Abstract
The Dutch East India Company (VOC) intended the Cape of Good Hope to be a refreshment stop for ships travelling between the Netherlands and its eastern colonies. The indigenous Khoisan, however, did not constitute an adequate workforce, therefore the VOC imported slaves from East Africa, Madagascar and Asia to expand the workforce. Cape Town became a cosmopolitan settlement with different categories of people, amongst them a non-European underclass that consisted of slaves, exiles, convicts and free-blacks. This study integrated new strontium isotope data with carbon and nitrogen isotope results from an 18th-19th century burial ground at Cobern Street, Cape Town, to identify non-European forced migrants to the Cape. The aim of the study was to elucidate individual mobility patterns, the age at which the forced migration took place and, if possible, geographical provenance. Using three proxies, 87Sr/86Sr, δ13Cdentine and the presence of dental modifications, a majority (54.5%) of the individuals were found to be born non-locally. In addition, the 87Sr/86Sr data suggested that the non-locally born men came from more diverse geographic origins than the migrant women. Possible provenances were suggested for two individuals. These results contribute to an improved understanding of the dynamics of slave trading in the Indian Ocean world.
Introduction
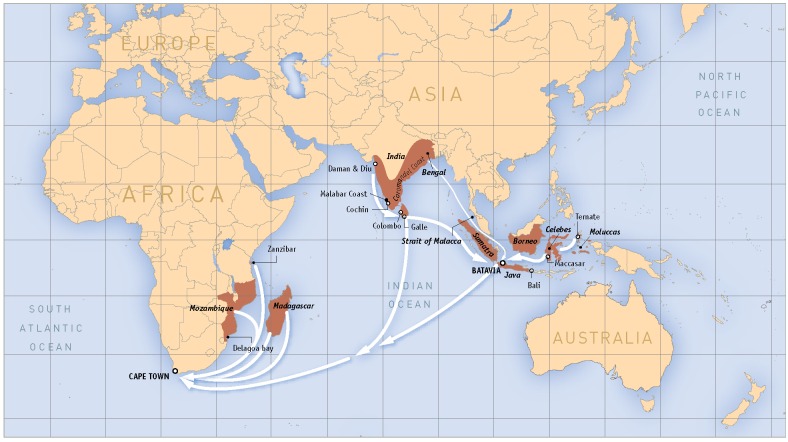
Between the years 1652 and 1795, the Dutch East India Company (VOC) governed the Cape Colony of present day South Africa. Save for a short-lived Batavian period (1803–1806), the British ruled the Cape from 1795 throughout the 19th-century. The VOC envisioned the Cape as a refreshment stop for company ships on their way to and from the East. Neither the VOC employees, nor the indigenous Khoisan produced adequate provisions to satisfy the demand of ships passing through the refreshment station. Thus, the VOC decided to import slaves to increase the available workforce. The VOC, however, could not rely on West Africa as a source of slaves because the Dutch West India Company had a monopoly on this trade [1]. In addition, the directors of the VOC, the Heren XVII or the Gentlemen Seventeen, did not support enslavement of indigenous people. Therefore, the VOC looked to eastern realms such as the east African coast, the middle South Asian circuit and the easternmost Asian circuit to supply the additional labour [2]. Records suggest that these three regions contributed approximately a quarter of the enslaved Cape population until 1808 when Britain outlawed the oceanic slave trade in its colonies. The remaining quarter of the Cape’s imported enslaved population originated from Madagascar [1]. These figures were not constant throughout this period as at any given time factors such as maritime conflicts and changing shipping patterns were at play [1]. Towards the end of the first Dutch administration, the colony relied less on imported Asian slaves and more on African slaves from the western Indian Ocean [3]. In their study of the 18th-century transoceanic informal trade in Asian slaves, Mbeki and van Rossum [4] reported that the majority of the enslaved transported by private persons to the Cape recorded toponyms from South Asia (65%) and the minority (33%) from the Indonesian archipelago (Fig 1). This study also suggested comparable numbers of men and women came from the Indonesian Archipelago, which is in contrast to the overwhelming preference for male slaves from south Asia. In general, men rather than women were favoured as slave labour at the Cape, particularly when agricultural production increased significantly in the hinterland. Of the enslaved that travelled on the Ceylon-Cape route, a large majority (89%) were from the Malabar Coast of India. The transportation patterns may have been affected by racial attitudes at the Cape that determined the kind of labour slaves were engaged in and the prices they fetched [4].
Fig 1. Indian Ocean slave trading routes.
Map generated based on images provided by Iziko Museums and Frans Huijzenveld (Faculty of Humanities, department of Art and Culture, History, and Antiquity, Vrije Universiteit Amsterdam).
There have been several excellent reviews of the history of the Dutch Cape Colony and Indian Ocean slavery to which the reader is referred [1, 2, 5–11]. A current impediment to a full understanding of Indian Ocean World slave trades stems from a shortcoming in the historical record that often only provides information about slaves’ points of departure or sale as opposed to their places of origin [12]. Researchers of the Atlantic Ocean slave trade have begun to address this issue by adopting a biomolecular approach to investigate the life histories of enslaved people. Isotopic analyses have proven to be useful in elucidating mobility, (childhood) diet, manumission, and industrialisation in slave populations in for example the Caribbean [13, 14], the United States [15–17], and Mexico [18].
One of the major differences between the Indian Ocean and Atlantic Ocean slave trades is that the latter primarily involved a triangular movement of money, commodities, and people between Europe, Africa and the Americas. Enslaved Africans from West and Central Africa were transported to the Americas to labour on plantations. In contrast, the Cape was far more cosmopolitan than any of these nodes, with slaves coming from several slaving regions from the Indian Ocean basin. The complexity of the Indian Ocean slave system, however, has yet to be fully quantified (see [19] and [11] for historical research attempting this difficult task), and would benefit from further extensive biogeochemical and biomolecular studies to establish the provenance of slaves.
Pioneering work by Cox and Sealy [20] employing carbon, nitrogen, and strontium isotopes in conjunction with historical documents demonstrated the contribution that isotopic research could make to the study of Indian Ocean slavery. This was followed by the isotopic investigation of the subaltern population (non-Europeans including slaves and free-blacks, and possibly convicts and exiles) discovered at an informal burial ground at Cobern Street, Cape Town (n = 53) [21]. Using carbon and nitrogen isotopes Cox and colleagues [21] differentiated locally and non-locally born individuals based on dietary shifts. They proposed that a significant dietary shift in δ13C and/or δ15N between childhood (dentine samples) and later diet (cancellous bone samples) exceeding 2‰ can provide strong confirmatory evidence for the presence of both first-generation slaves from Africa and the East and locally born people. The study presented here compliments and refines their findings through a statistical reassessment of the δ13C data to establish more relevant background data. Moreover, strontium isotope analyses were performed on a selection of individuals from the same population (n = 35). The study was designed to elucidate individual mobility patterns, the age at which the forced migration took place and, if possible, geographical provenance. Ultimately, the results will contribute to an improved understanding of the dynamics of slave trading in the Indian Ocean in world history.
Determination of Geological Origins through Isotope Analysis
The applicability of strontium isotopes to resolve environmental, ecological, archaeological, historical and forensic research questions has been illustrated by many scientific studies, e.g., [22–29]. The strontium isotope ratio 87Sr/86Sr serves as a powerful proxy to assign people and animals to specific geological areas [28, 30–33]. 87Sr is a radiogenic isotope, derived from the radioactive decay of 87Rb (t1/2 of 4.88 x 1010 years) [34]. The 87Sr content of a rock is a function of Rb content and the amount of time that has passed since its initial crystallisation [28, 35, 36]. Strontium passes from the geological bedrock into soil and is eventually taken up by vegetation [28, 35, 37, 38]. Since vegetation controls the 87Sr that enters the human and animal food chain [28, 39], it is noteworthy that the 87Sr/86Sr of vegetation differs slightly from the underlying geology, due to various soil-to-plant transfer factors, such as climate, fungi, root-depth and taxon [40, 41]. Moreover, in particular in coastal regions such as the Cape region, the effect of marine derived strontium on the deviation between floral 87Sr/86Sr ratios and geological 87Sr/86Sr ratios may be significant [14]. Crops in coastal regions may absorb marine derived strontium from rainwater, sea-spray and sea-splash, causing their 87Sr/86Sr ratio to shift towards a more marine signal (~0.7092 [14, 28, 35, 42, 43, 44]). Hence, the biologically available strontium may therefore deviate from the geological strontium isotope signature [14, 28, 45, 46].
Although mammals preferentially excrete ingested strontium via the kidneys and bile, a small proportion is retained in the body and incorporated into bone and dental enamel through diet. It then substitutes for calcium in the structure of hydroxyapatite (Ca10(PO4)6(OH)2), a calcium phosphate mineral [47]. Whereas bone constantly remodels during life, dental enamel develops during childhood and remains chemically unchanged in later life. The mineralisation age varies between dental elements, ranging from birth (first molars, M1) to approximately 16 years of age (third molars, M3) in permanent dentition [48–51]. Due to the difference in development, and age of incorporation of strontium, a difference in 87Sr/86Sr between bone and enamel could be interpreted as the result of migration in an individual’s lifetime. Diagenetic processes, however, lead to permanent alteration of the chemical and/or structural properties of bone and dentine [52]. Enamel is markedly less prone to diagenetic processes than dentine and bone, making it the preferred material for strontium isotope investigations [53–55]. Moreover, the study of different dental elements potentially allows the determination of the age at which migration took place provided it occurred in early life. Multi dental-elemental sampling, enabling inter-element comparison, therefore, offers a high-resolution manner to trace migratory patterns during early life (see Material and sampling strategy).
The interpretation of 87Sr/86Sr ratios and the ultimate determination of geological provenance is highly dependent on available local or regional (bioavailable Sr) background data. Regional bioavailable 87Sr/86Sr distribution maps have been constructed for a few countries across the world, such as the United States [56], the United Kingdom [57, 58], France [59], the Netherlands [60], Germany [61, 62], Denmark [63] and Greece [64], while other archaeological studies in e.g. New Zealand and Thailand rely on the known geological data [65, 66]. A wide selection of papers report archaeological, geological or modern biosphere and faunal data from South Africa (see [67] for references), but to date no systematic study has been undertaken to map the spatial distribution of one of these proxies. Based on the bioavailable (fauna) data provided in Sealy et al. and Balasse et al. [68, 69], a schematic of the spatial distribution of 87Sr/86Sr in the Western Cape Province was generated (Fig 2).
