Abstract
Background
Isolated hepatitis B core antibody (anti-HBc) is a common serologic finding in HIV-infected persons but the clinical significance is uncertain. We studied HIV/HCV infected women over time to determine if the trajectory of liver disease progression (LDP) is affected by isolated anti-HBc serologic status.
Methods
We performed serial Enhanced Liver Fibrosis (ELF) markers on HIV/HCV coinfected women to assess LDP trajectory over time comparing women with isolated anti-HBc to women with either negative HB serologies, anti-HBs alone or anti-HBc and anti-HBs. ELF, a serum marker that combines direct markers of extracellular matrix remodeling and fibrosis, was performed on serum stored biannually. Women with at least 3 ELF determinations and persistent HCV RNA positivity were included.
Results
344 women, including 132 with isolated anti-HBc and 212 with other serologic findings were included. A median of 6 (IQR 5–7) biannual ELF values were available for each woman, totaling 2119 visits. ELF increased over time from a median of 9.07 for women with isolated anti-HBc and 9.10 for those without to 9.83 and 9.88 respectively with no difference in degree of change or slope in the mixed effect model including age, race, CD4 count, antiretroviral therapy (ART), drug and alcohol use. Factors independently associated with LDP were older age, lower CD4, ART non-use and Hispanic ethnicity.
Conclusion
Isolated anti-HBc serologic status was not associated with accelerated liver disease progression over a median of 9.5 years among HIV/HCV coinfected women.
Keywords: HIV, Hepatitis C, Hepatitis B, Isolated anti-HBc, Liver disease progression, Hepatic fibrosis
Introduction
Antibody to hepatitis B core antigen (anti-HBc) in the absence of hepatitis B surface antigen (HBsAg) and hepatitis B surface antibody (anti-HBs) is a common serologic finding but the clinical significance is uncertain 1,2. Isolated anti-HBc could represent active hepatitis B (HBV) infection with undetectable or mutant HBsAg, resolved infection in which anti-HBs titers are below the level defining positive, a false positive test or, least likely, the “window period” between the resolution of HBs antigenemia and the development of anti-HBs 3–5. The significance of each of these causes of isolated anti-HBc is quite different and clinicians are faced with uncertainty about whether patients are susceptible to HBV, have active disease requiring monitoring or treatment, or have resolved disease.
Isolated anti-HBc is highly prevalent in HIV infection; in large cohorts, the prevalence of isolated anti-HBc has been found to range from 12 to 28% 6–8. In Western cohorts, isolated anti-HBc is consistently found to be associated with hepatitis C infection 3,7,9–12 and variably associated with higher HIV RNA, lower CD4+ T-lymphocyte count (CD4 count), and injection drug use 3,7,10,13. Long-term studies in the Women’s Interagency HIV Study (WIHS) and the Multicenter AIDS Cohort Study (MACS) have demonstrated that in approximately 80% of persons with isolated anti-HBc, serologic status is stable over time, with the remainder acquiring anti-HBs and a very small proportion acquiring HBsAg 7,14.
The HIV literature has numerous studies reporting the prevalence of HB viremia in persons with isolated anti-HBc. These studies are mostly cross-sectional and report low rates of detectable HB viremia, less than 5%, (though one outlier found that 89% of persons eventually demonstrated detectable viremia with repeated measures15)3,8,15,16. In the WIHS, for example, we found only eight (2%) of 400 HIV-infected women with isolated anti-HBc had detectable HB viremia 16. In contrast, it is clear from the liver transplant literature that a substantial proportion of those with anti-HBc have replication capable virus largely due to covalently closed circular DNA (cccDNA) in the nuclei of hepatocytes. In two large transplant series, the rates of de novo HBV infection were 58 and 66% when HBV naïve recipients received livers from anti-HBc positive donors without prophylaxis 17,18. Whether occult replication capable virus is present in the majority of HIV-infected persons with isolated anti-HBc is unknown.
In the face of the demonstrated persistence of isolated anti-HBc over time in HIV-infected persons and the uncertainty over its clinical significance, we sought to determine if the trajectory of liver disease progression in HIV/HCV co-infection is affected by isolated anti-HBc status.
Methods
This manuscript describes a nested study of HIV/HCV-infected women participating in the Women’s Interagency HIV Study (WIHS). The WIHS is a longitudinal study of HIV infected and uninfected at-risk women that enrolled 2054 HIV-infected women and 569 uninfected women at six sites, Chicago, San Francisco Bay Area, Brooklyn and Bronx/Manhattan, New York, Washington, DC and Los Angeles from October 1994 through November 1995. From October 2001 through September 2002, an additional 737 HIV-infected women and 406 uninfected women were enrolled. Informed consent was obtained from all participants in accordance with the US Department of Health and Human Services (DHHS) guidelines and the institutional review boards of participating institutions. The procedures followed were in accordance with the Helsinki Declaration of the World Medical Association. Women are seen semiannually for interview, physical exam and collection of blood and genital specimens. The cohort was designed to reflect the demographics of the HIV epidemic among US women. Details of cohort recruitment, retention and demographics are published elsewhere 19,20.
