Abstract
Oncogenic viruses reprogram host gene expression driving proliferation, ensuring survival, and evading the immune response. The recent appreciation of microRNAs (miRNAs) as small non-coding RNAs that broadly regulate gene expression has provided new insight into this complex scheme of host control. This review highlights the role of viral and cellular miRNAs during the latent and lytic phases of the EBV life cycle.
Keywords: Epstein-Barr virus, EBV, microRNA, miRNA, B cell lymphoma, LMP1, miR-155, miR-34, miR-146, miR-200, lytic reactivation, ZEB
Introduction
MicroRNAs (miRNAs) are small, ~21–25 nucleotide, non-coding RNAs expressed by all multicellular eukaryotes that negatively regulate gene expression by targeting complementary sequences in messenger RNAs [1]. These regulatory RNAs have been demonstrated to play a key role in a variety of processes including development, cell cycle regulation, and immunity and their malfunction has been associated with several human pathologies including cancer [2]. MiRNAs perform their gene regulatory function as the guide RNA component of the RNA-induced silencing complex (RISC) complex, which binds perfect or partially complementary sequences predominantly found in the 3 ’UTR of target mRNAs, causing mRNA translation inhibition or mRNA degradation. As miRNAs require only limited complementarity for mRNA binding, they are able to modulate the expression of multiple genes. Conversely, different miRNAs can control a single mRNA, making miRNA regulatory networks particularly complex to investigate. The region that dictates the specificity of the miRNA:mRNA target interaction corresponds to nt 2–8 from the miRNA 5’ end and is referred to as the “seed” sequence. Seed sequences can be shared by several distinct miRNAs, which are termed members of the same seed family [3].
MiRNAs are generally produced as RNA polymerase II-driven, capped, and poly-adenylated RNA precursors [1]. Stem-loop structures within these primary miRNAs (pri-miRNAs) are recognized by the enzyme Drosha and processed to yield ~65–70 nucleotide precursor miRNAs (pre-miRNAs), which are subsequently exported from the nucleus to the cytoplasm through an Exportin 5-dependent pathway. The pre-miRNA is then recognized by a complex containing the RNAse III enzyme Dicer, which liberates a duplex intermediate of ~22 base pairs. One strand of this duplex is then loaded into the RISC composed of Argonaute family proteins and accessories. The mature miRNA guides the RISC complex to target mRNAs through its seed sequence to enable suppression of target expression.
The identification and characterization of cellular as well as virally-encoded miRNAs have established their roles as broad and important regulators of the host/pathogen interface [4]. The major family of viruses that encode and modulate miRNAs is the Herpesviridae. These large double-stranded DNA viruses typically contain nearly one hundred protein coding genes and it is now evident that many miRNAs are also encoded in their genomes. In particular, the oncogenic γ-herpesviruses encode a large number of miRNAs and also modulate host miRNAs as a means of effecting cell transformation. An important human pathogen and model system for studying the role of miRNAs in viral oncogenesis is the γ-herpesvirus Epstein-Barr virus (EBV).
EBV infects greater than 90% of adults worldwide [5]. Despite the high rate of prevalence, disease is rarely manifested in infected individuals due to a strong cytotoxic T cell response. In immune-compromised individuals, such as those infected with HIV or following transplant, EBV-associated malignancies are more common. Furthermore, EBV is causally implicated in African endemic Burkitt’s lymphoma (BL) and the epithelial cancer nasopharyngeal carcinoma (NPC). Acute infection during adolescence also leads to infectious mononucleosis due to the uncontrolled expansion of poly-reactive B cells.
EBV is a large, enveloped virus containing a ~184kbp double stranded DNA genome. In vivo, B lymphocytes and epithelial cells are common targets, while rare infection of NK and T cells has also been observed [5]. Infection of primary B cells in vitro leads to a latent infection in which only a subset of viral genes are expressed including the latent membrane proteins 1, 2A, and 2B and Epstein-Barr nuclear antigens (EBNAs) 1, 2, 3A, 3B, 3C, and LP as well as 25 viral pre-miRNAs. This expression program is called latency III and drives the indefinite proliferation of primary B cells (Table 1). In other settings in vivo including BL tumors, Hodgkin’s lymphoma (HL), and NPC, EBV displays a more restricted form of latency. Finally, in normal infected individuals, the virus exists in memory B cells in the peripheral blood where no genes are expressed except for EBNA1 during cell division [6, 7]. Studies of EBV-infected B cells and tumor-derived cell lines have informed much of our understanding of the mechanisms by which EBV drives tumorigenesis [5].
Table 1.
EBV latency gene expression programs
| Latency I | Latency II | Wp-Restricted | Latency III | |
|---|---|---|---|---|
|
Viral Protein Expression |
EBNA1 | EBNA1, LMP1, LMP2A, 2B |
EBNA1, LP, 3A, 3B, 3C LMP1, 2A, 2B, BHRF1 |
EBNA1, 2, 3A, 3B, 3C, LP LMP1, 2A, 2B |
| EBERs | Yes | Yes | Yes | Yes |
| miRNAs | BART miRNAs (modest) |
BART miRNAs (high) |
BHRF1 miRNAs (modest) BART miRNAs (modest) |
BHRF1 miRNAs (high) BART miRNAs (modest) |
|
Diseases/ Cell States |
Burkitt’s lymphoma |
Nasopharyngeal carcinoma, Hodgkin’s lymphoma |
Burkitt’s lymphoma |
Post-Transplant Lymphoproliferative Disease, HIV lymphomas, Diffuse large B cell lymphomas, Lymphoblastoid cell lines |
Infection of either B lymphocytes or epithelial cells with EBV poses several barriers to long-term persistence in the host. Both the innate and adaptive immune response can prevent virus replication and the growth of virus-infected cells. Therefore, the virus ensures control of host physiology by regulating host cell gene expression. This occurs both through modulation of specific signaling pathways as well as by restricting its own gene expression. For example, LMP1 mimics a constitutively active CD40 (B cell co-stimulatory TNFR family member) [8], while LMP2A mimics the B-cell receptor (BCR) and antagonizes endogenous BCR signaling [9]. Fundamentally, restriction of viral gene expression, for example in latency I, prevents CD8+ T cell recognition of immune-dominant epitopes in the EBNA3 proteins, and enables long-term persistence of latently-infected cells. Lastly, EBV latent infection also depends on tight control of the viral lytic transactivator protein Zta.
The primary effects of EBV on host cell physiology are mediated through changes in host gene expression. Given the importance of miRNAs in regulating gene expression, many studies have now implicated miRNAs in mechanisms through which EBV modulates the host. These reports will be highlighted in this review covering five major areas: i) the expression of EBV-encoded miRNAs, ii) mRNA targets and functional significance of EBV miRNAs, iii) the regulation of cellular miRNA expression during EBV infection, iv) the functional role of cellular miRNAs in EBV latency and lytic reactivation, and v) genome-wide methods to identify mRNA targets of miRNAs in EBV-infected cells.
I. Expression of Epstein-Barr virus encoded miRNAs
A. EBV miRNA expression in infected cells and tumors
EBV was the first human virus shown to express miRNAs and to date is the virus that encodes more miRNAs than any other human virus, with twenty-five identified pre-miRNAs. Pfeffer et al. were the first to show that EBV expresses miRNAs by cloning small RNAs from an EBV- infected Burkitt’s lymphoma cell line [10]. In this study, 5 viral miRNAs, located in two distinct clusters were identified. One cluster is located near the mRNA of the BHRF1 (BamHI fragment H rightward open reading frame 1) gene, coding miR-BHRF1-1 to 3, while the other is located in intronic regions of the BART (Bam HI fragment A rightward transcript) giving origin to miR-BART1 and 2. Since this initial report, other groups have identified additional EBV miRNAs, all of them located within the BART cluster. Cai and colleagues identified 14 novel viral miRNAs using traditional cloning and sequencing of small RNAs from latently EBV-infected BC-1 cells [11]. Contemporaneously, Grundhoff et al., used a computational approach followed by a microarray analysis to identify possible miRNAs encoded by EBV that were further validated by Northern blot [12]. This study identified 18 pre-miRNAs and 22 mature miRNAs. The more than four-fold increase in the number of novel miRNAs identified by these groups was largely due to the fact that they interrogated EBV-infected cells containing “wild-type” strains, rather than the B95-8 strain of EBV. This prototype laboratory strain carries a deletion of about 12 kb that includes part of the EBV BART locus, where almost all of the viral miRNAs are located. Two additional BART miRNAs, miR-BART21 and 22, were subsequently identified by Zhu, et al. in NPC samples using small RNA deep sequencing [13]. Finally, four additional mature BART miRNAs were identified in another recent miRNA deep sequencing study of NPC tumor samples thereby identifying and rigorously characterizing the mature sequences of all 44 possible BART miRNAs (i.e. 22 miRNAs, both strands) in infected cells or tumor samples [14].