Fig 2. Schematic geological map of the southwestern Cape.
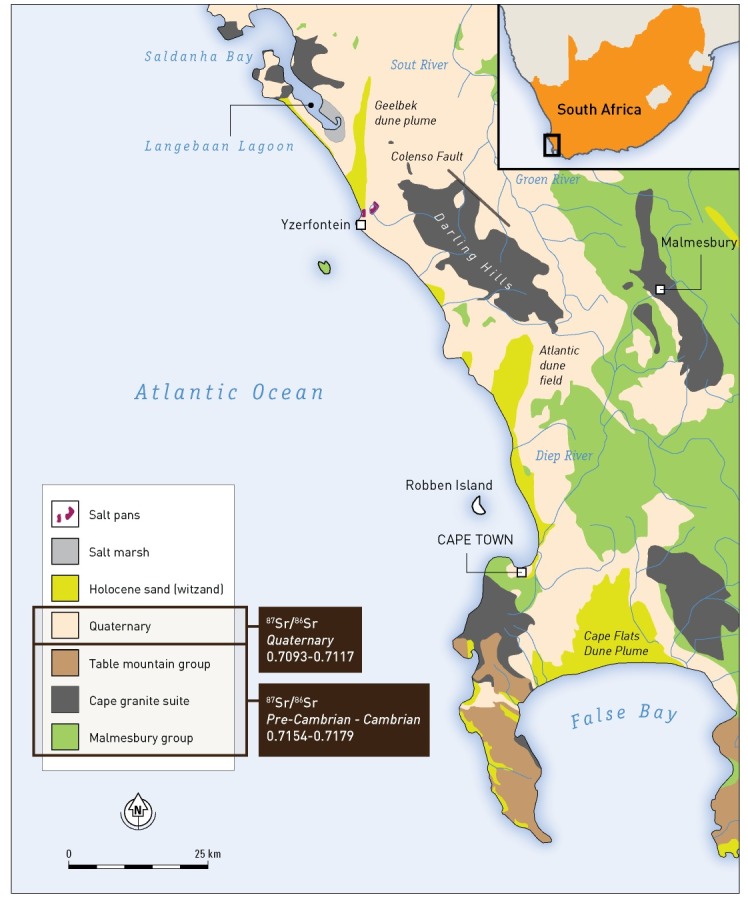
Figure adapted from Compton et al. [70]. Strontium isotope data as published by Sealy et al. [69] and Balasse et al. [68].
An in-depth, but regionally applicable study by Maurer et al. emphasized the difficulties in determining the sources of strontium that enter the local food chain [46]. Moreover, they confirmed the offset in 87Sr/86Sr between geological, biosphere and faunal samples [38, 45]. Both factors hamper the accurate interpretation of the data in terms of provenancing, especially if only geological analyses are available to investigate origins. Nevertheless, the available data did provide some insight into the expected regional strontium ratios in the Cape region, and contributed to understanding the local strontium isotope signature.
Material and Methods
The complete Cobern Street burial collection is curated at the Department of Human Biology, University of Cape Town. The collection is available for academic research. All necessary permits were obtained, which complied with all relevant regulations. Permission for sampling and analysis was granted by Heritage Western Cape (Ref.: 130129TS09) and an export permit for samples was obtained from the South African Heritage Resource Agency (Ref.: 9/2/018/0206. Permit ID: 219).
Material and sampling strategy
In 1994, excavations revealed 63 intact primary burials belonging to the Cobern Street informal burial ground in Cape Town, which was used between circa 1750 and 1827 AD [21, 71]. The carbon and nitrogen isotope study by Cox et al. [21] was performed on 53 individuals, dating to the pre-colonial (<1652 AD) and colonial eras. The material and sampling strategy of the bone samples are provided in Cox [72], and will be summarised here. Cancellous bone samples were taken, preferably, from rib bones. If ribs were not available vertebrae were taken. A detailed overview of the samples taken for stable isotope analysis per individual is presented in Cox [72]. The rate of bone remodelling is influenced by bone type, bone element, age and a number of physiological and pathological factors [73]. Little quantitative are available, however, about collagen turnover rates [74]. It is known to vary between 2% and 4%/yr in femoral cortical bone in adults, with much higher rates up to even 100% reached during growth [75]. The turnover rate of trabecular bone, and in particular that or ribs and vertebrae, is faster than cortical bone and collagen (on average 10%/yr in adults: [76]). The carbon isotope data published by Cox et al. [21] and reassessed in this study therefore provide information on the dietary intake of the last circa 5 years of life. In contrast to bone, there is no significant turnover or replacement of dentine [77]. As a result, the δ13C value of dentine is directly related to the dietary intake of carbon during the time of root formation, which is element dependant. In permanent dentition, the initiation of root formation (developmental stage Ri: [78]) can start as early as four years of age and finish as late as the early twenties (apex completed: Ac). The selected dental elements and the interpretational consequences of that selection are specifically discussed in Cox [72].
For this research, a complete overlap with the Cox et al. [21] dataset could not be achieved due to insufficient sample material. To enable meaningful comparison, and depending on availability, enamel was sampled from 35 individuals dating from the colonial era. Where possible, first molars were selected for single-elemental analysis to distinguish locally and non-locally born individuals.
Multi dental-elemental analysis was performed on preferably first, second and third molars of a subset of individuals (n = 17) to further investigate individual migration events in early life (Table 1). Slight variations in mineralisation age are observed between European, Asian and African populations [49, 79]. Despite the fact that persons of Asian ancestry were present in the colonial Cape [1], in this paper the tooth crown initiation (Ci) and completion (Crc) times for Southern African populations published by Reid and Dean [49] are used for reference (Table 2). First evidence of calcification of the crown (Ci) of the first molar is observed at birth. The last state of dental enamel formation, crown completion (Crc), finishes around the age of three. The observed 87Sr/86Sr ratio of this element, therefore, reflects the dietary 87Sr/86Sr intake during the first three years of life. Since the M1 mineralises while the infant is likely to be breastfed, the mother’s dietary 87Sr/86Sr intake will be (partly) reflected in the children’s deciduous teeth and molars, which mineralise in the womb (in utero), and the first permanent molar. The second and third permanent molars mineralised between the ages of circa 3 and 6, and 8 and 16 years respectively.
Table 1. Osteological data and selected teeth for strontium isotope analysis from 35 individuals from the Cobern Street informal burial site, Cape Town, dating to the colonial period.
| Burial type | Burial # | UCT # | Age (yr) | Sex | Dental modifications? | dI1 or dI2 | I2 | PM1 | M1 | M2 | M3 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| B | 3 | 460 | 20 | M | - | - | - | - | 16 | - | - |
| B | 4 | 458.1 | 17–18 | F | - | - | - | - | 16 | - | - |
| B | 10 | 498 | 35–40 | F | - | - | - | - | 26 | - | - |
| B | 12 | 500 | 35–40 | M | - | - | - | - | 46 | - | - |
| B | 13 | 501 | 30–40 | M | - | - | - | - | 46 | - | - |
| B | 14 | 502 | 45–55 | F | - | - | - | - | 46 | 47 | 48 |
| B | 15 | 504 | 25 | M | - | - | - | - | 46 | - | - |
| B | 18 | 508 | >50 | F | - | - | - | - | 26 | 47 | 18 |
| B | 20A | 510 | 25–30 | M | The maxillary central incisors were chipped mesially at the midline, and the lateral maxillary incisors were chipped distally to form inverted 'V' shapes | - | - | - | 16 | 17 | 18 |
| B | 20B | 511 | 16 | F | The maxillary central incisors were chipped mesially at the midline, and the lateral maxillary incisors were chipped distally to form inverted 'V' shapes | - | - | - | 46 | 47 | 38 |
| B | 20C | 512 | 1,5–2 | ? | - | 71/2 or 81/2 | - | - | - | - | - |
| B | 21 | 514 | 25–35 | F | - | - | - | - | 46 | 47 | 38 |
| B | 23 | 516 | 17–19 | F | - | - | - | - | 36 | 37 | 38 |
| B | 24 | 517 | 20–25 | M | - | - | - | - | 36 | - | - |
| B | 25 | 518 | 35–40 | M | - | - | - | - | 46 | - | - |
| B | 27B | 521 | 40–15 | M | - | - | - | - | 26 | - | - |
| B | 28 | 522 | 50 | F | - | - | - | - | 46 | - | - |
| C | 32 | 526 | 50–60 | M | - | - | - | - | 46 | 17 | 48 |
| B | 34 | 528 | 14–15 | F | - | - | - | - | 46 | - | - |
| B | 40 | 535 | 12 | F | The maxillary central incisors were chipped mesially at the midline | - | - | - | 46 | - | - |
| B | 41 | 536 | 35–50 | M | - | - | - | - | 36 | - | - |
| B | 42A | 539 | 40–50 | M | - | - | - | - | 16 | - | - |
| B | 42B | 540 | 40–50 | F | - | - | - | - | 26 | - | - |
| B | 44 | 542 | 40–50 | F | - | - | - | - | 46 | 47 | 48 |
| B | 45 | 543 | 50+ | M | - | - | - | - | - | 37 | - |
| B | 46 | 544 | 35–50 | F | - | - | - | - | 16 | - | - |
| B | 47 | 545 | 30–40 | M | - | - | - | - | 46 | - | - |
| B | 49 | 547 | 30–35 | M | - | - | - | - | 26 | 47 | - |
| B | 50 | 548 | 35–50 | M | The central maxillary incisors were chipped distally, and the lateral maxillary incisors were chipped mesially. | - | - | - | 46 | 47 | 48 |
| B | 51 | 549 | 35–40 | M | - | - | - | - | 26 | - | - |
| B | 52 | 550 | 25–35 | F | The chipping of the maxillary central and lateral incisors to points. | - | - | - | 46 | 47 | 48 |
| B | 53 | 551 | 35–40 | M | - | - | - | 44 | - | - | - |
| B | 54 | 552 | 30–35 | M | - | - | - | - | 46 | 47 | 48 |
| B | 56 | 554 | 35 | M | - | - | - | - | 46 | - | - |
| C | 57 | 555 | 20–30 | F | - | - | - | - | 36 | 47 | 18 |
| B | 58 | 556 | 35–40 | F | - | - | 32 | - | - | 17 | 48 |
| C | 59 | 557 | 40 | M | - | - | - | - | 36 | 37 | 18 |
| B | 60 | 558 | 30 | F | The central and lateral maxillary incisors are sharpened to a points, by chipping the incisors both mesially and distally. | - | - | - | 36 | 37 | 18 |
| B | 61 | 559 | 35 | M | - | - | - | - | 46 | - | - |
| C | 65 | 563 | 22–25 | F | - | - | - | - | 26 | 27 | 18 |
Key: Archaeological and osteological data from [71], dental modofication data from [71, 72]. Burial type refers to supine/Christian style (B) or facing Signal Hill/Muslim style (C). UCT number refers to the accession number. Dental element notation conforms to Fédération Dentaire Internationale (syntax: <quadrant code><tooth code>. Details presented in Table 2). dI1 of dI2: deciduous central or lateral incisor. I2: permanent lateral incisor. PM1: first premolar. M1: permanent first molar. M2: permanent second molar. M3: third molar.
