Abstract
The emergence and success of stereotactic body radiation therapy (SBRT) in lung cancer has led to its rapid adoption for liver cancers. SBRT can achieve excellent results for small liver tumors. However, the vast majority of physicians interpret SBRT as meaning doses of radiation (4 to 20 Gy) that may not be ablative, but are delivered within about 1 week (i.e., in 3 to 6 fractions). Adherence to this approach has limited the effectiveness of SBRT for large liver tumors (> 7 cm) owing to the need to reduce doses to meet organ constraints. The prognosis for patients who present with large liver tumors is poor with median survival times of 12 months or less, and most such patients die from tumor-related liver failure. Herein, we present a comprehensive solution to achieve stereotactic ablative body radiation (SABR) doses for patients with large liver tumors by using a combination of classical, modern, and novel concepts of radiotherapy: fractionation, dose painting, motion management, image guidance, and simultaneous integrated protection. We discuss these concepts in the context of large inoperable liver tumors and review how this approach can substantially prolong survival for patients, most of whom otherwise have a very poor prognosis and few effective treatment options.
Keywords: stereotactic ablative radiation therapy, hepatoma, intrahepatic cholangiocarcinoma, IGRT, gating
Background
As recently as the 1980’s, radiation therapy to the liver was thought to be unsafe, based on the inability of the whole liver to tolerate high radiation doses. Studies of whole abdominal irradiation for endometrial cancer, and whole liver irradiation for pancreatic cancer demonstrated that doses exceeding 30Gy at 2 to 3 Gy per fraction could lead to liver failure.1 Investigators from the University of Michigan subsequently pioneered the concept that partial liver volumes could tolerate very high doses, demonstrated evidence for a dose response relationship, and created objective parameters to evaluate dose and volume relationships2. A series of prospective trials helped to establish the role of radiotherapy as a treatment option for primary and metastatic liver tumors.3
Over the past decade, another paradigm shift has revolutionized radiotherapy and affected the approach to patients with liver tumors. Studies of lung4, 5 and liver6 cancer have shown that stereotactic body radiation therapy (SBRT) can effectively ablate small tumors. (Ablative SBRT has been called stereotactic ablative radiation therapy [SABR]. In contrast, the term SBRT is a term that is used when the treatment is not necessarily ablative. Here we define ablative doses as those that produce local tumor control rates of approximately 90% at 2 years.) SABR has been a major step forward in the treatment of tumors located within organs with parallel functional subunits such as the liver and lung. However, SABR in 3 to 6 fractions is challenging or impossible when the tumors are near critical organs at risk (OARs) whose functional subunits are arranged in series, such as the spinal cord and the gastrointestinal (GI) tract. In these cases, the total dose often must be reduced to meet normal tissue constraints. This strategy may be appropriate if the goal is palliation, but not if ablation of the tumor could lead to improved survival with a chance of cure. Doses of SBRT given in 3 to 6 fractions for liver tumors are often reduced by 20% to 50% from an ablative threshold to meet liver constraints, a practice that abandons well-established principles of fractionation and normal tissue repair and produces results similar to those achieved from standard fractionation using moderate doses (50–60Gy). The advantages are convenience and a lower risk of acute toxicity, albeit at a higher financial cost to the healthcare system.7 SBRT for pancreatic cancer is a prime example of low-dose SBRT that has produced median survival rates similar to or worse than fractionated regimens delivered with 3D conformal planning with less toxicity.8, 9
Delivering SBRT safely to large liver tumors (>7 cm) has been challenging. For example, in a report of sequential phase I and II trials of SBRT for 102 patients with hepatocellular carcinoma (HCC) who were not eligible for other locoregional therapies (median tumor size 7 cm), the locoregional control rate at 1 year was good (87%), but the rate of grade ≥3 was high (30%) and 7 patients may have died of treatment- related causes10. These results emphasize that the key to successfully controlling large liver tumors is achieving an ablative dose while staying within tolerance of the organs at risk (OARs). Use of an SBRT technique with control of organ motion and high quality image guidance is an essential starting point. However, even with these technologies, delivery of ablative doses without overdosing the liver, GI mucosa, or main bile ducts remains a challenge in many cases.
Our strategy to address this challenge involves (1) hyopfractionation that achieves biologically equivalent doses (BEDs) of approximately 100Gy in 15 or 25 fractions, (2) reducing the margin of the clinical target volume (CTV) or planning target volume (PTV), if needed, to stay within liver tolerance (3) creating a 5–10 mm area near adjacent OARs at the tolerance dose while ablating the rest of the tumor at BEDs that are twice as high, and (4) treating the hypoxic center of the tumor to extremely high doses (BED >140Gy) when possible. We call this approach simultaneous integrated boost (SIB) with simultaneous integrated protection (SIP).
In this review, we outline a pragmatic and rational approach to the definitive management of liver tumors. We present an alternative to the current common practice of using a short course of low-dose SBRT for large liver tumors and tumors near the bowel. We describe results of fractionated SABR consisting of a novel heterogeneous dosing paradigm with an SBRT technique that couples meticulous control of internal organ motion with diagnostic-quality CT image guidance to spare nearby OARs. We review the rationale, challenges, and solutions that allow the safe delivery of curative doses of radiotherapy for tumors that direct abut the GI tract or main bile ducts, or lesions where normal liver dose is a limiting factor.
WHY DO PATIENTS DIE FROM LARGE LIVER TUMORS?
The rationale for taking an aggressive approach to treating large liver tumors is that patients often die from liver failure related to disease progression regardless of the presence of extrahepatic disease. The clinical benefit of any local treatment option depends on the effectiveness of the modality and the a priori probability that local progression will lead to mortality. If a primary or metastatic tumor is likely to cause rapid mortality, an effective treatment will have a major survival benefit.
Understanding why patients die of disease is a crucial step in the development of an effective treatment strategy. Little has been reported on what causes tumor-related death in patients with inoperable liver tumors; most oncologists probably assume that distant metastatic disease is the culprit in most cases. The reality is quite different for tumors that are near the confluence of the hepatic veins and inferior vena cava or the hilum. Patients with such tumors die of tumor-related liver failure. For patients with HCC, underlying liver disease often leads to liver failure and death. For those with well-compensated liver function, intrahepatic cholangiocarcinoma, or liver-dominant metastases, death may result from inadequate control of intrahepatic disease leading to functional liver parenchymal loss and liver failure from biliary obstruction, portal venous obstruction, or obstruction of all 3 hepatic veins. The latter event causes Budd-Chiari syndrome, with acute liver congestion and ischemia leading to the rapid development of liver failure. Other causes of life-threatening complications of uncontrolled liver disease are discussed in more detail in subsequent sections.
We demonstrated that large liver tumors directly cause liver failure in a series of patients treated for with radiation for intrahepatic cholangiocarcinoma with radiation at The University of Texas MD Anderson Cancer Center from 2002 through 2014, an interval during which BEDs were increased from 60Gy to BEDs to up to 100 Gy. Death correlated strongly with the delivery of subablative doses and resulted from tumor-related liver failure in the 89% of patients whose cause of death could be determined. Half of those deaths were from tumor related biliary obstruction and the other half from vascular compromise or a combination11. Thus, using ablative doses to control large liver tumors confers a major survival benefit by preventing tumor-related liver failure.
WHAT ARE THE HEPATIC-DIRECTED TREATMENT OPTIONS?
Current practice includes many options for liver directed-therapy. The gold standard for tumors isolated to the liver is surgical resection. For inoperable patients, percutaneous image-guided ablative options are preferred, especially for peripheral tumors. Radiofrequency and microwave ablation are convenient and cost effective for appropriately selected patients (those with tumors <5 cm that are not near a segmental bile duct, liver surface, or major vessel; attempted ablation of larger tumors results in a higher marginal miss rate, the vessels act as a heat sink, and ablation of a branch of the biliary tree can result in a bile leak.) Additional options include arterial embolization with yttrium-90 and transarterial chemoembolization. Radiation therapy is a complementary option for patients with liver tumors and is a preferred option for tumors near the hilum of the liver, main portal vein, or inferior vena cava. Tumors in these locations are often not ideal for resection or percutaneous ablation but are at a high risk of causing liver failure related to vascular or biliary compromise. Ablation of these tumors with radiation can extend the median survival time by years and provide a chance for cure. Use of low-dose SBRT may have a more modest median survival benefit, but it represents a potential missed opportunity to substantially prolong survival. We explore in the following sections common pitfalls that undermine successful ablative radiation approaches in tumors near major bile ducts, the GI tract, and in patients with limited liver reserve and we describe principles and techniques that have led to greater success in liver tumors.