EBV miRNAs are differentially expressed in lymphoid and epithelial cells and under the different virus latency programs (Table 1). The BHRF1 miRNAs are expressed at high levels in cells displaying type III EBV latency, including LCLs as well as in a range of EBV-infected B-cell tumors [15]. The association with this specific latency program is due to the fact that these miRNAs are expressed from an EBNA transcript that is produced only in latency III starting from the viral Wp or Cp promoter. Consequently, they are not detected in other latency stages including latency I BL and latency II NPC cell lines [10, 11, 16], although they are expressed in Wp-restricted BL cell lines [17]. Two groups have further confirmed that BHRF1 miRNAs are not expressed in NPC by miRNA expression profiling and deep sequencing of NPC tumor biopsy samples [13, 18].
On the other hand, BART miRNAs are expressed mostly in epithelial cells undergoing type II EBV latency, including EBV-induced nasopharyngeal and gastric carcinomas [11, 18, 19] even though BART miRNAs are also expressed at reduced levels in lymphoid cell lines [11, 17, 20]. Although the level of BART miRNAs detected in epithelial cells is higher compared to lymphoid cell lines, it has been reported by Edwards et al. and, more recently, by Amoroso et al. that BART miRNA expression is not characteristic of a specific cell type, as some epithelial and lymphoid cell lines show high expression, while others not [17, 20]. Furthermore, and more surprisingly, extensive variation in the levels of individual BART miRNAs up to 50-fold was observed between as well as within epithelial and B cell lines infected with EBV [17, 21]. Since these miRNAs are processed from the same primary transcript, it was unexpected that their mature levels would vary so greatly. Amoroso, et al. found no differences in stability between the BART miRNAs and therefore suggested a role for alternative processing of these miRNAs from the primary BART transcript [17].
B. EBV miRNA expression during lytic reactivation
Both BART and BHRF1 miRNAs are expressed during lytic reactivation as demonstrated initially by Cai et al. [22]. This study found that the expression of some EBV miRNAs increased during lytic replication in LCL, BL, and PEL cell lines. Up-regulation was in part due to their location. In fact, miR-BHRF1-2 and -3 are located in the 3’UTR of the early lytic protein BHRF1 and also BART mRNA expression has been demonstrated to increase during lytic infection [23] so it is not surprising that BHRF1- and BART-derived miRNA levels also increase during the lytic cycle.
In the recent study by Amoroso, et al., a rigorous quantitative analysis of BART and BHRF1 miRNA levels in lytically induced Akata cells further clarified these initial findings [17]. The BHRF1-2 and 1-3 miRNAs increased as early as 24 hour post lytic induction, as the lytic BHRF1 promoter and mRNA were induced. However, BHRF1-1 was not induced until 48h or later as the viral Wp and Cp promoters became active. Furthermore, expression of BHRF1-1 dependended on viral DNA replication, as did Wp- and Cp-transcription, while lytic BHRF1 expression did not. Interestingly, despite robust primary BART transcription (>40-fold increase during lytic induction), relatively modest induction of BART miRNAs was observed during lytic reactivation. These data are consistent with the steady-state variation in BART miRNA levels further suggesting that miRNA processing during latency as well lytic reactivation plays a role in the accumulation of BART miRNAs.
C. EBV miRNAs are released in exosomes from EBV-positive cells
Recently, miRNAs have been found in a unique set of microvesicles called exosomes deriving from reverse budding of the limiting membrane of multivesicular endosomes (MVEs). Several studies have indicated that miRNAs are probably loaded onto exosomes by RISC, which has been shown to be associated with MVEs [24]. As exosomes are able to transfer from cell to cell and to be secreted by several different cell types in culture and human sera, Pegtel et al. hypothesized that EBV miRNAs could be transferred through exosomes thereby regulating mRNAs in neighboring cells [25]. Indeed, this group detected viral miRNAs in purified CD63-positive exosomes from the supernatant of EBV-infected cells. Interestingly, in co-culturing experiments, this group demonstrated that EBV miRNAs could be transferred to non-EBV infected cells where they repressed target mRNAs.
They first provided evidence that exosomes contain EBV miRNAs and can transfer from LCLs to monocyte-derived dendritic cells (MoDC). Indeed, the co-culturing of labeled, purified LCL exosomes and MoDC caused an increased fluorescence in MoDCs, indicating that LCLs are able to release exosomes that are then internalized in adjacent DCs. After demonstrating that viral miRNAs are actually present in MoDCs, Pegtel et al. also showed that these miRNAs are functional in the recipient cells. In fact, EBV miRNAs were specifically able to reduce luciferase levels of target 3’UTR constructs expressed in the uninfected cells. Interestingly, BART miRNAs were not only detected in exosomes from EBV infected B cells, but also in circulating, uninfected non-B cells, indicating the transfer of EBV miRNAs from infected to uninfected cells in vivo. Since the EBV genome was not present in recipient cells and these cells do not have primary transcripts encoding viral miRNAs, this group postulated that EBV miRNAs are functionally transferred in vivo in order to mediate intercellular communication during infection.
Two additional groups have recently reported on the release of EBV miRNAs from NPC cells [26, 27]. In these studies BART miRNAs were detected in CD63-positive exosomes purified from the supernatant of the EBV-positive C666-1 NPC cell line and cultures of the EBV-positive C15 and C17 NPC xenografts. Furthermore, BART miRNAs could be detected in the plasma of mice harboring the C15 NPC xenografts [26]. Critically, BART miRNAs could also be detected in the plasma of NPC patients [26]. These data are consistent with those of Pegtel and suggest that viral miRNAs may serve as both a bio-marker for EBV-associated cancers, and also implicate these molecules in intracellular communication that may be important for the pathogenesis of EBV-positive tumors.
D. Conservation of EBV miRNAs and cellular miRNA relatedness
Herpesviruses share conserved genes encoding structural proteins and enzymes important for the production of new virion particles. These genes share collinear homology across viruses and species and are highly conserved at the sequence level. However, despite modest genomic colinearity, viral miRNAs are rather poorly conserved across herpesviruses [28]. In fact, within the γ-herpesvirus subfamily, EBV and KSHV miRNAs share little sequence homology. The most related viral miRNAs are found within the genus of lymphocryptoviruses where EBV and the rhesus lymphocryptovirus (rLCV) share approximately 22 of 25 viral miRNAs by evolutionary comparison with only 7 miRNAs sharing seed sequence conservation [11, 28]. Therefore, viral miRNA seed sequences are not highly conserved, though conservation of mRNA targets often are (see below) and may prove to be a source of convergent evolution in viral pathogenesis.
Another intriguing aspect of EBV miRNA sequences that may provide insight into pathogenesis stems from the observation that the most abundantly expressed BART miRNAs share identical 6-mer seed sequences with cellular miRNAs [14]. Given the 642 unique 6-mer seed sequences for cellular miRNAs, approximately 15% of BART miRNAs would be expected to share seeds with cellular miRNAs. In contrast, nearly 30% of EBV BART miRNA seed sequences are identical to cellular miRNA seed sequences [14]. In fact, the most abundantly expressed BART miRNAs were significantly more likely to share a cellular seed than less abundantly expressed BART miRNAs. Therefore, Chen, et al. propose that viral miRNAs act as mimics or competitors of cellular miRNAs in EBV-infected cells [14]. This hypothesis was supported by the correlation in expression between several high abundance EBV BART miRNAs and their cellular seed-sharing orthologues (for example, miR-18/BART 5-5p and miR-29/BART 1-3p) in normal tissue versus NPC tumors [14].