Table 2. Chronology of human dentition in South African populations.
Average crown formation times from [49].
| FDI notation | Average crown formation times | ||||
|---|---|---|---|---|---|
| Dentition | Tooth | Right quadrant | Left quadrant | Initiation (Ci) | Completion (Crc) |
| Permanent maxillary teeth | Central incisor | 11 | 21 | 4 mon. | 4.1 yr. |
| Lateral incisor | 12 | 22 | 12.5 mon. | 4.8 yr. | |
| Canine | 13 | 23 | 9 mon. | 4.8 yr. | |
| First premolar | 14 | 24 | 1.5 yr.* | 6 yr.* | |
| Second premolar | 15 | 25 | 2 yr.* | 7 yr.* | |
| First molar | 16 | 26 | Birth | 2.9 yr. | |
| Second molar | 17 | 27 | 3 yr. | 6.4 yr. | |
| Third molar | 18 | 28 | 8 yr. | 11.3–16 yr.** | |
| Permanent mandibular teeth | Central incisor | 41 | 31 | 3 mon. | 3.4 yr. |
| Lateral incisor | 42 | 32 | 5 mon. | 3.8 yr. | |
| Canine | 43 | 33 | 6.5 mon. | 5.2 yr. | |
| First premolar | 44 | 34 | 1.75 yr.* | 6 yr.* | |
| Second premolar | 45 | 35 | 2.25 yr.* | 7 yr.* | |
| First molar | 46 | 36 | Birth | 3 yr. | |
| Second molar | 47 | 37 | 3 yr. | 6.2 yr. | |
| Third molar | 48 | 38 | 8 yr. | 11.2–16 yr.** | |
Key: Ci: FDI notation: a two-digit system (ISO 3950) developed by the Feédeération Dentaire Internationale (FDI) to associate information to a specific tooth. Syntax: <quadrant code><tooth code>. Deciduous teeth quadrant codes start with 5 (left quadrant maxilla), followed by 6 (right quadrant maxilla), 7 (right quadrant mandible), and 8 (left quadrant mandible); Ci: cusp initiated; Crc: crown completed (developmental stages conform to Moorrees [78]);
To test the hypothesis that a significant dietary shift is indicative of migration [21], multi-dental elemental sampling was performed on eight individuals who were assumed to represent migrants based on the presence of such a significant dietary shift in δ13C (burials 14, 18, 21, 32, 49, 54, 57, and 58). An additional five individuals (burials 20A, 20B, 50, 52, and 60) were selected for multi-dental elemental sampling based on the presence of dental modifications. Intentional dental modifications present in the Cobern Street collection (see [71, 72] for a summary of the unpublished data by Morris and Phillips [80]. Illustrative images are provided in Manyaapelo [81]) were not a tradition at the Cape at any time, and are therefore indicative of foreign origins [81]. Four individuals were selected for multi-dental elemental analysis based on the absence of such a significant dietary shift (random selection: burials 23, 44, 59, and 65). It is noteworthy that individuals 44, 49, and 65 showed a shift in δ15N exceeding 2.0‰. In contrast to Cox et al. [21], however, individuals with significant isotopic differences between δ15Ndentine and δ15Ncancellous were not assigned as migrants. This is due to the fact that there are multiple contributing factors that influence δ15N, such as sea spray, precipitation, fertilisers, disease, malnutrition and water stress [82–88]. Eighteen individuals, who were assumed to be locally born, or second- or subsequent generation slaves, were selected for single-elemental analysis. The individuals sampled for isotopic analysis represented two distinct patterns of burial placements, defined as type B (supine burial, Christian style) and type C (buried on their right side, Muslim style).
Analytical methods
Osteological methods and analytical details of the carbon isotope analyses were provided elsewhere [71, 72]. For the strontium isotope analysis, dental enamel samples were obtained at the Department of Human Biology, University of Cape Town. Mechanical cleaning was performed on the enamel using an acid-leached diamond-tipped drill bit to expose a dull white surface visually unaffected by diagenetic alterations. Approximately 1–3 milligrams of enamel powder were collected from the buccal or lingual surface, depending on the state of preservation of the dental element, sealed in acid-cleaned polyethylene Eppendorf centrifuge tubes and transported to the class 100 clean laboratory at the Vrije Universiteit Amsterdam. The samples were leached with 0.1N acetic acid (CH3CO2H) to remove labile diagenetic strontium [55], and eventually dissolved in 3.0N nitric acid (HNO3). Strontium was isolated by ion exchange chromatography using Sr-Resin (EIChroM) and collected in acid-leached Teflon vials (Savillex). Blanks were spiked with 84Sr. All samples were nitrated twice with concentrated HNO3 before isotopic analysis.
The samples were loaded on single annealed rhenium filaments with TaCl5. The measurements were performed on a MAT-Finnigan 262 RPQ-plus multicollector mass spectrometer (Finnigan Corp., San Jose, CA) at the Vrije Universiteit Amsterdam using a static routine. The isotope ratios were corrected for mass-fractionation to 86Sr/88Sr = 0.1194. All measurements were referenced to the NBS987 standard, which gave a mean 87Sr/86Sr value of 0.710241 (n = 10) over the period of the study. The samples were run to an internal precision of ± 0.000006 (1SE) or better. The total procedural blanks (n = 5) provided a negligible contribution (mean 65 pg). All statistical assessments were performed in SPSS 22.0 (IBM SPSS Statistics for Macintosh, Armonk, IBM Corp.).
Results
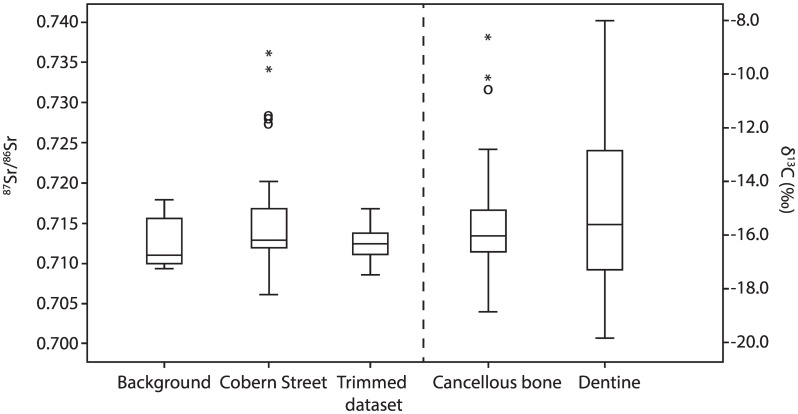
Strontium, carbon and nitrogen isotope ratios from 35 individuals are reported in Table 3. Knowledge of the local isotopic background signature is essential for accurate interpretation of the isotopic data. Available human δ13C isotope values from Cox et al. were therefore reassessed to gain (additional) insight into the carbon isotope range specific to the Cape non-European underclass. Intra-population comparisons of carbon isotopes seem to provide the best way to identify individuals as migrants based on a different isotopic composition compared to the majority [90]. Therefore, to calculate the range of non-European underclass δ13C values local to the Cape, we reanalysed all human δ13C data from Cox et al. [21]. A statistical assessment of the carbon isotope data showed that the variance in δ13Cdentine was three times as high as the variance in δ13Ccancellous (11.3 and 3.6 respectively), indicating more diverse childhood diets, which converged in later life to a narrower range. We interpret these data as indicative of the Cape diet of this group (Table 4). The data are presented in a Tukey’s schematic boxplot in Fig 3 to display the variations in δ13C of the dentine and cancellous bone samples. By interpreting the mild outliers and extreme outliers in δ13Ccancellous (indicative of diet in the last few months to years of life) as non-local to the Cape, a local dietary δ13C range between -18.8‰ to -13.5‰ can be inferred (Fig 3). This range corresponds to a diet consisting of predominantly C3 foods with an approximate 25% to 65% contribution from C4 and/or marine food resources (see Fig 6 in [91]).
