Abstract
Mutations in the KCNA1 gene are known to cause episodic ataxia/myokymia syndrome type 1 (EA1). Here, we describe two families with unique presentations who were enrolled in an IRB-approved study, extensively phenotyped, and whole exome sequencing (WES) performed. Family 1 had a diagnosis of isolated cataplexy triggered by sudden physical exertion in multiple affected individuals with heterogeneous neurological findings. All enrolled affected members carried a KCNA1 c.941T>C (p.I314T) mutation. Family 2 had an 8-year-old patient with muscle spasms with rigidity for whom WES revealed a previously reported heterozygous missense mutation in KCNA1 c.677C>G (p.T226R), confirming the diagnosis of EA1 without ataxia. WES identified variants in KCNA1 that explain both phenotypes expanding the phenotypic spectrum of diseases associated with mutations of this gene. KCNA1 mutations should be considered in patients of all ages with episodic neurological phenotypes, even when ataxia is not present. This is an example of the power of genomic approaches to identify pathogenic mutations in unsuspected genes responsible for heterogeneous diseases.
Keywords: KCNA1, Cataplexy, Episodic ataxia, Whole exome sequencing, Hypertonia
Introduction
Episodic ataxia type 1 (EA1, OMIM #160120), also known as episodic ataxia syndrome and myokymia syndrome, is a monogenic neurodevelopmental disorder that is characterized by sporadic bouts of ataxia (severe discoordination) with or without myokymia (continuous muscle movement) [1, 2]. EA1 presentation and severity can be quite heterogeneous even among monozygotic twins [3, 4].
Studies have implicated dominantly inherited or de novo mutations of potassium voltage-gated channel shaker related subfamily A member 1 (KCNA1, OMIM # 176260), also known as Kv1.1, to cause EA1) [2, 5]. Potassium channels represent the most complex class of voltage-gated ion channels. Their diverse functions include maintaining membrane potential, regulating cell volume, and modulating electrical excitability in neurons. The delayed rectifier function of potassium channels allows nerve cells to repolarize efficiently following an action potential.
We describe two rare KCNA1 variants, including one from a large family with clinical features consistent with isolated cataplexy, phenotypically discordant from the known phenotypes of EA1 due to KCNA1 mutations. We demonstrate that whole exome sequencing approach can delineate unusual presentations associated with KCNA1 mutations, broadening the phenotypic spectrum and providing insights into its pathophysiology. Interestingly, probands in both families were treated with acetazolamide and had significant negative reactions, suggesting that monogenic diseases such as EA1 are more complex than previously thought and there is an urgent need for finding appropriate therapies.
Methods
Participant enrollment
DNA and medical records were collected for the probands and family members by The Manton Center for Orphan Disease Research, Gene Discovery Core under informed consent governed by the Institutional Review Board of Boston Children’s Hospital. Participation in the study did not alter the standard of care.
For family 1, whole exome sequencing (WES) was performed on four affected family members by Baylor College of Medicine Human Genome Sequencing Center using a custom Nimblegen capture. For family 2, WES was performed by Axeq Technologies, Rockville, MD. Library preparation was performed by Axeq using Illumina TruSeq Exome Enrichment kits (62 Mb) with 16-sample indexing on an Illumina HiSeq platform.
Libraries were quantified and multiplexed into pools. Completed, indexed library pools were run on the Illumina HiSeq platform as paired-end 2×100-bp runs. FASTQ files were mapped against UCSC hg19 using BWA (BWA version 0.5.8), and SNPs and Indels were detected by SAMTOOLS (version 0.1.7). The product was a comprehensive report listing variants of phenotypic significance. Further analysis was performed by the Boston Children’s Hospital genomic analytic pipeline and via the Codified Genomics, LLC analytic pipeline (Houston, TX). Databases utilized included the NHLBI Exome Variant Server (EVS, http://evs.gs.washington.edu/EVS/), 1000 Genomes (http://www.1000genomes.org/data), and the Complete Genomics Public Genome Data Repository [6]. All variants of interest identified by WES were confirmed by Sanger sequencing. Numbering of all reported cDNA and protein sequence variants in KCNA1 is based on reference sequences hg19, NM_000217.2, and NP_ 000208.2, respectively.
