Abstract
Background
Rilpivirine pharmacokinetics are defined by its absorption, distribution, metabolism, and excretion. Pregnancy can affect these factors by changes in cardiac output, protein binding, volume of distribution, and cytochrome P450 (CYP) 3A4 activity. Rilpivirine is metabolized by CYP3A4. The impact of pregnancy on rilpivirine pharmacokinetics is largely unknown.
Methods
IMPAACT P1026s is a multicenter, non-blinded, prospective study evaluating antiretroviral pharmacokinetics in HIV-infected pregnant women that included a cohort receiving rilpivirine 25mg once daily as part of their combination antiretrovirals for clinical care. Thirty-two women were enrolled in this study. Intensive PK sampling was performed at steady state during the second trimester, third trimester and postpartum. Maternal and umbilical cord blood samples were obtained at delivery. Plasma rilpivirine concentration was measured using liquid chromatography-mass spectrometry; lower limit of quantitation was 10 ng/mL.
Results
Median (range) AUC0–24 were 1969 (867–4987, n=15), 1669 (556–4312, n=28) and 2387 (188–6736, n=28) ng*hr/mL in the second trimester, third trimester and postpartum, respectively (p<0.05 for either trimester vs postpartum). Median (range) C24 were 63 (37–225, n=17), 56 (<10–181, n=30), and 81 (<10–299, n=28) ng/mL (p<0.05 for either trimester vs postpartum). High variability in pharmacokinetic parameters was observed between subjects. Median (range) cord blood/maternal concentration ratio was 0.55 (0.4–0.8, n=9). Delivery HIV-1 RNA was ≤ 50 copies/mL in 70% and ≤ 400 copies/mL in 90% of women. Cmin were significantly lower at 14 visits with detectable HIV-1 RNA compared to 62 visits with undetectable HIV-1 RNA, 30 (<10 – 93) versus 63 (15 – 199) ng/mL (p=0.0004). Cmin was below the protein-binding adjusted EC90 concentration (12.2 ng/mL) at 4 visits in 3 of 31 women (10%).
Conclusions
Rilpivirine exposure is lower during pregnancy compared to postpartum, and highly variable. Ninety percent of women had minimum concentrations above the protein-binding adjusted EC90 for rilpivirine.
Keywords: rilpivirine, pregnancy, HIV, pharmacokinetics
Introduction
The number of newly infected children in 2013 was estimated at 240,000 and has decreased by 40% since 2009 due to more effective use of antiretroviral therapy and expansion of preventative mother-to-child transmission programs.[1] Pregnant women infected with HIV need to receive antiretroviral drugs both for their own health and to prevent mother-to-child transmission of HIV. Current US Department of Health and Human Services guidelines recommend that all antiretroviral naïve pregnant women receive a combination antiretroviral regimen including two nucleoside reverse transcriptase inhibitors and either a protease inhibitor, an integrase strand transfer inhibitor or a nonnucleoside reverse transcriptase inhibitor (NNRTI).[2, 3] Rilpivirine (Edurant) is a second generation NNRTI licensed for use in antiretroviral naïve adults with HIV-1 RNA less than 100,000 copies/mL.[3] Rilpivirine is dosed as a once daily, single-tablet regimen, either as an individual tablet or co-formulated with tenofovir-DF and emtricitabine.
During pregnancy, physiological changes occur which can impact systemic drug exposure and standard antiretroviral doses may result in reduced drug exposure. This has been seen for all protease inhibitors studied in pregnancy.[4] However, reduced or unchanged drug exposure was seen in some NNRTI, such as nevirapine and efavirenz, and increased drug exposure was seen in etravirine.[18–21] Examples of these physiological changes include increased cardiac output, increased volume of distribution, decreased protein binding, and altered cytochrome P450 activities.[5] Cytochrome P450 3A4 activity is increased by 35–38% during all stages of pregnancy.[17]As a result, use of standard antiretroviral doses in pregnant women may not be sufficient to suppress viral replication. Since rilpivirine is a substrate of cytochrome P450 3A4, the impact of pregnancy on rilpivirine exposure may be clinically significant.
Although rilpivirine is being used in HIV-infected pregnant women, no data are available describing rilpivirine pharmacokinetics and safety in this population. This study was designed to describe rilpivirine exposure during pregnancy and postpartum, and to evaluate transplacental passage of rilpivirine.
Methods
Study Design
Data were collected as part of International Maternal Pediatric Adolescent AIDS Clinical Trials (IMPAACT) Protocol 1026s (P1026s), an ongoing, multicenter, multi-arm, open-label prospective study evaluating the pharmacokinetics of clinically prescribed antiretrovirals in pregnant HIV-infected women [Clincialtrials.gov identifier NCT00042289]. HIV infected pregnant women receiving rilpivirine 25 mg orally once daily as part of clinical care before the beginning of the 35th week of pregnancy were eligible to enroll in the rilpivirine arm. Subjects received antiretroviral medications prescribed by their clinical care providers and all antiretrovirals were dispensed by local pharmacies. The choice of additional antiretrovirals and duration of treatment were determined by each subject and her clinical care provider. Subjects received rilpivirine for at least 2 weeks prior to pharmacokinetic sampling and planned to continue rilpivirine until at least 6 weeks postpartum. Maternal exclusion criteria were: current use of medications known to interfere with rilpivirine disposition, for example, dexamethasone, omeprazole, phenytoin, etc., multiple gestations, or clinical or laboratory toxicity that, in the opinion of the site investigator, would be likely to require a change in the antiretroviral regimen during the study. No requirements of HIV-1 RNA suppression were needed for enrollment. Local institutional review boards approved P1026s at all participating sites, and the study followed all relevant human subjects research guidelines. All subjects provided signed informed consent prior to participation.
Mothers and their infants continued on the study until 6 months after delivery. Infant HIV status was evaluated at 24 weeks of life by physical exam and chart abstraction. Intensive 24-hour rilpivirine pharmacokinetic sampling was performed during the second trimester if feasible, the third trimester and again postpartum.
Clinical and laboratory monitoring
HIV-related laboratory testing was performed as part of the study, if not available from routine clinical care. Maternal clinical data included in this analysis were age, ethnicity, weight, concomitant medications, CD4+ lymphocyte count and plasma HIV-1 RNA. Plasma HIV-1 RNA assays were done locally with lower limits of detection ranging from less than 20 copies/mL to less than 400 copies/mL. Maternal clinical toxicities were assessed through clinical evaluations of history and physical examination. Toxicity was assessed by monitoring maternal alanine aminotransferase, aspartate aminotransferase, creatinine, BUN, albumin, bilirubin, and hemoglobin on each pharmacokinetic sampling day and at delivery. Infant birth weight, gestational age at birth, and HIV infection status data were collected. Infants received physical examinations after birth and infant laboratory evaluations were done only as clinically indicated. Toxicity reports were reviewed by the study team on monthly conference calls, and the patient’s care provider was responsible for toxicity management. The Division of AIDS (DAIDS)/NIAID Toxicity Table for Grading Severity of Adult Adverse Experiences was used to report adverse events for study subjects.[7] All toxicities were followed through to resolution or 24 weeks postpartum.