Table 3. Osteological, carbon, nitrogen, and strontium isotope data from 35 individuals from the Cobern Street informal burial site, Cape Town, dating to the colonial period.
| Burial type | Burial # | UCT # | Age (yr) | Sex | δ13C dentine (‰) | δ13C cancellous (‰) | Δδ13C (‰) | δ15N dentine (‰) | δ15N cancellous (‰) | Δδ15N (‰) | Element # | 87Sr/86Sr | ± 2 S.E. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B | 3 | 460 | 20 | M | -16.4 | -16.5 | 0.1 | 11.8 | 11.1 | 0.7 | 16 | 0.71375 | 0.00001 |
| B | 4 | 458.1 | 17–18 | F | -18.8 | - | 12.7 | 16 | 0.71581 | 0.00001 | |||
| B | 10 | 498 | 35–40 | F | -16.6 | -15.9 | 0.7 | 13.3 | 12.9 | 0.4 | 26 | 0.71195 | 0.00001 |
| B | 12 | 500 | 35–40 | M | -17.1 | -16.4 | 0.7 | 11.2 | 12.4 | 1.2 | 46 | 0.70862 | 0.00001 |
| B | 13 | 501 | 30–40 | M | -16.3 | -16.2 | 0.1 | 12.4 | 13.2 | 0.8 | 46 | 0.71350 | 0.00001 |
| B | 14 | 502 | 45–55 | F | -19.2 | -16.9 | 2.3 | 8.8 | 8.7 | 0.1 | 46 | 0.71274 | 0.00001 |
| 47 | 0.71336 | 0.00001 | |||||||||||
| 48 | 0.71234 | 0.00001 | |||||||||||
| B | 15 | 504 | 25 | M | -17.4 | -16.6 | 0.8 | 10.9 | 12.4 | 1.5 | 46 | 0.71328 | 0.00001 |
| B | 18 | 508 | >50 | F | -13.9 | -16.2 | 2.3 | 11.8 | 12.6 | 0.8 | 26 | 0.71465 | 0.00001 |
| 47 | 0.71499 | 0.00001 | |||||||||||
| 18 | 0.71167 | 0.00001 | |||||||||||
| B | 20A | 510 + | 25–30 | M | -13.8 | -12.8 | 1.0 | 9.8 | 9.6 | 0.2 | 16 | 0.72738 | 0.00001 |
| 17 | 0.72858 | 0.00001 | |||||||||||
| 18 | 0.72278 | 0.00001 | |||||||||||
| B | 20B | 511 + | 16 | F | -12.2 | -8.6 | 3.6 | 7.5 | 7.5 | 0.0 | 46 | 0.71675 | 0.00001 |
| 47 | 0.71522 | 0.00001 | |||||||||||
| 38 | 0.71570 | 0.00001 | |||||||||||
| B | 20C | 512 | 1,5–2 | ? | -16.2 | -16.2 | 0.0 | 16.1 | 14.1 | 2.0 | 71/2 or 81/2 | 0.71194 | 0.00001 |
| B | 21 | 514 | 25–35 | F | -11.3 | -16.0 | 4.7 | 12.3 | 12.3 | 0.0 | 46 | 0.71219 | 0.00001 |
| 47 | 0.71158 | 0.00001 | |||||||||||
| 38 | 0.71047 | 0.00001 | |||||||||||
| B | 23 | 516 | 17–19 | F | -15.3 | -16.6 | 1.3 | 12.0 | 13.4 | 1.4 | 36 | 0.71211 | 0.00001 |
| 37 | 0.71109 | 0.00001 | |||||||||||
| 38 | 0.71199 | 0.00001 | |||||||||||
| B | 27B | 521 | 40–15 | M | -16.4 | -16.7 | 0.3 | 10.3 | 9.9 | 0.4 | 26 | 0.71900 | 0.00001 |
| B | 28 | 522 | 50 | F | -13.9 | - | 11.9 | 46 | 0.71267 | 0.00001 | |||
| B | 34 | 528 | 14–15 | F | -19.8 | -18.8 | 1.0 | 10.3 | 9.9 | 0.4 | 46 | 0.71555 | 0.00001 |
| B | 40 | 535 + | 12 | F | -9.9 | -10.5 | 0.6 | 6.1 | 7.3 | 1.2 | 46 | 0.72803 | 0.00001 |
| B | 41 | 536 | 35–50 | M | -15.3 | -16.2 | 0.9 | 12.1 | 10.8 | 1.3 | 36 | 0.71822 | 0.00001 |
| B | 44 | 542 | 40–50 | F | -13.4 | -14.1 | 0.7 | 14.3 | 11.7 | 2.6 | 46 | 0.73407 | 0.00001 |
| 47 | 0.74186 | 0.00001 | |||||||||||
| 48 | 0.71913 | 0.00001 | |||||||||||
| B | 45 | 543 | 50+ | M | -16.7 | -17.6 | 0.9 | 13.1 | 12.1 | 1.0 | 37 | 0.71343 | 0.00001 |
| B | 46 | 544 | 35–50 | F | -17.7 | -16.8 | 0.9 | 11.3 | 11.9 | 0.6 | 16 | 0.72015 | 0.00001 |
| B | 47 | 545 | 30–40 | M | -15.3 | -16.7 | 1.4 | 12.1 | 13.4 | 1.3 | 46 | 0.71260 | 0.00001 |
| B | 49 | 547 | 30–35 | M | -10.3 | -14.1 | 3.8 | 8.1 | 11.2 | 3.1 | 26 | 0.72830 | 0.00001 |
| 47 | 0.74143 | 0.00001 | |||||||||||
| B | 50 | 548 + | 35–50 | M | -9.8 | -16.0 | 6.2 | 7.6 | 12.6 | 5.0 | 46 | 0.70639 | 0.00001 |
| 47 | 0.70603 | 0.00001 | |||||||||||
| 48 | 0.70907 | 0.00001 | |||||||||||
| B | 51 | 549 | 35–40 | M | -16.0 | - | 12.8 | 26 | 0.71017 | 0.00001 | |||
| B? | 52 | 550 + | 25–35 | F | -12.0 | -10.1 | 1.9 | 7.2 | 8.6 | 1.4 | 46 | 0.70921 | 0.00001 |
| 47 | 0.70938 | 0.00001 | |||||||||||
| 48 | 0.71545 | 0.00001 | |||||||||||
| B | 54 | 552 | 30–35 | M | -18.6 | -15.8 | 2.8 | 9.9 | 12.3 | 2.4 | 46 | 0.71027 | 0.00001 |
| 47 | 0.71044 | 0.00001 | |||||||||||
| 48 | 0.71221 | 0.00001 | |||||||||||
| B | 56 | 554 | 35 | M | -16.5 | - | 11.2 | 46 | 0.71387 | 0.00001 | |||
| B | 58 | 556 | 35–40 | F | -11.9 | -14.2 | 2.3 | 11.7 | 12.0 | 0.3 | 32 | 0.71233 | 0.00001 |
| 17 | 0.71182 | 0.00001 | |||||||||||
| 48 | 0.71378 | 0.00001 | |||||||||||
| B | 60 | 558 + | 30 | F | -8.0 | -15.3 | 7.3 | 7.2 | 12.8 | 5.6 | 36 | 0.73605 | 0.00001 |
| 37 | 0.73566 | 0.00001 | |||||||||||
| 1/28 | 0.73608 | 0.00001 | |||||||||||
| B | 61 | 559 | 35 | M | -15.5 | -15.2 | 0.3 | 14.6 | 14.0 | 0.6 | 46 | 0.71183 | 0.00001 |
| C | 32 | 526 | 50–60 | M | -18.0 | -15.9 | 2.1 | 11.4 | 14.1 | 2.7 | 46 | 0.71225 | 0.00001 |
| 17 | 0.71093 | 0.00001 | |||||||||||
| 48 | 0.70923 | 0.00001 | |||||||||||
| C | 57 | 555 | 20–30 | F | -18.4 | -15.6 | 2.8 | 11.8 | 13.7 | 1.9 | 36 | 0.71011 | 0.00001 |
| 47 | 0.71023 | 0.00001 | |||||||||||
| 18 | 0.70947 | 0.00001 | |||||||||||
| C | 59 | 557 | 40 | M | -17.6 | -17.1 | 0.5 | 8.1 | 12.2 | 4.1 | 36 | 0.70600 | 0.00001 |
| 37 | 0.70618 | 0.00001 | |||||||||||
| 18 | 0.70602 | 0.00001 | |||||||||||
| C | 65 | 563 | 22–25 | F | -15.6 | -14.9 | 0.7 | 10.8 | 13.3 | 2.5 | 26 | 0.70921 | 0.00001 |
| 27 | 0.70921 | 0.00001 | |||||||||||
| 18 | 0.70910 | 0.00001 |
Key: Initial osteological investigations are executed and published by the Western Cape Physical Anthropology Group (see [71] for details). Carbon and nitrogen isotope data from Cox et al. [21]. Intra-individual differences between δ13Cdentine and δ13Ccancellous, and δ15Ndentine and δ15Ncancellous are expressed as Δδ13C and Δδ15N respectively. Burial type refers to supine/Christian style (B) or facing Signal Hill/Muslim style (C). UCT number refers to the accession number. “+” indicates the presence of intentional dental modifications (see Table 1 for details). Dental element notation conforms to Fédération Dentaire Internationale (details presented in Table 2).
Table 4. Statistical assessment of human 87Sr/86Sr data (this study) and δ13C [21] data from Cobern Street, Cape Town.
| Statistics | 87Sr/86Sr | Trimmed 87Sr/86Sr | δ13Cdentine | δ13Ccancellous |
|---|---|---|---|---|
| N | 33 | 23 | 36 | 50 |
| Mean | 0.71527 | 0.71239 | -14.98 | -15.54 |
| Median | 0.71274 | 0.71236 | -16.25 | -16.20 |
| Standard deviation (1σ) | 0.00696 | 0.00215 | 33.63 | 19.07 |
| Standard deviation (2σ) | 0.01393 | 0.00431 | 67.26 | 38.14 |
| Variance | 0.00001 | 0.00001 | 11.31 | 3.64 |
| Minimum | 0.70600 | 0.70862 | -19.80 | -18.80 |
| Maximum | 0.73605 | 0.71675 | -5.30 | -8.60 |
| Range | 0.03006 | 0.00813 | 14.50 | 10.20 |
Key: The trimmed dataset resembles a normally distributed dataset in which the statistical outliers (n = 10) are excluded.
Fig 3. Tukey’s schematic boxplot showing 87Sr/86Sr variation for the background data (n = 18: Sealy et al. [69] and Balasse et al. [68]), the (trimmed) Cobern Street data (this study), and the variation in δ13C for the dentine (n = 36) and cancellous bone (n = 50) samples from Cox et al. [21].
