Abstract
Lyme borreliosis is caused by the spirochete Borrelia burgdorferi sensu lato, a fastidious bacterium that replicates slowly and requires special conditions to grow in the laboratory. Borrelia isolation from clinical material is a golden standard for microbiological diagnosis of borrelial infection. Important factors that affect in vitro borrelia growth are temperature of incubation and number of borrelia cells in the sample. The aim of the study was to assess the influence of temperature on borrelia growth and survival by evaluation and comparison of growth of 31 different borrelia strains at five different temperatures and to determine the influence of different inoculums on borrelia growth at different temperatures. Borreliae were cultured in the MKP medium; the initial and final number of spirochetes was determined by dark field microscopy using Neubauer counting chamber. The growth of borrelia was defined as final number of cells/mL after three days of incubation. For all three Borrelia species, the best growth was found at 33°C, followed by 37, 28, and 23°C, while no growth was detected at 4°C (P<0.05). The growth of B. afzelii species was weaker in comparison to the other two species at 23, 28, 33 and 37°C (P<0.05), respectively. There was no statistically significant difference between the growth of B. garinii and B. burgdorferi sensu stricto at 28, 33, and 37°C (P>0.05), respectively. Inoculum had statistically significant influence on growth of all three Borrelia species at all tested temperatures except at 4°C.
Introduction
Lyme borreliosis is a multisystem disease caused by the spirochetes of the Borrelia burgdorferi sensu lato complex that are transmitted by the hard ticks of the Ixodes species complex [1, 2].
In Europe, at least four Borrelia species (B. afzelii, B. garinii, B. burgdorferi sensu stricto, B. spielmanii) can cause disease in humans, but some other species have also been reported to be a rare (B. bissettii and B. lusitaniae) or potential (B. valaisiana) causes of human disease. In contrast, B. burgdorferi sensu stricto is the only known species that cause human disease in North America [1, 3, 4].
Spirochetes can be isolated from skin, blood, cerebrospinal fluid (CSF), and other clinical materials during early as well as late Lyme borreliosis [1, 5, 6]. The clinical material for isolation should be transported to the laboratory as soon as possible; if feasible, specimens such as skin and CSF, must be inoculated immediately into the culture medium. Isolation, as well as cultivation are demanding procedures that are performed in a limited number of laboratories [6, 7].
Borreliae are fastidious, slow-growing, and biochemically inactive bacteria that need special care and optimal conditions for growth such as anaerobic environment and temperature between 30 and 34°C [8, 9]. Some borrelia strains grow well also at higher temperatures (35–39°C), but temperature ≥ 40°C substantially reduce or prevent their growth [10–13]. Generally, low temperatures (room or lower) are better tolerated than high temperatures (37–42°C) [9].
The in vitro generation time of borrelia ranges from 7 to 20 hours; it is influenced by available nutrients, conditions of cultivation and adaptation of borrelia to the artificial medium [9] Cultivation from clinical material may last up to 12 weeks, which is much longer than for the majority of other human bacterial pathogens [5, 7, 14]. Borrelia requires complex liquid media for in vitro cultivation, due to inability to synthesize any amino acids, nucleosides, nucleotides, fatty acids, and severalother cellular building blocks [15]. For a routine laboratory work, modified Kelly-Pettenkofer (MKP), Barbour-Stoenner-Kelly II (BSK-II) and commercially available BSK-H (Sigma, USA) are the most commonly used media [10, 16, 17].
In addition, temperature during clinical material transportation from patient to the laboratory is important for borrelia survival. Room temperature was reported as suitable for transport of samples infected with borrelia during the period from one to 11 days, while refrigerator temperature (5°C) was described as inadequate [18–20].
The aim of the study was to assess and compare the growth of B. afzelii, B. garinii, and B. burgdorferi sensu stricto strains at five different temperatures (4, 23, 28, 33, and 37°C) and to examine the influence of different inoculum on the growth at different temperatures.
Materials and Methods
Borrelia strains
Thirty-one strains, 10 B. afzelii, 10 B. garinii, and 11 B. burgdorferi sensu stricto were randomly selected from the collection of strains of the Institute of Microbiology and Immunology, Faculty of Medicine, University of Ljubljana, Slovenia. Isolates were obtained from 31 patients diagnosed with different clinical manifestations of Lyme borreliosis at the Department of Infection Diseases of the University Medical Center Ljubljana, Slovenia. Borrelia species of the isolated strains was determined by MluI-restriction fragment length polymorphism (Mlu-RFLP) as described previously [5, 21, 22]. Data regarding the origin of strains are listed in Table 1. Stock cultures of these low passage isolates had been stored at –80°C; for the study, we inoculated and cultured them in the MKP medium [5, 23].