Included in this study were HIV-infected women with chronic hepatitis C infection and negative HBsAg who had three or more available measurements of the Enhanced Liver Fibrosis (ELF) test to allow for evaluation of trends over time. We chose to study HCV co-infected women because the majority of WIHS women with isolated anti-HBc have HCV co-infection 11 and these women were predicted to be more likely to experience liver disease progression during the period included in this study. The primary comparison was the degree and timing of liver fibrosis progression between women with isolated anti-HBc and those with other hepatitis serologic findings (including no positive HBV serologies, anti-HBs alone and anti-HBs with anti-HBc). Active HCV was defined by HCV antibody positivity with detectable HCV RNA at the most recent available measurement. HBV DNA was measured once at WIHS baseline on the vast majority (400/434) of HIV-infected women with isolated anti-HBc; women with HBV DNA at baseline were excluded.
The Enhanced Liver Fibrosis test (ELF) is a serum marker that combines direct markers of extracellular matrix remodeling and fibrosis: tissue inhibitor of metalloproteinase-1 (TIMP-1), hyaluronic acid (HA) and propeptide type III collagen (PIIINP) using highly sensitive automated immunoassays. ELF was performed in the WIHS on stored serum every two years on HIV/HCV coinfected women with at least 5 years of follow-up in the WIHS, available serum from the first or second WIHS visit and varying levels of fibrosis by the APRI and FIB-4 non-invasive markers. Because limited funds were available for ELF measurements, the group was randomly selected from women with intermediate values of APRI and FIB-4 and enriched, again randomly, for women with APRI and FIB-4 representing the extremes of mild and more severe fibrosis. Overall 76% (376/499) of HIV/HCV infected women with 5 years of followup and available serum had serial ELF determinations.
Cutoffs recommended by the manufacturer and independent investigators are based on liver biopsy correlations studies; ELF levels defining cirrhosis, fibrosis and excluding fibrosis are ≥11.3, ≥9.8 and <7.7 respectively 21,22. The first visit where ELF was performed is the index visit for this study, as opposed to the baseline visit which is entry into the main WIHS study.
Variable classifications
The outcome for analysis was liver disease progression (LDP) as indicated by ELF score on a continuous scale and as a categorical variable with clinically significant cutoffs. The primary explanatory variable was presence of isolated anti-HBc (yes/no) at the index visit (first ELF visit). Other variables selected a priori for inclusion in multivariable analysis were based on previously identified associations with liver disease progression or conceptual or clinical relevance. These covariates included age at the index visit, race/ethnicity, CD4 count, use of and type of highly active antiretroviral therapy (HAART), any hepatitis C treatment, alcohol use, and ever use or recent use of injection drugs. CD4 count was analyzed as <200 vs. ≥200. Total number of alcoholic drinks per week was analyzed as a continuous variable and categorically (hazardous vs non-hazardous drinking with hazardous drinking defined as >7 drinks/week or >3 drinks on any occasion). CD4 count, HAART use, alcohol use, and recent injection drug use were treated as time-varying for analysis. HIV RNA was explored as a covariate in preliminary analyses and, though significantly associated with LDP, was consistently co-linear with CD4 count and HAART use and added little to the model, therefore we chose to use CD4 count and HAART use in the model.
Statistical Methods
Distributions of exposure variables at the index visit were compared by isolated anti-HBc status using Pearson Chi-Square tests for categorical variables and Wilcoxon rank-sum tests for continuous variables. Multivariable associations between the various HBV serologic findings and ELF were assessed using mixed effects linear regression models to account for correlation between repeated measurements on women over time. Models included random effects for intercept and trend to allow for individual variation about the intercept and in trajectories over time. Differences in LDP trends by isolated anti-HBc status over time were assessed by fitting models with a group by linear time interaction term with likelihood ratio testing to assess statistical significance. Higher order interaction terms were also explored but were not significant. In the absence of significant interactions, models were fit without the interaction term to estimate average group differences across all visits. Linear regression assumptions were assessed using standard model diagnostics, including graphical examination of normal probability and residual plots and by plotting residuals vs. predicted values. For analysis using categorical cutpoints for LDP, Cox proportional hazards regression was used to compare time to progression from no fibrosis to (fibrosis or cirrhosis) and progression from no cirrhosis to cirrhosis by isolated anti-HBc status among those without fibrosis or cirrhosis at the index visit. Analyses were conducted using SAS software version 9.3 (SAS Institute, Cary, NC).
Laboratory Methods
Hepatitis B serologies including anti-HBs, total anti-HBc and HBsAg were performed at the baseline WIHS visit (i.e. WIHS study entry) using Abbott Ausab enzyme immunoassay (EIA), Abbott Corzyme EIA and Abbott Auszyme Microparticle EIA, respectively (Abbott Laboratories, Abbott Park, IL) 23,24.
HCV infection was defined by HCV antibody and RNA positivity. Hepatitis C antibody testing was performed at WIHS baseline using EIA 2.0 or 3.0 (Abbott Laboratories and Ortho Diagnostics, respectively). Qualitative HCV RNA testing was performed at baseline on HCV antibody positive women using COBAS Amplicor HCV v2.0 (Roche Diagnostics, Branchburg, New Jersey, USA) with a lower limit of 100 IU/ml. Repeat HCV RNA testing was performed in 2008 on the last available sample for all women with anti-HCV. Only women who were HCV RNA positive at the most recent testing were included, thus women who were treated for HCV and had a documented sustained viral response are not included but women with unsuccessful HCV therapy are included.