Key: the boxes represent the interquartile range (IQR: Q3-Q1), the central line indicates the median. The whiskers represent Q1–1.5*IQR and Q3 + 1.5*IQR. The circles represent mild outliers (>1.5*IQR), the asterisks extreme outliers (>3*IQR). The trimmed dataset resembles a normally distributed dataset in which the statistical outliers are excluded.
The 87Sr/86Sr of the human enamel data range from 0.70600 to 0.73605 with a mean of 0.71527 ± 0.015 (2σ). The extremely wide range in strontium values with no apparent clustering of values indicated that the individuals in this study came from very diverse geological regions. A nonparametric Mann-Whitney U test was performed to quantify differences between males (n = 17) and females (n = 17). The results showed no statistically significant difference between the median 87Sr/86Sr ratios (U = 122, z = -0.775, p = 0.438) of males (0.714051 ± 0.00621) and females (0.716680 ± 0.00827).
Insight into the local strontium signal is gleaned from a statistical assessment of the first molar and deciduous teeth data from Cobern Street (n = 33). As in Wright [92], it is assumed that the 87Sr/86Sr ratios of a ‘local’ population are normally distributed. A Shapiro-Wilk test rejects the hypothesis that the Cobern Street dataset resembles a normal distribution (W = 830, df = 33, α = 0.000). Exclusion of statistical outliers (n = 10) results in a normally distributed dataset (W = 0.943, df = 23, α = 0.677) in which the mean and median coincide (0.71239 and 0.71236 respectively). Based on the human data, an approximate local range of 0.7086 to 0.7167 could be defined which was subject to later refinement (See section on Background 87Sr/86Sr data).
Discussion
Background 87Sr/86Sr data
A large amount of regional 87Sr/86Sr data, both biological and geological, are available for the Greater Cape Floral Region and surrounding areas of South Africa (e.g., see [67] for an extensive overview). These latter data are, however, not incorporated in this study due to A) the diverse nature of the sample types (biosphere/biological and geological); B) the distance of the samples location from Cape Town (up to ±150 kilometre); and/or C) the relatively low precision of the data due to choice of analytical method (±0.005, resulting in a large data range from similar sample types: Δ87Sr/86SrMIN-MAX = 0.023). Consequently, as explained in the section on determination of geological origins through isotope analysis, the regional strontium values were estimated solely on faunal samples. Local modern and archaeological faunal strontium data from wild and domestic animals from the south-western Cape within a 150 kilometre radius of Cape Town were reported by Balasse et al. [68] (n = 8) and Sealy et al. [69] (n = 10). Sealy and colleagues [69] found that humans and animals living on the Holocene coastal sands exhibited 87Sr/86Sr ratios between 0.7094 and 0.7117. The strontium ratios of modern fauna found in the (Pre-) Cambrian inland resource zones are characterised by higher ratios ranging between 0.7154 and 0.7179. The Cape granite areas surrounding Cape Town are characterized by bioavailable 87Sr/86Sr ratios ranging between 0.7099 and 0.7107 [68]. Based on the available modern and archaeological faunal data there appears to be an isotopic compositional gap in the local environment between 0.7117 and 0.7154 (Fig 2).
The human 87Sr/87Sr local signature was found to range from 0.7086 to 0.7167, simultaneously overlapping with the Sealy et al. [69] and Balasse et al. [68] datasets and filling the compositional gap left by the faunal data. Thus, in this study, a conservative local 87Sr/86Sr range, 0.7086 (human data) to 0.7179 (Pre-Cambrian bioavailable data), is used for the Cape (Fig 2). This range is remarkably wide due to the geological diversity of the region and hence not especially diagnostic. Moreover, the range will probably encompass the vast majority of the human data. As a result, the extent of human mobility is likely to be significantly underestimated, and the number of individuals assigned as non-local to the Western Cape should be considered a minimum.
Human isotopic data
Based on the strontium data, a minimum of ten individuals were found to have not been born at the Cape, three of whom were female (burials 44, 46 and 60), while six were male (burials 20A, 27B, 41, 49, 50 and 59) and one was a child (burial 40). It seems reasonable to speculate that their migrations might have coincided with enslavement or change of ownership. Despite the absence of significant Δδ13C and/or Δδ15N in four of the assumed local or second- or subsequent generation slaves (burials 27B, 40, 41 and 46, see Table 1 and [21]), these individuals appeared to be alien to the Western Cape based on their 87Sr/86Sr ratio. It was therefore concluded that the isotopic difference between δ13Cdentine and δ13Ccancellous and/or δ15Ndentine and δ15Ncancellous did not provide reliable evidence for the presence of migrants. The non-local male dataset was isotopically more varied; its standard deviation (0.0089) was almost 1.5 times as high as the non-local female dataset (0.0061). Although the sample sets are small, this difference may indicate a larger variety in geological origins of the male population.
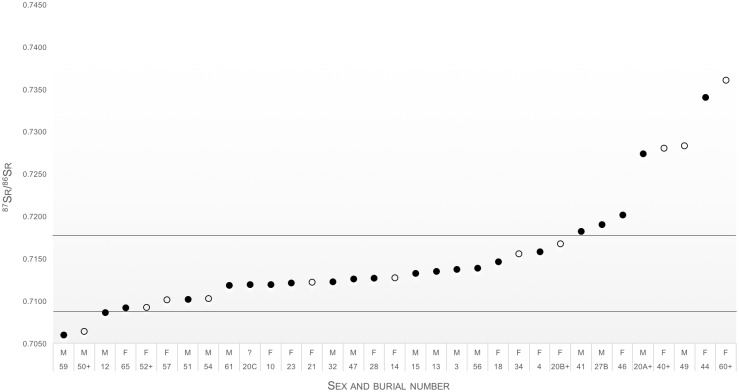
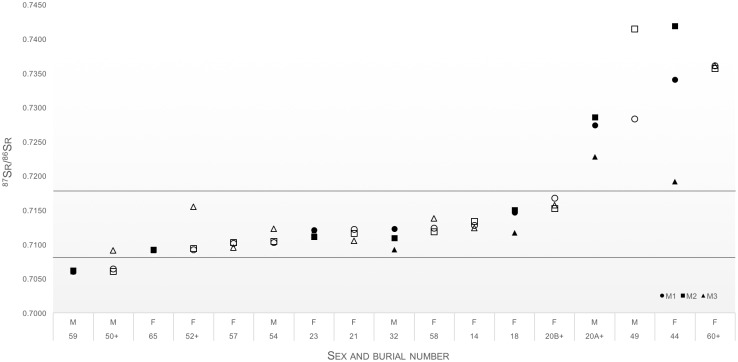
Figs 4 and 5 display the first molar and deciduous teeth 87Sr/86Sr data (n = 33) and multi-dental elemental 87Sr/86Sr data (n = 17) respectively. The data range from 0.7060 to 0.7419. The majority of these individuals (n = 22) also displayed a local childhood dietary carbon isotope signal (δ13Cdentine: -18.8 to -13.5‰). Only four individuals, two males, one female and one child, exhibited both non-local strontium and carbon isotope values (burials 40, 49, 50 and 60). Burial 40, a child aged 12, had a significantly different diet during life (δ13Cdentine: -9.9‰; δ13Ccancellous: -10.5‰), which was dominated by C4 food resources [21]. In addition, this individual displayed a type of intentional dental modification characteristic of people of Mozambican descent [20, 21, 93]. This individual’s elevated 87Sr/86Sr ratio, 0.72803, was consistent with provenance from the radiogenic Phanerozoic and Precambrian bedrocks in Mozambique, further supporting possible Mozambican origins. Seven individuals, burials 14, 20B, 21, 34, 52, 54 and 57, exhibit local 87Sr/86Sr ratios, but their δ13Cdentine values deviated from the local range. They may therefore also be assigned as non-local.
Fig 4. Strontium isotope data from M1 and deciduous teeth data from 33 individuals. The horizontal lines mark the local strontium range.
Key: “+” next to sample number indicates the presence of intentional dental modifications. Open symbols identify individuals who exhibit non-local δ13Cdentine values. The standard errors in the analytical data are smaller than the plotted symbols.
Fig 5. Multi-dental elemental strontium isotope data from 17 individuals. The horizontal lines mark the local strontium range.
Key: “+” next to sample number indicates the presence of intentional dental modifications. Open symbols identify individuals who exhibit non-local δ13Cdentine values. The standard errors in the analytical data are smaller than the plotted symbols.
All five individuals who exhibited culturally modified teeth (burials 20A, 20B, 50, 52 and 60; all type B burials) were found to be of non-local descent based on either 87Sr/86Sr (20A) or δ13Cdentine (20B and 52), or both proxies (50 and 60). These results verify the earlier assumption that the use of only one biogeochemical proxy may lead to the underestimation of the number of non-local individuals. The fact that all individuals with intentional dental modifications exhibited non-local 87Sr/86Sr and/or δ13Cdentine values was expected. It has been suggested that imported slaves quickly abandoned this practice to avoid recognition if they ran away [72, 94]. It would be unlikely that locally born individuals would continue this cultural practice. Based on the multi-dental elemental strontium data, individuals 20A and 60 lived in more radiogenic areas than the Cape in early life. Individual 50 originated from a geological area with less radiogenic strontium. Individual 20A appears to have experienced at least two migration events: one at the age of circa 8 (Δ87Sr/86SrM1-M2 = 0.0012), and one after 16 (Δ87Sr/86SrM2-M3 = 0.0058). In contrast, the isotopic data of individual 60 indicates residential stability until the age of at least 16 (Δ87Sr/86Sr < 0.001). The strontium isotope data for individuals 20B and 52 were compatible with the local range of strontium isotope ratios. The presence of dental modifications and a predominantly C4 based childhood diet (δ13Cdentine ~-12‰), however, precluded these individuals from being locally born. Burials 20A (a male between 25 and 30 years at time of death) and 20B (a young female of approximately 16 years of age) were buried together with a 1.5–2-year-old child (20C) in a common shaft and shared a single coffin [71]. The isotopic data and the presence of dental modifications showed that individuals 20A and 20B were of non-local descent, but did not share common geographical origins. Perhaps their dietary shift towards a more C4 signal, implied by the change in δ13C between dentine and cancellous bone, corresponded to their transportation to a central slave market from where they were bought and subsequently shipped to the Cape. They died shortly after arrival, before their bone was able to remodel and incorporate the local dietary isotopic signature. The 87Sr/86Sr ratio and δ13Cdentine value of the child, however, are consistent with the inferred local values.
