Abstract
Objective
A nucleosomal protein, HMGB1, can be secreted by activated immune cells or passively released by dying cells, thereby amplifying rigorous inflammatory responses. In this study we aimed to test the possibility that ionizing radiation similarly induces cytoplasmic HMGB1 translocation and extracellular release.
Method
Human skin fibroblast (GM0639) and bronchial epithelial (16HBE) cells and animals (rats) were exposed to X-ray radiation, and HMGB1 translocation and release were assessed by immunocytochemistry and immunoassay, respectively.
Results
At a wide dose range (4.0 – 12.0 Gy), X-ray radiation induced a dramatic cytoplasmic HMGB1 translocation, and triggered a time- and dose-dependent HMGB1 release both in vitro and in vivo. The radiation-mediated HMGB1 release was associated with noticeable chromosomal DNA damage and loss of cell viability.
Conclusion
radiation induces HMGB1 cytoplasmic translocation and extracellular release through active secretion and passive leakage processes.
Keywords: X-ray, HMGB1, tumor cells, inflammatory response, damage-associated molecule pattern molecules (DAMP)
HMGB1 is ubiquitously expressed in most cells to maintain a large “pool” of pre-formed protein in the nucleus [1;2] owing to the existence of two nuclear-localization sequences (NLS) that facilitate its nuclear transportation [3]. Within the nucleus, HMGB1 binds chromosomal DNA, and fulfills its nuclear functions in maintaining nucleosomal structures and regulating gene expression [4]. The localized depletion of HMGB1 expression renders HMGB1-deficient tissues more susceptible to infectious [5] or injurious insults [6;7], suggesting an overall protective role of intracellular HMGB1 against stresses [8].
In response to microbial toxins (such as CpG-DNA and endotoxin) [9;10], cytokines [e.g., interferon (IFN)-γ and Cold-inducible RNA-binding protein (CIRP)] [11–13] or oxidative free radicals (e.g., hydrogen peroxide) [14], macrophages/monocytes acetylate and/or phosphorylate the NLS of HMGB1 [8;15–17], enabling its sequestration into cytoplasmic vesicles destined for subsequent secretion [2;11;18]. Cytoplasmic HMGB1 can be secreted through several pathways, including the double-stranded RNA-activated protein kinase R (PKR)- and Caspase-1/Caspase-11-mediated inflammasome activation and pyroptosis. For instance, genetic disruption PKR expression or pharmacological inhibition of PKR phosphorylation similarly reduces NLRP3 or NLRP1 agonists-induced inflammasome activation [19;20], pyroptosis [19;20] and HMGB1 release [19].
In addition to active secretion, HMGB1 can be passively released from damaged cells [21] following ischemia/reperfusion [22;23], trauma [24;25] or toxemia [26–28], thereby serving as a damage-associated molecular pattern molecule (DAMP). Although ionizing (X-ray) radiation emits high energy photons that can ionize atoms to disrupt molecular bonds, it was previously unknown whether it similarly induces HMGB1 cytoplasmic translocation and release. Here we provided evidence that X-ray irradiation induces a time- and dose-dependent HMGB1 cytoplasmic translocation and release by tumor cells in vitro, and stimulates systemic HMGB1 accumulation in vivo.
1 MATERIAL AND METHODS
1.1 Cells
Human skin fibroblast GM0639 cell line was obtained from the Radiobiological Laboratory of the National Research Center for Environment and Health (GSF), Germany. Human bronchial epithelial 16HBE cell line was obtained from the Shanghai Cell Collection Center of the Chinese Academy of Sciences. These cells were maintained as a monolayer in low glucose DMEM culture medium supplemented with 10% fetal calf serum (Hyclone, Logan, UT, USA), 2 mmol/L glutamine, penicillin (100 U/ml) and streptomycin (100 U/ml). Cells were kept at 37 °C in an atmosphere of 5% carbon dioxide and 95% air, and subcultured twice a week to remain in exponential growth. Cells were washed twice with, and subsequently cultured in, serum-free OPTI-MEM I medium (Gibco BRL, Grand Island, NY) before X-ray irradiation.
1.2 Animal
Male and female Sprague-Dawley rats (8–12 weeks old, 220–250 g) were allowed to acclimate for 7 days before X-ray irradiation. All animal procedures were approved by the Institutional Animal Care and Use Committee of the Soochow University. Rats were housed in the animal facility of the Key Laboratory of Jiangsu Radiation Medicine and Protection under standard temperature, and light and dark cycles.
1.3 X-ray irradiation
As a form of ionizing radiation when charged electrons or ions of sufficient energy hit a material with high speed, X-ray was generated in high voltage electron tubes of X-ray generators (Primus High-Energy Siemens), which emitted high energy photons at a rate of 200 cGy/min. For cell irradiation, a locator with a source-cell distance of 100 mm, irradiation field of 40 × 40 cm was employed to expose cell cultures to X-ray at doses ranging from 0 to 8 Gy. For animal experiment, rats were irradiated using X-ray generator emitting at a fixed dose rate of 200 cGy/min, with an irradiation field of 40 × 40 cm, centered 100 mm above the animals. At various time points after X-ray radiation, 0.5 ml blood was collected from each animal (3 male and 3 female) by tail bleeding, and serum HMGB1 levels were determined using the Shino-Test Corporation ELISA kit.