WHAT ARE THE CHALLENGES TO IRRADIATING LARGE LIVER TUMORS?
Baseline Liver Reserve
The risk of radiation-induced liver disease is related mostly to both the dose and volume of “normal” liver that receives radiation.12, 13 The dose-volume relationship is altered when the “normal” liver has limited reserve.14–17 Limited liver reserve is commonly seen in patients with large hepatobiliary tumors and represents a significant challenge to safe and effective radiation treatment.
Common reasons for limited liver reserve include dysfunction due to cirrhosis, limited normal-liver volume due to previous resection or hepatotoxic chemotherapy, and tumor-related dysfunction due to biliary or vascular compromise. We will discuss each of these topics in the following sections.
Cirrhosis
Chronic damage to the liver parenchyma leads to progressive fibrosis and hepatic dysfunction. Etiologies of cirrhosis include alcoholic liver disease, chronic hepatitis B or C viral infection, nonalcoholic steatotic hepatitis, primary biliary cirrhosis, primary sclerosing cholangitis, autoimmune hepatitis, hereditary hemochromatosis, Wilson disease, and alpha-1 antitrypsin deficiency, among others.
Notably, the incidence of each etiology of cirrhosis is changing. Historically, the most common causes of cirrhosis in the United States were alcohol and hepatitis C. However, the number of adults with nonalcoholic steatotic hepatitis awaiting transplant has almost tripled in the past decade, and is predicted to become the leading indication for liver transplantation in the United States.18 Additional details on etiologies and medical management of cirrhosis are provided elsewhere.19
Several reports have noted that patients with advanced cirrhosis are at a higher risk of radiation-induced liver disease.15–17 Indeed, the tolerance dose of the liver has been shown to be different for patients with primary versus metastatic liver cancers.2 The reasons why cirrhotic livers are more sensitive to irradiation are poorly understood. A typical pathological finding in radiation-induced liver disease is veno-occlusive disease without inflammation.13, 20 A better understanding of the mechanisms of radiation-induced liver disease in patients with cirrhosis is needed.
Limited hepatic reserve due to prior treatment
Many patients with primary hepatobiliary tumors or liver metastases have received chemotherapy or liver-directed therapy before being evaluated for radiation. Such treatments reduce hepatic reserve through various mechanisms; oxaliplatin, for example, has been associated with perisinusoidal and veno-occlusive fibrosis, sinusoidal dilatation, and hemorrhage,21 and irinotecan with steatohepatitis22. These toxic effects of chemotherapy, particularly steatohepatitis, have been linked with increased 90-day mortality rates after hepatic surgery.22
The use of radiation after hepatotoxic chemotherapy is becoming more common, especially for colorectal liver metastases, for which oxaliplatin and irinotecan are the backbones of systemic therapy. Data are limited regarding the use of radiotherapy in such cases, warranting prospective evaluation to determine whether current dose constraints are appropriate for patients with steatosis or steatohepatitis from chemotherapy agents.
Surgery also reduces hepatic reserve through the removal of functional healthy liver. After limited resections, the liver’s regenerative capacity can mitigate this problem. However, large resections can substantially limit hepatic reserve. For example, 20%–25% of patients with bilobar liver metastases with planned two-stage hepatectomy cannot undergo the second stage owing to inadequate liver hypertrophy after portal vein embolization and a predicted inadequate liver remnant.23, 24 In such situations, the options for liver-directed treatment are severely limited because of the compromised liver reserve. The role of radiotherapy for patients with small liver remnants (<1000cm3) remains to be defined, and again prospective evaluation is warranted. We are currently conducting a clinical trial to evaluate the safety of SABR when liver volumes < 700cm3 will be treated below tolerance.
Tumor-related liver dysfunction
For many patients with HCC and intrahepatic cholangiocarcinoma, hepatic reserve is directly impaired by the tumor at diagnosis. Hilar or extrahepatic cholangiocarcinoma often presents as painless jaundice due to biliary obstruction, as can centrally located HCC. Rapid growth of peripherally located tumors can also lead to obstruction of the primary and secondary biliary tree. Biliary-tree obstruction can lead to cholangitis, abscess, and liver parenchymal loss, which can be life-threatening.
We use ursodiol for partial biliary obstruction and reserve stent placement for complete obstruction. Medical management of cirrhosis or other liver disease is always optimized before radiation therapy is begun.
Another mechanism of hepatobiliary cancer-related liver dysfunction is vascular compromise. The central vessels of the liver (inferior vena cava, superior vena cava, portal vein, and hepatic artery) supply and transport most of the blood of the liver parenchyma. Direct invasion of the veins is common in HCC and uncommon in cholangiocarcinoma. Tumor thrombi in the portal vein and inferior vena cava can propagate within these venous systems, leading to elevated portal pressures and ascites, which can put the patient at risk for peritonitis. Vascular compromise of the liver can also lead to infarction or atrophy of the liver parenchyma as well as causing thrombotic events in distant organs.
Portal vein tumor thrombosis25 represents a significant management challenge; the median survival time for patients with this condition is less than one year. Radiation has been investigated prospectively in combination with transarterial chemoembolization26 and as a single modality therapy27, 28 for portal vein tumor thrombosis. However, use of radiation can be complicated because of the degree of baseline liver dysfunction and the volume of irradiation that is often needed to cover thrombi involving the large central vessels of the liver. Moreover, ascites due to vascular compromise can affect the location of the radiation target, requiring advanced image guidance and the need for adaptive planning during treatment. These techniques are further discussed in subsequent sections.
Organ Motion
Controlling internal organ motion during radiotherapy is another challenge. The liver moves with the diaphragm during respiration; the amplitude of motion can vary significantly from patient to patient and depends on the location of the tumor within the liver or biliary tree. The range of motion generally is greatest in the cranio-caudal direction, with amplitude exceeding 2 cm in some patients [19]. Further, although breathing amplitudes can be different during four-dimensional computed tomography (4D CT) –based treatment planning versus during radiation delivery, but the direction of variability seems to be predictable [20]. Different parts of the liver move differently with respiration. In general, the closer a tumor is to the central diaphragm, the greater the motion. The liver also deforms throughout the respiratory cycle, especially in elderly patients with diminished abdominal wall muscle tone. Chest versus abdominal breathing also affects liver shape, and must remain consistent from the simulation to the treatment delivery.
Day-to-day differences in bowel position and shape are other uncertainties that must be accounted for and monitored to ensure safe treatment. The extent to which the luminal GI organs affect accurate radiation treatment delivery has not been well described and may not be predictable. Filling of the stomach can vary substantially from day to day, depending on how much air, liquid, and solid a patient ingests. The left lobe of the liver is susceptible to deformation caused by stomach filing, whereas the right lobe is less affected by surrounding organs. Generally, we instruct our patients to ingest nothing for at least 3 hours before radiation sessions in an attempt to reduce the variability of stomach filling and enhance the tendency of the stomach to pull away from the left lobe of the liver. Gallbladder filling and motility during radiation has not been thoroughly described, but this too is known to be variable outside the context of radiation therapy.29 Typically, a full gallbladder displaces the colon and duodenum. The amount of solid, liquid and gas in the ascending, transverse, and descending colon can vary from day to day. This variability should be monitored and assessed for position changes near the tumor. We use simethicone for patients who have significant amounts of gas in the large bowel. Reduction in bowel gas can often increase the separation between the tumor and colon.