The majority of the 12 individuals without dental modifications for whom multi-dental elemental analysis was performed exhibited strontium ratios compatible with the Cape indicating possible local provenance (n = 9). The carbon isotope data for individuals 18, 21 and 58, however, indicated a strong reliance on C4 food resources uncharacteristic of the Cape diet (all δ13Cdentine >-13.5‰). Thus despite strontium isotope ratios compatible with the estimated local range, local origins were unlikely. A similar conclusion was drawn for individuals 14, 54 and 57: their childhood diet consisted of C3 foods, but in much larger proportions than the inferred local diet at the Cape. Hence, the use of strontium isotope ratios alone as a diagnostic tool at the Cape will invariably lead to an underestimation of migrants. As a result, just four out of the seventeen individuals selected for multi-dental elemental sampling exhibited both δ13Cdentine values and 87Sr/86Sr ratios compatible with the inferred local ranges. From the available data, it was not possible to determine if these individuals were second or subsequent generation slaves, or had consumed a comparable diet to that of the Cape and hailed from a geologically similar region.
In addition to individual 20A, multiple migration events were also evident from the strontium isotope data of individual 44. This 40 to 50-year-old female was transhipped to more radiogenic regions after the age of 3 (Δ87Sr/86SrM1-M2 = 0.0078) and experienced another (forced) migration after the age of 7 (Δ87Sr/86SrM2-M3 = 0.023). As with individual 44, individual 49, a 30 to 35-year-old male, exhibited an M2 87Sr/86Sr ratio that was significantly more radiogenic than his M1 ratio (Δ87Sr/86SrM1-M2 = 0.013). Due to the absence of the 3rd molar, it could not be established whether this individual’s migration to the Cape occurred after the age of 7 or 16.
Individual 59 plotted outside of the Cape strontium range throughout life. The 87Sr/86Sr ratios for this individual were similar to those of the M1 and M2 of individual 50, however, their differing childhood dietary habits (δ13Cdentine -17.6‰ and -12.0‰ respectively) suggested different geographical origins for the two males.
Individuals 32 and 59 (males) and 57 and 65 (females) were buried facing Signal Hill (type C burials, see Table 3), a sacred place for Cape Muslims. Due to their orientation, it was deduced that these individuals were Muslims. During the period under investigation, Islam was present in all corners of the Indian Ocean basin, including the Cape where slaves favoured it to Christianity for its inclusiveness [95, 96]. Unsurprisingly individuals from burial type C showed a wide range of Sr isotope ratios, ranging from 0.70600 to 0.71225, implying origins from very different geological regions. Based on combined carbon and strontium isotope data, two individuals (57 and 59) were undoubtedly of non-local descent. Individual 59’s low 87Sr/86SrM1 ratio (0.70600) allows a tentative assignment to a region characterised by a young volcanic geology, such as the Indonesian archipelago, the Deccan traps region of India or volcanic islands in the Indian Ocean.
Conclusion
We demonstrated the utility of relating dietary isotope data to strontium isotope data to enable a more accurate identification of individuals as local or non-local. The approach is particularly useful for migrations in which the geographical relocation is associated with the adaptation to new dietary habits.
The variable geology and the current absence of a comprehensive biological or biosphere database from the region in the near proximity of Cape Town, however, prevents the accurate delineation of the local strontium signal for the Cape Town region. As a result, the relatively broad local 87Sr/86Sr signature undoubtedly led to an underestimation of the number of non-local individuals. We argue, however, that this outcome is preferable to the overestimation of migrants. The use of only one isotopic proxy for migration (either strontium or carbon) generates an incomplete and inaccurate number of non-locally born individuals. This study demonstrates the efficacy of using multiple lines of evidence to generate a more reliable assessment of migration.
We conclude that the absence or the presence of a significant dietary shift cannot be used alone as a reliable proxy for migration. In contrast, a δ13Cdentine value that deviates from the assumed local range seems to identify migrants remarkably well and is as indicative as the presence of intentional dental morphological modifications, a practice not reported at the Cape. As a result, based on the combined interpretation of the osteological, carbon isotope and strontium isotope data, a minimum of 54.5% of the investigated population can be identified as non-local to the Cape (18/35).
Further identification of non-locally born individuals might be feasible using additional isotopic proxies such as lead (206/207/208Pb/204Pb and 207/208Pb/206Pb) and oxygen (δ18O). Lead isotope analysis was not undertaken in this study, however, as it requires a minimum of 120 mg of enamel powder for archaeological samples, destroying most of the dental element. Recent analytical developments, however, now offer this possibility as sub nanogram amounts of Pb can now be analysed [97]. Although oxygen isotope analysis may give additional information, expected oxygen isotope values in coastal regions of South Africa and countries bordering the Indian Ocean basin partly overlap, hampering an accurate interpretation of a person’s geological provenance (see e.g. Fig 3 in [98]).
This is the first extensive isotopic study that elucidates the complexity of the multi-directional Indian Ocean slave trade and sheds light on possible provenances. To date, the history of the Indian Ocean slave trades after European involvement has been highly dependent on the historical record in which subaltern populations are not always well represented. Future interdisciplinary research (combining isotopic data with palaeogenetics) of non-Europeans at the Cape will offer a direct route to assess a neglected history.
Acknowledgments
The authors would like to thank Richard Smeets for analytical assistance. The department of Human Biology of the University of Cape Town kindly granted access to the Cobern Street skeletal remains for sample collection. Bert Brouwenstijn (Vrije Universiteit Amsterdam) kindly provided the map for Figs 1 and 2. This paper greatly benefited from the constructive comments and suggestions made by two anonymous reviewers.
Data Availability
All relevant data are within the paper.
Funding Statement
This research benefitted from funding from the National Research Foundation (South Africa: grant no. 74691), the Oppenheimer Memorial Trust (OMT ref. 19671/02), the former Institute for Geo- and Bioarchaeology of the Vrije Universiteit Amsterdam and the Centre for International Cooperation (CIS) of the Vrije Universiteit Amsterdam (Ref: Mbeki). GRD is funded by the European Research Council under the European Union's Seventh Framework Programme (FP7/2007-2013) / ERC Synergy grant agreement no 319209.
References
- 1.Shell RC-H. Children of bondage. A social history of the slave society at the Cape of Good Hope, 1652–1838. Johannesburg Witwatersrand University Press; 1994. [Google Scholar]
- 2.Vink M. "The world’s oldest trade”: Dutch slavery and slave trade in the Indian Ocean in the seventeenth Century. Journal of World History. 2003;14(2):131–77. [Google Scholar]
- 3.Worden N. Indian Ocean slavery and its demise in the Cape Colony In: Campbell G, editor. Abolition and its aftermath in the Indian Ocean Africa and Asia. London: Routledge; 2005. p. 29–49. [Google Scholar]
- 4.Mbeki L, Van Rossum M. Private slave trade in the Dutch Indian Ocean world: a study into the networks and backgrounds of the slavers and the enslaved in South Asia and South Africa. Slavery & Abolition: A Journal of Slave and Post-Slave Studies. 2016. 10.1080/0144039X.2016.1159004 [DOI] [Google Scholar]
- 5.De Kock V. Those in bondage. London: George Allen & Unwin LTD; 1950. [Google Scholar]
- 6.Elphick R, Shell RC-H. Intergroup relations: Khoikhoi, settlers, slaves and free blacks, 1652–1795 In: Elphick R, Giliomee H, editors. The Shaping of South African Society, 1652–1820. Cape Town: Longman; 1979. p. 116–69. [Google Scholar]
- 7.Worden N. Slavery in Dutch South Africa. Cambridge: Cambridge University Press; 1985. [Google Scholar]
- 8.Ross R. The last years of the slave trade to the Cape Colony. Slavery & Abolition: A Journal of Slave and Post-Slave Studies. 1988;9:209–19. [Google Scholar]
- 9.Worden N. Contingent lives: social identity and material culture in the VOC world. Worden N, editor. Cape Town: University of Cape Town; 2007. [Google Scholar]
- 10.Groenewald G. Slaves and free blacks in VOC Cape Town, 1652–1795. History Compass. 2010;8/9:964–83. [Google Scholar]
- 11.Allen RB. European slave trading in the Indian Ocean, 1500–1850. Athens: Ohio University Press; 2015. [Google Scholar]
- 12.Raben R. Batavia and Colombo. The ethnic and spatial order of two colonial cities 1600–1800 1996.