ELF includes three direct measures of extracellular matrix components: HA, PIIINP and TIMP-1 which were assayed on an automated IMMUNO 1 immunoanalyzer (Siemens Medical Solutions Diagnostics Tarrytown, NY). The assays are magnetic particle separation immunoassays. The TIMP-1 and PIIINP assays each use two monoclonal antibodies that bind to independent binding sites on their respective antigens. The HA assay uses HA binding protein which is isolated from bovine nasal septum. The ELF marker was analyzed individually and the results continually refereed to a set of quality standards to ensure accurate analysis. Tests were performed according to the manufacturer’s instructions at iQur Limited London, UK. The performance of ELF has been reported using the area under the receiver operating curve (AUROC) to describe its ability to detect histologically classified mild, moderate or severe fibrosis in chronic hepatitis C liver biopsies with excellent sensitivity and specificity 25,26.
Results
Characteristics of the Study Population
Three hundred eighty five HIV/HCV co-infected WIHS women had available ELF data. Of these 385, 41 were excluded from further analysis: three had unknown HBV antibody status, six had HBs antigenemia, 15 had fewer than three valid ELF measurements and 17 cleared hepatitis C (primarily due to treatment) during the study period. The final analytic sample consisted of 344 women with ELF measured at 2119 visits (median 6 visits, range 3–14 visits) over an average of 9.5 years of total follow-up. Of those included, 132 women had isolated anti-HBc and 212 had other serologic patterns, including 67 with negative hepatitis B serologies, 13 with only anti-HBs implying vaccination and 132 with anti-HBs and anti-HBc, implying resolved natural infection. Characteristics of the women are presented in Table 1. At the index visit, participants were median age 40 (IQR 36–44). By race/ethnicity, 14.5% self identified as white, 63.3% black, 20.3% Hispanic, and 1.7% other races. Only 12% were on HAART at the index visit, because most of these visits were in 1995 and 1996 before widespread availability of HAART.
Table 1.
Characteristics of HIV/HCV coinfected women by Hepatitis B Core Antibody Status
| Isolated Anti-HBc, n (%) or Median (IQR) | Non-isolated Anti-HBc, n (%) or Median (IQR) | p-value3 | |
|---|---|---|---|
| Total women, n | 132 | 212 | – |
| Total study visits, median (IQR) | 6 (5–7) | 6 (5–7) | 0.316 |
| Total follow-up time in years, median (IQR) | 9.87 (7.28–11.61) | 9.40 (7.21–11.36) | 0.300 |
| Year of index visit, median (IQR) | 1996 (1995–1997) | 1996 (1995–1997) | 0.807 |
| ELF at index visit, median (IQR) | 9.07 (8.45–9.71) | 9.10 (8.46–9.72) | 0.801 |
| ELF categories | 0.577 | ||
| <7.7 (no fibrosis) | 7 (5.3) | 11 (5.2) | |
| 7.7–9.7 | 96 (72.7) | 156 (73.6) | |
| 9.8–11.2 (fibrosis) | 24 (18.2) | 42 (19.8) | |
| ≥11.3 (cirrhosis) | 5 (3.8) | 3 (1.4) | |
| Age at index visit, median (IQR) | 40 (36–44) | 40 (36–43) | 0.587 |
| Race/Ethnicity | 0.932 | ||
| White | 18 (13.6) | 32 (15.1) | |
| Black | 84 (63.6) | 134 (63.2) | |
| Hispanic | 27 (20.5) | 43 (20.3) | |
| Other race | 3 (2.3) | 3 (1.4) | |
| CD4 count at index visit, median (IQR) | 362 (227.5–562.5) | 360 (229–530) | 0.561 |
| CD4 count, all visits (n=2098), median (IQR) | 379.5 (226.5–587.5) | 372 (216–558) | 0.842 |
| HAART use at index visit | 12 (9.1) | 29 (13.7) | 0.202 |
| Used Lamivudine | 26 (23.6) | 39 (20.5) | 0.529 |
| Used Emtricidabine | 0 (0.0) | 2 (0.9) | 0.526 |
| Used Tenofovir | 0 (0.0) | 0 (0.0) | — |
| HAART use, all visits (n=2119) | 369 (45.0) | 683 (52.6) | 0.070 |
| Used Lamivudine (n=2073) | 352 (44.1) | 574 (45.0) | 0.777 |
| Used Emtricidabine (n=2119) | 36 (4.4) | 71 (5.5) | 0.388 |
| Used Tenofovir (n=2119) | 86 (10.5) | 164 (12.6) | 0.342 |
| Hepatitis C treatment at index visit | 0 (0.0) | 0 (0.0) | — |
| Any treatment for Hepatitis C during study | 23 (17.4) | 34 (16.0) | 0.737 |
| # Drinks/week at index visit, median (IQR)1 | 0 (0–4.5) | 0 (0–2.5) | 0.248 |
| # Drinks/week, all study visits (n=2081), median (IQR)a | 0 (0–1.5) | 0 (0–1.5) | 0.512 |
| Hazardous alcohol use at index visit1,2 | 31 (24.0) | 38 (18.1) | 0.188 |
| Hazardous alcohol use, all visits2 (n=2082) | 145 (18.1) | 216 (16.9) | 0.796 |
| IDU, ever (at index visit) | 128 (97.0) | 174 (82.1) | <0.001 |
| IDU, recent use at index visit1 | 25 (19.1) | 33 (15.6) | 0.409 |
| IDU, recent use, all visitsa (n=2090) | 82 (10.2) | 116 (9.0) | 0.408 |
Time frame for exposure is since last WIHS visit
Hazardous alcohol use was defined as > 7 drinks per week or > 3 drinks on any occasion since the last WIHS visit.
