Abstract
Aloe vera is one of the most commonly used botanicals for various prophylactic and therapeutic purposes. Recently, NTP/NCTR has demonstrated a dose-dependent increase in large intestinal tumors in F344 rats chronically exposed to Aloe barbadensis Miller (Aloe vera) non-decolorized whole leaf extract (AVNWLE) in drinking water. The morphological and molecular pathways of AVNWLE-induced large intestinal tumors in the F344 rats were compared to human colorectal cancer (hCRC) literature. Defined histological criteria were used to compare AVNWLE-induced large intestinal tumors with hCRC. The commonly mutated genes (Kras, Ctnnb1, and Tp53) and altered signaling pathways (MAPK, WNT, and TGF-β) important in hCRC were evaluated within AVNWLE-induced large intestinal tumors. Histological evaluation of the large intestinal tumors indicated eight of twelve adenomas (Ads) and four of twelve carcinomas (Cas). Mutation analysis of eight Ads and four Cas identified point mutations in exons 1 and 2 of the Kras gene (two of eight Ads, two of four Cas), and in exon 2 of the Ctnnb1 gene (three of eight Ads, one of four Cas). No Tp53 (exons 5–8) mutations were found in Ads or Cas. Molecular pathways important in hCRC such as MAPK, WNT, and TGF-β signaling were also altered in AVNWLE-induced Ads and Cas. In conclusion, the AVNWLE-induced large intestinal tumors in F344 rats share several similarities with hCRC at the morphological and molecular levels.
Keywords: Aloe vera, colon, F344 rat, human, colorectal tumors
Introduction
There has been an increased popularity in using tradition-based botanicals as medicines and supplements. Increasingly, these medicinal herbs and their respective extracts are being used for newer arbitrary prophylactic and therapeutic purposes at various dosages and duration. There is insufficient scientific evidence on the indications and contraindications of many of these herbal supplements and medicines. Also, the importance of interactions of these herbal supplements and drugs cannot be overstated (Hu et al. 2005; Izzo and Ernst 2009). These herbal products are marketed as dietary supplements (rather than as drugs), thereby obviating the burden of providing safety and efficacy data to the United States Food and Drug Administration (USFDA), under the Dietary Supplement Health and Educational Act of 1994. The United States National Toxicology Program (NTP) is testing several commonly used medicinal herbs for their safety, for example, goldenseal, comfrey, pulegone, ginkgo, echinacea, Aloe vera, ginseng, kava kava, milk thistle, and thujone (Anonymous 1999).
Aloe barbadensis (Miller), Aloe vera (AV), is one of the most commonly used botanicals for a wide variety of ailments. The composition of Aloe products depends on several factors such as location, time of growth and harvest of Aloe crop, species of Aloe, as well as the extraction and purification process. Hence, aloe products may contain variable amounts of acemannans along with other polysaccharides, as well as mixtures of anthrones and anthraquinones (Boudreau and Beland 2006; Elsohly et al. 2007). Aloe is used as aloe gel, decolorized whole leaf extract, and non-decolorized whole leaf extract. The decolorization process involves treatment with activated carbon to remove the latex anthraquinones from the whole leaf extract. Aloe gel is used as a skin balm for minor burns and is also taken internally to treat constipation, colitis, peptic ulcers, diabetes, and for some other therapeutic purposes. In addition, aloe juice is marketed as a health tonic and as an ingredient of other fruit juice mixtures. As with some other herbal products, there is a paucity of safety data on the acute and chronic effects of aloe ingestion. Recently, the NTP and the National Center for Toxicological Research have conducted a subchronic (ninety-day) bioassay and a chronic (two-year) bioassay in F344 rats and B6C3F1 mice of both sexes by administering Aloe barbadensis Miller (Aloe vera) non-decolorized whole leaf extract (AVNWLE) in drinking water (NTP 2011). In the chronic studies using B6C3F1 mice, there was no significant tumor incidence. In contrast, there was a marked dose-related increase in the incidence of large intestinal adenomas (Ads) and carcinomas (Cas) in F344 rats of both sexes, with higher incidence in males than in females (NTP 2011).
Colorectal cancer (hCRC) is the third most frequently diagnosed cancer in men and women and the second leading cause of cancer-related deaths in the United States (Edwards et al. 2010). About 15% of CRC are hereditary, whereas the other 85% are considered to be sporadic (Markowitz and Bertagnolli 2009). Vogelstein and colleagues proposed a multistep genetic model involving accumulation of multiple, and in some cases sequential, genetic mutations and aberrant gene expression that lead to colorectal tumorigenesis (Fearon and Vogelstein 1990). The common signal transduction pathways and genes involved in CRC are WNT/CTNNB1, KRAS/BRAF, SMAD4/TGF-β, PI3K/AKT, and TP53/BAX (Markowitz and Bertagnolli 2009). In addition to these canonical pathways, novel interactions between these pathways as well as novel genes and pathways are being characterized in the pathogenesis of hCRC.
We hypothesized that the genetic changes within AVNWLE-induced large intestinal tumors in F344 rats would reflect major signaling pathways altered in hCRC. Hence, the objective of the current study was to evaluate MAPK, WNT/CTNNB1, and TGF-β signaling pathways in the large intestinal Ads and Cas from AVNWLE-exposed F344 rats, and compare them to the hCRC literature.
Materials and Methods
Sample Collection
Samples used in this study were collected during the two-year NTP/NCTR bioassay. All of the animals in the study were cared for and humanely handled according to the institutional guidelines (NTP 2011). Malic Acid and aloin-A were used as markers for evaluating the stability of the NTP test article AVNWLE and to confirm the effective dosages. In this study, drinking water solutions of 0.5%, 1.0%, and 1.5% AVNWLE had an average malic acid content of 975, 1945, and 2920 μg/g water, respectively, and an average aloin A content of 32.3, 65.6, and 98.3 μg/g water, respectively. The mean percentages of targeted values and standard deviations for malic acid and aloin A in dosed waters were 95% ± 7% and 100% ± 12%, respectively. The F344 rats that were exposed to AVNWLE in drinking water, ad libitum for two years, were necropsied and examined for treatment-related lesions. The twelve tumor samples larger than 0.5 cm in diameter were sectioned in half; one half was fixed in 10% neutral buffered formalin, and the other half was flash frozen in liquid nitrogen. The negative controls (four samples) for mutation analysis consisted of fresh frozen colons (transmural sections) from concurrent untreated control two-year-old F344 rats. The negative controls (four samples) for the quantitative real-time reverse-transcriptase polymerase chain reaction (RT-PCR) arrays consisted of fresh, gently scraped colonic “mucosa only,” without the outer muscular tunics, from adult untreated six-month-old F344 rats to accurately calculate the differential fold changes since the “tumor only” tissue (without the corresponding outer muscular tunics) was collected for molecular analysis. DNA was isolated from frozen tumor tissue (eight Ads and four Cas) as well as the frozen normal colonic tissue (four samples) from untreated rats for mutation analysis. RNA was isolated from frozen tumor tissue (four Ads and four Cas) as well as the normal fresh colonic tissue mucosa (four samples) from untreated rats for PCR arrays. The formalin-fixed tissue was routinely processed and stained with hematoxylin and eosin (H&E) for microscopic analysis.
Following defined histological criteria (Boivin et al. 2003; Elwell and McConnell 1990), three board-certified pathologists (ARP, MJH, and GPF) independently conducted microscopic analysis.