1.4 Immunocytochemistry and cell fractionation/Western blot
At 24 h after X-ray radiation, cellular HMGB1 was immunostained with HMGB1-specific polyclonal antibodies, and images were acquired using a fluorescent microscope as previously described [29]. Alternatively, the subcellular localization of HMGB1 was examined by a cell fractionation/Western blotting technique as previously described [29]. Cell fractionation is based on differential lysis of plasma and nuclear membranes by nonionic detergent (NP-40). Briefly, after selective lysis of the plasma membrane in low salt buffer (10 mM HEPES, pH 7.9; 10 mM KCl; 0.1 mM EDTA; 0.1 mM EGTA; 1 mM DTT; 0.5 mM PMSF, 1% NP-40), the intact nuclei was collected by a quick centrifugation step (7,000 g, 1 min, 4°C), leaving the cytoplasmic fraction in the supernatant. The nuclei pellet was resuspended in NP-40 high salt buffer (20 mM HEPES, pH 7.9; 0.4 M NaCl; 1 mM EDTA; 1 mM EGTA; 1 mM DTT; 1 mM PMSF, 1% NP-40), and briefly sonicated to generate the nuclear fraction. After fractionation, the protein content of different fractions was determined by a Bradford method, and each fraction was assayed for levels of various protein by Western blotting analysis using primary antibodies specific for HMGB1, a cytoplasmic protein (β-actin, Santa Cruz Biotechnology), and a nuclear protein (Lamin B1, BD Biosciences).
1.5 DNA damage assay
Immediately after irradiation, cells grown on covered slide chambers (Lab-Tek, Nunc, Napterville, IL, USA) were washed with PBS and fixed with 2% paraformaldehyde in PBS for 15 min. After three washes of PBS with 10 min each, the cells were treated with 0.2% Triton X-100 solution in PBS for 5 min, and stained with mouse monoclonal antibody for Serine 139 phospho-H2AX from Millipore (Cat. 05636, 1: 200) overnight at 4°C. After extensive washings, FITC-labeled rabbit anti-mouse antibodies were added, and fluorescent images of cells were captured using a fluorescence microscope.
1.6 MTT assay
Cell viability was measured by the reduction of yellow tetrazolium salt [MTT, 3-(4, 5-dimethylthiazolyl-2)-2, 5-diphenyltetrazolium bromide) by dehydrogenase of metabolically active cells, to generate reducing purple formazan that can be solubilized and quantified by spectrophotometry. The MTT substrate was prepared in a physiologically balanced solution, added to cell culture at a final concentration of 0.2 mg/ml, and incubated for 2 hours. The quantity of the resultant purple formazan was measured by recording changes in absorbance at 570 nm using a plate reading spectrophotometer.
1.7 Statistical analysis
Data are expressed as mean ± SEM of two independent experiments in triplicates. One-way analyses of variance (ANOVA) followed by the Tukey’s test for multiple comparisons were used to compare between different groups. A P value less than 0.05 was considered statistically significant.
2. RESULTS
2.1 Ionizing radiation induces HMGB1 cytoplasmic translocation
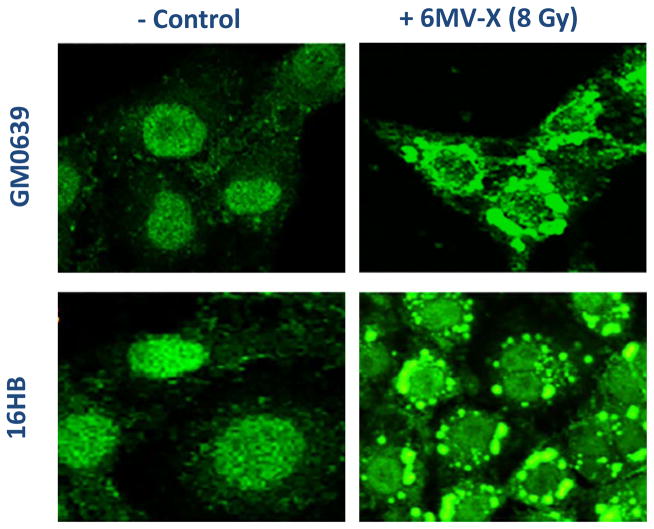
To assess the impact of X-ray irradiation on possible HMGB1 release, we first determined its effect on HMGB1 cytoplasmic translocation – an essential step for subsequent HMGB1 release. Quiescent tumor cells constitutively expressed HMGB1 and maintained an intracellular “pool” of HMGB1 predominantly in the nucleus (Fig. 1, left panels). At 24 h post X-ray irradiation (8.0 Gy), large amount of HMGB1 staining was also noticed in numerous cytoplasmic vesicles (Fig. 1, right panels), suggesting that ionizing radiation stimulated tumor cells to actively translocate nuclear HMGB1 into the cytoplasmic vesicles before releasing into the extracellular milieu.
Figure 1. Ionizing radiation induced cytoplasmic HMGB1 translocation in tumor cells.
Human skin fibroblast (GM0639) and bronchial epithelial (16HBE) cells were subjected to 6 MeV X-ray radiation at a dose of 8 Gy for 24 h, and assayed for HMGB1 cytoplasmic translocation by imunohistochemistry using HMGB1-specific antibodies. Note that HMGB1 was predominantly localized in the nuclear region of un-treated cells (“control”), but found in both cytoplasmic and nuclear regions of X-ray radiated cells (“6MeV-X”).
