Abstract
Hypomagnesemia can lead to cardiac arrhythmias. Recently, observational data has linked chronic proton pump inhibitor (PPI) exposure to hypomagnesemia. Whether PPI exposure increases the risk for arrhythmias has not been well studied. Using a large, single center inception cohort of critically ill patients, we examined whether PPI exposure was associated with admission electrocardiogram (ECG) readings of a cardiac arrhythmia in over 8000 patients. There were 24.5% PPI users while 6% were taking a histamine 2 antagonist. 14.3% had a cardiac arrhythmia. PPI use was associated with a 1.18 (95% CI=1.02–1.36, p=0.02) unadjusted and 0.96 (95% CI=0.83–1.12, p=0.62) adjusted risk of arrhythmia. Amongst diuretic users (n=2468), PPI use was similarly not associated with an increased risk of cardiac arrhythmia. In summary, in a large cohort of critically ill patients, PPI exposure is not associated with an increased risk of cardiac arrhythmia.
Introduction
Proton pump inhibitors (PPI), used widely by prescription and over the counter, have recently been linked to hypomagnesemia [1–9], although not consistently [10–12]. Risk factors for PPI associated hypomagnesemia include long-term PPI use and diuretic exposure [13, 14]. PPI may prevent the absorption of magnesium across the intestinal surface, leading to chronic magnesium deficiency [15].
Whereas many observational studies have found significant associations between chronic PPI use and hypomagnesemia, there remains no conclusive data. Residual confounding due to decreased dietary magnesium intake remains in these studies, and since magnesium is an intracellular ion, serum concentrations likely do not reflect magnesium homeostasis. Therefore, determining whether PPI use is associated with a known complication of magnesium depletion, such as arrhythmia, might clarify the relationship between PPI use and magnesium.
One of the most common adverse consequences of hypomagnesemia is cardiac arrhythmias. Low magnesium affects the modulation of the voltage-dependent L-type Ca2+ channels and decreases the membrane stabilizing action of Mg2+[16]. A small study has found that PPI use is associated with an increased risk of arrhythmias (including ventricular fibrillation, ventricular tachycardia, non-sustained ventricular tachycardia, atrial fibrillation, and atrial tachycardia)[17], but has not been studied more comprehensively.
Using a large cohort of critically ill patients, we determined whether premorbid use of PPI was associated with the risk of arrhythmia. In order to account for confounding by indication, we also evaluated for a potential association between histamine 2 antagonist (H2RA) and arrhythmias. In addition, since concomitant diuretic use is considered a risk factor for PPI associated hypomagnesemia, we evaluated whether diuretic exposure modified the association of PPI and arrhythmias.
Method
Study population
We used the Multiparameter Intelligent Monitoring in Intensive Care (MIMIC-II) research database, a joint venture of the Laboratory for Computational Physiology at Massachusetts Institute of Technology (MIT) and the Department of Medicine at the Beth Israel Deaconess Medical Center (BIDMC) [18], a large, urban, academic medical center. The database contains data of high temporal resolution obtained from clinical computing systems, including lab results, electronic documentation, and bedside monitor trends and waveforms, for all patients admitted to BIDMC ICUs between 2001 and 2008. Use of the MIMIC II database has been approved by the Institutional Review Boards of Beth Israel Deaconess Medical Center and the MIT.
