Abstract
We investigated the effects of mangiferin on the expression and activity of metalloproteinase (MMP)-9 and the invasion of tumor necrosis factor (TNF)-α-stimulated human LNCaP prostate carcinoma cells. Reverse-transcription polymerase chain reaction (RT-PCR) and western blot analysis showed that mangiferin significantly reversed TNF-α-induced mRNA and protein expression of MMP-9 expression. Zymography data confirmed that stimulation of cells with TNF-α significantly increased MMP-9 activity. However, mangiferin substantially reduced the TNF-α-induced activity of MMP-9. Additionally, a matrigel invasion assay showed that mangiferin significantly reduced TNF-α-induced invasion of LNCaP cells. Compared to untreated controls, TNF-α-stimulated LNCaP cells showed a significant increase in nuclear factor-κB (NF-κB) luciferase activity. However, mangiferin treatment markedly decreased TNF-α-induced NF-κB luciferase activity. Furthermore, mangiferin suppressed nuclear translocation of the NF-κB subunits p65 and p50. Collectively, our results indicate that mangiferin is a potential anti-invasive agent that acts by suppressing NF-κB-mediated MMP-9 expression. [BMB Reports 2015; 48(10): 559-564]
Keywords: Invasion, Mangiferin, Matrix metalloproteinase-9, Nuclear factor kappa B
INTRODUCTION
Matrix metalloproteinases (MMPs) are zinc-dependent endopeptidases, which belong to a larger family of zinc-finger proteins (1). Many studies over the last several decades have shown that MMPs play a crucial role in tumor invasion and metastasis, which involves tissue remodeling via the extracellular matrix, degradation of the basement membrane, and induction of angiogenesis (2, 3). Recently, 24 human MMP genes were identified including the MMP-9, which is a key effector molecule in the breakdown of the extracellular matrix in normal physiological processes such as embryonic development, reproduction, cell migration, and wound healing (4, 5). In particular, MMP-9 is expressed in various types of cancers, including bladder, brain, liver, prostate, and pancreatic cancers (6). Moreover, an increase in MMP-9 expression positively correlates with tumor stage, grade, and prognosis (7). Most complications associated with prostate cancer are attributable to metastasis to distant organs including the brain, liver, lungs, bones, and genitourinary sites (8, 9). Therefore, therapies that regulate MMP-9 expression can be used to treat prostate cancers.
Tumor necrosis factor (TNF)-α is involved in all stages of carcinogenesis, including cellular transformation, proliferation, survival, metastasis, and angiogenesis, and can be detected in many human cancer tissues such as ovarian and breast (10, 11). Following the binding of their ligand, TNF-α receptors induce recruitment of adaptor proteins, which activate various signal transduction pathways, including the nuclear factor-kappa B (NF-κB) pathway (12). NF-κB directly regulates diverse biological processes, such as cell growth and survival, tissue homeostasis, immune responses, and inflammation by regulating various target genes. Normally, NF-κB subunits are located in the cytoplasm as inactive dimers that consist of p65 and p50 subunits. In response to various stimuli, the inhibitor of kappa B (IκB) is phosphorylated and degraded, and then, NF-κB translocates to the nucleus. Subsequently, NF-κB binds to specific DNA sequences to regulate the expression of target genes such as MMP-9, which are related to tumor development and metastasis. Therefore, deregulation of NF-κB signaling is a plausible therapeutic approach in the treatment of inflammatory diseases and cancer invasion (13).
Mangiferin (Fig. 1A), which is C-glucopyranoside 1,3,6,7- tetrahydroxyxanthone, is a natural phytopolyphenol that exhibits various pharmacological properties (14, 15). Mangiferin is present in several plant species such as Mangifera indica, Iris unguicularis, and Anemarrhena asphodeloides (16). A recent study showed that mangiferin selectively inhibits MMP-9 gene expression in phorbol 12-myristate 13-acetate (PMA)-induced human astrogliomas by inhibiting the binding of NF-κB and activator protein (AP)-1 (15). Furthermore, mangiferin exerts antitumor activity in breast cancer cells by significantly inhibiting the activation of the β-catenin pathway, which is involved in the regulation of MMP-7, MMP-9, and epithelial-mesenchymal transition (14). However, to the best of our knowledge, there are no studies evaluating the effects of mangiferin on MMP-9 gene expression in TNF-α-stimulated non-invasive prostate cancer cells. Therefore, in this study, we evaluated the effects of mangiferin on MMP-9 expression in TNF-α-stimulated non-invasive LNCaP prostate carcinoma cells. Our results showed that mangiferin downregulates TNF-α-induced MMP-9 mRNA and protein expression by suppressing NF-κB activity, consequently suppressing the invasion of LNCaP cells.