Mutation Analysis
DNA was extracted using a DNeasy Tissue Kit (Qiagen, Valencia, CA) from snap-frozen AVNWLE-exposed tumors (eight Ads and four Cas) as well as the normal colonic mucosa (four samples) from untreated rats. The PCR primers used to amplify the hot spot exons of Kras (1 and 2), Ctnnb1 (2), and Tp53 (5–8) genes are presented in supplementary Table 1. (A supplemental appendix to this article is published electronically only at http://tpx.sagepub.com/supplemental.) Gene amplification reactions were performed by semi-nested PCR; controls lacking template DNA were run with all sets of reactions. Polymerase chain reaction–amplified products were purified using a QIAquick Gel Extraction Kit (Qiagen, Valencia, CA). The purified PCR products were cycled with Terminal Ready Reaction Mix-Big Dye (Perkin Elmer, Foster City, CA), and the extension products were purified with DyeEx 2.0 Spin Kit (Qiagen). The lyophilized PCR products were sequenced with an automatic sequencer (Perkin-Elmer ABI Model 3100).
Quantitative Real-Time RT-PCR Arrays
Two catalogued PCR arrays (MAP Kinase signaling [PARN-061] and WNT signaling [PARN-043]) containing eighty-four genes each (on a ninety-six–well plate) and one custom PCR array containing ninety-two genes were obtained from SABiosciences (Frederick, MD). The custom PCR array was designed to include the canonical TGF-β signaling pathway (thirty-two genes), as well as sixty other genes obtained from meta-analysis of ten human CRC microarray datasets (GSE7466, E-MTAB-57, GSE4183, GSE5350, GSE10972, GSE6988, GSE13471, GSE5364, GSE3294, and GSE4107) using NextBio software (www.nextbio.com) and data from the hCRC literature. Quantitative differential gene expression levels were detected using arrays containing corresponding PCR primers and SABiosciences SYBR Green qPCR master mix, and the reactions were run on an ABI PRISM 7900HT Sequence Detection System (Foster City, CA) using the manufacturer’s protocols.
Statistics and Data Analysis
Gene expression within each PCR array was normalized to the housekeeping gene Actb, and the fold changes were calculated based on the ΔΔCt method (Pfaffl 2001). The data were analyzed on the basis of the progressive colorectal tumorigenesis paradigm proposed by Vogelstein and colleagues (Fearon and Vogelstein 1990). According to this paradigm, a trend analysis was conducted to evaluate an increasing or decreasing trend in gene expression from normal colon tissue to adenoma to carcinoma. In addition to trend analysis, pairwise comparisons (controlling for direction errors) were also performed, for each gene, between Ads and normal, Cas and normal, and Cas and Ads. The gene expression data are not necessarily normally distributed, so the residual bootstrap-based methodology (Efron and Tibshirani 1994) was employed to compute the p values using 10,000 bootstrap samples. Since multiple statistical tests were performed, the statistical significance was determined by controlling the false discovery rate (FDR) at a nominal 5% level. The trend tests and pairwise comparisons were performed using the publicly available software ORIOGEN, version 4.01 (Peddada et al. 2005). The principal component analysis (PCA) and agglomerative hierarchical unsupervised cluster analysis (HCA) were done using the Partek Genomics Suite, version 6.3 (St. Louis, MO). The venn diagrams were created using the bioconductor R/limma program (Smyth 2004). Ingenuity Pathway Analysis (IPA) software was used to create maps of canonical pathways and to decipher interactions between various molecular pathways (Ingenuity Systems, www.ingenuity.com).
Results
Rat Large Intestinal Ads and Cas are Morphologically Similar to hCRC
The results of the microscopic evaluation of the tumors used in this study were in concordance with the NTP pathology data (NTP TR 577). The Ads were characterized by exophytic polypoid masses with distorted glands often lined by dysplastic epithelium. Occasional glandular lumina and lamina propria had mild to moderate mixed inflammatory cell infiltration. There was no evidence of stromal invasion of the stalk of the polyp or invasion into the muscularis mucosa (Figure 1A). The Cas were characterized by neoplastic polypoid lesions with anaplastic glandular epithelium invading the stalk stroma/muscularis mucosa (Figure 1B). Mitoses, desmoplasia, and mixed inflammatory cell infiltrates were often noted in the Cas.
FIGURE 1.
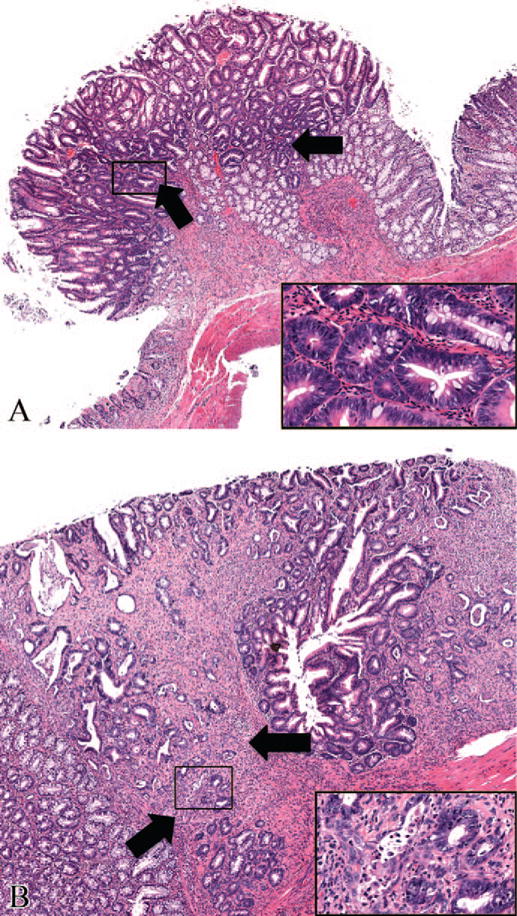
Photomicrographs of hematoxylin and eosin–stained colonic tumor sections from F344 rats exposed to Aloe vera non-decolorized whole leaf extract in drinking water, ad libitum, for two years. (A) Adenoma (50×). The exophytic mass with dysplastic hyperchromatic cells (arrows and inset box) was limited to the mucosa without evidence of invasion into the muscularis mucosa. (B) Carcinoma (60×). Note the neoplastic cells invading past the muscularis mucosa (arrows) and the fibroplasia surrounding the neoplastic cells (inset box).
Large Intestinal Tumors from AVNWLE-Exposed Rats Have Point Mutations within Kras or Ctnnb1, but Not in Tp53
Summary of the mutation analysis is presented in Table 1, and examples of the electropherogram indicating mutations in various codons of Kras and Ctnnb1 genes are presented in Figure 2. Point mutations within the Kras were observed in two of eight (25%) Ads and two of four (50%) Cas. Both the Ads samples had Kras mutations within codon 13 (G to C). The Kras mutations within the Cas samples were within codon 12 (G to A) and codon 61 (A to G). Point mutations within the regulatory domain (codons 29–50) of Ctnnb1 were observed in three of eight (38%) Ads and one of four (25%) Cas. The Ctnnb1 mutations within the Ads samples were observed within codon 32 (G to A), codon 41 (A to C), and codon 45 (C to T). The Ctnnb1 mutation within the Cas sample was in codon 34 (G to A). The mutations within Kras and Ctnnb1 were mutually exclusive within the tumor samples. No Tp53 (exons 5–8) mutations were observed within either the Ads or Cas. No mutations were observed within the Kras, Ctnnb1, or Tp53 in control colonic epithelial samples.
TABLE 1.
