Abstract
Purpose
We recently reported that corneal lymphatic vessels develop integrin alpha-9 (Itga-9)-positive valves during inflammatory lymphangiogenesis. The purpose of this study was to further investigate the role of Itga-9 in corneal lymphatic valve formation in vivo and lymphatic endothelial cell (LEC) functions in vitro.
Methods
Standard murine suture placement model was used to study the effect of Itga-9 blockade on lymphatic valve formation in vivo using Itga-9 neutralizing antibody. Whole-mount corneas were harvested for immunofluorescent microscopic analysis. Additionally, human LEC culture system was used to examine the effect of Itga-9 gene knockdown on cell functions using small interfering RNAs (siRNAs).
Results
Itga-9 blockade in vivo significantly reduced the number of lymphatic valves formed in the inflamed cornea. Moreover, Itga-9 gene knockdown in human LECs suppresses cell functions of proliferation, adhesion, migration, and tube formation.
Conclusions
Itga-9 is critically involved in corneal lymphatic valve formation. Further investigation of the Itga-9 pathway may provide novel strategies to treat lymphatic-related diseases occurring both inside and outside the eye.
Keywords: lymphatic valve, lymphangiogenesis, inflammation, integrin alpha-9
This study presents the first evidence showing that integrin α-9 (Itga-9) mediates inflammatory corneal lymphatic valve formation. Further investigation promises for divulging new therapies for lymphatic diseases.
The lymphatic network permeates most tissues and plays important functions in tissue fluid homeostasis and immune surveillance. A broad spectrum of diseases and conditions are associated with lymphatic dysfunction including inflammation, cancer metastasis, and transplant rejection.1–5 Despite the prevalence of diseases associated with the lymphatic system, there are still few treatments available for lymphatic disorders. It is therefore a field with an urgent demand for new therapeutic strategies.
With the discovery of several lymphatic-specific markers, such as lymphatic vessel endothelial hyaluronan receptor-1 (LYVE-1) and transcription factor prospero homeobox protein-1 (Prox-1), the field of lymphatic research has expanded rapidly in the last decade.6,7 There is increasing evidence pointing to the role of integrins in the lymphatic system though it is yet to be fully investigated.8 Integrins are a family of heterodimeric cell surface transmembrane glycoproteins involved in cell–cell and cell–matrix interactions.9–11 Several studies from us and other researchers have shown that integrin α5β1, α1β1, and α4β1 mediate inflammation- or tumor-associated lymphangiogenesis (LG, the formation of new lymphatic vessels).12–15 More recently, we have reported that corneal lymphatic vessels develop luminal valves as inflammatory LG proceeds, and these valves, consisting of endothelial layers, express integrin α9β1 (Itga-9), and function to direct lymph flow in inflamed corneas.16,17 However, the direct role of Itga-9 in corneal lymphatic valve formation and lymphatic endothelial cell (LEC) function still remain to be elucidated, which is the focus of this study. Answers to these questions are essential for developing new therapeutic strategies for lymphatic diseases.
In this paper, using in vivo murine model of corneal inflammatory LG with valve formation and in vitro human LEC culture system, we provide the first evidence showing that Itga-9 is critically involved in corneal lymphatic valve formation in vivo and LEC functions in vitro. Itga-9 blockade in vivo via neutralizing antibodies significantly suppresses lymphatic valve formation in inflamed cornea. Additionally, Itga-9 depletion in LECs via small interfering RNAs (siRNAs) halts critical cellular processes in vitro, such as proliferation, adhesion, migration, and tube formation. Taken together, this work reveals a critical role of Itga-9 in corneal lymphatic valve formation. The combined in vivo animal work and in vitro human cell research should provide highly translational information for future development of new therapeutic strategies to treat lymphatic disorders that occur both inside and outside the eye.
Methods
Animals
Six- to 8-week-old normal adult male BALB/c mice were purchased from Taconic Farms (Germantown, NY, USA). All mice were treated according to ARVO Statement for the Use of Animals in Ophthalmic and Vision Research and the protocols approved by the Animal Care and Use Committee of the institute. Mice were anesthetized using a mixture of ketamine, xylazine, and acepromazine (50-, 10-, and 1-mg/kg body weight, respectively) for each surgical procedure.
Induction of Corneal Lymphangiogenesis and Administration of Blocking Antibody
The suture placement model was used to induce corneal inflammatory LG with valve formation, as previously described.16 Briefly, three 11-0 nylon sutures (AROSurgical, Newport Beach, CA, USA) were placed into the corneal stroma without penetrating into the anterior chamber. Subsequently, mice were randomized to receive subconjunctival injections of either 6.4 μg hamster anti-mouse Itga-9 blocking antibody18 or isotype control hamster IgG (Jackson ImmunoResearch, West Grove, PA, USA) twice a week for 2 weeks beginning on day 0 after suturing. Experiments were repeated twice with a total of 10 mice in each group.