Table 1. Origin of Borrelia afzelii, Borrelia garinii, and Borrelia burgdorferi sensu stricto strains analyzed in the study.
| Borrelia afzelii | Borrelia garinii | Borrelia burgdorferi sensu sticto | |||
|---|---|---|---|---|---|
| Strain number | Source | Strain number | Source | Strain number | Source |
| 941/08 | Skin | 468/08 | Skin | 2249/07 | Skin |
| 819/08 | Skin | 1881/08 | Skin | 851/97 | Skin |
| 1131/08 | Skin | 1291/06 | Skin | 2093/06 | Skin |
| 1539/08 | Skin | 2507/06 | Skin | 1506/08 | Skin |
| 2357/08 | Skin | 531/08 | CSF | 2130/06 | Skin |
| 999/09 | Skin | 553/08 | CSF | 2213/06 | Skin |
| 884/09 | CSF | 892/08 | CSF | 1963/06 | Skin |
| 2498/08 | CSF | 932/09 | CSF | 2157/06 | Skin |
| 2588/03 | Blood | 13745/05 | CSF | 1442/99 | CSF |
| 1170/09 | Blood | 1459/09 | CSF | 953/03 | Blood |
| 2092/06 | Blood | ||||
CSF = cerebrospinal fluid.
Cultivation and numbering of spirochetes
The study was carried out in aseptic conditions provided by laminar flow box in order to reduce the risk of contamination. An aliquot of frozen spirochetes was thawed at room temperature, transferred into starting tubes with 7 mL of MKP medium and incubated at 33°C. Borrelia strains were allowed to multiply to cell density of about 106/mL (4-day-old cultures, with a cell motility >95%) that was determined by dark filed microscopy using Neubauer counting chamber.
The study was conducted using starting tubes with borrelia cells/mL in the range: 1x105-2.5x107 for B. afzelii, 3x105-5x107 for B.garinii, and 8.5x105-4.8x107 for B. burgdorferi sensu stricto strains. Within the range of individual species different concentrations of borrelia cells/mL were distinguished and further processed; generally, these concentrations were reported as low and high. Experiment with individual borrelia strain at particular temperature was repeated at least 10 times with low and high concentration, respectively.
After establishing the exact number of cells/mL in starting tubes, we inoculated aliquots of 50 μL into five new tubes with 7 mL of fresh MKP medium and determined the initial number of cells/mL in inoculated tubes. The tubes were incubated at five different temperatures: 4, 23, 28, 33 and 37°C. After 3 days of incubation we checked the number of spirochetes (final number). The initial and the final number of spirochetes were determined by dark filed microscopy using Neubauer counting chamber. Growth was defined as an increase in the number of cells/mL comparing the initial number and the number of cells/mL after three days of incubation (final number).
Statistical analysis
All statistical tests were performed using SigmaPlot 11.0 (Systat. Software Inc., Richmond, CA, USA). All graphics were created using MS Excel 2007.
Correlation between the initial and final number of borrelia cells/mL at particular temperature was assessed using Spearman’s rho correlation coefficient. A linear regression analysis was applied to evaluate the influence of initial number of cells/mL on the growth of borrelia.
Kruskal-Wallis and Mann-Whitney tests were used to compare growth of Borrelia species at five and two different temperatures, respectively. Values were expressed as median, 25th percentile (P25) and 75th percentile (P75).
P values less than 0.05 were considered statistically significant to all statistical analyses.
Ethics Statement
The study was approved by the National Medical Ethics Committee of the Republic of Slovenia (No: 35p/10/12).
Results
Results of the present study are based on the growth analysis of 31 different borrelia strains at five different temperatures.
Comparison of the influence of the initial number (inoculum) on Borrelia growth
Using Spearman’s rho correlation coefficient we found statistically significant positive correlation between the initial and final number for all three Borrelia species cultivated at 23, 28, 33 and 37°C, respectively, but not for the strains cultivated at 4°C (Table 2).
Table 2. Relationship between the initial and final number of borrelia cells/mL (Borrelia afzelii, Borrelia garinii, and Borrelia burgdorferi sensu stricto) assessed by Spearman’s rho correlation coefficient.
| Temperature | Initial/final number* of B. afzelii strains/mL (rho) | P value |
| 4°C | 0.105 | 0.291 |
| 23°C | 0.514 | < 0.001 |
| 28°C | 0.381 | < 0.001 |
| 33°C | 0.551 | < 0.001 |
| 37°C | 0.110 | 0.019 |
| Temperature | Initial/final number* of B. garinii strains/mL (rho) | P value |
| 4°C | 0.154 | 0.127 |
| 23°C | 0.329 | 0.001 |
| 28°C | 0.294 | 0.003 |
| 33°C | 0.454 | < 0.001 |
| 37°C | 0.269 | 0.007 |
| Temperature | Initial/final number* of B. burgdorfer isensu stricto/mL (rho) | P value |
| 4°C | 0.052 | 0.585 |
| 23°C | 0.434 | < 0.001 |
| 28°C | 0.446 | < 0.001 |
| 33°C | 0.460 | < 0.001 |
| 37°C | 0.330 | < 0.001 |
*Growth was defined as an increase of the number of cells comparing the initial and the final number of cells/mL after three days of incubation at individual temperature (4, 23, 28, 33, and 37°C) presenting as Spearman’s rho correlation coefficient.