Summary of gene mutations in F344 rat large intestinal tumors.a
| Animal ID | Dx | Codon 12 |
Kras Codon13 |
Codon 61 |
Ctnnb1 Exon 2 |
Tp53 Exons 5–8 |
|---|---|---|---|---|---|---|
| 962 | Ads | wt | wt | wt | wt | wt |
| 1712 | Ads | wt | GGC->CGC (Gly->Arg) | wt | wt | wt |
| 1711 | Ads | wt | wt | wt | Codon 32 GAT->AAT (Asp->Asn) | wt |
| 1812 | Ads | wt | wt | wt | wt | wt |
| 1301 | Ads | wt | wt | wt | Codon 45 TCC->TTC (Ser->Phe) | wt |
| 1102 | Ads | wt | wt | wt | wt | wt |
| 1602 | Ads | wt | GGC->CGC (Gly->Arg) | wt | wt | wt |
| 1922 | Ads | wt | wt | wt | Codon 41 ACC->CCC (Thr->Pro) | wt |
| 1052 | Cas | wt | wt | CAA->CGA (Glu->Arg) | wt | wt |
| 1151 | Cas | GGT->GAT (Gly->Asp) | wt | wt | wt | wt |
| 1402 | Cas | wt | wt | wt | Codon 34 GGA->GAA (Gly->Glu) | wt |
| 1921 | Cas | wt | wt | wt | wt | wt |
Note: There were no Kras, Ctnnb1, and Tp53 point mutations within the four age-matched untreated colon tissue from F344 control rats.
F344 rats were treated ad libitum with Aloe vera non-decolorized whole leaf extract in drinking water for 2 years.
Abbreviations: Ads, adenoma; Cas, carcinoma; Dx, diagnosis; N, normal colonic mucosal epithelium; wt, wild type.
FIGURE 2.
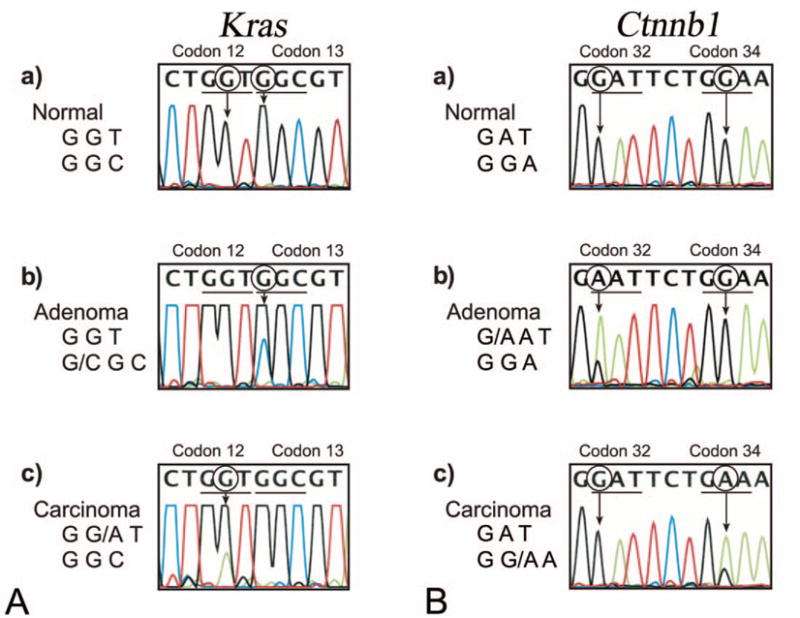
Examples of electropherograms indicating point mutations within Kras and Ctnnb1 genes amplified from large intestinal adenomas and carcinomas of F344 rats chronically exposed to Aloe vera non-decolorized whole leaf extract. (A) Identification of point mutations in codons 12 and 13 (exons 1 and 2) of the Kras gene. (a) Normal Kras codon 12 (GGT) and codon 13 (GGC); (b) adenoma with mutated codon 13 (GGC>CGC); (c) carcinoma with mutated codon 12 (GGT>GAT). (B) Identification of point mutations in codons 32 and 34 (exon 2) of Ctnnb1 gene. (a) Normal Ctnnb1 codon 32 (GAT) and codon 34 (GGA); (b) adenoma with mutated codon 32 (GAT>AAT); (c) carcinoma with mutated codon 34 (GGA->GAA).
Differential Gene Expression Indicates Up-Regulation of Pathways Associated with hCRC Principal Component Analysis (PCA) and Hierarchal Cluster Analysis (HCA)
Principal component analysis plots revealed a clear-cut clustering between normal mucosal epithelial samples (negative controls) and tumor samples. However, within the tumor samples, there was a slight overlap between Ads and Cas samples (Figure 3A). Likewise, HCA of all of the 110 statistically significant genes revealed a tight clustering of normal mucosal epithelium (negative controls) and Ads and Cas segregated reasonably well, with the exception of a single Cas that has clustered with the Ads (Figure 3B).
FIGURE 3.
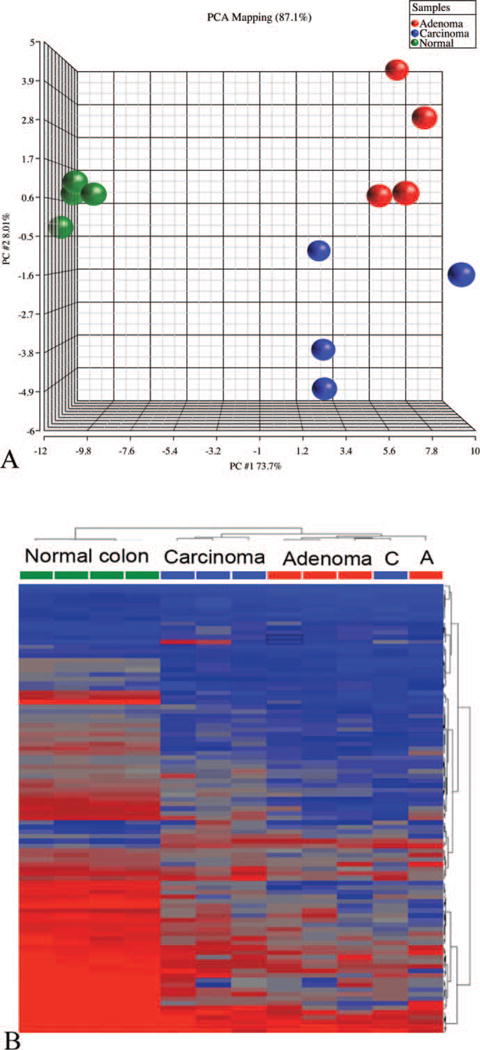
Principal component analysis (A) and hierarchical cluster analysis (B) of differentially expressed genes within untreated colon tissues as well as large intestinal adenomas and carcinomas from F344 rats exposed to Aloe vera non-decolorized whole leaf extract in drinking water, ad libitum, for two years.
