Abstract
Heat Shock Proteins (HSP) are expressed at high levels in cancer and form a fostering environment essential for tumor development. We have reviewed the recent data in this area, concentrating mainly on Hsp27, Hsp70 and Hsp90. The overriding role of the HSPs in cancer is to stabilize the active functions of overexpressed and mutated cancer gene. Elevated HSPs are thus required for many of the traits that underlie the morbidity of cancer, including increased growth, survival and formation of secondary cancers. In addition, HSPs participate in the evolution of cancer treatment resistance. HSPs are also released from cancer cells and influence malignant properties by receptor- mediated signaling. Current data strongly support efforts to target HSPs in cancer treatment.
Introduction to HSPs, Chaperones and Cancer
HSPs are the effector components of a universal, explosive response to cellular stresses, most notably heat shock itself [1, 2]. In fact, HSPs are expressed in such quantities after heat shock that they can be visualized as the dominant bands on one-dimensional SDS PAGE gels after Coomassie blue staining; their abundance astonished some of the early investigators in the field. These proteins are synthesized in response to proteomic damage and can be thought of as components of a protein repair kit (Table 1).
Table1.
Major HSP families in humans
| Protein | Function | Co-chaperones1 | Refs |
|---|---|---|---|
| Small HSP (Hsp27) | Chaperone | None | [12] |
| HSP60 | Chaperonin | Hsp10 | [7] |
| HSP70 | Chaperone | Hsp40, Grpe, Bag1, Bag3, Hip, Hop, CHIP | [4, 10, 14] |
| HSP90 | Chaperone | P23, Aha1, Hop, FKBP51, FKBP52, Cyp40, Cdc37 | [5, 17, 23, 34] |
| HSP110 | Holdase, Co-chaperone | None | [11, 13] |
These are the major HSPs induced by heat shock. Their roles in cancers of various morphologies have been reviewed previously[3]. Co-chaperones Hsp10, Hsp40, Grpe, Bag1, Bag3, Hip, Hop, CHIP, p23, Aha1FKBP51 and FKBP52, Cyp40 and Cdc37 facilitate interactions of the primary chaperone with client proteins.
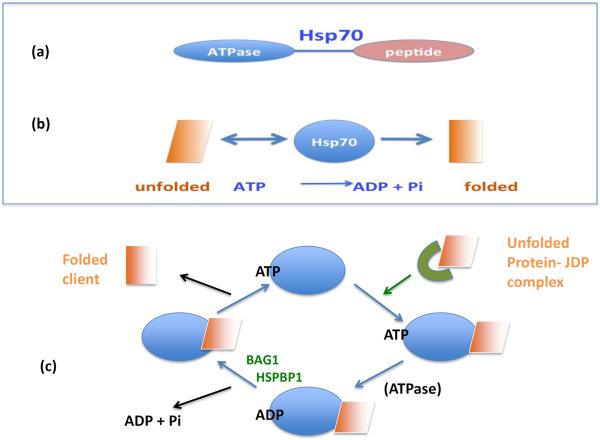
Two major types of HSP are synthesized in stressed cells, which are distinguished by their general mechanisms of protein folding. The first type includes Hsp27, Hsp70 and Hsp90, and they interact directly with the surfaces of unfolded proteins[4–6] (Box1). By contrast, the second type (which includes the chaperonin Hsp60) assembles into complexes resembling folding chambers, which form privileged environments that exclude bulk cytoplasm and favor recovery of active protein conformations[7]. As an example of the chaperone reactions, we consider the properties of Hsp70 in more depth in Fig. 1. Hsp70 contains two major functional domains including a C-terminal peptide binding sequence and an N-terminal ATPase domain that permits the folding of denatured clients (Fig. 1a)[4, 8, 9]. In brief, Hsp70 is able to sense the topology of and bind to unfolded proteins, thus enabling their folding (Fig. 1b, c). Hsp70 is then released from the folded client when ATP bound to the N-terminal domain is hydrolyzed by utilizing its intrinsic ATPase activity. These two domains are regulated by mutual allosteric control: ATP binding triggers polypeptide release, while peptide binding provokes ATPase activity[4]. It should be noted that within the environment of the cell most chaperones require the aid of a number of co-chaperones to amplify the rates of client association, ATPase activity and nucleotide exchange[9]. Hsp60, Hsp70, Hsp90 and Hsp110 each utilize ATP binding and hydrolysis to mediate cycles of polypeptide binding, folding and release [5, 7, 10, 11]. The small HSP, Hsp27 is an exception and appears to function in folding reactions by formation of large oligomers that can store proteins in an aggregation-independent state[12]. Lacking an ATPase domain, Hsp27 requires the activity of Hsp70 or Hsp90 to become released from bound client proteins[12]. Hsp110 although possessing abundant protein binding (holdase) activity appears to function largely as a co-chaperone for Hsp70 and a complex of Hsp110-Hsp70-J-domain co-chaperones possess the capacity to refold aggregated proteins[13]. Within the cell, proteins associated with Hp27 are passed on to Hsp70- co-chaperone complexes. Finally, the Hsp70-bound clients are passed to Hsp90 complexes, which carry out the finishing touches, producing a folded and functional client protein[9, 12]. Inhibition of any of these stages inhibits the folding reaction. Cells that survive one protein stress episode and become enriched in each of the HSPs described above and become “thermotolerant;” that is, markedly resistant to any further such damage [14].Due to the role of HSPs in accompanying cellular proteins and deterring inappropriate interactions, this class of proteins became known as chaperones.
Box 1.Molecular chaperones.
Molecular chaperones are an ancient class of proteins found in all cellular organisms, suggesting a fundamental role. Their level of conservation is quite remarkable. For instance, the human Hsp70.1 and E. coli Hsp70 protein DNAK are at least 50% conserved[8]. Molecular chaperones bind to “client” proteins and deter unfolding or aid in de novo folding[103]. A number of different chaperone families with unrelated sequences exist. Such heterogeneity among chaperones may indicate that they interact with different client proteins or that they perform subtly different tasks in folding.
Many chaperones are members of multigene families, often with similar DNA sequences. For instance, human cells have 12 members of the HSP70 family. The presence of multiple similar genes may permit rapid production of HSPs when cells are stressed[8]. However, there is increasing evidence for distinct functions often between quite similar chaperones, suggesting specialized functions within the cell[100].
Figure 1. The Basics of Hsp70 Chaperoning.
(a) Heat shock protein 70 (Hsp70) contains two main functional domains, including a peptide-binding domain that recognizes unfolded client proteins and an ATPase domain that can utilize the energy stored in ATP for the folding reaction (above). (b) Thus, the Hsp70 and ATP can promote protein folding (below).(c) Hsp70 associates with unfolded proteins that are themselves recruited by J domain co-chaperones (JDP). Binding of the client protein then triggers the intrinsic ATPase domain of Hsp70 to hydrolyze ATP, resulting in a higher affinity association of ADP-bound Hsp70 with the client. Client release from Hsp70 is triggered by co-chaperones such as HSP-BP1 (Heat Shock Protein-Binding Protein 1) and Bag1 (BCl2-associated athanogene 1). These proteins mediate dissociation of ADP from Hsp70, followed by the binding of ATP. ATP-bound Hsp70 has a reduced affinity for the client that can now dissociate in a more folded conformation.
HSPs can also bind more stably to some protein clients; presumably the more dynamic and structurally unstable proteins[15]. In this case, at the end of the folding cascade the client-protein complex either fails to dissociate or there are repeated cycles of chaperone release followed by re-association. Such persistent interactions have regulatory functions within cells. For example, molecular chaperones form complexes with steroid hormone receptors [15, 16], which appear to stabilize the receptors and permit them to adopt anticipatory conformations that are primed to be activated by ligand binding. It is an interesting theme that many proteins that are persistently associated with Hsp90 complexes, although stable and fully folded, are inactive[16, 17]. Activation of proteins complexed to Hsp90 generally leads to their release; the released client is then transiently functional prior to its unfolding and deactivation. By contrast, Hsp70 association was shown to lead to loss of activity in the client protein partner through partial unfolding and Hsp70 could thus function as an inhibitor of some processes [15]. Elevated levels of Hsp70 inhibit the apoptotic cascade by such a mechanism.