Results of linear regression showed that the initial number of cells/mL had statistically significant influence on the growth of all three Borrelia species at 23, 28, 33 and 37°C, but not at 4°C. With the initial number of B. afzelii cells/mL 0.8% [adjusted (R2 0.008)], 23% (R2 0.230), 14.8% (R2 0.148), 34.5% (R2 0.345), and 12% (R2 0.12) of growth at 4, 23, 28, 33, and 37, respectively, can be explained. The corresponding findings for B. garinii were 0.2% [adjusted (R2 0.002)], 9.7% (R2 0.097), 6.8% (R2 0.068), 27.4% (R2 0.274), and 7.8% (R2 0.078), respectively; and for B. burgdorferi sensu stricto 0.4% [adjusted (R2 0.004)], 12.7% (R2 0.127), 15% (R2 0.150), 10.7% (R2 0.107), and 3% (R2 0.03), respectively.
Moreover, positive β coefficient indicated a statistically positive relationship between the initial and final number of cells/mL for all three Borrelia species growing at 23, 28, 33 and 37°C, but not at 4°C. Results are presented in Table 3.
Table 3. Influence of the initial number of cells/mL (inoculum) on growth of Borrelia afzelii, Borrelia garinii, and Borrelia burgdorferi sensu stricto strains at five different temperatures (4, 23, 28, 33, and 37°C).
| Species | Dependent variable* Growth** at | F-value | P-value | AdjustedR2 | Standardized Coefficient (β) | P-value |
|---|---|---|---|---|---|---|
| B. afzelii | 4°C | 0.16 | 0.685 | 0.008 | 0.040 | 0.685 |
| 23°C | 31.15 | <0.001 | 0.230 | 0.487 | <0.001 | |
| 28°C | 18.48 | <0.001 | 0.148 | 0.395 | <0.001 | |
| 33°C | 50.51 | <0.001 | 0.345 | 0.593 | <0.001 | |
| 37°C | 12.45 | 0.012 | 0.12 | 0.289 | 0.012 | |
| B. garinii | 4°C | 1.165 | 0.281 | 0.002 | 0.109 | 0.281 |
| 23°C | 11.64 | 0.001 | 0.097 | 0.326 | 0.001 | |
| 28°C | 8.34 | 0.004 | 0.068 | 0.278 | 0.004 | |
| 33°C | 35.76 | <0.001 | 0.274 | 0.531 | <0.001 | |
| 37°C | 9.27 | 0.003 | 0.078 | 0.295 | 0.003 | |
| B.burgdorferi sensu stricto | 4°C | 0.53 | 0.464 | 0.004 | 0.070 | 0.464 |
| 23°C | 17.02 | < 0.001 | 0.127 | 0.368 | < 0.001 | |
| 28°C | 20.46 | < 0.001 | 0.150 | 0.398 | < 0.001 | |
| 33°C | 13.74 | < 0.001 | 0.107 | 0.340 | < 0.001 | |
| 37°C | 4.36 | 0.039 | 0.03 | 0.198 | 0.039 |
*Dependent variable is the final number of borrelia cells/mL. Independent variable is the initial number of borrelia cells/mL at particular temperature.
**Growth was defined as an increase of the number of cells comparing the initial and final number of cells/mL after three days of incubation at five different temperatures (4, 23, 28, 33, and 37°C)
Growth of strains belonging to individual Borrelia species at different temperatures
Growth of individual Borrelia species at particular temperature is shown on Figs 1–3.
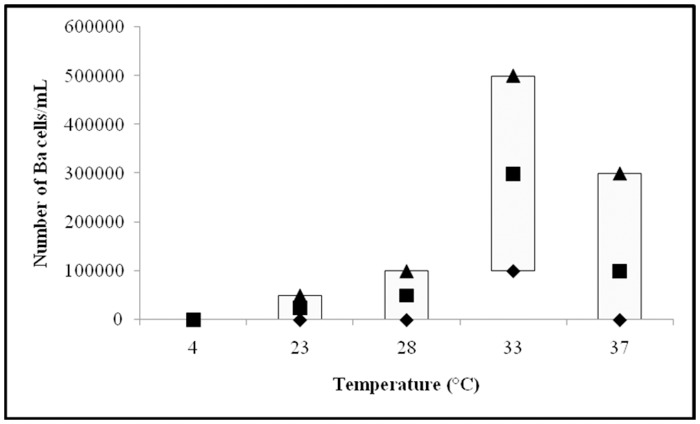
Fig 1. Growth of Borrelia afzelii (Ba) strains at 4, 23, 28, 33, and 37°C. Median (■), P25 (♦) and P75 (▲) are expressed as number of cells/mL.
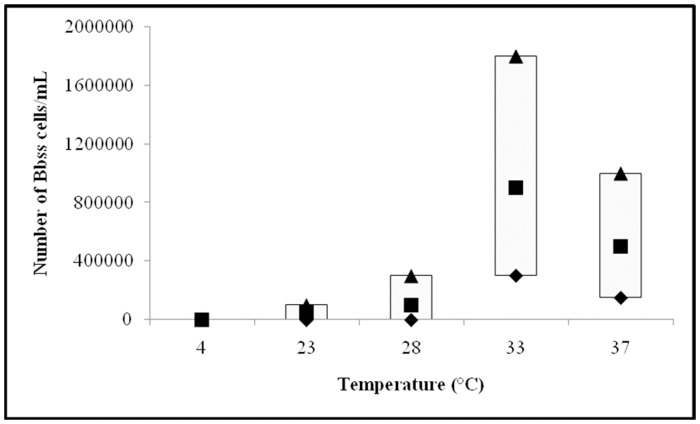
Fig 3. Growth of Borrelia burgdorferi sensu stricto (Bbss) strains at 4, 23, 28, 33 and 37°C. Median (■), P25 (♦) and P75 (▲) are expressed as number of cells/mL.