Sample collection and drug assays
Plasma samples were drawn during the second trimester, third trimester and postpartum pharmacokinetic evaluation visits. Blood samples were drawn at pre-dose and 1, 2, 4, 6, 8, 12, and 24 hours after an observed dose. On the day of sampling, rilpivirine was given with a meal consisting of at least 500 calories. In addition, a single maternal plasma sample and an umbilical cord sample after the cord was clamped were collected at delivery. Samples obtained during pregnancy were assayed and each subject’s care provider was notified of the subject’s rilpivirine plasma concentrations and AUC within two weeks of collection. Individual care providers could elect a dosing modification if the AUC was below 880 ng*hr/mL, the 10th percentile rilpivirine AUC in non-pregnant adults.[6]
Rilpivirine concentrations in plasma were measured by the University of California, San Diego IMPAACT Pharmacology Laboratory using high performance liquid chromatography with UV detection. Mean recovery of drug from plasma was 99.1%. The method was linear over the concentration range of 10 to 2560 ng/mL, with a lower limit of quantitation of 10 ng/mL. Linearity was evaluated over three days, and had an average correlation coefficient of 0.9992 from three curves. For all validation samples, the between assay precision and accuracy ranged from 4.06 to 9.04 % coefficient of variation, and −9.39 to 7.62 % deviation, respectively. Rilpivirine is stable in plasma stored at −70°C for 2 years.
Pharmacokinetic analyses
The pharmacokinetic parameters during pregnancy and postpartum for each patient were determined using standard non-compartmental methods. The pre-dose concentration (C0), maximum concentration (Cmax), and time of maximum concentration (Tmax) were directly observed. Cmax was defined as highest concentration after the observed dose (time = 0). For concentrations below the assay limit of detection, a value of one-half of the detection limit was used in the calculations. The area under the concentration time curve from 0–24 hours (AUC0–24) was estimated using the trapezoidal rule. The terminal slope (λz) was calculated from declining concentrations at the end of the dose interval. The apparent oral clearance (CL/F) was calculated by dose (25mg) divided by AUC0–24. The apparent volume of distribution (Vd/F) was determined by CL/F divided by λz.
Statistical analyses
Target enrollment for the rilpivirine arm of P1026s was at least 25 women with evaluable third trimester pharmacokinetic data. Enrollment was allowed to continue until evaluable pharmacokinetic data from 12 women during the second trimester were available. To prevent ongoing enrollment of subjects receiving inadequate dosing, enrollment was to be stopped early if 6 study subjects had third trimester rilpivirine AUC0–24 below 880 ng*hr/mL, which is the estimated 10th percentile AUC for non-pregnant adults.
Rilpivirine pharmacokinetic parameters during second and third trimester, second trimester and postpartum, and third trimester and postpartum were compared at the within subject level using the Wilcoxon signed-rank test, with a two-sided p < 0.05 considered statistically significant. Descriptive statistics were calculated for pharmacokinetic parameters of interest during each study period. Within-subject geometric mean ratios and 90% confidence intervals based on the student’s t distribution were calculated for pharmacokinetic parameters between time periods. Minimum concentrations were compared between visits with detectable versus undetectable HIV-1 RNA with the Wilcoxon rank-sum test. Statistical analyses were conducted using Excel © (Microsoft, Redmond, WA) and STATA © (Statacorp, College Station, TX).
Results
Characteristics
This study enrolled a total of 32 women between May 2013 and February 2015. Nineteen women in the second trimester (one woman only had second trimester data available), 31 in the third trimester, and 28 women postpartum completed pharmacokinetic sampling. Plasma HIV-1 RNA at delivery was ≤ 50 copies/mL in 21 of 30 subjects (70%) and ≤ 400 copies/mL in 27 of 30 subjects (90%). Table 1 summarizes the clinical characteristics of the women and their pregnancy outcomes.