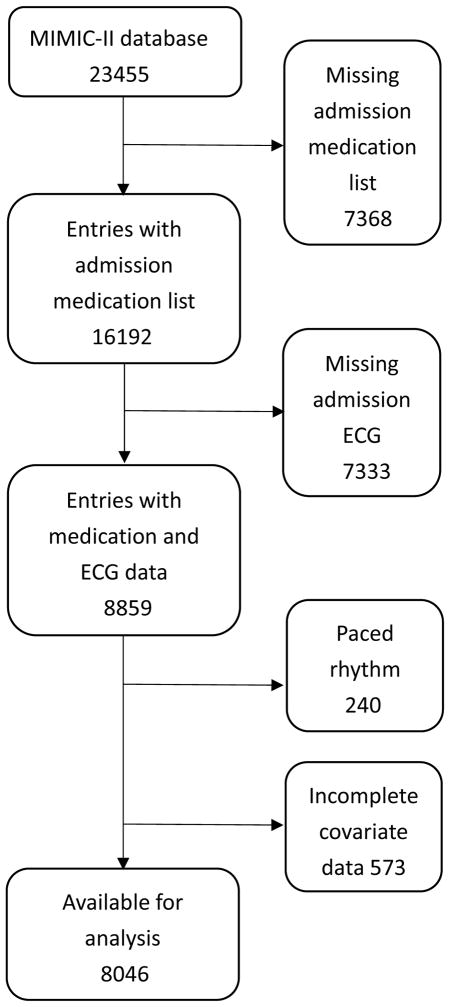
Of the 23,455 unique ICU admissions retrieved from MIMIC-II data base, 16192 have an identifiable medication section of the discharge summary, indicating their premorbid medication exposure. Of these, 7333 did not have documented ECG rhythm, 240 had a paced rhythm, and 573 lacked clinical data and were further excluded [Fig. 1]. 8046 patients remained for analysis.
Figure 1.
Selection of study population.
Primary exposure
PPI or H2RA exposure was defined as any PPI or H2RA listed as a pre-admission medication. We evaluated medications on admission using Natural Language Processing (NLP) of discharge summaries. We used an NLP algorithm that searched for a discrete home medication section in the discharge summary and then processed the medications to find individual entries of PPI, H2RA, and diuretics, as described and previously validated [13].
Outcome
Electrocardiograms centered within twelve hours of a patient’s admission to the ICU were used to document the presence of an arrhythmia. NLP was developed to read the automatic ECG rhythm interpretation, and upon refinement, was manually tested by review of 200 randomly selected ECG’s. From this sample, 98% of ECGs were interpreted accurately by the NLP code.
Any arrhythmia was considered the primary endpoint, but we also stratified by atrial and ventricular origins. Arrhythmia included one of the following rhythms: atrial bradycardia, atrial fibrillation, atrial flutter, premature atrial contraction, atrial rhythm, atrial tachycardia, atrial-ventricular dissociation, junctional rhythm, supraventricular bradycardia, ventricular rhythm, or ventricular tachycardia. Atrial arrhythmia included atrial bradycardia, atrial fibrillation, atrial flutter, premature atrial contraction, atrial rhythm, and atrial tachycardia. Ventricular arrhythmia included rhythms of ventricular rhythm and ventricular tachycardia.
Statistical analysis
Patients were separated into those with PPI exposure, with H2RA exposure, and patients with neither PPI nor H2RA exposure [Table 1]. (There were 51 patients on both PPI and H2RA were included in the group of PPI exposure). To assess whether PPI exposure was related to arrhythmia, we developed sequential multivariable linear regression models. PPI and H2RA exposure were included as binary variables. Binary indicator variables were also created for all Elixhauser comorbidities (except for arrhythmia), ICU types, and ethnicity. Age and SAPS score were included as continuous variables. Multivariable regression was done separately for arrhythmia, atrial arrhythmia, and ventricular arrhythmia and adjusted for age, sex, race, ICU type, comorbidities, and SAPS scores. To determine whether the association of PPI exposure and outcome was modified by premorbid diuretic exposure, we created an interaction term between premorbid diuretic exposure and PPI exposure, and present the stratified results.
Table 1.
Characteristics of study population.