B. afzelii strains
Comparison of the growth of 10 B. afzelii strains is presented on Fig 1. The median, P25 and P75 final number of cells/mL for these strains after three days of incubation were as follows: at 4°C median = 0, P25 = 0, and P75 = 0; at 23°C median = 2.5x104, P25 = 0, and P75 = 5x104; at 28°C median = 5x104, P25 = 0, and P75 = 1x105; at 33°C median = 3x105, P25 = 1x105, and P75 = 5x105; and at 37°C median = 1x105, P25 = 0 and P75 = 3x105. Based on final number of B. afzelii cells, the fastest growth was found at 33°C, followed by 37, 28 and 23°C, while no growth was detected at 4°C (Fig 1); using Kruskal-Wallis test, that compares median values, these differences were statistically significant (P<0.001).
The Mann Whitney test that compares growth at two particular temperatures showed statistically significant differences in B. afzelii growth between particular and all other tested temperatures (P <0.05).
B. garinii strains
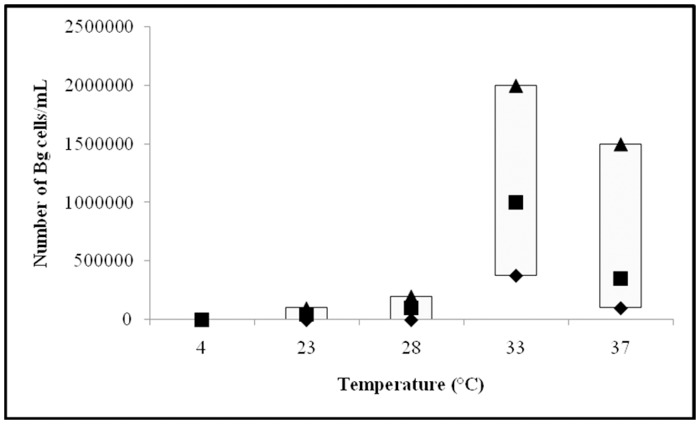
Comparison of the growth of 10 B.garinii strains is shown on Fig 2. The median, P25 and P75 final number of 10 B. garinii strains expressed in cells/mL were as follows: incubation at 4°C median = 0, P25 = 0 and P75 = 0; at 23°C median = 5x104, P25 = 0, and P75 = 1x105; at 28°C median = 1x105, P25 = 0, and P75 = 2x105; at 33°C median = 1x106, P25 = 3.75x105, and P75 = 2x106; and at 37°C median = 3.5x105, P25 = 1x105, and P75 = 1.5x106. Based on final number of B. garinii cells, the most successful growth was found at 33°C, followed by 37, 28 and 23°C, while no growth was detected at 4°C (Fig 2); using Kruskal-Wallis test for comparison of the median values, these differences were statistically significant (P<0.001).
Fig 2. Growth of Borrelia garinii (Bg) strains at 4, 23, 28, 33 and 37°C. Median (■), P25 (♦) and P75 (▲) are expressed as number of cells/mL.
Comparison of B. garinii growth at two particular temperatures with Mann Whitney test showed statistically significant differences in growth between particular and all other tested temperatures (P <0.05).
B. burgdorferi sensu stricto strains
Comparison of the growth of 11 B. burgdorferi sensu stricto strains is depicted on Fig 3. The median, P25 and P75 final number presented in cells/mL were as follows: at 4°C median = 0, P25 = 0, and P75 = 0; at 23°C median = 5x104, P25 = 0, and P75 = 1x105; at 28°C median = 1x105, P25 = 0, and P75 = 3x105; at 33°C median = 9x105, P25 = 3x105, and P75 = 1.8x106; and at 37°C median = 5x105, P25 = 1.5x105, and P75 = 1x106. Based on final number of borrelia cells, the best growth was found at 33°C, followed by 37, 28 and 23°C, while no growth was detected at 4°C (Fig 3); comparison of the median values (Kruskal-Wallis test) showed that the differences were statistically significant (P<0.001). Statistically significant differences were found also comparing the gowth of B. burgdorferi sensu stricto strains at different temperatures (P <0.05).