Table 1.
Study Population Characteristics and Pregnancy Outcomes
| Characteristic | N (%) or Median (Range) |
|---|---|
| Race/ethnicity | |
| Black, Non-Hispanic | 18 (56%) |
| Hispanic | 12 (38%) |
| White, Non-Hispanic | 1 (3%) |
| Asian, Pacific Islander | 1 (3%) |
| Concomitant Antiretroviral Comedications | |
| Emtricitabine | 32 (100%) |
| Tenofovirdisoproxil fumarate | 32 (100%) |
| Zidovudine | 5 (16%) |
| Darunavir/ritonavir | 1 (3%) |
| Age at delivery (years) | 26.7 (17.2 – 37.5) |
| Duration of rilpivirine therapy at the 3rd trimester visit (weeks) | 28 (3 – 337) |
| Weight at delivery (kg) | 90.7 (60.9 – 171) |
| CD4+ at delivery (cells/mm3) | 550 (112 – 1149) |
| Weeks after delivery at postpartum study visit | 9.3 (6.1 – 12.4) |
| Weight at postpartum visit (kg) | 83.1 (50.9 – 161.2) |
| HIV-1 RNA at 2nd trimester (copies/mL) | < 40 (<20 – 2770) |
| HIV-1 RNA at 3rd trimester (copies/mL) | < 20 (<20 – 48700) |
| HIV-1 RNA at Delivery (copies/mL) | < 20 (<16 – 48700) |
| HIV-1 RNA at Delivery (copies/mL) | |
| ≤ 50 copies/mL | 21/30 (70%) |
| ≤ 400 copies/mL | 27/30 (90%) |
| Gestational age at delivery (weeks) | 38.9 (32.3 – 41.4) |
| Number of preterm deliveries* | 3 (10%) |
| Infant Birth weight (g) | 3095 (1570 – 4570) |
Preterm delivery in 3 subjects at 32, 35, and 36 weeks
Rilpivirine exposure
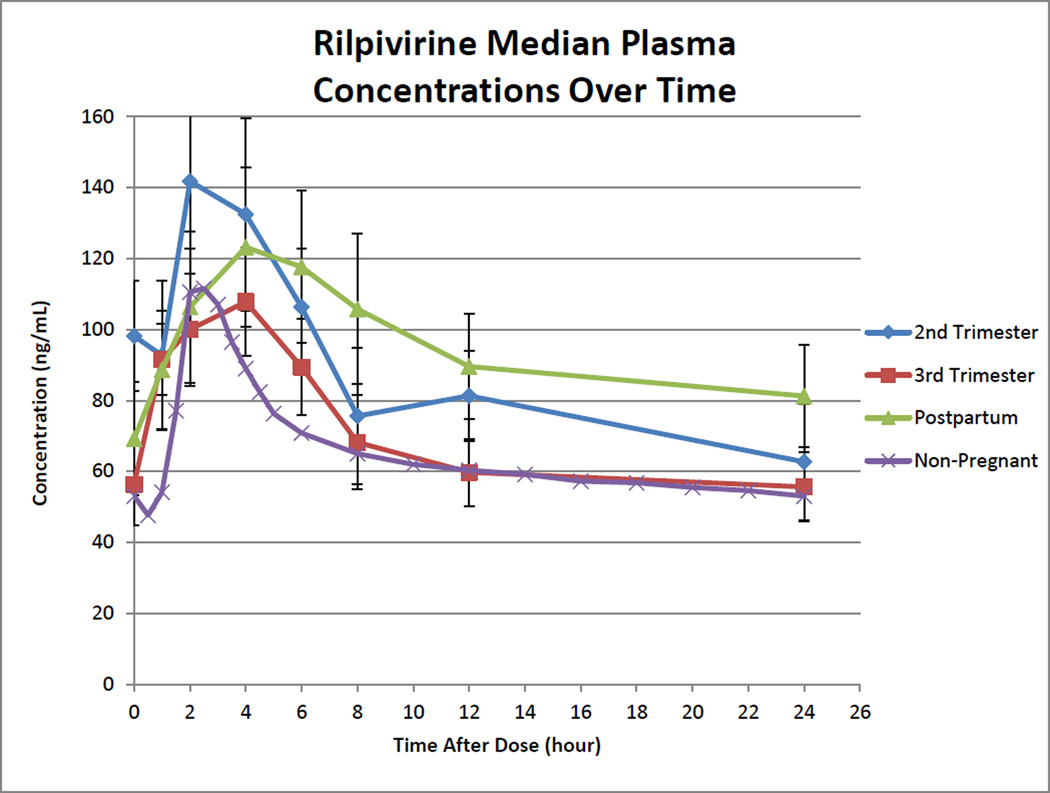
Rilpivirine pharmacokinetic parameters with standard adult dosing are presented in Table 2. Rilpivirine median concentration versus time curves for second trimester, third trimester, and postpartum are presented in Figure 1. Absorption lags were noted when 1 hour post-dose rilpivirine concentration was less than pre-dose concentration (Figure 1). Lags were observed in 9 of 18 (50%) women during the second trimester, 13 of 30 (43%) during the third trimester, and 11 of 28 (39%) during postpartum.
Table 2.
Rilpivirine Non-compartmental Pharmacokinetic Parameters
| Parameters | Second Trimester Median (Range) n=18 |
Third Trimester Median (Range) n = 30 |
Postpartum Median (Range) n = 28 |
|---|---|---|---|
| AUC24 (ng*hr/mL) | 1969(867 – 4987)* | 1669(556 – 4312)† | 2387 (188 – 6736) |
| C0 (ng/mL) | 98(31 – 216)‡ | 56(<10 – 210) | 69 (<10 – 285) |
| Cmax (ng/mL) | 145(43 – 347) | 134 (49 – 267) | 134 (42 – 407) |
| Tmax (hr)** | 4 (1 – 6) | 2 (1 – 24) | 4 (1 – 8) |
| C24 (ng/mL) | 63(37 – 225)* | 56(<10 – 181)† | 81 (<10 – 299) |
| Cmin (ng/mL) | 65(29 – 178) | 51(<10 – 136) | 58 (<10 – 200) |
| Tmin(hr)** | 24 (0 – 24) | 0(0 – 24) | 0 (0 – 24) |
| Vd/F (L) | 750 (148 – 12035)‡ | 1210 (155 – 30626) | 705 (74 – 23571) |
| CL/F (L/hr) | 13 (5 – 29) | 15 (6 – 45)† | 10 (4 – 133) |
Abbreviations: AUC24, area under the concentration-time curve through 24 hours post-dose; C0, pre-dose concentration; Cmax, maximum concentration; Tmax, time post-dose at which the maximum concentration occurs; C24, 24 hour post-dose concentration; Cmin, minimum observed concentration at any time post-dose; Tmin, time post-dose at which the minimum concentration occurs; Vd/F, apparent volume of distribution; CL/F, oral clearance.
P<0.05 for second trimester versus postpartum with the Wilcoxon signed-rank test.
P<0.05 for third trimester versus postpartum with the Wilcoxon signed-rank test.
P<0.05 for second trimester versus third trimester with the Wilcoxon signed-rank test.
Tmax (hr) and Tmin(hr) are expressed in mode (range)
Figure 1. Rilpivirine Median Plasma Concentrations Over Time.
Second trimester, blue line with diamonds; Third trimester, red line with squares; Postpartum, green line with triangles; Non-pregnant reference population, purple line with x’s. Non-pregnant reference exposure was determined from package insert.[6] Error bars are standard error of the median.
