Abstract
Objective
We aimed to determine the association between the stepwise increase in the sustained viral response (SVR) and Swiss and United States (US) market prices of drug regimens for treatment-naive, genotype 1 chronic hepatitis C virus (HCV) infection in the last 25 years. We identified the following five steps in the development of HCV treatment regimens: 1) interferon (IFN)-α monotherapy in the early '90s, 2) IFN-α in combination with ribavirin (RBV), 3) pegylated (peg) IFN-α in combination with RBV, 4) the first direct acting antivirals (DAAs) (telaprevir and boceprevir) in combination with pegIFN-α and RBV, and 5) newer DAA-based regimens, such as sofosbuvir (which is or is not combined with ledipasvir) and fixed-dose combination of ritonavir-boosted paritaprevir and ombitasvir in combination with dasabuvir.
Design
We performed a linear regression and mean cost analysis to test for an association between SVRs and HCV regimen prices. We conducted a sensitivity analysis using US prices at the time of US drug licensing. We selected randomized clinical trials of drugs approved for use in Switzerland from 1997 to July 2015 including treatment-naïve patients with HCV genotype 1 infection.
Results
We identified a statistically significant positive relationship between the proportion of patients achieving SVRs and the costs of HCV regimens in Switzerland (with a bivariate ordinary least square regression yielding an R2 measure of 0.96) and the US (R2 = 0.95). The incremental cost per additional percentage of SVR was 597.14 USD in Switzerland and 1,063.81 USD in the US.
Conclusion
The pricing of drugs for HCV regimens follows a value-based model, which has a stable ratio of costs per achieved SVR over 25 years. Health care systems are struggling with the high resource use of these new agents despite their obvious long-term advantages for the overall health of the population. Therefore, the pharmaceutical industry, health care payers and other stakeholders are challenged with finding new drug pricing schemes to treat the entire population infected with HCV.
Introduction
The development and market approval of novel direct-acting antivirals (DAAs) have dramatically changed the hepatitis C virus (HCV) treatment landscape. For the first time, chronic HCV infection, a major human pathogen responsible for cirrhosis and hepatocellular carcinoma and a leading indication for liver transplantation, may be eradicated in all patients using an all-oral, short-duration, well-tolerated, safe, and highly efficacious treatment.[1]
A new nosological entity first identified in the 1970s, the so-called non-A, non-B hepatitis, was discovered as HCV in 1989.[2, 3] The first treatment consisted of recombinant interferon (IFN)-α monotherapy and was characterized by a low cure percentage (<10%) and severe side effects.[3] In subsequent decades, new drugs gradually increased the percentage of cure in a stepwise fashion.[2, 3] The IFN-α in combination with ribavirin (RBV) resulted in 34–42% cure rates. The substitution of standard IFN-α with its pegylated (peg) form, administered once per week, resulted in an increase in cure rates from 45% to 80% depending on the HCV genotype.[4, 5] The first DAAs were telaprevir (TVR) and boceprevir (BOC), which increased the cure rate up to 70–80% for HCV genotype 1.[6–8] Finally, the arrival of the new DAAs, such as sofosbuvir (SOF) by itself or in combination with ledipasvir (LDV), and the triple fixed-dose combination of ritonavir-boosted paritaprevir, ombitasvir (PTV/r/OBV) in combination with dasabuvir (DSV) led to unprecedented ~100% cure rates in several patient subgroups.[9–11] At the same time, two additional DAAs, simeprevir (SMV) and daclatasvir (DCV), were added to the already available armamentarium.[12–15] However, the new drug regimens were marketed at very high prices for each treatment compared to their predecessors, increasing the financial challenge for health care systems aiming to provide the entire HCV-infected population with access to those medicines. The cost of a 12-week SOF-based regimen in the United Sates (US) that contains pegIFN-α and RBV exceeded 90,000 USD at registration, which is much higher than the standard cost of 20,000 USD for historical IFN-α monotherapy.[16, 17]
Therefore, a better understanding of the determinants of HCV drug pricing is essential.[18] Key elements of drug pricing include the amount of money invested in research and development (R&D), production costs, efficacy, safety, ease of administration, duration of treatment, features of treatment comparators, innovation, international benchmarking, market size and market value.[19–22] Thus, we aimed to determine the potential association between the stepwise increase in the sustained viral response (SVR) and treatment regimen prices. We assessed the prices of different regimens at their market entry and the respective SVR rates, which are listed in the official Swiss label (available at http://www.swissmedicinfo.ch) for treatment-naive, genotype 1 chronic hepatitis C patients, corresponding to the most prevalent patient subgroup in our country.[1, 23] We also conducted a sensitivity analysis using US prices at the time of drug licensing.