ADDRESSING THE CHALLENGES OF IRRADIATING LARGE LIVER TUMORS
Solutions for Organ Motion
Image-guided radiation therapy (IGRT) has advanced considerably during the past decade. Multiple options for target verification and motion control enable greater certainty in target alignment, which can be used to reduce dose to normal tissues and escalate dose to tumors. The most common IGRT strategies are reviewed in this section.
Tracking
Liver tumor targets can be tracked in real time by using implanted fiducials. For example, the ExacTrac system visualizes target motion by tracking radiopaque fiducials that are implanted in or near the tumor. Multiple non-coplanar X-rays are directed in the region of the isocenter, and automated computer algorithms provide alignment shifts. For hypofractionated treatments to liver tumors, this method reportedly is able to track a moving target with an accuracy of within 1 mm.30 The CyberKnife system also allows real-time tracking of tumors, and has been used for stereotactic radiosurgery for liver cancers31. Other tracking systems can also be used for real-time tracking of tumors32, 33. Fiducial-based alignment can be achieved with orthogonal films or cone-beam CT on-board imaging that most linear accelerators now have.
Breath Hold
Several systems have been developed to enable treatment during inspiratory or expiratory breath hold, including the Varian RPM system and the Active Breathing Control system. Interfractional variations in breath hold position can exceed 4 mm,34, 35 and so a breath hold technique is usually coupled with image guidance to verify the target position with each fraction. Image guidance can be achieved by using 2D image sets using metallic fiducials or other soft tissue surrogate or with 3D images obtained in the breath hold position.
Gating
Respiratory gating is another method for delivering radiation to liver cancers.36 Respiratory gating involves turning the beam on during specified points in the breathing cycle. Successful use of gating techniques requires a regular breathing pattern; gating at end expiration is usually best because there is less motion during that point in the respiratory cycle. Most gating systems are based on monitoring abdominal motion. We use an external fiducial placed on the patient’s abdomen that is tracked by couch mounted camera. The camera reports the height of the fiducial with respect to a baseline level established based on the patient’s end-expiration level. The treatment beam can be directly triggered when the height of the fiducial is within a fixed range, this is referred to as amplitude based gating. The height of the fiducial vs time can also be fit to a function that approximates a normal breathing cycle and that function can then be used to trigger the beam when the patient is in a certain phase range of the breathing cycle, this is referred to as phase based gating. Phase-based gating is most common, but amplitude-based gating (beam on at expiration) can also be used if the breathing pattern is irregular. Treatment with a respiratory gating technique requires placement of fiducial markers as well as 4D simulation and contouring the target and avoidance structures at end expiration. Positions of the respiratory cycle surrounding end expiration move the least and are a good starting point to assess the gating window. A narrower gating window means a longer treatment time, but may be preferred to minimize motion. Similar to breath hold, respiratory gating requires that the fiducial position be verified between intensity-modulated radiation therapy (IMRT) beams to assess intrafraction variability.
Abdominal compression
Restricting the movement of the abdomen by using a compression device can also minimize respiratory-associated motion. This method is commonly used while treating patients with SBRT. Several commercial devices are available for this application. The most common technique uses an abdominal compression plate that is placed 3 to 4 cm below the costal margin. The plate is connected to a load cell that can measure how much force is being applied to the abdomen. This device is usually used when the superior-inferior movement of the tumor exceeds 1 cm, but it may also be needed for tumors within 1 cm of the GI tract.37 Because compression plates can cause variable deformation of the liver, an alternative solution for liver tumors is use of a pneumatic compression belt. This emerging option has been reported to reduce respiratory motion to less than 5mm38. Notably, although compression does not require a regular breathing pattern, it only minimizes rather than eliminates motion, and can move bowel closer to large or extrahepatic cancers.
Image Guidance
As described in the previous paragraphs, some options for image guidance include fiducial-based kilovoltage X-ray solutions used in tumor tracking, deep inspiration breath hold, inspiration breath hold, and free-breathing gating techniques such as end-expiratory gating and abdominal compression. Another option, soft tissue imaging via CT-on-rails or magnetic resonance imaging (MRI) have the advantage of being able to clearly visualize the interface of the liver with the GI tract, and, most of the time, the tumor within the liver.
The CyberKnife system allows real-time tracking of tumors, but the dose rate is too low to efficiently treat large tumors. The ExacTrac system is a linac-based system that allows tracking with kilovoltage IGRT. Both of these solutions require placement of fiducials and may not be optimal for delivering ablative doses to tumors near the GI tract due to the uncertainty of stomach duodenum and colon position
Among the soft tissue imaging options, cone-beam CT is the most widely available. Because images are acquired over 60-second periods, motion artifact is significant. Respiratory motion artifact and tissue density homogeneity undermine image quality in the upper abdomen. A 3-D breath hold or 4-D cone-beam CT can be obtained to help improve image quality.
Cone-beam CT is often used for SBRT of small liver metastases. Metallic fiducial placement is helpful to align to the tumors within the liver, which are otherwise not visible on cone-beam CT. Most small liver tumors can be treated with SBRT by using a free-breathing internal target volume that accounts for respiratory motion and aligning the patient to bony landmarks. For larger tumors, a breath-hold soft tissue set-up to liver shape can be used with gated cone-beam CT and 3D-3D registration. The challenge arises when the tumor is near the GI tract. CT image guidance overcomes this limitation. We use CT-on-rails IGRT, coupled with inspiration breath hold. With a diagnostic non-contrast CT, we can visualize and set up to large tumors within the liver, visualize the interface of the tumor and GI structures, and monitor tumor response. We also use these images for adaptive planning. Most adaptive planning is actually triggered by movement of OARs from simulation to treatment. CT-on-rails is not being marketed commercially at this time. Its limitations include space requirements, registration and positioning of the patient. MRI linacs seem to be the way of the future for optimal IGRT in these challenging cases, with potential advantages over CT such as better visualization of tumors within the liver and improved image registration accuracy owing to better soft-tissue contrast than CT.
Strategies to Assess Underlying Liver Function
Our patients undergo appropriate baseline testing to assess the degree of underlying liver dysfunction and the risk of toxicity from radiation therapy. This testing can include laboratory and clinical assessments as well as functional imaging in selected patients before treatment is begun.
Child Pugh score
The most commonly used classification of liver function is the Child-Pugh score, which accounts for the presence or absence of ascites and encephalopathy and measurements of bilirubin, albumin, and prothrombin, the latter as an international normalized ratio. Historically speaking, this convention was developed to assess prognosis after surgery for variceal bleeding in patients with cirrhosis and portal hypertension.39, 40 This classification has proven useful for many decades and is often used to decide whether patients are eligible for radiation therapy. Generally speaking, patients with Child-Pugh Class A cirrhosis can safely receive radiation, but patients with Class B or C cirrhosis are not considered candidates.
The limitation of using Child Pugh scoring in radiation treatment decision-making is related to its subjective elements of encephalopathy and ascites, as well as its inability to distinguish underlying cirrhosis from tumor-related liver dysfunction. For these reasons, other grading systems have been proposed, including using just the albumin and bilirubin measurements (ALBI)41 or using measurements of insulin-like growth factor 1 (IGF-I) and vascular endothelial growth factor (VEGF).42 However, these other grading systems have yet to be applied to radiotherapy.
Imaging-based assessment of liver function
Several imaging modalities can be used to assess liver function, by providing assessments of overall hepatic metabolic function or spatial/anatomic location of functional liver parenchyma verses cirrhotic areas. The modalities evaluated and used in the clinic most commonly are described briefly below.
Indocyanine green
Indocyanin green (ICG) is a water-soluble inert compound that binds to albumin in the plasma after intravenous injection. ICG is selectively taken up by hepatocytes and excreted unmetabolized into the bile in an ATP-dependent fashion. Since ICG is not recirculated into the enterohepatic system, its excretion rate in bile reflects the hepatic excretory function and energy status. Hepatic function can be assessed by measuring ICG clearance and ICG retention. Patients with low ICG clearance have a higher 30-day mortality rates than do patients with high ICG clearance.43 ICG has been used to assess individual functional liver reserve in patients undergoing radiation, to identify those with impaired function early so that adaptive planning can be used to optimize radiation doses and toxicity.44
Sulfur colloid Technetium 99 SPECT/CT
Sulfur colloid is preferentially taken up by the Kupffer cells, which are generally present in functional liver parenchyma.45, 46 Thus, their location can be a surrogate for the location of healthy hepatocytes. Labeling the colloid with Technetium 99m can allow the localization of functional liver by single positron emission CT (SPECT), which can be combined with a standard CT scan. Sulphur colloid and ICG measurements correlate with each other, indicating that sulphur colloid SPECT can be used to assess liver function.47 Indeed, sulphur colloid SPECT has been used to quantify functional liver and to predict clinical outcomes for patients with cirrhosis46, 48.