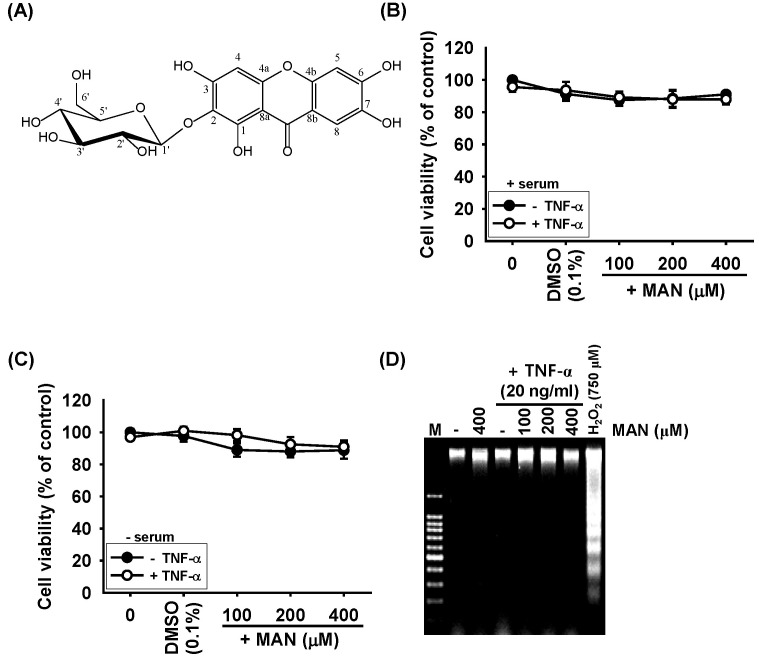
Fig. 1. Effects of mangiferin (MAN) on the viability of LNCaP prostate carcinoma cells. (A) Chemical structure of MAN. (B) LNCaP cells treated with the indicated concentrations of MAN or 20 ng/ml TNF-α in the presence or (C) absence of serum for 24 h. Cell viability was measured using an MTT assay after 24 h. (D) Total DNA was extracted from the treated cells, and a DNA fragmentation assay was performed on a 1.5% agarose gel. MTT, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium briomide.
RESULTS
Effects of mangiferin on cell viability
To assess whether mangiferin influences the viability of LNCaP cells, we performed a 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay 24 h after treatment with different concentrations of mangiferin in the presence or absence of TNF-α in serum and serum-free conditions. There were no cytotoxic evident in LNCaP cells treated with up to 400 μM of mangiferin alone under both serum and serum-free conditions (Fig. 1B and 1C). Additionally, in the presence of TNF-α (20 ng/ml), mangiferin did not affect cell viability. Furthermore, to elucidate the effect of mangiferin on cytotoxicity, we analyzed DNA fragmentation as an apoptotic marker. Compared to the hydrogen peroxide (H2O2)-treated positive group, treatment with mangiferin alone or in the presence of TNF-α showed no fragmentation of DNA (Fig. 1D). Therefore, the concentration of mangiferin used in the subsequent experiments was this range.