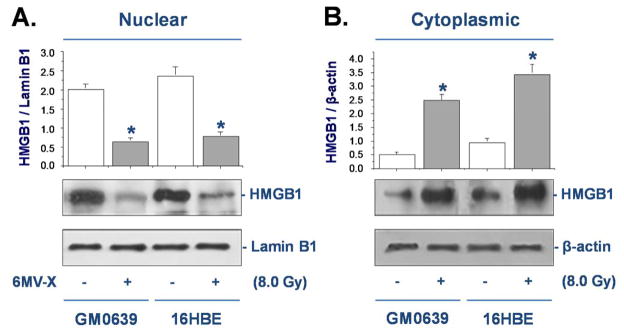
To confirm the cytoplasmic HMGB1 translocation, whole cell lysates were fractionated and the levels of HMGB1 in the cytoplasmic and nuclear fractions were determined by Western blotting analysis. The relative levels of HMGB1 (with reference to Lamin B1) in the nuclear fractions were significantly reduced in both GM0639 and 16HBE tumor cells after X-ray irradiation (Fig. 2A). In parallel, the relative levels of HMGB1 (with reference to β-actin) in the cytoplasmic fraction were significantly elevated after irradiation (Fig. 2B), confirming that X-ray irradiation induced significant HMGB1 cytoplasmic translocation in these tumor cells.
Figure 2. Ionizing radiation inversely altered nuclear and cytoplasmic HMGB1 levels.
Following X-ray radiation, cytoplasmic and nuclear fractions were isolated, and assayed for HMGB1 levels with reference to a nuclear (Lamin B1) or cytoplasmic (β-actin) marker by Western blotting analysis. Equal loading of samples was confirmed by Western blotting analysis of respective fractions with cytoplasmic (β-actin) or nuclear (Lamin B1) protein markers.
2.2 Ionizing irradiation induces HMGB1 release
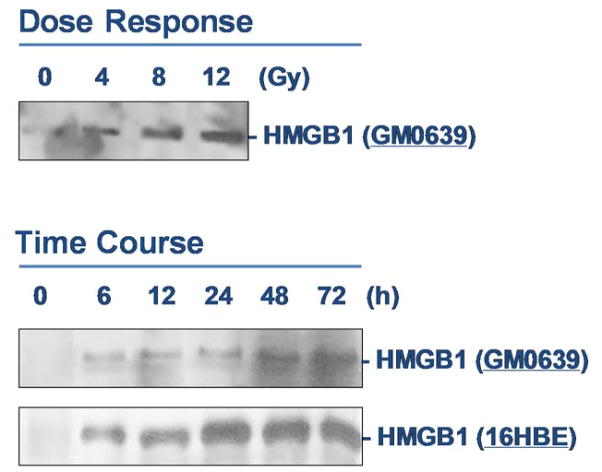
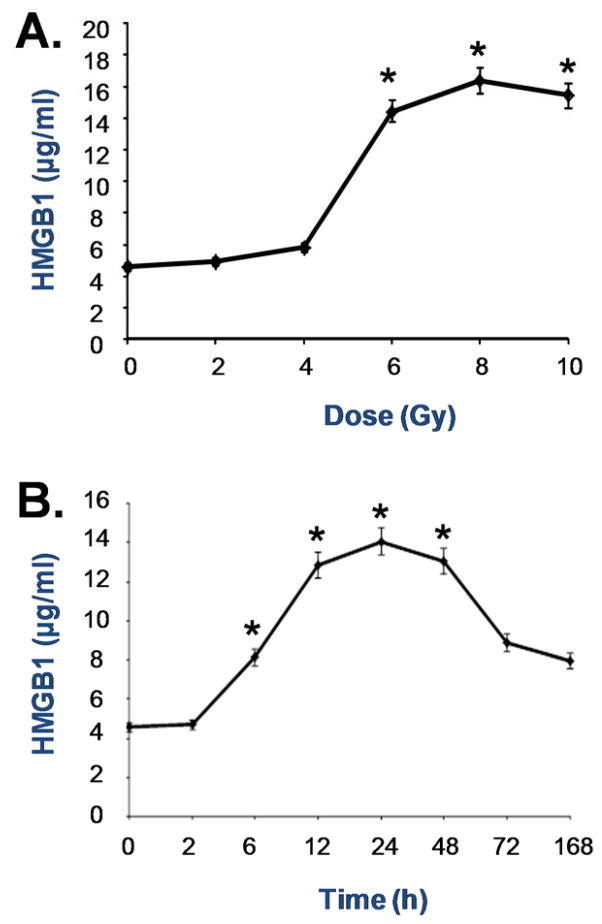
To determine whether X-ray irradiation induces HMGB1 release, extracellular levels of HMGB1 in the cell-conditioned culture medium were determined by Western blotting analysis. The levels of HMGB1 in the culture medium conditioned by the quiescent tumor cells were relatively low. Following X-ray irradiation, extracellular HMGB1 levels were elevated in a dose- and time-dependent fashion (Fig. 3). At doses as low as 4–8 Gy, X-ray irradiation induced HMGB1 release as early as 6 h post stimulation (Fig. 3).
Figure 3. Ionizing radiation induced a dose- and time-dependent HMGB1 release.
Human skin fibroblast (GM0639) and/or bronchial epithelial (16HBE) were exposed to X-ray radiation at various doses for different time periods, and extracellular HMGB1 levels were determined by Western blotting analysis. Note that proteins were recovered from equal volume of cell-conditioned medium, and sample loading was normalized by equal volume of cell-conditioned medium.