MAPK Signaling
The MAPK signaling pathway was highly represented within both the Ads and Cas samples (Table 2, Figure 4A, supplementary Figure 1). Literature citing the roles played by each of the significantly altered genes within CRC is presented in the references column of supplementary Table 2. Trend analysis of differential gene expression after normalizing to the housekeeping gene Actb indicated twenty-five genes and two genes with increasing and decreasing differential gene expression patterns, respectively (supplementary Table 3). Pairwise comparisons (controlling for directional errors and FDR at a 5% nominal level) indicated that twenty-two genes and thirteen genes were significantly altered within the Ads and Cas samples, respectively (Figure 4A, supplementary Table 2). There was an excellent agreement between the trend test and pairwise comparisons. There were only five of twenty-seven unique genes within the trend test and one of twenty-three unique genes within the pairwise comparisons. There was up-regulation of transcription regulators (Ccne1, Creb1, Ets1, and Tp53), kinases (Cdk4, Cdk6, and Ksr1), and cell cycle genes (Ccnb2, Ccnd1, and Ccnd2) in both Ads and Cas. In contrast, some transcription factors (Cdnk2c and Myc), and kinases (Cdc42, Egfr, Mapk7, Mapk2k1, Mapk2k4, Mapk12, and Mapk13) were significantly up-regulated only in Ads (they did not meet the FDR criteria for significance in Cas). Heat shock protein Hspb1 was up-regulated in Ads and Cas, whereas Hspa5 was down-regulated only in Cas. Col1a1, a profibrogenic extracellular matrix gene, was highly expressed both within Ads and Cas.
TABLE 2.
Summary of differentially expressed genes in the large intestinal tumors of F344 rats chronically exposed to Aloe vera non-decolorized whole leaf extract in drinking water.
| Group | Differentially expressed genes in Aloe vera non-decolorized whole leaf extract–induced tumors |
|---|---|
| MAPK signaling | |
| Ras/Raf1/MEK/ERK | Map2k1, Mapk1 |
| Stress-activated MAPKs | Map2k4, Mapk7, Mapk12, Mapk13, Mapk8ip3 |
| Transcription regulators | Ets1, Tp53, Creb1, Ccne1, Myc, Cdkn2c |
| Cell cycle genes | Ccnd1, Ccnd2, Ccnd3, Ccnb2, Cdc42, Cdk6, Cdk4, Csnk2a1, Csnk2b |
| Others | Egfr, Ksr1, Col1a1, Hspa5a, Hspb1 |
| WNT signaling | |
| WNT ligands and receptors | Wnt 2b, Wnt3, Wnt3a, Wnt4, Wnt7b, Frzb, Fzd2, Fzd5a, Fzd6 |
| APC complex | Dvl1, Axin1, Axin2, Ctnnb1 |
| Non-canonical WNT signaling | Wnt5a, Fzd6, Dvl1, RhoA, Nkd1, Nkd2 |
| Others | Tcf3, Tcf4, Tle1, RhoA, Bcl9, Dkk3, Nkd2, Sfrp1, Sfrp4, Fbxw2, Lef1, Lrp5, Pitx2, Porcn, Rgd1561440, Ppp2ca |
| TGF-β Signaling | |
| Growth factors | Smad1, Smad2, Smad5 |
| Transcription regulators | Tgfb1, Tgfb2, Tgfb3, Bmp1, Bmp4, Bmpr2, Inhba |
| Kinases | Tgfbr1, Tgfbr2, Tgfbr3 |
| Other genes important in hCRC | |
| Transcription regulators | Klf4a, Sox4, Sox9, Stat1, Stat3, Tcf7l2 |
| Kinases | Pik3cb, Pik3r1, Pik3r2, Akt1, Akt2, Akt3, Fgfr1, Sgk1a, Stk11, Fgfr1 |
| Phosphatases | Cdc25a, Dusp4, Ptpro, Ptprs, |
| Apoptosis | Birc5, Bax, Casp3a |
| Others | Tnf-α, Nos2, Ca2a, Prdx6, Hpgda, Hsd17b2a, Msh2, Psat1, Timp1a, Hspd1, Top2a, Sparc |
Down-regulated gene. All other genes are up-regulated compared to untreated control colonic epithelium.
FIGURE 4.
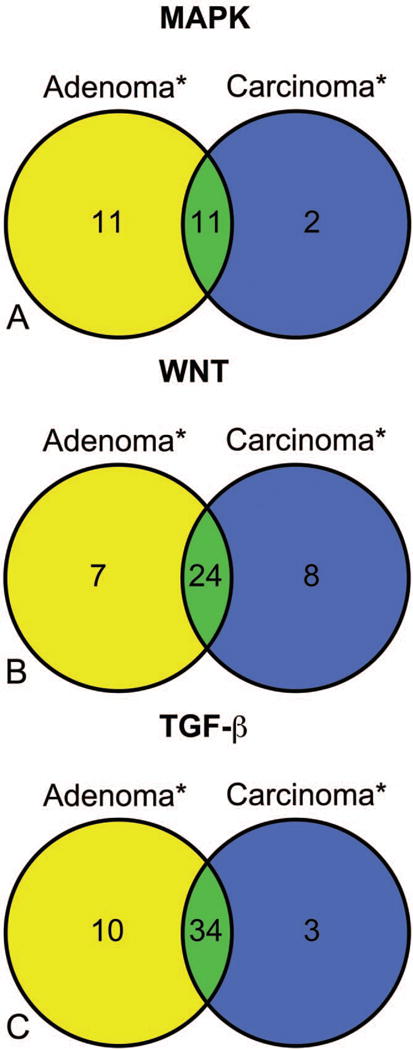
The venn diagrams represent the number of differentially expressed genes (*), within the Aloe vera non-decolorized whole leaf extract exposed F344 large intestinal adenoma and carcinoma samples compared to control colon tissue, by multiple pairwise comparisons and the statistical significance was determined by controlling the false discovery rate (FDR) at a nominal 5% level. (A) MAPK signaling; (B) WNT signaling; (C) TGF-β signaling.
WNT Signaling
The WNT signaling pathway was highly represented within both the Ads and Cas samples (Table 2, Figure 4B, supplementary Figure 2). Literature citing the roles played by each of the significantly altered genes within CRC is presented in the references column of supplementary Table 4. Trend analysis of differential gene expression after normalizing to the housekeeping genes Actb indicated thirty-four genes and one gene with increasing and decreasing differential gene expression patterns, respectively (supplementary Table 5). Pairwise comparisons (controlling for directional errors and FDR at a 5% nominal level) indicated that thirty-one genes and thirty-two genes were significantly altered within the Ads and Cas samples, respectively (Figure 4B, supplementary Table 4). There were only two of thirty-four unique genes within the trend test and nine of thirty-nine unique genes within the pairwise comparisons. There was up-regulation of transcription regulators (Ctnnb1, Lef1, Tcf3, Tcf4, and Tcf7), WNT genes (Wif1, Wnt3, Wnt4, and Wnt7b), APC complex genes (Axin1, Dvl1, Frzb, Fzd2, Fzd5, and Fzd6), cell cycle genes (Csnk2a1 Ccnd1, Ccnd2, and Ccnd3), and other important WNT pathway genes (RhoA, Bcl9, Dkk3, Nkd2, Sfrp1, and Sfrp4) in both Ads and Cas. Transcription regulators Myc, Pitx2, and Tle2 and WNT pathway genes Fbxw2, Axin2, Nkd1, RGD1561440, and Ppp2ca were up-regulated only in Ads. Conversely, WNT genes Lrp5, Porcn, Wnt2b, Wnt3a, and Wnt5b were up-regulated only within Cas.
TGF-β Signaling and Other Genes Relevant for Human Colorectal Cancers
Several important genes within the TGF-β signaling pathway were altered in both Ads and Cas (Table 2, Figure 4C, supplementary Figure 3). Literature citing the roles played by each of the significantly altered genes within CRC is presented in the references column of supplementary Table 6. Trend analysis of differential gene expression after normalizing to the housekeeping genes Actb indicated thirty-eight genes and ten genes with increasing and decreasing differential gene expression patterns, respectively (supplementary Table 7). Pairwise comparisons (controlling for directional errors and FDR at a 5% nominal level) indicated that forty-four genes and thirty-seven genes were significantly altered within the Ads and Cas samples, respectively (Figure 4C, supplementary Table 6). There were only seven of forty-eight unique genes within the trend test and six of forty-seven unique genes within the pairwise comparisons. Relevant TGF-β pathway transcription regulators (Smad2), growth factors (Bmp1, Bmp4, Inhba, Tgfb1, Tgfb2, and Tgfb3), and kinases (Tgfbr1, Tgfbr2, and Tgfbr3) were altered in both Ads and Cas. Smad5 was altered only in Ads, and Smad1 was altered only in Cas.