Comparison of growth at particular temperatures according to Borrelia species (B. afzelii, B. garinii, and B. burgdorferi sensu stricto)
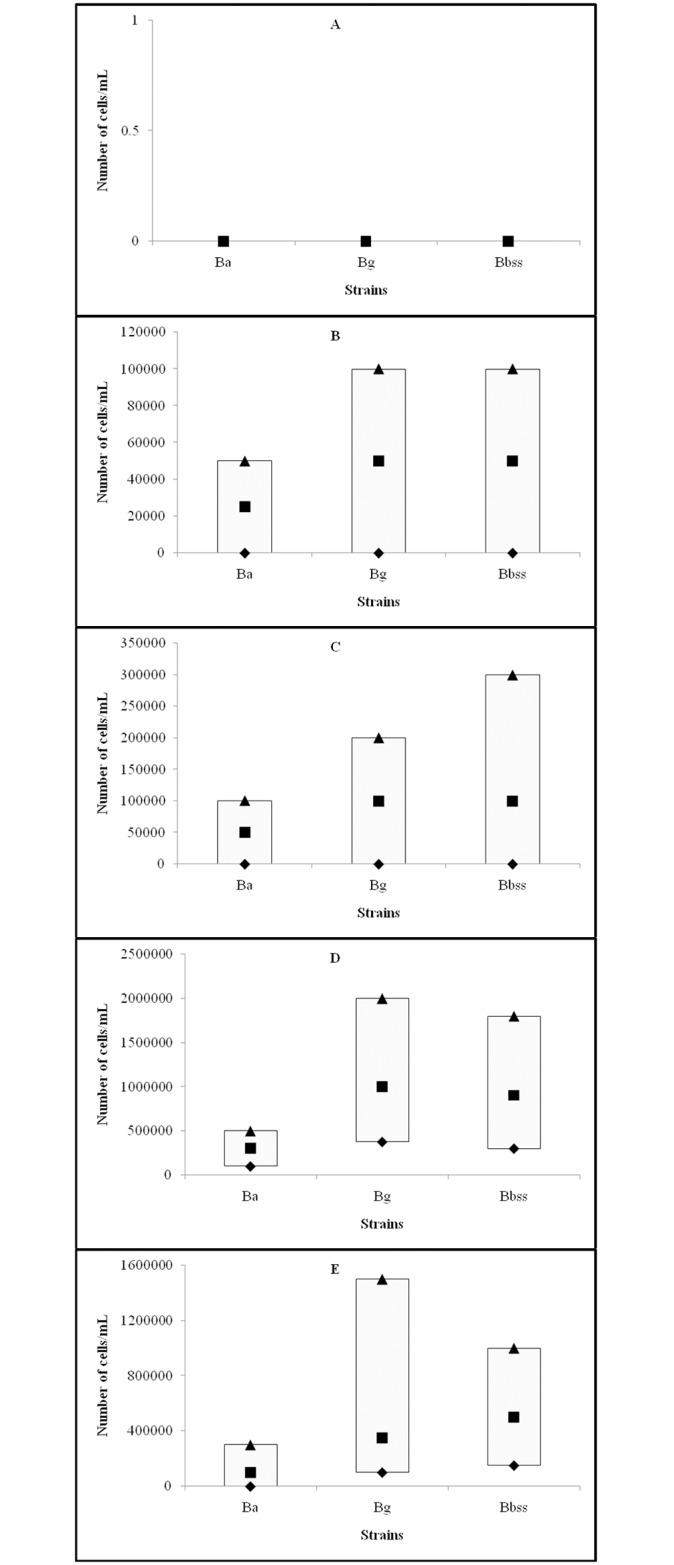
Comparison of growth of B. afzelii, B. garinii, and B. burgdorferi sensu stricto strains at different temperatures is shown on Fig 4A–4E.
Fig 4. Growth of Borrelia afzelii, Borrelia garinii, and Borrelia burgdorferi sensu stricto at different temperatures expressed as number of Borrelia cells/mL and presented as median (■), P25 (♦) and P75 (▲); A: growth at 4°C, B: 23°C, C: 28°C D: 33°C and E: 37°C.
Comparison of growth at 4°C
The median, P25, and P75 final number (in cells/mL) of all three Borrelia species (B.afzelii, B. garinii, and B. burgdorferi sensu stricto) were 0; the Kruskal-Wallis test did not show statistically significant differences in growth at 4°C between the three Borrelia species (P = 0.263). Results are presented on Fig 4A.
Comparison of growth at 23°C
The median, P25, and P75 final number (in cells/mL) of three Borrelia species at 23°C were as follows: B. afzelii median = 2.5x104, P25 = 0 and P75 = 5x104; B. garinii median = 5x104, P25 = 0, and P75 = 1x105; and B. burgdorferi sensu stricto median = 5x104, P25 = 0, and P75 = 1x105, respectively. Results are presented on Fig 4B.
The Kruskal-Wallis test showed statistically significant differences in the growth at 23°C between B. afzelii, B. garinii, and B. burgdorferi sensu stricto after three days of incubation (P = 0.021).
The Mann-Whitney test demonstrated statistically significant differences between the growth of B. afzelii and B. garinii strains (P = 0.023), as well as between B. afzelii and B. burgdorferi sensu stricto strains (P = 0.001), while no statistically significant difference was found comparing the growth of B. garinii and B. burgdorferi sensu stricto strains (P = 0.807).
Comparison of growth at 28°C
The median, P25, and P75 final number (in cells/mL) of three Borrelia species at 28°C were as follows: B. afzelii median = 5x104, P25 = 0, and P75 = 1x105; B. garinii median = 1x105, P25 = 0 and P75 = 2x105; and B.burgdorferi sensu stricto median = 1x105, P25 = 0 and P75 = 3x105 (Fig 4C).