Immunofluorescent Microscopic Assay and Lymphatic Valve Quantification
The experiments were performed as previously reported.16,19 Briefly, whole-mount corneas were fixed in acetone for immunofluorescent staining. Samples were sequentially incubated with purified rabbit-anti-mouse LYVE-1 (Abcam, Cambridge, MA, USA) and goat-anti-mouse Itga-9 antibodies (R&D Systems, Minneapolis, MN, USA), which were visualized by FITC-conjugated donkey-anti-rabbit and Cy3-conjugated donkey-anti-goat secondary antibodies (Jackson ImmunoResearch), respectively. Samples were mounted with Vector Shield mounting medium (Vector Laboratories; Burlingame, CA, USA) and examined using a Zeiss LSM 710 AxioObserver Inverted Confocal microscope with ZEN Digital Imaging software (Carl Zeiss, Inc., Germany). Itga-9+ focal areas along the length of LYVE-1+ lymphatic vessels were identified as valves. The number of lymphatic valves per cornea was quantified and the percentage scores were obtained by normalizing to control groups where the scores were defined as being 100%.16,19
Lymphatic Endothelial Cell Culture and Immunocytofluorescent Microscopic Assay
The experiments were performed as previously described.13,19 Briefly, human neonatal microdermal LECs were purchased from Lonza (Walkersville, MD, USA) and maintained in EGM-2MV cell culture medium (Lonza) according to manufacturer's instructions. For LEC staining, cells were incubated with rabbit anti-human-Itga-9 antibody (Novus Biologicals, Littleton, CO, USA) and visualized by Cy3-conjugated donkey-anti-rabbit secondary antibody (Jackson ImmunoResearch). Samples were mounted with DAPI mounting medium (Vectashield; Vector Laboratories). Digital images were taken with a Zeiss Axioplan 2 epifluorescence microscope (Carl Zeiss, Inc.).
siRNA Transfection
The experiments were performed as previously reported.13,19 Custom-designed siRNA duplexes were synthesized by Qiagen (Valencia, CA, USA) against human Itga-9 mRNA (5′-AAG AAG AAA GTC GTA CTA TAG-3′). Scrambled siRNA control was purchased from Ambion (Austin, TX, USA). Transfections were carried out according to manufacturer's instructions with a transfection reagent (RNAiMax; Invitrogen, Carlsbad, CA, USA) and opti-MEM–reduced serum medium at 37°C in a 5% CO2 humidified air incubator.
Reverse Transcription and Real-Time PCR
The experiments were performed as previously described and based on the minimum information for publication of quantitative real-time PCR experiments (MIQE) guidelines.13,19,20 Total RNA was extracted and purified from LECs 48 hours after siRNA transfection with an RNAeasy mini-kit from Qiagen. Reverse transcription was performed using the SuperScript VILO cDNA synthesis kit from Invitrogen. Primer sequences were as follows: human Itga-9, forward 5′-CGG AAT CAT GTC TCC AAC CT-3′ and 5′-TCT CTG CAC CAC CAG ATG AG-3′; human β-actin, forward 5′-GAT CTG GCA CCA CAC CTT CT-3′, reverse 5′-GGG GTG TTG AAG GTC TCA AA-3.′ Real-time PCR was performed using SsoFast EvaGreen with the CFX96 sequence detection system (Bio-Rad Laboratories, Inc., Hercules, CA, USA). Relative expression of the Itga-9 gene was calculated from the Δ-Ct (threshold cycle) of the targeted gene normalized to the Δ-Ct of actin.
Flow Cytometry
Forty-eight hours after LEC transfection, LEC viability was evaluated by Guava ViaCount assay (Millipore, Billerica, MA, USA). Data were acquired using a Guava easyCyte HT cytometer (Millipore) and the InCyte 2.6 software (Millipore). Viable and nonviable cells were assessed by the differential permeability of two DNA-binding dyes in the Guava ViaCount reagent. One dye stains all nucleated cells (red fluorescent channel), while the other dye stains nonviable cells (yellow fluorescent channel). Live cells were gated (R1) and the percentage over the total population was calculated. Experiments were repeated three times and the percentage scores were normalized to the control condition where the scores were defined as being 100%.
Proliferation Assay
As described previously,13,19 LECs were seeded into 96-well plates. Forty-eight hours following siRNA transfection with either Itga-9 or scrambled siRNA, cells were subjected to a MTS proliferation assay from Promega (Madison, WI, USA) according to the manufacturer's protocol. Assays were performed in triplicate and repeated three times.
Adhesion Assay
As described previously,13 forty-eight hours following siRNA transfection with either Itga-9 or scrambled siRNA, LECs were seeded into 96-well plates coated with fibronectin. Plates were incubated for 1 hour at 37°C, washed twice and incubated with calcein (1 μg/mL) in HBSS for 30 minutes at room temperature. Plates were washed with PBS and fluorescence intensity was measured with a microplate reader (Spectramax M5e; Molecular Devices, Sunnyvale, CA, USA). Assays were performed in triplicate and repeated three times.