Among other relevant human CRC genes, there was differential expression of transcription regulators (Klf4, Sox9, Stat1, Stat3, and Tcf7l2), kinases (Akt1, Akt2, Akt3, Sgk1, and Stk11), phosphatases Cdc25a, Dusp4, Ptpro and Ptprs), and other colorectal cancer genes (Tnf, Ca2, Hpgd, Hsd17b2, Msh2, Nos2, Psat1, Bax, and Sparc) in both Ads and Cas. Some kinases (Fgfr1, Pik3cb, Pik3r1, and Pik3r1) and other colorectal cancer genes (Hspd1, Top2a, Birc5, and Timp1) were up-regulated only in Ads, and Casp3 was down-regulated only within Cas.
Discussion
We have demonstrated that the AVNWLE-induced large intestinal tumors in F344 rats share several similarities with human CRC at the morphological and molecular level. There was excellent correlation between the trend analysis and pairwise comparisons and the resulting genes from each pathway yielded a very meaningful molecular insight into the possible pathogenesis of AVNWLE-induced colorectal tumors in F344 rats. There was no single molecular pathway that was unique to Ads or Cas samples. Within the PCA and HCA, the segregation between Ads and Cas samples was not very discrete and there was a slight overlap. These findings were not surprising since these plots were based on a limited set of genes (not based on whole genome arrays) and may also be due to the fact that the pathogenesis of the Ads and Cas is part of a continuum. Histological re-examination of the Cas sample that had clustered with the Ads (in the HCA plot) unequivocally confirmed our previous diagnosis of Cas.
In this study, the ideal samples for differential gene expression would have been age-matched controls, since it is known that there can be differences in gene expression between intestinal mucosa of control animals of different ages. However, because of logistical issues (described in sample collection), this method was not possible. Nevertheless, the differential gene expression between tumor tissue (from two-year-old rats) and control colonic epithelium (from six-month-old rats) is highly suggestive of a tumor signature.
Kras mutations are an early event in chemical-induced colorectal carcinogenesis and are seen with increasing frequency within aberrant crypt foci<adenoma<carcinoma. As illustrated in Table 3, the incidence of Kras mutations in AVNWLE-treated F344 rats was 33% and was comparable to human colorectal cancers (40%), 1,2-dimethylhydrazine (1,2-DMH)–induced colon tumors in albino rats (66%), and azoxymethane (AOM)-induced colon tumors in F344 rats (37%; Bos et al. 1987; Jacoby et al. 1991; Vivona et al. 1993). Point mutations in exon 1 of Kras observed in this study were similar to those reported in 1,2-DMH and AOM-induced colon tumors in rats as well as in human CRC. Ctnnb1 mutations are also potential early events in human CRC (Markowitz and Bertagnolli 2009). The incidence of Ctnnb1 mutations in this study was 33% (Table 3) and was comparable to human CRC (10–26%), but significantly lower than that reported in rats exposed to AOM (80%) and cooked meat–derived heterocyclic amines (75%; Dashwood et al. 1998; Morin et al. 1997; Takahashi et al. 1998). AVNWLE-induced Ctnnb1 mutations observed in codons 32, 34, 41, and 45 are in the proximity of threonine and serine sites that are important for phosphorylation. The mutations within Kras and Ctnnb1 were mutually exclusive within the tumor samples, that is, no single tumor sample had mutations in both Kras and Ctnnb1. In addition, the Kras mutations in adenomas (codon 13) and carcinomas (codons 12 and 61) were also mutually exclusive. These seemingly mutually exclusive mutations may be a result of the small sample size, especially considering the Vogelstein’s paradigm of adenoma-carcinoma continuum, where the carcinoma samples should include the mutations observed within the adenoma samples. In human CRC, the frequency of mutations in the Tp53 gene is about 50% (Nigro et al. 1989). However, no Tp53 mutations (in exons 5–8) were found within the AVNWLE-induced large intestinal tumors. Interestingly, no Tp53 mutations were observed in rodent models of colon cancer, such as 2-amino-1-methyl-6-phenylimidazo-[4,5-b]pyridine (PhIP) and azoxymethane (Takahashi and Wakabayashi 2004). These data suggest that AVNWLE-induced large intestinal tumors in the rat develop independently of the TP53 signal transduction pathway, but they involve other significant genetic pathways that are relevant to human colon cancer.
TABLE 3.
Comparison of incidence of point mutations within large intestinal tumors of F344 rats chronically exposed to Aloe vera non-decolorized whole leaf extract with human colorectal cancera and rodent models of chemically induced colon cancer.b
| Group | % Kras mutations | % Ctnnb1 mutations | % Tp53 mutations |
|---|---|---|---|
| Aloe vera non-decolorized whole leaf extract | 33 | 33 | 0 |
| Human colon cancer | 40–60 | 15–26 | 50* |
| Azoxymethane | 30–60 | 50–80 | 0 |
| Heterocyclic amines | 0–14 | 50–75 | 0 |
Fisher exact test, p < .008.
The MAPK signal transduction pathway is very important in colon cancer. It consists of four main pathways: extracellular signal-related kinases (Ras/Raf1/MEK/ERK), ERK5 (BMK1 or MAPK7), c-Jun N-terminal kinases or stress activated protein kinases (JNK or SAPK), and p38 kinases (p38α, p38β, p38γ, p38δ; Johnson and Lapadat 2002). These pathways may be activated through consistent activation of Kras and by various stimuli such as growth factors, cytokines, G-protein–coupled receptor ligands, stress, carcinogens, and transforming agents (Johnson and Lapadat 2002). In this study, several members of the MAPK pathway were significantly altered, such as the Map2k1, Map2k4, Mapk1, Mapk12, Mapk13, Mapk7, and Mapk8ip3. Map2k1 activates Mapk3 and Mapk1, and plays an important role in the epithelial-to-mesenchymal transition (Lemieux et al. 2009). Map2k4, an upstream kinase of the p38 and JNK pathway, is primarily activated by environmental stress. Mapk12 and Mapk13 are members of the p38 MAPK signaling and Mapk7 is a member of the ERK5 signaling. The up-regulation of Map2k1 and Mapk1 may be related to mitogenic stimulation by the mutated Kras, and the up-regulation of Mapk7, Map2k4, Mapk12, Mapk13, and Mapk8ip3 may be caused by various cellular stresses. Thus, the overrepresentation of the MAPK pathway within these large intestinal tumors is likely a result of a multi-pronged stimulation caused by the mutated Kras as well as cellular stress.
In addition to activation of the MAPK pathway, there was alteration of several downstream cell cycle genes (Cdc42, Cdk4, Cdk6, Ccnb2, Ccnd1, and Ccnd2) that play an important role in human CRC. Creb1, up-regulated in both Ads and Cas, inhibits apoptosis in colon cancer cells by inducing cellular inhibitor of apoptosis protein 2 (Nishihara et al. 2004). Ets1, a proto-oncogene, was highly up-regulated in AVNWLE tumors, and its expression was correlated with tumor invasion in human CRC (Nakayama et al. 2001). Consistent with the role of inflammation in hCRC, ETS1 can also regulate COX2 promoter activity (Zhang et al. 2007).