| Baseline characteristics stratified by acid suppression medication exposure | ||||
|---|---|---|---|---|
| Group | Proton pump inhibitors (N=1973)a | Histamine 2 receptor antagonists (N=483) | NONE (N=5590) | p value* |
| Characteristics | ||||
| Age (mean ± Std) | 68.14 ± 14.69 | 68.84 ± 14.23 | 64.49 ± 16.92 | <0.001** |
| Male | 55.4 % | 57.9 % | 59.0 % | 0.016** |
| SAPS1 | 14.23 ± 5.30 | 13.96 ± 5.31 | 13.75 ± 5.47 | 0.0036** |
| Race | ||||
| White | 71.81 % | 68.60 % | 68.51 % | 0.017** |
| Black | 7.97 % | 8.33 % | 7.10 % | 0.30 |
| Hispanic | 2.31 % | 2.71 % | 2.51 % | 0.82 |
| Asian | 1.65 % | 2.33 % | 2.11 % | 0.38 |
| Other | 1.84 % | 1.55 % | 2.93 % | 0.008** |
| Comorbidities | ||||
| Congestive Heart Failure | 24.98% | 22.09% | 16.87% | <0.001** |
| Renal disease | 7.55% | 6.78% | 4.07% | <0.001** |
| Hypertension | 34.47% | 34.11% | 33.35% | 0.63 |
| Diabetes Mellitus | 29.51% | 30.81% | 24.10% | <0.001** |
| ICU type | ||||
| MICU2 | 42.90 % | 35.47 % | 31.96% | <0.001** |
| CCU3 | 18.25 % | 24.42 % | 22.47 % | <0.001** |
| CARDIOTHORACIC ICU | 22.87 % | 25.00 % | 28.35 % | <0.001** |
| SICU4 | 15.98 % | 15.12 % | 17.22 % | 0.25 |
Abbreviations:
SAPS, Simplified Acute Physiologic Score;
MICU, Medical Intensive Care Unit;
CCU, Coronary Care Unit;
SICU, Surgical Intensive Care Unit
P-values reflect across-group differences.
P-values that are smaller than 0.05.
Patients with both proton pump inhibitor use and histamine 2 receptor antagonist use (N=55) are included in the proton pump inhibitor group
Results
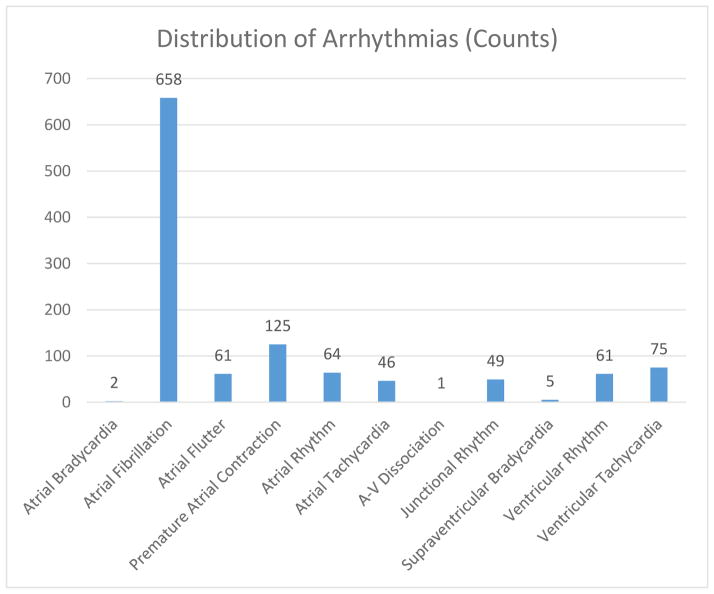
As seen in table 1, PPIs users tended to be older, with more comorbidities, and a higher level of illness acuity than non-PPI users. 14.3 (n=1147) percent of admission ECG had a non-sinus source of cardiac origin. [Figure 2]. Of these, 83.3 (n=956) percent were atrial in origin, and 11.9 (n=136) percent were ventricular. The remaining 4.8 (n=55) percent included atrial-ventricular dissociation, junctional rhythm, and supraventricular bradycardia [Figure 2].
Figure 2.
Types of cardiac arrhythmias
Although PPI use was associated with an increased adjusted risk of arrhythmia (OR=1.18, 95% CI=1.02–1.36, p=0.02), adjustment for comorbidities and illness severity reduced this to non-significance (OR=0.96, 95% CI=0.83–1.12, p=0.62). Similarly, PPI use was not associated with an increased risk of either atrial or ventricular arrhythmias. H2RA exposure was not associated with cardiac arrhythmias.
Amongst the 2468 patients concurrently taking a PPI and a diuretic, PPI exposure was not a significant predictor of cardiac arrhythmia on admission to the ICU [table III]. A multiplicative interaction term between PPI and diuretics exposure in multi-variable regression was not significant (p=0.93).