Migration Assay
Forty-eight hours following siRNA transfection with either Itga-9 or scrambled siRNA, a 10-μL pipette tip was used to create linear wounds within LEC monolayers. Differential interference contrast (DIC) phase images were taken at 0, 24, and 72 hours post scratch to visualize wound closure in cell monolayers. Scratches were analyzed for wound healing using the TScratch program (Tobias Gebäck and Martin Schulz, ETH Zürich). Cells were stained using TRITC-conjugated phalloidin (Millipore, Temecula, CA, USA) for visualization of cell migration during wound closure.
Tube Formation Assay
As described previously,13,19 48 hours following siRNA transfection with either Itga-9 or scrambled siRNA, LECs were seeded (2 × 104 cells/well) onto 96-well plates containing solidified Matrigel (BD Biosciences, San Jose, CA, USA). Tube formation was imaged at 24 hours post seeding using a Zeiss Axio Observer A1 inverted microscope (Carl Zeiss, Inc.). Phase images of tubes were taken (Qcapture; Qimaging, Surrey, BC, Canada) and total tube length per well was calculated using ImageJ software (http://imagej.nih.gov/ij/; provided in the public domain by the National Institutes of Health, Bethesda, MD, USA). Assays were performed in triplicate and repeated at least three times.
Statistical Analysis
The results are reported as mean ± SEM and Student t-test was used for the determination of significance levels between different groups using Prism software (GraphPad, La Jolla, CA, USA). The differences were considered statistically significant when P < 0.05.
Results
Effect of Itga-9 Blockade on Corneal Lymphatic Valve Formation In Vivo
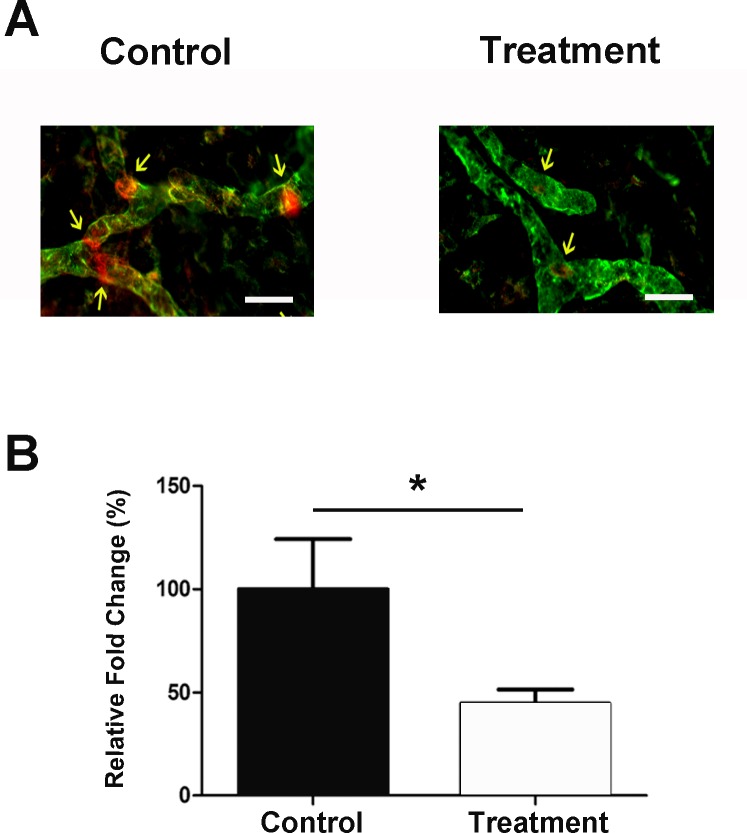
We first set out to study the effect of Itag-9 blockade on corneal lymphatic valve formation in inflammatory LG. The standard suture placement model was used to evaluate the effect of subconjunctival delivery of Itga-9 neutralizing antibody on the number of valves formed in inflamed corneas with LG. As presented in Figure 1A, following treatment with the Itga-9 blocking antibody, corneal lymphatic vessels contained significantly fewer valves compared with the control condition. Summarized data from repetitive experiments are shown in Figure 1B (*P < 0.05).
Figure 1.
Itga-9 blockade inhibits corneal valve formation in vivo. (A) Representative immunofluorescent microscopic images showing significantly fewer valves in the Itga-9 blocking antibody treated cornea, as compared with control isotype treated sample. Red: Itga-9; Green: LYVE-1. Scale bars, 50 μm. (B) Summarized data quantifying valve formation. *P < 0.05.
