Abstract
PURPOSE
To characterize the extent and location of macular thinning in patients with unilateral optic nerve hypoplasia (ONH) as compared to the contralateral normal eye.
METHODS
The medical records of patients with unilateral ONH who underwent spectral domain optical coherence tomography (SD-OCT) of the macula were retrospectively reviewed. SD-OCT scans were manually segmented by 3 observers in 3 macular regions (superior, central, inferior). Boundaries identified included the inner limiting membrane, the junction between the inner nuclear layer and outer plexiform layer, and the neural retina–retinal pigment epithelium interface. Using custom MATLAB software, inner and outer retinal thickness profiles were quantified. A paired t test was used to compare the retinal thickness between the ONH eye and the contralateral normal eyes.
RESULTS
Inner retinal thickness of the ONH eye was decreased in all areas of the macula (superior, central, and inferior) compared to the contralateral normal eye (P < 0.05). Outer retinal thicknesses were also decreased in the central and inferior sections compared with the normal eye (P < 0.05).
CONCLUSIONS
Optic nerve hypoplasia is a congenital disease known to result in thinning of the nerve fiber and ganglion cell layer. Our small cohort demonstrated thinning of the inner retinal layers as well as the outer retinal layers in the ONH eye compared with the contralateral normal eye.
Optic nerve hypoplasia (ONH) is a developmental anomaly represented by a decrease in the number of axons within the optic nerve.1–4 Correspondingly, there is a reduced number of retinal ganglion cells and thinning or absence of the retinal nerve fiber layer (RNFL).5,6 Visual acuity in patients with ONH can range from light perception to normal visual acuity.6,7 Various theories have been proposed to explain the pathogenic mechanism of ONH, including a developmental failure of the retinal ganglion cell,8 defective cell migration,9 axon guidance,10 exaggerated apoptosis,11 or destructive events.12 Environmental toxins and genetic insults have also been implicated.13
Several of these theories are supported by histologic reports showing thinning of the ganglion cells and RNFL with preservation of normal inner nuclear, outer plexiform, outer nuclear, and photoreceptor layers.11,14 However, many of these observations have been documented from histologic sections of stillbirths or patients with other developmental and central nervous system anomalies and may not be representative of isolated optic nerve hypoplasia. Furthermore, older children may demonstrate changes in the retina that are secondary, degenerative, or acquired with time, as opposed to a nonprogressive insult in utero.
With spectral domain optical coherence tomography (SD-OCT), structural changes in the optic nerve and retina can be observed and studied quantitatively in situ. The use of OCT to detect retinal nerve fiber layer abnormalities has been useful in ONH, including segmental forms of optic nerve hypoplasia.15,16 OCT has been used to study macular thickness in patients with optic nerve pathway gliomas,17 foveal hypoplasia,18 achromatopsia,19 and glaucoma20 to offer insight into functional outcomes in patients with structural optic nerve and retinal anomalies. The present study aimed to identify, through SD-OCT, quantitative differences in macular thickness in all retinal layers between ONH eyes and normal contralateral eyes in patients with unilateral ONH.
Subjects and Methods
Institutional review board exemption was obtained prior to initiation of research. All procedures conducted were in compliance with regulations outlined in the US Health Insurance Portability and Accountability Act of 1996. The medical records of patients with a history of unilateral optic nerve hypoplasia, determined by clinical evaluation by the managing pediatric ophthalmologist (LMK), seen between January 2010 and June 2011 at the University of Illinois at Chicago, were retrospectively reviewed. All patients had undergone SD-OCT (Spectralis, Heidelberg Engineering, Heidelberg, Germany) of the macula in both eyes as part of routine care in order to quantify possible visual potential. Inclusion criteria included a contralateral eye that appeared structurally normal without asymmetric ONH and visual acuity of at least 20/20. Patients with concomitant optic nerve or retinal disease and patients with an unreadable SD-OCT were excluded. All patients were further evaluated with brain and orbital MRI imaging and by pediatric neurology and pediatric endocrinology specialists: all patients were determined to have only isolated unilateral ONH.