II. The mRNA targets and functional significance of EBV miRNAs
A. Viral mRNA targets of EBV miRNAs
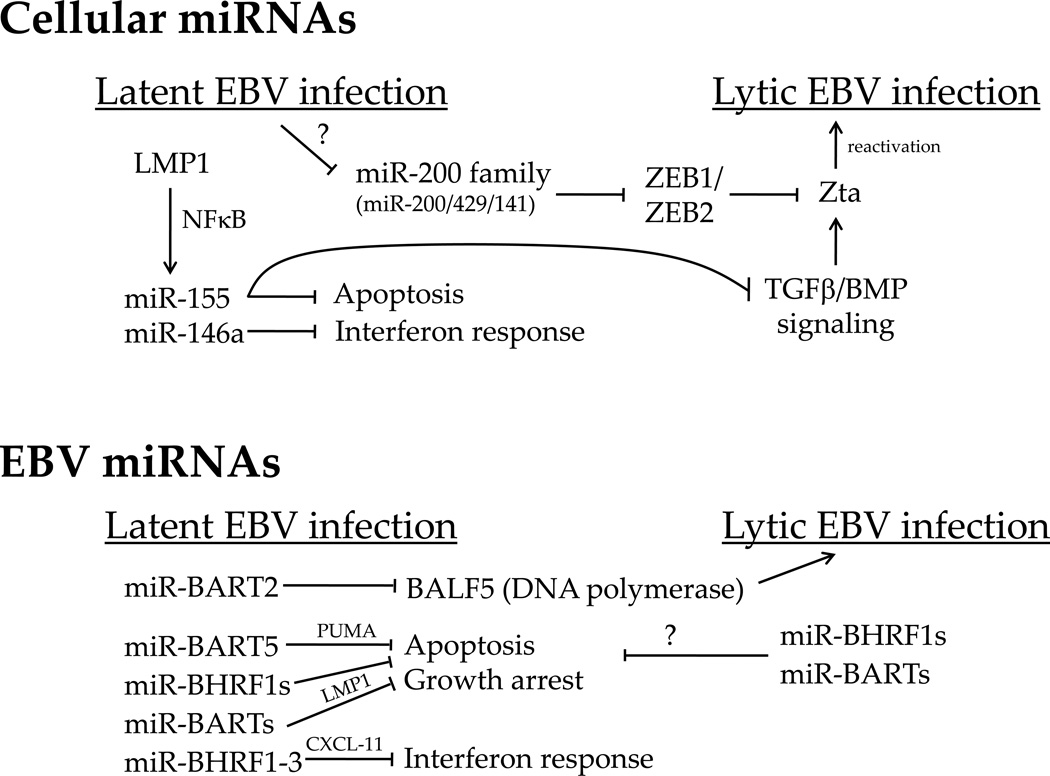
Our knowledge of EBV miRNA function has been steadily increasing since their discovery (Fig. 1). Pfeffer et al. reported that the miR-BART2 transcript is antisense to the viral DNA polymerase BALF5 and its sequence is exactly complementary to the BALF5 3’UTR. This observation led to the hypothesis that this viral miRNA could lead to degradation of the BALF5 mRNA during EBV infection [9]. This hypothesis was later confirmed by Barth et al. who demonstrated that miR-BART2 enhances BALF5 mRNA cleavage, down-regulates the BALF5 3’UTR in luciferase assays, and suppresses BALF5 protein expression [29]. While miR-BART2 over-expression only modestly suppressed lytic replication, its expression levels decrease on lytic reactivation as BALF5 mRNA and protein levels increase. These data suggest a possible functional interaction between miR-BART2 and BALF5 in regulating viral lytic reactivation.
Figure 1. Summary of cellular (top) and EBV (bottom) miRNA functions and targets in EBV latency and reactivation.
The names above inhibitory arrows are targets of the given miRNA (e.g. miR-BHRF1-3 and CXCL-11). Question marks indicate unknown mechanisms of action or speculative activities (such as the EBV miRNAs suppressing apoptosis during lytic reactivation). These interactions are largely derived from work in B cells, although they may be true in epithelial cells as well (e.g. miR-200 family and ZEB interaction, miR-BART5 and PUMA interaction, and miR-155 and BMP interaction).
In addition to BALF5, two additional viral proteins are reported targets of EBV miRNAs: LMP1 and LMP2A. Lo, et al. found that several miRNAs from the BART cluster can target the 3’UTR of LMP1, thereby inhibiting LMP1 protein expression by translation repression in EBV infected epithelial cell lines [30]. However, two of these miRNAs (BART16-3p and -17-5p) were originally mis-annotated and matches to their seed sequences do not actually appear in the LMP1 3’UTR. Additionally, the BART1-5p interaction with the LMP1 mRNA cannot be confirmed, at least in LCLs (B. Cullen and R. Skalsky, personal communication). Nevertheless, miRNA expression from the BART cluster decreased LMP1 protein levels, and consequently, decreased NFκB activity. Although LMP1 induces transformation, high levels of LMP1 expression can inhibit proliferation and increase sensitivity to pro-apoptotic stresses [31, 32]. Indeed, BART miRNA-mediated LMP1 suppression reduced the sensitivity of epithelial cells to cisplatin and consequently, mitigated the apoptotic response [30].
Another latent protein to be regulated by miRNAs is LMP2A. Lung, et al. reported that miR-BART22 is the only EBV miRNA able to target LMP2A in 3’UTR luciferase assays despite its 3’UTR containing binding sites for other BART miRNAs [33]. Furthermore, over-expression of miR-BART22 caused a reduction of LMP2A protein expression without affecting mRNA levels, indicating that LMP2A is a direct target of this miRNA and its regulation occurs at the level of translation. Since LMP2A is highly immunogenic, it was proposed that miR-BART22 limits its levels in order to escape the host immune response.
B. Cellular targets of EBV miRNAs
Xia et al. reported that miR-BHRF1-3, which is highly expressed in type III latency cell lines and primary EBV-associated AIDS-related diffuse large B cell lymphoma (DLBCL), targets the interferon-inducible T cell attracting chemokine, CXCL-11/I-TAC [15]. This chemokine is a potent T cell chemoattractant known to activate the chemokine receptor CXCR3 and it is plausible that by down-regulating CXCL-11/I-TAC, miR-BHRF1-3 could inhibit activation of the host interferon response upon EBV infection (Fig. 1).
Choy et al. showed that miR-BART5 regulates p53 up-regulated modulator of apoptosis (PUMA) [34]. Over-expression of miR-BART5 in epithelial cells suppressed the 3’UTR of PUMA as well as endogenous PUMA protein and mRNA levels. Consistently, miR-BART5 depletion led to up-regulation of endogenous PUMA protein. Importantly, loss of miR-BART5-mediated suppression of PUMA in NPC cell line enhanced susceptibility to apoptotic stimuli. Given these findings in vitro, it was also interesting to note that an inverse correlation exists between PUMA expression and miR-BART5 levels in NPC tumors. This study was the first to indicate that an EBV miRNA might be important in promoting tumor cell survival.
Nachmani et al., made the interesting observation that multiple herpesvirus miRNAs converge on a similar mRNA target, MICB, a natural killer (NK) cell ligand [35]. EBV BART3-5p, human cytomegalovirus (HCMV) UL112-1, and KSHV miR-K7 target MICB thereby preventing efficient recognition of virally infected cells by NK cells. Furthermore, each viral miRNA targets MICB through a unique seed sequence implying convergent evolution by herpesviruses used to solve a common functional problem in viral immune evasion.
C. Functional role of EBV miRNAs
Two recent studies have defined the role of the EBV miRNAs in B cell immortalization. [36, 37]. Seto et al. generated several mutant EBV recombinants modulating expression of the two clusters of viral miRNAs. They constructed mutants in the B95-8 strain that either: i) lacked all BHRF1 miRNAs, ii) lacked all viral miRNAs (BHRF1 and BARTs), or iii) expressed all possible EBV miRNAs (add back of BARTs deleted from B95-8). While all mutant were able to transform primary B cells into LCLs, those lacking all miRNAs or only deleted for the BHRF1 miRNAs were compromised in their ability to induce B cell proliferation and suppress spontaneous apoptosis [36]. This phenotype persisted in LCLs generated from these mutants, which less efficiently entered S phase and retained higher levels of spontaneous apoptosis than control or revertant virus-infected cells. Similar findings were observed by Feederle, et al. [37], who specifically knocked out the BHRF1 miRNA locus. These authors observed a compromise in B cell immortalization efficiency, S phase progression, and increased apoptosis in infected cells. Neither group observed an effect on lytic reactivation in any of the miRNA-deficient recombinants. Therefore, the EBV miRNAs play a role in promoting the latency promoted cell cycle and protect B cells from spontaneous apoptosis.
III. EBV regulation of cellular miRNA expression
A. Expression changes of host miRNAs in EBV-positive tumors
EBV is associated with several human lymphoid- and epithelial-cell cancers including African endemic BL, HL, post-transplant lymphoproliferative disease (PTLD), diffuse large B cell lymphoma (DLBCL), and NPC. The role of miRNAs in these tumors is unclear, however recent studies suggest a contribution of EBV to the miRNA expression profile of primary tumors.
Navarro et al., demonstrated that EBV could influence miRNA expression in classic Hodgkin lymphoma (cHL) [38]. Analysis of 30 cHL tumors, 3 cHL cell lines, and 5 reactive lymph nodes (RLNs) defined a 25 miRNA signature that distinguished cHL from RLNs and 36 miRNAs were differentially expressed between cHL of the nodular sclerosing versus mixed cellularity types. Importantly, the comparison between EBV positive and EBV negative cHL identified 10 differentially expressed miRNAs: miR-128a, -128b, -129, and miR-205 were down-regulated by EBV, while miR-28, -130b, -132, -140, and miR-330 were up-regulated. The importance of these differences in HL and regulation by EBV latency gene products remains to be validated.
Another group investigated changes in miRNA expression in EBV-positive and EBV-negative BL cases [39]. They analyzed the levels of 4 miRNAs that have been associated with B cell differentiation regulation: miR-125a, -125b, -127, and 9*. miR-127 was the only miRNA whose expression was altered by the presence of EBV in BL tumors. Indeed, miR-127 up-regulation in EBV-positive BL cell lines was responsible for down-regulation of BLIMP-1 leading to persistence of BCL-6 expression, thereby blocking germinal center exit and consequently the B cell differentiation process.