WNT/CTNNB1 signaling is altered in almost all cases of CRC (Kinzler and Vogelstein 1996; Morin et al. 1997). Several genes within the WNT/CTNNB1 pathway were also altered within the AVNWLE-induced large intestinal tumors in F344 rats, including up-regulation of Ctnnb1. The catalog of differentially expressed genes within AVNWLE-induced large intestinal tumors in rats indicates that all stages of the canonical WNT/CTNNB1 pathway were represented. However, several negative regulators (Dkk3, Wif1, Sfrp1, Sfrp4, Nkd1, and Nkd2) of the canonical WNT/CTNNB1 pathway were up-regulated within our dataset, perhaps because of negative feedback up-regulation of these genes (Cebrat et al. 2004; Katoh 2001). In addition, some of these canonical WNT antagonists and agonists may regulate CTNNB1-independent WNT signaling pathways, such as the WNT/Ca2+ pathway or the WNT/planar cell polarity (PCP) pathway (Katoh 2005; Katoh 2007). Several non-canonical WNT signaling mediators that were up-regulated within our dataset included Wnt5a, Fzd6, Dvl1, RhoA, Nkd1, and Nkd2. Also, some of these genes may interact with the MAPK signaling through non-canonical WNT signaling pathways (Katoh 2005; 2007).
The TGF-β pathway is a critical pathway that is usually altered during the later stages of human CRC pathogenesis (Markowitz and Bertagnolli 2009). A potent pleotrophic cytokine, TGF-β has myriad functions based on the cell type, stage, and context of signaling. It acts as a tumor suppressor in normal intestinal cells but acts as a potent tumor promoter in colon cancer (Wakefield and Roberts 2002). In tumor cells, TGF-β promotes uncontrolled cell proliferation, epithelial-mesenchymal transition (EMT), invasion, metastasis, angiogenesis, and dysregulated immunosurveillance (Tian et al. 2010).
Based on the differentially expressed genes within the canonical TGF-β signaling pathway in AVNWLE-induced large intestinal tumors, there was up-regulation of ligands (Tgfb1, Tgfb2, and Tgfb3) that bind and activate TGFβ receptors (Tgfbr1 and Tgfbr2). The activated Tgfr1 phosphorylates Smad2, but its heteromeric complex partners Smad3 and Smad4 were not represented within the AVNWLE-induced rat large intestinal tumors. Overexpression of tumor-derived Smad2.D450E, an unphosphorylable form of Smad2 found in colorectal cancers, induces enhanced cellular invasion in the presence of TGF-β but does not inhibit TGFβ-mediated growth arrest (Prunier et al. 1999). The expression of Smad4, a bonafide tumor suppressor, is lost in several colon cancers and was not detected in this study. In addition, it has been shown the SMAD4 silencing and Ras hyperstimulation can overcome TGF-β’s tumor suppressor effects (Calonge and Massague 1999). BMP-mediated SMAD signaling appears to be down-regulated within our data set since Bmpr2 is down-regulated with corresponding up-regulation of Bmp1 and Bmp4, which is possibly a consequence of a feedback loop. In human colorectal tumors, down-regulation of BMPRII and up-regulation of BMP1 and BMP4 has been demonstrated (Beppu et al. 2008; Kim et al. 2002). Non-canonical SMAD-independent TGF-β signaling occurs through several signal transduction pathways such as MAPK (ERK1/ERK2, p38, and JNK), growth and survival kinases (PI3K, AKT/PKB, and mTOR), and small GTP-binding proteins (RAS, RHOA, RAC1, and CDC42; Tian et al. 2010). Several of these mediators are up-regulated within AVNWLE-induced intestinal tumors, suggesting an interplay between multiple signal transduction pathways.
Several mediators that play important roles in inflammation as well as cellular proliferation were altered in AVNWLE-induced intestinal tumors. It is well known that inflammation plays a promoter role in carcinogenesis, and examples include CRC within ulcerative colitis, dextran sodium sulfate rodent colitis-tumor models, and genetically engineered mouse models such as IL-2/β2M DKO, IL-10 KO, and Rag2 KO (Itzkowitz and Yio 2004). Within these models, many of the genes that were altered within colon tumors were also altered within inflamed colonic mucosa without any histological evidence of dysplasia (Itzkowitz and Yio 2004). Tnf, an important pleotrophic ligand for activation of NFκB, stress related-MAPK JNK, and p38 signaling, and apoptosis signaling was markedly up-regulated within both AVNWLE-induced Ads and Cas samples (Beissert et al. 1989; Yoshimi et al. 1994). Nos2, an important inducer of TNF, was also markedly up-regulated within all colon tumors examined in this study (Ambs et al. 1998; Zhang et al. 1998). STAT1 and STAT3 are important mediators within the JAK-STAT signaling pathway and are activated by interferon γ and IL-6 ligands, respectively. Activated STAT1 and STAT3 regulate several target genes important in inflammation, and innate and adaptive immune responses. In addition, activation of STAT3 has been reported in several tumors in which it has been associated with growth promotion and anti-apoptosis (Aaronson and Horvath 2002). Several mediators of the PI3K/Akt signaling pathway (Pik3cb, Pik3r1, Pik3r2, Akt1, Akt2, Akt3, and Stk11) were up-regulated in this study. This pathway is important in effecting inflammatory response, anti-apoptosis, and cell proliferation (Paez and Sellers 2003). Evaluation of IPA pathway interactions based on the differentially expressed genes in this study indicated altered canonical inflammatory pathways such as IL-8, NFκB, and glucocorticoid receptor signaling (supplementary Figure 4). Further prospective studies are warranted to examine the roles played by these inflammatory pathways in colon carcinogenesis in greater detail.
Most anthranoid laxatives, and probably those from AVNWLE as well, exert their laxative effects by inhibition of Na+-K+ ATPase, damaging colonic epithelial cells, and increasing intestinal motility. In addition, the damaged epithelial cells release numerous proinflammatory cytokines, resulting in recruitment of inflammatory cells that subsequently release additional inflammatory mediators with myriad functions in inflammation and cell proliferation (van Gorkom et al. 1999). In this study, there was moderate mixed inflammatory cell infiltration within the tumors (NTP 2011). However, it is uncertain whether the inflammatory infiltrate preceded or is the result of tumorigenesis. The genotoxicity of anthraquinone compounds or mixtures is dependent on the dose, type of assay, and chemical nature and structure of the anthraquinones (Mueller et al. 1996; Westendorf et al. 1990). Though AVNWLE was found to be non-genotoxic in the NTP’s Ames test, it does not necessarily mean that the test article is indeed non-genotoxic at the tissue level (colonic epithelial interface) because of the numerous variables that can influence the genotoxic status of the test compound. Thus, it is possible that genotoxicity along with chronic inflammation may have contributed to AVNWLE-induced large intestinal tumorigenesis in F334 rats.
In conclusion, we have demonstrated that the molecular pathways important in the pathogenesis of hCRC such as MAPK, WNT, and TGF-β signaling were also altered in large intestinal tumors from F344 rats chronically exposed to AVNWLE in drinking water. Further research, particularly earlier pre-disease time points, may provide data on potential predictors or biomarkers of disease that can be useful in hazard identification and protecting human health.