Another application of this functional imaging assessment is to avoid irradiation of normal tissues. Technical aspects of registering the SPECT images for treatment planning can be achieved with fiducial markers.49 One approach to designing radiation plans for differential avoidance of functional liver50, has demonstrated the dosimetric advantages of this rational treatment-planning approach. However, prospective evaluations are needed.
Eovist. Gadolinium-ethoxybenzyl-deithylenetriamine pentaacetic acid (Gadolinium-EOB-DTPA, Eovist) is selectively taken up by hepatocytes and thus can be used to assess hepatocyte function without some of the limitations of purely extracellular contrast agents. For example, Eovist has been shown to increase the signal intensity of normal liver parenchyma on T1-weighted MRI and thus can help define lesions in the liver,51 distinguishing between benign and malignant processes based on imaging characteristics at each phase of a liver protocol MRI (i.e., the T1-weighted precontrast, arterial, and hepatocyte phases).
Radiation damage to the liver can result in distinct imaging findings. For example, Eovist has been used to estimate the distal fall off range for proton radiotherapy52, and lack of Eovist uptake has been correlated with histopathological evidence of radiation-induced liver damage53.
HOW CAN THESE SOLUTIONS BE INTEGRATED INTO A COMPREHENSIVE TREATMENT PLAN?
Simultaneous Integrated Boost with Simultaneous Integrated Protection Technique
We started our novel treatment planning approach with the assumptions that high and low dose inhomogeneity was necessary to control large tumors located <1 cm from the gastrointestinal tract or main bile ducts. First, regarding the protection aspect, the whole tumor does not need to receive an ablative dose. We have not seen recurrences in areas abutting the GI tract that were restricted to a microscopic dose while the rest of the tumor received an ablative dose. Second, regarding the boost aspect, the center of the tumor can safely receive very high doses (up to 140 Gy BED, Fig. 1). We do not know yet whether the use of this central SIB improves tumor control. However, we have not seen significant toxicity from its use.
Figure 1.
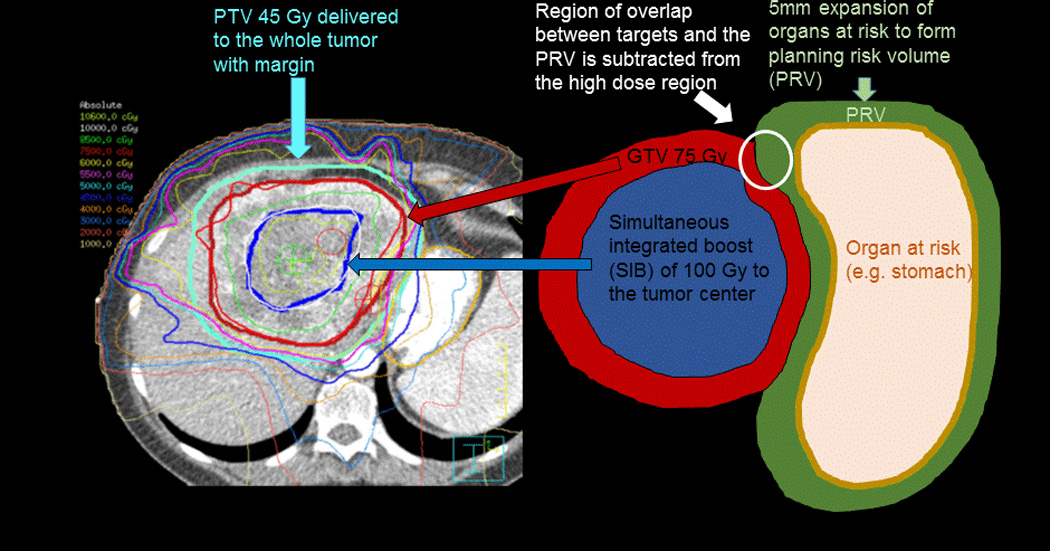
Radiation treatment plan and illustration of the Simultaneous Integrated Boost/Protection (SIB/SIP) technique. A dose of 100 Gy in 25 fractions is delivered to the center of the tumor while the planning target volume (PTV) receives 75 Gy in 25 fractions. This does not overlap with the planning risk volume (PRV) created by a 5 mm expansion of adjacent organs at risk for SIP.] A microscopic dose of 45 Gy that is within the tolerance of the gastric mucosa is delivered to the whole tumor with margin.
Our treatment planning approach involves using IMRT or proton therapy with an SIB technique, typically with 3 different PTVs (a microscopic dose, an SIB to the GTV, and an SIB to a higher dose to the hypoxic center). Areas of potential microscopic extension are treated with 37.5 Gy in 15 fractions or 45 Gy in 25 fractions with a 5-to 10-mm CTV + a 5-mm PTV, for a total of 10–15 mm from GTV to PTV). The SIB to the GTV (67.5 Gy in 15 fractions or 75 Gy in 25 fractions) is treated with a 0- to 5-mm PTV. The SIP technique involves subtracting a planning-OAR-volume for all luminal structures and the central biliary tree (created by taking the 4D contour of the OAR and adding 5 mm) from this high-dose PTV, and then contracting that volume by 10 mm to create the PTV to the hypoxic center. This volume receives 75 Gy in 15 fractions or 100 Gy in 25 fractions. The decision to use 5 versus 10 mm for the CTV or 0 versus 5 mm for the PTV is based on the mean dose to the normal liver (i.e., the liver minus the GTV). We start the planning process with the larger expansions and reduce the volumes rather than the total doses to achieve a plan that is within liver tolerance. In other words, we use the larger margins if we can meet the dose constraints.
For large liver tumors, the choice of fraction number is based on the proximity of luminal GI structures, bile duct, and liver tolerance considerations. When the tumor is within 1 cm of the GI tract, we always use 25 fractions to optimally spare those structures while achieving the highest minimum dose to the tumor. If the tumor is >1 cm from the bowel, we use 15 fractions, provided liver tolerance can be achieved. For the NRG GI Oncology 001 trial, we recommend 67.5 Gy in 15 fractions for central lesions. Finally, if we cannot meet liver constraints (Table 1) with 67.5 Gy in 15 fractions, we use 75 Gy in 25 fractions, which allows a higher mean dose to be delivered to the uninvolved liver. Thus far, these doses have been under the threshold for biliary stricture, but further investigation and longer follow up are necessary.
TABLE 1.
Liver Dose Constraints
| Standard Fractionation | Hypofractionation (>3 Gy/fraction) | |||
|---|---|---|---|---|
| Metric | No Cirrhosis | Cirrhosis | No Cirrhosis | Cirrhosis |
| Mean dose | 28 Gy | 24 Gy | 24 Gy | 24 Gy |
| Absolute volume | >700 cm3 <30 Gy | >700 cm3 <25 Gy | >700 cm3 <25 Gy | >700 cm3 <20 Gy |
| Relative volume | V30 < 1/3 | V30 < 1/3 | V25 < 1/3 | V20 < 1/3 |
Deciding Whether to Use Proton Therapy or IMRT
The decision of whether to use proton therapy or IMRT is based on anatomic considerations. For most patients, we do treatment planning plans for each modality and compare them to select the best plan. This practice is necessary for insurance approval. Generally, patients with large tumors that are >2 cm from the GI tract are treated with protons because proton therapy can spare more normal liver than can IMRT. Because all such patients have compromised liver function due to underlying liver disease or tumor, we attempt to spare as much liver as possible. This practice also leaves more options for retreatment of intrahepatic recurrences, which can be life-threatening. However, the not-so-often discussed disadvantage of protons is their wider penumbra due to lateral scatter. Because of this, proton therapy is not the best choice for tumors that are close to a critical structure. We generally treat patients with IMRT if the tumor is within 2 cm of adjacent bowel (Fig. 2). We can treat almost any large liver tumor to doses that are twice the tolerance of the GI tract because we use daily CT image-guided soft tissue alignment and inspiration breath hold gating. We do not currently have CT image guidance at our proton facility, which influences our clinical decision-making.
