Abstract
Alcohol consumption is associated with a modest increased risk of colon cancer, but its relationship with colon cancer survival has not been elucidated. Using data from a phase III randomized adjuvant trial, we assessed the association of alcohol consumption with colon cancer outcomes. Patients completed a risk factor questionnaire before randomization to FOLFOX or FOLFOX+cetuximab (N=1984). Information was collected on lifestyle factors, including smoking, physical activity, and consumption of different types of alcohol. Cox models assessed the association between alcohol consumption and outcomes of disease-free survival (DFS), time-to-recurrence (TTR) and overall survival (OS), adjusting for age, sex, study arm, body mass, smoking, physical activity, and performance status. No statistically significant difference in outcomes between ever and never drinkers were noted [hazard ratio (HR)DFS=0.86, HRTTR=0.87, HROS=0.86, p-values=0.11 to 0.17]. However, when considering alcohol type, ever consumers of red wine (n=628) had significantly better outcomes than never consumers (HRDFS=0.80, HRTTR=0.81, HROS=0.78, p-values=0.01 to 0.02). Favorable outcomes were confirmed in patients who consumed 1–30 glasses/month of red wine (n=601, HR=0.80 to 0.83, p-values=0.03 to 0.049); there was a suggestion of more favorable outcomes in patients who consumed >30 glasses/month of red wine (n=27, HR=0.33 to 0.38, p-values=0.05 to 0.06). Beer and liquor consumption were not associated with outcomes. Although alcohol consumption was not associated with colon cancer outcomes overall, mild to moderate red wine consumption was suggestively associated with longer OS, DFS, and TTR in stage III colon cancer patients.
Keywords: colon cancer, alcohol, survival, recurrence, red wine
INTRODUCTION
Despite recent gains in prevention and early detection, colon cancer remains a leading cause of cancer-related mortality in the United States.1 Although risk factors for colon cancer are relatively well-established, comparatively little is known about epidemiologic factors associated with colon cancer prognosis. In particular, the role of lifestyle factors, such as alcohol consumption, in relation to colon cancer outcomes remains unclear.
The consumption of alcoholic beverages has been classified as a Class I carcinogen by the International Agency for Research on Cancer (IARC).2 In particular, studies of colon cancer have noted a modest increased risk of disease among moderate or heavy drinkers compared to non-drinkers, primarily among men.3–8 Similarly, alcohol consumption has been associated with an increased risk for liver metastasis after colon cancer and higher stage at diagnosis.9,10 The few studies that have considered the possible relationship between alcohol consumption and colon cancer survival have noted no significant association;11–13 however, in a recent analysis of patients with early-stage rectal cancers, Patel et al. found no association between alcohol consumption and overall survival, but an increased risk of disease recurrence and shorter time to disease recurrence, among drinkers relative to non-drinkers.14 In light of such findings, and given the established association between moderate alcohol consumption, particularly wine consumption, and lower risk of cardiovascular disease mortality,15 it is plausible that studies of overall survival and longer-term mortality outcomes may obscure associations between alcohol consumption and shorter-term colon cancer outcomes, such as disease recurrence. Similarly, studies of overall alcohol consumption may obscure associations between consumption of specific types of alcohol and disease outcomes.
Using data from a clinical trial of adjuvant chemotherapy for stage III colon cancer, we evaluated the relationship between pre-diagnostic alcohol consumption, disease-free survival, overall survival, and time-to-recurrence, with consideration for alcohol type and possible heterogeneity by tumor and patient attributes.