The coordinate induction of individual HSPs by stress suggested a shared mechanism regulating their expression. Indeed all HSP genes have been shown to contain at least one binding site (heat shock element) for heat shock transcription factor 1 (HSF1) in their proximal promoter regions[18]. HSF1 is a sequence specific transcription factor that is triggered by proteotoxic stress to form trimers and bind to HSP promoters within a few seconds, leading to a prompt and massive transcriptional response. HSP gene induction involves the reversal of Hsp90-mediated product inhibition as well as posttranslational modifications such as phosphorylation, sumoylation and acetylation[18, 19]. Considerable effort is being exerted in determining mechanisms for HSF1 activation in cancer, although a definitive mechanism has not yet been identified. For the purposes of this review, we have only briefly discussed HSF1 as this rapidly expanding subject merits consideration of its own.
This review is a follow-up of our article on heat shock proteins (HSPs) in cancer published in TIBS in 2006, and is concentrated now largely on work published in the past five years [20]. Since 2006 the volume of publications in this area has increased dramatically and HSPs have come to be seen as playing significant roles in of many of the attributes of cancer. We have prepared an overall review of the properties of HSPs that may influence the defining traits of cancer. More detailed reviews regarding the mechanisms, in tumor development of HSPs in general[21], Hsp27[12], Hsp70[22], Hsp90 and its inhibitors[23] are available. We have concentrated on Hsp27, Hsp70 and Hsp90 as the majority of reports encountered by us in the literature involved these chaperones. While there is some evidence for a role for hsp60, particularly in GI cancers, we could find little information available for Hsp110[24]. It is not clear at this stage whether the paucity of information regarding Hsps 60 and 110 reflects their minor status in tumorigenesis or if it is the result of the preferences of investigators for study of individual HSPs.
Cancer cells therefore resemble thermotolerant cells, in that they are enriched in chaperones and consequently in protein folding power
We might next ask how the enriched folding environment of the tumor cell participates in cancer The molecular components that drive the development of cancer are largely proteins that permit increased cell accumulation, tumor formation and the escape of cells from primary tumors to cause secondary cancers [25–29]. Intuitively one would consider HSPs unlikely to play a direct causal role in any of these processes. However, elevated HSP levels do appear to provide an enabling environment for tumor progression to take place [20]. How does this work?
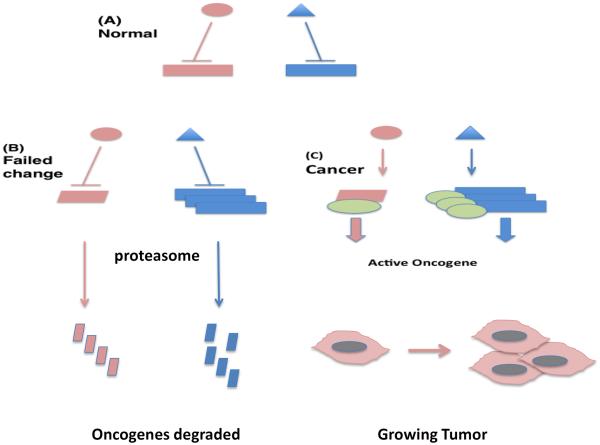
Cancer development involves a radical breakdown in cellular regulation, as most differentiated cells in the body have ceased growth and are enmeshed within the tissue architecture[26]. Overcoming tissue homeostasis and resuming growth and mobility in cancer involves multistep changes to overwhelm the capacity of the regulatory proteins [26]. Such cancer-promoting changes involve increases in oncogene levels, gain of function mutations in such oncogenes and loss of function mutations in tumor suppressor genes. Thus we can envisage a role for elevated protein folding power in managing the amplified proteome and in accompanying mutant proteins that are essentially “unfoldable” due to covalent changes in structure (Figure 2). Indeed inhibition of Hsp90 in cancer cells leads to wholesale degradation of oncogenic proteins[23]. Furthermore expression of the oncogene Her2 in the absence of Hsp70 in lead to cell inactivation indicating the important functions of chaperones in oncogenesis[30].
Figure 2. HSPs in Cancer Signaling.
(A) We depict potentially oncogenic proteins (rectangles) being suppressed in normal cells by regulatory proteins (oval, triangle). (B) Potentially oncogenic changes including mutation (pink parallelogram) or increased expression (multiple blue rectangles) fail to transform cells in the absence of elevated HSPs due to insufficient chaperoning power. These mutated and overexpressed oncoproteins are depicted as then undergoing proteasome-mediated degradation. (C) Elevated levels of chaperones (green ovals) permit transformation by stabilizing the mutated or hyperexpressed oncogenic proteins and permitting tumor growth. Cells with elevated HSP levels as in (C) can be reverted to those with a failed oncogenic change as in (B), with oncogene degradation, by chaperone antagonism using drugs such as Hsp90 inhibitors (not shown).
HSPs and the Defining Traits of Cancer Cells
When the United States President Richard Nixon signed the National Cancer Act in 1971, few would have predicted the complexities that such a “war on cancer” would turn up. However, studies carried out by the cancer research community over the next 30 or so years led Douglas Hanahan and Robert Weinberg to compile in 2000 a list of accepted “Hallmarks” essential in tumorigenesis including: (1) unregulated proliferation (2) evasion of anti-growth signals, (3) escape from programmed cell death, (4) avoidance of cell senescence, (5) de novo angiogenesis and (6) cell invasion and metastasis [25]. We consider here how the HSP-rich tumor environment contributes to these traits.
Chaperones of Cell Proliferation
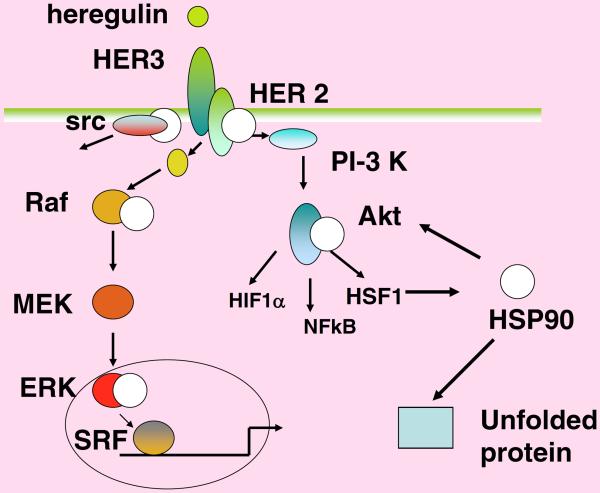
Cell proliferation in adult tissues is not a default state and most cells have long ceased growing at this stage. In cancer, growth control is deregulated and proliferation resumes. When growth is required, normal cells receive instruction for proliferation from secreted growth factors[31]. The factors then bind to high affinity receptor proteins that have extracellular binding surfaces as well as intracellular signaling domains[31]. Receptors thus accept the growth signal and transmit it into the cell's interior. Signals then pass through a series of relay proteins that amplify the message leading ultimately to cell proliferation[32]. Deregulation at each of the signal transduction stages can be oncogenic due to unscheduled proliferation. Figure 3 shows the growth promoting, branched cascade leading from the cancer-causing growth factor heregulin[33]. Most of the receptors and enzymes that constitute the cascade are oncogenic when expressed at elevated levels or activated through mutation. Many of these proteins are clients of Hsp90; therefore amplification of Hsp90 is permissive for unrestrained proliferation[34]. Hsp90 chaperone complexes thus maintain the signaling circuitry that underlies the capacity of many cancers for independent growth[35]. There is some suggestion that Hsp70 may also be required in a similar fashion, because inactivation of this chaperone led to inhibition of proliferation in murine mammary tumor cells[36]. Indeed, Hsp27>Hsp70>Hsp90 may operate in relay manner in maintaining the integrity of the activated proteins involved in proliferative signaling cascades. By analogy with electrical circuitry, HSPs may be thought of as providing the insulation necessary for prompt and abundant passage of signals for growth.
Figure 3. Hsp90 enhances the heregulin-HER3 Signaling Cascade.