Results
Clinical description of the affected families
Family 1
The proband 1 is a 24-year-old female with history of episodic loss of coordination, dysarthria, and visual disturbance resembling cataplexy. She was asymptomatic until 5 years when an episode of slurred speech and “wobbly” legs without falling to the ground associated with fever was noted. This first episode resolved spontaneously after a few minutes and without sequelae. Following this, she had very frequent, brief (30 s–2 min) episodes occurring often with playing sports like basketball and cross-country running and associated with the excitement of returning to the basketball court, starting or finishing a cross-country race. During the spells, she could not handle the basketball or felt her knees become weak and her legs “rubbery” causing her to slow down or fall. She also reported spells when sitting in class feeling stressed. She did not take naps and reported no excessive sleepiness. Because of the resemblance of the spells to cataplexy, at age 12, the patient was prescribed clomipramine, 25 mg every night at bedtime. She responded well to treatment without side effects and with abatement of spells and improved sports performance. At age 16, she took herself off clomipramine (75 mg/day) because of depression and emotional lability. History of feeling tired and sleepiness in class prompted a polysomnogram followed by a Multiple Sleep Latency Test (MSLT), while off clomipramine, to investigate the possibility of narcolepsy. Her polysomnogram was essentially normal, and she was given a presumptive clinical diagnosis of isolated cataplexy. Her brain MRI at 12 years age was normal except for a small right subcortical frontal area of gliosis of uncertain significance, essentially unchanged on a follow-up brain MRI 13 months later. At age 21, she presented again with renewed difficulty competing in track events after a relative stability during her first 3 years of college. She was asked to do a strenuous exercise in the office, and a spell was elicited. She had difficulty extending her legs, developed a head tremor, bilateral dysmetria in the arms and legs, tremulous voice, needed to sit down, and was without nystagmus. She was diagnosed with episodic ataxia and started on acetazolamide with a decrease in the frequency of spells, but this was discontinued because of depression. She was then evaluated by the genetics team and serum creatine phosphokinase (CPK), and acylcarnitine and amino acids along with urine organic acids were performed that returned within normal limits.
She has a significant family history of similar episodes on her maternal side, which appears to be inherited in a dominant fashion. Other family members had a similar age of onset as well as a decrease in severity and frequency of episodes over time. In all affected members of this family, the precipitating factors for these episodes appeared to be rapid-onset muscle contractions. The patient and her family members reported an increase in the frequency of these episodes related to the menstrual cycle (for females), inter-current illnesses, particularly with fever, and at times of emotional stress. Caffeine and alcohol also exacerbated the episodes. Additional triggers included cold weather and postural change. Headache, tinnitus, hearing loss, vertigo, and scotomas were not reported (Table 1).
Table 1.
Phenotypic variability of cataplexy episodes in members of family 1 carrying KCNA1 mutations
| Affected family members | II:1 | II:2 | II:3 | III:2 | III:6 (proband) | III:7 |
|---|---|---|---|---|---|---|
| Gender/age in years (age of onset) | F/60 (childhood) | F/58 (childhood) | F/53 (childhood) | M/33 (10 years) | F/24 (5 years) | M/17 (5 years) |
| Isolated episodes | Present | Present | Present | Present | Present | Present |
| Features during isolated episodes | ||||||
| Imbalance | + | + | + | + | + | + |
| Slurred speech | + | + | + | − | + | +/− |
| Coordination difficulties in arms | + | + | + | − | + | − |
| Visual disturbance | − | + | + | − | + | + |
| Myokymia | − | + | − | − | + | − |
| Weakness | + | + | + | + | − | + |
| Stiffness | − | +/− | − | − | − | − |
| Dizziness/vertigo | − | + | + | + | − | − |
| Frequency of isolated episodes | ||||||