METHODS AND MATERIALS
Study Population
N0147 is a multicenter phase III randomized trial of adjuvant 5-fluorouracil, oxaliplatin, and leucovorin (FOLFOX) with or without cetuximab in patients with resected stage III colon cancer, led by the North Central Cancer Treatment Group (NCCTG) (now a part of the Alliance for Clinical Trials in Oncology).16 Eligible patients had histologically confirmed adenocarcinoma of the colon, at least one pathologically confirmed positive lymph node, complete surgical resection performed ≤56 days before randomization, and Eastern Cooperative Oncology Group performance status ≤2. Patients with evidence of metastatic disease, other prior or concurrent malignancies, previous EGFR therapy, aged <18 years, or with exclusionary co-morbid conditions at the time of randomization were excluded from participation. Enrollment began on February 10, 2004, and closed on November 25, 2009. In total, 3,397 patients were enrolled, of whom 2,686 were randomized to the primary treatment comparison arms (FOLFOX vs. FOLFOX plus cetuximab). The present study is limited to patients enrolled in the primary treatment comparison arms who had completed a patient questionnaire and/or brief food questionnaire at the baseline study visit (N=1,984) (Figure 1). All participants signed Institutional Review Board-approved, protocol-specific informed consent forms in accordance with federal and institutional guidelines.
Figure 1.
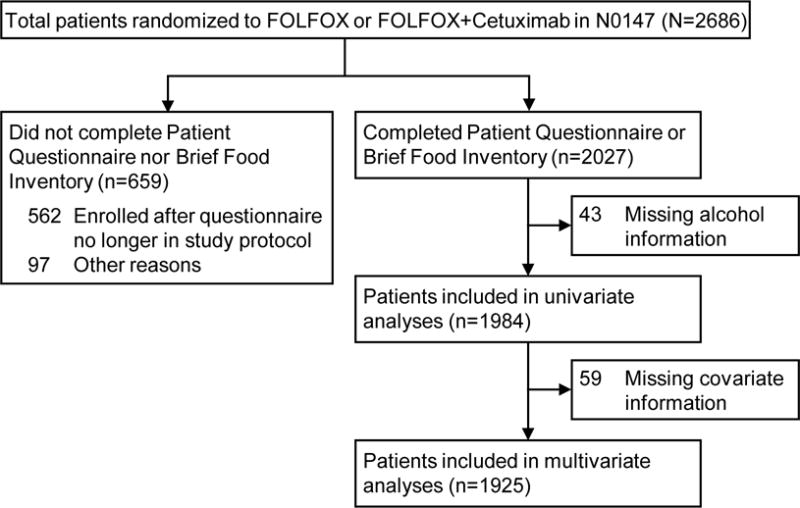
Derivations of translational analytic cohort in North Central Cancer Treatment Group Phase III Trial N0147
Ascertainment of Alcohol Consumption
A patient questionnaire eliciting information on risk factors, including smoking history and lifetime alcohol consumption patterns, and a brief food frequency questionnaire, assessing recent dietary habits, was administered to N0147 participants prior to treatment. On the patient questionnaire, participants were asked if they had ever consumed alcohol on a regular basis (≥1 drink/month at any time prior to study enrollment); those who reported having ever been a regular consumer of alcohol were asked to report the total number of drinks they had typically consumed per month, their typical monthly consumption of red and white wine, and, as applicable, the age at which they quit drinking on a regular basis. In the brief food questionnaire, participants were asked to report how often they had consumed beer, wine, and liquor in the past year, with separate questions for each alcohol type, and their typical number of servings each time they consumed these beverages.
Tumor Characterization
Diagnostic tumor tissue blocks were collected from the original surgical resection for study participants. Centralized testing for KRAS-mutation status, BRAF-mutation status, and DNA mismatch repair (MMR) status was conducted at the Mayo Clinic. Specifically, DNA isolated from tumor specimens was used to test for 7 mutations in codons 12 and 13 of KRAS exon 2 and the BRAF V600E mutation.17 MMR status was determined by immunohistochemical assessment of hMLH-1, hMSH-2, and hMSH-6;18 patients with tumors exhibiting a loss of expression for any of these proteins were classified as having defective MMR (dMMR), whereas patients with no loss of expression were classified as having proficient MMR (pMMR).
Classification of Outcomes
The primary clinical endpoint for N0147 and the present analysis was disease-free survival (DFS), defined as the time from randomization to the first documented cancer recurrence or death from any cause, whichever came first. Associations were also evaluated with respect to overall survival (OS) and time-to-recurrence (TTR). OS was defined as the time from randomization to death from any cause. TTR was defined as the time from randomization to first documented disease recurrence; participants who died before a recurrence were censored at their last study visit. Patients were censored at 5 years post-randomization in analyses of DFS and TTR; patients were censored at 8 years post-randomization for OS analyses. Analyses were based on follow-up through Dec 3, 2014.