Suppression of MMP-9 activity and invasion by mangiferin
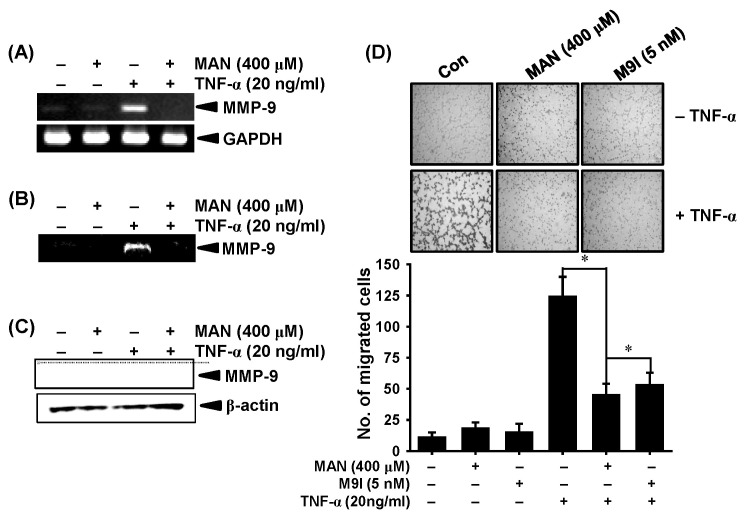
To assess whether mangiferin regulates MMP-9 expression, we performed RT-PCR, zymography, and western blot analysis. The RT-PCR showed that mangiferin significantly suppressed the TNF-α-induced MMP-9 expression in LNCaP cells (Fig. 2A). In addition, zymography and western blot analysis showed that stimulation of cells with TNF-α significantly increased MMP-9 expression; whereas, pretreatment with mangiferin significantly suppressed this effect (Fig. 2B and 2C). Furthermore, we examined the inhibitory effects of mangiferin on the invasion of LNCaP cells. Compared to that in the untreated control (11 ± 4 pg/ml), TNF-α-treated LNCaP cells (124 ± 16 pg/ml) showed a marked increase in cell invasion. However, pretreatment with mangiferin and the MMP-9 inhibitor similarly decreased the TNF-α-induced penetration of cells through a matrigel-coated membrane by approximately 60% (45 ± 9 pg/ml and 53 ± 11 pg/ml, respectively, Fig. 2D). These results confirmed that mangiferin inhibits TNF-α-induced invasion in LNCaP cells.
Fig. 2. Effects of mangiferin (MAN) on TNF-α-induced matrix metalloproteinase (MMP)-9 expression and activity in LNCaP prostate carcinoma cells. (A) LNCaP cells (2 × 105 cells/ml) were incubated with the indicated concentrations of MAN for 1 h before TNF-α (20 ng/ml) treatment for 6 h. Total RNA was isolated and reverse transcription-polymerase chain reaction (RT-PCR) was performed using MMP-9 specific primers. (B) In a parallel experiment, cell-free supernatants were collected after 24 h, followed by gelatin zymography. (C) Equal amounts of cell lysates were resolved on sodium dodecyl sulfate (SDS)-polyacrylamide gels, transferred to nitrocellulose membranes, and probed with antibodies against MMP-9. GAPDH and β-actin were used as internal controls for RT-PCR and western blot analysis, respectively. (D) The upper compartments of trans wells were coated with matrigel for the invasion assay. The cells were cultured in serum-free media for 3 h before treatment with 400 μM MAN and 5 nM MMP-9 inhibitor (M9I) in the absence or presence of 20 ng/ml TNF-α. After 24-h incubation, the cells that passed through the matrigel to the membrane were stained using 0.125% Coomassie blue in ethanol. Statistical significance was determined using one-way analysis of variance (ANOVA) (*P < 0.05 vs. TNF-α-treated group). GAPDH, glyceraldehyde 3-phosphate dehydrogenase.
Inhibition of TNF-α-induced NF-κB activity by mangiferin
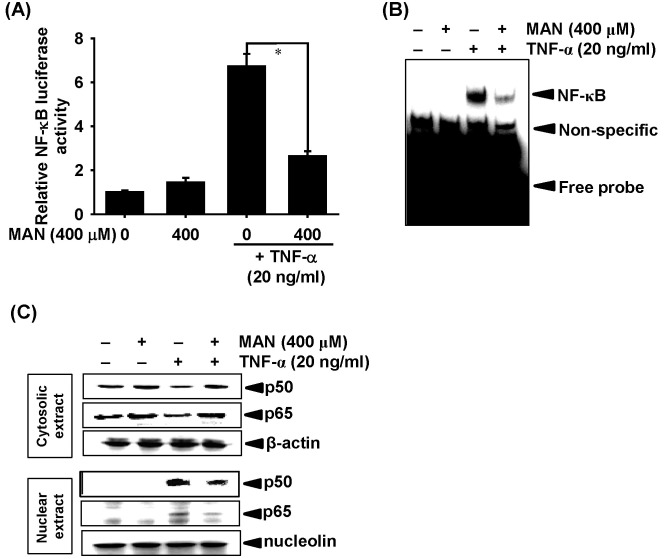
To determine the importance of the role MMP-9 expression in NF-κB activity, we performed a promoter assay using LNCaP cells transiently transfected with a luciferase reporter vector expressing NF-κB-binding sites. Compared to the untreated control cells, TNF-α-stimulated LNCaP cells showed approximately a 7-fold increase in NF-κB luciferase activity (Fig. 3A). Mangiferin treatment significantly decreased the NF-κB luciferase activity in TNF-α-stimulated LNCaP cells. Then, to determine whether mangiferin inhibits MMP-9 activity by suppressing NF-κB, we determined the specific DNA-binding activity of NF-κB. The electrophoretic mobility shift assay (EMSA) showed that TNF-α-stimulation markedly increased the binding complexes between NF-κB and specific-binding DNA after 30 min. However, pretreatment with mangiferin for 1 h significantly decreased TNF-α-induced NF-κB activity (Fig. 3B). We simultaneously determined the cytoplasmic and nuclear expression levels of p65 and p50 after TNF-α stimulation in the absence or presence of mangiferin. Treatment with TNF-α significantly decreased the cytoplasmic and increased the nuclear expression of p65 and p50 in the LNCaP cells (Fig. 3C). However, treatment with mangiferin did not alter the TNF-α- induced expression of p65 and p50 in the cytoplasm (Fig. 3C). These data indicate that mangiferin downregulates the TNF-α- induced NF-κB activity.