Supplementary Material
Acknowledgments
The authors would like to thank Dr. Darlene Dixon and Dr. Michael Devito for their review of this manuscript; Dr. Keith Shockley and Dr. Grace Kissling for help with statistics; and Ms. Natasha Clayton and Ms. Beth Mahler for their excellent technical assistance. This study was supported in part by IAG #224-07-0007 between the FDA and the NTP as well as by the intramural research program at NIEHS/DHHS.
This article may be the work product of an employee or group of employees of the National Institute of Environmental Health Sciences (NIEHS), National Institutes of Health (NIH); however, the statements, opinions or conclusions contained therein do not necessarily represent the statements, opinions, or conclusions of NIEHS, NIH, or the United States government.
Abbreviations
- Ads
adenomas
- AVNWLE
Aloe barbadensis Miller (Aloe vera) non-decolorized whole leaf extract
- Cas
carcinomas
- CTNNB1
β-catenin
- KRAS
Kirsten rat sarcoma viral oncogene homolog
- MAPK
mitogen-activated protein kinase
- PCR arrays
quantitative real-time reverse-transcriptase polymerase chain reaction arrays
- NTP
National Toxicology Program
- NCTR
National Center for Toxicological Research
- USFDA
United States Food and Drug Administration
- WNT
Wg (wingless) and Int
Footnotes
The authors received no financial support for the research and/or authorship of this article.
References
- Aaronson DS, Horvath CM. A road map for those who don’t know JAK-STAT. Science. 2002;296:1653–55. doi: 10.1126/science.1071545. [DOI] [PubMed] [Google Scholar]
- Ambs S, Merriam WG, Bennett WP, Felley-Bosco E, Ogunfusika MO, Oser SM, Klein S, Shields PG, Billiar TR, Harris CC. Frequent nitric oxide synthase-2 expression in human colon adenomas: Implication for tumor angiogenesis and colon cancer progression. Cancer Res. 1998;58:334–41. [PubMed] [Google Scholar]
- Anonymous. Medicinal herbs: NTP extracts the facts. Environ Health Perspect. 1999;107:A604–5. doi: 10.1289/ehp.107-1566815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beissert S, Bergholz M, Waase I, Lepsien G, Schauer A, Pfizenmaier K, Kronke M. Regulation of tumor necrosis factor gene expression in colorectal adenocarcinoma: in vivo analysis by in situ hybridization. Proc Natl Acad Sci U S A. 1989;86:5064–68. doi: 10.1073/pnas.86.13.5064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beppu H, Mwizerwa ON, Beppu Y, Dattwyler MP, Lauwers GY, Bloch KD, Goldstein AM. Stromal inactivation of BMPRII leads to colorectal epithelial overgrowth and polyp formation. Oncogene. 2008;27:1063–70. doi: 10.1038/sj.onc.1210720. [DOI] [PubMed] [Google Scholar]
- Boivin GP, Washington K, Yang K, Ward JM, Pretlow TP, Russell R, Besselsen DG, Godfrey VL, Doetschman T, Dove WF, Pitot HC, Halberg RB, Itzkowitz SH, Groden J, Coffey RJ. Pathology of mouse models of intestinal cancer: Consensus report and recommendations. Gastroenterology. 2003;124:762–77. doi: 10.1053/gast.2003.50094. [DOI] [PubMed] [Google Scholar]
- Bos JL, Fearon ER, Hamilton SR, Verlaan-de Vries M, van Boom JH, van der Eb AJ, Vogelstein B. Prevalence of ras gene mutations in human colorectal cancers. Nature. 1987;327:293–97. doi: 10.1038/327293a0. [DOI] [PubMed] [Google Scholar]
- Boudreau MD, Beland FA. An evaluation of the biological and toxicological properties of Aloe barbadensis (miller), Aloe vera. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev. 2006;24:103–54. doi: 10.1080/10590500600614303. [DOI] [PubMed] [Google Scholar]
- Calonge MJ, Massague J. Smad4/DPC4 silencing and hyperactive Ras jointly disrupt transforming growth factor-beta antiproliferative responses in colon cancer cells. J Biol Chem. 1999;274:33637–43. doi: 10.1074/jbc.274.47.33637. [DOI] [PubMed] [Google Scholar]
- Cebrat M, Strzadala L, Kisielow P. Wnt inhibitory factor-1: A candidate for a new player in tumorigenesis of intestinal epithelial cells. Cancer Lett. 2004;206:107–13. doi: 10.1016/j.canlet.2003.10.024. [DOI] [PubMed] [Google Scholar]
- Dashwood RH, Suzui M, Nakagama H, Sugimura T, Nagao M. High frequency of beta-catenin (ctnnb1) mutations in the colon tumors induced by two heterocyclic amines in the F344 rat. Cancer Res. 1998;58:1127–29. [PubMed] [Google Scholar]
- Edwards BK, Ward E, Kohler BA, Eheman C, Zauber AG, Anderson RN, Jemal A, Schymura MJ, Lansdorp-Vogelaar I, Seeff LC, van Ballegooijen M, Goede SL, Ries LA. Annual report to the nation on the status of cancer, 1975–2006, featuring colorectal cancer trends and impact of interventions (risk factors, screening, and treatment) to reduce future rates. Cancer. 2010;116:544–73. doi: 10.1002/cncr.24760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efron B, Tibshirani R. An Introduction to Bootstrap. Chapman and Hall; New York, NY: 1994. [Google Scholar]
- Elsohly MA, Gul W, Avula B, Khan IA. Determination of the anthraquinones aloe-emodin and aloin-A by liquid chromatography with mass spectrometric and diode array detection. J AOAC Int. 2007;90:28–42. [PubMed] [Google Scholar]
- Elwell MR, McConnell EE. Small and large intestine. In: Boorman GA, Eustis SL, Elwell MR, Montgomery J Jr, A C, Mackenzie WF, editors. Pathology of the Fisher rat: Reference and Atlas. Academic Press, Inc; San Diego, CA: 1990. pp. 43–62. [Google Scholar]
- Fearon ER, Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990;61:759–67. doi: 10.1016/0092-8674(90)90186-i. [DOI] [PubMed] [Google Scholar]
- Hu Z, Yang X, Ho PC, Chan SY, Heng PW, Chan E, Duan W, Koh HL, Zhou S. Herb-drug interactions: A literature review. Drugs. 2005;65:1239–82. doi: 10.2165/00003495-200565090-00005. [DOI] [PubMed] [Google Scholar]
- Itzkowitz SH, Yio X. Inflammation and cancer IV. Colorectal cancer in inflammatory bowel disease: the role of inflammation. Am J Physiol Gastrointest Liver Physiol. 2004;287:G7–17. doi: 10.1152/ajpgi.00079.2004. [DOI] [PubMed] [Google Scholar]
- Izzo AA, Ernst E. Interactions between herbal medicines and prescribed drugs: An updated systematic review. Drugs. 2009;69:1777–98. doi: 10.2165/11317010-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Jacoby RF, Llor X, Teng BB, Davidson NO, Brasitus TA. Mutations in the K-ras oncogene induced by 1,2-dimethylhydrazine in preneoplastic and neoplastic rat colonic mucosa. J Clin Invest. 1991;87:624–30. doi: 10.1172/JCI115039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson GL, Lapadat R. Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science. 2002;298:1911–12. doi: 10.1126/science.1072682. [DOI] [PubMed] [Google Scholar]