Statistical Analysis
Based on patient questionnaire data, we evaluated associations with the following aspects of lifetime alcohol consumption: history of ever being a regular alcohol consumer (ever, never), current alcohol consumption status at the time of enrollment (never, former, current drinker), typical overall monthly alcohol consumption (0, 1–10, 11–30, ≥30 drinks/month), typical monthly red wine consumption (0, 1–30, >30 glasses/month), and typical monthly white wine consumption (0, 1–30, >30 glasses/month). Based on information from the brief food questionnaire, we considered the following aspects of recent alcohol consumption in the year before study enrollment: typical monthly servings of beer (0, 1–30, >30), typical wine (0, 1–30, >30), and liquor (0, 1–30, >30).
Descriptive statistics of patient demographics and disease characteristics were tabulated by lifetime regular alcohol consumption status, and compared by Wilcoxon rank sum test and chi-squared test when applicable. The distributions of survival times by lifetime wine consumption patterns were also assessed using Kaplan-Meier methods19 and log-rank tests.20 Associations of lifetime and recent alcohol consumption patterns with outcomes were evaluated by Cox proportional hazards regression. All models were adjusted for the following factors selected a priori: ECOG performance score, age at diagnosis, sex, frequency of vigorous physical activity, body mass index (BMI), smoking history at enrollment, race, and treatment assignment. Participants in two treatment groups were pooled since there was no difference in the association between alcohol consumption and DFS by treatment (Pinteraction = 0.41). Proportional hazards assumptions were verified by testing for a non-zero slope of the scaled Schoenfeld residuals on ranked failure times.
In addition to primary analyses in the overall population, we evaluated possible interaction and stratum-specific associations with lifetime alcohol consumption variables by patient and tumor attributes. Tests for interaction were conducted with respect to sex, age, T-stage, lymph node involvement, tumor subsite, KRAS- and BRAF-mutation, and MMR status, using categorizations as listed in Table 1.
TABLE 1.
Characteristics of study participants from North Central Cancer Treatment Group Trial N0147 according to lifetime history of alcohol consumption at the time of study enrollment*
| Ever Drinker (N=1389) N (row%) | Never Drinker (N=595) N (row%) | Total (N=1984) N (row%) | P† | |
|---|---|---|---|---|
| Treatment | 0.56 | |||
| FOLFOX | 699 (71%) | 291 (29%) | 990 (50%) | |
| FOLFOX+cetuximab | 690 (69%) | 304 (31%) | 994 (50%) | |
| Age at randomization | 0.0042 | |||
| N | 1389 | 595 | 1984 | |
| Mean (SD) | 57.6 (11.1) | 59.0 (11.4) | 58.0 (11.2) | |
| Range | (23.0–85.0) | (19.0–86.0) | (19.0–86.0) | |
| Race | <0.0001 | |||
| White | 1242 (73%) | 471 (27%) | 1713 (86%) | |
| Non-white | 129 (53%) | 115 (47%) | 244 (12%) | |
| Unknown | 18 (67%) | 9 (33%) | 27 (1%) | |
| Gender | <0.0001 | |||
| Female | 559 (59%) | 385 (41%) | 944 (48%) | |
| Male | 830 (80%) | 210 (20%) | 1040 (52%) | |
| Tumor site | 0.94 | |||
| Missing | 21 | 6 | 27 | |
| Proximal | 722 (70%) | 312 (30%) | 1034 (53%) | |
| Distal | 646 (70%) | 277 (30%) | 923 (47%) | |
| T-Stage | 0.32 | |||
| Missing | 0 | 1 | 1 | |
| T1/T2 | 203 (71%) | 84 (29%) | 287 (15%) | |
| T3 | 1022 (69%) | 453 (31%) | 1475 (74%) | |
| T4 | 164 (74%) | 57 (26%) | 221 (11%) | |
| Lymph node involvement | 0.36 | |||
| 1–3 | 810 (69%) | 360 (31%) | 1170 (59%) | |