Heregulin binds to its receptor and launches growth-promoting signaling through the Erk (left), src (far left) and Akt (right) branches. Numerous high affinity partners for Hsp90 (white circle) exist along these pathways, indicating the long reach of Hsp90 in cancer signaling. These pathways can be blocked by Hsp90 inhibitors at each individual Hsp90 chaperoned step, leading to multitargeted cancer therapy.
Evading Anti-Growth Signals with HSPs
One of the principal factors that control the development of cancer is p53, a protein with a role in mediating growth arrest and apoptosis in response to DNA damage. The potency of p53 in preventing carcinogenesis is illustrated by the findings that germline mutation in p53 is the principal causal agent in Li-Fraumeni syndrome, a genetic disorder that involves pronounced susceptibility to breast cancer, osteosarcoma, gastric cancers and soft tissue sarcomas [37]. Inactivating mutations in Tp53 (the gene encoding p53) appear to be dominant to the other, normal allele, perhaps through imposing a defective conformation on wild-type p53 protein through dimerization. Expression of hsp70 and hsp90 increases to high levels in tumors with mutated p53 and both chaperones may play roles in stabilizing the altered conformation of mutant p53 [38, 39]. Hsp27 interacts with wild type p53 in a pathway that leads to functional inactivation and interruption of senescence[40].
Escape from Cell Death May Be Aided by Elevated HSP Levels
The chaperones Hsp70 and Hsp27 appear to be powerful inhibitors of cell death pathways and thus can, when they become elevated in cancer, indirectly stimulate tumor growth. Programmed cell death (PCD) is an intrinsic mechanism that all cells are capable of executing, leading to loss of cells that either pose a danger to the organism such as activated T cells or cells sacrificed during tissue remodeling [41]. Such death pathways can also be triggered by stresses. Cancer cells, have been shown to deploy a range of mechanisms to evade PCD [42]. Remarkably, Hsp70 and Hsp27 have both been shown to interact directly with protein intermediates in the apoptosis pathways and are potent inhibitors of PCD[43, 44]. Hsp27 inhibits PCD through its capacity to block multiple steps in these death pathways. These effects include inhibiting cytochrome C and SMAC Diablo release from mitochondria as well as antagonizing caspases 3 and 9[45–47]. In addition the alternative death receptor pathways including the Fas, TNFα and TRAIL pathways are targets for inhibition by Hsp27[48]. Hsp70 is likewise a versatile inhibitor, blocking the c-jun kinase pathway of PCD and interrupting cytochrome C release from mitochondria[44, 47]. The effectiveness of Hsp27 and Hsp70 in inhibiting PCD suggests that such a mechanism could in fact be a component of the ancestral heat shock response to limit cell death caused by protein stress, which would permit an interlude in which HSPs could be synthesized and proteins could be refolded. PCD can also be prompted by unscheduled expression of oncogenes or by cytotoxic therapies [49]. The chaperone-rich cytoplasm of the cancer cell thus contributes to the resistance to PCD and killing by cytotoxins [49]. Chaperones appear to act additively in the restraint of cell death and for instance dual targeting of Hsp70 and Hsp90 promoted chemotherapy-induced death in bladder cancer[50].
HSPs Contribute to Limitless Proliferation and Avoidance of Senescence
HSPs are also very effective at interrupting another pathway of cell inactivation- in this case by inhibiting cell senescence. Normal cells resist transformation by having a limited number of permitted divisions [51]. This system is based on the lack of replication of chromosome ends at each cell division; the capping structures at the chromosome ends become progressively shortened, leading to arrest of further division and cell senescence[52, 53]. Cancer cells evade the senescence program by deploying the enzyme telomerase, which replaces the shortening ends of telomeres[53].
Hsp90 binds to telomerase and is required for its efficient function[54]. Thus, Hsp90 might deter senescence by chaperoning telomerase and overcoming the erosion of telomeres over time when expressed to high levels as in cancer. Indeed, chemical targeting of Hsp90 inhibits telomerase function, confirming a role for the chaperone in limiting senescence in cancer [55]. Interestingly, Hsp90 was also able interact with the promoter of the human telomerase gene and enhance expression of telomerase in human cancer cells [56]. In addition, Hsp27 and Hsp70 inhibit the effector arm of the senescence pathway by reducing the effectiveness of p53 in promoting cell senescence [40, 49, 57, 58]. p53 transcriptionally upregulates cell cycle protein p21, which directly arrests proliferation, and this process is inhibited by high levels of Hsp70[57]. The exaggerated levels of HSPs in cancer thus provide an environment that is conducive to maintaining the status of the potentially immortal cancer cell.
Do HSPs Contribute to Angiogenesis?
Growing tumors inevitably outgrow the local blood supply as they increase in size, and begin to starve for oxygen[59]. However, tumor cells are able to deploy Hypoxia Inducible Factors (HIF), proteins that can sense the low O2 environment and mediate the expression of growth factors such as vascular endothelial growth factor (VEGF) that increase the growth of the tumor capillary network[60]. Although there are few reports regarding the role of HSPs in tumor hypoxia and HIF1 activity, one study indicated HIF1–α degradation in the presence of Hsp90 inhibitors, suggesting a role for the chaperone in stabilizing this factor [61]. Recent reports also indicate that Hsp27 becomes pro-angiogenic when released from tumor cells and can bind to receptors, stimulating VEGF transcription through an alternative pathway involving the factor NFkB[62]. Extracellular Hsp27 also exerts pro-angiogenic properties through direct interactions with VEGF in the medium[63].
HSPs Fuel Tumor Cell Invasion and Metastasis
Metastasis is a complex behavior in which transformed cells acquire the capabilities to: (i) Remodel the local microenvironment and detach from neighbor cells (ii) Migrate within the primary tumor locus, (iii) Invade across the tumor capillary wall and enter the bloodstream and (iv) Survive the journey through the circulation to invade distant organs[26]. As this is a complex process, changes in a number of genes are associated with the acquisition of a metastatic phenotype[64]. Elevated expression of each of the HSPs strongly promotes metastasis. An increase in Hsp90 is associated with metastasis largely due its capacity to chaperone focal adhesion kinase, integrin linked kinase and the receptor tyrosine kinases ErbB2 and MET [65]. MET appears to be an important client for HSPs in cancer and Hsp70 inactivation leads to decreased MET expression and loss of MET autophosphorylation in mammary tumors [36, 66]. Hsp27 expression also favors metastasis though its effects on a process known as the epithelial-mesenchymal transition, in which cells switch from a compact shape to a spindle shape and gain enhanced cell motility [67–69]. In addition, elevated co-chaperone levels also promote (prostate) cancer; increased levels of the Hsp90 co-chaperone p23 increases metastasis in prostate cancer[70]. The ability of HSPs to respond to and protect cells from stress may also permit metastasizing cells to survive the trauma involved in passage through blood vessels.
HSP90: A Pivotal Factor in Evolution, Tumor Progression and the Origin of Treatment-Resistant Phenotypes
In 1998, the Lindquist Lab made a groundbreaking discovery: that Hsp90 could play a role in Darwinian evolution in cells and organisms[71] (Box 2). Hsp90 appeared to participate in `canalization;' that is, it smoothed over changes in phenotype despite the accumulation of genetic changes that occur naturally. Selection of new traits could be brought about by heat shock in which Hsp90 is sequestered by denatured proteins, or by Hsp90 inhibitors, presumably when mutant conformations are unmasked by chaperone inactivation and new phenotypes can thus be tested for fitness[71]. These changes also bred true after selection stress and inhibiting Hsp90 thus led to novel heritable traits. If there were a tissue environment where Darwinian evolution would be expected to operate in an accelerated manner, it would be the nutrient deprived –but viable cells in the center of the tumor milieu. The tumor milieu is generally in a critical state for energy production, with reduced tumor glucose levels and hypoxia in cells remote from the microcirculation[72]. These are conditions in which Hsp90, which requires a high ATP level and low ADP level to function at physiologically significant rates, becomes inactive [73]. The initial emergence of cancer also involves selection for genetic mutations and this process accelerates as defects in mismatch repair and non-homologous end joining repair accelerate along with tumor progression and the traits encoded by these altered genes might be further unmasked when Hsp90 is inhibited[74]. Thus one might speculate that the emergence of new phenotypes close to the hypoxic cores at the center of more advanced tumors might be at least partially a consequence of inactivated Hsp90 and loss of phenotypic buffering [75]. Chemotherapy and radiation therapy also put an extra level of stress on these cells and may further lead to the emergence of chemoresistance and radiation resistance. This is a compelling mechanism for the rapid evolution of treatment resistance in tumors and suggests further investigation. These considerations suggested the profound strategy of inhibiting Hsp90 with non-toxic amounts of drugs in combination with standard therapies to combat the evolution of drug-resistant traits[76].