Materials and Methods
Therapy stepping stones
We classified the different treatment regimens into five steps, as suggested by the literature (Table 1).[3, 24, 25]
Table 1. Approved drugs for HCV infection in Switzerland and the United States.
| Steps | Regimen | Drugs | In combination with Interferon | Swissmedic approval | FDA approval |
|---|---|---|---|---|---|
| Step 1 | Interferon-α monotherapy | IFN-α-2a | - | 1997 | |
| IFN-α-2b | - | 1998 | |||
| Step 2 | Interferon-α associated with ribavirin | RBV | Yes | 2002 | 2001 |
| Step 3 | Pegylated interferon-α associated with ribavirin | pegIFN-α-2a | 2001 | ||
| pegIFN-α-2b | - | 2003 | 2002 | ||
| Step 4 | First DAAs (serine protease inhibitors) associated with pegylated interferon-α and ribavirin | TVR, BOC | Yes | 2011 | 2011 |
| SMV | Yes | 2015 | 2013 | ||
| Step 5 | Second wave DAAs: nucleotidic and non-nucleosidic polymerase inhibitors, NS5A inhibitors, more serine protease inhibitors that are or are not associated with pegylated interferon-α and ribavirin | SOF | Yes | 2014 | 2013 |
| SOF/LDV | No | 2015 | 2014 | ||
| PTV/r/OBV +DSV | No | 2014 | 2015 |
IFN: interferon; RBV: ribavirin; pegIFN: pegylated interferon; TVR telaprevir; BOC: boceprevir; SMV: simeprevir; SOF: sofosbuvir; LDV: ledipasvir; PTV/r/OBV: fixed dose combination of ritonavir-boosted paritaprevir and ombitasvir; DSV: dasabuvir
We selected all phase 3 randomized clinical trials (RCT) that enrolled previously untreated patients with chronic HCV genotype 1 infection and tested drugs that were approved by Swissmedic, which were therefore marketed in Switzerland from January 1st 1997 to July 31st 2015. For each of these drugs regimens, we used the SVR rate as reported in the studies at the time of the Swissmedic marketing authorization request (http://www.swissmedic.org); Swissmedic is the Swiss agency that authorizes and supervises drugs. The drug price, dosage and duration were based on the recommended Swissmedic guidelines for HCV treatment-naive patients with genotype 1 infection during the study period. For older drug regimens that are not listed in the Swissmedic database, we used data from the corresponding US agency (Food and Drug Administration, FDA). Finally, because data for IFN-α were not recorded in either database, we contacted the pharmaceutical companies to determine which published studies contributed to drug marketing. [4,5] SVR was defined as undetectable HCV RNA in the serum 12 weeks after the end of treatment for DAA-based regimens and 24 weeks for IFN-α based regimens.[26]
Swiss market access
In Switzerland, market access is conditional on a two-step decision process. First, a drug must be approved by Swissmedic.[27] Then, the Federal Office of Public Health (FOPH) decides whether or not to include the medication in the list of drugs reimbursed by the mandatory health insurance scheme based on a recommendation by the Federal Drug Commission (FDC). The FDC evaluates the value of new drugs according to the following three criteria: effectiveness, appropriateness, and efficiency. Furthermore, the FDC applies reference pricing using a basket of nine European countries. Finally, the FDC considers the budget impact by comparing the total costs of the new treatment regimen with the standard of care. The FOPH then negotiates the final price with the manufacturer and re-evaluates the drug price every three years. We performed analysis from a third-party payer perspective.
Cost calculation
The market prices of IFN-α, pegIFN-α and RBV have changed throughout the past 25 years. Therefore, we controlled for inflation by using the market price of IFN-α for step 1 in 1997, which was adjusted by the inflation rate for both Swiss and US pricing using an online calculator (fxtop.com/en/inflation-calculator.php). Likewise, for steps 2 and 3, we used the January 2003 prices of IFN-α, pegIFN-α and RBV, which were adjusted by the inflation rate. For the first and second wave DAAs included in steps 4 and 5, we used the launching market price and current price for pegIFN-α and RBV without adjusting for inflation. For the comparison of prices adjusted to the 2015 level, we considered an exchange rate of one CHF equal to one USD. Although RBV administration is usually weight-adapted, we arbitrarily considered a standard daily dose of 1,000 mg for all recipients. We assumed that the US drug costs were equal to the wholesale acquisition costs (WAC) as listed in the Red Book Online (http://www.redbook.com/redbook/online). For regimens requiring RBV, and when many manufacturers shared the RBV market, we selected the cheapest regimen. We also calculated the ratio of costs per SVR (costs/SVR) for each regimen, representing the costs to cure one patient.
Statistical analysis
We used two methods to assess the HCV therapy pricing model. First, we plotted the costs per treatment of the twenty-two regimens against the rate of SVR for both Switzerland and the US. It is noteworthy that ordering according to the SVR rate corresponds to the stepwise increase in the cure over time described in the literature.[2, 3] Because the scatter diagram indicates a linear relationship, we tested for the linear correlation between the two variables by the standard Pearson correlation coefficient and R2 of a bivariate linear regression.[28] Both represent the overall fit of a linear model. Second, we measured the mean and standard deviation of the costs and costs per SVR for the five HCV treatment steps.[3, 24] We used Eviews 8 software (QMS) for the statistical analysis.
Results
Twenty-two RCTs were included in our study, comprising a total of 5,900 patients (Table 2).