Itga-9 Expression and Depletion in LECs
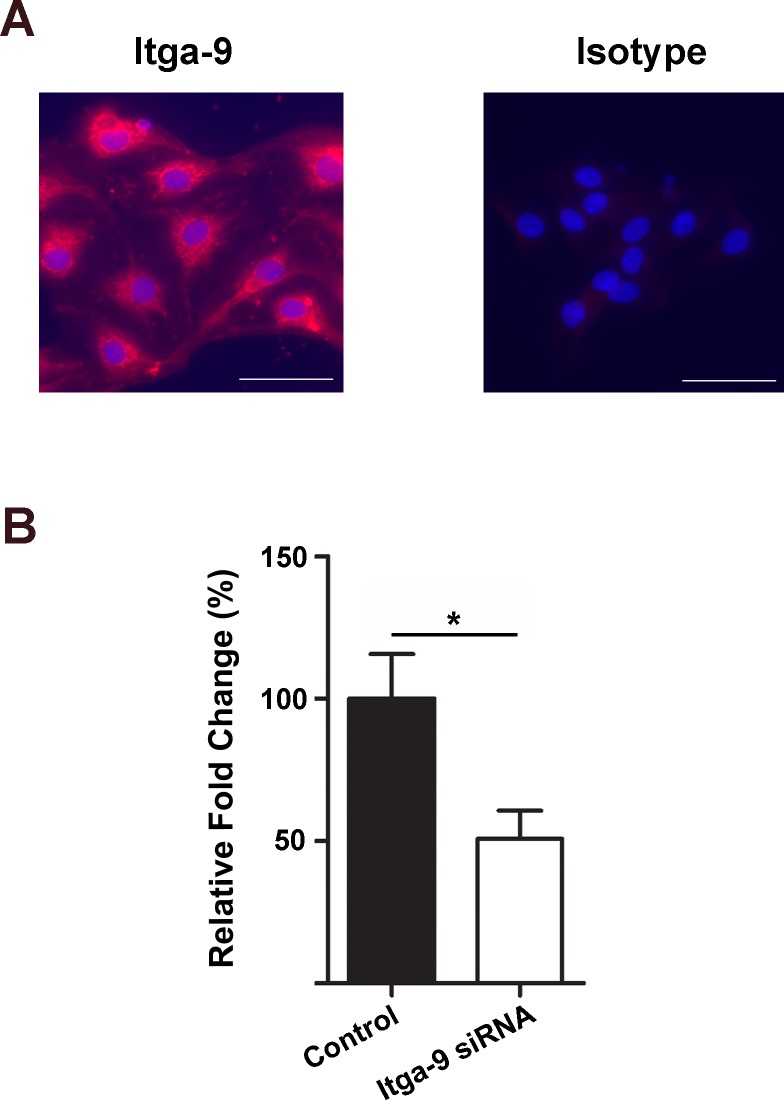
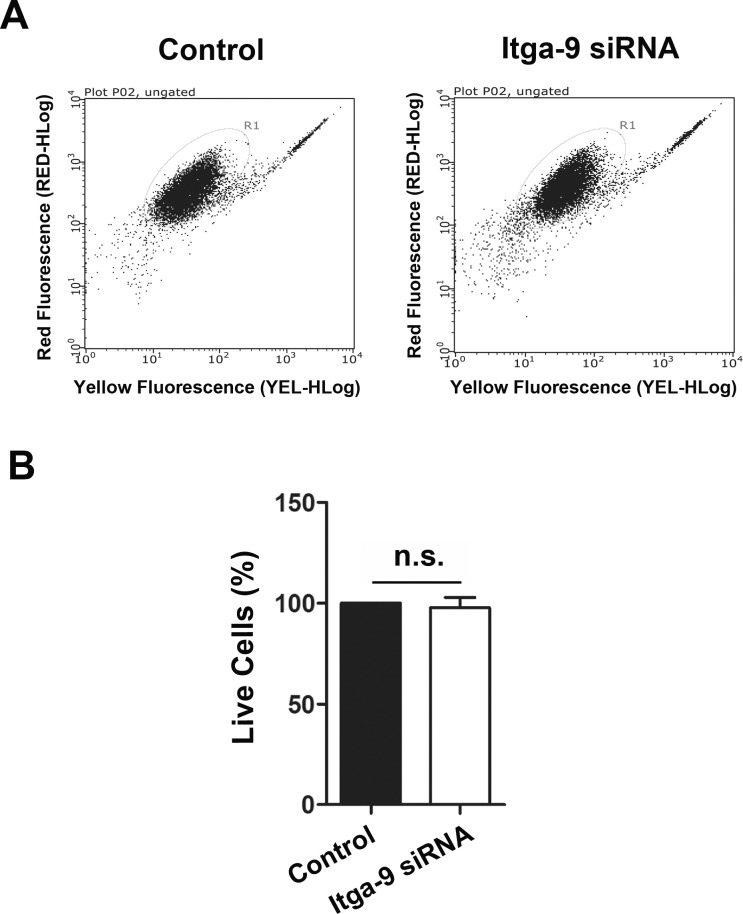
Lymphatic valves are made of the extracellular matrix sandwiched by two layers of endothelial cells. To further investigate the specific role of Itga-9 in lymphatic endothelial cells in vitro, we next employed the human LEC culture system. As shown in Figure 2A, we first confirmed the expression of Itga-9 on these cells by the immunocytofluorescent microscopic analysis. We then assessed whether Itga-9 expression in these LECs can be downregulated by a siRNA-mediated gene silencing approach (Fig. 2B). Our data from real-time PCR analysis showed that following the transfection with Itga-9 siRNA, the Itga-9 expression in LECs was significantly reduced by around 50%. We also confirmed that the transfection had no direct effect on LEC viability by flow cytometric analysis with the Guava ViaCount assay, as shown in Figure 3. Together, these data indicate that transfection with siRNA can be used to study the functional role of Itga-9 in LECs in vitro.