Box 2. Hsp90 as a facilitator of tumor cell evolution.
It has been hypothesized that Hsp90 plays a unique role as a “capacitor for evolution” based on its ability to chaperone quasi-stable structures resulting from point mutations and even more gross alterations in structure as in the fusion genes formed from domain swapping between distinct proteins[71]. Cells in human tumors undergo a process similar to natural selection as they compete to overcome the many physiological barriers to growth of malignant cells, struggle to survive in the hostile, energy-deprived milieu of the tumor[59, 72], and intermittently undergo massive levels of cytotoxic stress during cancer therapy[81]. In addition, the process of tumor progression is accompanied by increasing genomic instability, an alteration that generates the point mutations and translocations mentioned above[74, 75]. Hsp90 may thus permit the survival of such altered proteins, which may drive the emergence of cryptic phenotypes that can be expressed when cells are stressed by therapy or by deterioration in the tumor milieu.
HSF1 and HSPs in Cancer Stem Cells and Tumor Initiation
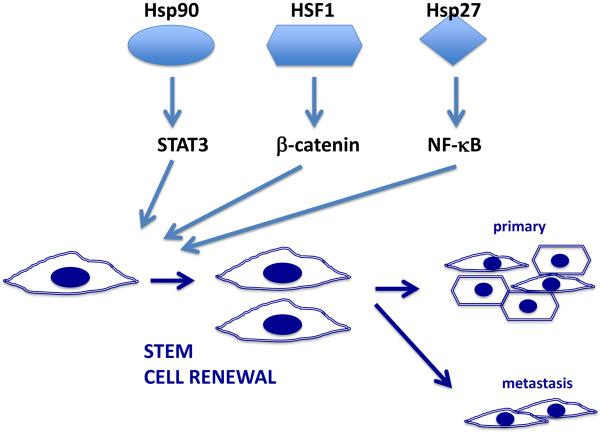
It has recently become apparent that tumors are heterogeneous at the cellular level and that only a fraction of the cell population is able to seed a new tumor [77]. The small tumor initiating sub-populations possess phenotypes similar to those of tissue stem cells, and are referred to as cancer stem cells (CSCs) (Box 3). The remainder of the tumor cells (non-stem cells, NSC) although continuing to proliferate, have reduced tumor initiating capacity and therapy resistance and are thus not the primary drivers of cancer growth and recurrence [78]. Many of the NSC may however be required for maintenance properties such as releasing growth factors and generating the stem cell niche and may possess the plasticity required to be recruited into the CSC fraction during episodes of cell death[79]. Thus the role of HSPs in both CSC and NSC may be informative. Stem cell renewal can be triggered by a number of signaling pathways including the Wnt / β-catenin, JAK-STAT, Hedgehog, Notch, TGF-β and Hippo-YAP/TAZ pathways that are often aberrantly regulated in cancer[79]. Interestingly, CSC seem to be enriched in HSPs, which undergo a decline upon differentiation [80]. CSCs are a particularly problematic subpopulation because they exhibit resistance to most therapies, drive tumor growth and metastasis and mediate tumor recurrence[79, 81]. It was shown recently that murine and human mammary CSCs are enriched in Hsp70[36]. Knockout of the genes encoding Hsp70 or reduction in HSP levels through knockdown of HSF1 markedly depleted CSC levels[36]. CSCs in this model system were the major drivers of metastasis and loss of Hsp70 led to a striking reduction in secondary lung tumors[36]. The molecular mechanisms underlying the role of Hsp70 in stemness and metastasis are still under study,[36] although a role for the oncogene MET was noted. HSF1 and HSPs may play a key role in activating the Wnt / β-catenin pathway of stem cell renewal through permitting elevated translation of β-catenin itself[82]. As with Hsp70, Hsp27 was also required for mammary CSC maintenance and cell migration. In this case, the mechanism for CSC renewal involved another pathway, one involving the factor NFkB, a known factor in stemness[83]. Inhibition of Hsp90 also led to a reduction in stemness in a number of tumor types [84, 85] (Figure 4). Hsp90 is required for activity of the CSC renewal pathway involving the JAK-STAT system[86, 87]. In normal tissues, the stem cell inducing pathways become inactivated during differentiation while in CSC, loss of regulation, a process involving elevated HSP levels leads to sustained CSC survival and promotion of cancer[36, 79].
Box 3. Cancer Stem Cells.
In most tissues, renewal or growth of the population is due to a specialized class of stem cells[78]. Stem cells divide and lead to a lineage of cells that differentiate progressively to finally produce the specialized effector cells that carry out organ-specific functions. It came as a surprise to most oncologists when the common solid tumors were found to contain cells that resembled stem cells (cancer stem cells)[79]. These cells are of high significance as they almost exclusively initiate the primary and secondary tumors. The majority of the remaining tumor cells thus have properties in common with differentiated cells and have much less pronounced tumor-initiating potential. Relative roles as stem or differentiated cells involve divergent fate-determining gene expression profiles. The stem cell fractions of tumors have powerful intrinsic resistance to drugs, ionizing radiation and immune attack, consonant with their essential roles as repositories of cell lineage[81].
Figure 4. Elevated levels of HSF1 and HSPs promote renewal of tumor-initiating cancer stem cells.
HSPs and HSF1 interact with effectors of CSC renewal and reinforce stemness in a non-canonical manners. Hsp90 and HSF1 promote stemness by, respectively, the STAT3 and β-catenin CSC pathways while Hsp27 promotes stemness through activating NFκB. Expanded populations of CSC then initiate primary and metastatic tumor growth. CSC divide either symmetrically to produce CSC daughter cells or asymmetrically to produce one daughter CSC and one daughter that is a progenitor cell capable of forming different types of tumor tissue. We depict CSCs with a spindle-shaped mesenchymal morphology and NSCs, formed by asymmetric division in the tumor with a more epithelial-type, polygonal morphology.
Although we have discussed the properties of HSPs in cancer under various headings, these processes are intimately linked. HSPs are important promoters of the CSC phenotype and such cells are highly invasive, support tumor repopulation, are profoundly metastatic and are resistant to treatment[36, 85]. Tumor hypoxia may play a part in the evolution of new cancer cell properties through its effects on Hsp90 activities in cells remote from the circulation. The combined effects of increases in sustained proliferation and reduction in PCD and senescence, each dependent on elevated levels of HSPs, may be key to promotion of tumor growth.
HSPS in Transgenic Tumor Models: In Vivo Veritas?
Within tumors, one or more dominant mutation(s) generally occur and these mutated proteins drive the malignant process [88]. Much may be learned about the roles of HSPs in tumorigenesis by studying transgenic mice that have been engineered to express dominant oncogenes For instance, Sherman et al. discovered an essential role for Hsp70 in the Her2 mouse model[30]. Mammary tumors arising in such mice are a model for the Her2 positive subtype of human breast cancer[30]. In this case the primary role of Hsp70 was to deter senescence in the emerging mammary tumors and to permit survival of Her2 transformed cells[49]. By contrast, in the hsp70−/− MMT spontaneous mouse mammary model, in which animals are transformed by the Polyoma Middle T antigen expressed from the mouse mammary tumor virus long terminal repeat sequence, the primary effects of Hsp70 were to encourage tumor initiation and metastasis [36]. Mammary tumors that develop in MMT mice mimic a number of aspects of human breast cancer including ability to metastasize and expression of estrogen receptors[77]. Interestingly Hsp70 appeared to interact with a specific molecular target in this model: the transforming oncogene MET[36]. Therefore, Hsp70 was essential in both systems investigated to date but may have quite different ways of interacting with the individual drivers of carcinogenesis. As multiple dominant oncogenes have been shown to fuel the growth of tumors of many morphologies, future studies using transgenic animals that express other driver oncogenes may permit us to ask fundamental questions regarding the role of HSPs throughout the tumorigenic process.