| Times per week during childhood (age <20 years) | 1–5 | 1–5 | 1–5 | 1–5 | Daily | 1–5 |
| During adulthood (times per week) | <1 | <1 | <1 | 1–5 | 1–5 | N/A |
| Duration of longest attack | 10 min. | 3–5 min. | 3–5 min. | 10 min. | 6–7 hrs. | 3–5 min. |
| Triggers for isolated episodes | ||||||
| Physical activity or exercise | − | + | + | − | + | + |
| Stress | + | + | + | + | + | + |
| Startle | − | + | + | + | + | + |
| Emotion | − | + | + | + | + | + |
| Illness/fever | + | + | + | +/− | + | + |
| Postural change (e.g., bending, standing up, looking down) | − | + | + | + | + | + |
| Environmental temperature | + | +/− | + | +/− | + | + |
| Health issues between episodes | ||||||
| Involuntary muscle contractions | − | − | + | + | + | − |
| Ataxia/difficulties with balance | − | − | + | − | − | − |
| Tremors | − | +/− | + | + | − | − |
Family 2
The now 10-year-old boy was born at 41 weeks without complications with normal weight and length. At 8 months, he developed femoral retroversion and bilateral hip external rotation with normal pelvis and femur X-rays. Subsequently, a hiatal hernia and bilateral congenital vertical talus were identified and corrected by 2 years of age. By 5 years, he had developmental delay, generalized hypertonia particularly in lower extremities, with periodic muscle spasms. Because of fatigue out of proportion to his neuromuscular issues, a sleep study was obtained. It showed prolonged sleep latency, reduced sleep efficiency, and episodes of obstructive sleep apnea and hypopnea with episodes of oxygen desaturation in the 80 % range. Meanwhile, his height dropped below the third centile (107.5 cm) and weight at fifth centile (19.5 kg). He received supplemental oxygen and a gastrostomy tube for the majority of his feedings. An electroretinograph was noted to be “generally abnormal”, and a muscle biopsy revealed abnormal mitochondria. A diagnosis of limb-girdle muscular dystrophy was considered. His genetic work up was essentially normal. A chromosomal microarray analysis, karyotype, mitochondrial genome analysis, testing for disorders of glycosylation, and Noonan spectrum chip did not reveal any significant findings.
KCNA1 is mutated in the affected family members
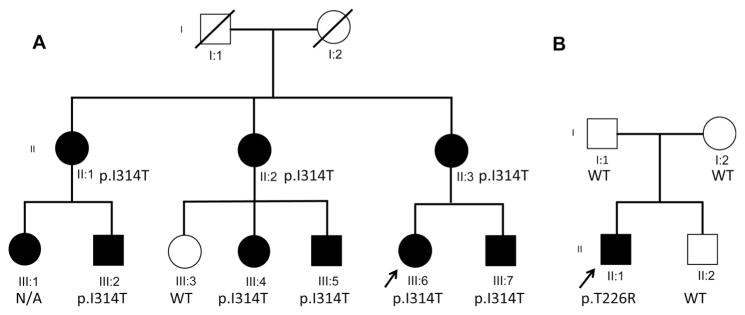
For family 1, exome sequencing was performed on four affected family members (III:2, III:4, III:6, III:7). These individuals had 82 rare variants (mean allele frequency <0.01 in publicly available databases described in methods) of which 25 were exonic, splicing, or indel changes. Eighteen of the 25 variants had a batch effect (seen in additional samples of the sequenced batch). After reviewing the remaining seven variants in more detail including expression patterns and function, mutation in KCNA1 was the most likely candidate causing the phenotype. All affected individuals carried the same novel mutation in KCNA1 (chr12:5021485 (hg19), c.941T>C; p.I314T). Sanger sequencing confirmed that all affected members of the family (except III:1 who did not enroll) carried the variant, while the only unaffected family member (III:3) did not carry it (Fig. 1a).
Fig. 1.
Pedigrees for families 1 and 2. a The affected proband (III:6), her affected brother (III:7) and mother (II:3), her two affected aunts (II:1 and II:2), and her four affected cousins (III:1, III:2, III:4, III:5) are shown along with their p.I314T variant status. b In family 2, the affected proband (II:1), his two unaffected parents (I:1 and I:2), and sibling (II:2) are shown along with the de novo variant p.T226R present in the proband. The square represents a male individual, a circle represents a female individual, and the arrow indicates the probands. Filled symbols represent clinically affected individuals
For family 2, exome sequencing was performed on the proband and parent trio. After similar filtering as described above, Proband 2 was found to have a de novo mutation in KCNA1 (chr12:5021221 (hg19), c. 677C>G; p.T226R) (dbSNP 138 rs28933383). This SNP has been previously clinically associated with EA1 [7] (Fig. 1b).
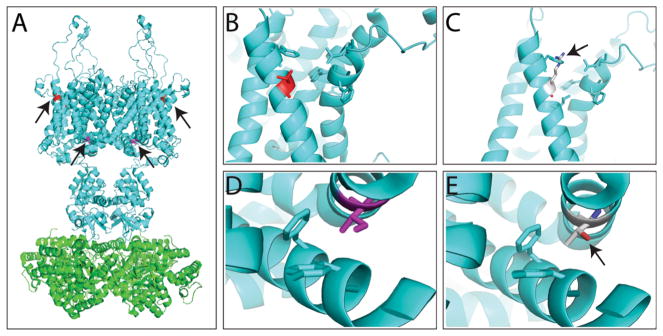
Molecular modeling was performed for both protein variants as shown in Fig. 2. The wild-type KCNA1 and the location of both the variants are shown in Fig. 2a.