| ≥4 | 579 (71%) | 235 (29%) | 814 (41%) | |
| Performance stage | 0.066 | |||
| 0 | 1078 (71%) | 439 (29%) | 1517 (76%) | |
| 1–2 | 311 (67%) | 156 (33%) | 467 (24%) | |
| Mismatch repair status (MMR) | 0.80 | |||
| Missing | 62 | 21 | 83 | |
| Proficient | 1162 (70%) | 505 (30%) | 1667 (88%) | |
| Deficient | 165 (71%) | 69 (29%) | 234 (12%) | |
| KRAS/BRAF mutation status | 0.19 | |||
| Missing | 112 | 36 | 148 | |
| KRAS-wildtype/BRAF-wildtype | 661 (71%) | 273 (29%) | 934 (51%) | |
| KRAS-mutated/BRAF-wildtype | 446 (69%) | 201 (31%) | 647 (35%) | |
| KRAS-wildtype/BRAF-mutated | 170 (67%) | 85 (33%) | 255 (14%) | |
| Body mass index (kg/m2) | 0.0818 | |||
| Missing | 4 | 3 | 7 | |
| <20 | 58 (76%) | 18 (24%) | 76 (4%) | |
| 20–24.9 | 344 (72%) | 137 (28%) | 481 (24%) | |
| 25–29.90 | 511 (70%) | 215 (30%) | 726 (37%) | |
| ≥30.0 | 472 (68%) | 222 (32%) | 694 (35%) | |
| Smoking history | <0.0001 | |||
| Missing | 32 | 1 | 33 | |
| Never | 516 (56%) | 411 (44%) | 927 (48%) | |
| Former | 728 (82%) | 157 (18%) | 885 (45%) | |
| Current | 113 (81%) | 26 (19%) | 139 (7%) | |
| Physical activity | 0.027 | |||
| Missing | 41 | 5 | 46 | |
| Almost none | 55 (60%) | 36 (40%) | 91 (5%) | |
| Mild | 556 (69%) | 245 (31%) | 801 (41%) | |
| Moderate | 558 (69%) | 254 (31%) | 812 (42%) | |
| Heavy | 179 (76%) | 55 (24%) | 234 (12%) |
Ever drinkers include those study participants who reported having ever regularly consumed ≥1 drink/month at any time prior to study enrollment.
p-value based on Wilcoxon rank sum test or chi-square test where applicable
Analyses were conducted using SAS version 9.3 (SAS Institute, Cary, NC). Two-sided p-values <0.05 were considered statistically significant. All data collection and statistical analyses were performed by the Alliance Statistics and Data Center.
RESULTS
Characteristics of the study population are provided in Table 1, according to lifetime history of alcohol consumption. Compared to those who never regularly consumed alcohol, ever drinkers were younger (P<0.01), more physically active (P=0.03), and more likely to be white, male, and a former or current smoker at study baseline (P<0.0001).
Over a median follow-up of 6.5 years (range: 0–8.0 years), n=649 study participants experienced a disease recurrence and/or died. After multivariate adjustment, DFS was suggestively, but not statistically significantly, more favorable in participants who reported ever having been regular alcohol consumers (HR=0.86, 95% confidence interval [CI]: 0.71–1.03, versus never drinkers); associations were similar for former and current drinkers (Table 2). No pattern of increasing or decreasing risk was noted with respect to typical lifetime levels of consumption for alcohol overall. DFS was, however, significantly more favorable in those who had ever been regular consumers of red wine (HR=0.80, 95% CI: 0.67–0.96). When considering the levels of red wine consumption, minimal to moderate drinkers (1–30 glasses/month) had longer DFS than never drinkers (HR=0.82, 95% CI: 0.69–0.98). Participants who reported consuming >30 glasses/month exhibited the most favorable DFS (HR=0.38, 95% CI: 0.14–1.02), although this association was not statistically significant, possibly due to small numbers. Similar patterns of association were noted with respect to OS and TTR, indicating more favorable outcomes in ever-consumers of red wine (Figure 2). Minimal to moderate consumption of white wine was also associated with more favorable OS (HR=0.75, 95% CI: 0.61–0.92), but was not significantly associated with other outcomes.