P-values were calculated using Pearson Chi-Square tests, Fisher’s exact tests, or Wilcoxon rank-sum tests for baseline comparisons and generalized estimating equations with adjustment for clustering among repeated measures for comparisons across multiple visits.
Abbreviations: IQR: interquartile range; IDU: injection drug use; ELF: Enhanced Liver Fibrosis score; HAART: highly active antiretroviral therapy.
At the index visit, patients with isolated anti-HBc were more likely than those with other serologic patterns to report a history of injection drug use (p<0.001). No differences were observed at baseline for ELF, age, race/ethnicity, CD4 count, HAART use, alcohol use, or recent injection drug use (Table 1). Over the visits studied, women with isolated anti-HBc had fewer visits on HAART but there was no difference in median CD4 count for all visits studied. (Table 1). Fifty seven study women received any treatment for hepatitis C during the study period but those with sustained viral response were not included, so only women who continued to be viremic are included in the study.
Liver Disease Progression over Time
Overall, ELF increased over time from a median of 9.07 for women with isolated anti-HBc and 9.10 for those with other HBV serologies to 9.83 and 9.88 respectively (Figure 1) with no difference in degree of change or slope of change by anti-HBc status in the unadjusted model (p=0.638 and p=0.178 respectively). There was no significant difference in liver disease progression (LDP) as defined by ELF in the mixed effects model that included age, race, history of injection drug use at the index visit, CD4, HAART use, and alcohol use (p=0.949) (Table 2). Factors independently associated with LDP were more elapsed time, older age, lower CD4 and HAART non-use (p≤0.001 for all). We also found a marginally significant difference in liver disease progression for Hispanic/Latino women compared to white women (p=0.038).
Figure 1. Liver Disease Progression over Time among HIV/HCV Coinfected Women by Isolated Anti-HBc Status.
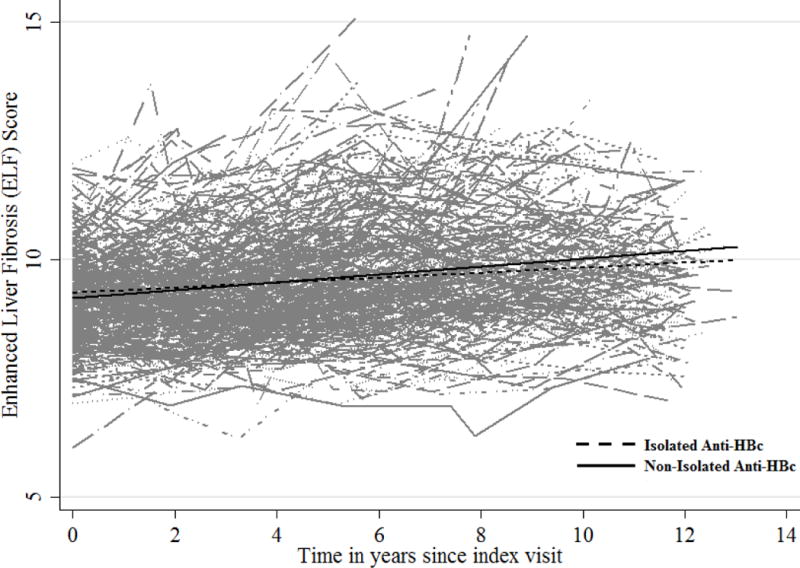
There was no difference in ELF change over time by isolated Anti-HBc status; p=0.178 for the group by time interaction in the final model that included age, race, CD4<200, HAART use, Hepatitis C treatment, hazardous alcohol use, and history of IDU + group, time, and group × time interaction
Table 2.
