Complement-mediated regulation of tissue factor activity in endothelium. J Exp Med. 1995;182:1807–1814. doi: 10.1084/jem.182.6.1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bustos M, Coffman TM, Saadi S, Platt JL. Modulation of eicosanoid metabolism in endothelial cells in a xenograft model: role of cyclooxygenase-2. J Clin Invest. 1997;100:1150–1158. doi: 10.1172/JCI119626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Malyguine AM, Saadi S, Holzknecht RA, Patte CR, Sud N, Platt JL, et al. Induction of procoagulant function in porcine endothelial cells by human NK cells. J Immunol. 1997;159:4659–4664. [PubMed] [Google Scholar]
- 29.Saadi S, Holzknecht RA, Patte CP, Platt JL. Endothelial cell activation by pore forming structures: pivotal role for IL-1a. Circulation. 2000;101:1867–1873. doi: 10.1161/01.cir.101.15.1867. [DOI] [PubMed] [Google Scholar]
- 30.Bustos M, Saadi S, Platt JL. Platelet-mediated activation of endothelial cells: implications for the pathogenesis of transplant rejection. Transplantation. 2001;72:509–515. doi: 10.1097/00007890-200108150-00025. [DOI] [PubMed] [Google Scholar]
- 31.Ihrcke NS, Parker W, Reissner KJ, Platt JL. Regulation of platelet heparanase during inflammation: role of pH and proteinases. J Cell Physiol. 1998;175:255–267. doi: 10.1002/(SICI)1097-4652(199806)175:3<255::AID-JCP3>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 32.Ihrcke NS, Platt JL. Shedding of heparan sulfate proteoglycan by stimulated endothelial cells: evidence for proteolysis of cell surface molecules. J Cell Physiol. 1996;168:625–637. doi: 10.1002/(SICI)1097-4652(199609)168:3<625::AID-JCP15>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 33.Saadi S, Platt JL. Endothelial cell responses to complement activation. In: Volanakis JE, Frank MM, editors. The human complement system in health and disease. New York: Marcel Dekker, Inc.; 1998. pp. 335–353. [Google Scholar]
- 34.Naparstek Y, Cohen IR, Fuks Z, Vlodavsky I. Activated T-lymphocytes produce a matrix-degrading heparan sulfate endoglycosidase. Nature. 1984;310:241–244. doi: 10.1038/310241a0. [DOI] [PubMed] [Google Scholar]
- 35.Fridman R, Lider O, Naparstek Y, Fuks Z, Vlodavsky I, Cohen IR. Soluble antigen induces T lymphocytes to secrete an endoglycosidase that degrades the heparan sulfate moiety of subendothelial extracellular matrix. J Cell Physiol. 1987;130:85–92. doi: 10.1002/jcp.1041300113. [DOI] [PubMed] [Google Scholar]
- 36.Raats CJ, Bakker MA, van den Born J, Berden JH. Hydroxyl radicals depolymerize glomerular heparan sulfate in vitro and in experimental nephrotic syndrome. J Biol Chem. 1997;272(42):26734–26741. doi: 10.1074/jbc.272.42.26734. [DOI] [PubMed] [Google Scholar]
- 37.Lider O, Baharav E, Mekori YA, Miller T, Naparstek Y, Vlodavsky I, et al. Suppression of experimental autoimmune diseases and prolongation of allograft survival by treatment of animals with low doses of heparins. J Clin Invest. 1989;83:752–756. doi: 10.1172/JCI113953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lider O, Mekori YA, Miller T, Bar-Tana R, Vlodavsky I, Baharav E, et al. Inhibition of T lymphocyte heparanase by heparin prevents T cell migration and T cell-mediated immunity. Eur J Immunol. 1990;20:493–499. doi: 10.1002/eji.1830200306. [DOI] [PubMed] [Google Scholar]
- 39.Wrenshall LE, Cerra FB, Carlson A, Bach FH, Platt JL. Regulation of murine splenocyte responses by heparan sulfate. J Immunol. 1991;147:455–459. [PubMed] [Google Scholar]
- 40.Wrenshall LE, Carlson A, Cerra FB, Platt JL. Modulation of cytolytic T cell responses by heparan sulfate. Transplantation. 1994;57:1087–1094. [PubMed] [Google Scholar]
- 41.Wrenshall LE, Cerra FB, Singh RK, Platt JL. Heparan sulfate initiates signals in murine macrophages leading to divergent biological outcomes. J Immunol. 1995;154:871–880. [PubMed] [Google Scholar]