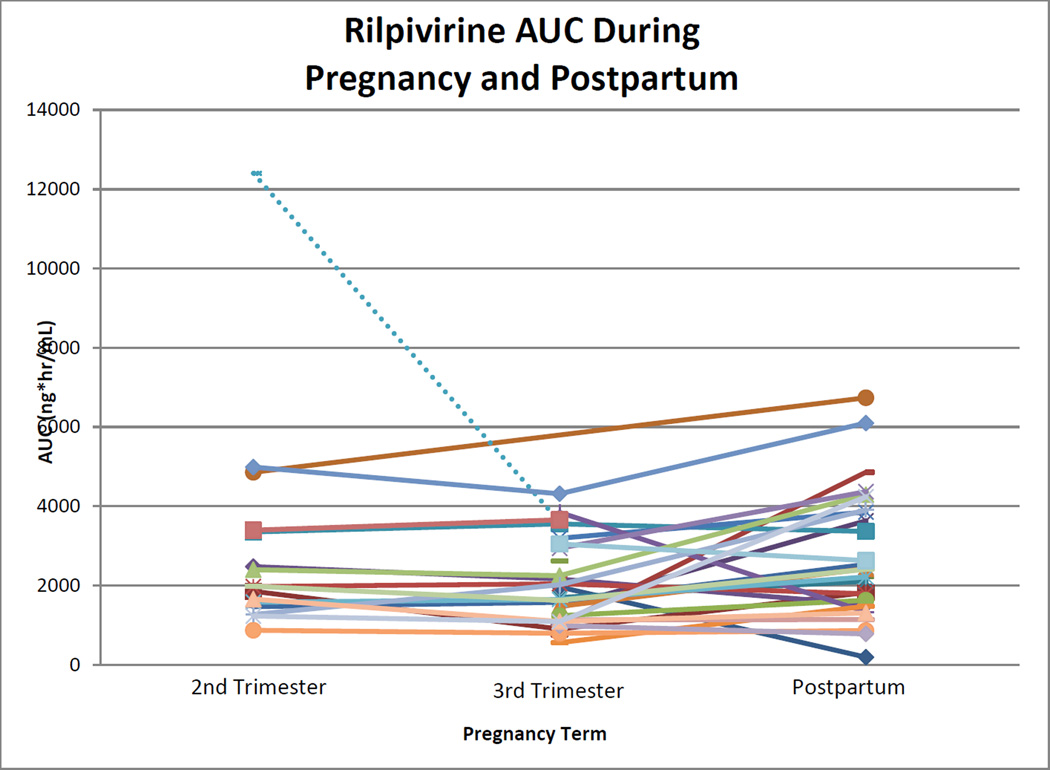
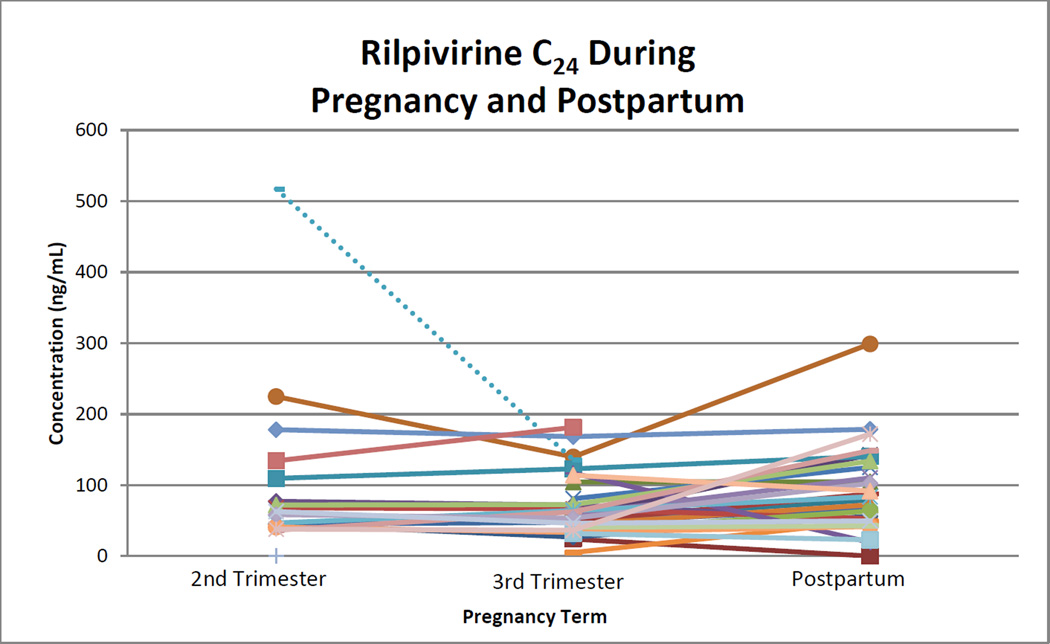
Rilpivirine median (range) AUC0–24 during second trimester, third trimester, and postpartum were 1969 (867 to 4987), 1669 (556 to 4312), and 2387 (188 to 6736) ng*hr/mL. Within-subject comparisons of AUC0–24 showed statistically significant differences between second trimester and postpartum (p=0.048) and third trimester and postpartum (p=0.012; Figure 2). No significant difference was seen between second and third trimester (p=0.272). Rilpivirine AUC0–24 could not be estimated in three women in the second trimester and in two women in the third trimester because several samples from each of their profiles could not be quantitated due to assay technical or interference issues which were subsequently resolved. Rilpivirine was below the AUC0–24 10th percentile target in 1 of 15 (7%) women during the second trimester, 2 of 28 (7%) during the third trimester, and 3 of 28 (11%) postpartum. Second and third trimester C24 were significantly lower when compared to postpartum (p=0.013 and 0.003; Figure 3). One woman in the third trimester and a different woman postpartum had an observed C24 below detection (<10 ng/mL). Two women in the third trimester had C0 below detection. One of these women also had the C24 below detection, after the observed dose, and appeared to have poor absorption, high clearance or a combination. This woman had much higher concentrations at all time points postpartum. The second woman with the C0 below detection had a C24 of 24 ng/mL. The lower C0 may have indicated poor adherence. However, this woman also had concentrations below detection at the postpartum visit at C0 and after the observed dose at C6 through C24. Whether this woman’s low concentrations were due to pharmacokinetics or poor adherence is unclear. Postpartum, one additional woman had a C0 below detection, with a C24 of 72 ng/mL, suggesting poor adherence. Oral clearance was significantly higher in the third trimester compared to postpartum (p=0.04). No other significant differences were seen in pharmacokinetic parameters during pregnancy compared to postpartum. Parameters were significantly increased (C0) or decreased (Vd/F) in the second trimester compared to the third trimester.
Figure 2. Rilpivirine AUC during Pregnancy and Postpartum.
Area under the rilpivirine concentration time curve during the second and third trimesters, and again postpartum. Each line represents a single subject. Dashed line represents subject on concomitant darunavir/ritonavir.
P=0.048 for second trimester versus postpartum with the Wilcoxon signed-rank test.
P=0.012 for third trimester versus postpartum with the Wilcoxon signed-rank test.
P=0.272 for second trimester versus third trimester with the Wilcoxon signed-rank test.
Ten women at 14 study visits had detectable HIV-1 RNA: 2 in the second trimester, 4 in the third trimester, and 6 postpartum. Median (range) minimum plasma concentrations were 30 ng/mL (<10 – 93) at the 14 visits with detectable HIV-1 RNA compared to 63 ng/mL (15 – 199) at the 62 visits with undetectable HIV-1 RNA (p=0.0004).
One subject was on concomitant darunavir/ritonavir, which is expected to increase rilpivirine exposure. This subject’s rilpivirine AUC in the second trimester was approximately six times higher than the median AUC in this study (12401 versus 1969 ng*hr/mL). Her third trimester AUC was double the median value (3602 versus 1669 ng*hr/mL). Her trough concentrations followed a similar pattern. This subject’s data were excluded from statistical analyses, Table 2, Table 3, and Figure 1. This subject did not complete a postpartum visit. No other subjects were taking concomitant antiretrovirals expected to interact with rilpivirine.