Results from Multivariable Mixed Effects Linear Regression Models: Factors Associated with Liver Disease Progression as Measured by Enhanced Liver Fibrosis (ELF) Score
| Estimate** | 95% CI | p-value | |
|---|---|---|---|
| Isolated Anti-HBc vs other serologies | 0.01 | −0.19, 0.20 | 0.949 |
| Age in years at index visit (per year increase) | 0.04 | 0.02, 0.06 | <0.001 |
| Race/Ethnicity | |||
| [Reference=White] | |||
| Black | 0.05 | −0.22, 0.32 | 0.716 |
| Hispanic | 0.34 | 0.02, 0.65 | 0.038 |
| Other race | −0.15 | −0.90, 0.60 | 0.691 |
| CD4 <200 vs > 200 | 0.38 | 0.28, 0.49 | <0.001 |
| HAART use vs non-use | −0.15 | −0.24, −0.06 | 0.001 |
| Any Hepatitis C treatment* | −0.08 | −0.28, 0.11 | 0.410 |
| Hazardous alcohol use vs non-use or non-hazardous use | −0.02 | −0.12, 0.09 | 0.746 |
| IDU ever vs never | 0.16 | −0.13, 0.46 | 0.274 |
| Follow-up time in years (per year) | 0.11 | 0.09, 0.13 | <0.001 |
Estimate reflects mean ELF compared to the reference category for each variable and is adjusted for other variables shown. CD4 count, HAART use, Hepatitis C treatment, and hazardous alcohol use were treated as time-varying in analysis; age, race/ethnicity, and history of injection drug use were treated as fixed covariates using the value at the index study visit.
Those with sustained viral response were not included in the study
Abbreviations: IDU: injection drug use; HAART: highly active antiretroviral therapy; CI: confidence interval.
When we analyzed LDP comparing women with isolated anti-HBc to women with specific HBV serologic findings, we found no difference in ELF trajectory for women with isolated anti-HBc compared with anti-HBc negative women (women with negative HBV serologies and vaccinated women) (p=0.39). Of interest, women with anti-HBs and anti-HBc had more rapid liver disease progression by ELF than anti-HBc negative women (p=0.029).
Progression to Cirrhosis over Time
We also analyzed the data using ELF as a categorical variable using the cutoffs above for cirrhosis (≥11.3) fibrosis (≥9.8) and excluding fibrosis (<7.7). Similar to the model using ELF as a continuous variable, using ELF as a categorical variable looking at progression between liver fibrosis stages, we found no difference by the Cox regression model in progression from no fibrosis to (fibrosis or cirrhosis) or progression from no cirrhosis to cirrhosis between women with isolated anti-HBc and those with all other serologic findings (p=0.518 and 0.225) respectively) or those with negative HBV serologies (p=0.540 and 0.525 respectively).
Effect of Antiretrovirals on Liver Disease Progression
We examined associations between antiretroviral medications with HBV activity and ELF. However in the vast majority (961/1121) of visits during which HAART was used, the regimen contained at least one drug with HBV activity. In multivariable models, ELF levels were inversely correlated with use of tenofovir, lamivudine, and emtricitabine-containing regimens when the effect of each was examined separately but this was not different from the effect of being on any HAART. Presented results are based on the composite HAART use variable (any vs. none) due to similarity in direction and magnitude of that variable to each of the individual antiretrovirals.
Discussion
We have demonstrated that, over a median of more than 9.5 years, liver disease progression as measured by the Enhanced Liver Fibrosis marker did not differ between HIV/HCV coinfected women with isolated anti-HBc and those with all other serologic findings or those with negative HBV serologies. While it is clear from the transplant literature and case reports of reactivation HBV that some cases of isolated anti-HBc represent occult HBV, our findings are reassuring that isolated anti-HBc status is not relevant to the trajectory of liver disease progression in the majority of cases. 7,17,18,27,28. Further, in the era of antiretrovirals whose antiviral spectrum includes HBV activity, the potential clinical significance of occult HBV infection on liver disease progression is even less important to consider.
Numerous investigators have attempted to ascertain the clinical significance of isolated anti-HBc in HIV. However most of these investigations have centered around occult active hepatitis B rather than liver disease progression. To our knowledge, ours is the first study to ascertain the trajectory of liver disease progression over time among HIV-infected person with hepatitis C and isolated anti-HBc. A cross-sectional study using the Veterans Aging Cohort Study data found that isolated anti-HBc status among persons with anti-HCV was associated with more advanced liver disease by APRI and FIB-4 compared to persons with no positive HBV serologies 29. One small study investigated liver stiffness values in HIV-infected persons with isolated anti-HBc without a control group and found that liver stiffness was associated with HCV infection and lipodystrophy but the investigation was unable to shed light on the effect of isolated anti-HBc serologic status because of lack of a control group30.
The Enhanced Liver Fibrosis marker has proved a valuable tool to non-invasively assess liver disease progression. In our study, the marker was useful both as a continuous and as a categorical variable to look at the effect of isolated anti-HBc on fibrosis progression. Although hepatitis C is the most well-studied, ELF has been shown to correlate well with fibrosis severity in other hepatic diseases including hepatitis B, fatty liver, alcoholic liver disease and primary biliary cirrhosis 25,26,31–33. ELF has been found to correlate with clinical outcomes as well. In 457 European patients with a variety of liver diseases and followed for a median of 7 years after ELF determination, baseline ELF predicted all liver outcomes (including liver-related death, manifestations of decompensated cirrhosis, hepatocellular carcinoma and transplantation). In this study, a one point increase in ELF was associated with a twofold increase in risk of clinical hepatic outcome across the range of ELF scores 34. In a study of 170 patients with hepatitis B followed for a median of 41 months, the area under the receiver operating curve (AUROC) for ELF predicting liver related outcomes was higher (though not significantly) than transient elastography or liver biopsy 35. In the WIHS, ELF was found to predict all cause mortality better than APRI and FIB-4 36.