Figure 2.
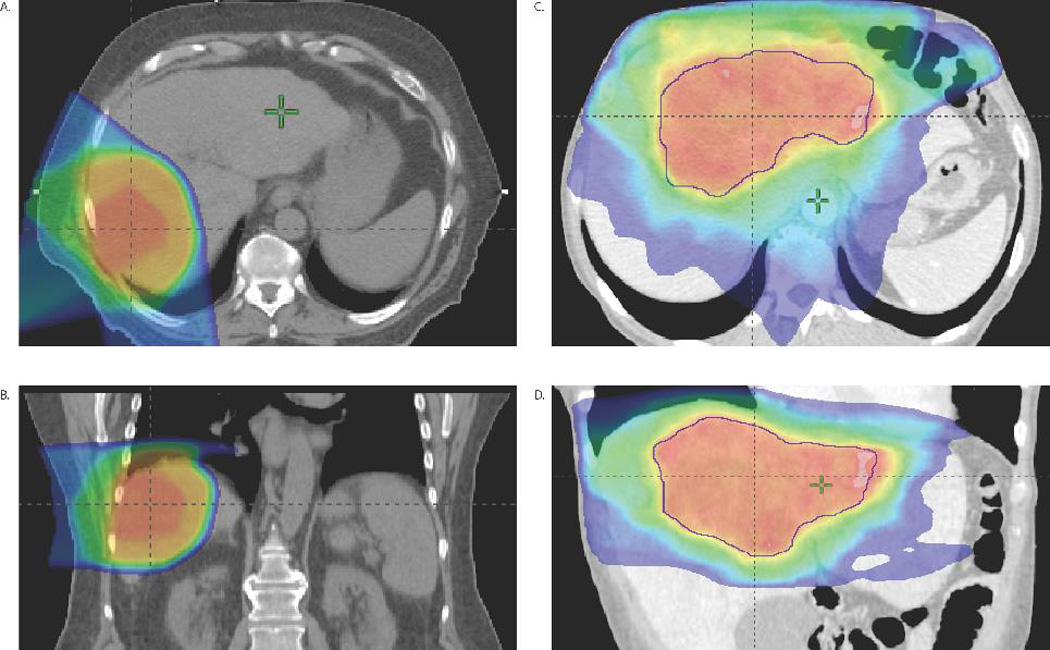
Ablative proton and IMRT plans for large liver tumors. Shown are axial (A) and sagittal (B) views of a proton plan for a right-sided HCC receiving 75 CGE in 15 fractions to the center of the tumor and 67.5 CGE in 15 fractions to the entire tumor. Another case of fractionated SABR is shown for a massive IHCC that received 75Gy in 25 fractions using IMRT (axial (C) and sagittal (D)) to the center of the tumor, 60Gy to the GTV, and a microscopic dose of 45Gy. Due to the small remnant of normal liver and location of this massive IHCC, the tumor the central SIB and GTV doses had to be reduced from initial goals of 100Gy and 75Gy, respectively.
Dose constraints for OARs
When ablative doses are considered, the radiation dose-limiting structures for patients with large hepatobiliary cancers are the bile ducts, the normal liver (i.e., the liver minus the GTV), the chest wall, and most commonly the bowel, including the stomach, duodenum, and colon.
Our approach to limiting the risk of mucosal bleeding, radiation-induced liver disease, biliary strictures, and chest wall pain is to return to the principles of dose fractionation by using an SBRT technique. When necessary, we use 15 or 25 fractions rather than reducing the total BED to protect these structures.
Biliary tree
The threshold dose for biliary stricture is not well known, but some generalizations can be made from clinical observations. Use of SBRT in 3–5 fractions has caused high biliary stricture rates54 and thus SBRT in 3–5 fractions should not be used anywhere near the common, right main, or left main hepatic ducts. Investigators at Tsukuba reported 3 cases of biliary strictures after delivery of 79.2 CGE in 16 fractions (i.e., 5.0 CGE/fraction); they subsequently changed that regimen to 72.6 CGE in 22 fractions (3.3 CGE/fraction) and saw no further strictures.55 We have patterned our practice from this observation. We use 75 Gy in 25 fractions (with a maximum point dose of 80Gy) to the central biliary tree. The Tsukuba experience suggests that this regimen should be under the threshold for biliary stricture, but longer follow-up will be necessary. We can state with certainty that 58 Gy in 15 fractions is a safe dose to the central biliary tree. However, that dose has led to inadequate tumor control for IHCC11 and is too low for liver metastases56. On the other hand, we have found 67.5 Gy in 15 fractions to be very effective for local tumor control (4-yr Local tumor control 85% for IHCC11. Initially we used 67.5 Gy in 15 fractions only for peripheral tumors, but we have changed our policy after observing high rates of tumor progression with lower doses and now use 67.5Gy in 15 fractions for tumors near the hilum. Our biliary constraints are found in Table 2. We have not observed grade 3 or higher chest wall pain or biliary stricture after 67.5 Gy in 15 fractions or 75 Gy in 25 fractions. So, for the central bile ducts and chest wall, these ablative doses seem to be under the threshold for complications.
TABLE 2.
Dose Constraints per Number of Fractions for Bowel and Biliary Tree
| Maximum Point Dose, Gy | ||
|---|---|---|
| No. of Fractions | Biliary Tree | Bowel |
| 25–28 | 80 | 60 |
| 15 | 70 | 45 |
| 10 | 60 | 40 |
| 3–5 | 45 | 30 |
Bowel
Strict adherence to bowel dose constraints is necessary because the bowel surrounds most surfaces of the liver (Table 2). The risk of moderately severe radiation-related small bowel toxicity seems to be related to the volume of bowel that exceeds a threshold dose. The estimate of the threshold dose and volume of bowel depends on the fractionation schedule of the radiation and the method of structure segmentation (the actual OAR vs. the potential space that the OAR occupies.57 We have found that bleeding events also may depend on the primary tumor, such as an intrahepatic cholangiocarcinoma58 versus a pancreatic cancer59. Our current dose constraints for the duodenum and stomach for 25 to 28 fractions is a maximum point dose of 60 Gy. For hypofractionated schedules (10–15 fractions), we keep the maximum dose to the duodenum and stomach to less than 45 Gy. For patients with liver primary tumors receiving 25 fractions, we also keep the volume of stomach receiving more than 40 Gy to <60 cm3 and more than 50 Gy to 40 cm3.58
Liver
The most important OAR to be spared with ablative radiation approaches is the liver. We evaluate the mean dose, the relative dose, and the volume of liver spared to keep all below the maximum tolerance dose, as shown in Table 1.
WHAT ARE THE RESULTS OF USING THESE PRINCIPLES FOR LARGE LIVER TUMORS?
Initial experiences with ablative proton therapy in Japan
A key paradigm shift occurred with the introduction of proton therapy in Japan, where HCC is endemic and quite common. Protons have allowed larger treatment volumes to be treated to larger doses per fraction. Results from hypofractionated regimens (16–25 fractions) to ablative doses for large tumors are similar to those after surgical resection, with 5-year local tumor control rates of 90% and overall survival (OS) rates of 50% among some patients.60–62 The key has been selecting patients with relatively small, isolated tumors with well-compensated cirrhosis. Starting in 2007, we patterned our dose fractionation regimens in the liver treatment program at MD Anderson Cancer Center after these successes.