Figure 2.
Expression and depletion of Itga-9 in human LECs. (A) Representative immunocytofluorescent microscopic images showing Itga-9 expression in human LECs. Red: Itga-9; Blue: DAPI. Scale bars, 50 μm. (B) Real-time PCR analysis showing Itga-9 gene knockdown with Itga-9 siRNA, compared with scrambled siRNA. *P < 0.05.
Figure 3.
Itga-9 knockdown has no effect on LEC viability. (A) Flow cytometric analysis with the Guava ViaCount assay showing no significant difference in cell viability between the control and Itga-9 siRNA transfected cells at 48 hours posttransfection. Summarized data from repetitive experiments are presented in panel B. n.s., not significant.
Effect of Itga-9 Depletion on Proliferation of LECs
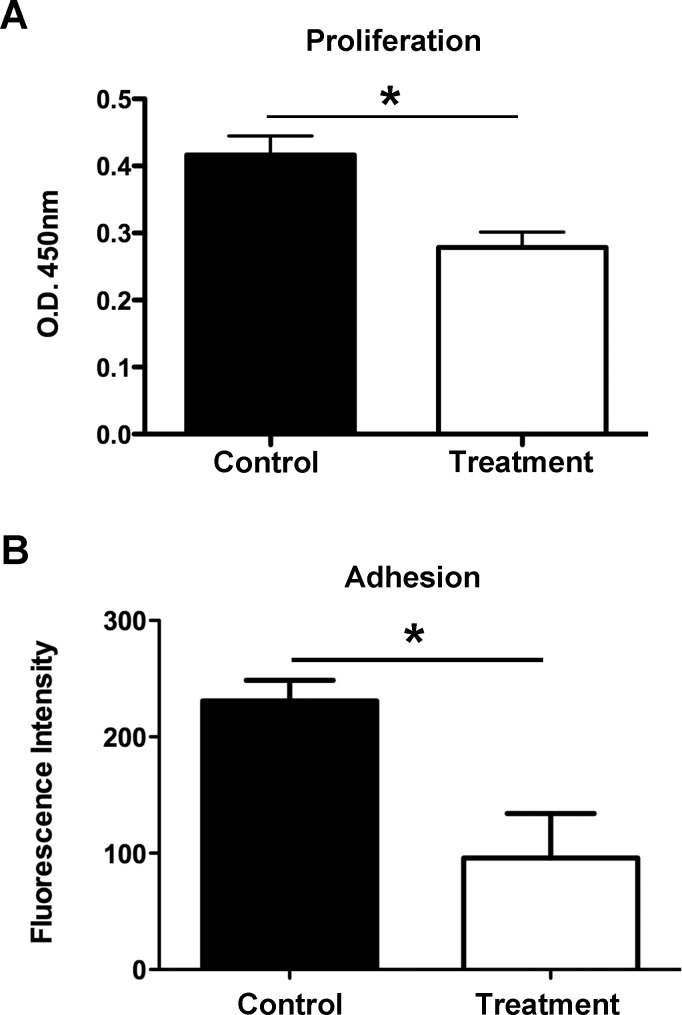
We next delved deeper and assessed the role of Itga-9 in the LEC function of proliferation using the siRNA approach. Forty-eight hours after siRNA transfection with either Itga-9 or scrambled siRNA, LECs were subjected to a MTS proliferation assay, as reported previously.19 Our data, as presented in Figure 4A, showed that Itga-9 siRNA treatment led to a significant reduction in LEC proliferation in comparison with control condition (*P < 0.05). This result suggests that Itga-9 engagement regulates LEC proliferation.
Figure 4.
Itga-9 knockdown inhibits LEC functions of proliferation and adhesion. (A) Summarized data showing significant suppression of LEC proliferation following transfection with Itga-9 siRNA using MTS proliferation assay. *P < 0.05. (B) Summarized data showing significant reduction of LEC adhesion to fibronectin after transfection with Itga-9 siRNA. *P < 0.05.