Table 2. Treatments, duration, costs and costs per SVR of HCV treatments over time in Switzerland and the US (Table A in S1 File).
| Treatment | Study | Treatment duration, weeks | Patients, n SVR achieved/n total | SVR, % | Swiss Costs USD | Costs per SVR in Switzerland USD | US costs USD | US costs per SVR in US USD |
|---|---|---|---|---|---|---|---|---|
| Step 1 | ||||||||
| IFN-α-2a | FDA Roferon label[29] | 48 | 20/173 | 11.56% | 9,544 | 82,561 | 6,148 | 53,183 |
| IFN-α-2b | Reference[4] | 48 | 37/303 | 12.21% | 9,706 | 79,492 | 6,046 | 49,517 |
| Step 2 | ||||||||
| IFN-α-2b+RBV | CARRY FORWARD[30] | 48 | 111/328 | 33.84% | 23,799 | 70,328 | 25,543 | 75,482 |
| IFN-α-2a+RBV | NV15801[31] | 48 | 100/285 | 35.09% | 20,951 | 59,706 | 24,584 | 70,060 |
| Step 3 | ||||||||
| pegIFN-α-2a+RBV | ADVANCE[6] | 48 | 158/361 | 43.77% | 34,575 | 78,992 | 37,222 | 85,040 |
| pegIFN-α-2a+RBV | NV15801[31] | 48 | 132/298 | 44.30% | 34,575 | 78,047 | 37,222 | 84,023 |
| pegIFN-α-2b+RBV | CARRY FORWARD[30] | 48 | 58/122 | 47.54% | 33,637 | 70,755 | 32,979 | 69,371 |
| pegIFN-α-2a+RBV | QUEST-1[13] | 48 | 65/130 | 50.00% | 34,575 | 69,150 | 37,222 | 74,444 |
| pegIFN-α-2a+RBV | QUEST-2[14] | 48 | 67/134 | 50.00% | 34,575 | 69,150 | 37,222 | 74,444 |
| pegIFN-α-2a+RBV | NV15942[31] | 48 | 142/271 | 52.40% | 34,575 | 65,983 | 37,222 | 71,034 |
| Step 4 | ||||||||
| BOC+ pegIFN-α-2a+RBV | SPRINT-2[7] | 4+44 (73%)* | 233/368 | 63.32% | 42,566 | 67,224 | 57,741 | 91,189 |
| TVR+ pegIFN-α-2a+RBV | OPTIMIZE[32] | 12+36 (67%)† | 270/371 | 72.78% | 47,304 | 64,996 | 75,210 | 103,339 |
| TVR+ pegIFN-α-2a+RBV | OPTMIZE[32] | 12+36 (69%)† | 274/369 | 74.25% | 47,091 | 63,422 | 74,818 | 100,765 |
| TVR+ pegIFN-α-2a+RBV | ADVANCE[6] | 12+36 (68%)† | 285/363 | 78.51% | 47,240 | 60,171 | 75,092 | 95,646 |
| SMV+ pegIFN-α+RBV | QUEST-1[13] | 12+12 (85%)¶ | 210/264 | 79.55% | 44,833 | 56,358 | 85,891 | 107,971 |
| SMV+ pegIFN-α+RBV | QUEST-2[14] | 12+12 (91%)¶ | 209/257 | 81.32% | 44,512 | 54,737 | 87,741 | 107,896 |
| Step 5 | ||||||||
| SOF+ pegIFN-α-2a+RBV | NEUTRINO[11] | 12 | 262/292 | 89.73% | 62,955 | 70,160 | 93,808 | 104,545 |
| SOF/LDV | ION-3[10] | 12 | 208/216 | 96.30% | 62,363 | 64,759 | 94,500 | 98,131 |
| PTV/r/OBV+DSV+ RBV | SAPHIRE-I[12] | 12 | 455/473 | 96.19% | 63,946 | 66,479 | 86,215 | 89,630 |
| PTV/r/OBV+DSV+ RBV | PEARL-IV[9] | 12 | 97/100 | 97.00% | 63,946 | 65,924 | 86,215 | 88,881 |
| SOF/LDV | ION-1[33] | 12 | 210/213 | 98.59% | 62,363 | 63,255 | 94,500 | 95,852 |
| PTV/r/OBV+DSV | PEARL-III[9] | 12 | 209/209 | 100% | 61,956 | 61,956 | 83,319 | 83,319 |
* The stopping rule for response-guided therapy BOC included patients with undetectable HCV RNA from treatment weeks 8 to 24
† A patient qualified for a shortened TVR therapy duration for HCV RNA <25 IU/ml at weeks 4 and 12.
¶ SMV response-guided therapy involved stopping the treatment after 24 weeks for patients with HCV RNA <25 IU/ml at week 4 (undetectable or detectable) and <25 IU/ml at week 12 (undetectable)
Two clinical trials were included for the IFN-α monotherapy step (n = 476 patients)[4, 29], two studies for the association between IFN-α and RBV (n = 613 patients)[5, 31], six for step 3 between pegIFN-α and RBV (n = 1,316 patients)[5, 6, 13, 14, 31], six for the first DAAs (n = 1,992 patients)[6, 7, 13, 14, 32], and six for the newer DAAs (n = 1,503 patients).[9–12, 33]
Table 2 provides the costs and costs per SVR for each individual treatment regimen and shows a steady cost increase paralleling the increase in the SVR rate for each treatment step. From step 4 onward, the treatment durations were shortened to 24 or even 12 weeks, and pegIFN-α could be omitted for most regimens at step 5.