B. Host miRNA profiling of EBV latently infected B cells
In order to identify cellular miRNAs regulated during EBV infection, several groups have performed miRNA profiling experiments of EBV latently infected cell lines. The first such study was performed by Mrazek, et al. using a subtractive hybridization approach [40]. A comparison of small non-coding RNAs expressed in the EBV-negative Burkitt’s lymphoma cell line BL41 versus an EBV-transformed lymphoblastoid cell line (LCL) identified a core set of differentially expressed miRNAs. Latency III gene expression in LCLs was associated with increased levels of miR-155, miR-146a, miR-21, miR-34a, miR-29b, miR-23a, and miR-27a and decreased levels of miR-20b, miR-15a, and miR-15b. Accumulating evidence at the time suggested that the latency III-induced miRNAs were growth-promoting oncomiRs, i.e. miRNAs whose expression is positively associated with tumorigenesis, while those that were latency III-repressed were growth suppressive miRNAs. These changes were confirmed and extended by other groups using miRNA microarray approaches.
Cameron et al. compared the miRNA expression differences between transformed B cell lines expressing either EBV latency III or latency I transcriptional programs relative to EBV-negative cell lines [41]. EBV latency III altered the expression of 41 cellular miRNAs where the most up-regulated miRNAs were miR-155 and miR-146a in latency III. However, the expression levels of miR-21, miR-28, miR-34, miR-146b and members of the miR23 family were also elevated in latency III cell lines.
Godshalk et al. also reported a study of global EBV-regulated changes in miRNA expression [42]. However, these investigators studied the changes in expression following primary B cell infection with EBV compared to anti-Ig and CD40 ligand (i.e., mimicry of antigen receptor and T-cell help, respectively) mediated B cell activation and used qRT-PCR to measure mature miRNA levels rather than microarray. In contrast to the data from Cameron, et al., this group observed a dramatic down-regulation of almost all detectable miRNAs in EBV infected cells relative to primary resting B cells. They observed the suppression of several miRNAs previously described as tumor suppressors, including some let-7 family members, miR-1 and miR-196b. Surprisingly, excluding miR-155 that was modestly up-regulated, other miRNAs considered onco-miRs were down-regulated after EBV infection in their system, including miR-17-5p, miR-20 and miR-21. However, they argued that this effect was not maintained over time and, indeed, expression of a subset of miRNAs including miR-17 and miR-20 increased at later times after infection. It is possible that the discrepancy in findings between this and other studies is due to the QPCR-based format for expression detection or possibly the heterogeneity of the infected cells at the time of analysis.
C. EBV latency protein regulation of host miRNA expression
While several studies have identified EBV latency III-regulated changes in cellular miRNA expression, only the potent signaling molecule LMP1 has thus far been directly implicated in these changes (Fig. 1). Motsch at al. observed that the expression of miR-146a increases in Burkitt’s lymphoma (BL) cell lines after EBV infection and in EBV latency III BL cell lines compared to latency I BL cell lines, which do not or poorly express LMP1 [43]. Furthermore, LMP1 ectopic expression in B cells stimulated the expression of miR-146a. This evidence together with the observation of the presence of two NFκB response elements in the miR-146a promoter led them to hypothesize that LMP-1 could regulate miR-146a through the NFκB pathway. Indeed, through luciferase reporter assays they demonstrated that the miR-146a promoter responds to LMP1 both in EBV-negative B lymphoma cell lines and that this activation was NFκB-mediated.
Cameron et al. also identified miR-146a as robustly LMP1 induced following a miRNA expression profiling experiment performed on EBV negative BL cell line transduced with a LMP1-expressing retrovirus [44]. They found that miR-146a was one of 35 miRNAs up-regulated in presence of LMP1, both at the level of primary and mature transcripts. Other significantly induced miRNAs in LMP1-expressing cells included miR-222, -99a, -342, -221, -125b, -100, -330, and -629 while miR-15a, 663, -150, -638, 199a* were LMP1-repressed. Since miR-146a was the most strongly regulated miRNA by LMP1, they followed up with promoter analysis and confirmed the observation by Motsch, et al. that LMP1 activated the miR-146a promoter through NFκB elements. Further, they identified a role for Oct-1 in basal regulation of the miR-146a promoter.
Along with miR-146a, a number of groups found that the primary miR-155 transcript, BIC, and mature miR-155 were both strongly up-regulated in LCLs or latency III-expressing BL cells compared to uninfected B cells or latency I-expressing BL cells [45–48]. This induction was not due to epigenetic differences between these cell lines but specifically depended on EBV latency III gene expression [49]. In fact, several groups demonstrated that LMP1 directly increased BIC/miR155 levels [47, 48, 50]. Lu et al. also observed that the BIC RNA is modestly up-regulated by EBNA2, but to a lesser extent than LMP1. However, LMP1-mediated BIC induction was specific for B cells, in fact LMP1 expression in epithelial cells failed to activate BIC transcription [47].
Analysis of BIC promoter activity in latency III-expressing cell lines indicated that an AP-1 site located 40 bp upstream of the transcriptional start site was critical and an upstream NFκB element was important for BIC expression [49]. Previous reports indicated that BIC was upregulated through AP-1 activity downstream of the B cell receptor [51]. However, LMP1 was still able to activate the BIC promoter in the absence of this site in luciferase assays. This result suggests the existence of additional regulatory mechanisms controlling the BIC promoter in EBV-infected cells. However, the p38 MAPK and/or NFκB pathways are likely functionally important as pharmacological inhibition of these two pathways reduced BIC promoter activity [48, 49]. Thus, while LMP1 certainly plays a key role in BIC/miR155 regulation, it may not be the only EBV latency gene involved in its induction.
Recently, Anastasiadou et al. analyzed miRNA expression changes induced by LMP1 expression in the DLBCL cell line U2932 [52]. Their goal was to identify miRNAs regulated by LMP1 that suppress expression of the T-cell leukemia gene (TCL-1), an oncogene over-expressed in T-cell leukemia and previously known to be suppressed by LMP1. They identified several miRNAs regulated by LMP1 and confirmed that miR-146a was the most robustly induced as previously reported [43, 44]. However, among the LMP1 induced miRNAs was the miR-29 family. Previous studies implicated miR-29b in reducing TCL1 expression [53]. Anastasiadou et al. subsequently demonstrated that LMP1 down-regulates TCL1 expression by inducing miR-29b levels through its two key cytoplasmic signaling domains. Furthermore, they showed that LMP1 induces miR-29b expression by increasing the level of its primary transcript.
IV. The role of cellular miRNAs during EBV latency and lytic reactivation
A. miR-155 is a key regulator of EBV-transformed cells growth and survival
MiR-155 is strongly up-regulated during latent EBV infection of B cells [45–48], is the most abundant miRNA expressed in LCLs [54], and is highly expressed in B cell lymphomas [55]. These data, coupled with the fact that two other oncogenic herpesviruses (Kaposi’s Sarcoma-Associated Herpesvirus and Marek’s Disease Virus) both encode an orthologue of miR-155 [56–59], strongly suggested that this miRNA plays a key role in EBV-associated tumorigenesis. Consequently, several groups have focused on the identification of miR-155 targets towards elucidating its function in the setting of EBV infection.
Yin et al. analyzed the mRNA expression profile of EBV latency I-expressing Akata cells, which normally lack miR-155 expression, upon reintroduction of this miRNA at levels normally found in LCLs [49]. They found 84 increased mRNAs upon miR-155 expression and 78 repressed mRNAs. Of the suppressed mRNAs, 17 contained miR-155 seed sequences in their 3’UTRs and 8 of these mRNAs were functionally validated as direct miR-155 targets by 3’UTR-luciferase assays. Interestingly, all of them (BACH1, ZIC3, ZNF652, ARID2, SMAD5, HIVEP2, CEBPB, and DET) are transcription factors, indicating that EBV-induced expression of miR-155 likely supports EBV signaling by regulating transcriptional regulatory mechanisms. One of these targets in particular, the transcriptional repressor BACH1, is a well-known miR-155 target that is also suppressed by the KSHV miR-155 orthologue, miR-K12-11, and has been demonstrated to inhibit AP1-mediated transcriptional activity. Consequently, the reduction of the inhibitory BACH1 activity could make AP1 sites more accessible to EBV regulatory elements, such as downstream signaling from LMP1, a known AP1 inducer, ultimately supporting viral and host gene expression.
Recent evidence from Linnstaedt, et al. demonstrates the importance of miR-155 in LCL proliferation and survival. This group used miRNA “sponge” technology [60] to specifically suppress the activity of miR-155 in freshly derived LCLs and the AIDS-DLBCL line IBL-1 [61]. In each case, miR-155 depletion completely abolished growth of the EBV-transformed cell line. This loss of proliferative capacity was accompanied by the suppression of S phase progression and massive induction of apoptosis [54] (Fig. 1).