Ablative radiation for intrahepatic cholangiocarinoma at MDACC
A retrospective dose response analysis of patients given definitive radiation therapy for inoperable intrahepatic cholangiocarcinoma in 2002–2014 at MD Anderson was recently published.11 That study identified 79 consecutive patients, most of whom had large tumors (median diameter 7.9 cm [range 2.2–17 cm]). Seventy patients (89%) had received systemic chemotherapy before radiation, which was given to doses of 35–100 Gy (median 58.05), for a median BED of 80.5 Gy (range 43.75–180 Gy). At a median follow-up time of 33 months (range 11–93 months), the median OS time after diagnosis was 30 months and the 3-year OS rate was 44%. Radiation dose was the single most important prognostic factor; higher doses correlated with improved local control and OS. The 3-year OS rate for those receiving BED >80.5 was 73% versus 38% for those receiving lower doses (P=0.017, Figure 3), and the 3-year local control rate was significantly higher (78%) after a BED >80.5 Gy than after lower doses (45%, P=0.04). As a continuous variable, BED also significantly influenced local control (P=0.0097) and OS (P=0.0045). No significant treatment-related toxicity was noted. These results suggest that a BED >80.5 Gy seems to be ablative for large intrahepatic cholangiocarcinomas, with long-term survival rates that compare favorably to resection.
Figure 3.
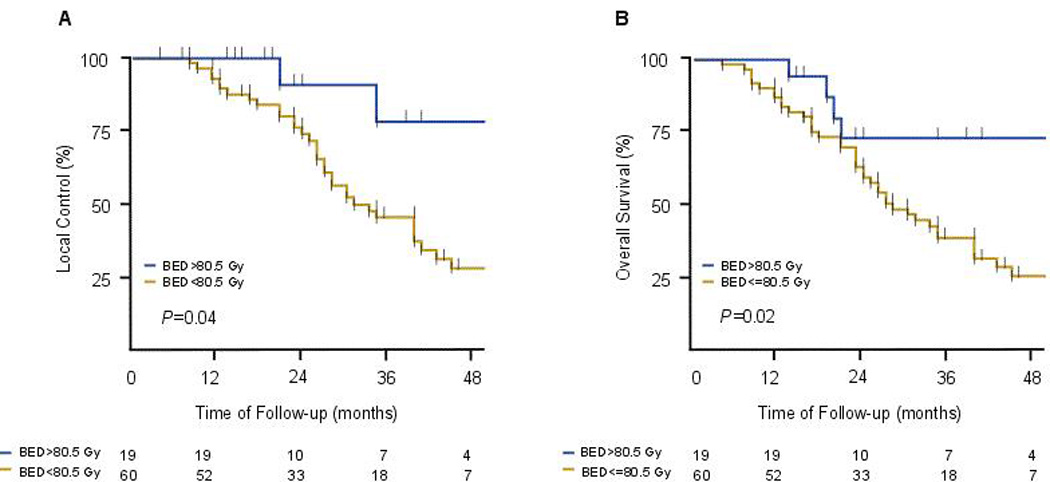
Kaplan-Meier estimates by treatment with radiation doses greater than a Biologic Equivalent Dose (BED) of 80.5 Gy. The lower dose regimens were 58Gy in 15 fractions and 50.4 Gy in 28 fractions and the higher dose regimens were either 75 Gy in 25 fractions or 67.5 Gy in 15 fractions. Effect of radiation dose of local control (A) and overall survival (B).
At MD Anderson, we treat all patients dispositioned for radiation with ablative doses, regardless of the location of the tumor relative to OARs such as bowel. This practice requires solutions for organ motion and high-quality images guidance in most, if not all cases.
CONCLUSIONS
Large liver tumors are among the most challenging cases to treat because of the proximity of the duodenum, colon, stomach, main bile ducts; the presence of underlying liver disease; the sensitivity of the liver parenchyma to radiation; and the respiratory and interfraction motion of the surrounding bowel. However, these challenges may be overcome by adhering to the following three principles: (1) Control respiratory motion. Fiducial-based solutions are most common, but without soft-tissue imaging, the position of the GI tract is unknown. We use inspiration breath hold with CT-on-rails image guidance, which is an effective solution. (2) Fractionation to achieve ablative radiation doses. An SBRT technique coupled with the time-honored principle of fractionation permits ablative doses to be given (90–100 Gy BED) and leads to a substantial survival benefit for patients with large liver tumors. For most large central tumors, giving 15–25 fractions with an SBRT technique is necessary to deliver an ablative dose and stay within the tolerance of the OARs. The alternative is to give 5 fractions and reduce the dose, which may no longer be ablative. (3) Heterogeneous dose distribution. For tumors near the GI tract, 90% GTV tumor coverage seems to be sufficient. Also, delivering BEDs of up to 140 Gy to the hypoxic core of the tumor is safe and may lead to improved tumor control. These solutions, principles, and techniques have led to encouraging results, with local control rates of 85%–90% for patients with large HCC and intrahepatic cholangiocarcinomas. We continue to follow our patients to monitor for late effects of this approach, and anticipate that following these three principles will enable a safe ablative approach that can substantially prolong survival.
Acknowledgments
Supported in part by Cancer Center Support (Core) Grant CA016672 from the National Cancer Institute to The University of Texas MD Anderson Cancer Center
Eugene Koay is supported by grants from the University of Texas MD Anderson Cancer Center, Center for Radiation Oncology Research, and by a sponsored research agreement with Philips Healthcare
Footnotes
The authors have no conflicts of interest
References
- 1.Austin-Seymour MM, Chen GT, Castro JR, et al. Dose volume histogram analysis of liver radiation tolerance. Int J Radiat Oncol Biol Phys. 1986;12:31–35. doi: 10.1016/0360-3016(86)90412-8. [DOI] [PubMed] [Google Scholar]
- 2.Lawrence TS, Ten Haken RK, Kessler ML, et al. The use of 3-D dose volume analysis to predict radiation hepatitis. Int J Radiat Oncol Biol Phys. 1992;23:781–788. doi: 10.1016/0360-3016(92)90651-w. [DOI] [PubMed] [Google Scholar]
- 3.Dawson LA, McGinn CJ, Normolle D, et al. Escalated focal liver radiation and concurrent hepatic artery fluorodeoxyuridine for unresectable intrahepatic malignancies. J Clin Oncol. 2000;18:2210–2218. doi: 10.1200/JCO.2000.18.11.2210. [DOI] [PubMed] [Google Scholar]
- 4.Chang JY, Senan S, Paul MA, et al. Stereotactic ablative radiotherapy versus lobectomy for operable stage I non-small-cell lung cancer: a pooled analysis of two randomised trials. Lancet Oncol. 2015;16:630–637. doi: 10.1016/S1470-2045(15)70168-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fakiris AJ, McGarry RC, Yiannoutsos CT, et al. Stereotactic body radiation therapy for early-stage non-small-cell lung carcinoma: four-year results of a prospective phase II study. Int J Radiat Oncol Biol Phys. 2009;75:677–682. doi: 10.1016/j.ijrobp.2008.11.042. [DOI] [PubMed] [Google Scholar]
- 6.Kavanagh BD, Schefter TE, Cardenes HR, et al. Interim analysis of a prospective phase I/II trial of SBRT for liver metastases. Acta Oncol. 2006;45:848–855. doi: 10.1080/02841860600904870. [DOI] [PubMed] [Google Scholar]
- 7.Haley ML, Gerszten PC, Heron DE, Chang YF, Atteberry DS, Burton SA. Efficacy and cost-effectiveness analysis of external beam and stereotactic body radiation therapy in the treatment of spine metastases: a matched-pair analysis. J Neurosurg Spine. 2011;14:537–542. doi: 10.3171/2010.12.SPINE10233. [DOI] [PubMed] [Google Scholar]
- 8.Herman JM, Chang DT, Goodman KA, et al. Phase 2 multi-institutional trial evaluating gemcitabine and stereotactic body radiotherapy for patients with locally advanced unresectable pancreatic adenocarcinoma. Cancer. 2015;121:1128–1137. doi: 10.1002/cncr.29161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crane CH, Varadhachary GR, Yordy JS, et al. Phase II trial of cetuximab, gemcitabine, and oxaliplatin followed by chemoradiation with cetuximab for locally advanced (T4) pancreatic adenocarcinoma: correlation of Smad4(Dpc4) immunostaining with pattern of disease progression. J Clin Oncol. 2011;29:3037–3043. doi: 10.1200/JCO.2010.33.8038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bujold A, Massey CA, Kim JJ, et al. Sequential Phase I and II Trials of Stereotactic Body Radiotherapy for Locally Advanced Hepatocellular Carcinoma. Journal of Clinical Oncology. 2013;31:1631–1639. doi: 10.1200/JCO.2012.44.1659. [DOI] [PubMed] [Google Scholar]