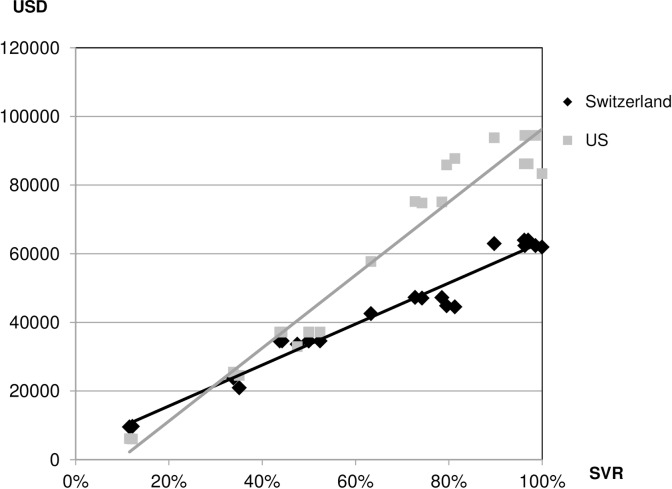
The scatter plot in Fig 1 suggests a positive linear association between SVR and the cost of HCV treatment regimens using Swiss cost figures. A high degree of positive linear dependence is indicated by a Pearson correlation coefficient that is close to unity (ρ = 0.98 for Switzerland and ρ = 0.98 for the US), corresponding to an R2 of 0.96 for Switzerland and 0.95 for the US. (Note that the two statistical measures are related by the square-root function.) The incremental costs per additional percentage point of SVR were estimated as 597.14 USD in Switzerland and 1,063.81 USD in the US by the slope coefficient of the respective regression model (Tables B-E in S1 File).
Fig 1. Scatter plot of costs against SVR of HCV therapies in Switzerland (ρ = 0.98, R2 = 0.96) and the US (ρ = 0.98, R2 = 0.95).
Table 3 details the mean costs and costs per SVR for each new HCV treatment step.
Table 3. Mean SVR, mean costs, costs per SVR and confidence intervals (CI) of HCV treatments over time in Switzerland and the US (Tables F-J in S1 File).
| Treatment regimens | Mean SVR, % (95% CI) | Mean costs, in Switzerland, USD (95% CI) | Mean costs per SVR in Switzerland, USD (95% CI) | Mean costs in US, USD (95% CI) | Mean costs per SVR in US USD (95% CI) |
|---|---|---|---|---|---|
| Step 1: IFN-α monotherapy | 11.89% (5.24%-18.53%) | 9,625 (7,736–11,514) | 81,026 (74,128–87,924) | 6,097 (-3,580–15,771) | 51,350 (41,406–61,295) |
| Step 2: IFN-α and RBV | 34.47% (27.82%-41.11%) | 22,375 (20,486–24,264) | 65,017 (58,119–71,915) | 25,064 (15,869–35,217) | 72,771 (64,826–82,715) |
| Step 3: pegIFN-α and RBV | 48.00% (44.16%-51.84%) | 34,419 (33,328–35,509) | 72,013 (68,030–75,996) | 36,515 (30,928–42,102) | 76,393 (70,651–82,134) |
| Step 4: First DAA protease inhibitors*, pegIFN-α and RBV | 74.96% (71.12%-78.79%) | 45,591 (44,500–46,682) | 61,151 (57169–65,134) | 76,082 (70-495-81,669) | 101,134 (95,393–106,876) |
| Step 5: New DAA† ± pegIFN-α and RBV | 96.30% (92.46%-1.00%) | 62,922 (61,831–64,012) | 65,422 (61,440–69,405) | 89,760 (84,172–95,347) | 93,393 (87,651–99,134) |
CI: Confidence Interval
* boceprevir; telaprevir; simeprevir
† sofosbuvir; ledipasvir; ritonavir-boosted paritaprevir, ombitasvir, and dasabuvir
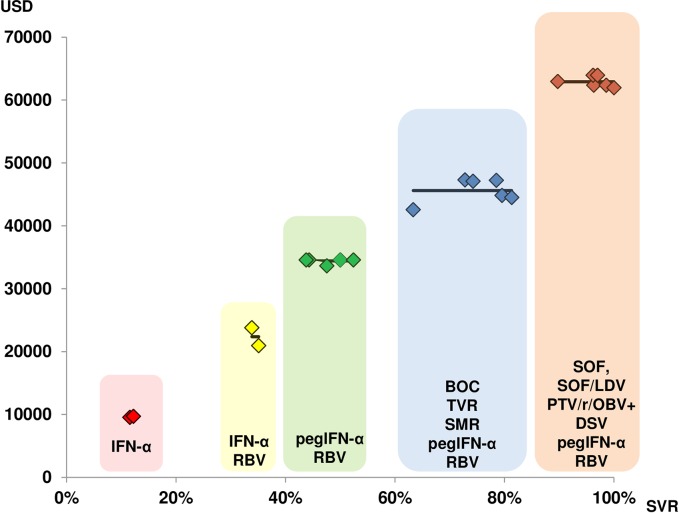
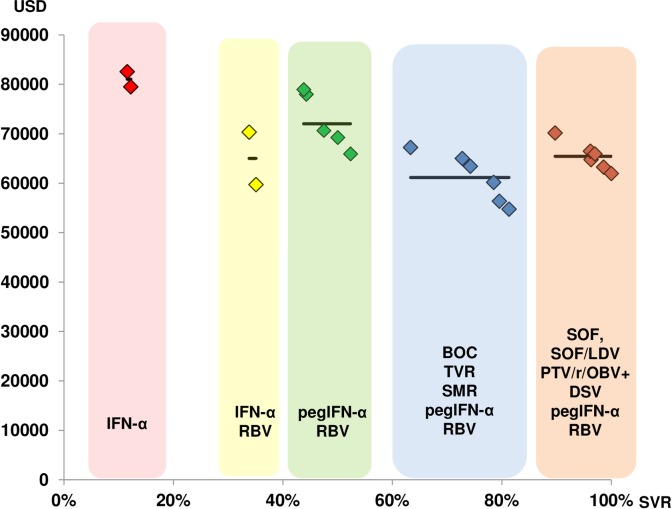
These results confirmed the close association between the mean costs per treatment step and the mean SVR rate. Fig 2 shows both the individual and the mean costs for each treatment step, according to the increasing SVR in Switzerland. Finally, Fig 3 shows that the costs per achieved HCV cure, expressed as the SVR, are relatively stable.