Fig. 3. Effects of mangiferin (MAN) on nuclear factor kappa B (NF-κB) activity. LNCaP cells were treated with 400 μM MAN in the presence of TNF-α (20 ng/ml). (A) Cells were transfected with wild-type NF-κB promoter-containing reporter vector, and luciferase activity of NF-κB was measured after 24 h. (B) The nuclear extracts were prepared after 30 min, and NF-κB binding to its DNA promoter region in the extract was measured using an electrophoretic mobility shift assay (EMSA). (C) Levels of p65 and p50 were determined in the cytosolic (top) and nuclear (bottom) extracts using western blot analysis after 30 min. β-Actin and nucleolin were used as internal controls for the western blot analysis. Statistical significance was determined using a one-way analysis of variance (ANOVA) (*P < 0.05 vs. TNF-α-treated group).
DISCUSSION
Mangiferin is a well-known plant-derived natural polyphenol, which exhibits various pharmacological properties such as antitumor, anticarcinogenesis, and hepatoprotection (17, 18). However, whether mangiferin exerts its anti-invasive effects on prostate carcinoma cells by regulating MMP-9 expression has not been determined. Our results indicate that mangiferin inhibited the TNF-α-induced MMP-9 expression and invasion of LNCaP cells by suppressing NF-κB activity.
Invasion and metastasis are complex processes that involve multiple host-tumor interactions. These interactions lead to the separation of a cell or a group of cells from a primary tumor, invasion of the host tissue, and survival of the tumor cells at secondary sites for successful proliferation (19, 20). MMPs are normally involved in the digestive and tissue repair processes of the extracellular matrix (21). Nevertheless, the inhibition of MMP-9 may still be an ideal strategy to control tumor growth, invasion, and metastasis because MMP-9 is a crucial molecule involved in promoting the progression of carcinogenesis (22, 23). Our results showed that mangiferin inhibits TNF-α-induced MMP-9 activity and suppresses MMP-9 transcription in LNCaP cells. Moreover, the matrigel invasion assay showed significantly decreased invasion of LNCaP cells. MMPs are regulated by the activation of precursor zymogens and inhibited by specific tissue inhibitors of MMPs (TIMPs) (24). Thus, the balance between MMPs and TIMPs is critical for the remodeling of the extracellular matrix in tissues. Therefore, it is important to consider that TIMPS may regulate the mangiferin-induced MMP-9 suppression in the extracellular matrix.
NF-κB transcription factor has been the focus of numerous studies aimed at elucidating its role in regulating MMP-9 expression in human prostate carcinoma cells (25, 26). In the past decades, considerable progress was made in elucidating the contribution of NF-κB to oncogenesis and carcinogenesis. In particular, hyperactivation of the NF-κB signaling cascade, mutations that inactivate the inhibitory IκB subunits and aberrations in the chromosomes involving various NF-κB genes have been investigated in many human carcinomas (27). Therefore, researchers have been attempting to develop anti-invasive agents from natural compounds that downregulate MMP-9 expression by suppressing NF-κB activity. In addition, a previous study showed that the tumorigenic and metastatic properties of human prostate cancer cells in nude mice were inhibited via downregulation of MMP-9 by the suppression of NF-κB activity (25). In this study, we found that mangiferin inhibited TNF-α-induced NF-κB activity in LNCaP cells, which indicates that mangiferin inhibits the invasive activity of LNCaP cells by suppressing NF-κB-dependent MMP-9 expression. However, Crowe and Brown reported that AP-1 sites in the MMP-9 promoter region are important in the MMP-9 expression regulated by molecules of the c-Jun and c-fos family (21). Therefore, further studies are required to determine whether other transcriptional elements are involved in MMP-9 expression regulated by mangiferin.