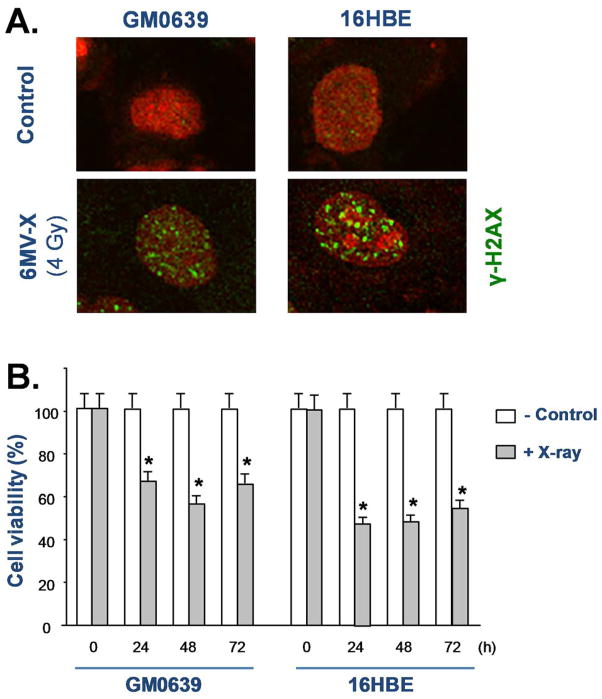
In addition to active secretion, HMGB1 could also be passively released from injured cells. It is known that ionizing radiation can cause double-stranded breaks of chromosomal DNA, which activates histone γ-H2AX phosphorylation, and results in the recruitment of DNA repair proteins to form the γ-H2AX foci, a biomarker for chromosomal DNA damage. To test the impact of ionizing radiation on DNA damage, we examined the effect of X-ray irradiation on the formation of γ-H2AX foci in both tumor cell lines. As indicated in Fig. 4A, X-ray irradiation, at a dose as low as 4 Gy, induced marked DNA damage as judged by the formation of γ-H2AX foci (Fig. 4A). Consistently, the cell viability was significantly reduced by X-ray irradiation in both GM0639 and 16HBE tumor cells (Fig. 4B), suggesting that X-ray irradiation induced HMGB1 release partly through passive leakage from these dying cells.
Figure 4. Ionizing radiation caused DNA damage and loss of cell viability.
Human skin fibroblast (GM0639) and bronchial epithelial (16HBE) cells were exposed to X-ray at a dose of 4 Gy, and cells were stained with γ-H2AX-specific antibodies to detect DNA damage. In parallel, the cell viability was determined by MTT assay, and expressed as a % of controls in the absence of X-ray radiation. *, P < 0.05 versus untreated control at respective time points.
2.3 Ionizing radiation induces systemic HMGB1 accumulation in vivo
To examine whether X-ray irradiation induces HMGB1 release in vivo, we subjected male and female rats to X-ray irradiation at different doses, and measured circulating levels of HMGB1 by ELISA. In agreement with the in vitro findings, X-ray irradiation induced systemic HMGB1 accumulation in a dose- and time-dependent fashion (Fig. 5). At a dose as low as 6 Gy, X-ray irradiation induced significant HMGB1 accumulation in the circulation as early as 6 h post stimulation (Fig. 5).
Figure 5. Ionizing radiation elevated circulating HMGB1 levels in vivo.
Male Sprague-Dawley rats were exposed to X-ray radiation at various doses and for different time periods, and blood samples were collected to measure serum HMGB1 levels by ELISA. *, P < 0.05 versus untreated controls (no radiation, Panel A; or immediately prior to X-ray radiation, Panel B).
3. DISCUSSION
As a form of ionizing radiation, X-rays emit high energy photons that can donate energy to cellular molecules, kicking out atomic electrons from the inner orbit to produce unstable and highly reactive free radicals. These radicals quickly react with nearby molecules, resulting in breakage of chemical bonds and oxidation (addition of oxygen atoms) of the affected molecules. In the present study, we demonstrated that X-ray irradiation induced DNA damage as manifested by the formation of γ-H2AX foci in the nuclei, and induced cytoplasmic HMGB1 translocation and release in human skin fibroblast (GM0639) and bronchial epithelial (16HBE) cell lines. Similar findings are made in breast (MCF-7, data not shown), lung (NCI-H1703), prostate (DU-145 and PC-3), colorectal (HCT 15 and SW480) [30] and glioblastoma (T98G and U251MG) [31] tumor cell lines.
As aforementioned, cytoplasmic HMGB1 translocation also occurs in innate immune cells following stimulation with cytokines (IFN-γ) [11] or hydrogen peroxide [14;32]. Because cytoplasmic HMGB1 translocation was not closely associated with the occurrence of cell death [11;14;32], it was believed that cytoplasmic HMGB1 translocation might be an active process regulated by chemical modifications of HMGB1 NLS. Notably, ionizing radiation also induces water radiolysis to produce free radicals, which can be converted into hydrogen peroxide (H2O2). Because hydrogen peroxide can induce active HMGB1 secretion or passive leakage [14;32], we propose that X-ray induces HMGB1 release through multiple mechanisms that are dependent on both active cytoplasmic translocation and passive leakage.