In contrast to Linnstaedt et al., Lu et al. reported that the inhibition of miR-155 activity using a specific inhibitor of this miRNA in LCL does not affect cell cycle profile, cellular proliferation or apoptosis induction [47]. Instead, they argued that miR-155 stabilizes EBV latency through the down-regulation of NFκB and interferon signaling in order to modulate the cellular antiviral immune response. IKKε, an IκB kinase that has been demonstrated to phosphorylate activators of both pathways, is described as a key miR-155 target mediating this putative phenotype. Furthermore, they showed that this miRNA is involved in EBV genome maintenance in latently infected cells as miR-155 inhibition causes a reduction of EBV copy number possibly due to a decrease in EBNA-1 levels. The discrepancy between the findings of these two groups with regard to cell growth and survival may be due to the technology used for suppressing miRNA function. Lu et al. used transient suppression with inhibitory RNA oligonucleotides, which may not have been sufficient to observe the potent growth phenotype observed upon stable suppression that was achieved by Linnstaedt et al. using a miR-155 “sponge”.
B. The miR-200 family as master regulators of the EBV latent/lytic switch
Although much of the published miRNA literature in the EBV field currently focuses on cellular miRNAs in EBV-infected B cells, no less important is an understanding of how this herpesvirus modulates miRNA expression in epithelial cells where it can drive the development of nasopharyngeal and gastric carcinomas. Of particular interest is the miR-200 miRNA family that has been recently recognized to function as a putative tumor suppressor due to its involvement in the suppression of epithelial to mesenchymal transition (EMT) during tumor progression and metastasis [62]. The miR-200 seed family contains five members located on two clusters: miR-200a, miR-200b and miR-429 situated on chromosome 1 and miR-200c and miR-141 on chromosome 12. Members of the same cluster are transcribed from the same primary transcript and share very similar seed sequences that differ by only one nucleotide. Both subgroups of this family have been shown to be important for maintaining the epithelial phenotype by targeting ZEB1 and ZEB2, two E-cadherin repressors and EMT activators which trigger cellular mobility and promote metastasis [63–66].
Interestingly, some components of this family are down regulated after EBV infection and their expression is reduced in several human cancers including EBV associated gastric carcinoma [67]. Indeed, Shinozaki et al. recently reported that miR-200a and miR-200b expression levels were decreased in EBV-associated gastric carcinoma as well as in EBV infected gastric carcinoma cell lines [67]. Furthermore, they demonstrated that this down regulation was mainly caused by the transcriptional repression of the primary miRNA, partially mediated by EBV latent genes BARF0, EBNA1, and LMP2A through unknown mechanisms. This down regulation resulted in the reduction of E-cadherin due to the presence of higher ZEB1 and ZEB2 expression, which ultimately led to the loss of cell adhesion and dramatic changes in epithelial morphology, promoting abnormal cell migration and invasion.
As both ZEB1 and ZEB2 have been shown to play a key role in the regulation of the EBV latent-lytic switch by repressing transcription from the EBV immediate-early BZLF1 gene promoter [68, 69], two groups recently investigated the role of the miR-200 family in the process of lytic reactivation [70, 71] (Fig. 1). Ellis-Connel et al. found that expression of miR-200b and miR-429 both in EBV infected epithelial and B cells was able to induce lytic replication by targeting ZEB1 and ZEB2 and blocking their repressing activity on the BZLF1 promoter Zp. Consistently, the down regulation of these miRNAs or the over expression of ZEB1 or ZEB2 led to a decrease in lytic reactivation. Likewise, Lin et al. arrived at the same conclusion. They showed that miR-429 expression in EBV infected fibroblasts and B cells shifted the latent/lytic equilibrium toward the lytic phase through repression of ZEB1.
C. miR-155 regulation of BMP signaling suppresses EBV lytic reactivation
Analysis of the 3’ UTRs containing miR-155 seed sequence led to the identification of several proteins belonging to the BMP signaling pathway as possible targets. BMPs (Bone morphogenetic proteins) are growth factors belonging to the transforming growth factor-beta (TGF-β) family that have been demonstrated to play a key role in a variety of developmental processes. BMPs signal through serine/threonine kinase receptors and transduce signals through Smad and non-Smad signaling pathways eventually modulating gene transcription. miR-155 was demonstrated to inhibit BMP signaling in EBV latency I cells transduced with miR155 expressing retrovirus by targeting two SMAD proteins (SMAD1 and SMAD 5), several transcriptional cofactors including RUNX2 and HIVEP2, as well as downstream BMP targets including MYO10 [72] (Fig. 1). After demonstrating that BMP signaling activation, similar to TGF-β, is able to reactivate EBV-infected B cells, Yin et al. showed evidence that miR-155 inhibits BMP-mediated lytic reactivation. These data suggest that one function of miR-155 could be to keep EBV infected cells in latency to ensure their survival by blocking the anti-tumor function of BMP signaling.
V. Genome-wide methods to identify mRNA targets of viral and cellular miRNAs in EBV-infected cells
A. mRNA expression profiling of miRNA expressing cells
One approach to identify putative miRNA targets and pathways affected by a given miRNA is to compare mRNA expression levels in cells expressing a given miRNA versus control cells not expressing that specific miRNA. In the case of miR-146a, which is highly EBV-induced, Cameron et al. performed a microarray-based gene expression comparison of wild-type Akata cells, which do not express miR-146a, and Akata in which miR-146a expression was induced by transduction with a retroviral vector expressing the primary miR-146a transcript. Interestingly, they found that miR-146a down-regulates several interferon stimulated genes (ISGs), though many of these changes were independent of direct miR-146a targeting [44]. A possible explanation for this down-regulation could be that EBV modulates the interferon-mediated response in order to preserve virus-infected cells and reduce the inflammatory response in vivo.
Other groups have also used microarray-based detection of mRNA changes looking for miRNA targets [56]. However, the shortcomings of this approach are the lack of miRNA seed specificity in many of the mRNA changes, as observed for the ISGs above, and the lack of robust quantitation of changes in either mRNA abundance or isoform change. Therefore, recently additional methods have been developed that account for these caveats and generate higher confidence mRNA target lists.
B. mRNA-seq of miRNA expressing cells
One such approach that addresses the shortcomings of the above method is deep sequencing of mRNAs (mRNA-Seq) in the context of specific miRNA expression or depletion. Specifically, mRNA-Seq addresses the problems of differential mRNA isoform usage and quantitation of mRNA abundance of putative miRNA targets. Recently, Xu, et al. performed mRNA-Seq in miR-155 expressing Mutu I cells, which normally do not express miR-155 [73]. This approach relies on deep sequencing of mRNAs followed by a computationally intensive mapping of these reads back to the expressed mRNAs from the human genome. The large number of sequence reads provides a broader dynamic range than oligonucleotide hybridization on microarrays. Furthermore, sequencing reads are derived from across the entire mRNA transcript which provides high resolution detail on mRNA isoform differences, an important attribute when characterizing the effects of miRNAs on the heterogeneous pool of mRNA species.
The experiments performed in miR-155 expressing Mutu I cells identified over 150 mRNAs with 7-mer or greater seed matching in their 3’UTRs. 76% of the 171 miR-155 3’UTRs identified by deep sequencing were quantitatively as sensitive to miR-155 in luciferase assays. Interestingly, several of the mR-155 targets from sequencing that did not conform in luciferase indicator assays were, in fact, expressed as shorter isoforms that did not contain the miR-155 seed match. These data are reminiscent of the recent findings by the Bartel and Burge laboratories describing a correlation between cell proliferation, miRNA expression, and shortening of 3’ UTRs containing miRNA seed matches [74, 75]. Therefore, this technology will be powerful to identify mRNA isoform changes induced by miRNA expression or depletion.
C. Immunoprecipitation of Argonaute-containing RISC complexes followed by mRNA abundance analysis on microarrays (Ago RIP-Chip)
An alternative approach to correlating miRNA expression with mRNA abundance focuses on identifying the mRNAs associated with miRNA-guided RISC complexes. Recently, Dolken, et al. used RIP-Chip to identify putative transcripts targeted by viral and cellular miRNAs in EBV latently infected B cells [76]. For this purpose, they used the EBV-negative Burkitt’s lymphoma cell line, BL41, and its infected counterpart, BL41/B95-8, which expresses a subset of viral miRNAs, as well as Jijoye, a cell line expressing all the viral miRNAs. They found 44 cellular miRNAs expressed and identified 2337 significantly enriched transcripts with predicted miRNA binding sites present mainly in coding region and 3’UTR. Among the identified transcripts there are some that have been already described to be targeted by specific miRNAs, as BACH1, FOS, IKBKE, RFK, RPS6KA3 and SPl1 for miR-155 [56, 77]. However, not all the already described targets are in the list of the putative transcripts obtained by RIP-Chip, as in the case of miR-21 and miR-146 targets. Furthermore, they observed 44 putative EBV miRNA targets with binding sites predominantly in 3’UTRs. Among the identified EBV miRNA targets, two genes were validated that are involved in cellular transport, IPO7 and TOMM22. These genes contain predicted binding sites for miR-BART16 and miR-BART3, respectively. The inhibition of these two proteins has been associated with prevention of apoptosis and reduction of cytokine production. Consequently, Dolken, et al. argued that EBV miRNAs are a tool for regulating trafficking and protein localization in order to block apoptosis and innate immunity. Moreover, their approach for identifying miRNA binding sites in RISC-associated mRNAs was an improvement over the less-specific mRNA abundance analysis described above.