- 42.Wrenshall LE, Stevens RB, Cerra FB, Platt JL. Modulation of macrophage and B cell function by glycosaminoglycans. J Leukoc Biol. 1999;66(3):391–400. doi: 10.1002/jlb.66.3.391. [DOI] [PubMed] [Google Scholar]
- 43.Steinman RM. Dendritic cells: understanding immunogenicity. Eur J Immunol. 2007;37(Suppl 1):S53–S60. doi: 10.1002/eji.200737400. [DOI] [PubMed] [Google Scholar]
- 44.Gilboa E. DC-based cancer vaccines. J Clin Invest. 2007;117(5):1195–1203. doi: 10.1172/JCI31205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Unanue ER. Antigen presentation in the autoimmune diabetes of the NOD mouse. Annu Rev Immunol. 2014;32:579–608. doi: 10.1146/annurev-immunol-032712-095941. [DOI] [PubMed] [Google Scholar]
- 46.Kodaira Y, Nair SK, Wrenshall LE, Gilboa E, Platt JL. Phenotypic and functional maturation of dendritic cells modulated by heparan sulfate. J Immunol. 2000;165:1599–1604. doi: 10.4049/jimmunol.165.3.1599. [DOI] [PubMed] [Google Scholar]
- 47.van Montfoort N, Camps MG, Khan S, Filippov DV, Weterings JJ, Griffith JM, et al. Antigen storage compartments in mature dendritic cells facilitate prolonged cytotoxic T lymphocyte cross-priming capacity. Proc Natl Acad Sci U S A. 2009;106(16):6730–6735. doi: 10.1073/pnas.0900969106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Steinman RM, Nussenzweig MC. Avoiding horror autotoxicus: the importance of dendritic cells in peripheral T cell tolerance. Proc Natl Acad Sci U S A. 2002;99(1):351–358. doi: 10.1073/pnas.231606698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hawiger D, Inaba K, Dorsett Y, Guo M, Mahnke K, Rivera M, et al. Dendritic cells induce peripheral T cell unresponsiveness under steady state conditions in vivo. J Exp Med. 2001;194(6):769–779. doi: 10.1084/jem.194.6.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Thomson AW, Robbins PD. Tolerogenic dendritic cells for autoimmune disease and transplantation. Ann Rheum Dis. 2008;67(Suppl 3):iii90–iii96. doi: 10.1136/ard.2008.099176. [DOI] [PubMed] [Google Scholar]
- 51.Frick JS, Grunebach F, Autenrieth IB. Immunomodulation by semi-mature dendritic cells: a novel role of Toll-like receptors and interleukin-6. Int J Med Microbiol. 2010;300(1):19–24. doi: 10.1016/j.ijmm.2009.08.010. [DOI] [PubMed] [Google Scholar]
- 52.Johnson DJ, Ohashi PS. Molecular programming of steady-state dendritic cells: impact on autoimmunity and tumor immune surveillance. Ann N Y Acad Sci. 2013;1284:46–51. doi: 10.1111/nyas.12114. [DOI] [PubMed] [Google Scholar]
- 53.Morelli AE, Thomson AW. Orchestration of transplantation tolerance by regulatory dendritic cell therapy or in-situ targeting of dendritic cells. Curr Opin Organ Transplant. 2014;19(4):348–356. doi: 10.1097/MOT.0000000000000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wrenshall LE, Platt JL. Regulation of T cell homeostasis by heparan sulfate-bound IL-2. J Immunol. 1999;163:3793–3800. [PubMed] [Google Scholar]
- 55.Wrenshall LE, Platt JL, Elliot TS, Wight TN, Miller JD. Propagation and control of T cell responses by heparan sulfate-bound IL-2. J Immunol. 2003;170:5470–5474. doi: 10.4049/jimmunol.170.11.5470. [DOI] [PubMed] [Google Scholar]
- 56.Platt JL, Johnson GB, Kodaira Y, Wrenshall LE. Tolerance and the microenvironment. Transplantation. 2001;72:S23–S24. [PubMed] [Google Scholar]
- 57.Jordan ML, Hoffman RA, Debe EF, West MA, Simmons RL. Prostaglandin E2 mediates subset- specific effects on the functional responses of allosensitized T lymphocyte clones. Transplantation. 1987;43(1):117–123. doi: 10.1097/00007890-198701000-00026. [DOI] [PubMed] [Google Scholar]
- 58.Brunn GJ, Saadi S, Platt JL. Differential regulation of endothelial cell activation by complement and interleukin 1alpha. Circ Res. 2006;98:793–800. doi: 10.1161/01.RES.0000216071.87981.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Poltorak A, He X, Smirnova I, Liu MY, Huffel CV, Du X, et al. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science. 1998;282:2085–2088. doi: 10.1126/science.282.5396.2085. [DOI] [PubMed] [Google Scholar]