LNCaP is non-metastatic cancer cell line that does not express MMP-9 in normal and PMA-stimulated conditions (28). However, TNF-α triggers cell invasion and metastasis by activating MMP-9 in various cancer cells. Therefore, this study suggests that mangiferin may suppress TNF-α-mediated cancer invasion and metastasis in vitro by inhibiting MMP-9 expression. Collectively, these data suggest that mangiferin is a potential therapeutic candidate for the prevention of prostate cancer invasion.
MATERIALS AND METHODS
Reagent and antibodies
Mangiferin and 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphnyl-2H-tetrazolium bromide (MTT) were purchased from Sigma (St. Louis, MO) and dissolved in DMSO (vehicle). Antibodies against p65, p50, MMP-9, nucleolin, and β-actin were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Roswell Park Memorial Institute Medium (RPMI), antibiotics mixture, and fetal bovine serum (FBS) were obtained from WelGENE Inc. (Daegu, Republic of Korea). Peroxidase-labeled goat anti-rabbit immunoglobulin was purchased from KOMA Biotechnology (Seoul, Republic of Korea). MMP-9 inhibitor I was obtained from Merck (Darmstadt, Germany). Other chemicals were purchased as Sigma grades.
Cell culture and cell viability assay
LNCaP cells (American Type Culture Collection, Manassas, VA) were cultured at 37℃ in 5% CO2 in RPMI supplemented with 10% FBS and antibiotics. Cell viability was determined by an MTT assay. Briefly, LNCaP cells (1 × 105 cells/ml) were plated onto 24 well plates and incubated overnight in serum and serum-free RPMI media. The cells were treated with the indicated concentrations of mangiferin for 1 h and then stimulated with TNF-α (20 ng/ml) for 24 h. Then, the cells were incubated with a solution of 0.5 mg/ml MTT and incubation for 45 min at 37℃ and 5% CO2. Supernatant was removed and the formation of formazan was observed by monitoring the signal at 540 nm using a microplate reader.
DNA fragmentation assay
LNCaP cells were treated with the indicated chemicals and then lysed on ice in a buffer containing 10 mM Tris-HCl (pH 7.4), 150 mM NaCl, 5 mM EDTA, and 0.5% Triton X-100 for 30 min. Lysates were vortexed and cleared by centrifugation at 10,000 g for 20 min. Fragmented DNA in the supernatant was extracted with an equal volume of neutral phenol:chloroform:isoamylalcohol (25:24:1, v/v/v) and analyzed electrophoretically on a 1.5% agarose gel containing ethidium bromide.
Western blot analysis
Total cell extracts were prepared using PROPREP protein extraction kit (iNtRON Biotechnology, Sungnam, Republic of Korea). Cytoplasmic and nuclear extracts were prepared using NE-PER nuclear and cytosolic extraction reagents (Pierce, Rockford, IL). Briefly, after treatment with the indicated concentrations of mangiferin, cells were harvested, washed once with ice-cold PBS, and gently lysed for 15 min in 100 μl ice-cold PRO-PREP lysis buffer. Lysates were centrifuged at 14,000 g for 10 min. Supernatants were collected and protein concentrations determined using a Bio-Rad protein assay kit (Bio-Rad, Hercules, CA). The samples were stored at −80℃ or immediately used for western blot analysis. The proteins were separated on sodium dodecyl sulfate (SDS)-polyacrylamide gels and transferred to nitrocellulose membranes (Schleicher & Schuell, Keene, NH). Proteins were detected using an enhanced chemiluminescence detection system (Amersham, Arlington Heights, IL).