Effect of Itga-9 Depletion on Adhesion of LECs
Integrins are essential for cellular adhesion to extracellular proteins. To investigate whether Itga-9 is important for LEC adhesion, cells were transfected with Itga-9 or scrambled siRNA and subjected to an adhesion assay on wells coated with Itga-9 ligand, fibronectin. As presented in Figure 4B, Itga-9 depletion in LECs resulted in a significant reduction of cell adhesion to fibronectin (*P < 0.05), indicating that Itga-9 is also crucial for LEC interaction with the extracellular matrix.
Effect of Itga-9 Depletion on Migration of LECs
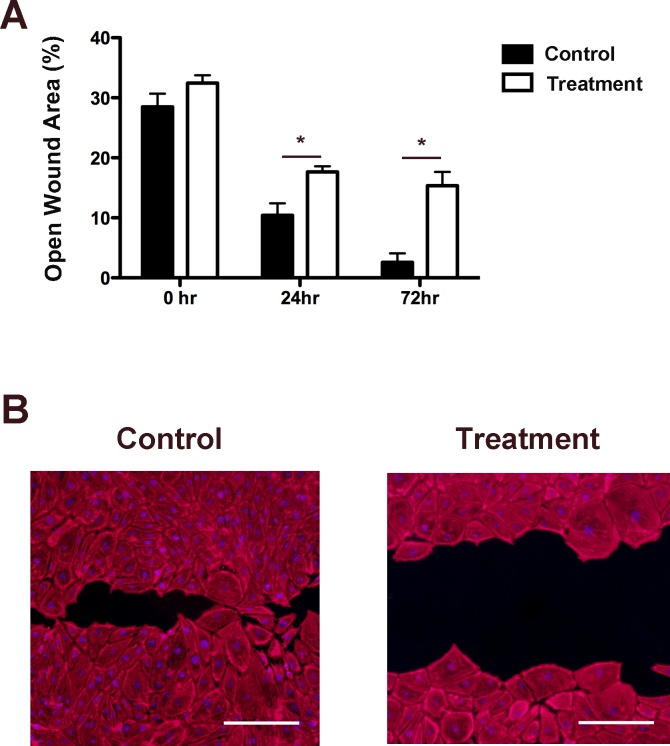
Cell migration is a process mainly dependent on the interaction between integrins and extracellular matrix proteins, we next investigated the importance of Itga-9 on LEC migration using a scratch assay of wound healing. Following siRNA transfection with either Itga-9 or scrambled siRNA, LECs were monitored over 72 hours. As shown in Figure 5A, LECs transfected with Itga-9 siRNA showed larger open wound area than control cells transfected with scramble siRNA at both time points studied, 24 and 72 hours after wounding (*P < 0.05). Moreover, the inhibition effect of Itga-9 on wound healing was greater at the later time point when cells in the control group had grown over the majority of the field of view (Fig. 5B). These results clearly indicate that Itga-9 is essential for LEC migration.
Figure 5.
Itga-9 knockdown inhibits LEC migration. (A) Summarized data showing significant reduction in LEC migration over a 72-hour period using scratch assay. *P < 0.05. (B) Representative images of cell migration at 72 hours. Scale bars, 100 μm. Red: TRITC-conjugated phalloidin.
Effect of Itga-9 Depletion on Tube Formation of LECs
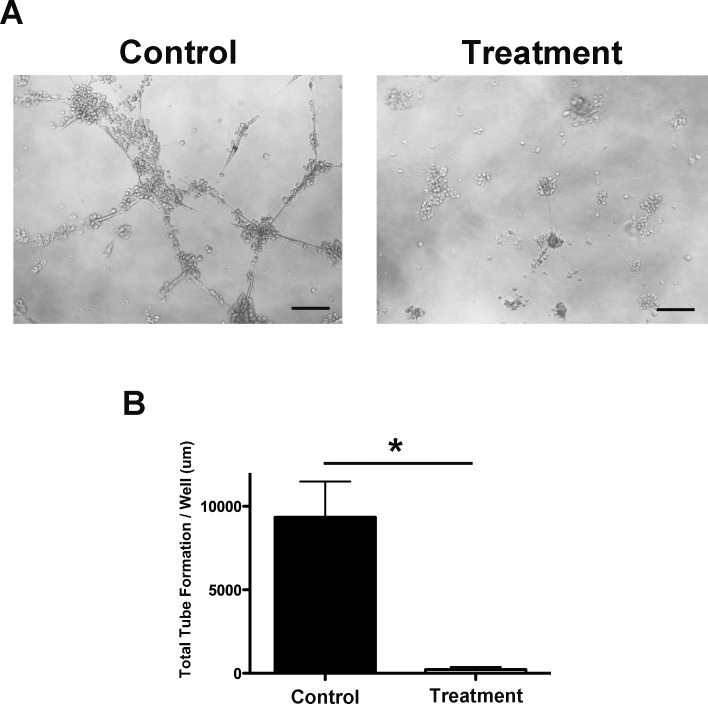
We also investigated the effect of Itga-9 depletion on the ability of LECs to organize into capillary-type tubes using a three-dimensional culture system. Forty-eight hours after transfection with either Itga-9 or scramble siRNA, LECs were seeded on solidified basement membrane matrix, Matrigel, to allow for tube formation. Results from this assay showed that Itga-9 depletion in LECs resulted in a dramatic reduction in total tube length, as demonstrated in Figure 6A. Summarized data from repetitive experiments are presented in Figure 6B (*P < 0.05). These results imply a critical role of Itga-9 in LEC organization into macroscale structures.