Table 3.
Risk of cardiac arrhythmia on admission ECG. Sub-group analysis stratified by diuretics exposure.
| Risk of cardiac arrhythmia on admission ECG | ||||
|---|---|---|---|---|
| Odds ratio | 95% CI | P value | ||
| Diuretics (N=2468) | Arrhythmia | |||
| PPI | 0.94 | 0.74 – 1.18 | 0.57 | |
| H2 blocker | 0.83 | 0.55–1.25 | 0.39 | |
| Atrial Arrhythmia | ||||
| PPI | 0.85 | 0.65–1.10 | 0.21 | |
| H2 blocker | 0.81 | 0.49–1.27 | 0.37 | |
| Ventricular Arrhythmia | ||||
| PPI | 1.09 | 0.57–2.10 | 0.79 | |
| H2 blocker | 0.31 | 0.04–2.29 | 0.25 | |
| No Diuretics (N=5578) | Arrhythmia | |||
| PPI | 0.94 | 0.77 – 1.16 | 0.58 | |
| H2 blocker | 0.78 | 0.54–1.13 | 0.18 | |
| Atrial Arrhythmia | ||||
| PPI | 0.89 | 0.70–1.13 | 0.35 | |
| H2 blocker | 0.73 | 0.47–1.12 | 0.15 | |
| Ventricular Arrhythmia | ||||
| PPI | 0.91 | 0.53–1.55 | 0.72 | |
| H2 blocker | 0.91 | 0.38–2.20 | 0.84 | |
Discussion
Since PPI exposure is potentially thought to decrease magnesium intestinal intake [19], thereby leading to magnesium deficiency, and since magnesium deficiency is associated with the risk of arrhythmias, we hypothesized that PPI use would increase the risk of arrhythmias. However, in our study of a large single center critically ill cohort, PPI use prior to hospital admission was not associated with the risk of arrhythmias. Our study provides differing results from a smaller previously published study [17].
Magnesium has well-described antiarrhythmic effects and is widely used for the prevention and treatment of cardiac arrhythmias. The electrophysiological effects of Mg2+ include decreasing the automaticity of cardiomyocytes [20], increasing atrial and AV nodal conduction time [21], increasing atrial and AV nodal refractory period [21, 22], blocking conduction via accessory pathways [23, 24], decreasing early/delayed after-depolarizations [25, 26], and prolonging His-ventricular conduction [27]. Hypomagnesemia is therefore an important arrhythmogenic factor.
Interestingly, in our study, we found a non-significant trend towards protection from arrhythmias with PPI use, as suggested previously by some studies [28, 29]. PPI have anti-oxidative [30] and anti-inflammatory [31] effects, and potentially could decrease the damage and remodeling of cardiomyocytes from various causes, therefore, decrease the risk of developing arrhythmia. This remains speculative, however.
Limitation
The limitations of this study include its retrospective and observational design. However, it’s unlikely that arrhythmias would influence the decision to prescribe a PPI medication, and we accessed premorbid PPI use to separate exposure and outcome. In addition, since PPI can be obtained without a prescription, bias due to unrecognized exposure is likely, and the length of PPI use was not available.
Conclusion
In summary, PPI exposure is not associated with an increased risk of arrhythmias in critically ill patients.
Table 2.
Risk of cardiac arrhythmia on admission ECG
| Risk of cardiac arrhythmia on admission ECG | |||
|---|---|---|---|
| Odds ratio | 95% CI | P value | |
| Arrhythmia | |||
| PPI | 0.96 | 0.83 – 1.12 | 0.62 |
| H2 blocker | 0.81 | 0.62–1.06 | 0.12 |
| Atrial Arrhythmia | |||
| PPI | 0.89 | 0.75–1.06 | 0.19 |
| H2 blocker | 0.76 | 0.55–1.04 | 0.087 |
| Ventricular Arrhythmia | |||
| PPI | 0.96 | 0.64–1.44 | 0.86 |
| H2 blocker | 0.71 | 0.32–1.55 | 0.39 |
Acknowledgments
Funding: R01 EB017205
Footnotes
Conflict of interest:
The contributing authors declare that there was no conflict of interest.