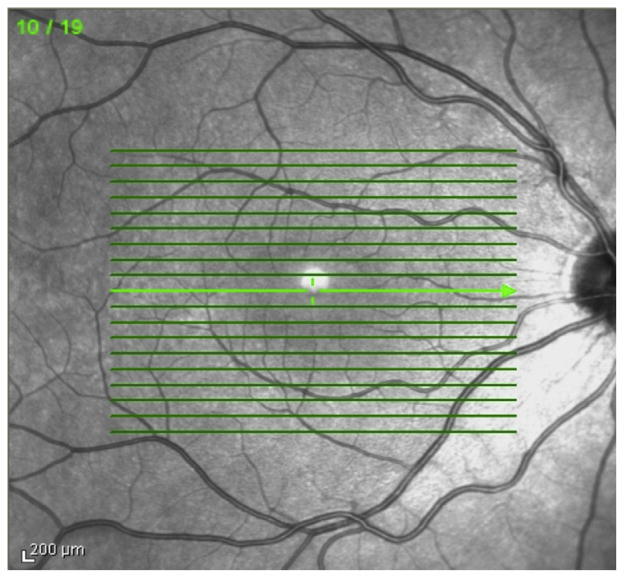
Manual segmentation of OCT images was conducted by three independent observers (JA, MB, NB) using ImageJ software (http://imagej.nih.gov/ij/). From the 19 SD-OCT raster images for each eye, B-scan images obtained at three macular regions (superior, central, inferior) were selected for analysis. Superior and inferior macular regions comprised of 2 B-scans located at 1250 μm and 1500 μm superior or inferior, respectively, to the foveal center (Figure 2). The central macular region was represented by 3 B-scans, through the foveal center, in addition to scans 250 μm superior and inferior to the foveal center. Segmentation lines were drawn in three retinal layer interfaces: at the internal limiting membrane, at the junction between the inner nuclear layer (INL) and the outer plexiform layer (OPL), and at the neural retina–retinal pigment epithelium (RPE) interface (Figure 1A,B). Inner and outer retinal thicknesses were quantified using an automated custom Matlab software program (Mathworks Inc, Natick, MA). Inner retinal thickness (IRT) was defined as the difference between segmentation lines drawn at the internal limiting membrane and the junction of the INL and OPL. The IRT included the retinal nerve fiber layer and ganglion cell layer as well as the inner plexiform and inner nuclear layers. Outer retinal thickness (ORT) was defined as the difference between segmentation lines drawn at the junction of the INL and OPL and the RPE interface. The INL-OPL interface was chosen due to variability of the apparent thickness of the outer nuclear layer (ONL) due to the shifting boundary of the OPL-ONL interface, which we noted in a previous study.21 This variability has subsequently been shown to represent variable detection of Henle layer based on off-axis orientation of scan acquisition.22 Thus ORT in this study represented a summation of the thicknesses of the outer plexiform and outer nuclear layers, the photoreceptor layer, and the RPE. Thickness profiles were generated by averaging thicknesses over 100 μm intervals along the B-scans. Thicknesses were then further averaged to quantify the IRT and ORT for each of the macular regions for each observer (JA, MB, NB). Comparisons were then made between the eye with ONH and its contralateral normal eye rather than to a control group so as to avoid known inter-racial and age-related OCT differences. 23
FIG 2.
Three macular regions selected for analysis: superior macula, central macula including presumed central fovea, and inferior macula. “Central” sections represent segmentation through the presumed foveal center as well as one section 250 μm inferior and superior to the foveal center. “Superior” and “inferior” sections delineate 2 segments of retina 1250 μm and 1500 μm superior or inferior to the foveal center, respectively.