TABLE 2.
Lifetime patterns of alcohol consumption and outcomes in patients with stage III colon cancer*
| N (column%) | Disease-Free Survival | Overall Survival | Time-to-Recurrence | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| HR (95% CI)† | Wald-P | P‡ | HR (95% CI)† | Wald-P | P‡ | HR (95% CI)† | Wald-P | P‡ | ||
| Alcohol overall | ||||||||||
| Never | 586 (30%) | 1.0 (ref) | 0.11 | 1.0 (ref) | 0.12 | 1.0 (ref) | 0.17 | |||
| Ever | 1339(70%) | 0.86 (0.71–1.03) | 0.10 | 0.85 (0.69–1.04) | 0.12 | 0.87 (0.72–1.06) | 0.17 | |||
| Former | 570 (30%) | 0.87 (0.70–1.07) | 0.18 | 0.27 | 0.92 (0.73–1.16) | 0.47 | 0.13 | 0.86 (0.68–1.08) | 0.18 | 0.39 |
| Current | 746 (40%) | 0.85 (0.69–1.05) | 0.34 | 0.80 (0.63–1.00) | 0.051 | 0.89 (0.71–1.10) | 0.28 | |||
| Red wine | ||||||||||
| Never | 1244 (67%) | 1.0 (ref) | 0.013 | 1.0 (ref) | 0.011 | 1.0 (ref) | 0.021 | |||
| Ever | 628 (34%) | 0.80 (0.67–0.96) | 0.015 | 0.78 (0.64–0.95) | 0.012 | 0.81 (0.67–0.97) | 0.023 | |||
| White wine | ||||||||||
| Never | 1300 (71%) | 1.0 (ref) | 0.041 | 1.0 (ref) | 0.0050 | 1.0 (ref) | 0.063 | |||
| Ever | 542 (29%) | 0.83 (0.69–0.99) | 0.043 | 0.75 (0.61–0.92) | 0.0061 | 0.83 (0.69–1.01) | 0.067 | |||
| Red wine servings/month | ||||||||||
| Never | 1244 (67%) | 1.0 (ref) | 0.010 | 1.0 (ref) | 0.0089 | 1.0 (ref) | 0.012 | |||
| 1–30 | 601 (32%) | 0.82 (0.69–0.98) | 0.031 | 0.80 (0.66–0.98) | 0.027 | 0.83 (0.69–1.00) | 0.049 | |||
| >30 | 27 (1%) | 0.38 (0.14–1.02) | 0.055 | 0.34 (0.11–1.05) | 0.060 | 0.33 (0.10–1.01) | 0.053 | |||
| White wine servings/month | ||||||||||
| Never | 1300 (71%) | 1.0 (ref) | 0.13 | 1.0 (ref) | 0.019 | 1.0 (ref) | 0.17 | |||
| 1–30 | 531 (29%) | 0.83 (0.69–1.00) | 0.045 | 0.75 (0.61–0.93) | 0.0061 | 0.83 (0.69–1.01) | 0.065 | |||
| >30 | 11 (1%) | 0.81 (0.26–2.54) | 0.72 | 0.84 (0.27–2.63) | 0.77 | 0.93 (0.30–2.92) | 0.91 | |||
Ever drinkers include those study participants who reported having ever regularly consumed ≥1 drink/month at any time prior to study enrollment; ever drinkers of red wine and white wine include those who reported having ever regularly consumed ≥1 glass of red wine/month at any time prior to study enrollment or ≥1 glass of white wine/month at any time prior to study enrollment, respectively.
Adjusted for treatment, sex, BMI, smoke, physical activity, performance score, race.
Global Type-3 Likelihood Ratio
Abbreviations: HR=hazard ratio; CI=confidence interval
Figure 2.