References
- 1.Cundy T, Dissanayake A. Severe hypomagnesaemia in long-term users of proton-pump inhibitors. Clin Endocrinol (Oxf) 2008;69(2):338–41. doi: 10.1111/j.1365-2265.2008.03194.x. [DOI] [PubMed] [Google Scholar]
- 2.Shabajee N, et al. Omeprazole and refractory hypomagnesaemia. BMJ. 2008;337:a425. doi: 10.1136/bmj.39505.738981.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Broeren MA, et al. Hypomagnesemia induced by several proton-pump inhibitors. Ann Intern Med. 2009;151(10):755–6. doi: 10.7326/0003-4819-151-10-200911170-00016. [DOI] [PubMed] [Google Scholar]
- 4.Kuipers MT, Thang HD, Arntzenius AB. Hypomagnesaemia due to use of proton pump inhibitors--a review. Neth J Med. 2009;67(5):169–72. [PubMed] [Google Scholar]
- 5.Hoorn EJ, et al. A case series of proton pump inhibitor-induced hypomagnesemia. Am J Kidney Dis. 2010;56(1):112–6. doi: 10.1053/j.ajkd.2009.11.019. [DOI] [PubMed] [Google Scholar]
- 6.Mackay JD, Bladon PT. Hypomagnesaemia due to proton-pump inhibitor therapy: a clinical case series. Qjm. 2010;103(6):387–95. doi: 10.1093/qjmed/hcq021. [DOI] [PubMed] [Google Scholar]
- 7.Regolisti G, et al. Severe hypomagnesemia during long-term treatment with a proton pump inhibitor. Am J Kidney Dis. 2010;56(1):168–74. doi: 10.1053/j.ajkd.2010.03.013. [DOI] [PubMed] [Google Scholar]
- 8.Negri AL, Valle EE. Hypomagnesaemia/hypokalemia associated with the use of esomeprazole. Curr Drug Saf. 2011;6(3):204–6. doi: 10.2174/157488611797579320. [DOI] [PubMed] [Google Scholar]
- 9.Quasdorff M, et al. Recurrent hypomagnesemia with proton-pump inhibitor rechallenge. Ann Intern Med. 2011;155(6):405–7. doi: 10.7326/0003-4819-155-6-201109200-00022. [DOI] [PubMed] [Google Scholar]
- 10.Faulhaber GA, et al. Serum magnesium and proton-pump inhibitors use: a cross-sectional study. Rev Assoc Med Bras. 2013;59(3):276–9. doi: 10.1016/j.ramb.2012.12.007. [DOI] [PubMed] [Google Scholar]
- 11.Koulouridis I, et al. Out-of-hospital use of proton pump inhibitors and hypomagnesemia at hospital admission: a nested case-control study. Am J Kidney Dis. 2013;62(4):730–7. doi: 10.1053/j.ajkd.2013.02.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Van Ende C, et al. Proton-pump inhibitors do not influence serum magnesium levels in renal transplant recipients. J Nephrol. 2014 doi: 10.1007/s40620-014-0105-9. [DOI] [PubMed] [Google Scholar]
- 13.Danziger J, et al. Proton-pump inhibitor use is associated with low serum magnesium concentrations. Kidney Int. 2013;83(4):692–9. doi: 10.1038/ki.2012.452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zipursky J, et al. Proton pump inhibitors and hospitalization with hypomagnesemia: a population-based case-control study. PLoS Med. 2014;11(9):e1001736. doi: 10.1371/journal.pmed.1001736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.William JH, et al. Proton-Pump Inhibitor use is associated with lower Urinary Magnesium Excretion. Nephrology (Carlton) 2014 doi: 10.1111/nep.12330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kolte D, et al. Role of magnesium in cardiovascular diseases. Cardiol Rev. 2014;22(4):182–92. doi: 10.1097/CRD.0000000000000003. [DOI] [PubMed] [Google Scholar]