- 11.Tao ea. Ablative radiotherapy doses lead to a substantial prolongation of survival in patients with inoperable intrahepatic cholangiocarcinoma. Journal of Clinical Oncology. 2015 doi: 10.1200/JCO.2015.61.3778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dawson LA, Ten Haken RK. Partial volume tolerance of the liver to radiation. Semin Radiat Oncol. 2005;15:279–283. doi: 10.1016/j.semradonc.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 13.Sempoux C, Horsmans Y, Geubel A, et al. Severe radiation-induced liver disease following localized radiation therapy for biliopancreatic carcinoma: activation of hepatic stellate cells as an early event. Hepatology. 1997;26:128–134. doi: 10.1002/hep.510260117. [DOI] [PubMed] [Google Scholar]
- 14.Cheng JC, Wu JK, Huang CM, et al. Radiation-induced liver disease after three-dimensional conformal radiotherapy for patients with hepatocellular carcinoma: dosimetric analysis and implication. Int J Radiat Oncol Biol Phys. 2002;54:156–162. doi: 10.1016/s0360-3016(02)02915-2. [DOI] [PubMed] [Google Scholar]
- 15.Cheng JC, Wu JK, Huang CM, et al. Radiation-induced liver disease after radiotherapy for hepatocellular carcinoma: clinical manifestation and dosimetric description. Radiother Oncol. 2002;63:41–45. doi: 10.1016/s0167-8140(02)00061-0. [DOI] [PubMed] [Google Scholar]
- 16.Cheng JC, Wu JK, Lee PC, et al. Biologic susceptibility of hepatocellular carcinoma patients treated with radiotherapy to radiation-induced liver disease. Int J Radiat Oncol Biol Phys. 2004;60:1502–1509. doi: 10.1016/j.ijrobp.2004.05.048. [DOI] [PubMed] [Google Scholar]
- 17.Liang SX, Zhu XD, Xu ZY, et al. Radiation-induced liver disease in three-dimensional conformal radiation therapy for primary liver carcinoma: the risk factors and hepatic radiation tolerance. Int J Radiat Oncol Biol Phys. 2006;65:426–434. doi: 10.1016/j.ijrobp.2005.12.031. [DOI] [PubMed] [Google Scholar]
- 18.Wong RJ, Aguilar M, Cheung R, et al. Nonalcoholic steatohepatitis is the second leading etiology of liver disease among adults awaiting liver transplantation in the United States. Gastroenterology. 2015;148:547–555. doi: 10.1053/j.gastro.2014.11.039. [DOI] [PubMed] [Google Scholar]
- 19.Schuppan D, Afdhal NH. Liver cirrhosis. Lancet. 2008;371:838–851. doi: 10.1016/S0140-6736(08)60383-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khozouz RF, Huq SZ, Perry MC. Radiation-induced liver disease. J Clin Oncol. 2008;26:4844–4845. doi: 10.1200/JCO.2008.18.2931. [DOI] [PubMed] [Google Scholar]
- 21.Rubbia-Brandt L, Audard V, Sartoretti P, et al. Severe hepatic sinusoidal obstruction associated with oxaliplatin-based chemotherapy in patients with metastatic colorectal cancer. Ann Oncol. 2004;15:460–466. doi: 10.1093/annonc/mdh095. [DOI] [PubMed] [Google Scholar]
- 22.Vauthey JN, Pawlik TM, Ribero D, et al. Chemotherapy regimen predicts steatohepatitis and an increase in 90-day mortality after surgery for hepatic colorectal metastases. J Clin Oncol. 2006;24:2065–2072. doi: 10.1200/JCO.2005.05.3074. [DOI] [PubMed] [Google Scholar]
- 23.Adam R, Laurent A, Azoulay D, Castaing D, Bismuth H. Two-stage hepatectomy: A planned strategy to treat irresectable liver tumors. Ann Surg. 2000;232:777–785. doi: 10.1097/00000658-200012000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jaeck D, Oussoultzoglou E, Rosso E, Greget M, Weber JC, Bachellier P. A two-stage hepatectomy procedure combined with portal vein embolization to achieve curative resection for initially unresectable multiple and bilobar colorectal liver metastases. Ann Surg. 2004;240:1037–1049. doi: 10.1097/01.sla.0000145965.86383.89. discussion 1049–1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Minagawa M, Makuuchi M. Treatment of hepatocellular carcinoma accompanied by portal vein tumor thrombus. World J Gastroenterol. 2006;12:7561–7567. doi: 10.3748/wjg.v12.i47.7561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yamada K, Izaki K, Sugimoto K, et al. Prospective trial of combined transcatheter arterial chemoembolization and three-dimensional conformal radiotherapy for portal vein tumor thrombus in patients with unresectable hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 2003;57:113–119. doi: 10.1016/s0360-3016(03)00434-6. [DOI] [PubMed] [Google Scholar]
- 27.Chen SC, Lian SL, Chang WY. The effect of external radiotherapy in treatment of portal vein invasion in hepatocellular carcinoma. Cancer Chemother Pharmacol. 1994;33(Suppl):S124–S127. doi: 10.1007/BF00686683. [DOI] [PubMed] [Google Scholar]
- 28.Yamada K, Soejima T, Sugimoto K, et al. Pilot study of local radiotherapy for portal vein tumor thrombus in patients with unresectable hepatocellular carcinoma. Jpn J Clin Oncol. 2001;31:147–152. doi: 10.1093/jjco/hye029. [DOI] [PubMed] [Google Scholar]
- 29.Schiedermaier P, Neubrand M, Hansen S, Sauerbruch T. Variability of gallbladder emptying after oral stimulation. Scand J Gastroenterol. 1997;32:719–724. doi: 10.3109/00365529708996524. [DOI] [PubMed] [Google Scholar]
- 30.Wurm RE, Gum F, Erbel S, et al. Image guided respiratory gated hypofractionated Stereotactic Body Radiation Therapy (H-SBRT) for liver and lung tumors: Initial experience. Acta Oncol. 2006;45:881–889. doi: 10.1080/02841860600919233. [DOI] [PubMed] [Google Scholar]
- 31.Brock KK, Dawson LA. Adaptive management of liver cancer radiotherapy. Semin Radiat Oncol. 2010;20:107–115. doi: 10.1016/j.semradonc.2009.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Verellen D, Depuydt T, Gevaert T, et al. Gating and tracking, 4D in thoracic tumours. Cancer Radiother. 2010;14:446–454. doi: 10.1016/j.canrad.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 33.Iizuka Y, Matsuo Y, Ishihara Y, et al. Dynamic tumor-tracking radiotherapy with real-time monitoring for liver tumors using a gimbal mounted linac. Radiother Oncol. 2015 doi: 10.1016/j.radonc.2015.08.033. [DOI] [PubMed] [Google Scholar]
- 34.Eccles C, Brock KK, Bissonnette JP, Hawkins M, Dawson LA. Reproducibility of liver position using active breathing coordinator for liver cancer radiotherapy. Int J Radiat Oncol Biol Phys. 2006;64:751–759. doi: 10.1016/j.ijrobp.2005.05.066. [DOI] [PubMed] [Google Scholar]
- 35.Dawson LA, Brock KK, Kazanjian S, et al. The reproducibility of organ position using active breathing control (ABC) during liver radiotherapy. Int J Radiat Oncol Biol Phys. 2001;51:1410–1421. doi: 10.1016/s0360-3016(01)02653-0. [DOI] [PubMed] [Google Scholar]
- 36.Tina Marie B, Sam B, Peter B, et al. Respiratory gating with EPID-based verification: the MDACC experience. Physics in Medicine and Biology. 2009;54:3379. doi: 10.1088/0031-9155/54/11/007. [DOI] [PubMed] [Google Scholar]