Mechanisms: Do We Understand How HSP Levels Increase in Cancer?
We next ask - is there an overall hypothesis to account for the abundance of HSP expression in cancer cells and do such cells have reduced capacity to fold proteins? This question was directly addressed in studies by Sherman et al., who monitored several criteria associated with cell stress and found that cancer cells do not seem to be in folding deficit[89]. However, there seems little doubt that cancer cells have an increased folding demand. This is evidenced by the studies showing that inhibition of Hsp90 in cancer cells leads to loss of a wide range of proteins essential for tumor growth[34]. It was however shown in a study of prostate cancer cell lines, each rich in chaperone expression, that heat shock led to rapid induction of HSP mRNAs, indicating that the heat shock response was far from saturated in such cells[90]. The prevailing hypothesis is, then, that as the expression levels of oncogenes rises, the heat shock response becomes progressively activated and HSP expression rises.
Other Regulatory Roles for HSPs in Driving Cancer: Beyond Chaperoning?
-
(i)
Do HSPs influence RNA expression? It was shown recently that inactivation of Hsp70 in mouse mammary cancer led to significant changes in transcripts of a number of potentially significant genes[36]. The roles of HSPs in regulating the tumor transcriptome are still unclear, although with the widespread availability of RNA-deep sequencing capabilities one predicts much new information in this area.
-
(ii)
Co-chaperones do not merely influence the rate of protein folding by chaperones but can also regulate their cellular functions. In addition, the interaction goes both ways: the primary chaperone also regulates co-chaperone function. Hsp70 can couple to the co-chaperone BAG3[89]. This may be highly significant as BAG3 is an important signaling protein and, when coupled with Hsp70, BAG3 influences the activities of a range of proteins with regulatory roles in cancer[89].
Extracellular Chaperones: the Jokers in the Pack
Although their existence was originally treated with some skepticism, the biological significance of extracellular HSPs is now growing rapidly. Extracellular HSPs can be proinflammatory in some contexts or show the opposite nature in others[91, 92]. When used as vaccines to chaperone tumor antigens, they can stimulate specific tumor immunity and lead to tumor regression[81]. The direction of their effects thus appears to depend on the environment. When cells are exposed to elevated Hsp70 levels in the context of inflammatory cell killing, potent immunity is observed[92]. When HSPs are released from tumors in the absence of such killing, a class of immune suppressor cells is stimulated and reduced immunity is observed[93]. Extracellular Hsp27 also influences the behavior of surrounding cells. Like Hsp70, this chaperone can influence immunity in both directions through pro-inflammatory cytokines IL-1β and TNF-α and anti-inflammatory cytokine IL-10 [94]. Extracellular Hsp27 also positively influences angiogenesis by increasing VEGF transcription in vascular endothelial cells [62].
In addition to its immune properties, extracellular Hsp90 plays a remarkably powerful role in stimulating wound healing[95]. As many of the properties of cancer resemble those found in wound healing this suggested a potential role in malignancy. Indeed, a number of studies suggest a role for extracellular Hsp90 in stimulation of metastasis, involving a range of mechanisms[96, 97]. Hsp90 appears to have a profound influence on would healing, EMT and metastasis through this “inside out” signaling cascade (Figure 5).
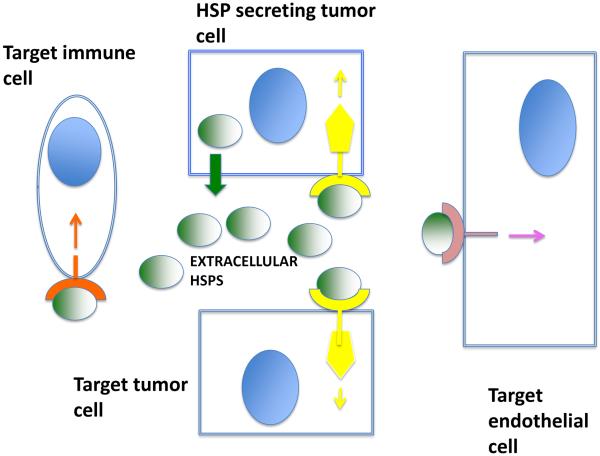
Figure 5. Intracellular and extracellular HSPs in cancer.
HSPs are released from tumor cells and can act in an autocrine manner on the secreting cancer cell. Here, we show an extracellular HSP binding a receptor and stimulating cancer signaling. In addition, extracellular HSPs may also function in a paracrine manner in the milieu, binding to other tumor cells, to immune cells such as macrophages, and to vascular endothelial cells. Extracellular HSPs may thus influence tumor immunity, invasion and metastasis and angiogenesis. Blue ovals are cell nuclei.
The expanding levels of molecular chaperones in the progressing tumor may thus precede the release of these molecules into the extracellular microenvironment. It is not clear by which mechanisms these molecules, lacking in secretion leader sequences are released from tumor cells. However, leakage of HSPs from dying cells in the necrotic cores of large tumors or from cells damaged in cancer therapy could be involved. In addition Hsp70 has been shown to be actively secreted from tumor cells[98]. Extracellular HSPs appear to be able to bind to cells of many morphologies, suggesting pleiotropic effects of the extracellular HSPs within the tumor milieu, which contains multiple competing or cooperating cell types[99]
Concluding Remarks
There is overwhelming evidence that molecular chaperones such as Hsp27, Hsp70 and Hsp90 are expressed at elevated levels in a wide range of cancers and their elevated expression indicates a poor prognosis in most diseases. HSP expression appears essential in many of the distinctive traits of malignant cells, including uncontrolled growth, reduced tumor suppression, enhanced cell survival and the acquisition of powerful capacities for angiogenesis and metastasis. It was also recently shown that HSPs play key roles in promoting the CSC phenotype, including a capacity for cell renewal, invasion and metastasis. Hsp90 appears to permit dynamic changes in tumors and aid in rapid evolution of new treatment-resistant phenotypes, a feature of tumor progression. Within malignant disease types, individual oncogenes are known drive the progression of individual cancers, and Hsp70 can interact distinctly with different driver oncogenes. In addition, extracellular HSPs are of growing importance in the etiology of cancer and may mediate powerful effects on tumor immunity and metastasis.
Outstanding Questions.
-
(1)
The HSP gene families have multiple members. However, it is not clear whether this redundancy serves mainly to increase gene dose, or whether individual HSP family members have significantly different functions. Determining the distinctive properties for each HSP family member would also help in the design of future drugs. Current drugs target properties common to the whole family, such as the ATPase domain of Hsp90 proteins. Thus, essential chaperoning properties that are required by normal cells are also inhibited and toxic complications can ensue. This will be a challenging project, as the structures of the proteins within individual HSP families can be very similar.
-
(2)
One area that has been relatively neglected is study of HSP regulation by posttranslational modification (PTM). However, the Neckers lab has recently determined a number of key PTMs in Hsp90, including phosphorylation and SUMOylation sites that may influence the susceptibility of cancer cells to Hsp90 drugs. Further study in this area might offer significant insights into how HSPs are regulated in cancer and in designing optimal new drugs.
-
(3)
Inside or outside? The relative importance of intracellular and extracellular HSPs in tumor growth and metastasis is not yet determined, and remains a key question for the future.
-
(4)
Do HSPs have properties beyond the chaperoning of proteins in cancer cells? Recent studies suggest so, and these properties may offer new areas that we can exploit for new therapies.
TRENDS BOX.
Molecular chaperones such as Hsp27, Hsp70 and Hsp90 have elevated expression in a wide range of cancers, which indicates a poor prognosis in most cases.
HSP expression is essential to many of the distinctive traits of malignant cells including uncontrolled growth, reduced tumor suppression, enhanced cell survival and angiogenic and metastatic properties.
Recent studies show that HSPs support cancer stem cell identity including the capacity for cell renewal, invasion and metastasis.