The Kruskal-Wallis test showed statistically significant differences in the growth at 28°C between three Borrelia species (B. afzelii, B. garinii, and B. burgdorferi sensu stricto) after three days of incubation (P<0.001).
The Mann-Whitney test demonstrated statistically significant difference between the growth of B. afzelii and B. garinii (P = 0.014) as well as comparing the growth of B. afzelii and B. burgdorferi sensu stricto (P<0.001) strains, while comparison of B. garinii and B. burgdorferi sensu stricto strains revealed no significant difference in the growth (P = 0.123).
Comparison of growth at 33°C
The median, P25, and P75 final number (in cells/mL) of three Borrelia species at 33°C were as follows: B. afzelii median = 3x105, P25 = 1x105, and P75 = 5x105; B. garinii median = 1x106, P25 = 3.75x105, and P75 = 2x106; and B. burgdorferi sensu stricto median = 9x105, P25 = 3x105 and P75 = 1.8x106, respectively. Results are presented on Fig 4D.
The Kruskal-Wallis test showed statistically significant differences in growth at 33°C between three Borrelia species (B. afzelii, B. garinii, and B. burgdorferi sensu stricto) after three days of incubation (P<0.001).
The Mann-Whitney test demonstrated statistically significant difference between the growth of B. afzelii and B. garinii (P<0.001) as well as B. burgdorferi sensu stricto (P<0.001), while no significant difference was found between B. garinii and B. burgdorferi sensu stricto (P = 0.868).
Comparison of growth at 37°C
The median, P25 and P75 final number (in cells/mL) of three Borrelia species at 33°C were as follows: B. afzelii median = 1x105, P25 = 0, and P75 = 3x105; B. garinii median = 3.5x105, P25 = 1x105, and P75 = 1.5x106; and B. burgdorferi sensu stricto median = 5x105, P25 = 1.5x105, and P75 = 1x106, respectively. Results are presented on Fig 4E.
The Kruskal-Wallis test showed statistically significant differences in growthat 37°C between three Borrelia species (B. afzelii, B. garinii, and B. burgdorferi sensu stricto) after three days of incubation (P<0.001).
The Mann-Whitney test demonstrated statistically significant difference comparing the growth of B. afzelii and B. garinii (P<0.001) as well as B. afzelii and B. burgdorferi sensu stricto strains (P<0.001), while no significant difference was found comparing the growth of B. garinii and B. burgdorferi sensu stricto strains (P = 0.490).
Discussion
Several factors influence in vitro borrelia growth such as medium ingredients and its pH, temperature of incubation, the presence of contaminants, sample’s cell density, number of borrelia strains in the sample, antecedent antibiotic therapy, capacity of particular Borrelia species to grow, etc. [10, 12, 14, 22, 24, 25]. Since there are many differences between bacterial growth in vivo and in vitro, the in vitro conditions and consequently the findings of the in vivo studies are only a rough approximation of the in vivo situation [26]. Therefore, the results of the in vitro studies can be used as a basis for understanding of the in vivo events but not as a proof that in vitro and in vivo findings are equivalent.
In the present study, we analyzed in vitro growth of 10 B. afzelii, 10 B. garinii, and 11 B. burgdorferi sensu stricto strains at different temperatures within the temperature range from 4 to 37°C. Even though similar in vitro studies have been reported previously [12, 13] their findings were limited due to a very small number of examined strains.
Temperature is one of the most important factors influencing bacterial growth. The optimal temperature for borrelia in vitro growth was reported to be in a rather wide range from 30 to 37°C [10]; borreliae are normally cultivated at temperatures 30 to 34°C, most often at 32 to 33°C [9, 13, 14, 16, 18, 22, 27].
Hubálek et al. [12] reported on differences in optimal growth temperatures for distinct Borrelia species. The authors tested only three borrelia strains (one B. afzelii, B. garinii and B. burgdorferi sensu stricto, respectively) and found that the optimal temperature for strain B31 (B. burgdorferi sensu stricto) was 33°C, for strain BR75 (B. afzelii) 35°C, and for strain BR14 (B. garinii) 37°C. Heroldová et al. [13] tested the same B31 strain and confirmed that 33°C is the optimal growth temperature for the strain, but the strain showed good growth also at 28, 30, 35 and 37°C. Since results based on one strain may not represent a typical finding for individual Borrelia species we analyzed larger number of strains that included 10 B. afzelii, 10 B. garinii, and 11 B. burgdorferi sensu stricto strains (Table 1). The best growth for all three Borrelia species was ascertained at 33°C; a good growth was observed at 37°C, while growth was weak at 28 and 23°C, and not detectedat 4°C (Figs 1–3). The finding that all three Borrelia species survived at 23, 28, 33, and 37°C during three days' incubation suggests that temperature range 23 to 37°C can be suitable for transport of clinical material to the laboratory and that these temperatures should not have a substantial negative impact on the survival of borreliae in the sample as well as on their further isolation and cultivation.