D. Photo-activatable ribonucleoside cross-linking immunoprecipitatoin (PAR-CLIP) of Argonaute-containing RISC complexes followed by deep sequencing of associated RNAs
Despite its ability to identify mRNAs bound to RISC, RIP-Chip analysis has several disadvantages. These include being limited to the characterization of kinetically stable interactions and the inability to identify the specific miRNA binding site in each mRNA. A new approach, called PAR-CLIP (photoactivatable ribonucleoside-enhanced crosslinking and immunoprecipitation) for the identification at high resolution and transcriptome-wide of binding sites of cellular RNA binding proteins (RBP) and microRNA-containing ribonucleoprotein complexes was recently described [78]. PAR-CLIP is performed by first incorporating photo-reactive ribonucleoside analogs into nascent RNA transcripts followed by UV exposure at 365 nm, which induces efficient crosslinking of photoreactive nucleoside-labeled cellular RNAs to interacting RBPs. The isolated RNA is then converted into a cDNA library and deep sequenced [78]. This technique also has the advantage of enabling the identification of the precise location of the RBP recognition element making possible to distingue the crosslinked sequences from the background. Considering the many strengths of the PAR-CLIP technique, future studies aimed at identifying miRNA targets in EBV-infected cells with this approach will be quite informative. In fact, Linnstaedt et al. recently reported to have used this system to identify nearly 200 transcripts directly bound by miR-155 in LCLs [54].
Concluding remarks
Viral and cellular miRNAs are now recognized as important contributors to the pathogenesis of EBV in different cell types. EBV infection manipulates the expression of cellular miRNAs and drives expression of a large set of viral miRNAs. Targeting of these small non-coding RNAs to host and viral mRNAs have a profound effect on gene expression in the host cell by modulating the efficiency of immortalization in B cells, the switch between latency and lytic infection of B and epithelial cells, and possibly even targeting of transcripts in non-infected cells in vivo. The story has only just begun and the field is now poised for discovery with robust tools to analyze not only the contribution of miRNAs during infection, but also the mechanisms used by these miRNAs to achieve this through identifying specific target recognition sites. The rapid development of technologies to interrogate miRNAs over the coming years will only speed our understanding of this essential aspect of EBV biology.
Acknowledgments
The authors thank Bryan Cullen and Rebecca Skalsky for sharing unpublished data as well as reviewing the manuscript prior to submission. We also acknowledge the support of the Stewart Trust, the Duke Center for AIDS Research, and the American Cancer Society as well as a joint NIH award to Bryan Cullen and Micah Luftig (P30-AI045008) for collaborations in the study of HIV-associated malignancies.
References
- 1.Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136:215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Garzon R, Calin GA, Croce CM. MicroRNAs in Cancer. Annu. Rev. Med. 2009;60:167–179. doi: 10.1146/annurev.med.59.053006.104707. [DOI] [PubMed] [Google Scholar]
- 3.Lewis BP, Burge CB, Bartel DP. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell. 2005;120:15–20. doi: 10.1016/j.cell.2004.12.035. [DOI] [PubMed] [Google Scholar]
- 4.Skalsky RL, Cullen BR. Viruses, microRNAs, and host interactions. Annu. Rev. Microbiol. 2010;64:123–141. doi: 10.1146/annurev.micro.112408.134243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kieff E, Rickinson A. Epstein-Barr virus and its replication. In: Knipe DM, Howley PM, editors. Fields Virology. Philadelphia: Lippincott, Williams, and Wilkins; 2006. pp. 2603–2654. [Google Scholar]
- 6.Babcock GJ, Hochberg D, Thorley-Lawson AD. The expression pattern of Epstein-Barr virus latent genes in vivo is dependent upon the differentiation stage of the infected B cell. Immunity. 2000;13:497–506. doi: 10.1016/s1074-7613(00)00049-2. [DOI] [PubMed] [Google Scholar]
- 7.Babcock GJ, Decker LL, Volk M, Thorley-Lawson DA. EBV persistence in memory B cells in vivo. Immunity. 1998;9:395–404. doi: 10.1016/s1074-7613(00)80622-6. [DOI] [PubMed] [Google Scholar]
- 8.Uchida J, Yasui T, Takaoka-Shichijo Y, Muraoka M, Kulwichit W, Raab-Traub N, Kikutani H. Mimicry of CD40 signals by Epstein-Barr virus LMP1 in B lymphocyte responses. Science. 1999;286:300–303. doi: 10.1126/science.286.5438.300. [DOI] [PubMed] [Google Scholar]
- 9.Miller CL, Lee JH, Kieff E, Longnecker R. An integral membrane protein (LMP2) blocks reactivation of Epstein-Barr virus from latency following surface immunoglobulin crosslinking. Proc. Natl. Acad. Sci. U. S. A. 1994;91:772–776. doi: 10.1073/pnas.91.2.772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pfeffer S, Zavolan M, Grasser FA, Chien M, Russo JJ, Ju J, John B, Enright AJ, Marks D, Sander C, Tuschl T. Identification of virus-encoded microRNAs. Science. 2004;304:734–736. doi: 10.1126/science.1096781. [DOI] [PubMed] [Google Scholar]
- 11.Cai X, Schafer A, Lu S, Bilello JP, Desrosiers RC, Edwards R, Raab-Traub N, Cullen BR. Epstein-Barr virus microRNAs are evolutionarily conserved and differentially expressed. PLoS Pathogens. 2006;2:e23. doi: 10.1371/journal.ppat.0020023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grundhoff A, Sullivan CS, Ganem D. A combined computational and microarray-based approach identifies novel microRNAs encoded by human gamma-herpesviruses. RNA. 2006;12:733–750. doi: 10.1261/rna.2326106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhu JY, Pfuhl T, Motsch N, Barth S, Nicholls J, Grasser F, Meister G. Identification of novel Epstein-Barr virus microRNA genes from nasopharyngeal carcinomas. J. virol. 2009;83:3333–3341. doi: 10.1128/JVI.01689-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen SJ, Chen GH, Chen YH, Liu CY, Chang KP, Chang YS, Chen HC. Characterization of Epstein-Barr virus miRNAome in nasopharyngeal carcinoma by deep sequencing. PLoS One. 2010;5 doi: 10.1371/journal.pone.0012745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xia T, O'Hara A, Araujo I, Barreto J, Carvalho E, Sapucaia JB, Ramos JC, Luz E, Pedroso C, Manrique M, Toomey NL, Brites C, Dittmer DP, Harrington WJ., Jr EBV microRNAs in primary lymphomas and targeting of CXCL-11 by ebv-mir-BHRF1-3. Cancer Res. 2008;68:1436–1442. doi: 10.1158/0008-5472.CAN-07-5126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xing L, Kieff E. Epstein-Barr virus BHRF1 micro- and stable RNAs during latency III and after induction of replication. J. virol. 2007;81:9967–9975. doi: 10.1128/JVI.02244-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Amoroso R, Fitzsimmons L, Thomas WA, Kelly GL, Rowe M, Bell AI. Quantitative studies of Epstein-Barr virus-encoded microRNAs provide novel insights into their regulation. J. virol. 2011;85:996–1010. doi: 10.1128/JVI.01528-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cosmopoulos K, Pegtel M, Hawkins J, Moffett H, Novina C, Middeldorp J, Thorley-Lawson DA. Comprehensive profiling of Epstein-Barr virus microRNAs in nasopharyngeal carcinoma. J. Virol. 2009;83:2357–2367. doi: 10.1128/JVI.02104-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim DN, Chae HS, Oh ST, Kang JH, Park CH, Park WS, Takada K, Lee JM, Lee WK, Lee SK. Expression of viral microRNAs in Epstein-Barr virus-associated gastric carcinoma. J. virol. 2007;81:1033–1036. doi: 10.1128/JVI.02271-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Edwards RH, Marquitz AR, Raab-Traub N. Epstein-Barr virus BART microRNAs are produced from a large intron prior to splicing. J. virol. 2008;82:9094–9106. doi: 10.1128/JVI.00785-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pratt ZL, Kuzembayeva M, Sengupta S, Sugden B. The microRNAs of Epstein-Barr Virus are expressed at dramatically differing levels among cell lines. Virology. 2009;386:387–397. doi: 10.1016/j.virol.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cai X, Schäfer A, Lu S, Bilello JP, Desrosiers RC, Edwards R, Raab-Traub N, Cullen BR. Epstein-Barr virus microRNAs are evolutionarily conserved and differentially expressed. PLoS Pathogens. 2006;2:e23. doi: 10.1371/journal.ppat.0020023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yuan J, Cahir-McFarland E, Zhao B, Kieff E. Virus and cell RNAs expressed during Epstein-Barr virus replication. J. virol. 2006;80:2548–2565. doi: 10.1128/JVI.80.5.2548-2565.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gibbings DJ, Ciaudo C, Erhardt M, Voinnet O. Multivesicular bodies associate with components of miRNA effector complexes and modulate miRNA activity. Nat. Cell. Biol. 2009;11:1143–1149. doi: 10.1038/ncb1929. [DOI] [PubMed] [Google Scholar]