Table 3.
Rilpivirine Pharmacokinetic Parameters Geometric Mean (90% CI) Ratios
| Parameters | Second Trimester/ Third Trimester, n = 15 |
Second Trimester/ Postpartum, n = 15 |
Third Trimester/ Postpartum, n = 28 |
|---|---|---|---|
| AUC24 (ng*hr/mL) | 1.09 (0.96 – 1.23) | 0.77 (0.61 – 0.96) | 0.80 (0.62 – 1.03) |
| C0 (ng/mL) | 1.30 (1.06 – 1.60) | 0.91 (0.61 – 1.38) | 0.94 (0.59 – 1.50) |
| Cmax (ng/mL) | 1.13 (0.96 – 1.34) | 0.84 (0.69 – 1.02) | 0.83 (0.68 – 1.01) |
| C24 (ng/mL) | 1.09 (0.95 – 1.26) | 0.70 (0.55 – 0.90) | 0.84 (0.57 – 1.23) |
| Cmin (ng/mL) | 1.22(1.02 – 1.45) | 0.88 (0.63 – 1.21) | 1.11 (0.70 – 1.74) |
| Vd/F (L) | 0.36(0.22 – 0.58) | 1.50 (0.58 – 3.93) | 1.42 (0.68 – 2.97) |
| CL/F (L/hr) | 0.92 (0.81 – 1.05) | 1.33 (1.04 – 1.70) | 1.26 (0.97 – 1.64) |
Abbreviations: AUC24, area under the concentration-time curve through 24 hours post-dose; C0, pre-dose concentration; Cmax, maximum concentration; C24, 24 hour post-dose concentration; Cmin, minimum observed concentration at any time post-dose; Vd/F, apparent volume of distribution; CL/F, oral clearance.
Rilpivirine pharmacokinetic parameters were highly variable between subjects. During second trimester, third trimester, and postpartum, CL/F ranged from 5 to 29, 6 to 45, and 4 to 133 L/hr. AUC0–24 ranged from 867 to 4987, 556 to 4312, and 188 to 6736 ng*hr/mL, respectively. The elimination phase was not clear (the last observed concentration was greater than the prior concentration, and no consistent decline in concentrations was observed) in 4 of 18 (22.2%) women during second trimester, 4 of 30 (13.3%) during third trimester, and 6 of 28 (21.4%) during postpartum, so λz was not estimated for those profiles. Additional time points prior to the 12 and 24 hour post-dose samples were used to estimate λz in 1 of 18 (5.5%) during second trimester, 4 of 30 (13.3%) during third trimester, and 6 of 28 (21.4%) during postpartum.
Maternal plasma and umbilical cord samples were collected at delivery for 9 women. One subject had maternal plasma and umbilical cord sample below the assay detection limit. The median (range) for maternal plasma and umbilical cord blood rilpivirine concentrations were 103.3 (<10 to 273.4) and 53.78 (<10 to 219.7) ng/mL. The median (range) for umbilical cord blood/maternal sample concentration ratios was 0.55 (0.4 to 0.8).
Safety and Infant outcomes
Rilpivirine was well tolerated. Adverse events were reported for six women. Two women had moderate increases in alanine amino transferase and one woman had oligohydramnios that were deemed possibly related to treatment. Other adverse events were not related to study drug. Two women and infants were lost to follow up.
Thirty infants born at a median 39 weeks had a median birth weight of 3095 grams (Table 1). All 25 infants with determinate HIV infection status were uninfected, 3 are pending HIV infection status, and 2 are HIV indeterminate. Congenital anomalies or other findings were reported in five infants, and included Mongolian spot, polydactyly, labial fusion, genitourinary, testicular torsion and bilateral hydrocele. In addition to the congenital anomalies, adverse events were reported for five infants. One infant had high bilirubin on day 1 and 2 of life; one infant had probable sepsis; one infant had low absolute neutrophil count; one infant had low blood glucose; and one infant had vomiting. One infant was born at 32 weeks gestation; the rest of the infants were born between 35 and 41 weeks gestation. The infant born at 32 weeks gestation had the Mongolian spot, but no other reported congenital anomalies or adverse effects and is HIV uninfected.
Discussion
This is the first intensive pharmacokinetic study of rilpivirine during pregnancy and postpartum. In this study, drug exposure from the standard rilpivirine dose of 25mg once daily was significantly reduced by 20% during the third trimester of pregnancy when compared to postpartum. Second trimester AUC was also significantly lower compared to postpartum, by 23%, while no significant difference was found between second and third trimester AUCs. Similarly, a case study of two women taking rilpivirine during pregnancy also showed a 30% and a 43% reduction in exposure during pregnancy compared to postpartum.[8]
The median EC50 and EC90 for rilpivirine are 0.27 ng/mL and 0.66 ng/mL.[6] All but three of the observed Cmin in this study were higher than the EC50 and the EC90. Even when Cmin were compared to rilpivirine protein binding-adjusted EC50 and EC90 (5.0 ng/mL and 12.2 ng/mL, respectively), all but three Cmin were still above the protein binding-adjusted EC50 and all but four were above the EC90.[27, 28] This suggests that the rilpivirine dose may not need to be increased routinely in all women during pregnancy, even though drug exposure was significantly reduced during third trimester when compared to postpartum.
Rilpivirine is a substrate of cytochrome P450 3A4 (CYP 3A4).[9] HIV protease inhibitors, including atazanavir, darunavir, fosamprenavir, indinavir, nelfinavir, lopinavir, and saquinavir, are also substrates of CYP 3A4, and have consistently shown significant decreases in AUC during pregnancy compared to postpartum.[10–16] During pregnancy, induction of this pathway most likely contributes to the decreases seen in CYP 3A4 substrate drug exposure, including rilpivirine.[17]
Information about pharmacokinetics during pregnancy are available for other NNRTIs, including nevirapine, efavirenz and etravirine. In an intensive pharmacokinetic study of nevirapine in pregnant Ugandan women, nevirapine AUC, Cmin, Cmax were reduced by 20% during third trimester compared to postpartum.[18] On the contrary, efavirenz exposure during third trimester was similar to postpartum, although a slightly lower trough concentration was observed.[19] In other studies, efavirenz clearance was increased during pregnancy, risk of low trough concentration increased especially in women who were extensive CYP2B6 metabolizers, and exposure decreased.[25,26] These differences may be explained by host genetic polymorphisms in CYP2B6. Efavirenz AUC, Cmax and Cmin decreased in women with CYP2B6 516GG genotype, AUC and Cmax decreased in women with CYP2B6 516TT genotype, and no apparent changes were seen in women with CYP2B6 516GG genotype during pregnancy compared to postpartum. [26 Surprisingly, etravirine exposure is increased during pregnancy compared to postpartum, but metabolism of etravirine is more complex and involves multiple CYP pathways.[20,21] Etravirine is primarily metabolized by CYP2C19 and during pregnancy CYP2C19 expression and activity are decreased. [27] A decrease in enzyme activity would result in an increased concentration.