- 37.Heinzerling JH, Anderson JF, Papiez L, et al. Four-dimensional computed tomography scan analysis of tumor and organ motion at varying levels of abdominal compression during stereotactic treatment of lung and liver. Int J Radiat Oncol Biol Phys. 2008;70:1571–1578. doi: 10.1016/j.ijrobp.2007.12.023. [DOI] [PubMed] [Google Scholar]
- 38.Lovelock DM, Zatcky J, Goodman K, Yamada Y. The effectiveness of a pneumatic compression belt in reducing respiratory motion of abdominal tumors in patients undergoing stereotactic body radiotherapy. Technol Cancer Res Treat. 2014;13:259–267. doi: 10.7785/tcrt.2012.500379. [DOI] [PubMed] [Google Scholar]
- 39.Child CG, Turcotte JG. Surgery and portal hypertension. Major Probl Clin Surg. 1964;1:1–85. [PubMed] [Google Scholar]
- 40.Pugh RN, Murray-Lyon IM, Dawson JL, Pietroni MC, Williams R. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg. 1973;60:646–649. doi: 10.1002/bjs.1800600817. [DOI] [PubMed] [Google Scholar]
- 41.Johnson PJ, Berhane S, Kagebayashi C, et al. Assessment of liver function in patients with hepatocellular carcinoma: a new evidence-based approach-the ALBI grade. J Clin Oncol. 2015;33:550–558. doi: 10.1200/JCO.2014.57.9151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kaseb AO, Morris JS, Hassan MM, et al. Clinical and prognostic implications of plasma insulin-like growth factor-1 and vascular endothelial growth factor in patients with hepatocellular carcinoma. J Clin Oncol. 2011;29:3892–3899. doi: 10.1200/JCO.2011.36.0636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hemming AW, Scudamore CH, Shackleton CR, Pudek M, Erb SR. Indocyanine green clearance as a predictor of successful hepatic resection in cirrhotic patients. Am J Surg. 1992;163:515–518. doi: 10.1016/0002-9610(92)90400-l. [DOI] [PubMed] [Google Scholar]
- 44.Stenmark MH, Cao Y, Wang H, et al. Estimating functional liver reserve following hepatic irradiation: adaptive normal tissue response models. Radiother Oncol. 2014;111:418–423. doi: 10.1016/j.radonc.2014.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wisse E, Braet F, Luo D, et al. Structure and function of sinusoidal lining cells in the liver. Toxicol Pathol. 1996;24:100–111. doi: 10.1177/019262339602400114. [DOI] [PubMed] [Google Scholar]
- 46.Hoefs JC, Wang F, Kanel G. Functional measurement of nonfibrotic hepatic mass in cirrhotic patients. Am J Gastroenterol. 1997;92:2054–2058. [PubMed] [Google Scholar]
- 47.Zuckerman E, Slobodin G, Sabo E, Yeshurun D, Naschitz JE, Groshar D. Quantitative liver-spleen scan using single photon emission computerized tomography (SPECT) for assessment of hepatic function in cirrhotic patients. J Hepatol. 2003;39:326–332. doi: 10.1016/s0168-8278(03)00296-4. [DOI] [PubMed] [Google Scholar]
- 48.Everson GT, Shiffman ML, Hoefs JC, et al. Quantitative liver function tests improve the prediction of clinical outcomes in chronic hepatitis C: results from the Hepatitis C Antiviral Long-term Treatment Against Cirrhosis Trial. Hepatology. 2012;55:1019–1029. doi: 10.1002/hep.24752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gayou O, Day E, Mohammadi S, Kirichenko A. A method for registration of single photon emission computed tomography (SPECT) and computed tomography (CT) images for liver stereotactic radiotherapy (SRT) Med Phys. 2012;39:7398–7401. doi: 10.1118/1.4766877. [DOI] [PubMed] [Google Scholar]
- 50.Bowen SR, Saini J, Chapman TR, et al. Differential hepatic avoidance radiation therapy: Proof of concept in hepatocellular carcinoma patients. Radiother Oncol. 2015;115:203–210. doi: 10.1016/j.radonc.2015.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cruite I, Schroeder M, Merkle EM, Sirlin CB. Gadoxetate disodium-enhanced MRI of the liver: part 2, protocol optimization and lesion appearance in the cirrhotic liver. AJR Am J Roentgenol. 2010;195:29–41. doi: 10.2214/AJR.10.4538. [DOI] [PubMed] [Google Scholar]
- 52.Yuan Y, Andronesi OC, Bortfeld TR, et al. Feasibility study of in vivo MRI based dosimetric verification of proton end-of-range for liver cancer patients. Radiother Oncol. 2013;106:378–382. doi: 10.1016/j.radonc.2013.01.016. [DOI] [PubMed] [Google Scholar]
- 53.Seidensticker M, Burak M, Kalinski T, et al. Radiation-induced liver damage: correlation of histopathology with hepatobiliary magnetic resonance imaging, a feasibility study. Cardiovasc Intervent Radiol. 2015;38:213–221. doi: 10.1007/s00270-014-0872-7. [DOI] [PubMed] [Google Scholar]
- 54.Osmundson EC, Wu Y, Luxton G, Bazan JG, Koong AC, Chang DT. Predictors of toxicity associated with stereotactic body radiation therapy to the central hepatobiliary tract. Int J Radiat Oncol Biol Phys. 2015;91:986–994. doi: 10.1016/j.ijrobp.2014.11.028. [DOI] [PubMed] [Google Scholar]
- 55.Nakayama H, Sugahara S, Tokita M, et al. Proton beam therapy for hepatocellular carcinoma: the University of Tsukuba experience. Cancer. 2009;115:5499–5506. doi: 10.1002/cncr.24619. [DOI] [PubMed] [Google Scholar]
- 56.Andratschke NHJ, Nieder C, Heppt F, Molls M, Zimmermann F. Stereotactic radiation therapy for liver metastases: factors affecting local control and survival. Radiation Oncology. 2015;10:69. doi: 10.1186/s13014-015-0369-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kavanagh BD, Pan CC, Dawson LA, et al. Radiation Dose–Volume Effects in the Stomach and Small Bowel. International Journal of Radiation Oncology*Biology*Physics. 2010;76:S101–S107. doi: 10.1016/j.ijrobp.2009.05.071. [DOI] [PubMed] [Google Scholar]
- 58.Das P, Abboud MT, Haque W, et al. Gastric bleeding after radiation therapy for intrahepatic cholangiocarcinoma. Pract Radiat Oncol. 2013;3:344–348. doi: 10.1016/j.prro.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 59.Kelly P, Das P, Pinnix CC, et al. Duodenal Toxicity After Fractionated Chemoradiation for Unresectable Pancreatic Cancer. International Journal of Radiation Oncology*Biology*Physics. 2013;85:e143–e149. doi: 10.1016/j.ijrobp.2012.09.035. [DOI] [PubMed] [Google Scholar]
- 60.Fukumitsu N, Sugahara S, Nakayama H, et al. A prospective study of hypofractionated proton beam therapy for patients with hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 2009;74:831–836. doi: 10.1016/j.ijrobp.2008.10.073. [DOI] [PubMed] [Google Scholar]
- 61.Kawashima M, Furuse J, Nishio T, et al. Phase II study of radiotherapy employing proton beam for hepatocellular carcinoma. J Clin Oncol. 2005;23:1839–1846. doi: 10.1200/JCO.2005.00.620. [DOI] [PubMed] [Google Scholar]
- 62.Mizumoto M, Okumura T, Hashimoto T, et al. Proton Beam Therapy for Hepatocellular Carcinoma: A Comparison of Three Treatment Protocols. International Journal of Radiation Oncology • Biology • Physics. 81:1039–1045. doi: 10.1016/j.ijrobp.2010.07.015. [DOI] [PubMed] [Google Scholar]