RNA extraction and RT-PCR
Total RNA was isolated using Trizol reagent (GIBCO-BRL, Gaithersburg, MD) according to the manufacturer's instructions. Genes of interest were amplified from cDNA that was reverse-transcribed from 1 μg of total RNA using the One-Step RT-PCR Premix (iNtRON Biotechnology, Sungnam, Republic of Korea). Primers for MMP-9 sense (5'- CCT GGA GAC CTG AGA ACC AAT CT-3') and MMP-9 antisense (5'- CCA CCC GAG TGT AAC CAT AGC-3'); and glyceraldehydes-3-phosphate dehydrogenase (GAPDH) sense (5'-CCA CCC ATG GCA AAT TCC ATG GCA-3') and GAPDH antisense (5'-TCT AGA CGG CAG GTC AGG TCC ACC-3') were used. PCR reaction was initiated at 94℃ for 2 min followed by 31 cycles of 94℃ for 30 sec, 30-sec annealing temperature, 72℃ for 30 sec followed by final extension at 72℃ for 5 min. Annealing temperatures for MMP-9 and GAPDH were 63℃ and 61℃, respectively. After amplification, PCR products were separated on 1.5% agarose gels and visualized by ethidium bromide fluorescence.
EMSA
EMSA was performed with the nuclear extract. Synthetic complementary NF-κB (5'-AGT TGA GGG GAC TTT CCC AGG C-3') binding oligonucleotides (Santa Cruz Biotechnology) were 3'-biotinylated using the biotin 3'-end DNA labeling kit (Pierce) according to the manufacturer's instructions, and annealed for 30 min at room temperature. Assays were loaded onto native 4% polyacrylamide gels pre-electrophoresed for 60 min in 0.5× Tris borate/EDTA before being transferred onto a positively charged nylon membrane (HybondTM-N+) in 0.5× Tris borate/EDTA at 100 V for 30 min. The transferred DNAs were cross-linked to the membrane at 120 mJ/cm2. Horseradish peroxidase-conjugated streptavidin was used according to the manufacturer's instructions to detect the transferred DNA.
Gelatin substrate gel zymography
LNCaP cells were incubated at 37℃ in 5% CO2 in serum-free RPMI medium with or without mangiferin for 24 h. Supernatants were collected and then subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) copolymerized with gelatin. After electrophoresis, the gels were washed several times with 2.5% Triton X-100 for 1 h at room temperature to remove the SDS and then incubated for 24 h at 37℃ in reaction buffer containing 5 mM CaCl2 and 1 μM ZnCl2. The gels were stained with 0.25% Coomassie blue for 30 min and then destained for 1 h in a solution of acetic acid and methanol. The proteolytic activity was evidenced as clear bands (zones of gelatin degradation) against the blue background of stained gelatin.
Invasion assay
Five × 104 cells/chamber were used for each invasion assy. Invasion assays were performed using modified Boyden chambers with polycarbonate nucleopore membrane (Corning, Corning, NY). Precoated filters (6.5 mm in diameter, 8 μm pore-size, matrigel 100 μg/cm2) were rehydrated and 5 × 104 cells in medium with or without mangiferin or MMP-9 inhibitor I (5 nM) in the presence of TNF-α were seeded into the upper part of each chamber. After incubation of 24 h, nonmigratory cells on the upper surface of the filter were wiped with a cotton swab and migrated cells on the lower surface of the filter were fixed and stained with 0.125% Coomassie Blue in a methanol:acetic acid:water mixture (45 : 10 : 45, v/v/v). Random fields were counted under a light microscope.
Luciferase assay
NF-κB reporter construct were purchased from Clontech (Palo Alto, CA). Briefly, cells were plated onto six-well plates at a density of 5 × 105 cells/well and grown overnight. Cells were transfected with 2 μg of each plasmid construct for 6 h by the Lipofectamine method. After transfection, the cells were cultured in 10% FBS with the indicated concentrations of mangiferin in the presence of 20 ng/ml TNF-α for 24 h. Cells were lysed with lysis buffer (20 mM Tris-HCl, pH 7.8, 1% Triton X-100, 150 mM NaCl, and 2 mM DTT). The cell lysate was mixed with luciferase activity assay reagent and luminescence produced for 5 s was measured using GLOMAX luminometer (Promega, Madison, WI).
Statistical analysis
All data were derived from at least three independent experiments. The images were visualized with Chemi-Smart 2000 (Vilber Lourmat, Cedex, France). Images were captured using Chemi-Capt (Vilber Lourmat) and transported into Adobe Photoshop (version 8.0). All data are presented as mean ± SE. Significant differences between the groups were determined using two-way ANOVA test. A value of *P < 0.05 was accepted as an indication of statistical significance.