- Katoh M. Molecular cloning, gene structure, and expression analyses of NKD1 and NKD2. Int J Oncol. 2001;19:963–69. [PubMed] [Google Scholar]
- Katoh M. WNT/PCP signaling pathway and human cancer (review) Oncol Rep. 2005;14:1583–88. [PubMed] [Google Scholar]
- Katoh M. WNT signaling pathway and stem cell signaling network. Clin Cancer Res. 2007;13:4042–45. doi: 10.1158/1078-0432.CCR-06-2316. [DOI] [PubMed] [Google Scholar]
- Kim JS, Crooks H, Dracheva T, Nishanian TG, Singh B, Jen J, Waldman T. Oncogenic beta-catenin is required for bone morphogenetic protein 4 expression in human cancer cells. Cancer Res. 2002;62:2744–48. [PubMed] [Google Scholar]
- Kinzler KW, Vogelstein B. Lessons from hereditary colorectal cancer. Cell. 1996;87:159–70. doi: 10.1016/s0092-8674(00)81333-1. [DOI] [PubMed] [Google Scholar]
- Lemieux E, Bergeron S, Durand V, Asselin C, Saucier C, Rivard N. Constitutively active MEK1 is sufficient to induce epithelial-to-mesenchymal transition in intestinal epithelial cells and to promote tumor invasion and metastasis. Int J Cancer. 2009;125:1575–86. doi: 10.1002/ijc.24485. [DOI] [PubMed] [Google Scholar]
- Markowitz SD, Bertagnolli MM. Molecular origins of cancer: Molecular basis of colorectal cancer. N Engl J Med. 2009;361:2449–60. doi: 10.1056/NEJMra0804588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin PJ, Sparks AB, Korinek V, Barker N, Clevers H, Vogelstein B, Kinzler KW. Activation of beta-catenin-Tcf signaling in colon cancer by mutations in beta-catenin or APC. Science. 1997;275:1787–90. doi: 10.1126/science.275.5307.1787. [DOI] [PubMed] [Google Scholar]
- Mueller SO, Eckert I, Lutz WK, Stopper H. Genotoxicity of the laxative drug components emodin, aloe-emodin and danthron in mammalian cells: Topoisomerase II mediated? Mutat Res. 1996;371:165–73. doi: 10.1016/s0165-1218(96)90105-6. [DOI] [PubMed] [Google Scholar]
- Nakayama T, Ito M, Ohtsuru A, Naito S, Sekine I. Expression of the ets-1 proto-oncogene in human colorectal carcinoma. Mod Pathol. 2001;14:415–22. doi: 10.1038/modpathol.3880328. [DOI] [PubMed] [Google Scholar]
- Nigro JM, Baker SJ, Preisinger AC, Jessup JM, Hostetter R, Cleary K, Bigner SH, Davidson N, Baylin S, Devilee P, et al. Mutations in the p53 gene occur in diverse human tumour types. Nature. 1989;342:705–8. doi: 10.1038/342705a0. [DOI] [PubMed] [Google Scholar]
- Nishihara H, Hwang M, Kizaka-Kondoh S, Eckmann L, Insel PA. Cyclic AMP promotes cAMP-responsive element-binding protein-dependent induction of cellular inhibitor of apoptosis protein-2 and suppresses apoptosis of colon cancer cells through ERK1/2 and p38 MAPK. J Biol Chem. 2004;279:26176–83. doi: 10.1074/jbc.M313346200. [DOI] [PubMed] [Google Scholar]
- NTP-TR-577. NTP Toxicology and carcinogenesis studies of Aloe vera non-decolorized whole leaf extract (8001-97-6) drinking water studies in F344/N rats and B6C3F1 mice. Natl Toxicol Program Tech Rep Ser. 2011;577 in press. [Google Scholar]
- Paez J, Sellers WR. PI3K/PTEN/AKT pathway. A critical mediator of oncogenic signaling. Cancer Treat Res. 2003;115:145–67. [PubMed] [Google Scholar]
- Peddada S, Harris S, Zajd J, Harvey E. ORIOGEN: Order restricted inference for ordered gene expression data. Bioinformatics. 2005;21:3933–34. doi: 10.1093/bioinformatics/bti637. [DOI] [PubMed] [Google Scholar]
- Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prunier C, Mazars A, Noe V, Bruyneel E, Mareel M, Gespach C, Atfi A. Evidence that Smad2 is a tumor suppressor implicated in the control of cellular invasion. J Biol Chem. 1999;274:22919–22. doi: 10.1074/jbc.274.33.22919. [DOI] [PubMed] [Google Scholar]
- Smyth GK. Linear models and empirical bayes methods for assessing differential expression in microarray experiments. Stat Appl Genet Mol Biol. 2004;3 doi: 10.2202/1544-6115.1027. Article 3. [DOI] [PubMed] [Google Scholar]
- Takahashi M, Fukuda K, Sugimura T, Wakabayashi K. Beta-catenin is frequently mutated and demonstrates altered cellular location in azoxymethane-induced rat colon tumors. Cancer Res. 1998;58:42–46. [PubMed] [Google Scholar]
- Takahashi M, Wakabayashi K. Gene mutations and altered gene expression in azoxymethane-induced colon carcinogenesis in rodents. Cancer Sci. 2004;95:475–80. doi: 10.1111/j.1349-7006.2004.tb03235.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian M, Neil JR, Schiemann WP. Transforming growth factor-beta and the hallmarks of cancer. Cell Signal. 2011;23:951–62. doi: 10.1016/j.cellsig.2010.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Gorkom BA, de Vries EG, Karrenbeld A, Kleibeuker JH. Review article: Anthranoid laxatives and their potential carcinogenic effects. Aliment Pharmacol Ther. 1999;13:443–52. doi: 10.1046/j.1365-2036.1999.00468.x. [DOI] [PubMed] [Google Scholar]
- Vivona AA, Shpitz B, Medline A, Bruce WR, Hay K, Ward MA, Stern HS, Gallinger S. K-ras mutations in aberrant crypt foci, adenomas and adenocarcinomas during azoxymethane-induced colon carcinogenesis. Carcinogenesis. 1993;14:1777–81. doi: 10.1093/carcin/14.9.1777. [DOI] [PubMed] [Google Scholar]
- Wakefield LM, Roberts AB. TGF-beta signaling: Positive and negative effects on tumorigenesis. Curr Opin Genet Dev. 2002;12:22–29. doi: 10.1016/s0959-437x(01)00259-3. [DOI] [PubMed] [Google Scholar]
- Westendorf J, Marquardt H, Poginsky B, Dominiak M, Schmidt J. Genotoxicity of naturally occurring hydroxyanthraquinones. Mutat Res. 1990;240:1–12. doi: 10.1016/0165-1218(90)90002-j. [DOI] [PubMed] [Google Scholar]
- Yoshimi N, Sato S, Makita H, Wang A, Hirose Y, Tanaka T, Mori H. Expression of cytokines, TNF-alpha and IL-1 alpha, in MAM acetate and 1-hydroxyanthraquinone-induced colon carcinogenesis of rats. Carcinogenesis. 1994;15:783–85. doi: 10.1093/carcin/15.4.783. [DOI] [PubMed] [Google Scholar]
- Zhang X, Zhang J, Yang X, Han X. Several transcription factors regulate COX-2 gene expression in pancreatic beta-cells. Mol Biol Rep. 2007;34:199–206. doi: 10.1007/s11033-007-9085-3. [DOI] [PubMed] [Google Scholar]
- Zhang XJ, Thompson JH, Mannick EE, Correa P, Miller MJ. Localization of inducible nitric oxide synthase mRNA in inflamed gastrointestinal mucosa by in situ reverse transcriptase-polymerase chain reaction. Nitric Oxide. 1998;2:187–92. doi: 10.1006/niox.1998.0177. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