Fig. 2.
Molecular modeling of KCNA1 (KV1.1) variants. a Molecular structure of rat KCNA2 (KV1.2) (PDB code: 3LUT), a protein very similar to KCNA1, and for which crystal structure is available. The structural modeling and illustration were prepared by the software PyMOL (The PyMOL Molecular Graphics System, Version 1.7.4 Schrödinger, LLC). The channel forms a tetramer, with Thr226 residue in red and Ile314 colored in purple (marked by arrows). b, c WT Thr226 residue (b) and variant Thr226Arg (c) modeling. c The variant Thr226Arg conflicts with a Phe residue on the same helix (marked by arrow) and likely affects the hydrophobic packing with another helix through the interaction with the aromatic residues. d, e WT Ile314 residue (d) and variant Ile314Thr (e) modeling. e The variant Ile314Thr likely disrupts the hydrophobic packing between two transmembrane helices (marked by arrow)
The Thr226Arg variant present in family 2 conflicted with a Phe residue on the same helix likely affecting the hydrophobic packing with another helix through interaction with the aromatic residues (Fig. 2b, c). The variant Ile314Thr present in family 1 likely disrupts the hydrophobic packing between two transmembrane helices (Fig. 2d, e).
Discussion
Episodic ataxia 1 is caused by dominantly inherited or de novo missense or nonsense mutations in the KCNA1 gene [1, 2, 5]. The two cases described here demonstrate variable expressivity both within a family and between two different variants in KCNA1. While a spectrum of features has been previously described for EA1, the fact that both families were not diagnosed despite being evaluated by several clinical teams, including neurology and genetics, is a consequence of the extensive clinical heterogeneity in presentation. Further, the clinical presentation of isolated cataplexy, unique to proband 1, further confounded the diagnostic process for this family. Similarly, the presence of multiple congenital anomalies, including vertical talus and hiatal hernia, is an unusual constellation of clinical features in the proband from family 2. We reviewed the literature for potential relationships between KCNA1 and known cataplexy genes such as HCRT or MOG but did not find any evidence for physical or physiological interactions between them.
This study demonstrates the utility of WES to gain insight into the variable presentation in a previously well-described monogenic disease such as EA1. As seen in Table 1, the individuals identified with KCNA1 variants in this family show great variability in terms of their episodes and triggers. An individual who may seem severe in one category (i.e., amount of triggers) may not show a consistently “severe” phenotype throughout all categories. For example, individual III-7 presented with all reported triggers but had a milder phenotype than III-6 during the actual episodes. Due to their unusual and variable presentations, affected members of family 1 remained undiagnosed for three generations, and family 2 waited for 7 years before a diagnosis was made. Interestingly, while both families showed episodic onset of various neurological features, neither presented with ataxia as a primary problem. The extent to which episodic “ataxia” may be a misnomer for many families with EA1 may be revealed as many more patients undergo genomic sequencing as part of their diagnostic evaluations. The KCNA1 variant seen in proband 2 has been previously described to be associated with ataxia, neuromyotonia, contractures, skeletal deformities, and epilepsy [7, 8], but abnormal muscle biopsy, and multiple congenital anomalies and absence of ataxia confounded the diagnosis. Some of the clinical findings including abnormal mitochondria in muscle have not been described with KCNA1 mutations, and it is unclear whether these are related to KCNA1 or to another unrelated genetic cause.
It is an interesting finding that the proband in family 1 responded well to clomipramine. This was an accidental discovery based on the thinking that those spells represented cataplexy and needs further exploration. Further, correctly diagnosing an individual with EA1 is crucial as certain medical interventions may help these individuals. Acetazolamide and other anti-seizure medications have been reported to decrease the frequency and severity of episodic attacks, even in the absence of seizures [9]. There is a great variability in the efficacy of these treatments, and there have been no reported associations between the genotype and effectiveness of treatment. Interestingly, both probands were started on acetazolamide, but they had very negative emotional and behavioral reactions. While the families reported amelioration of the episodic attacks while on acetazolamide, the drug was discontinued due to neuropsychiatric manifestations.