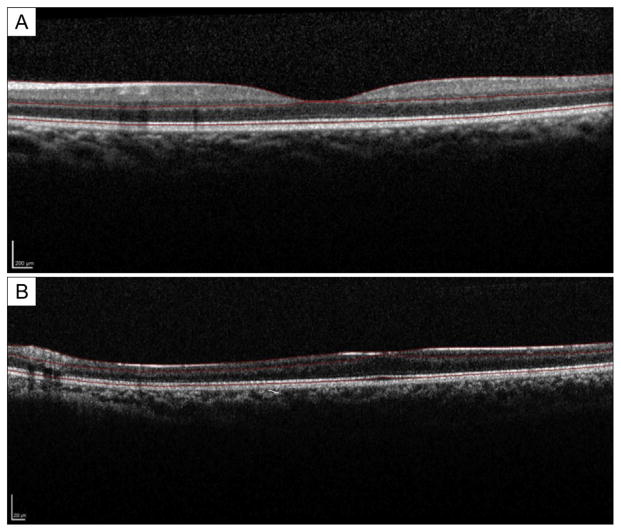
FIG 1.
Horizontal macular optical coherence tomography (OCT) of normal eye (A) and pathologic eye (B) with optic nerve hypoplasia. Manual segmentation delineates internal limiting membrane, posterior border of the outer plexiform layer, and the neural retina–retinal pigment epithelial junction.
Statistical Analysis
The agreement of the retinal thickness measurement of each region among the three observers was first assessed by using the intraclass correlation coefficient and its 95% confidence intervals. Analysis of variance (ANOVA) was also performed to compare the differences between the three observers in retinal thickness measures; the correlation of measures from the same eye by three observers were accounted for by using generalized estimating equations.
Because of high intraobserver agreement in measures of retinal thickness, the average of retinal thickness from the three observers was calculated and used for statistical comparisons. The retinal thickness (IRT, ORT, and IRT+ORT) was compared between eyes with ONH and the contralateral normal eyes using paired t test for each region and across all three regions. Their mean difference along with its 95% confidence interval was calculated. All the statistical analyses were performed using SAS v9.2 (SAS Institute Inc, Cary, NC), and two-sided P value of < 0.05 was considered to be statistically significant.
Results
A total of 5 patients (3 boys) met inclusion criteria. Demographic data are provided in Table 1. The visual acuity in the ONH eyes ranged from 20/150 to counting fingers, and all had unilateral severely hypoplastic optic nerves by clinical examination. Representative SD-OCT B-scan images at the foveal center with segmentation lines are shown in Figure 1. The contralateral eye (Figure 1A) shows normal retinal thickness of all layers, both IRT and ORT. In contrast, the ONH eye (Figure 1B) shows decreased thickness and hypoplasia in all retinal layers, including the outer retinal layers. Macular regions (superior, central, inferior) delineated in this analysis are shown in Figure 2.
Table 1.
Demographics of patients with unilateral optic nerve hypoplasia
| Patient | Age, years | Sex | Race | Visual acuity in hypoplastic eye |
|---|---|---|---|---|
| 1 | 11 | M | African American | HM |
| 2 | 11 | F | Hispanic | CF |
| 3 | 12 | M | Hispanic | 20/150 |
| 4 | 11 | F | Asian | CF |
| 5 | 8 | M | Hispanic | CF |
CF, counting fingers; HM, hand motions.
In all three macular regions (superior, central and inferior), IRT was thinner in ONH eyes compared to control eyes (P < 0.05; Table 2). The mean difference in IRT, averaged over three graders and three macular regions, between the ONH and control eyes was 61.7 μm (95% CI, 47.4–71.9). In the inferior and central macular regions, ORT was thinner in ONH eyes compared to control eyes (P< 0.05; Table 2). ORT, averaged over three graders and three macular regions, was also significantly thinner in the ONH eyes compared to control eyes (P < 0.05), with a mean difference of 8.14 μm (95% CI, 3.49–12.8).