Increased levels of Hsp90 aides the rapid evolution of new treatment-resistant phenotypes by permitting new traits to arise within tumors.
HSPs can interact distinctly with different driver oncogenes, which drive the progression of individual cancers.
Extracellular HSPs are of growing importance in the etiology of cancer and may mediate powerful effects on tumor immunity and metastasis.
Acknowledgments
*Support provided by NIH research grants, RO-1CA119045 and RO-1CA094397
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Subjeck J.R.a.S., James J. Coexpression of Thermotolerance and Heat Shock Proteins in Mammalian Cells. Cold Spring Harbor Laboratory Press; 1982. [Google Scholar]
- 2.Richter K, et al. The heat shock response: life on the verge of death. Mol Cell. 2010;40:253–266. doi: 10.1016/j.molcel.2010.10.006. [DOI] [PubMed] [Google Scholar]
- 3.Ciocca DR, Calderwood SK. Heat shock proteins in cancer: diagnostic, prognostic, predictive, and treatment implications. Cell stress & chaperones. 2005;10:86–103. doi: 10.1379/CSC-99r.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kityk R, et al. Pathways of allosteric regulation in Hsp70 chaperones. Nat Commun. 2015;6:8308. doi: 10.1038/ncomms9308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lorenz OR, et al. Modulation of the Hsp90 chaperone cycle by a stringent client protein. Mol Cell. 2014;53:941–953. doi: 10.1016/j.molcel.2014.02.003. [DOI] [PubMed] [Google Scholar]
- 6.Kayser J, et al. The small heat shock protein Hsp27 affects assembly dynamics and structure of keratin intermediate filament networks. Biophys J. 2013;105:1778–1785. doi: 10.1016/j.bpj.2013.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haldar S, et al. Chaperonin-Assisted Protein Folding: Relative Population of Asymmetric and Symmetric GroEL:GroES Complexes. J Mol Biol. 2015;427:2244–2255. doi: 10.1016/j.jmb.2015.04.009. [DOI] [PubMed] [Google Scholar]
- 8.Lindquist S, Craig EA. The heat-shock proteins. Annu Rev Genet. 1988;22:631–677. doi: 10.1146/annurev.ge.22.120188.003215. [DOI] [PubMed] [Google Scholar]
- 9.Calderwood SK. Molecular cochaperones: tumor growth and cancer treatment. Scientifica. 2013;2013:217513. doi: 10.1155/2013/217513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kityk R, et al. Structure and dynamics of the ATP-bound open conformation of Hsp70 chaperones. Mol Cell. 2012;48:863–874. doi: 10.1016/j.molcel.2012.09.023. [DOI] [PubMed] [Google Scholar]
- 11.Manjili MH, et al. HSP110-HER2/neu chaperone complex vaccine induces protective immunity against spontaneous mammary tumors in HER-2/neu transgenic mice. Journal of immunology. 2003;171:4054–4061. doi: 10.4049/jimmunol.171.8.4054. [DOI] [PubMed] [Google Scholar]
- 12.Arrigo AP, et al. Immense Cellular Implications Associated to Small Heat Shock Protein Expression: Impacts on Human Pathologies. Springer; 2015. [Google Scholar]
- 13.Nillegoda NB, et al. Crucial HSP70 co-chaperone complex unlocks metazoan protein disaggregation. Nature. 2015;524:247–251. doi: 10.1038/nature14884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Calabria G, et al. Hsp70 protein levels and thermotolerance in Drosophila subobscura: a reassessment of the thermal co-adaptation hypothesis. J Evol Biol. 2012;25:691–700. doi: 10.1111/j.1420-9101.2012.02463.x. [DOI] [PubMed] [Google Scholar]
- 15.Kirschke E, et al. Glucocorticoid receptor function regulated by coordinated action of the Hsp90 and Hsp70 chaperone cycles. Cell. 2014;157:1685–1697. doi: 10.1016/j.cell.2014.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yoshida S, et al. Molecular chaperone TRAP1 regulates a metabolic switch between mitochondrial respiration and aerobic glycolysis. Proc Natl Acad Sci U S A. 2013;110:E1604–1612. doi: 10.1073/pnas.1220659110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Picard D, et al. Reduced levels of hsp90 compromise steroid receptor action in vivo. Nature. 1990;348:166–168. doi: 10.1038/348166a0. [DOI] [PubMed] [Google Scholar]
- 18.Akerfelt M, et al. Heat shock factors: integrators of cell stress, development and lifespan. Nature reviews. Molecular cell biology. 2010;11:545–555. doi: 10.1038/nrm2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zou J, et al. Repression of heat shock transcription factor HSF1 activation by HSP90 (HSP90 complex) that forms a stress-sensitive complex with HSF1. Cell. 1998;94:471–480. doi: 10.1016/s0092-8674(00)81588-3. [DOI] [PubMed] [Google Scholar]
- 20.Calderwood SK, et al. Heat shock proteins in cancer: chaperones of tumorigenesis. Trends in biochemical sciences. 2006;31:164–172. doi: 10.1016/j.tibs.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 21.Ciocca DR, et al. Heat shock proteins and heat shock factor 1 in carcinogenesis and tumor development: an update. Archives of toxicology. 2013;87:19–48. doi: 10.1007/s00204-012-0918-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sherman MY, Gabai VL. Hsp70 in cancer: back to the future. Oncogene. 2015;34:4153–4161. doi: 10.1038/onc.2014.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Neckers L, Workman P. Hsp90 molecular chaperone inhibitors: are we there yet? Clinical cancer research : an official journal of the American Association for Cancer Research. 2012;18:64–76. doi: 10.1158/1078-0432.CCR-11-1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Campanella C, et al. Heat shock protein 60 levels in tissue and circulating exosomes in human large bowel cancer before and after ablative surgery. Cancer. 2015;121:3230–3239. doi: 10.1002/cncr.29499. [DOI] [PubMed] [Google Scholar]
- 25.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 26.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 27.Wang Z, et al. N terminus of ASPP2 binds to Ras and enhances Ras/Raf/MEK/ERK activation to promote oncogene-induced senescence. Proc Natl Acad Sci U S A. 2013;110:312–317. doi: 10.1073/pnas.1201514110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Whelan KA, et al. The oncogene HER2/neu (ERBB2) requires the hypoxia-inducible factor HIF-1 for mammary tumor growth and anoikis resistance. J Biol Chem. 2013;288:15865–15877. doi: 10.1074/jbc.M112.426999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vander Griend DJ, et al. Conversion of androgen receptor signaling from a growth suppressor in normal prostate epithelial cells to an oncogene in prostate cancer cells involves a gain of function in c-Myc regulation. Int J Biol Sci. 2014;10:627–642. doi: 10.7150/ijbs.8756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Meng L, et al. Heat shock protein Hsp72 plays an essential role in Her2-induced mammary tumorigenesis. Oncogene. 2011;30:2836–2845. doi: 10.1038/onc.2011.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Momeny M, et al. Heregulin-HER3-HER2 signaling promotes matrix metalloproteinase-dependent blood-brain-barrier transendothelial migration of human breast cancer cell lines. Oncotarget. 2015;6:3932–3946. doi: 10.18632/oncotarget.2846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gray PJ, Jr., et al. Targeting the oncogene and kinome chaperone CDC37. Nature reviews. Cancer. 2008;8:491–495. doi: 10.1038/nrc2420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Khaleque MA, et al. Induction of heat shock proteins by heregulin beta1 leads to protection from apoptosis and anchorage-independent growth. Oncogene. 2005;24:6564–6573. doi: 10.1038/sj.onc.1208798. [DOI] [PubMed] [Google Scholar]
- 34.Kamal A, et al. A high-affinity conformation of Hsp90 confers tumour selectivity on Hsp90 inhibitors. Nature. 2003;425:407–410. doi: 10.1038/nature01913. [DOI] [PubMed] [Google Scholar]