Acknowledgments
This work was supported by the research grant from the Chuongbong Academic Research Fund of Jeju National University in 2014.
References
- 1.Zucker S, Vacirca J. Role of matrix metalloproteinases (MMPs) in colorectal cancer. Cancer Metastasis Rev. (2004);23:101–117. doi: 10.1023/A:1025867130437. [DOI] [PubMed] [Google Scholar]
- 2.McCawley LJ, Matrisian LM. Matrix metalloproteinases: multifunctional contributors to tumor progression. Mol Med Today. (2000);6:149–156. doi: 10.1016/S1357-4310(00)01686-5. [DOI] [PubMed] [Google Scholar]
- 3.Zhang S, Li L, Lin JY, et al. Imbalance between expression of matrix metalloproteinase-9 and tissue inhibitor of metalloprtienase-1 in invasiveness and metastasis of human gastric carcinoma. World J Gastroenterol. (2003);9:899–904. doi: 10.3748/wjg.v9.i5.899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen L, Nakai M, Belton RJ, Jr, et al. Expression of extracellular matrix metalloproteinase inducer and matrix metalloproteinases during mouse embryonic development. Reproduction. (2007);133:405–414. doi: 10.1530/rep.1.01020. [DOI] [PubMed] [Google Scholar]
- 5.Liu Y, Min D, Bolton T, et al. Increased matrix metalloproteinase-9 predicts poor wound healing in diabetic foot ulcers. Diabetes Care. (2009);32:117–119. doi: 10.2337/dc08-0763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yao J, Xiong S, Klos K, et al. Multiple signaling pathways involved in activation of matrix metalloproteinase-9 (MMP-9) by heregulin-beta1 in human breast cancer cells. Oncogene. (2001);20:8066–8074. doi: 10.1038/sj.onc.1204944. [DOI] [PubMed] [Google Scholar]
- 7.Ibtissam C, Hassane R, José ML, et al. Screening of antibacterial activity in marine green and brown macroalgae from the coast of Morocco. Afr J Biotechnol. (2009);8:1258–1262. [Google Scholar]
- 8.de Oliveira Barros EG, Palumbo A, Jr, Mello PL, et al. The reciprocal interactions between astrocytes and prostate cancer cells represent an early event associated with brain metastasis. Clin Exp Metastasis. (2014);31:461–474. doi: 10.1007/s10585-014-9640-y. [DOI] [PubMed] [Google Scholar]
- 9.Sammon JD, Kaczmarek BF, Ravi P, et al. Effect of metastatic site on emergency department disposition in men with metastatic prostate cancer. Can J Urol. (2013);20:7008–7014. [PubMed] [Google Scholar]
- 10.Charles KA, Kulbe H, Soper R, et al. The tumor-promoting actions of TNF-α involve TNFR1 and IL-17 in ovarian cancer in mice and humans. J Clin Invest. (2009);119:3011–3023. doi: 10.1172/JCI39065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stuelten CH, Byfield SDC, Arany PR, et al. Breast cancer cells induce stromal fibroblasts to express MMP-9 via secretion of TNF-α and TGF-β. J Cell Sci. (2005);118:2143–2153. doi: 10.1242/jcs.02334. [DOI] [PubMed] [Google Scholar]
- 12.Balkwill F. TNF-α in promotion and progression of cancer. Cancer Metastasis Rev. (2006);25:409–416. doi: 10.1007/s10555-006-9005-3. [DOI] [PubMed] [Google Scholar]
- 13.Nguyen DP, Li J, Yadav SS, et al. Recent insights into NF-κB signaling pathways and the link between inflammation and prostate cancer. BJU Int. (2014);114:168–176. doi: 10.1111/bju.12488. [DOI] [PubMed] [Google Scholar]
- 14.Das J, Ghosh J, Roy A, et al. Mangiferin exerts hepatoprotective activity against D-galactosamine induced acute toxicity and oxidative/nitrosative stress via Nrf2- NFκB pathways. Toxicol Appl Pharmacol. (2012);260:35–47. doi: 10.1016/j.taap.2012.01.015. [DOI] [PubMed] [Google Scholar]