- 13.Schroeder H, O’Connell TC, Evans JA, Shule KA, Hedges REM. Trans-Atlantic slavery: isotopic evidence for forced migrations. Am J Phys Anthropol. 2009;139(4):547–57. 10.1002/ajpa.21019 [DOI] [PubMed] [Google Scholar]
- 14.Laffoon JE, Davies GR, Hoogland MLP, Hofman CL. Spatial variation of biologically available strontium isotopes (87Sr/86Sr) in an archipelagic setting: a case study from the Caribbean. J Archaeol Sci. 2012;39(7):2371–84. [Google Scholar]
- 15.Goodman AH, Jones J, Reid J, Mack ME, Blakey ML, Amarasiriwardena D, et al. Isotopic and elemental chemistry of teeth: implications for places of birth, forced migration patterns, nutritional status, and pollution In: Blakey ML, Rankin-Hill LM, editors. Skeletal biology of the New York African burial ground Part 1. Washington D.C: Howard University; 2009. p. 95–118. [Google Scholar]
- 16.Nystrom KC, Amato LA, Jankowitz LA. Strontium isotopic reconstruction of the composition of an urban free black population from the 19th century United States. J Archaeol Sci. 2011;38:3505–17. [Google Scholar]
- 17.Shea MK, Yann LT, DeSantis L, Tung TA. Carbon and oxygen isotope analysis to document childhood diet and local vs. non-local status among African slave burials from the Grassmere Plantation, Nashville, Tennessee. YoungScientist. 2015;5:42–5. [Google Scholar]
- 18.Price TD, Tiesler V, Burton JH. Early African diaspora in colonial Campeche, Mexico: strontium isotopic evidence. Am J Phys Anthropol. 2006;130(4):485–90. 10.1002/ajpa.20390 [DOI] [PubMed] [Google Scholar]
- 19.Van Rossum M. Kleurrijke tragiek De geschiedenis van slavernij in Azië onder de VOC. Hilversum: Verloren BV; 2015. [Google Scholar]
- 20.Cox G, Sealy JC. Investigating identity and life histories: isotopic analysis and historical documentation of slave skeletons found on the Cape Town foreshore, South Africa. International Journal of Historical Archaeology. 1997;1(3):207–24. 10.1023/A:1027349115474 [DOI] [Google Scholar]
- 21.Cox G, Sealy JC, Schrire C, Morris A. Stable carbon and nitrogen isotopic analyses of the underclass at the colonial Cape of Good Hope in the eighteenth and nineteenth centuries. World Archaeology. 2001;33(1):73–97. [DOI] [PubMed] [Google Scholar]
- 22.Trincherini PR, Baffi C, Barbero P, Pizzoglio E, Spalla S. Precise determination of strontium isotope ratios by TIMS to authenticate tomato geographical origin. Food Chemistry. 2014;145:349–55. 10.1016/j.foodchem.2013.08.030 [DOI] [PubMed] [Google Scholar]
- 23.Font L, Nowell GM, Graham Pearson D, Ottley CJ, Willis SG. Sr isotope analysis of bird feathers by TIMS: a tool to trace bird migration paths and breeding sites. Journal of Analytical Atomic Spectrometry. 2007;22(5):513–22. 10.1039/B616328A [DOI] [Google Scholar]
- 24.Åberg G. The use of natural strontium isotopes as tracers in environmental studies. Water, Air, and Soil Pollution. 1995;79(1):309–22. 10.1007/bf01100444 [DOI] [Google Scholar]
- 25.Voerkelius S, Lorenz GD, Rummel S, Quétel CR, Heiss G, Baxter M, et al. Strontium isotopic signatures of natural mineral waters, the reference to a simple geological map and its potential for authentication of food. Food Chemistry. 2010;118(4):933–40. [Google Scholar]
- 26.Erickson J. Strontium isotope characterization in the study of prehistoric human ecology. Journal of Human Evolution. 1985;14(5):503–14. [Google Scholar]
- 27.Font L, van der Peijl G, van Leuwen C, van Wetten I, Davies GR. Identification of the geographical place of origin of an unidentified individual by multi-isotope analysis. Science & Justice. 2015;55(1):34–42. 10.1016/j.scijus.2014.06.011 [DOI] [PubMed] [Google Scholar]
- 28.Bentley RA. Strontium isotopes from the Earth to the archaeological skeleton: A review. Journal of Archaeological Method and Theory. 2006;13(3):135–87. [Google Scholar]
- 29.Degryse P, De Muynck D, Delporte S, Boyen S, Jadoul L, De Winne J, et al. Strontium isotopic analysis as an experimental auxiliary technique in forensic identification of human remains. Analytical Methods. 2012;4(9):2674–9. [Google Scholar]
- 30.Pye K. Isotope and trace element analysis of human teeth and bones for forensic purposes. Geological Society, London, Special Publications. 2004;232:215–36. [Google Scholar]
- 31.Hobson K, Barnett-Johnson R, Cerling T. Using isoscapes to track animal migration In: West JB, Bowen GJ, Dawson TE, Tu KP, editors. Isoscapes: Springer Netherlands; 2010. p. 273–98. [Google Scholar]
- 32.Schwarcz HP, White CD, Longstaffe FJ. Stable and radiogenic isotopes in biological archaeology: some applications In: West J.B. B GJ, Dawson T.E. & Tu K.P., editor. Understanding movement, pattern, and process on Earth through isotope mapping. Netherlands: Springer Science; 2010. p. 335–56. [Google Scholar]
- 33.Slovak NM, Paytan A. Applications of Sr isotopes in archaeology In: Baskaran M, editor. Handbook of Environmental Isotope Geochemistry. Advances in Isotope Geochemistry. 1 Heidelberg: Spinger-Verlag; 2011. p. 743–68. [Google Scholar]
- 34.Steiger RH, Jäger E. Subcommission on geochronology: Convention on the use of decay constants in geo- and cosmochronology. Earth and Planetary Science Letters. 1977;36 (3):359–62. [Google Scholar]
- 35.Capo RC, Stewart BW, Chadwick OA. Strontium isotopes as tracers of ecosystem processes: theory and methods. Geoderma. 1998;82:197–225. [Google Scholar]
- 36.Faure G. Principles of isotope geology (2nd edition). U.S.A: John Wiley and Sons Inc; 1986. [Google Scholar]
- 37.Miller EK, Blum JD, Friedland AJ. Determination of soil exchangeable-cation loss and weathering rates using Sr isotopes. Nature. 1993;362:438–41. [Google Scholar]
- 38.Price TD, Burton JH, Bentley RA. The characterization of biologically available strontium isotope ratios for the study of prehistoric migration. Archaeometry. 2002;44:117–35. ISI:000174025600008. [Google Scholar]
- 39.Burton JH, Price TD, Middleton WD. Correlation of bone Ba/Ca and Sr/Ca due to biological purification of calcium. J Archaeol Sci. 1999;26(6):609–16. 10.1006/jasc.1998.0378 [DOI] [Google Scholar]
- 40.Dijkstra FA, Van Breemen N, Jongmans AG, Davies GR, Likens GE. Calcium weathering in forested soils and the effect of different tree species. Biogeochemistry. 2003;62(3):253–75. 10.1023/A:1021132132199 [DOI] [Google Scholar]
- 41.Isermann K. Uptake of stable strontium by plants and effects on plant growth In: Skoryna SC, editor. Handbook of Stable Strontium: Springer US; 1981. p. 65–86. [Google Scholar]
- 42.Veizer J. Strontium isotopes in seawater through time. Annu Rev Earth Planet Sci. 1989;17:141–67. [Google Scholar]
- 43.Whipkey CE, Capo RC, Chadwick OA, Stewart BW. The importance of sea spray to the cation budget of a coastal Hawaiian soil: a strontium isotope approach. Chemical Geology. 2000;168(1–2):37–48. 10.1016/S0009-2541(00)00187-X [DOI] [Google Scholar]
- 44.Montgomery J. Passports from the past: Investigating human dispersals using strontium isotope analysis of tooth enamel. Annals of human biology. 2010;37(3):325–46. 10.3109/03014461003649297 [DOI] [PubMed] [Google Scholar]
- 45.Price TD, Wahl J, Knipper C, Burger-Heinrich E, Kurtz G, Bentley RA. Das bandkeramische Gräberfeld vom „Viesenhäuser Hof”bei Stuttgart-Mühlhausen: Neue Untersuchungsergebnisse zum Migrationsverhalten im frühen Neolithikum In: Funda DT, editor. Fundberichte aus Baden-Württemberg. Stuttgart: Kommissionsverlag Konrad Theiss Verlag: Stuttgart, Germany; 2003. p. 23–58. [Google Scholar]
- 46.Maurer A-F, Galer SJG, Knipper C, Beierlein L, Nunn EV, Peters D, et al. Bioavailable 87Sr/86Sr in different environmental samples—effects of anthropogenic contamination and implications for isoscapes in past migration studies. Science of The Total Environment. 2012;433:216–29. 10.1016/j.scitotenv.2012.06.046 [DOI] [PubMed] [Google Scholar]
- 47.Nielson FH. Other elements: Sb, Ba, B, Br, Cs, Ge, Rb, Ag, Sr, Sn, Ti, Zr, Be, Bi, Ga, Au, In, Nb, Sc, Te, Tl, W In: Mertz W, editor. Trace elements in human and animal nutrition—Volume 2. Orlando: Academic Press, Inc; 1986. [Google Scholar]
- 48.Woelfel JB, Scheid RC. Dental anatomy: its relevance to dentistry (6th edition). Philadelphia: Lippincott Williams & Wilkins; 2002. [Google Scholar]
- 49.Reid DJ, Dean MC. Variation in modern human enamel formation times. Journal of Human Evolution. 2006;50(3):329–46. 10.1016/j.jhevol.2005.09.003 [DOI] [PubMed] [Google Scholar]
- 50.Nelson SJ, Ash MM. Wheeler's dental anatomy, physiology, and occlusion (9th edition). St. Louis, Missouri: Saunders Elsevier; 2010. [Google Scholar]
- 51.Szostek K, Stepańczak B, Szczepanek A, Kępa M, Głąb H, Jarosz P, et al. Diagenetic signals from ancient human remains—bioarchaeological applications. Mineralogia. 2011;42(2):93–112. 10.2478/v10002-011-0009-4 [DOI] [Google Scholar]
- 52.Nielsen-Marsh CM, Hedges REM. Patterns of diagenesis in bone I: the effects of site environments. J Archaeol Sci. 2000;27:1139–51. [Google Scholar]
- 53.Budd P, Montgomery J, Barreiro B, Thomas RG. Differential diagenesis of strontium in archaeological human dental tissues. Appl Geochem. 2000;15:687–94. [Google Scholar]
- 54.Hedges REM. Bone diagenesis: an overview of processes. Archaeometry. 2002;44(3):319–28. 10.1111/1475-4754.00064 [DOI] [Google Scholar]
- 55.Hoppe KA, Koch PL, Furutani TT. Assessing the preservation of biogenic strontium in fossil bones and tooth enamel. International Journal of Osteoarchaeology. 2003;13:20–8. [Google Scholar]
- 56.Hedman KM, Curry BB, Johnson TM, Fullagar PD, Emerson TE. Variation in strontium isotope ratios of archaeological fauna in the Midwestern United States: a preliminary study. J Archaeol Sci. 2009;36(1):64–73. [Google Scholar]
- 57.Evans JA, Montgomery J, Wildman G. Isotope domain mapping of 87Sr/86Sr biosphere variation on the Isle of Skye, Scotland. Journal of the Geological Society. 2009;166:617–31. [Google Scholar]
- 58.Evans JA, Montgomery J, Wildman G, Boulton N. Spatial variations in biosphere 87Sr/86Sr in Britain. Journal of the Geological Society. 2010;167:1–4. [Google Scholar]
- 59.Willmes M, McMorrow L, Kinsley L, Armstrong R, Aubert M, Eggins S, et al. The IRHUM (Isotopic Reconstruction of Human Migration) database–bioavailable strontium isotope ratios for geochemical fingerprinting in France. Earth Syst Sci Data. 2014;6: 117–22. 10.5194/essd-6-117-2014 [DOI] [Google Scholar]
- 60.Kootker LM, Van Lanen RJ, Kars H, Davies GR. Strontium isoscapes in The Netherlands. Spatial variations in 87Sr/86Sr as a proxy for palaeomobility. Journal of Archaeological Science: Reports. 2016;6:1–13. 10.1016/j.jasrep.2016.01.015 [DOI] [Google Scholar]
- 61.Bentley RA, Knipper C. Geographical patterns in biologically available strontium, carbon and oxygen isotope signatures in prehistoric SW Germany. Archaeometry. 2005;47:629–44. ISI:000231737600009. [Google Scholar]
- 62.Oelze VM, Koch JK, Kupke K, Nehlich O, Zäuner S, Wahl J, et al. Multi-isotopic analysis reveals individual mobility and diet at the Early Iron Age monumental tumulus of Magdalenenberg, Germany. Am J Phys Anthropol. 2012;148(3):406–21. 10.1002/ajpa.22063 [DOI] [PubMed] [Google Scholar]
- 63.Frei KM, Frei R. The geographic distribution of strontium isotopes in Danish surface waters: A base for provenance studies in archaeology, hydrology and agriculture. Appl Geochem. 2011;26(3):326–40. [Google Scholar]
- 64.Nafplioti A. Tracing population mobility in the Aegean using isotope geochemistry: a first map of local biologically available 87Sr/86Sr signatures. J Archaeol Sci. 2011;38(7):1560–70. [Google Scholar]
- 65.Bentley RA, Cox K, Tayles N, Higham C, Macpherson C, Nowell G, et al. Community diversity at Ban Lum Khao, Thailand: isotopic evidence from the skeletons. Asian Perspectives. 2009;48:79–97. 10.1353/asi.0.0017 [DOI] [Google Scholar]
- 66.Kinaston RL, Walter RK, Jacomb C, Brooks E, Tayles N, Halcrow SE, et al. The first New Zealanders: patterns of diet and mobility revealed through isotope analysis. PLoS ONE. 2013;8(5):e64580 10.1371/journal.pone.0064580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Copeland SR, Copeland SR, Cawthra HC, Fisher EC, Lee-Thorp JA, Cowling RM, et al. Strontium Isotope investigation of ungulate movement patterns on the Pleistocene 34 Paleo-Agulhas plain of the Greater Cape Floristic Region, South Africa. subm.