Fig 2. Costs associated with the five steps in HCV therapy development over time (SVR steadily increases with time).
Fig 3. Cost per SVR associated with SVR rates corresponding to the five steps in the development of HCV therapies over time (SVR steadily increases with time).
Discussion
Our results show that the costs of drug regimens for treating HCV increased steadily over time, both in Switzerland and in the US, according to the stepwise approval of new antiviral drugs, and in close correlation with the corresponding HCV cure rates. This resulted in relatively stable costs to cure a treatment-naive patient with HCV genotype 1 infection, irrespective of the fact that drug costs were higher in the US than in Switzerland. Our results are consistent with a value-based pricing model in which drug costs are closely related to their clinical efficacy. The relatively stable ratio of costs per achieved SVR, with the exception of the first treatment step, suggests that the societal willingness to pay for a specific health gain (i.e., one SVR) have remained relatively stable over time, at least in Switzerland.
For the HCV market, pharmaceutical companies price their drugs based on the value of SVR and profit maximization rather than the costs of R&D, production, marketing and distribution or the volume of drugs used.[19, 34, 35] These were also the conclusions of a recent bipartisan US Congress investigation on the SOF drug pricing strategy.[36] Newer antiviral drugs for HCV infection have significantly benefited from an improved understanding of viral biology and drug targets in the field of HIV infection.[2] Moreover, and in contrast to biologic agents in the fields of cancer and immunology, HCV drug production costs are very low.[37]
Drug prices evolved in line with the cure rate from a mere 10% to almost 100% over a 25-year period. Therefore, we could argue that the “very high” nominal prices of the recently introduced HCV DAAs are justifiable. On the other hand, DAAs have other significant advantages, such as improved safety and tolerability, shorter treatment durations, and entirely oral administration. These strong advantages–not present at the time of poorly tolerated IFN-α-based regimens–were not factored into the price of the first DAAs or into the price of the DAAs for the newer wave. Additionally, the purported innovation conferred by the introduction of long-awaited, IFN-free regimens was not responsible for boosting the market price.[38]
Some antiviral therapies have not only unprecedented high cure rates but are also safe and well tolerated, which makes them theoretically useful for treating nearly the entire infected population, at variance with the IFN-based therapies used in the early 1990s, which were associated with many safety issues and therefore of relatively limited use.[24] Also, antiviral therapies potentially prevent the dramatic and costly long-term complications of HCV infection, such as cirrhosis and hepatocellular carcinoma, loss of productivity and reduced quality of life.[23, 17] However, nominal drug costs fall short of emphasizing the true financial burden of large-scale treatment, despite their cost-effectiveness. [39] A number of analyses on novel HCV treatments have been published, demonstrating that expensive drugs can still be cost-effective.[1, 16, 40–43] In a recent analysis based on US costs, the incremental cost effectiveness ratios of DAAs compared to a reference treatment strategy, based on BOC, RBV and pegIFN-α, varied between 14,432 and 70,097 USD per additional QALY for genotype 1 infection, and they were highly sensitive to nominal drug prices.[41] For instance, a combination of SOF/LVD could be cost-saving as long as the weekly cost of SOF was reduced from 7,000 USD to less than 5,500 USD. Nevertheless, such projections take into account the long-term savings of curing patients of HCV, while the weight of treatment costs on health systems is immediate. Additionally, a drug that is considered cost-effective for an individual patient may still be unaffordable for the health care system, which depends on the disease prevalence in the general population.[44, 45] Thus, the cost-effectiveness of a drug that is measured at the patient level does not correspond to the cost-affordability of the drug at the population level.[39, 43–45] For this reason, varying degrees of restrictions have been introduced. In Switzerland, only patients with advanced fibrosis (Metavir F3), compensated cirrhosis (Metavir F4) or who are awaiting liver transplantation initially had access to reimbursement for DAAs by the mandatory health insurance at the time of their first approval. To address this situation, European countries implemented different policies.[46] One of them is price negotiation as more than one pharmaceutical company enters the hepatitis C market.[43] From August 2015, the Swiss FOPH extended the use of DAAs to patients with fibrosis stage F2, a lower stage of fibrosis. This extension was accompanied by a market price reduction. The Tuscany region (Italy) has proposed a more sophisticated tool, i.e., a tendering scheme that extends the accessibility to DAA for approximately 20,000 patients who have milder disease.[47] As a further example, in the field of other diseases, Novartis recently declared the launch of their new heart failure drug, Entresto® (sacubitril associated to valsartan), offering a high rebate while subsequently increasing prices if the new drug reduces hospitals visits.[48]
Our study had limitations. First, we included only treatment-naive patients infected with HCV genotype 1. However, this is the largest subgroup of hepatitis C patients in Switzerland, and it is sufficiently representative for studying the pricing business model.[25] Moreover, HCV genotype 1 has been the most difficult genotype to treat for the last two decades, justifying the efforts to develop novel genotype-specific drugs. Second, prices are negotiated over time, and the market price at the time of licensing may not necessarily reflect the future, evolving situation.