In animals, X-ray radiation causes injury initially at the skin, but goes beyond the surface and continues to damage inner tissues in the body. Although many irradiated cells could repair DNA and protein damage, some cells would die of necrosis or apoptosis. It is thus possible that ionizing radiation may induce HMGB1 secretion and leakage to amplify an inflammatory response. Indeed, HMGB1 carries three redox-sensitive cysteine residues (C23, C45 and C106), and can exist in three isoforms termed “HMGB1” (all thiol form), “disulfide HMGB1” (partially oxidized), and oxidized HMGB1 [33;34]. The “all-thiol” HMGB1 binds to other chemokines (e.g., CXCL12) to facilitate leukocyte recruitment via the CXCR4 receptor [35] or other signaling molecules [36–38] to the sites of injury [39;40]. In a sharp contrast, the “disulfide” HMGB1 can activate immune cells to produce cytokines/chemokines via TLR4 or other receptors such as RAGE [41], TLR2, TLR4 [42–44], TLR9 [10;41], cluster of differentiation 24 (CD24)/Siglec-10 [45], Mac-1 [38], thrombomodulin [46], or single transmembrane domain proteins (e.g., syndecans) [47]. Once fully oxidized, HMGB1 is devoid of either chemokine or cytokine activities [33;34]. Thus, extracellular HMGB1 could serve as a proinflammatory signal to recruit and activate innate immune cells to sustain a potentially injurious inflammatory response to ionizing radiation.
It has been well established that excessive HMGB1 release adversely contributes to the pathogenesis of infection- and injury-elicited inflammatory diseases, because HMGB1-neutralizing antibodies are protective in animal models of sepsis [9;48–51], ischemia/reperfusion [22;52;53], trauma [54;55], chemical toxemia [26;56;57], atherosclerosis [58], gastric ulcer [59] and hyperoxia [60]. Even during lethal infection [9;48–51], tissue injury is accompanied by massive HMGB1 release that further amplifies the cytokine storm to precipitate organ dysfunction. In fact, HMGB1 itself could trigger caspase-1-dependent programmed cell death, pyroptosis, which is characterized by rapid plasma membrane rupture, and release of proinflammatory intracellular contents (including HMGB1) [61]. Although it is difficult to distinguish between microbial infection-induced sepsis from injury-elicited systemic inflammatory response syndrome [62;63], it might be important to develop strategies to specifically attenuate radiation-mediated inflammatory responses.
Recently, many herbal components such as the Green tea polyphenolic catechins [64], tanshinones [29], carbenoxolone [65] have been shown to inhibit HMGB1 release through multiple mechanisms. These divergent mechanisms include the stimulation of autophagic degradation of cytoplasmic HMGB1 [66], the enhancement of endocytosis of exogenous HMGB1 into cytoplasmic vesicles [67], or inhibition of key signaling molecules (e.g., PKR) involved in the regulation of HMGB1 release [68]. It may be important to assess whether these herbal HMGB1 inhibitors can similarly prevent radiation-induced excessive inflammation without compromising the efficacy of ionizing radiation in the treatment of malignant cancers.
Acknowledgments
Fund program: H.W. is supported by the U.S. National Center of Complementary and Alternative Medicine (NCCAM, R01AT005076) and the U.S. National Institute of General Medical Sciences (NIGMS, R01GM063075).
References
- 1.Chen G, Li J, Qiang X, et al. Suppression of HMGB1 release by stearoyl lysophosphatidylcholine: an additional mechanism for its therapeutic effects in experimental sepsis. J Lipid Res. 2005;46:623–7. doi: 10.1194/jlr.C400018-JLR200. [DOI] [PubMed] [Google Scholar]
- 2.Bonaldi T, Talamo F, Scaffidi P, et al. Monocytic cells hyperacetylate chromatin protein HMGB1 to redirect it towards secretion. EMBO J. 2003;22:5551–60. doi: 10.1093/emboj/cdg516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kang R, Chen R, Zhang Q, et al. HMGB1 in health and disease. Mol Aspects Med. 2014:10. doi: 10.1016/j.mam.2014.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bustin M. At the crossroads of necrosis and apoptosis: signaling to multiple cellular targets by HMGB1. Sci STKE. 2002;2002:E39. doi: 10.1126/stke.2002.151.pe39. [DOI] [PubMed] [Google Scholar]
- 5.Yanai H, Matsuda A, An J, et al. Conditional ablation of HMGB1 in mice reveals its protective function against endotoxemia and bacterial infection. Proc Natl Acad Sci U S A. 2013;110:20699–704. doi: 10.1073/pnas.1320808110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kang R, Zhang Q, Hou W, et al. Intracellular Hmgb1 inhibits inflammatory nucleosome release and limits acute pancreatitis in mice. Gastroenterology. 2014;146:1097–107. doi: 10.1053/j.gastro.2013.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang H, Nace GW, McDonald KA, et al. Hepatocyte-specific high-mobility group box 1 deletion worsens the injury in liver ischemia/reperfusion: a role for intracellular high-mobility group box 1 in cellular protection. Hepatology. 2014;59:1984–97. doi: 10.1002/hep.26976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lu B, Wang C, Wang M, et al. Molecular mechanism and therapeutic modulation of high mobility group box 1 release and action: an updated review. Expert Rev Clin Immunol. 2014;10:713–27. doi: 10.1586/1744666X.2014.909730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang H, Bloom O, Zhang M, et al. HMG-1 as a late mediator of endotoxin lethality in mice. Science. 1999;285:248–51. doi: 10.1126/science.285.5425.248. [DOI] [PubMed] [Google Scholar]