- 68.Balasse M, Ambrose SH, Smith AB, Price TD. The seasonal mobility model for prehistoric herders in the south-western Cape of South Africa assessed by isotopic analysis of sheep tooth enamel. J Archaeol Sci. 2002;29(9):917–32. [Google Scholar]
- 69.Sealy JC, van der Merwe NJ, Sillen A, Kruger FJ, Krueger HW. 87Sr/86Sr as a dietary indicator in modern and archaeological bone. J Archaeol Sci. 1991;18(3):399–416. 10.1016/0305-4403(91)90074-Y [DOI] [Google Scholar]
- 70.Compton JS, White RA, Smith M. Rare earth element behavior in soils and salt pan sediments of a semi-arid granitic terrain in the Western Cape, South Africa. Chemical Geology. 2003;201(3–4):239–55. 10.1016/S0009-2541(03)00239-0 [DOI] [Google Scholar]
- 71.Apollonio H. Identifying the dead: eighteen century mortuary practices at Cobern Street, Cape Town Cape Town: University of Cape Town; 1998. [Google Scholar]
- 72.Cox G. Cobern Street burial ground: Investigating the identity and life histories of the underclass of eighteenth century Cape Town. Unpublished MSc. thesis. Cape Town: University of Cape Town; 1999.
- 73.Waterlow JC. Protein turnover. Wallingford: CABI Publishing; 2006. [Google Scholar]
- 74.Babraj J, Cuthbertson DJ, Rickhuss P, Meier-Augenstein W, Smith K, Bohé J, et al. Sequential extracts of human bone show differing collagen synthetic rates. Biochemical Society Transactions. 2002;30(2):61–5. 10.1042/bst0300061 [DOI] [PubMed] [Google Scholar]
- 75.Hedges REM, Clement JG, Thomas CDL, O'Connell TC. Collagen turnover in the adult femoral mid-shaft: Modeled from anthropogenic radiocarbon tracer measurements. Am J Phys Anthropol. 2007;133(2):808–16. 10.1002/ajpa.20598 [DOI] [PubMed] [Google Scholar]
- 76.International Commission on Radiological Protection. Alkaline Earth metabolism in adult man John H. Marshall, Chairman, Task Group of Committee 2, Pergamon Press, Oxford and New York: 1973. [PubMed] [Google Scholar]
- 77.Richards MP, Mays S, Fuller BT. Stable carbon and nitrogen isotope values of bone and teeth reflect weaning age at the Medieval Wharram Percy site, Yorkshire, UK. Am J Phys Anthropol. 2002;119(3):205–10. 10.1002/ajpa.10124 [DOI] [PubMed] [Google Scholar]
- 78.Moorrees CF, Fanning EA, Hunt EE. Age variation of formation stages for 10 permanent teeth. Journal of Dental Research. 1963;42:490–502. [DOI] [PubMed] [Google Scholar]
- 79.Kajiyama S. Total number of regular incremental lines (Regulare Parallelstreifen nach Asper) in the enamel of human permanent teeth. Journal of Nihon University School of Dentistry. 1965;39:77–83. [Google Scholar]
- 80.Morris AG, Phillips V. Dental health and dental practises amongst the people of Cobern Street. Paper presented at the 27th annual Congress of the Anatomical Society of Southern Africa, Cape Town, South Africa1997.
- 81.Manyaapelo T. An odentological analysis of 18th and 19th century burial sites from in and around Cape Town. Unpublished MSc. thesis. Cape Town: University of Cape Town; 2007.
- 82.Schumm DE. Essentials of biochemistry (2nd edition). Boston: Little, Brown and Company; 1995. [Google Scholar]
- 83.Pate FD, Anson TJ, Noble AH, Schoeninger MJ. Bone collagen stable carbon and nitrogen isotope variability in modern South Australian mammals: a baseline for palaeoecological inferences. Quaternary Australasia. 1998;16 (1):43–51. [Google Scholar]
- 84.Adams TS, Sterner RW. The effect of dietary nitrogen content on trophic level δ15N enrichment. Limnology and Oceanography. 2000;45:601–7. [Google Scholar]
- 85.Bogaard A, Heaton THE, Poulton P, Merbach I. The impact of manuring on nitrogen isotope ratios in cereals: archaeological implications for reconstruction of diet and crop management practices. J Archaeol Sci. 2007;34(3):335–43. [Google Scholar]
- 86.Sharp Z. Principles of stable isotope geochemistry. New York: Pearson Education, Inc; 2007. [Google Scholar]
- 87.Leatherdale AJ. Interpreting stable carbon and nitrogen isotope ratios in archaeological remains: An overview of the processes influencing the δ13C and δ15N values of type I collagen. Totem: The University of Western Ontario Journal of Anthropology. 2013;21 (1):40–50. [Google Scholar]
- 88.Schwarcz HP, Dupras TL, Fairgrieve SI. 15N enrichment in the Sahara: in search of a global relationship. J Archaeol Sci. 1999;26:629–36. [Google Scholar]
- 89.Liversidge HM. Dental age revisited Technique and application in dental anthropology. Cambridge: Cambridge University Press; 2008. [Google Scholar]
- 90.Hakenbeck S, McManus E, Geisler H, Grupe G, O'Connell T. Diet and mobility in Early Medieval Bavaria: A study of carbon and nitrogen stable isotopes. Am J Phys Anthropol. 2010;143(2):235–49. 10.1002/ajpa.21309 [DOI] [PubMed] [Google Scholar]
- 91.Van der Merwe NJ. Carbon isotopes, photosynthesis, and archaeology. American Scientist. 1982;70:596–606. [Google Scholar]
- 92.Wright LE. Identifying immigrants to Tikal, Guatemala: defining local variability in strontium isotope ratios of human tooth enamel. J Archaeol Sci. 2005;32(4):555–66. [Google Scholar]
- 93.Cox G. Historical background and isotopic analysis of skeletons found near the site of Fort Knokkem Cape Town Foreshore: University of Cape Town; 1995. [Google Scholar]
- 94.Handler JS, Corruccini RS, Mutaw RJ. Tooth mutilation in the Carribean: Evidence from a slave burial population in Barbados. Journal of Human Evolution. 1982;11:297–313. [Google Scholar]
- 95.Beyers J. Beyond denial and exclusion: The history of relations between Christians and Muslims in the Cape Colony during the 17th–18th centuries with lessons for a post-colonial theology of religions. HTS Teologiese Studies / Theological Studies. 2016;72 (1):a3117 10.4102/hts.v72i1.3117 [DOI] [Google Scholar]
- 96.Alpers EA. The Indian Ocean in world history. Oxford: Oxford University Press; 2014. [Google Scholar]
- 97.Klaver M, Smeets RJ, Koornneef JM, Davies GR, Vroon PZ. Pb isotope analysis of ng size samples by TIMS equipped with a 1013Ω resistor using a 207Pb/204Pb double spike. Journal of Analytical Atomic Spectrometry. 2016;31(1):171–8. 10.1039/C5JA00130G [DOI] [Google Scholar]
- 98.Bowen GJ. Isoscapes: spatial pattern in isotopic biogeochemistry. Annual Review of Earth and Planetary Sciences. 2010;38(1):161–87. 10.1146/annurev-earth-040809-152429 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.