In conclusion, there is a relatively stable ratio of costs per cured patient over time, resulting in a strong positive correlation between the HCV cure rate and costs per treatment. This is an indication that pharmaceutical companies used a value-based pricing model for HCV treatments. The finding is in line with a claim by Ezekiel J. Emanuel, who stated that high HCV prices are not fully accounted by high costs of R&D or risks associated with drug development. [49] Health care systems, even of wealthy countries, such as Switzerland and the US, are struggling with the high budget impact of these new agents. Ironically, the issue is caused by the very high effectiveness of the DAAs and the willingness to pay for a specific health gain (i.e. one SVR) set 25 years ago, with the consequence of high prices due to the high patient value. Nevertheless, the current pricing of antiviral drugs against HCV does not allow treatment of all HCV-infected patients despite the obvious long-term advantages in terms of population health. Therefore, the pharmaceutical industry, health care payers and stakeholders are challenged with finding new pricing schemes to treat the entire population for new drugs that are highly effective when the disease prevalence is high.
Supporting Information
Table A in S1 File: Data set: SVR, Swiss costs (USD), United States (US) costs (USD), cost per SVR in Switzerland (USD), costs per SVR in US (USD), stepping stones dummy variables. Table B in S1 File: The Swiss incremental costs per additional percentage point of SVR regression coefficients. Table C in S1 File: The Swiss incremental costs per additional percentage point of SVR regression table output. Table D in S1 File: The US incremental costs per additional percentage point of SVR regression coefficients. Table E in S1 File: The US incremental costs per additional percentage point of SVR table output. Table F in S1 File: Mean SVR, 95% CI output. Table G in S1 File: Mean costs and 95% CI in Switzerland output. Table H in S1 File: Mean costs per SVR and 95% CI in Switzerland output. Table I in S1 File: Mean costs and 95% CI in the US output. Table J in S1 File: Mean costs per SVR and 95% CI in the US output.
(DOCX)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Pfeil AM, Reich O, Guerra IM, Cure S, Negro F, Mullhaupt B, et al. Cost-effectiveness analysis of sofosbuvir compared to current standard treatment in swiss patients with chronic hepatitis C. PloS one. 2015;10(5):e0126984 10.1371/journal.pone.0126984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Manns MP, Foster GR, Rockstroh JK, Zeuzem S, Zoulim F, Houghton M. The way forward in HCV treatment—finding the right path. Nat Rev Drug Discov. 2007;6(12):991–1000. [DOI] [PubMed] [Google Scholar]
- 3.Marinho RT, Barreira DP. Hepatitis C, stigma and cure. World J Gastroenterol. 2013;19(40):6703–9. 10.3748/wjg.v19.i40.6703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lindsay KL, Trepo C, Heintges T, Shiffman ML, Gordon SC, Hoefs JC, et al. A randomized, double-blind trial comparing pegylated interferon alfa-2b to interferon alfa-2b as initial treatment for chronic hepatitis C. Hepatology. 2001;34(2):395–403. [DOI] [PubMed] [Google Scholar]
- 5.Manns MP, McHutchison JG, Gordon SC, Rustgi VK, Shiffman M, Reindollar R, et al. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet. 2001;358(9286):958–65. [DOI] [PubMed] [Google Scholar]
- 6.Jacobson IM, McHutchison JG, Dusheiko G, Di Bisceglie AM, Reddy KR, Bzowej NH, et al. Telaprevir for previously untreated chronic hepatitis C virus infection. N Engl J Med. 2011;364(25):2405–16. 10.1056/NEJMoa1012912 [DOI] [PubMed] [Google Scholar]
- 7.Poordad F, McCone J, Bacon BR, Bruno S, Manns MP, Sulkowski MS, et al. Boceprevir for untreated chronic HCV genotype 1 infection. N Engl J Med. 2011;364(13):1195–206. 10.1056/NEJMoa1010494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Silva MO, Treitel M, Graham DJ, Curry S, Frontera MJ, McMonagle P, et al. Antiviral activity of boceprevir monotherapy in treatment-naive subjects with chronic hepatitis C genotype 2/3. J Hepatol. 2013;59(1):31–7. 10.1016/j.jhep.2013.02.018 [DOI] [PubMed] [Google Scholar]