Functional studies have shown that pathogenic EA1 mutations in KCNA1 lead to production of nonfunctional ion channels and are associated with episodic attacks resulting from increased neuronal excitability due to defective membrane repolarization after an action potential [10]. Kv channels are tetrameric structures, with six subunit transmembrane-spanning α-helicies and cytoplasmic N- and C-terminal domains per subunit (Fig. 2). Voltage sensors are in the S1–S4 segments, and channels respond to membrane depolarization [11]. Previous studies have demonstrated that negatively charged clusters in the S2 and S3 segments, together with the positive charges in S4, are involved in the opening and closing of Kv1 channels [12–14]. The mutations discussed here are located in S2 (T226R, family 2) and intracellular linker between transmembrane domains S4 and S5 (I314T, family 1). The T226R mutation has been previously shown to enhance neurotransmitter release without detectable effect on neuronal excitability [15]. Further, the I314 residue has been extensively studied (reported as residue I384 on Shaker gene in drosophila) and has been found to be critically important in coupling of the voltage sensor to pore opening [16]. When residue 314 is mutated from isoleucine to asparagine, it completely uncouples voltage sensor movement from pore opening [16]. Interestingly, while well conserved across various species and many Kv channels, the Kv5.1 and Kv6.1 channels encoded by KCNF1 and KCNG1 respectively contain a T instead of I at that location, similar to the I314T mutation carried by family 1. The location of the mutations and potential compensatory effect mediated by other isoforms (e.g., Kv1.2) may account for the transient episodes and the variability in phenotypes.
The study of familial cases with EA1 offers the opportunity to identify the different mutations involved in the expression of discordant phenotypes. Together with the previous descriptions of KCNA1 mutations, our report suggests that KCNA1 aberrations may be implicated in discordant phenotypes regardless of ataxia presentation. We would advocate for performing WES in patients with undiagnosed central or peripheral nervous system diseases that should include careful interrogation of KCNA1. This study further demonstrates the value of WES in the identification of a genetic cause of rare and unusual disease phenotypes, and this approach may reduce the time and money spent in diagnostic odyssey that many families and caretakers have to endure.
Acknowledgments
The authors would like to thank the two families who volunteered for the research study. This work was supported by K08 AR055072 (PBA) from the National Institute of Arthritis and Musculoskeletal and Skeletal Diseases (NIAMS) of National Institute of Health (NIH), U19HD077671 (PBA and AHB) and P30 HD018655 from Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) and National Human Genome Research Institute (NHGRI) of the NIH, the Research Connection at Boston Children’s Hospital (BCH), and The Manton Center for Orphan Disease Research at BCH.
Funding/support This work was supported by K08 AR055072 (PBA) from the National Institute of Arthritis and Musculoskeletal and Skeletal Diseases (NIAMS) of National Institute of Health (NIH), U19HD077671 (PBA and AHB), and P30 HD018655 from Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) and National Human Genome Research Institute (NHGRI) of the NIH, the Research Connection at Boston Children’s Hospital (BCH), GETTYLAB, and The Manton Center for Orphan Disease Research at BCH.
Footnotes
Conflict of interest The funding source had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication. The authors have no conflict of interest to declare.
Author contributions Drs. Agrawal and Brownstein had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Brownstein, Beggs, and Agrawal.
Acquisition of Data: Beggs, Rodan, Towne, Pelletier, Rosenberg, Urion, Picker, Cao, Tan, and Agrawal.
Analysis and interpretation of data: Brownstein and Agrawal.
Drafting of the manuscript: Brownstein, Pelletier, Beggs, Rosenberg, Towne, and Agrawal.
Critical revision of the manuscript for important intellectual content: all authors.
Statistical analysis: none needed.
Obtained funding: Agrawal and Beggs.
Administrative, technical, or material support: Beggs and Agrawal.
Study supervision: Beggs and Agrawal.
Role of funder/sponsor The funding source had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Contributor Information
Catherine A. Brownstein, Email: catherine.brownstein@childrens.harvard.edu.
Pankaj B. Agrawal, Email: pagrawal@enders.tch.harvard.edu.