We found that markers of HIV disease status, CD4 count and HAART use were the most potent predictors of liver disease progression. This has been demonstrated in other longitudinal studies; control of HIV has been consistently found to slow liver disease progression in HIV/HCV coinfection 37. Also in our model, Hispanic ethnicity was associated with more rapid liver disease progression. Several studies in hepatitis C mono-infected persons have noted accelerated liver disease progression. Proposed explanations for these findings were higher prevalence of hepatic steatosis, obesity and elements of the metabolic syndrome 38–40. In the WIHS, Sarkar and colleagues demonstrated that HIV/HCV coinfected Hispanics had a greater risk of liver-related mortality than African-Americans and more accelerated liver disease progression by FIB-4 41. More in-depth analysis of this observation using genetic and epidemiologic data is ongoing.
Our study should be evaluated in light of its limitations. We evaluated the significance of isolated anti-HBc in women with HIV and HCV; given the complex interaction of these viruses, our results may not be generalizable to all instances of isolated anti-HBc. We did not have contemporaneous measurements of hepatitis B DNA for the timepoints where ELF was measured. We were also unable to measure cccDNA in the hepatic parenchyma. However, our intent was to use many longitudinal datapoints to draw clinically useful conclusions and these measures are often not measured clinically.
Our study has many strengths, including more than 9 years of follow-up of women who began the study period with a wide range of liver fibrosis severity and a well-characterized and diverse cohort with the ability to control for known confounders, including drug and alcohol use and indicators of HIV disease progression. The lack of accelerated liver disease progression demonstrated in this longitudinal study supports the assertion that the presence of anti-HBc does not portend a worse liver disease prognosis in HIV/HCV coinfection.
Acknowledgments
Source of Funding: The Women’s Interagency HIV Study (WIHS) is funded primarily by the National Institute of Allergy and Infectious Diseases (NIAID) (U01-AI-103401, U01-AI-103408, UO1-AI-35004, UO1-AI-31834, UO1-AI-34994, U01-AI-103397, U01-AI-103390, UO1-AI-34989, and UO1-AI-42590), with additional co-funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), the National Cancer Institute (NCI), the National Institute on Drug Abuse (NIDA), and the National Institute on Mental Health (NIMH). Targeted supplemental funding for specific projects is also provided by the National Institute of Dental and Craniofacial Research (NIDCR), the National Institute on Alcohol Abuse and Alcoholism (NIAAA), the National Institute on Deafness and other Communication Disorders (NIDCD), and the NIH Office of Research on Women’s Health.
William Rosenberg is an inventor of the ELF test, which is owned by Siemens, he is a consultant for Siemens but receives no income from ELF testing
Footnotes
The data were presented in part at the 2014 Conference on Retroviruses and Opportunistic Infections, Boston MA, March 2014
Conflicts of Interest: All other authors, no conflicts of interest.
References
- 1.Grob P, Jilg W, Bornhak H, et al. Serological pattern “anti-HBc alone”: report on a workshop. J Med Virol. 2000;62(4):450–455. doi: 10.1002/1096-9071(200012)62:4<450::aid-jmv9>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 2.Knoll A, Hartmann A, Hamoshi H, Weislmaier K, Jilg W. Serological pattern “anti-HBc alone”: characterization of 552 individuals and clinical significance. World J Gastroenterol. 2006;12(8):1255–1260. doi: 10.3748/wjg.v12.i8.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Neau D, Winnock M, Jouvencel AC, et al. Occult hepatitis B virus infection in HIV-infected patients with isolated antibodies to hepatitis B core antigen: Aquitaine cohort, 2002–2003. Clin Infect Dis. 2005;40(5):750–753. doi: 10.1086/427882. [DOI] [PubMed] [Google Scholar]
- 4.Gandhi RT, Wurcel A, McGovern B, et al. Low prevalence of ongoing hepatitis B viremia in HIV-positive individuals with isolated antibody to hepatitis B core antigen. J Acquir Immune Defic Syndr. 2003;34(4):439–441. doi: 10.1097/00126334-200312010-00013. [DOI] [PubMed] [Google Scholar]
- 5.Quaglio G, Lugoboni F, Vento S, et al. Isolated presence of antibody to hepatitis B core antigen in injection drug users: do they need to be vaccinated? Clin Infect Dis. 2001;32(10):E143–144. doi: 10.1086/320162. [DOI] [PubMed] [Google Scholar]