Table 2.
Comparison of inner and outer retinal thickness between eyes with ONH and their fellow eyes in subjects with unilateral ONH
| Measures | Thickness, μm, mean ± SE
|
Mean thickness differencea (95% CI) | P valuea | |
|---|---|---|---|---|
| Control eyes (n = 5) | ONH eyes (n = 5) | |||
| IRT | 146.5 ± 6.8 | 84.8 ± 2.6 | 61.7 (38.0 to 85.4) | 0.002 |
| Superior | 159.7 ± 13.3 | 87.8 ± 5.1 | 71.9 (43.5 to 100.2) | 0.002 |
| Central | 132.2 ± 5.6 | 84.7 ± 6.0 | 47.4 (29.4 to 65.4) | 0.002 |
| Inferior | 147.6 ± 13.7 | 81.9 ± 2.3 | 65.7 (29.2 to 102.3) | 0.008 |
| ORT | 139.8 ± 2.0 | 131.6 ± 1.9 | 8.14 (3.49 to 12.8) | 0.008 |
| Superior | 132.5 ± 2.1 | 129.6 ± 4.1 | 2.88 (−3.38 to 9.15) | 0.27 |
| Central | 145.7 ± 3.2 | 135.5 ± 2.4 | 10.2 (1.47 to 19.0) | 0.032 |
| Inferior | 141.1 ± 2.7 | 129.8 ± 3.0 | 11.3 (1.27 to 21.3) | 0.035 |
| IRT+ORT | 286.3 ± 7.0 | 216.4 ± 3.7 | 69.8 (46.2 to 93.4) | 0.001 |
| Superior | 292.1 ± 14.4 | 217.4 ± 8.6 | 74.8 (50.1 to 99.5) | 0.001 |
| Central | 277.9 ± 8.4 | 220.2 ± 5.9 | 57.7 (34.7 to 80.7) | 0.002 |
| Inferior | 288.8 ± 14.7 | 211.7 ± 4.7 | 77.0 (39.5 to 114.6) | 0.005 |
CI, confidence interval; IRT, inner retinal thickness; ONH, optic nerve hypoplasia; ORT, outer retinal thickness; SE, standard error.
Difference and P value were calculated from paired t test.
Retinal thickness measures from all three graders agreed well, with an intraclass correlation coefficient of 0.99 (95% CI, 0.97–0.99) for IRT and 0.82 (95% CI, 0.58–0.87) for ORT (Table 3). Mean difference between any pairs of graders was within 6.8 μm for IRT and within 13.4 μm for ORT (data not shown).
Table 3.
Agreement between three graders in the measurement of macular thicknesses of the inner and outer retina (n = 10 eyes of 5 patients)
| Intraclass correlation (95% CI) | P value for difference between graders | |
|---|---|---|
| IRT | 0.99 (0.96–1.00) | 0.13 |
| Superior | 0.99 (0.98–1.00) | 0.09 |
| Central | 0.98 (0.93–0.99) | 0.13 |
| Inferior | 0.97 (0.92–0.99) | 0.23 |
| ORT | 0.82 (0.57–0.95) | 0.09 |
| Superior | 0.73 (0.41–0.92) | 0.09 |
| Central | 0.87 (0.68–0.96) | 0.08 |
| Inferior | 0.58 (0.20–0.86) | 0.11 |
| IRT+ORT | 0.99 (0.97–1.00) | 0.11 |
| Superior | 0.99 (0.97–1.00) | 0.14 |
| Central | 0.98 (0.95–1.00) | 0.12 |
| Inferior | 0.99 (0.97–1.00) | 0.11 |
CI, confidence interval; IRT, inner retinal thickness; ORT, outer retinal thickness.