Figure 6.
Itga-9 knockdown inhibits LEC tube formation. (A) Representative microscopic images showing significant reduction of tube formation on Matrigel. Scale bars, 500 μm. (B) Summarized data on total tube length. *P < 0.05.
Discussion
In the present study, we have demonstrated the essential role of Itga-9 in corneal lymphatic valve formation and LECs using both in vivo animal and in vitro human cell models. We are able to draw two interdependent conclusions from this study. Firstly, lymphatic valve formation in vivo is tightly regulated by Itga-9, as shown through the experiments using Itga-9 blocking antibody. Secondly, Itga-9 is critically involved in LEC processes in vitro, such as proliferation, migration, and adhesion, as revealed by the siRNA knockdown approach. While the in vivo data suggest a new strategy to interfere with lymphatic valve formation in inflamed corneas, the in vitro results provide more details on possible mechanisms underlying the role of Itga-9 in various functions of LECs, which together orchestrate the process of valve formation.
Cell anchorage to extracellular matrix is primarily mediated through integrin-based linkages, creating a strong connection between the intracellular and extracellular environments.11 Integrins have specificity in extracellular matrix proteins depending on the α and β subunits. One of the main ligands for Itga-9 is fibronectin, as reported in a human cancer cell model.21 In this study with human LECs, using Itga-9 targeting siRNA, we are able to show that upon depletion of Itga-9, LEC adhesion to fibronectin is significantly inhibited. Furthermore, at the core of cell migration are cell–matrix adhesion complexes, which are composed principally of integrins that link extracellular matrix to intracellular actin.22 To identify the role of Itga-9 in LEC migration, we investigated the effect of Itga-9 loss on cell migration through a wound healing scratch assay. We are able to show that LECs lacking Itga-9 never fully migrates over the scratch wound in comparison to control LECs, which completely grows over the scratch surface over the course of time. Collectively with the proliferation data, the significance of Itga-9 in processes associated with LEC linkage to the extracellular matrix becomes unquestionable. Further investigation on these basic processes may divulge new targets for therapeutic intervention.
Moreover, our in vitro data demonstrate that LECs with Itga-9 depletion lost their ability to form capillary type tubes in culture. However, our in vivo treatment regimen with the Itga-9 blocking antibody shows no significant reduction of lymphatic vessels but valves. Nevertheless, this discrepancy phenomenon is also observed in a previous study on lymphatic valve formation in nonocular tissues during development. Particularly, Bazigou et al.23 examined lymphatic valve formation in Itga-9 knockout mice and reported reduced number of valves, but not lymphatic vessels, in Itga-9 homozygotes. One possible explanation for our in vivo data with the neutralizing antibody is that Itga-9 is more highly expressed on corneal lymphatic valves than vessels,16,17 which may render the valves more sensitive to the anti–Itga-9 treatment. This warrants further investigation. Taken together, these results designate Itga-9 as an ideal target to manipulate lymphatic valve formation without disturbing the vessels. It is also suggested that a combined valve/vessel targeting approach is necessary when an intervention of both valves and vessels are required in disease management, which demands further research as well. Because the lymphatic vessels constitute the afferent arm of the immune reflex arc,1 and their luminal valves function to direct lymph (containing immune cells) flow inside the vessels, it is speculated that by manipulating the number of vessels and/or valves formed inside the corneas, we could possibly interfere with the degree of the immune responses and the progression and resolution of the diseases at various stages. In summary, this study not only offers new insights into corneal valve formation but may also shed some light on future development of new Itga-9–based therapies for the broad spectrum of lymphatic diseases occurring inside or outside the eye.
Acknowledgments
Supported in part by grants from the National Institutes of Health (Bethesda, MD, USA), Department of Defense (Arlington, VA, USA), and University of California at Berkeley (LC; Berkeley, CA, USA).