- 6.Tien PC, Kovacs A, Bacchetti P, et al. Association between syphilis, antibodies to herpes simplex virus type 2, and recreational drug use and hepatitis B virus infection in the Women’s Interagency HIV Study. Clin Infect Dis. 2004;39(9):1363–1370. doi: 10.1086/424879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Witt MD, Lewis RJ, Rieg G, Seaberg EC, Rinaldo CR, Thio CL. Predictors of the isolated hepatitis B core antibody pattern in HIV-infected and -uninfected men in the multicenter AIDS cohort study. Clin Infect Dis. 2013;56(4):606–612. doi: 10.1093/cid/cis908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Di Lello FA, Macias J, Cifuentes CC, Vargas J, Palomares JC, Pineda JA. Low prevalence of occult HBV infection among HIV-infected patients in Southern Spain. Enferm Infecc Microbiol Clin. 2012;30(6):312–314. doi: 10.1016/j.eimc.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 9.Berger A, Doerr HW, Rabenau HF, Weber B. High frequency of HCV infection in individuals with isolated antibody to hepatitis B core antigen. Intervirology. 2000;43(2):71–76. doi: 10.1159/000025026. [DOI] [PubMed] [Google Scholar]
- 10.Davaro RE, Cheeseman SH, Keroack MA, Ellison RT., 3rd The significance of isolated antibody to hepatitis B core antigen seropositivity in patients infected with human immunodeficiency virus. Clin Infect Dis. 1996;23(1):189–190. doi: 10.1093/clinids/23.1.189. [DOI] [PubMed] [Google Scholar]
- 11.French AL, Operskalski E, Peters M, et al. Isolated hepatitis B core antibody is associated with HIV and ongoing but not resolved hepatitis C virus infection in a cohort of US women. J Infect Dis. 2007;195(10):1437–1442. doi: 10.1086/515578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Perez-Rodriguez MTSB, Crespo M, et al. Clinical significance of anti-HBc alone in HIV positive patients. World J Gastroenterol. 2009;15(10):1237–1241. doi: 10.3748/wjg.15.1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cohen Stuart JW, Velema M, Schuurman R, Boucher CA, Hoepelman AI. Occult hepatitis B in persons infected with HIV is associated with low CD4 counts and resolves during antiretroviral therapy. J Med Virol. 2009;81(3):441–445. doi: 10.1002/jmv.21422. [DOI] [PubMed] [Google Scholar]
- 14.French AL, Lin MY, Evans CT, et al. Long-term serologic follow-up of isolated hepatitis B core antibody in HIV-infected and HIV-uninfected women. Clin Infect Dis. 2009;49(1):148–154. doi: 10.1086/599610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hofer M, Joller-Jemelka HI, Grob PJ, Luthy R, Opravil M. Frequent chronic hepatitis B virus infection in HIV-infected patients positive for antibody to hepatitis B core antigen only. Swiss HIV Cohort Study. Eur J Clin Microbiol Infect Dis. 1998;17(1):6–13. doi: 10.1007/BF01584356. [DOI] [PubMed] [Google Scholar]
- 16.Tsui JI, French AL, Seaberg EC, et al. Prevalence and long-term effects of occult hepatitis B virus infection in HIV-infected women. Clin Infect Dis. 2007;45(6):736–740. doi: 10.1086/520989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Avelino-Silva VI, D’Albuquerque LA, Bonazzi PR, et al. Liver transplant from Anti-HBc-positive, HBsAg-negative donor into HBsAg-negative recipient: is it safe? A systematic review of the literature. Clin Transplant. 2010;24(6):735–746. doi: 10.1111/j.1399-0012.2010.01254.x. [DOI] [PubMed] [Google Scholar]
- 18.Skagen CL, Jou JH, Said A. Risk of de novo hepatitis in liver recipients from hepatitis-B core antibody-positive grafts - a systematic analysis. Clin Transplant. 2011;25(3):E243–249. doi: 10.1111/j.1399-0012.2011.01409.x. [DOI] [PubMed] [Google Scholar]
- 19.Barkan SE, Melnick SL, Preston-Martin S, et al. The Women’s Interagency HIV Study. WIHS Collaborative Study Group. Epidemiology. 1998;9(2):117–125. [PubMed] [Google Scholar]
- 20.Hessol NA, Schneider M, Greenblatt RM, et al. Retention of women enrolled in a prospective study of human immunodeficiency virus infection: impact of race, unstable housing, and use of human immunodeficiency virus therapy. Am J Epidemiol. 2001;154(6):563–573. doi: 10.1093/aje/154.6.563. [DOI] [PubMed] [Google Scholar]
- 21.Lichtinghagen R, Pietsch D, Bantel H, Manns MP, Brand K, Bahr MJ. The Enhanced Liver Fibrosis (ELF) score: normal values, influence factors and proposed cut-off values. J Hepatol. 2013;59(2):236–242. doi: 10.1016/j.jhep.2013.03.016. [DOI] [PubMed] [Google Scholar]
- 22.Advia. Advia Centaur Enhanced liver. Fibrosis Test Specifications. 2015:2015. [Google Scholar]
- 23.Ismail N, Fish GE, Smith MB. Laboratory evaluation of a fully automated chemiluminescence immunoassay for rapid detection of HBsAg, antibodies to HBsAg, and antibodies to hepatitis C virus. J Clin Microbiol. 2004;42(2):610–617. doi: 10.1128/JCM.42.2.610-617.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.La’ulu SL, Roberts WL. The analytic sensitivity and mutant detection capability of six hepatitis B surface antigen assays. Am J Clin Pathol. 2006;125(5):748–751. doi: 10.1309/K5EM-795V-NGGF-GBXX. [DOI] [PubMed] [Google Scholar]