- 10.Ivanov S, Dragoi AM, Wang X, et al. A novel role for HMGB1 in TLR9-mediated inflammatory responses to CpG-DNA. Blood. 2007;110:1970–81. doi: 10.1182/blood-2006-09-044776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rendon-Mitchell B, Ochani M, Li J, et al. IFN-gamma Induces High Mobility Group Box 1 Protein Release Partly Through a TNF-Dependent Mechanism. J Immunol. 2003;170:3890–7. doi: 10.4049/jimmunol.170.7.3890. [DOI] [PubMed] [Google Scholar]
- 12.Kim JH, Kim SJ, Lee IS, et al. Bacterial endotoxin induces the release of high mobility group box 1 via the IFN-beta signaling pathway. J Immunol. 2009;182:2458–66. doi: 10.4049/jimmunol.0801364. [DOI] [PubMed] [Google Scholar]
- 13.Qiang X, Yang WL, Wu R, et al. Cold-inducible RNA-binding protein (CIRP) triggers inflammatory responses in hemorrhagic shock and sepsis. Nat Med. 2013;19:1489–95. doi: 10.1038/nm.3368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tang D, Shi Y, Kang R, et al. Hydrogen peroxide stimulates macrophages and monocytes to actively release HMGB1. J Leukoc Biol. 2007;81:741–7. doi: 10.1189/jlb.0806540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lu B, Antoine DJ, Kwan K, et al. JAK/STAT1 signaling promotes HMGB1 hyperacetylation and nuclear translocation. Proc Natl Acad Sci U S A. 2014;111:3068–73. doi: 10.1073/pnas.1316925111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Youn JH, Shin JS. Nucleocytoplasmic shuttling of HMGB1 is regulated by phosphorylation that redirects it toward secretion. J Immunol. 2006;177:7889–97. doi: 10.4049/jimmunol.177.11.7889. [DOI] [PubMed] [Google Scholar]
- 17.Wang H, Ward MF, Sama AE. Targeting HMGB1 in the treatment of sepsis. Expert Opin Ther Targets. 2014;18:257–68. doi: 10.1517/14728222.2014.863876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gardella S, Andrei C, Ferrera D, et al. The nuclear protein HMGB1 is secreted by monocytes via a non-classical, vesicle-mediated secretory pathway. EMBO Rep. 2002;3:955–1001. doi: 10.1093/embo-reports/kvf198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lu B, Nakamura T, Inouye K, et al. Novel role of PKR in inflammasome activation and HMGB1 release. Nature. 2012;488:670–4. doi: 10.1038/nature11290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hett EC, Slater LH, Mark KG, et al. Chemical genetics reveals a kinase-independent role for protein kinase R in pyroptosis. Nat Chem Biol. 2013;9:398–405. doi: 10.1038/nchembio.1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Scaffidi P, Misteli T, Bianchi M. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature. 2002;418:191–5. doi: 10.1038/nature00858. [DOI] [PubMed] [Google Scholar]
- 22.Tsung A, Sahai R, Tanaka H, et al. The nuclear factor HMGB1 mediates hepatic injury after murine liver ischemia-reperfusion. J Exp Med. 2005;201:1135–43. doi: 10.1084/jem.20042614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Andrassy M, Volz HC, Igwe JC, et al. High-mobility group box-1 in ischemia-reperfusion injury of the heart. Circulation. 2008;117:3216–26. doi: 10.1161/CIRCULATIONAHA.108.769331. [DOI] [PubMed] [Google Scholar]
- 24.Cohen MJ, Brohi K, Calfee CS, et al. Early release of high mobility group box nuclear protein 1 after severe trauma in humans: role of injury severity and tissue hypoperfusion. Crit Care. 2009;13:R174. doi: 10.1186/cc8152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peltz ED, Moore EE, Eckels PC, et al. HMGB1 is markedly elevated within 6 hours of mechanical trauma in humans. Shock. 2009;32:17–22. doi: 10.1097/shk.0b013e3181997173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhou RR, Liu HB, Peng JP, et al. High mobility group box chromosomal protein 1 in acute-on-chronic liver failure patients and mice with ConA-induced acute liver injury. Exp Mol Pathol. 2012;93:213–9. doi: 10.1016/j.yexmp.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 27.Antoine DJ, Dear JW, Lewis PS, et al. Mechanistic biomarkers provide early and sensitive detection of acetaminophen-induced acute liver injury at first presentation to hospital. Hepatology. 2013;58:777–87. doi: 10.1002/hep.26294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Seo YS, Kwon JH, Yaqoob U, et al. HMGB1 recruits hepatic stellate cells and liver endothelial cells to sites of ethanol induced parenchymal cell injury. Am J Physiol Gastrointest Liver Physiol. 2013 doi: 10.1152/ajpgi.00151.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li W, Li J, Ashok M, et al. A cardiovascular drug rescues mice from lethal sepsis by selectively attenuating a late-acting proinflammatory mediator, high mobility group box 1. J Immunol. 2007;178:3856–64. doi: 10.4049/jimmunol.178.6.3856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schildkopf P, Frey B, Mantel F, et al. Application of hyperthermia in addition to ionizing irradiation fosters necrotic cell death and HMGB1 release of colorectal tumor cells. Biochem Biophys Res Commun. 2010;391:1014–20. doi: 10.1016/j.bbrc.2009.12.008. [DOI] [PubMed] [Google Scholar]