- 9.Ferenci P, Bernstein D, Lalezari J, Cohen D, Luo Y, Cooper C, et al. ABT-450/r-ombitasvir and dasabuvir with or without ribavirin for HCV. N Engl J Med. 2014;370(21):1983–92. 10.1056/NEJMoa1402338 [DOI] [PubMed] [Google Scholar]
- 10.Kowdley KV, Gordon SC, Reddy KR, Rossaro L, Bernstein DE, Lawitz E, et al. Ledipasvir and Sofosbuvir for 8 or 12 Weeks for Chronic HCV without Cirrhosis. N Engl J Med. 2014;370(20):1879–88. 10.1056/NEJMoa1402355 [DOI] [PubMed] [Google Scholar]
- 11.Lawitz E, Mangia A, Wyles D, Rodriguez-Torres M, Hassanein T, Gordon SC, et al. Sofosbuvir for previously untreated chronic hepatitis C infection. N Engl J Med. 2013;368(20):1878–87. 10.1056/NEJMoa1214853 [DOI] [PubMed] [Google Scholar]
- 12.Feld JJ, Kowdley KV, Coakley E, Sigal S, Nelson DR, Crawford D, et al. Treatment of HCV with ABT-450/r-ombitasvir and dasabuvir with ribavirin. N Engl J Med. 2014;370(17):1594–603. 10.1056/NEJMoa1315722 [DOI] [PubMed] [Google Scholar]
- 13.Jacobson IM, Dore GJ, Foster GR, Fried MW, Radu M, Rafalsky VV, et al. Simeprevir with pegylated interferon alfa 2a plus ribavirin in treatment-naive patients with chronic hepatitis C virus genotype 1 infection (QUEST-1): a phase 3, randomised, double-blind, placebo-controlled trial. Lancet. 2014;384(9941):403–13. 10.1016/S0140-6736(14)60494-3 [DOI] [PubMed] [Google Scholar]
- 14.Manns M, Marcellin P, Poordad F, de Araujo ES, Buti M, Horsmans Y, et al. Simeprevir with pegylated interferon alfa 2a or 2b plus ribavirin in treatment-naive patients with chronic hepatitis C virus genotype 1 infection (QUEST-2): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2014;384(9941):414–26. 10.1016/S0140-6736(14)60538-9 [DOI] [PubMed] [Google Scholar]
- 15.Sulkowski MS, Gardiner DF, Rodriguez-Torres M, Reddy KR, Hassanein T, Jacobson I, et al. Daclatasvir plus sofosbuvir for previously treated or untreated chronic HCV infection. N Engl J Med. 2014;370(3):211–21. 10.1056/NEJMoa1306218 [DOI] [PubMed] [Google Scholar]
- 16.Kamal-Yanni M. Hepatitis C drug affordability. Lancet Glob Health. 2015;3(2):e73–4. 10.1016/S2214-109X(14)70365-1 [DOI] [PubMed] [Google Scholar]
- 17.Kmietowicz Z. Thousands of patients in England to get new hepatitis C drugs. BMJ. 2015;350:h3222 10.1136/bmj.h3222 [DOI] [PubMed] [Google Scholar]
- 18.Andrieux-Meyer I CJ, Aff onso de Araújo E, Hamid S. Disparity in market prices for hepatitis C virus direct-acting drugs. Lancet Glob Health. 2015;3:676–7. [DOI] [PubMed] [Google Scholar]
- 19.DiMasi JA, Hansen RW, Grabowski HG. The price of innovation: new estimates of drug development costs. J Health Econ. 2003;22(2):151–85. [DOI] [PubMed] [Google Scholar]
- 20.Mailankody S, Prasad V. Five Years of Cancer Drug Approvals: Innovation, Efficacy, and Costs. JAMA Oncol. 2015;1(4):539–40. 10.1001/jamaoncol.2015.0373 [DOI] [PubMed] [Google Scholar]
- 21.Munos B. Lessons from 60 years of pharmaceutical innovation. Nat Rev Drug Discov. 2009;8(12):959–68. 10.1038/nrd2961 [DOI] [PubMed] [Google Scholar]
- 22.Paris V, Docteur E. Pharmaceutical pricing and reimbursement policies in Switzerland. OCDE. 2007;27. [Google Scholar]
- 23.Mullhaupt B, Bruggmann P, Bihl F, Blach S, Lavanchy D, Razavi H, et al. Modeling the Health and Economic Burden of Hepatitis C Virus in Switzerland. PloS one. 2015;10(6):e0125214 10.1371/journal.pone.0125214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Twenty-Five Years of Progress against Hepatitis C: setbacks and Stepping Stones. PRMA. December 2014. [Google Scholar]
- 25.Girardin FG, N., Vernaz N., Negro F. Rethinking the reimbursement policy of direct acting antivirals against chronic hepatitis C. Rev Med Suisse. September 2 2015;484:1610–5. [PubMed] [Google Scholar]
- 26.Morisco F, Granata R, Stroffolini T, Guarino M, Donnarumma L, Gaeta L, et al. Sustained virological response: a milestone in the treatment of chronic hepatitis C. World J Gastroenterol. 2013;19(18):2793–8. 10.3748/wjg.v19.i18.2793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Müller KR. Ispor Global Health Care Systems Road Map: Switzerland. International Society for Pharmacoeconomics and Outcomes Research. April 2011. [Google Scholar]
- 28.Sedgwick P. Correlation versus linear regression. BMJ. 2013;346:f2686. [Google Scholar]
- 29.FDA. Roferon label.
- 30.Swissmedic. Rebetol label.
- 31.http://www.fda.gov/ohrms/dockets/ac/02/briefing/3909B1_01_Hoffman-LaRoche.pdf.