- 25.Rosenberg WM, Voelker M, Thiel R, et al. Serum markers detect the presence of liver fibrosis: a cohort study. Gastroenterology. 2004;127(6):1704–1713. doi: 10.1053/j.gastro.2004.08.052. [DOI] [PubMed] [Google Scholar]
- 26.Xie Q, Zhou X, Huang P, Wei J, Wang W, Zheng S. The performance of enhanced liver fibrosis (ELF) test for the staging of liver fibrosis: a meta-analysis. PLoS One. 2014;9(4):e92772. doi: 10.1371/journal.pone.0092772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sheng WH, Kao JH, Chen PJ, et al. Evolution of hepatitis B serological markers in HIV-infected patients receiving highly active antiretroviral therapy. Clin Infect Dis. 2007;45(9):1221–1229. doi: 10.1086/522173. [DOI] [PubMed] [Google Scholar]
- 28.Chakvetadze C, Bani-Sadr F, Le Pendeven C, Lamontagne F, Vincensini JP, Pialoux G. Reactivation of hepatitis B virus replication during peginterferon-ribavirin therapy in an HIV/hepatitis C virus-co-infected patient with isolated anti-hepatitis B core antibodies. AIDS. 2007;21(3):393–394. doi: 10.1097/QAD.0b013e328012b5d3. [DOI] [PubMed] [Google Scholar]
- 29.Bhattacharya DC-hT, Tate J, et al. Isolated hepatitis B core antibody is associated with advanced hepatitic fibrosis; Twentieth Conference on Retroviruses and Opportunistic Infections; 2014; Boston, MA. [Google Scholar]
- 30.Chakvetadze C, Bani-Sadr F, Lescure FX, et al. Liver stiffness values in HIV-infected patients with isolated anti-hepatitis B core antibodies. Med Mal Infect. 2014;43(6):222–225. doi: 10.1016/j.medmal.2013.02.011. [DOI] [PubMed] [Google Scholar]
- 31.Nobili V, Parkes J, Bottazzo G, et al. Performance of ELF serum markers in predicting fibrosis stage in pediatric non-alcoholic fatty liver disease. Gastroenterology. 2009;136(1):160–167. doi: 10.1053/j.gastro.2008.09.013. [DOI] [PubMed] [Google Scholar]
- 32.Mayo MJ, Parkes J, Adams-Huet B, et al. Prediction of clinical outcomes in primary biliary cirrhosis by serum enhanced liver fibrosis assay. Hepatology. 2008;48(5):1549–1557. doi: 10.1002/hep.22517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Morling JR, Fallowfield JA, Guha IN, et al. Using non-invasive biomarkers to identify hepatic fibrosis in people with type 2 diabetes mellitus: the Edinburgh type 2 diabetes study. J Hepatol. 2014;60(2):384–391. doi: 10.1016/j.jhep.2013.10.017. [DOI] [PubMed] [Google Scholar]
- 34.Parkes J, Roderick P, Harris S, et al. Enhanced liver fibrosis test can predict clinical outcomes in patients with chronic liver disease. Gut. 2010;59(9):1245–1251. doi: 10.1136/gut.2009.203166. [DOI] [PubMed] [Google Scholar]
- 35.Kim BK, Kim HS, Yoo EJ, et al. Risk assessment of clinical outcomes in Asian patients with chronic hepatitis B using enhanced liver fibrosis test. Hepatology. 2014;60(6):1911–1919. doi: 10.1002/hep.27389. [DOI] [PubMed] [Google Scholar]
- 36.Peters M, Bacchetti P, Boylan R, et al. Utility of enhance liver fibrosis marker as a non-invastive predictor of mortality in HIV/HCV coinfected women in the WIHS. Sixth Conference on HIV Pathogenesis and Treatment; 2011; Rome, Italy. [Google Scholar]
- 37.Chen J, Feeney ER, Chung RT. HCV and HIV co-infection: mechanism and management. Nat Rev Gastroenterol Hepatol. 2014;11:362–371. doi: 10.1038/nrgastro.2014.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Verma S, Bonacini M, Govindarajan S, Kanel G, Lindsay KL, Redeker A. More advanced hepatic fibrosis in hispanics with chronic hepatitis C infection: role of patient demographics, hepatic necroinflammation, and steatosis. Am J Gastroenterol. 2006;101(8):1817–1823. doi: 10.1111/j.1572-0241.2006.00682.x. [DOI] [PubMed] [Google Scholar]
- 39.Kallwitz ER, Layden-Almer J, Dhamija M, et al. Ethnicity and body mass index are associated with hepatitis C presentation and progression. Clin Gastroenterol Hepatol. 2010;8(1):72–78. doi: 10.1016/j.cgh.2009.08.009. [DOI] [PubMed] [Google Scholar]
- 40.Carrion AF, Ghanta R, Carrasquillo O, Martin P. Chronic liver disease in the Hispanic population of the United States. Clin Gastroenterol Hepatol. 2011;9(10):834–841. doi: 10.1016/j.cgh.2011.04.027. quiz e109–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sarkar MBP, French AL, et al. Lower liver-related death in African-American women with HIV/hepatitis C coinfection compared to Caucasian and Hispanic women. Hepatology. 2012;56:1799–1705. doi: 10.1002/hep.25859. [DOI] [PMC free article] [PubMed] [Google Scholar]