- 15.Jung JS, Jung K, Kim DH, et al. Selective inhibition of MMP-9 gene expression by mangiferin in PMA-stimulated human astroglioma cells: involvement of PI3K/Akt and MAPK signaling pathways. Pharmacol Res. (2012);66:95–103. doi: 10.1016/j.phrs.2012.02.013. [DOI] [PubMed] [Google Scholar]
- 16.Qin J, Deng J, Feng X, et al. Quantitative RP-LC analysis of mangiferin and homomangiferin in Mangifera indica L. leaves and in Mangifera persiciforma C.Y. Wu et T.L. ming leaves. Chromatographia. (2008);68:955–960. doi: 10.1365/s10337-008-0842-9. [DOI] [Google Scholar]
- 17.Li H, Huang J, Yang B, et al. Mangiferin exerts antitumor activity in breast cancer cells by regulating matrix metalloproteinases, epithelial to mesenchymal transition, and β-catenin signaling pathway. Toxicol Appl Pharmacol. (2013);272:180–190. doi: 10.1016/j.taap.2013.05.011. [DOI] [PubMed] [Google Scholar]
- 18.Rajendran P, Ekambaram G, Sakthisekaran D. Protective role of mangiferin against benzo(a)pyrene induced lung carcinogenesis in experimental animals. Biol Pharm Bull. (2008);31:1053–1058. doi: 10.1248/bpb.31.1053. [DOI] [PubMed] [Google Scholar]
- 19.Chaffer CL, Weinberg RA. A perspective on cancer cell metastasis. Science. (2011);331:1559–1564. doi: 10.1126/science.1203543. [DOI] [PubMed] [Google Scholar]
- 20.Nabeshima K, Inoue T, Shimao Y, et al. Matrix metalloproteinases in tumor invasion: role for cell migration. Pathol Int. (2002);52:255–264. doi: 10.1046/j.1440-1827.2002.01343.x. [DOI] [PubMed] [Google Scholar]
- 21.Crowe DL, Brown TN. Transcriptional inhibition of matrix metalloproteinase 9 (MMP-9) activity by a c-fos/estrogen receptor fusion protein is mediated by the proximal AP-1 site of the MMP-9 promoter and correlates with reduced tumor cell invasion. Neoplasia. (1999);1:368–372. doi: 10.1038/sj.neo.7900041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Folgueras AR, Pendás AM, Sánchez LM, et al. Matrix metalloproteinases in cancer: from new functions to improved inhibition strategies. Int J Dev Biol. (2004);48:411–424. doi: 10.1387/ijdb.041811af. [DOI] [PubMed] [Google Scholar]
- 23.Nelson AR, Fingleton B, Rothenberg ML, et al. Matrix metalloproteinases: biologic activity and clinical implications. J Clin Oncol. (2000);18:1135–1149. doi: 10.1200/JCO.2000.18.5.1135. [DOI] [PubMed] [Google Scholar]
- 24.Visse R, Nagase H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: structure, function, and biochemistry. Circ Res. (2003);92:827–839. doi: 10.1161/01.RES.0000070112.80711.3D. [DOI] [PubMed] [Google Scholar]
- 25.Andela VB, Gordon AH, Zotalis G, et al. NFkappaB: a pivotal transcription factor in prostate cancer metastasis to bone. Clin Orthop Relat Res. (2003);415:S75–S85. doi: 10.1097/01.blo.0000093048.96273.aa. [DOI] [PubMed] [Google Scholar]
- 26.Vayalil PK, Mittal A, Katiyar SK. Proanthocyanidins from grape seeds inhibit expression of matrix metalloproteinases in human prostate carcinoma cells, which is associated with the inhibition of activation of MAPK and NFκB. Carcinogenesis. (2004);25:987–995. doi: 10.1093/carcin/bgh095. [DOI] [PubMed] [Google Scholar]
- 27.Diaz-Meco MT, Moscat J. Akt regulation and lung cancer: a novel role and mechanism of action for the tumor suppressor Par-4. Cell Cycle. (2008);7:2817–2820. doi: 10.4161/cc.7.18.6735. [DOI] [PubMed] [Google Scholar]
- 28.Roomi MW, Monterrey JC, Kalinovsky T, et al. Patterns of MMP-2 and MMP-9 expression in human cancer cell lines. Oncol Rep. (2009);21:1323–1333. doi: 10.3892/or_00000358. [DOI] [PubMed] [Google Scholar]