- 35.Agorreta J, et al. TRAP1 regulates proliferation, mitochondrial function, and has prognostic significance in NSCLC. Mol Cancer Res. 2014;12:660–669. doi: 10.1158/1541-7786.MCR-13-0481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gong J, et al. Targeting the hsp70 gene delays mammary tumor initiation and inhibits tumor cell metastasis. Oncogene. 2015 doi: 10.1038/onc.2015.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Masciari S, et al. Gastric cancer in individuals with Li-Fraumeni syndrome. Genet Med. 2011;13:651–657. doi: 10.1097/GIM.0b013e31821628b6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pinhasi-Kimhi O, et al. Specific interaction between the p53 cellular tumour antigen and major heat shock proteins. Nature. 1986;320:182–184. doi: 10.1038/320182a0. [DOI] [PubMed] [Google Scholar]
- 39.Wiech M, et al. Molecular mechanism of mutant p53 stabilization: the role of HSP70 and MDM2. PloS one. 2012;7:e51426. doi: 10.1371/journal.pone.0051426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.O'Callaghan-Sunol C, et al. Hsp27 modulates p53 signaling and suppresses cellular senescence. Cancer Res. 2007;67:11779–11788. doi: 10.1158/0008-5472.CAN-07-2441. [DOI] [PubMed] [Google Scholar]
- 41.Al-Chaqmaqchi H, et al. The role of programmed cell death ligand-1 (PDL1/CD274) in the development of graft versus host disease. PloS one. 2013;8:e60367. doi: 10.1371/journal.pone.0060367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li L, et al. MicroRNA-21 stimulates gastric cancer growth and invasion by inhibiting the tumor suppressor effects of programmed cell death protein 4 and phosphatase and tensin homolog. J BUON. 2014;19:228–236. [PubMed] [Google Scholar]
- 43.Lanneau D, et al. Apoptosis versus cell differentiation: role of heat shock proteins HSP90, HSP70 and HSP27. Prion. 2007;1:53–60. doi: 10.4161/pri.1.1.4059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Beere HM, et al. Heat-shock protein 70 inhibits apoptosis by preventing recruitment of procaspase-9 to the Apaf-1 apoptosome. Nat Cell Biol. 2000;2:469–475. doi: 10.1038/35019501. [DOI] [PubMed] [Google Scholar]
- 45.Chauhan D, et al. Hsp27 inhibits release of mitochondrial protein Smac in multiple myeloma cells and confers dexamethasone resistance. Blood. 2003;102:3379–3386. doi: 10.1182/blood-2003-05-1417. [DOI] [PubMed] [Google Scholar]
- 46.Paul C, et al. Dynamic processes that reflect anti-apoptotic strategies set up by HspB1 (Hsp27) Experimental cell research. 2010;316:1535–1552. doi: 10.1016/j.yexcr.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 47.Garrido C, et al. Heat shock proteins 27 and 70: anti-apoptotic proteins with tumorigenic properties. Cell cycle. 2006;5:2592–2601. doi: 10.4161/cc.5.22.3448. [DOI] [PubMed] [Google Scholar]
- 48.Arrigo AP, Gibert B. HspB1 dynamic phospho-oligomeric structure dependent interactome as cancer therapeutic target. Current molecular medicine. 2012;12:1151–1163. doi: 10.2174/156652412803306693. [DOI] [PubMed] [Google Scholar]
- 49.Gabai VL, et al. Heat shock protein Hsp72 controls oncogene-induced senescence pathways in cancer cells. Mol Cell Biol. 2009;29:559–569. doi: 10.1128/MCB.01041-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ma L, et al. Dual targeting of heat shock proteins 90 and 70 promotes cell death and enhances the anticancer effect of chemotherapeutic agents in bladder cancer. Oncol Rep. 2014;31:2482–2492. doi: 10.3892/or.2014.3132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Campisi J. Aging, cellular senescence, and cancer. Annu Rev Physiol. 2013;75:685–705. doi: 10.1146/annurev-physiol-030212-183653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hao XD, et al. Correlation of telomere length shortening with TP53 somatic mutations, polymorphisms and allelic loss in breast tumors and esophageal cancer. Oncol Rep. 2013;29:226–236. doi: 10.3892/or.2012.2098. [DOI] [PubMed] [Google Scholar]
- 53.Jaskelioff M, et al. Telomerase deficiency and telomere dysfunction inhibit mammary tumors induced by polyomavirus middle T oncogene. Oncogene. 2009;28:4225–4236. doi: 10.1038/onc.2009.268. [DOI] [PubMed] [Google Scholar]
- 54.Toogun OA, et al. The hsp90 molecular chaperone modulates multiple telomerase activities. Mol Cell Biol. 2008;28:457–467. doi: 10.1128/MCB.01417-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chaklader M, et al. 17-AAG mediated targeting of Hsp90 limits tert activity in peritoneal sarcoma related malignant ascites by downregulating cyclin D1 during cell cycle entry. Exp Oncol. 2012;34:90–96. [PubMed] [Google Scholar]
- 56.Kim RH, et al. Association of hsp90 to the hTERT promoter is necessary for hTERT expression in human oral cancer cells. Carcinogenesis. 2008;29:2425–2431. doi: 10.1093/carcin/bgn225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yaglom JA, et al. High levels of heat shock protein Hsp72 in cancer cells suppress default senescence pathways. Cancer Res. 2007;67:2373–2381. doi: 10.1158/0008-5472.CAN-06-3796. [DOI] [PubMed] [Google Scholar]
- 58.Kim G, et al. The heat shock transcription factor Hsf1 is downregulated in DNA damage-associated senescence, contributing to the maintenance of senescence phenotype. Aging Cell. 2012;11:617–627. doi: 10.1111/j.1474-9726.2012.00827.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wijsman R, et al. Hypoxia and tumor metabolism in radiation oncology: targets visualized by positron emission tomography. Q J Nucl Med Mol Imaging. 2013;57:244–256. [PubMed] [Google Scholar]
- 60.Joseph JV, et al. Hypoxia enhances migration and invasion in glioblastoma by promoting a mesenchymal shift mediated by the HIF1alpha-ZEB1 axis. Cancer Lett. 2015;359:107–116. doi: 10.1016/j.canlet.2015.01.010. [DOI] [PubMed] [Google Scholar]
- 61.Okui T, et al. Antitumor effect of novel HSP90 inhibitor NVP-AUY922 against oral squamous cell carcinoma. Anticancer Res. 2011;31:1197–1204. [PubMed] [Google Scholar]
- 62.Thuringer D, et al. Extracellular HSP27 mediates angiogenesis through Toll-like receptor 3. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2013;27:4169–4183. doi: 10.1096/fj.12-226977. [DOI] [PubMed] [Google Scholar]
- 63.Choi SH, et al. MMP9 processing of HSPB1 regulates tumor progression. PloS one. 2014;9:e85509. doi: 10.1371/journal.pone.0085509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Prensner JR, et al. RNA biomarkers associated with metastatic progression in prostate cancer: a multi-institutional high-throughput analysis of SChLAP1. Lancet Oncol. 2014;15:1469–1480. doi: 10.1016/S1470-2045(14)71113-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tsutsumi S, et al. Impact of heat-shock protein 90 on cancer metastasis. Future Oncol. 2009;5:679–688. doi: 10.2217/fon.09.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Miyajima N, et al. The HSP90 inhibitor ganetespib synergizes with the MET kinase inhibitor crizotinib in both crizotinib-sensitive and -resistant MET-driven tumor models. Cancer Res. 2013;73:7022–7033. doi: 10.1158/0008-5472.CAN-13-1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pavan S, et al. HSP27 is required for invasion and metastasis triggered by hepatocyte growth factor. Int J Cancer. 2014;134:1289–1299. doi: 10.1002/ijc.28464. [DOI] [PubMed] [Google Scholar]
- 68.Shiota M, et al. Hsp27 regulates epithelial mesenchymal transition, metastasis, and circulating tumor cells in prostate cancer. Cancer Res. 2013;73:3109–3119. doi: 10.1158/0008-5472.CAN-12-3979. [DOI] [PubMed] [Google Scholar]
- 69.Gibert B, et al. Targeting heat shock protein 27 (HspB1) interferes with bone metastasis and tumour formation in vivo. British journal of cancer. 2012;107:63–70. doi: 10.1038/bjc.2012.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cano LQ, et al. The co-chaperone p23 promotes prostate cancer motility and metastasis. Mol Oncol. 2015;9:295–308. doi: 10.1016/j.molonc.2014.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rutherford SL, Lindquist S. Hsp90 as a capacitor for morphological evolution. Nature. 1998;396:336–342. doi: 10.1038/24550. [DOI] [PubMed] [Google Scholar]