In this study, many of the pharmacokinetic parameters were not significantly different between pregnancy and postpartum, including Cmax, Tmax, C0, Cmin,, and Vd/F. Almost half the women demonstrated absorption lags. These were commonly seen at each sampling time point (50%, 43.3%, and 39.3% of women at second trimester, third trimester and postpartum, respectively), which contributes to the variability of this drug’s absorption. C24 was significantly lower during pregnancy compared to postpartum (30% lower in the second trimester and 16% lower in the third trimester vs. postpartum). This finding is consistent with the increased CYP 3A4 activity during pregnancy, which results in lower trough concentration with the same drug dose.
While variability in rilpivirine pharmacokinetic parameters has been evident in studies in non-pregnant adults, we found higher between-subject variability in the pregnant women in this study. In a phase 3 clinical trial of rilpivirine in non-pregnant HIV-infected individuals, the coefficient of variation (CV) for AUC was 38%.[6] More recently, in a study of pharmacokinetic parameters of rilpivirine in healthy subjects, the CV reported was 29% for AUC, 28% for Cmax, and 31% for Cmin.[22] Among the pregnant subjects in this study, CV during 2nd trimester, 3rd trimester, and postpartum for AUC was 186%, 194%, 60%, for Cmax was 53%, 212%, 58%, and for Cmin was 55%, 154%, 71%, respectively. Previously reported studies were clinical trials with tightly controlled enrollment of patients, which represented a more homogenous population than this study population. In addition, prior studies consisted of healthy subjects or HIV-infected non-pregnant subjects, while this patient population consisted of HIV-infected women during pregnancy and postpartum. In non-pregnant subjects, coadministering rilpivirine with food significantly increases systemic exposure compared to fasting; relative bioavailability is increased with food (absolute bioavailability is unknown). Typical explanations for increased bioavailability when medications are given with food include improved drug solubility due to any of several factors – more time spent in the stomach, mechanical mixing in stomach, bile acids help solubilize the drug – and more contact time at the major site of absorption in upper intestines because of more gradual release of drug from stomach. Any factors that alter these processes can alter not just the rate of drug absorption but also the extent. Even though rilpivirine was taken with a standardized meal in this study, the extent to which food delays gastric emptying between individuals is extremely variable. Furthermore, during pregnancy, physiological changes occur that alter gastric emptying and gastric motility.[23] Changes in gastric motility result in increased variability in absorption time, which could contribute to the within- and between-subject variability seen in the pharmacokinetic parameters in this study. The relative influence of genetics on the pharmacokinetics of some antiretroviral drugs was studied in non-pregnant adults. The study found greater genetic contributions to pharmacokinetic variability in NNRTIs and nucleoside reverse-transcriptase inhibitors than in protease inhibitors and integrase inhibitors, relative to each other.[30] Although rilpivirine was not included in the study, it is an NNRTI and the between-subject variability seen in the pharmacokinetic parameters could be partially explained by each subject’s individual genetics.
In a previous case study, rilpivirine used during pregnancy in two different women resulted in no transmission of HIV from mother-to-child.[8] Similarly, in this study, all infants for which data are available are HIV uninfected, suggesting that rilpivirine exposure along with other antiretrovirals was adequate to suppress the HIV-1 RNA in the mother and to prevent HIV transmission. In this study, the median ratio of cord blood to maternal plasma rilpivirine concentrations was 0.55. In the reported case study, rilpivirine crossed the placenta with a cord blood to maternal plasma ratio in one woman of 0.74.[8] Thus, rilpivirine seems to moderately crosses the placenta, and rilpivirine cord blood concentrations were below maternal concentrations. Rilpivirine umbilical cord blood concentrations were lower than maternal plasma concentrations, but the median umbilical cord blood concentration was still above protein binding-adjusted EC90, which could contribute to the prevention of perinatal HIV transmission.
One of the limitations of this study is that it evaluated pregnant women receiving rilpivirine for clinical care enrolled at various stages of pregnancy and with different histories of antiretroviral therapy use, which resulted in a heterogeneous population. Another limitation is that only total plasma rilpivirine concentrations were measured. Rilpivirine is highly bound to protein (99.7%).[6] During pregnancy, protein binding can decrease which increases the free fraction of drug. The active concentration of drug may not change in all stages of pregnancy to the same extent as the total concentrations.
This study evaluated steady-state 24-hour pharmacokinetic profiles of rilpivirine in 32 pregnant women. A standard dose of 25 mg of rilpivirine once daily showed lower overall exposure and trough concentrations during pregnancy with high between-subject variability in both the second and third trimesters. The median protein-binding adjusted EC90 for rilpivirine is 12.2 ng/mL.[6] All but four of the minimum observed concentrations in this study were higher than this EC90, suggesting that rilpivirine doses do not need to be routinely increased during pregnancy in all women. However, the high between-subject variability observed in rilpivirine exposure during pregnancy highlights the need for close monitoring for each individual patient.
Figure 3. Rilpivirine C24 during Pregnancy and Postpartum.
24 hour post-dose rilpivirine concentration during the second and third trimesters, and again postpartum. Each line represents a single subject. Dashed line represents subject on concomitant darunavir/ritonavir.
P=0.013 for second trimester versus postpartum with the Wilcoxon signed-rank test.
P=0.003 for third trimester versus postpartum with the Wilcoxon signed-rank test.
P=0.309 for second trimester versus third trimester with the Wilcoxon signed-rank test.