- 60.Johnson GB, Brunn GJ, Kodaira Y, Platt JL. Receptor-mediated monitoring of tissue well-being via detection of soluble heparan sulfate by toll-like receptor 4. J Immunol. 2002;168(10):5233–5239. doi: 10.4049/jimmunol.168.10.5233. [DOI] [PubMed] [Google Scholar]
- 61.Johnson GB, Brunn GJ, Tang AH, Platt JL. Evolutionary clues to the functions of the Toll-like family as surveillance receptors. Trends Immunol. 2003;24(1):19–24. doi: 10.1016/s1471-4906(02)00014-5. [DOI] [PubMed] [Google Scholar]
- 62.Goldstein DR, Tesar BM, Akira S, Lakkis FG. Critical role of the Toll-like receptor signal adaptor protein MyD88 in acute allograft rejection. J Clin Invest. 2003;111(10):1571–1578. doi: 10.1172/JCI17573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Janeway CA, Jr, Medzhitov R. Lipoproteins take their toll on the host. Curr Biol. 1999;9(23):R879–R882. doi: 10.1016/s0960-9822(00)80073-1. [DOI] [PubMed] [Google Scholar]
- 64.Medzhitov R. Toll-like receptors and innate immunity. Nat Rev Immunol. 2001;1:135. doi: 10.1038/35100529. [DOI] [PubMed] [Google Scholar]
- 65.Rapraeger A, Bernfield M. Cell surface proteoglycan of mammary epithelial cells: protease releases a heparin sulfate-rich extodomain from a putative membrane-associated domain. J Biol Chem. 1985;260:4103–4109. [PubMed] [Google Scholar]
- 66.Johnson GB, Riggs BL, Platt JL. A genetic basis for the “Adonis” phenotype of low adiposity and strong bones. FASEB J. 2004;18(11):1282–1284. doi: 10.1096/fj.04-1572fje. [DOI] [PubMed] [Google Scholar]
- 67.Johnson GB, Brunn GJ, Platt JL. An endogenous pathway to systemic inflammatory response syndrome (SIRS)-like responses through toll-like receptor 4. J Immunol (Cutting Edge) 2004;172(1):20–24. doi: 10.4049/jimmunol.172.1.20. [DOI] [PubMed] [Google Scholar]
- 68.Brunn GJ, Wijdicks MF, Platt JL. Toward a modern concept of sepsis: new answers to ancient questions. Discov Med. 2006;6(31):11–17. [PubMed] [Google Scholar]
- 69.Brunn GJ, Platt JL. The etiology of sepsis: turned inside out. Trends Mol Med. 2006;12(1):10–16. doi: 10.1016/j.molmed.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 70.Opal SM, Laterre PF, Francois B, LaRosa SP, Angus DC, Mira JP, et al. Effect of eritoran, an antagonist of MD2-TLR4, on mortality in patients with severe sepsis: the ACCESS randomized trial. JAMA. 2013;309(11):1154–1162. doi: 10.1001/jama.2013.2194. [DOI] [PubMed] [Google Scholar]
- 71.Koch CA, Khalpey ZI, Platt JL. Accommodation: preventing injury in transplantation and disease. J Immunol. 2004;172(9):5143–5148. doi: 10.4049/jimmunol.172.9.5143. [DOI] [PubMed] [Google Scholar]
- 72.Lynch RJ, Platt JL. Accommodation in organ transplantation. Curr Opin Organ Transplant. 2008;13:165–170. doi: 10.1097/MOT.0b013e3282f6391e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cascalho MI, Chen BJ, Kain M, Platt JL. The paradoxical functions of B cells and antibodies in transplantation. J Immunol. 2013;190(3):875–879. doi: 10.4049/jimmunol.1100120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Koch CA, Khalpey ZI, Platt JL. Humoral immunity in xenotransplantation: B-cell tolerance and accommodation. Curr Opin Organ Transplant. 2004;9(2):170–175. [Google Scholar]
- 75.Koch CA, Kanazawa A, Nishitai R, Knudsen BE, Ogata K, Plummer TB, et al. Intrinsic resistance of hepatocytes to complement-mediated injury. J Immunol. 2005;174:7302–7309. doi: 10.4049/jimmunol.174.11.7302. [DOI] [PubMed] [Google Scholar]
- 76.Williams JM, Holzknecht ZE, Plummer TB, Lin SS, Brunn GJ, Platt JL. Acute vascular rejection and accommodation: divergent outcomes of the humoral response to organ transplantation. Transplantation. 2004;78(10):1471–1478. doi: 10.1097/01.tp.0000140770.81537.64. [DOI] [PubMed] [Google Scholar]