- 31.Pasi F, Paolini A, Nano R, et al. Effects of single or combined treatments with radiation and chemotherapy on survival and danger signals expression in glioblastoma cell lines. Biomed Res Int. 2014;2014:453497. doi: 10.1155/2014/453497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tang D, Kang R, Xiao W, et al. Nuclear Heat Shock Protein 72 as a Negative Regulator of Oxidative Stress (Hydrogen Peroxide)-Induced HMGB1 Cytoplasmic Translocation and Release. J Immunol. 2007;178:7376–84. doi: 10.4049/jimmunol.178.11.7376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Venereau E, Casalgrandi M, Schiraldi M, et al. Mutually exclusive redox forms of HMGB1 promote cell recruitment or proinflammatory cytokine release. J Exp Med. 2012;209:1519–28. doi: 10.1084/jem.20120189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang H, Lundback P, Ottosson L, Erlandsson-Harris H, Venereau E, Bianchi ME, Al Abed Y, Andersson U, Tracey KJ, Antoine DJ. Redox modification of cysteine residues regulates the cytokine activity of high mobility group box-1 (HMGB1) Mol Med. 2012;18:250–9. doi: 10.2119/molmed.2011.00389. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 35.Schiraldi M, Raucci A, Munoz LM, et al. HMGB1 promotes recruitment of inflammatory cells to damaged tissues by forming a complex with CXCL12 and signaling via CXCR4. J Exp Med. 2012;209:551–63. doi: 10.1084/jem.20111739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang D, Chen Q, Yang H, et al. High mobility group box-1 protein induces the migration and activation of human dendritic cells and acts as an alarmin. J Leukoc Biol. 2007;81:59–66. doi: 10.1189/jlb.0306180. [DOI] [PubMed] [Google Scholar]
- 37.Dumitriu IE, Bianchi ME, Bacci M, et al. The secretion of HMGB1 is required for the migration of maturing dendritic cells. J Leukoc Biol. 2007;81:84–91. doi: 10.1189/jlb.0306171. [DOI] [PubMed] [Google Scholar]
- 38.Orlova VV, Choi EY, Xie C, et al. A novel pathway of HMGB1-mediated inflammatory cell recruitment that requires Mac-1-integrin. EMBO J. 2007;26:1129–39. doi: 10.1038/sj.emboj.7601552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Degryse B, Bonaldi T, Scaffidi P, et al. The high mobility group (HMG) boxes of the nuclear protein HMG1 induce chemotaxis and cytoskeleton reorganization in rat smooth muscle cells. J Cell Biol. 2001;152:1197–206. doi: 10.1083/jcb.152.6.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Degryse B, de Virgilio M. The nuclear protein HMGB1, a new kind of chemokine? FEBS Lett. 2003;553:11–7. doi: 10.1016/s0014-5793(03)01027-5. [DOI] [PubMed] [Google Scholar]
- 41.Tian J, Avalos AM, Mao SY, et al. Toll-like receptor 9-dependent activation by DNA-containing immune complexes is mediated by HMGB1 and RAGE. Nat Immunol. 2007;8:487–96. doi: 10.1038/ni1457. [DOI] [PubMed] [Google Scholar]
- 42.Yu M, Wang H, Ding A, et al. HMGB1 SIGNALS THROUGH TOLL-LIKE RECEPTOR (TLR) 4 AND TLR2. Shock. 2006;26:174–9. doi: 10.1097/01.shk.0000225404.51320.82. [DOI] [PubMed] [Google Scholar]
- 43.Ha T, Xia Y, Liu X, et al. Glucan phosphate attenuates myocardial HMGB1 translocation in severe sepsis through inhibiting NF-kappaB activation. Am J Physiol Heart Circ Physiol. 2011;301:H848–H855. doi: 10.1152/ajpheart.01007.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xiang M, Shi X, Li Y, et al. Hemorrhagic shock activation of NLRP3 inflammasome in lung endothelial cells. J Immunol. 2011;187:4809–17. doi: 10.4049/jimmunol.1102093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen GY, Tang J, Zheng P, et al. CD24 and Siglec-10 selectively repress tissue damage-induced immune responses. Science. 2009;323:1722–5. doi: 10.1126/science.1168988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Abeyama K, Stern DM, Ito Y, et al. The N-terminal domain of thrombomodulin sequesters high-mobility group-B1 protein, a novel antiinflammatory mechanism. J Clin Invest. 2005;115:1267–74. doi: 10.1172/JCI22782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Salmivirta M, Rauvala H, Elenius K, et al. Neurite growth-promoting protein (amphoterin, p30) binds syndecan. Exp Cell Res. 1992;200:444–51. doi: 10.1016/0014-4827(92)90194-d. [DOI] [PubMed] [Google Scholar]