Acknowledgments
We thank the women enrolled in this study and the investigators at the following IMPAACT Sites: 3801 Texas Children’s Hospital (Shelley Buschur, RN, CNM; Chivon Jackson, RN, BSN, ADN; Mary Paul, MD); 4001 Lurie Children’s Hospital of Chicago (Donna McGregor APN; Ram Yogev MD; Rohit Kalra CRC); 4201 University of Miami Pediatric Perinatal HIV/AIDS (Claudia Florez, MD; Patricia Bryan, BSN, MPH; Monica Stone, MD); 4601 University of California San Diego Mother-Child-Adolescent Program (Andrew D. Hull, MD FRCOG FACOG; Mary Caffery, RN, MSN; Stephen A. Spector, MD); 4701 Duke University Medical Center (Joan Wilson, RN, BSN, MPH; Julieta Giner, RN, ACRN; Margaret A. Donnelly, PA-C); 5011 Boston Medical Center Pediatric HIV Program (Ellen R. Cooper MD; Debra A. McLaud RN; Lisa F. Tucker, BFA); 5017 Seattle Children’s Hospital (Jane Hitti, MD, MPH; Amanda Robson-Nuss, BS; Ann J. Melvin, MD, MPH); 5045 Harbor UCLA Medical Center (Margaret A. Keller, MD, Michael A. Bolaris, MD, Judy Hayes, RN); 5048 University of Southern California School of Medicine– Los Angeles County (Françoise Kamer, MD; LaShonda Spencer, MD; James Homans, MD); 5052 University of Colorado Denver (Torri Metz, MD; Jenna Wallace, MSW; Alisa Katai, MHA); 5083 Rush University Cook County Hospital Chicago (Mariam Aziz, MD; Maureen McNichols RN MSN CRC; Julie Schmidt MD);5091 University of California San Francisco (Diane Wara, MD; Kristinalisa Maka, RN; Deborah Cohan, MD); 5093 Miller Children’s Hospital (Audra Deveikis MD; Jagmohan Batra MD; Janielle Jackson Alvarez RN); 5112 David Geffen School of Medicine at UCLA (Michele Carter, RN; Jaime Deville, MD; Carla Janzen, MD); 5114 Bronx-Lebanon Hospital Center (Jenny Gutierrez, MD; Martha Cavallo, NP; Murli Purswani, MD); 6501 St Jude Children’s Research Hospital (Katherine M. Knapp, MD; Nina Sublette, FNP, PhD; Thomas Wride, MS).
Source Funding: M.M. is a member of a DSMB for studies funded by ViiV and Merck and is a member of an Advisory Board for Merck. Overall support for the International Maternal Pediatric Adolescent AIDS Clinical Trials Group (IMPAACT) was provided by the National Institute of Allergy and Infectious Diseases (NIAID) of the National Institutes of Health (NIH) under Award Numbers UM1AI068632 (IMPAACT LOC), UM1AI068616 (IMPAACT SDMC) and UM1AI106716 (IMPAACT LC), with co-funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) and the National Institute of Mental Health (NIMH). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Conflicts of Interest: All other co-authors have no conflicts (A.H.T., B.M.B., A.S., J.W., E.V.C., S.K.B., R.K., K.R., K.G., T.R.C., N.C., E.S., D.E.S.).
These data were presented in part at the Conference on Retroviruses and Opportunistic Infections (CROI 2015); Seattle, WA, USA; Feb 23 – 26, 2015.
Contributor Information
Anna Hoang Tran, Skaggs School of Pharmacy and Pharmaceutical Sciences, University of California, San Diego, 92093, USA.
Brookie M. Best, Skaggs School of Pharmacy and Pharmaceutical Sciences, Pediatrics Department-Rady Children’s Hospital, University of California, San Diego, 92093, USA.
Alice Stek, University of Southern California, Los Angeles, 90089, USA.
Jiajia Wang, Harvard School of Public Health, Boston, 02115, USA.
Edmund V. Capparelli, Skaggs School of Pharmacy and Pharmaceutical Sciences, Pediatrics Department-Rady Children’s Hospital, University of California, San Diego, 92093, USA.
Sandra K. Burchett, Department of Medicine, Children’s Hospital Boston, Boston, 02115, USA.
Regis Kreitchmann, Irmandade da Santa Casa de Misericordia de Porto Alegre, HIV/AIDS Research Department, Porto Alegre, 90020090, Brazil.
Kittipong Rungruengthanakit, Research Institute for Health Sciences, Chiang Mai University, Chiang Mai, 50202, Thailand.
Kathleen George, FHI 360, IMPAACT Operations Office, Durham, 22701, USA.
Tim R. Cressey, Program for HIV Prevention and Treatment, Department of Medical Technology, Faculty of Associated Medical Sciences, Chiang Mai University, Chiang Mai, 50200, Thailand.
Nahida Chakhtoura, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institute of Health, DHHS, Bethesda, 20852, USA.
Elizabeth Smith, National Institute of Allergy and Infectious Diseases, Bethesda, 20852, USA.
David E. Shapiro, Harvard School of Public Health, Boston, 02115, USA.
Mark Mirochnick, Boston University School of Medicine, Boston, 02118, USA.
References
- 1. [Accessed January 5, 2016];Global Update on the Health Sector Response to HIV. 2014 Available at http://apps.who.int/iris/bitstream/10665/128494/1/9789241507585_eng.pdf?ua=1. [Google Scholar]
- 2.Panel on Treatment of HIV-Infected Pregnant Women and Prevention of Perinatal Transmission. [Accessed April 3, 2014];Recommendations for Use of Antiretroviral Drugs in Pregnant HIV-1-Infected Women for Maternal Health and Interventions to Reduce Perinatal HIV Transmission in the United States. Available at http://aidsinfo.nih.gov/contentfiles/lvguidelines/PerinatalGL.pdf.
- 3.Panel on Antiretroviral Guidelines for Adults and Adolescents. [Accessed April 9, 2014];Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services. Available at http://aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf.