Among three Borrelia species, the growth of B. afzelii was weaker than the growth of B. garinii or B. burgdorferi sensu stricto, while the growth of B. garinii and B. burgdorferi sensu stricto was comparable. These findings were valid for temperatures 23, 28, 33 and 37°C. As expected, no growth was observed at 4°C (Fig 4A–4E).
It is well known that B. afzelii and B. garinii are mostly associated with skin manifestations (erythema migrans and acrodermatitis chronica atrophicans) and nervous system involvement (Lyme neuroborreliosis), respectively, while B. burgdorferi sensu stricto is associated with joint involvement (Lyme arthritis), which is more often observed in North America than in Europe [1, 2]. Some authors suggested that differences in Borrelia species organotropism may be due to distinct tissue temperature [11, 12]. Hubalek et al. [12] tested in vitro growth of three different pathogenic Borrelia species at different temperatures and showed that B. garinii was the most thermotolerant species, followed by B. afzelii, while B. burgdorferi sensu stricto was the least theromtolerant. Thus, “heat-stable” Borrelia species affect warmer regions of the body (e.g. B. garinii is mostly associated with Lyme neuroborreliosis), while “heat-sensitive” borrelia strains involve body regions with lower temperature (e.g. B. afzelii is mostly associated with skin manifestations) [11]. However, the results of the present study do not support previous hypothesis that different Borrelia species organotropism could be a consequence of distinct tissue temperature.
Generally, difference in growth between borrelia strains at different temperatures may be interpreted as natural borrelia characteristic.
We demonstrated that the initial number of borrelia cells/mL had statistically significant positive influence on the growth of all three Borrelia species (B. afzelii, B. garinii, and B. burgdorferi sensu stricto) at all tested temperatures, except at 4°C (Table 3). This findings suggest that a large number of borrelia cells/mL present in a clinical sample has good chances to survive, adapt and grow during in vitro incubation while specimen with small number of borrelia/mL may adversely influence borrelial isolation rate and the outcome of cultivation [14, 22].
Conclusions
Our study revealed that 23, 28, 33 and 37°C were suitable for borrelia growth and survival. For all three Borrelia species (B. afzelii, B. garinii, and B. burgdorferi sensu stricto), the optimal temperature was 33°C, followed by 37, 28, and 23°C. There was no statistical significant difference between the growth of B. garinii and B. burgdorferi sensu stricto at 23, 28, 33 and 37°C, respectively, while the growth of B. afzelii species was weaker in comparison to the other two species. The initial number of borrelia cells/mL influenced on growth of all three Borrelia species at all tested temperatures, except at 4°C, suggesting that the large number of borrelia is important for successful cultivation. Reported difference in growth of borrelia strains at different temperature may be interpreted as natural borrelia characteristic.
Data Availability
All relevant data are within the paper.
Funding Statement
This study was supported by the Ministry of Higher Education, Science and Sport of Slovenia (grant no. P3-0083).
References
- 1.Stanek G, Wormser GP, Gray J, Strle F. Lyme borreliosis. Lancet. 2012;379(9814):461–73. Epub 2011/09/10. 10.1016/S0140-6736(11)60103-7 . [DOI] [PubMed] [Google Scholar]
- 2.Steere AC. Lyme disease. N Engl J Med. 2001;345(2):115–25. Epub 2001/07/14. 10.1056/NEJM200107123450207 . [DOI] [PubMed] [Google Scholar]
- 3.Bergström S NL, Gylfe Ä, Östberg Y. Molecular and cellular biology of Borrelia burgdorferi sensu lato In: Gray J KO, Lane RS, Stanek G, editor. Lyme borreliosis: biology, epidemiology and control. New York: CABI Publishing; 2002. p. 47–90. [Google Scholar]
- 4.Fingerle V, Schulte-Spechtel UC, Ruzic-Sabljic E, Leonhard S, Hofmann H, Weber K, et al. Epidemiological aspects and molecular characterization of Borrelia burgdorferi s.l. from southern Germany with special respect to the new species Borrelia spielmanii sp. nov. Int J Med Microbiol. 2008;298(3–4):279–90. Epub 2007/07/10. 10.1016/j.ijmm.2007.05.002 . [DOI] [PubMed] [Google Scholar]
- 5.Ruzic-Sabljic E, Maraspin V, Lotric-Furlan S, Jurca T, Logar M, Pikelj-Pecnik A, et al. Characterization of Borrelia burgdorferi sensu lato strains isolated from human material in Slovenia. Wien Klin Wochenschr. 2002;114(13–14):544–50. Epub 2002/11/09. . [PubMed] [Google Scholar]
- 6.Wilske B P-M V. Microbiological diagnosis of Lyme borreliosis In: Weber K B W, Shiery G, editor. Aspects of Lyme borreliosis. Berlin: Springer-Verlag; 1993. p. 268–99. [Google Scholar]