- 72.Viale A, Draetta GF. Sugar? No Thank You, Just a Deep Breath of Oxygen for Cancer Stem Cells. Cell Metab. 2015;22:543–545. doi: 10.1016/j.cmet.2015.09.020. [DOI] [PubMed] [Google Scholar]
- 73.Peng X, et al. Heat shock protein 90 stabilization of ErbB2 expression is disrupted by ATP depletion in myocytes. J Biol Chem. 2005;280:13148–13152. doi: 10.1074/jbc.M410838200. [DOI] [PubMed] [Google Scholar]
- 74.Mateo J, et al. DNA-Repair Defects and Olaparib in Metastatic Prostate Cancer. N Engl J Med. 2015;373:1697–1708. doi: 10.1056/NEJMoa1506859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mihaylova VT, et al. Decreased expression of the DNA mismatch repair gene Mlh1 under hypoxic stress in mammalian cells. Mol Cell Biol. 2003;23:3265–3273. doi: 10.1128/MCB.23.9.3265-3273.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Whitesell L, et al. HSP90 empowers evolution of resistance to hormonal therapy in human breast cancer models. Proc Natl Acad Sci U S A. 2014;111:18297–18302. doi: 10.1073/pnas.1421323111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Weng D, et al. Metastasis is an early event in mouse mammary carcinomas and is associated with cells bearing stem cell markers. Breast cancer research : BCR. 2012;14:R18. doi: 10.1186/bcr3102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sreekumar A, et al. The mammary stem cell hierarchy: a looking glass into heterogeneous breast cancer landscapes. Endocr Relat Cancer. 2015;22:T161–176. doi: 10.1530/ERC-15-0263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ajani JA, et al. Cancer stem cells: the promise and the potential. Seminars in oncology. 2015;42(Suppl 1):S3–17. doi: 10.1053/j.seminoncol.2015.01.001. [DOI] [PubMed] [Google Scholar]
- 80.Torigoe T, et al. Constitutive expression and activation of stress response genes in cancer stem-like cells/tumour initiating cells: potent targets for cancer stem cell therapy. Int J Hyperthermia. 2013;29:436–441. doi: 10.3109/02656736.2013.814809. [DOI] [PubMed] [Google Scholar]
- 81.Weng D, et al. Immunotherapy of radioresistant mammary tumors with early metastasis using molecular chaperone vaccines combined with ionizing radiation. Journal of immunology. 2013;191:755–763. doi: 10.4049/jimmunol.1203286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chou SD, et al. HSF1 regulation of beta-catenin in mammary cancer cells through control of HuR/elavL1 expression. Oncogene. 2014 doi: 10.1038/onc.2014.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wei L, et al. Hsp27 participates in the maintenance of breast cancer stem cells through regulation of epithelial-mesenchymal transition and nuclear factor-kappaB. Breast cancer research : BCR. 2011;13:R101. doi: 10.1186/bcr3042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Newman B, et al. HSP90 inhibitor 17-AAG selectively eradicates lymphoma stem cells. Cancer Res. 2012;72:4551–4561. doi: 10.1158/0008-5472.CAN-11-3600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sobhan PK, et al. Identification of heat shock protein 90 inhibitors to sensitize drug resistant side population tumor cells using a cell based assay platform. Cancer Lett. 2012;317:78–88. doi: 10.1016/j.canlet.2011.11.009. [DOI] [PubMed] [Google Scholar]
- 86.Nagaraju GP, et al. Targeting the Janus-activated kinase-2-STAT3 signalling pathway in pancreatic cancer using the HSP90 inhibitor ganetespib. European journal of cancer. 2015;52:109–119. doi: 10.1016/j.ejca.2015.10.057. [DOI] [PubMed] [Google Scholar]
- 87.Tao W, et al. HSP90 inhibitor AUY922 induces cell death by disruption of the Bcr-Abl, Jak2 and HSP90 signaling network complex in leukemia cells. Genes & cancer. 2015;6:19–29. doi: 10.18632/genesandcancer.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Calderwood SK. Tumor heterogeneity, clonal evolution, and therapy resistance: an opportunity for multitargeting therapy. Discovery medicine. 2013;15:188–194. [PMC free article] [PubMed] [Google Scholar]
- 89.Colvin TA, et al. Hsp70-Bag3 interactions regulate cancer-related signaling networks. Cancer research. 2014;74:4731–4740. doi: 10.1158/0008-5472.CAN-14-0747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tang D, et al. Expression of heat shock proteins and heat shock protein messenger ribonucleic acid in human prostate carcinoma in vitro and in tumors in vivo. Cell stress & chaperones. 2005;10:46–58. doi: 10.1379/CSC-44R.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Fong JJ, Sreedhara K, Deng L, Varki NM, Angata T, Liu Q, Nizet V, Varki A. Immunomodulatory activity of extracellular Hsp70 mediated via paired receptors Siglec-5 and Siglec-14. EMBO J. 2015 doi: 10.15252/embj.201591407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kottke T, et al. Induction of hsp70-mediated Th17 autoimmunity can be exploited as immunotherapy for metastatic prostate cancer. Cancer research. 2007;67:11970–11979. doi: 10.1158/0008-5472.CAN-07-2259. [DOI] [PubMed] [Google Scholar]
- 93.Chalmin F, et al. Membrane-associated Hsp72 from tumor-derived exosomes mediates STAT3-dependent immunosuppressive function of mouse and human myeloid-derived suppressor cells. J Clin Invest. 2010;120:457–471. doi: 10.1172/JCI40483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Salari S, et al. Extracellular HSP27 acts as a signaling molecule to activate NF-kappaB in macrophages. Cell stress & chaperones. 2013;18:53–63. doi: 10.1007/s12192-012-0356-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Jayaprakash P, et al. Hsp90alpha and Hsp90beta together operate a hypoxia and nutrient paucity stress-response mechanism during wound healing. J Cell Sci. 2015;128:1475–1480. doi: 10.1242/jcs.166363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hance MW, et al. Secreted Hsp90 is a novel regulator of the epithelial to mesenchymal transition (EMT) in prostate cancer. J Biol Chem. 2012;287:37732–37744. doi: 10.1074/jbc.M112.389015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Nolan KD, et al. Tumor-secreted Hsp90 subverts polycomb function to drive prostate tumor growth and invasion. J Biol Chem. 2015;290:8271–8282. doi: 10.1074/jbc.M115.637496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mambula SS, Calderwood SK. Heat shock protein 70 is secreted from tumor cells by a nonclassical pathway involving lysosomal endosomes. Journal of immunology. 2006;177:7849–7857. doi: 10.4049/jimmunol.177.11.7849. [DOI] [PubMed] [Google Scholar]
- 99.Theriault JR, et al. Extracellular HSP70 binding to surface receptors present on antigen presenting cells and endothelial/epithelial cells. FEBS letters. 2005;579:1951–1960. doi: 10.1016/j.febslet.2005.02.046. [DOI] [PubMed] [Google Scholar]
- 100.Mollapour M, Neckers L. Post-translational modifications of Hsp90 and their contributions to chaperone regulation. Biochimica et biophysica acta. 2012;1823:648–655. doi: 10.1016/j.bbamcr.2011.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Mollapour M, et al. Casein kinase 2 phosphorylation of Hsp90 threonine 22 modulates chaperone function and drug sensitivity. Oncotarget. 2011;2:407–417. doi: 10.18632/oncotarget.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Mollapour M, et al. Asymmetric Hsp90 N domain SUMOylation recruits Aha1 and ATP-competitive inhibitors. Molecular cell. 2014;53:317–329. doi: 10.1016/j.molcel.2013.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ellis RJ. Protein misassembly: macromolecular crowding and molecular chaperones. Adv Exp Med Biol. 2007;594:1–13. doi: 10.1007/978-0-387-39975-1_1. [DOI] [PubMed] [Google Scholar]