- 4.Colbers A, Greupink R, Burger D. Pharmacological considerations on the use of antiretrovirals in pregnancy. Curr Opin Infect Dis. 2013;26:575–588. doi: 10.1097/QCO.0000000000000017. [DOI] [PubMed] [Google Scholar]
- 5.Helldén A, Madadi P. Pregnancy and pharmacogenomics in the context of drug metabolism and response. Pharmacogenomics. 2013;14:1779–1791. doi: 10.2217/pgs.13.176. [DOI] [PubMed] [Google Scholar]
- 6.EDURANT package insert. Janssen Products, LP; Available at http://www.edurant.com/sites/default/files/EDURANT-PI.pdf. [Google Scholar]
- 7. [Accessed May 5, 2010];The Division of AIDS (DAIDS) Standardized Toxicity Table for Grading Severity of Adult Adverse Experiences. 1992 Aug; Available at http://rsc.techres.com/safetyandpharmacovigilance. [Google Scholar]
- 8.Colbers A, Gingelmaier A, van der Ende M, et al. Pharmacokinetics, safety and transplacental passage of rilpivirine in pregnancy: two cases. AIDS. 2014;20:288–290. doi: 10.1097/QAD.0000000000000100. [DOI] [PubMed] [Google Scholar]
- 9.Ripamonti D, Bombana E, Rizzi M. Rilpivirine: drug profile of a second-generation non-nucleoside reverse transcriptase HIV-inhibitor. Expert Review of Anti-infective Therapy. 2014;12:13–29. doi: 10.1586/14787210.2014.863708. [DOI] [PubMed] [Google Scholar]
- 10.Mirochnick M, Best BM, Stek AM, et al. Atazanavir pharmacokinetics with and without tenofovir during pregnancy. J Acquir Immune Defic Syndr. 2011;56:412–419. doi: 10.1097/QAI.0b013e31820fd093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stek A, Best BM, Wang J, et al. Pharmacokinetics of Once versus Twice Daily Darunavir In Pregnant HIV-Infected Women. J Acquir Immune Defic Syndr. 2015 May 6; doi: 10.1097/QAI.0000000000000668. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cespedes MS, Castor D, Ford SL, et al. Steady-state pharmacokinetics, cord blood concentrations, and safety of ritonavir-boosted fosamprenavir in pregnancy. J Acquir Immune Defic Syndr. 2013;62:550–554. doi: 10.1097/QAI.0b013e318285d918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cressey TR, Best BM, Achalapong J, et al. Reduced indinavir exposure during pregnancy. Br J Clin Pharmacol. 2013;76:475–483. doi: 10.1111/bcp.12078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stek AM, Mirochnick M, Capparelli E, et al. Reduced lopinavir exposure during pregnancy. AIDS. 2006;20:1931–1939. doi: 10.1097/01.aids.0000247114.43714.90. [DOI] [PubMed] [Google Scholar]
- 15.Fang A, Valluri SR, O'Sullivan MJ, et al. Safety and pharmacokinetics of nelfinavir during the second and third trimesters of pregnancy and postpartum. HIV Clin Trials. 2012;13:46–59. doi: 10.1310/hct1301-046. [DOI] [PubMed] [Google Scholar]
- 16.Martinez-Rebollar M, Lonca M, Perez I, et al. Pharmacokinetic study of saquinavir 500 mg plus ritonavir (1000/100 mg twice a day) in HIV-positive pregnant women. Ther Drug Monit. 2011;33:772–777. doi: 10.1097/FTD.0b013e318236376d. [DOI] [PubMed] [Google Scholar]
- 17.Tracy TS, Venkataramanan R, Glover DD, et al. Temporal changes in drug metabolism (CYP1A2, CYP2D6, and CYP3A activity) during pregnancy. Am J Ob Gyn. 2005;192:633–639. doi: 10.1016/j.ajog.2004.08.030. [DOI] [PubMed] [Google Scholar]
- 18.Lamorde M, Byakika-Kibwika P, Okaba-Kayom V, et al. Suboptimal nevirapine steady-state pharmacokinetics during intrapartum compared with postpartum in HIV-1-seropositive Ugandan women. J Acquir Immune Defic Syndr. 2010;55:345–350. doi: 10.1097/QAI.0b013e3181e9871b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cressey TR, Stek A, Capparelli E, et al. Efavirenz pharmacokinetics during the third trimester of pregnancy and postpartum. J Acquir Immune Defic Syndr. 2013;63:59–66. doi: 10.1097/QAI.0b013e31823ff052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ramgopal M, Osiyemi O, Zorrilla C, et al. Pharmacokinetics of etravirine (ETV) in HIV-1 infected pregnant women [abstract 893]. Program and abstracts for CROI 2015, Conference on Retroviruses and Opportunistic Infections; 23–26 Feb 2015; Seattle WA. [Google Scholar]
- 21.Best BM, Colbers A, Wang J, et al. Etravirine pharmacokinetics during pregnancy and postpartum [abstract 892]. Program and abstracts for CROI 2015; Conference on Retroviruses and Opportunistic Infections Annual Meeting; 2015 Feb 23 – 26; Seattle, WA. [Google Scholar]
- 22.Crauwels H, Vingerhoets J, Ryan R, et al. Pharmacokinetic parameters of once-daily rilpivirine following administration of efavirenz in healthy subjects. Antivir Ther. 2012;17:439–446. doi: 10.3851/IMP1959. [DOI] [PubMed] [Google Scholar]
- 23.Mirochnick M, Capparelli E. Pharmacokinetics of antiretrovirals in pregnant women. Clin Pharmacokinet. 2004;43:1071–1087. doi: 10.2165/00003088-200443150-00002. [DOI] [PubMed] [Google Scholar]
- 24.Tracy TS, Venkataramanan R, Glover DD, Caritis SN. Temporal changes in drug metabolism (CYP1A2, CYP2D6, and CYP3A activity) during pregnancy. Am J Ob Gyn. 2005;192:633–639. doi: 10.1016/j.ajog.2004.08.030. [DOI] [PubMed] [Google Scholar]
- 25.Dooley KE, Denti P, Martison N, et al. Pharmacokinetics of efavirenz and treatment of HIV-1 among pregnant women with and without tuberculosis coinfection. J Infect Dis. 2015 Jan 15;211(2):197–205. doi: 10.1093/infdis/jiu429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Olagunju A, Bolaji O, Amara A, et al. Pharmacogenetics of pregnancy-induced changes in efavirenz pharmacokinetics. Clin Pharmacol Ther. 2015 Mar;97(3):298–306. doi: 10.1002/cpt.43. [DOI] [PubMed] [Google Scholar]
- 27.Olagunju A, Owen A, Cressey TR, et al. Potential effect of pharmacogenetics on maternal, fetal and infant antiretroviral drug exposure during pregnancy and breastfeeding. Pharmacogenomics. 2012 Oct;13(13):1501–1522. doi: 10.2217/pgs.12.138. [DOI] [PubMed] [Google Scholar]
- 28. [Assessed January 7, 2016];Edurant (Rilpivirine) CHMP Assessment Report. 2011 Available at http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Public_assessment_report/human/002264/WC500118872.pdf. [Google Scholar]
- 29.Azijn H, Tirry I, Vingerhoets J, et al. TMC278, a Next-Generation Nonnucleoside Reverse Transcriptase Inhibitor (NNRTI), Active against Wild-Type and NNRTI-Resistant HIV-1. Antimicrob Agents Chemother. 2010;54:718–727. doi: 10.1128/AAC.00986-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Siccardi M, Olagunju A, Simiele M, et al. Class-specific relative genetic contribution for key antiretroviral drugs. J Antimicrob Chemother. 2015 Nov;70(11):3074–3079. doi: 10.1093/jac/dkv207. [DOI] [PubMed] [Google Scholar]