Discussion
To our knowledge, this is the first case series in the literature to show quantitative thinning of both inner and outer layers of the retina in ONH, as evidenced by SD-OCT, in patients with isolated unilateral ONH. By quantifying macular thickness with manual segmentation, we found macular thinning in all three macular sections of the IRT (superior, central, inferior) and two macular sections of the ORT (central, inferior). Using OCT for quantitative assessment of the optic nerve head, retinal nerve fiber layer, and macular thickness, Srinivasan and colleagues24 reported a decrease in overall macular thickness in a patient with unilateral ONH; however, specific retinal layers were not studied. Hoang and colleagues25 reported thinning of the outer retinal layers in the central and inferior macular regions on SD-OCT in an 8-year-old with unilateral ONH, similar to our results. In contrast, Park and colleagues26 reported the macular OCT analysis of a single case of presumed unilateral ONH and found no statistically significant difference between the outer retinal thickness in the ONH eye and the contralateral normal eye. However, their patient’s normal eye showed segmental peripapillary retinal thinning by OCT and may have had subtle or segmental ONH. Sampling bias must also be considered in reports of a single case.
Outer retinal thinning has been shown in other retinal diseases, such as achromatopsia19; however, this would be an unexpected finding in ONH if ONH was strictly limited to a loss of only ganglion cells and their axons.
That both the inner and outer retinal layers are involved in ONH requires an expanded definition of optic nerve hypoplasia, one that includes a deficit of all layers of the retina as well as the optic nerve. In the present study, eyes with ONH had an overall thinner ORT than their contralateral eyes, possibly representing an embryologic arrest in this layer or a targeted secondary degeneration. The reason for this thinning of the ORT is uncertain, although there are emerging theories involving complex signaling pathways 27 and retinal waves28 that seek to explain layer-specific development of the retina. Alternatively, there could be secondary degeneration of the outer retina due to lack of neurotrophic factors from the largely absent inner retina. Although the central and inferior ORT was significantly thinner in eyes with ONH than in normal eyes, the superior section of the ORT was not found to differ significantly. Given that the thickness of the superior retina in eyes with ONH was thinner that of the control eyes, but not significantly so, it seems possible that a larger sample size may have detected a significant difference; however, we cannot exclude a difference in development or degeneration between the superior and inferior outer retina.
Previous OCT studies have evaluated ongoing degeneration 15 in diseases such as optic pathway gliomas. Our report provides information about anatomic and potentially developmental changes in the retina in a disease known to exist at birth. Previous work has demonstrated an association between ONH and peripheral retinal avascularity, at least in a subset of patients.29 Our findings demonstrate that ONH can be associated with subclinical outer retinal abnormalities. It is unclear whether these arise as part of the primary condition or as secondary contiguous effects of transsynaptic degeneration.
Limitations of the current study include the small sample size and the retrospective nature of the study design. In addition, quantitative assessment of the nerve fiber layer was not performed, and this may account for the majority of the thinning of the IRT thickness. The RNFL in the ONH eyes in this study was extremely thin or even absent; reliable segmentation of this layer was not possible and was therefore omitted from the study design. Also, it was not possible to identify the exact center of the anatomic fovea on the scans. We would have preferred to exclude the scans through the center of the fovea, where there is no IRT in both normal and ONH eyes. The inclusion of some near zero values for the IRT in the control eyes blunted the asymmetry of our IRT comparisons (Table 2). Regardless, a significant difference was found in the IRT despite not excluding the fovea region. We hope to conduct a prospective study involving SD-OCT at different time points in children with unilateral ONH in order to provide more information on the anatomic changes over time.
Acknowledgments
Supported by NIH EY001792, Research to Prevent Blindness.
Footnotes
Literature Search
PubMed was last searched, without language or date restriction, in September 2014 using the following search terms alone and in combination: optic nerve hypoplasia, optical coherence tomography, macular OCT, and retinal nerve fiber layer analysis.