Disclosure: E. Altiok, None; T. Ecoiffier, None; R. Sessa, None; D. Yuen, None; S. Grimaldo, None; C. Tran, None; D. Li, None; M. Rosner, None; N. Lee, None; T. Uede, None; L. Chen, None
References
- 1. Chen L. Ocular lymphatics: state-of-the-art review. Lymphology. 2009; 42: 66–76. [PMC free article] [PubMed] [Google Scholar]
- 2. Christiansen A,, Detmar M. Lymphangiogenesis and cancer. Genes Cancer. 2011; 2: 1146–1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Alitalo K. The lymphatic vasculature in disease. Nat Med. 2011; 17: 1371–1380. [DOI] [PubMed] [Google Scholar]
- 4. Zhang H,, Grimaldo S,, Yuen D,, Chen L. Combined blockade of VEGFR-3 and VLA-1 markedly promotes high-risk corneal transplant survival. Invest Ophthalmol Vis Sci. 2011; 52: 6529–6535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dietrich T,, Bock F,, Yuen D,, et al. Cutting edge: lymphatic vessels, not blood vessels, primarily mediate immune rejections after transplantation. J Immunol. 2010; 184: 535–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Banerji S,, Ni J,, Wang SX,, et al. LYVE-1, a new homologue of the CD44 glycoprotein, is a lymph-specific receptor for hyaluronan. J Cell Biol. 1999; 144: 789–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wigle JT,, Oliver, G. Prox1 function is required for the development of the murine lymphatic system. Cell. 1999; 98: 769–778. [DOI] [PubMed] [Google Scholar]
- 8. Avraamides CJ,, Garmy-Susini B,, Varner JA. Integrins in angiogenesis and lymphangiogenesis. Nat Rev Cancer. 2008; 8: 604–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Berman AE,, Kozlova NI,, Morozevich GE. Integrins: structure and signaling. Biochemistry Biokhimiia. 2003; 68: 1284–1299. [DOI] [PubMed] [Google Scholar]
- 10. Stepp MA. Corneal integrins and their functions. Exp Eye Res. 2006; 83: 3–15. [DOI] [PubMed] [Google Scholar]
- 11. Giancotti FG. Integrin signaling. Science. 1999; 285: 1028–1033. [DOI] [PubMed] [Google Scholar]
- 12. Dietrich T,, Onderka J,, Bock F,, et al. Inhibition of inflammatory lymphangiogenesis by integrin α5 blockade. Am J Pathol. 2007; 171: 361–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Grimaldo S,, Yuen D,, Ecoiffier T,, Chen L. Very late antigen-1 mediates corneal lymphangiogenesis. Invest Ophthalmol Vis Sci. 2011; 52: 4808–4812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Garmy-Susini B,, Makale M,, Fuster M,, Varner JA. Methods to study lymphatic vessel integrins. Methods Enzymol. 2007; 426: 415–438. [DOI] [PubMed] [Google Scholar]
- 15. Chen L,, Huq S,, Gardner H,, de Fougerolles AR,, Barabino S,, Dana MR. Very late antigen 1 blockade markedly promotes survival of corneal allografts. Arch Ophthalmol. 2007; 125: 783–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Truong T,, Altiok E,, Yuen D,, Ecoiffier T,, Chen L. Novel characterization of lymphatic valve formation during corneal inflammation. PloS One. 2011; 6: e21918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Truong T,, Huang E,, Yuen D,, Chen L. Corneal lymphatic valve formation in relation to lymphangiogenesis. Invest Ophthalmol Vis Sci. 2014; 55: 1876–1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kanayama M,, Kurotaki D,, Morimoto J,, et al. Alpha9 integrin and its ligands constitute critical joint microenvironments for development of autoimmune arthritis. J Immunol. 2009; 182: 8015–8025. [DOI] [PubMed] [Google Scholar]
- 19. Yuen D,, Grimaldo S,, Sessa R,, et al. Role of angiopoietin-2 in corneal lymphangiogenesis. Invest Ophthalmol Vis Sci. 2014; 55: 3320–3327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bustin SA,, Benes V,, Garson JA,, et al. The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clin Chem. 2009; 55: 611–622. [DOI] [PubMed] [Google Scholar]
- 21. Liao YF,, Gotwals PJ,, Koteliansky VE,, Sheppard D,, Van De Water L. The EIIIA segment of fibronectin is a ligand for integrins alpha 9beta 1 and alpha 4beta 1 providing a novel mechanism for regulating cell adhesion by alternative splicing. J Biol Chem. 2002; 277: 14467–14474. [DOI] [PubMed] [Google Scholar]
- 22. Lock JG,, Wehrle-Haller B,, Stromblad S. Cell-matrix adhesion complexes: master control machinery of cell migration. Semin Cancer Biol. 2008; 18: 65–76. [DOI] [PubMed] [Google Scholar]
- 23. Bazigou E,, Xie S,, Chen C,, et al. Integrin-alpha9 is required for fibronectin matrix assembly during lymphatic valve morphogenesis. Dev Cell. 2009; 17: 175–186. [DOI] [PMC free article] [PubMed] [Google Scholar]