- 32.Buti M, Agarwal K, Horsmans Y, Sievert W, Janczewska E, Zeuzem S, et al. Telaprevir twice daily is noninferior to telaprevir every 8 hours for patients with chronic hepatitis C. Gastroenterology. 2014;146(3):744–53 e3. 10.1053/j.gastro.2013.11.047 [DOI] [PubMed] [Google Scholar]
- 33.Afdhal N, Zeuzem S, Kwo P, Chojkier M, Gitlin N, Puoti M, et al. Ledipasvir and sofosbuvir for untreated HCV genotype 1 infection. N Engl J Med. 2014;370(20):1889–98. 10.1056/NEJMoa1402454 [DOI] [PubMed] [Google Scholar]
- 34.Vernaz N, Haller G, Girardin F, Huttner B, Combescure C, Dayer P, et al. Patented drug extension strategies on healthcare spending: a cost-evaluation analysis. PLoS Med. 2013;10(6):e1001460 10.1371/journal.pmed.1001460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Neumann PJ, Chambers JD, Simon F, Meckley LM. Risk-sharing arrangements that link payment for drugs to health outcomes are proving hard to implement. Health Aff (Millwood). 2011;30(12):2329–37. [DOI] [PubMed] [Google Scholar]
- 36.McCarthy M. US Senate committee launches investigation into drug pricing. BMJ. 2015;351:h5989 10.1136/bmj.h5989 [DOI] [PubMed] [Google Scholar]
- 37.van de Ven N, Fortunak J, Simmons B, Ford N, Cooke GS, Khoo S, et al. Minimum target prices for production of direct-acting antivirals and associated diagnostics to combat hepatitis C virus. Hepatology. 2015;61(4):1174–82. 10.1002/hep.27641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bichoupan K, Martel-Laferriere V, Sachs D, Ng M, Schonfeld EA, Pappas A, et al. Costs of telaprevir-based triple therapy for hepatitis C: $189,000 per sustained virological response. Hepatology. 2014;60(4):1187–95. 10.1002/hep.27340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Messori A, Maratea D, Fadda V, Trippoli S. Letter: estimating the cost-neutral price of sofosbuvir-based triple therapy for the treatment of naive patients with genotype 1 HCV infection in Italy. Aliment Pharmacol Ther. 2014;40(2):217–8. 10.1111/apt.12823 [DOI] [PubMed] [Google Scholar]
- 40.Chhatwal J, Kanwal F, Roberts MS, Dunn MA. Cost-effectiveness and budget impact of hepatitis C virus treatment with sofosbuvir and ledipasvir in the United States. Ann Intern Med. 2015;162(6):397–406. 10.7326/M14-1336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Najafzadeh M, Andersson K, Shrank WH, Krumme AA, Matlin OS, Brennan T, et al. Cost-effectiveness of novel regimens for the treatment of hepatitis C virus. Ann Intern Med. 2015;162(6):407–19. 10.7326/M14-1152 [DOI] [PubMed] [Google Scholar]
- 42.van de Vooren K, Curto A, Garattini L. Pricing of forthcoming therapies for hepatitis C in Europe: beyond cost-effectiveness? Eur J Health Econ. 2015;16(4):341–5. 10.1007/s10198-014-0653-x [DOI] [PubMed] [Google Scholar]
- 43.Zoulim F, Liang TJ, Gerbes AL, Aghemo A, Deuffic-Burban S, Dusheiko G, et al. Hepatitis C virus treatment in the real world: optimising treatment and access to therapies. Gut. 2015;64(11):1824–33. 10.1136/gutjnl-2015-310421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bach PB. New Math on Drug Cost-Effectiveness. N Engl J Med. 2015;373(19):1797–9. 10.1056/NEJMp1512750 [DOI] [PubMed] [Google Scholar]
- 45.McCarthy M. New drug for hepatitis C contributes to 13% rise in spending on prescription drugs in US. BMJ. 2015;350:h2055 10.1136/bmj.h2055 [DOI] [PubMed] [Google Scholar]
- 46.van de Vooren K, Duranti S, Curto A, Garattini L. A critical systematic review of budget impact analyses on drugs in the EU countries. Appl Health Econ Health Policy. 2014;12(1):33–40. 10.1007/s40258-013-0064-7 [DOI] [PubMed] [Google Scholar]
- 47.Brunetto MR, De Luca A, Messori A, Zignego AL. Reducing the price of new hepatitis C drugs in the Tuscany region of Italy. BMJ. 2015;350:h3363 10.1136/bmj.h3363 [DOI] [PubMed] [Google Scholar]
- 48.Novartis to test new pricing model with heart failure drug. Reuters. 30 June 2015. [cited: April 24 2016]. Available: http://reuters.com/article/us-novartis-heart-idUSKCN0PA1N720150630.
- 49.Ezekiel J. Emanuel: The Solution to Drug Prices. New York Times; 9 September 2015. [cited: April 24 2016]. Available: http://nytimes.com/2015/09/09/opinion/the-solution-to-drug-prices.html. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table A in S1 File: Data set: SVR, Swiss costs (USD), United States (US) costs (USD), cost per SVR in Switzerland (USD), costs per SVR in US (USD), stepping stones dummy variables. Table B in S1 File: The Swiss incremental costs per additional percentage point of SVR regression coefficients. Table C in S1 File: The Swiss incremental costs per additional percentage point of SVR regression table output. Table D in S1 File: The US incremental costs per additional percentage point of SVR regression coefficients. Table E in S1 File: The US incremental costs per additional percentage point of SVR table output. Table F in S1 File: Mean SVR, 95% CI output. Table G in S1 File: Mean costs and 95% CI in Switzerland output. Table H in S1 File: Mean costs per SVR and 95% CI in Switzerland output. Table I in S1 File: Mean costs and 95% CI in the US output. Table J in S1 File: Mean costs per SVR and 95% CI in the US output.
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.