- 48.Abraham E, Arcaroli J, Carmody A, et al. HMG-1 as a mediator of acute lung inflammation. J Immunol. 2000;165:2950–4. doi: 10.4049/jimmunol.165.6.2950. [DOI] [PubMed] [Google Scholar]
- 49.Yang H, Ochani M, Li J, et al. Reversing established sepsis with antagonists of endogenous high-mobility group box 1. Proc Natl Acad Sci U S A. 2004;101:296–301. doi: 10.1073/pnas.2434651100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Qin S, Wang H, Yuan R, et al. Role of HMGB1 in apoptosis-mediated sepsis lethality. J Exp Med. 2006;203:1637–42. doi: 10.1084/jem.20052203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Suda K, Kitagawa Y, Ozawa S, et al. Anti-high-mobility group box chromosomal protein 1 antibodies improve survival of rats with sepsis. World J Surg. 2006;30:1755–62. doi: 10.1007/s00268-005-0369-2. [DOI] [PubMed] [Google Scholar]
- 52.Wu H, Ma J, Wang P, et al. HMGB1 contributes to kidney ischemia reperfusion injury. J Am Soc Nephrol. 2010;21:1878–90. doi: 10.1681/ASN.2009101048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Qiu J, Nishimura M, Wang Y, et al. Early release of HMGB-1 from neurons after the onset of brain ischemia. J Cereb Blood Flow Metab. 2008;28:927–38. doi: 10.1038/sj.jcbfm.9600582. [DOI] [PubMed] [Google Scholar]
- 54.Okuma Y, Liu K, Wake H, et al. Anti-high mobility group box-1 antibody therapy for traumatic brain injury. Ann Neurol. 2012;72:373–84. doi: 10.1002/ana.23602. [DOI] [PubMed] [Google Scholar]
- 55.Shimazaki J, Matsumoto N, Ogura H, et al. Systemic involvement of high-mobility group box 1 protein and therapeutic effect of anti-high-mobility group box 1 protein antibody in a rat model of crush injury. Shock. 2012;37:634–8. doi: 10.1097/SHK.0b013e31824ed6b7. [DOI] [PubMed] [Google Scholar]
- 56.Yang R, Zhang S, Cotoia A, et al. High mobility group B1 impairs hepatocyte regeneration in acetaminophen hepatotoxicity. BMC Gastroenterol. 2012;12:45. doi: 10.1186/1471-230X-12-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nadatani Y, Watanabe T, Tanigawa T, et al. High mobility group box 1 promotes small intestinal damage induced by nonsteroidal anti-inflammatory drugs through Toll-like receptor 4. Am J Pathol. 2012;181:98–110. doi: 10.1016/j.ajpath.2012.03.039. [DOI] [PubMed] [Google Scholar]
- 58.Hirata Y, Kurobe H, Higashida M, et al. HMGB1 plays a critical role in vascular inflammation and lesion formation via toll-like receptor 9. Atherosclerosis. 2013;231:227–33. doi: 10.1016/j.atherosclerosis.2013.09.010. [DOI] [PubMed] [Google Scholar]
- 59.Nadatani Y, Watanabe T, Tanigawa T, et al. High-Mobility Group Box 1 Inhibits Gastric Ulcer Healing through Toll-Like Receptor 4 and Receptor for Advanced Glycation End Products. PLoS One. 2013;8:e80130. doi: 10.1371/journal.pone.0080130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Patel VS, Sitapara RA, Gore A, et al. High Mobility Group Box-1 mediates hyperoxia-induced impairment of Pseudomonas aeruginosa clearance and inflammatory lung injury in mice. Am J Respir Cell Mol Biol. 2013;48:280–7. doi: 10.1165/rcmb.2012-0279OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Xu J, Jiang Y, Wang J, et al. Macrophage endocytosis of high-mobility group box 1 triggers pyroptosis. Cell Death Differ. 2014;21:1229–39. doi: 10.1038/cdd.2014.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sursal T, Stearns-Kurosawa DJ, Itagaki K, et al. Plasma bacterial and mitochondrial DNA distinguish bacterial sepsis from sterile systemic inflammatory response syndrome and quantify inflammatory tissue injury in nonhuman primates. Shock. 2013;39:55–62. doi: 10.1097/SHK.0b013e318276f4ca. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vincent JL, Opal SM, Marshall JC, Tracey KJ. Sepsis definitions: time for change. Lancet. 2013;381:774–5. doi: 10.1016/S0140-6736(12)61815-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Li W, Ashok M, Li J, et al. A Major Ingredient of Green Tea Rescues Mice from Lethal Sepsis Partly by Inhibiting HMGB1. PLoS ONE. 2007;2:e1153. doi: 10.1371/journal.pone.0001153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Li W, Li J, Sama AE, Wang H. Carbenoxolone Blocks Endotoxin-Induced Protein Kinase R (PKR) Activation and High Mobility Group Box 1 (HMGB1) Release. Mol Med. 2013;19:203–11. doi: 10.2119/molmed.2013.00064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Li W, Zhu S, Li J, et al. EGCG stimulates autophagy and reduces cytoplasmic HMGB1 levels in endotoxin-stimulated macrophages. Biochem Pharmacol. 2011;81:1152–63. doi: 10.1016/j.bcp.2011.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhang Y, Li W, Zhu S, et al. Tanshinone IIA sodium sulfonate facilitates endocytic HMGB1 uptake. Biochem Pharmacol. 2012;84:1492–500. doi: 10.1016/j.bcp.2012.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.MacMicking JD, Nathan C, Hom G, et al. Altered responses to bacterial infection and endotoxic shock in mice lacking inducible nitric oxide synthase. Cell. 1995;81:641–50. doi: 10.1016/0092-8674(95)90085-3. [DOI] [PubMed] [Google Scholar]