- 17.El-Charabaty E, et al. Effects of proton pump inhibitors and electrolyte disturbances on arrhythmias. Int J Gen Med. 2013;6:515–8. doi: 10.2147/IJGM.S46932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Saeed M, et al. Multiparameter Intelligent Monitoring in Intensive Care II: a public-access intensive care unit database. Crit Care Med. 2011;39(5):952–60. doi: 10.1097/CCM.0b013e31820a92c6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Swaminathan K, Wilson J. Elusive cause of hypomagnesaemia. BMJ. 2011;343:d5087. doi: 10.1136/bmj.d5087. [DOI] [PubMed] [Google Scholar]
- 20.Iseri LT, et al. Ionic biology and ionic medicine in cardiac arrhythmias with particular reference to magnesium. Am Heart J. 1992;123(5):1404–9. doi: 10.1016/0002-8703(92)91059-a. [DOI] [PubMed] [Google Scholar]
- 21.Rasmussen HS, Thomsen PE. The electrophysiological effects of intravenous magnesium on human sinus node, atrioventricular node, atrium, and ventricle. Clin Cardiol. 1989;12(2):85–90. doi: 10.1002/clc.4960120204. [DOI] [PubMed] [Google Scholar]
- 22.DiCarlo LA, Jr, et al. Effects of magnesium sulfate on cardiac conduction and refractoriness in humans. J Am Coll Cardiol. 1986;7(6):1356–62. doi: 10.1016/s0735-1097(86)80157-7. [DOI] [PubMed] [Google Scholar]
- 23.Viskin S, et al. Clinical and electrophysiologic effects of magnesium sulfate on paroxysmal supraventricular tachycardia and comparison with adenosine triphosphate. Am J Cardiol. 1992;70(9):879–85. doi: 10.1016/0002-9149(92)90731-d. [DOI] [PubMed] [Google Scholar]
- 24.Christiansen EH, et al. Dose-related cardiac electrophysiological effects of intravenous magnesium. A double-blind placebo-controlled dose-response study in patients with paroxysmal supraventricular tachycardia. Europace. 2000;2(4):320–6. doi: 10.1053/eupc.2000.0123. [DOI] [PubMed] [Google Scholar]
- 25.Bailie DS, et al. Magnesium suppression of early afterdepolarizations and ventricular tachyarrhythmias induced by cesium in dogs. Circulation. 1988;77(6):1395–402. doi: 10.1161/01.cir.77.6.1395. [DOI] [PubMed] [Google Scholar]
- 26.Kaseda S, Gilmour RF, Jr, Zipes DP. Depressant effect of magnesium on early afterdepolarizations and triggered activity induced by cesium, quinidine, and 4-aminopyridine in canine cardiac Purkinje fibers. Am Heart J. 1989;118(3):458–66. doi: 10.1016/0002-8703(89)90258-5. [DOI] [PubMed] [Google Scholar]
- 27.Satoh Y, et al. Effect of magnesium sulfate on the haloperidol-induced QT prolongation assessed in the canine in vivo model under the monitoring of monophasic action potential. Jpn Circ J. 2000;64(6):445–51. doi: 10.1253/jcj.64.445. [DOI] [PubMed] [Google Scholar]
- 28.Zellerhoff S, Lenze F, Eckardt L. Prophylactic proton pump inhibition after atrial fibrillation ablation: is there any evidence? Europace. 2011;13(9):1219–21. doi: 10.1093/europace/eur139. [DOI] [PubMed] [Google Scholar]
- 29.Lin K, et al. Proton pump inhibitors as also inhibitors of atrial fibrillation. Eur J Pharmacol. 2013;718(1–3):435–40. doi: 10.1016/j.ejphar.2013.07.043. [DOI] [PubMed] [Google Scholar]
- 30.Schulz-Geske S, et al. Molecular mechanism and functional consequences of lansoprazole-mediated heme oxygenase-1 induction. World J Gastroenterol. 2009;15(35):4392–401. doi: 10.3748/wjg.15.4392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.De Jonge PJ, et al. Proton pump inhibitor therapy in gastro-oesophageal reflux disease decreases the oesophageal immune response but does not reduce the formation of DNA adducts. Aliment Pharmacol Ther. 2008;28(1):127–36. doi: 10.1111/j.1365-2036.2008.03699.x. [DOI] [PubMed] [Google Scholar]