References
- 1.Whinery RD, Blodi FC. Hypoplasia of the optic nerve—a clinical and histopathologic correlation. Trans Am Acad Ophthalmol Otolaryngol. 1963;67:733–8. [PubMed] [Google Scholar]
- 2.Hellstrom A, Wiklund LM, Svensson E. The clinical and morphologic spectrum of optic nerve hypoplasia. J AAPOS. 1999;3:212–20. doi: 10.1016/s1091-8531(99)70005-4. [DOI] [PubMed] [Google Scholar]
- 3.Garcia ML, Ty EB, Taban M, David Rothner A, Rogers D, Traboulsi EI. Systemic and ocular findings in 100 patients with optic nerve hypoplasia. J Child Neurol. 2006;21:949–56. doi: 10.1177/08830738060210111701. [DOI] [PubMed] [Google Scholar]
- 4.Capo H, Repka MX, Edmond JC, Drack AV, Blumenfeld L, Siatkowski RM. Optic nerve abnormalities in children: a practical approach. J AAPOS. 2011;15:281–90. doi: 10.1016/j.jaapos.2011.02.010. [DOI] [PubMed] [Google Scholar]
- 5.Skarf B, Hoyt CS. Optic nerve hypoplasia in children: association with anomalies of the endocrine and CNS. Arch Ophthalmol. 1984;102:62–7. doi: 10.1001/archopht.1984.01040030046032. [DOI] [PubMed] [Google Scholar]
- 6.Frisén L, Holmegaard L. Spectrum of optic nerve hypoplasia. Br J Ophthalmol. 1978;62:7–15. doi: 10.1136/bjo.62.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gardner HB, Irvine AR. Optic nerve hypoplasia with good visual acuity. Arch Ophthalmol. 1972;88:255–8. doi: 10.1001/archopht.1972.01000030257005. [DOI] [PubMed] [Google Scholar]
- 8.Macgregor S, Hewitt AW, Hysi PG, et al. Genome-wide association identifies ATOH7 as a major gene determining human optic disc size. Hum Mol Genet. 2010;19:2716–24. doi: 10.1093/hmg/ddq144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shenoy AM, Markowitz JA, Bonnemann CG, Krishnamoorthy K, Bossler AD, Tseng BS. Muscle-eye-brain disease. J Clin Neuromuscul Dis. 2010;11:124–6. doi: 10.1097/CND.0b013e3181c5054d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ogata-Iwao M, Inatani M, Iwao K, et al. Heparan sulfate regulates intraretinal axon pathfinding by retinal ganglion cells. Invest Ophthalmol Vis Sci. 2011;52:6671–9. doi: 10.1167/iovs.11-7559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saadati HG, Hsu HY, Heller KB, Sadun AA. A histopathologic and morphometric differentiation of nerves in optic nerve hypoplasia and Leber hereditary optic neuropathy. Arch Ophthalmol. 1998;116:911–16. doi: 10.1001/archopht.116.7.911. [DOI] [PubMed] [Google Scholar]
- 12.Taylor D. Congenital tumours of the anterior visual system with dysplasia of the optic discs. Br J Ophthalmol. 1982;66:455–63. doi: 10.1136/bjo.66.7.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kelberman D, Dattani MT. Septo-optic dysplasia—novel insights into the aetiology. Horm Res. 2008;69:257–65. doi: 10.1159/000114856. [DOI] [PubMed] [Google Scholar]
- 14.Mosier MA, Lieberman MF, Green WR, Know DL. Hypoplasia of the optic nerve. Arch Ophthalmol. 1978;96:1437–42. doi: 10.1001/archopht.1978.03910060185017. [DOI] [PubMed] [Google Scholar]
- 15.Hayashi K, Tomidokoro A, Konno S, Mayama C, Aihara M, Araie M. Evaluation of optic nerve head configuration of superior segmental optic hypoplasia by spectral-domain optical coherence tomography. Br J Ophthalmol. 2010;94:768–72. doi: 10.1136/bjo.2009.168690. [DOI] [PubMed] [Google Scholar]