- 25.Pegtel DM, Cosmopoulos K, Thorley-Lawson DA, van Eijndhoven MA, Hopmans ES, Lindenberg JL, de Gruijl TD, Wurdinger T, Middeldorp JM. Functional delivery of viral miRNAs via exosomes. Proc. Natl. Acad. Sci. U. S. A. 2010;107:6328–6333. doi: 10.1073/pnas.0914843107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gourzones C, Gelin A, Bombik I, Klibi J, Verillaud B, Guigay J, Lang P, Temam S, Schneider V, Amiel C, Baconnais S, Jimenez AS, Busson P. Extra-cellular release and blood diffusion of BART viral micro-RNAs produced by EBV-infected nasopharyngeal carcinoma cells. Virol. J. 2010;7:271. doi: 10.1186/1743-422X-7-271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meckes DG, Jr, Shair KH, Marquitz AR, Kung CP, Edwards RH, Raab-Traub N. Human tumor virus utilizes exosomes for intercellular communication. Proc. Natl. Acad. Sci. U. S. A. 2010;107:20370–20375. doi: 10.1073/pnas.1014194107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Walz N, Christalla T, Tessmer U, Grundhoff A. A global analysis of evolutionary conservation among known and predicted gammaherpesvirus microRNAs. J. virol. 2010;84:716–728. doi: 10.1128/JVI.01302-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barth S, Pfuhl T, Mamiani A, Ehses C, Roemer K, Kremmer E, Jaker C, Hock J, Meister G, Grasser FA. Epstein-Barr virus-encoded microRNA miR-BART2 down-regulates the viral DNA polymerase BALF5. Nucleic Acids Res. 2008;36:666–675. doi: 10.1093/nar/gkm1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lo AK, To KF, Lo KW, Lung RW, Hui JW, Liao G, Hayward SD. Modulation of LMP1 protein expression by EBV-encoded microRNAs. Proc. Natl. Acad. Sci. U. S. A. 2007;104:16164–16169. doi: 10.1073/pnas.0702896104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lu JJ, Chen JY, Hsu TY, Yu WC, Su IJ, Yang CS. Induction of apoptosis in epithelial cells by Epstein-Barr virus latent membrane protein 1. J. Gen. Virol. 1996;77(Pt 8):1883–1892. doi: 10.1099/0022-1317-77-8-1883. [DOI] [PubMed] [Google Scholar]
- 32.Liu Y, Wang X, Lo AK, Wong YC, Cheung AL, Tsao SW. Latent membrane protein-1 of Epstein-Barr virus inhibits cell growth and induces sensitivity to cisplatin in nasopharyngeal carcinoma cells. J. Med. Virol. 2002;66:63–69. doi: 10.1002/jmv.2112. [DOI] [PubMed] [Google Scholar]
- 33.Lung RW, Tong JH, Sung YM, Leung PS, Ng DC, Chau SL, Chan AW, Ng EK, Lo KW, To KF. Modulation of LMP2A expression by a newly identified Epstein-Barr virus-encoded microRNA miR-BART22. Neoplasia. 2009;11:1174–1184. doi: 10.1593/neo.09888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Choy EY, Siu KL, Kok KH, Lung RW, Tsang CM, To KF, Kwong DL, Tsao SW, Jin DY. An Epstein-Barr virus-encoded microRNA targets PUMA to promote host cell survival. J. Exp. Med. 2008;205:2551–2560. doi: 10.1084/jem.20072581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nachmani D, Stern-Ginossar N, Sarid R, Mandelboim O. Diverse herpesvirus microRNAs target the stress-induced immune ligand MICB to escape recognition by natural killer cells. Cell Host Microbe. 2009;5:376–385. doi: 10.1016/j.chom.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 36.Seto E, Moosmann A, Gromminger S, Walz N, Grundhoff A, Hammerschmidt W. Micro RNAs of Epstein-Barr virus promote cell cycle progression and prevent apoptosis of primary human B cells. PLoS Pathogens. 2010;6 doi: 10.1371/journal.ppat.1001063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Feederle R, Linnstaedt SD, Bannert H, Lips H, Bencun M, Cullen BR, Delecluse HJ. A viral microRNA cluster strongly potentiates the transforming properties of a human herpesvirus. PLoS Pathogens. 2011;7:e1001294. doi: 10.1371/journal.ppat.1001294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Navarro A, Gaya A, Martinez A, Urbano-Ispizua A, Pons A, Balague O, Gel B, Abrisqueta P, Lopez-Guillermo A, Artells R, Montserrat E, Monzo M. MicroRNA expression profiling in classic Hodgkin lymphoma. Blood. 2008;111:2825–2832. doi: 10.1182/blood-2007-06-096784. [DOI] [PubMed] [Google Scholar]
- 39.Leucci E, Onnis A, Cocco M, De Falco G, Imperatore F, Giuseppina A, Costanzo V, Cerino G, Mannucci S, Cantisani R, Nyagol J, Mwanda W, Iriso R, Owang M, Schurfeld K, Bellan C, Lazzi S, Leoncini L. B-cell differentiation in EBV-positive Burkitt lymphoma is impaired at posttranscriptional level by miRNA-altered expression. Int. J. Cancer. 2010;126:1316–1326. doi: 10.1002/ijc.24655. [DOI] [PubMed] [Google Scholar]
- 40.Mrazek J, Kreutmayer SB, Grasser FA, Polacek N, Huttenhofer A. Subtractive hybridization identifies novel differentially expressed ncRNA species in EBV-infected human B cells. Nucleic Acids Res. 2007;35:e73. doi: 10.1093/nar/gkm244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cameron JE, Fewell C, Yin Q, McBride J, Wang X, Lin Z, Flemington EK. Epstein-Barr virus growth/latency III program alters cellular microRNA expression. Virology. 2008;382:257–266. doi: 10.1016/j.virol.2008.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Godshalk SE, Bhaduri-McIntosh S, Slack FJ. Epstein-Barr virus-mediated dysregulation of human microRNA expression. Cell Cycle. 2008;7:3595–3600. doi: 10.4161/cc.7.22.7120. [DOI] [PubMed] [Google Scholar]
- 43.Motsch N, Pfuhl T, Mrazek J, Barth S, Grasser FA. Epstein-Barr virus-encoded latent membrane protein 1 (LMP1) induces the expression of the cellular microRNA miR-146a. RNA Biol. 2007;4:131–137. doi: 10.4161/rna.4.3.5206. [DOI] [PubMed] [Google Scholar]
- 44.Cameron JE, Yin Q, Fewell C, Lacey M, McBride J, Wang X, Lin Z, Schaefer BC, Flemington EK. Epstein-Barr virus latent membrane protein 1 induces cellular MicroRNA miR-146a, a modulator of lymphocyte signaling pathways. J. Virol. 2008;82:1946–1958. doi: 10.1128/JVI.02136-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jiang J, Lee EJ, Schmittgen TD. Increased expression of microRNA-155 in Epstein-Barr virus transformed lymphoblastoid cell lines. Genes Chromosomes Cancer. 2006;45:103–106. doi: 10.1002/gcc.20264. [DOI] [PubMed] [Google Scholar]
- 46.Kluiver J, Haralambieva E, de Jong D, Blokzijl T, Jacobs S, Kroesen BJ, Poppema S, van den Berg A. Lack of BIC and microRNA miR-155 expression in primary cases of Burkitt lymphoma. Genes Chromosomes Cancer. 2006;45:147–153. doi: 10.1002/gcc.20273. [DOI] [PubMed] [Google Scholar]
- 47.Lu F, Weidmer A, Liu CG, Volinia S, Croce CM, Lieberman PM. Epstein-Barr virus-induced miR-155 attenuates NF-kappaB signaling and stabilizes latent virus persistence. J. virol. 2008;82:10436–10443. doi: 10.1128/JVI.00752-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rahadiani N, Takakuwa T, Tresnasari K, Morii E, Aozasa K. Latent membrane protein-1 of Epstein-Barr virus induces the expression of B-cell integration cluster, a precursor form of microRNA-155, in B lymphoma cell lines. Biochem. Biophys. Res. Commun. 2008;377:579–583. doi: 10.1016/j.bbrc.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 49.Yin Q, McBride J, Fewell C, Lacey M, Wang X, Lin Z, Cameron J, Flemington EK. MicroRNA-155 is an Epstein-Barr virus-induced gene that modulates Epstein-Barr virus-regulated gene expression pathways. J. virol. 2008;82:5295–5306. doi: 10.1128/JVI.02380-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gatto G, Rossi A, Rossi D, Kroening S, Bonatti S, Mallardo M. Epstein-Barr virus latent membrane protein 1 trans-activates miR-155 transcription through the NF-kappaB pathway. Nucleic Acids Res. 2008;36:6608–6619. doi: 10.1093/nar/gkn666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yin Q, Wang X, McBride J, Fewell C, Flemington E. B-cell receptor activation induces BIC/miR-155 expression through a conserved AP-1 element. J. Biol. Chem. 2008;283:2654–2662. doi: 10.1074/jbc.M708218200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Anastasiadou E, Boccellato F, Vincenti S, Rosato P, Bozzoni I, Frati L, Faggioni A, Presutti C, Trivedi P. Epstein-Barr virus encoded LMP1 downregulates TCL1 oncogene through miR-29b. Oncogene. 2010;29:1316–1328. doi: 10.1038/onc.2009.439. [DOI] [PubMed] [Google Scholar]