References
- 1.Baloh RW. Episodic ataxias 1 and 2. Handb Clin Neurol. 2012;103:595–602. doi: 10.1016/B978-0-444-51892-7.00042-5. [DOI] [PubMed] [Google Scholar]
- 2.Graves TD, Cha YH, Hahn AF, et al. Episodic ataxia type 1: clinical characterization, quality of life and genotype-phenotype correlation. Brain J Neurol. 2014;137(Pt 4):1009–1018. doi: 10.1093/brain/awu012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Graves TD, Rajakulendran S, Zuberi SM, et al. Nongenetic factors influence severity of episodic ataxia type 1 in monozygotic twins. Neurology. 2010;75(4):367–372. doi: 10.1212/WNL.0b013e3181ea9ee3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tan SV, Wraige E, Lascelles K, Bostock H. Episodic ataxia type 1 without episodic ataxia: the diagnostic utility of nerve excitability studies in individuals with KCNA1 mutations. Dev Med Child Neurol. 2013;55(10):959–962. doi: 10.1111/dmcn.12236. [DOI] [PubMed] [Google Scholar]
- 5.Browne DL, Gancher ST, Nutt JG, et al. Episodic ataxia/myokymia syndrome is associated with point mutations in the human potassium channel gene, KCNA1. Nat Genet. 1994;8(2):136–140. doi: 10.1038/ng1094-136. [DOI] [PubMed] [Google Scholar]
- 6.Drmanac R, Sparks AB, Callow MJ, et al. Human genome sequencing using unchained base reads on self-assembling DNA nanoarrays. Science. 2010;327(5961):78–81. doi: 10.1126/science.1181498. [DOI] [PubMed] [Google Scholar]
- 7.Zuberi SM, Eunson LH, Spauschus A, et al. A novel mutation in the human voltage-gated potassium channel gene (Kv1.1) associates with episodic ataxia type 1 and sometimes with partial epilepsy. Brain J Neurol. 1999;122(Pt 5):817–825. doi: 10.1093/brain/122.5.817. [DOI] [PubMed] [Google Scholar]
- 8.Kinali M, Jungbluth H, Eunson LH, et al. Expanding the phenotype of potassium channelopathy: severe neuromyotonia and skeletal deformities without prominent Episodic Ataxia. Neuromuscul Disord NMD. 2004;14(10):689–693. doi: 10.1016/j.nmd.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 9.Kotagal V. Acetazolamide-responsive ataxia. Semin Neurol. 2012;32(5):533–537. doi: 10.1055/s-0033-1334475. [DOI] [PubMed] [Google Scholar]
- 10.Lassche S, Lainez S, Bloem BR, et al. A novel KCNA1 mutation causing episodic ataxia type I. Muscle Nerve. 2014;50(2):289–291. doi: 10.1002/mus.24242. [DOI] [PubMed] [Google Scholar]
- 11.Jan LY, Jan YN. Cloned potassium channels from eukaryotes and prokaryotes. Annu Rev Neurosci. 1997;20:91–123. doi: 10.1146/annurev.neuro.20.1.91. [DOI] [PubMed] [Google Scholar]
- 12.Zhu J, Alsaber R, Zhao J, Ribeiro-Hurley E, Thornhill WB. Characterization of the Kv1.1 I262T and S342I mutations associated with episodic ataxia 1 with distinct phenotypes. Arch Biochem Biophys. 2012;524(2):99–105. doi: 10.1016/j.abb.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 13.van der Wijst J, Glaudemans B, Venselaar H, et al. Functional analysis of the Kv1.1 N255D mutation associated with autosomal dominant hypomagnesemia. J bBiol Chem. 2010;285(1):171–178. doi: 10.1074/jbc.M109.041517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Long SB, Tao X, Campbell EB, MacKinnon R. Atomic structure of a voltage-dependent K+ channel in a lipid membrane-like environment. Nature. 2007;450(7168):376–382. doi: 10.1038/nature06265. [DOI] [PubMed] [Google Scholar]
- 15.Heeroma JH, Henneberger C, Rajakulendran S, Hanna MG, Schorge S, Kullmann DM. Episodic ataxia type 1 mutations differentially affect neuronal excitability and transmitter release. Dis Model Mech. 2009;2(11–12):612–619. doi: 10.1242/dmm.003582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haddad GA, Blunck R. Mode shift of the voltage sensors in Shaker K+ channels is caused by energetic coupling to the pore domain. J Gen Physiol. 2011;137(5):455–472. doi: 10.1085/jgp.201010573. [DOI] [PMC free article] [PubMed] [Google Scholar]