- 16.Epstein AE, Cavuoto KM, Change TC. Utilizing optical coherence tomography in diagnosing a unique presentation of chiasmal hypoplasia variant of septo-optic dysplasia. J Neuroophthalmol. 2014;34:103–4. doi: 10.1097/WNO.0000000000000092. [DOI] [PubMed] [Google Scholar]
- 17.Chang L, El-Dairi MA, Frempong TA, et al. Optical coherence tomography in the evaluation of neurofibromatosis type-1 subjects with optic pathway gliomas. J AAPOS. 2010;14:511–17. doi: 10.1016/j.jaapos.2010.08.014. [DOI] [PubMed] [Google Scholar]
- 18.Thomas MG, Kumar A, Mohammad S, et al. Structural grading of foveal hypoplasia using spectral-domain optical coherence tomography a predictor of visual acuity? Ophthalmology. 2011;118:1653–60. doi: 10.1016/j.ophtha.2011.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thomas MG, Kumar A, Kohl S, Proudlock FA, Gottlob I. High-resolution in vivo imaging in achromatopsia. Ophthalmology. 2011;118:882–7. doi: 10.1016/j.ophtha.2010.08.053. [DOI] [PubMed] [Google Scholar]
- 20.Rao HL, Babu JG, Addepalli UK, Senthil S, Garudadri CS. Retinal nerve fiber layer and macular inner retina measurements by spectral domain optical coherence tomograph in Indian eyes with early glaucoma. Eye. 2012;26:133–9. doi: 10.1038/eye.2011.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bagci AM, Shahidi M, Ansari R, Blair M, Blair NP, Zelkha R. Thickness profiles of retinal layers by optical coherence tomography image segmentation. Am J Ophthalmol. 2008;146:679–87. doi: 10.1016/j.ajo.2008.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lujan BJ, Roorda A, Knighton RW, Carroll J. Revealing Henle’s fiber layer using spectral domain optical coherence tomography. Invest Ophthalmol Vis Sci. 2011;52:1486–92. doi: 10.1167/iovs.10-5946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Girkin CA, McGwin G, Jr, Sinai MJ, et al. Variation in optic nerve and macular structure with age and race with spectral-domain optical coherence tomography. Ophthalmology. 2011;118:2403–8. doi: 10.1016/j.ophtha.2011.06.013. [DOI] [PubMed] [Google Scholar]
- 24.Srinivasan G, Venkatesh P, Garg S. Optic nerve head, retinal nerve fiber layer, and macular thickness characteristics on optical coherence tomography in optic disk hypoplasia [letter] J Pediatr Ophthalmol Strabismus. 2007;44:140–41. doi: 10.3928/0191-3913-20070301-01. [DOI] [PubMed] [Google Scholar]
- 25.Hoang QV, Chau FY, Shahidi M, Miller MT, Blair MP. Macular thinning associated with unilateral optic nerve hypoplasia. Ophthalmic Surg Lasers Imaging. 2011;42:e6–9. doi: 10.3928/15428877-20110125-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moon D, Park TK. Optical coherence tomographic findings in optic nerve hypoplasia. Indian J Ophthalmol. 2013;61:596–8. doi: 10.4103/0301-4738.121088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baye LM, Link BA. Nuclear migration during retinal development. Brain Res. 2008;1192:29–36. doi: 10.1016/j.brainres.2007.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Feller MB. Retinal waves are likely to instruct the formation of eye-specific retinogeniculate projections. Neural Dev. 2009;4:24. doi: 10.1186/1749-8104-4-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shapiro MJ, Chow CC, Blair MP, et al. Peripheral nonperfusion and tractional retinal detachment associated with congenital optic nerve anomalies. Ophthalmology. 2013;120:607–15. doi: 10.1016/j.ophtha.2012.08.027. [DOI] [PubMed] [Google Scholar]