- 53.Pekarsky Y, Santanam U, Cimmino A, Palamarchuk A, Efanov A, Maximov V, Volinia S, Alder H, Liu CG, Rassenti L, Calin GA, Hagan JP, Kipps T, Croce CM. Tcl1 expression in chronic lymphocytic leukemia is regulated by miR-29 and miR-181. Cancer Res. 2006;66:11590–11593. doi: 10.1158/0008-5472.CAN-06-3613. [DOI] [PubMed] [Google Scholar]
- 54.Linnstaedt SD, Gottwein E, Skalsky RL, Luftig MA, Cullen BR. Virally induced cellular miR-155 plays a key role in B-cell immortalization by EBV. J. Virol. 2010 doi: 10.1128/JVI.01248-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Eis PS, Tam W, Sun L, Chadburn A, Li Z, Gomez MF, Lund E, Dahlberg JE. Accumulation of miR-155 and BIC RNA in human B cell lymphomas. Proc. Natl. Acad. Sci. U. S. A. 2005;102:3627–3632. doi: 10.1073/pnas.0500613102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gottwein E, Mukherjee N, Sachse C, Frenzel C, Majoros WH, Chi JT, Braich R, Manoharan M, Soutschek J, Ohler U, Cullen BR. A viral microRNA functions as an orthologue of cellular miR-155. Nature. 2007;450:1096–1099. doi: 10.1038/nature05992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Skalsky RL, Samols MA, Plaisance KB, Boss IW, Riva A, Lopez MC, Baker HV, Renne R. Kaposi's sarcoma-associated herpesvirus encodes an ortholog of miR-155. J. virol. 2007;81:12836–12845. doi: 10.1128/JVI.01804-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Morgan R, Anderson A, Bernberg E, Kamboj S, Huang E, Lagasse G, Isaacs G, Parcells M, Meyers BC, Green PJ, Burnside J. Sequence conservation and differential expression of Marek's disease virus microRNAs. J. virol. 2008;82:12213–12220. doi: 10.1128/JVI.01722-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhao Y, Xu H, Yao Y, Smith LP, Kgosana L, Green J, Petherbridge L, Baigent SJ, Nair V. Critical role of the virus-encoded microRNA-155 ortholog in the induction of Marek's disease lymphomas. PLoS Pathogens. 2011;7:e1001305. doi: 10.1371/journal.ppat.1001305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ebert MS, Neilson JR, Sharp PA. MicroRNA sponges: competitive inhibitors of small RNAs in mammalian cells. Nat. Methods. 2007;4:721–726. doi: 10.1038/nmeth1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lu P, Yang C, Guasparri I, Harrington W, Wang YL, Cesarman E. Early events of B-cell receptor signaling are not essential for the proliferation and viability of AIDS-related lymphoma. Leukemia. 2009;23:807–810. doi: 10.1038/leu.2008.304. [DOI] [PubMed] [Google Scholar]
- 62.Kong D, Li Y, Wang Z, Banerjee S, Ahmad A, Kim HR, Sarkar FH. miR-200 regulates PDGF-D-mediated epithelial-mesenchymal transition, adhesion, and invasion of prostate cancer cells. Stem Cells. 2009;27:1712–1721. doi: 10.1002/stem.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Burk U, Schubert J, Wellner U, Schmalhofer O, Vincan E, Spaderna S, Brabletz T. A reciprocal repression between ZEB1 and members of the miR-200 family promotes EMT and invasion in cancer cells. EMBO Rep. 2008;9:582–589. doi: 10.1038/embor.2008.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Korpal M, Lee ES, Hu G, Kang Y. The miR-200 family inhibits epithelial-mesenchymal transition and cancer cell migration by direct targeting of E-cadherin transcriptional repressors ZEB1 and ZEB2. J. Biol. Chem. 2008;283:14910–14914. doi: 10.1074/jbc.C800074200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Park SM, Gaur AB, Lengyel E, Peter ME. The miR-200 family determines the epithelial phenotype of cancer cells by targeting the E-cadherin repressors ZEB1 and ZEB2. Genes Dev. 2008;22:894–907. doi: 10.1101/gad.1640608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gregory PA, Bert AG, Paterson EL, Barry SC, Tsykin A, Farshid G, Vadas MA, Khew-Goodall Y, Goodall GJ. The miR-200 family and miR-205 regulate epithelial to mesenchymal transition by targeting ZEB1 and SIP1. Nat. Cell. Biol. 2008;10:593–601. doi: 10.1038/ncb1722. [DOI] [PubMed] [Google Scholar]
- 67.Shinozaki A, Sakatani T, Ushiku T, Hino R, Isogai M, Ishikawa S, Uozaki H, Takada K, Fukayama M. Downregulation of microRNA-200 in EBV-associated gastric carcinoma. Cancer Res. 2010;70:4719–4727. doi: 10.1158/0008-5472.CAN-09-4620. [DOI] [PubMed] [Google Scholar]
- 68.Yu X, Wang Z, Mertz JE. ZEB1 regulates the latent-lytic switch in infection by Epstein-Barr virus. PLoS Pathogens. 2007;3:e194. doi: 10.1371/journal.ppat.0030194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ellis AL, Wang Z, Yu X, Mertz JE. Either ZEB1 or ZEB2/SIP1 can play a central role in regulating the Epstein-Barr virus latent-lytic switch in a cell-type-specific manner. J. virol. 2010;84:6139–6152. doi: 10.1128/JVI.02706-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lin Z, Wang X, Fewell C, Cameron J, Yin Q, Flemington EK. Differential expression of the miR-200 family microRNAs in epithelial and B cells and regulation of Epstein-Barr virus reactivation by the miR-200 family member miR-429. J. virol. 2010;84:7892–7897. doi: 10.1128/JVI.00379-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ellis-Connell AL, Iempridee T, Xu I, Mertz JE. Cellular microRNAs 200b and 429 regulate the Epstein-Barr virus switch between latency and lytic replication. J. virol. 2010;84:10329–10343. doi: 10.1128/JVI.00923-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yin Q, Wang X, Fewell C, Cameron J, Zhu H, Baddoo M, Lin Z, Flemington EK. MicroRNA miR-155 inhibits bone morphogenetic protein (BMP) signaling and BMP-mediated Epstein-Barr virus reactivation. J. virol. 2010;84:6318–6327. doi: 10.1128/JVI.00635-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Xu G, Fewell C, Taylor C, Deng N, Hedges D, Wang X, Zhang K, Lacey M, Zhang H, Yin Q, Cameron J, Lin Z, Zhu D, Flemington EK. Transcriptome and targetome analysis in MIR155 expressing cells using RNA-seq. RNA. 2010;16:1610–1622. doi: 10.1261/rna.2194910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mayr C, Bartel DP. Widespread shortening of 3'UTRs by alternative cleavage and polyadenylation activates oncogenes in cancer cells. Cell. 2009;138:673–684. doi: 10.1016/j.cell.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sandberg R, Neilson JR, Sarma A, Sharp PA, Burge CB. Proliferating cells express mRNAs with shortened 3' untranslated regions and fewer microRNA target sites. Science. 2008;320:1643–1647. doi: 10.1126/science.1155390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dolken L, Malterer G, Erhard F, Kothe S, Friedel CC, Suffert G, Marcinowski L, Motsch N, Barth S, Beitzinger M, Lieber D, Bailer SM, Hoffmann R, Ruzsics Z, Kremmer E, Pfeffer S, Zimmer R, Koszinowski UH, Grasser F, Meister G, Haas J. Systematic analysis of viral and cellular microRNA targets in cells latently infected with human gamma-herpesviruses by RISC immunoprecipitation assay. Cell Host Microbe. 2010;7:324–334. doi: 10.1016/j.chom.2010.03.008. [DOI] [PubMed] [Google Scholar]
- 77.Vigorito E, Perks KL, Abreu-Goodger C, Bunting S, Xiang Z, Kohlhaas S, Das PP, Miska EA, Rodriguez A, Bradley A, Smith KG, Rada C, Enright AJ, Toellner KM, Maclennan IC, Turner M. microRNA-155 regulates the generation of immunoglobulin class-switched plasma cells. Immunity. 2007;27:847–859. doi: 10.1016/j.immuni.2007.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hafner M, Landthaler M, Burger L, Khorshid M, Hausser J, Berninger P, Rothballer A, Ascano M, Jr, Jungkamp AC, Munschauer M, Ulrich A, Wardle GS, Dewell S, Zavolan M, Tuschl T. Transcriptome-wide identification of RNA-binding protein and microRNA target sites by PAR-CLIP. Cell. 2010;141:129–141. doi: 10.1016/j.cell.2010.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]