- 7.Aguero-Rosenfeld ME, Wang G, Schwartz I, Wormser GP. Diagnosis of lyme borreliosis. Clin Microbiol Rev. 2005;18(3):484–509. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barbour AG, Hayes SF. Biology of Borrelia species. Microbiological reviews. 1986;50(4):381–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Preac-Mursic V W B. Biology of Borrelia burgdorferi In: Weber K B W, editor. Aspects of Lyme borreliosis. Berlin: Springer Verlag; 1993. p. 44–58. [Google Scholar]
- 10.Barbour AG. Isolation and cultivation of Lyme disease spirochetes. The Yale journal of biology and medicine. 1984;57(4):521–5. [PMC free article] [PubMed] [Google Scholar]
- 11.Reisinger E, Wendelin I, Gasser R, Halwachs G, Wilders-Truschnig M, Krejs G. Antibiotics and increased temperature against Borrelia burgdorferi in vitro. Scand J Infect Dis. 1996;28(2):155–7. . [DOI] [PubMed] [Google Scholar]
- 12.Hubalek Z, Halouzka J, Heroldova M. Growth temperature ranges of Borrelia burgdorferi sensu lato strains. J Med Microbiol. 1998;47(10):929–32. Epub 1998/10/27. . [DOI] [PubMed] [Google Scholar]
- 13.Heroldova M, Nemec M, Hubalek Z. Growth parameters of Borrelia burgdorferi sensu stricto at various temperatures. Zentralblatt fur Bakteriologie: international journal of medical microbiology. 1998;288(4):451–5. . [DOI] [PubMed] [Google Scholar]
- 14.Wormser GP, Bittker S, Cooper D, Nowakowski J, Nadelman RB, Pavia C. Comparison of the yields of blood cultures using serum or plasma from patients with early Lyme disease. J Clin Microbiol. 2000;38(4):1648–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fraser CM, Casjens S, Huang WM, Sutton GG, Clayton R, Lathigra R, et al. Genomic sequence of a Lyme disease spirochaete, Borrelia burgdorferi. Nature. 1997;390(6660):580–6. 10.1038/37551 . [DOI] [PubMed] [Google Scholar]
- 16.Preac-Mursic V, Wilske B, Schierz G. European Borrelia burgdorferi isolated from humans and ticks culture conditions and antibiotic susceptibility. Zentralblatt fur Bakteriologie, Mikrobiologie, und Hygiene Series A, Medical microbiology, infectious diseases, virology, parasitology. 1986;263(1–2):112–8. . [DOI] [PubMed] [Google Scholar]
- 17.Pollack RJ, Telford SR 3rd, Spielman A. Standardization of medium for culturing Lyme disease spirochetes. J Clin Microbiol. 1993;31(5):1251–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Berger BW, Johnson RC, Kodner C, Coleman L. Cultivation of Borrelia burgdorferi from erythema migrans lesions and perilesional skin. J Clin Microbiol. 1992;30(2):359–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Campbell GL, Piesman J, Mitchell PD, Quan TJ, Reed KD, Dennis DT. An evaluation of media for transport of tissues infected with Borrelia burgdorferi. American journal of clinical pathology. 1994;101(2):154–6. . [DOI] [PubMed] [Google Scholar]
- 20.O'Rourke M, Traweger A, Lusa L, Stupica D, Maraspin V, Barrett PN, et al. Quantitative detection of Borrelia burgdorferi sensu lato in erythema migrans skin lesions using internally controlled duplex real time PCR. PLoS One. 2013;8(5):e63968 10.1371/journal.pone.0063968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ruzic-Sabljic E, Strle F. Comparison of growth of Borrelia afzelii, B. garinii, and B. burgdorferi sensu stricto in MKP and BSK-II medium. Int J Med Microbiol. 2004;294(6):407–12. Epub 2004/12/15. 10.1016/j.ijmm.2004.08.002 . [DOI] [PubMed] [Google Scholar]
- 22.Ruzic-Sabljic E, Lotric-Furlan S, Maraspin V, Cimperman J, Logar M, Jurca T, et al. Comparison of isolation rate of Borrelia burgdorferi sensu lato in MKP and BSK-II medium. Int J Med Microbiol. 2006;296 Suppl 40:267–73. Epub 2006/03/15. 10.1016/j.ijmm.2006.01.005 . [DOI] [PubMed] [Google Scholar]
- 23.Strle F, Nelson JA, Ruzic-Sabljic E, Cimperman J, Maraspin V, Lotric-Furlan S, et al. European Lyme borreliosis: 231 culture-confirmed cases involving patients with erythema migrans. Clin Infect Dis. 1996;23(1):61–5. Epub 1996/07/01. . [DOI] [PubMed] [Google Scholar]
- 24.Jobe DA, Callister SM, Schell RF. Recovery of Borrelia burgdorferi by filtration. J Clin Microbiol. 1993;31(7):1896–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang X, Popova TG, Goldberg MS, Norgard MV. Influence of cultivation media on genetic regulatory patterns in Borrelia burgdorferi. Infect Immun. 2001;69(6):4159–63. 10.1128/IAI.69.6.4159-4163.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lorian V. In vitro simulation of in vivo conditions: physical state of the culture medium. J Clin Microbiol. 1989;27(11):2403–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nadelman RB, Pavia CS, Magnarelli LA, Wormser GP. Isolation of Borrelia burgdorferi from the blood of seven patients with Lyme disease. Am J Med. 1990;88(1):21–6. . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.