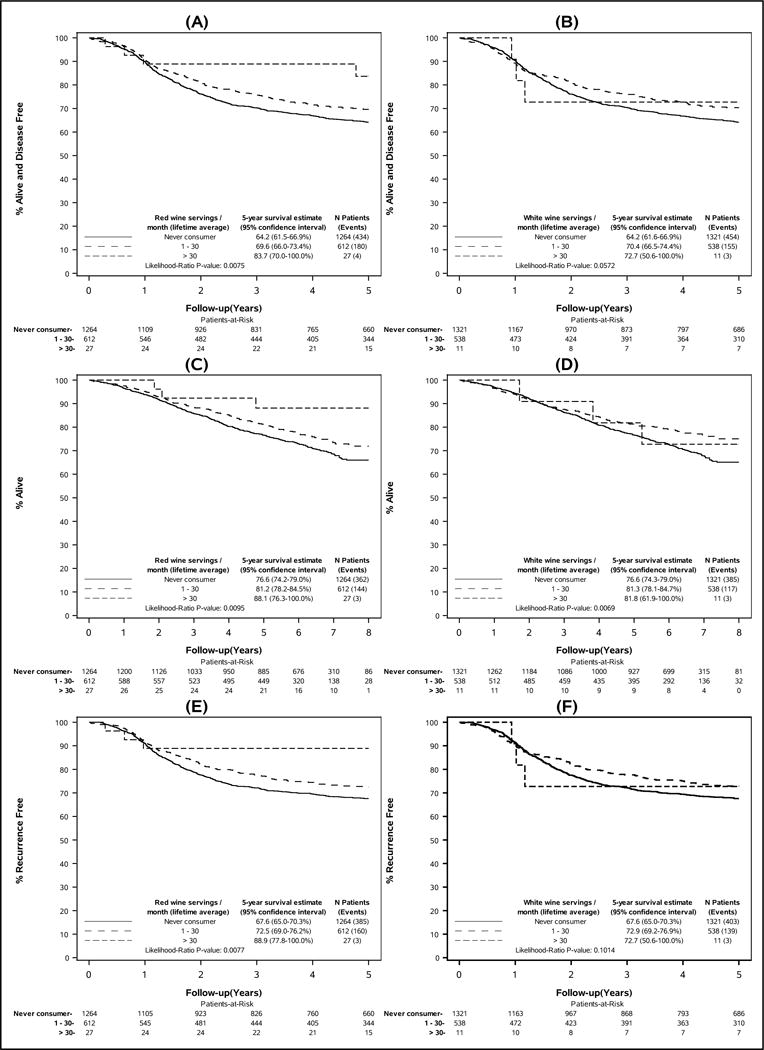
Colon cancer outcomes by lifetime average monthly wine consumption: (A) disease-free survival by red wine consumption, (B) disease-free survival by white wine consumption, (C) overall survival by red wine consumption, (D) overall survival by white wine consumption, (E) time-to-recurrence by red wine consumption, (F) time-to-recurrence by white wine consumption. Disease-free survival is defined as the time from randomization to the first documented cancer recurrence or death from any cause, whichever came first. Overall survival was defined as the time from randomization to death from any cause. Time-to-recurrence was defined as the time from randomization to first documented disease recurrence. Never consumers include those who reported that they had never consumed red wine or white wine on a regular basis at any time prior to study enrollment.
In stratified analyses, the association between lifetime red wine consumption (ever/never) and DFS was most pronounced among those with KRAS-mutated/BRAF-wildtype colon cancer (HR=0.62, 95% CI: 0.46–0.84), whereas no association was noted among those with KRAS-wildtype/BRAF-mutated tumors (HR=1.22, 95% CI: 0.81–1.85) (Pinteraction=0.05) (Figure 3). The favorable association of red wine with DFS also appeared to be stronger among, or limited to, individuals with stage T3 versus T4 cancers, those with pMMR but not dMMR tumors, those with tumors in the distal colon, obese patients, and never smokers; however, differences in associations with red wine consumption by these factors and other patient or tumor attributes did not attain statistical significance. In stratified analyses of white wine consumption, the association of ever consumption with DFS was, again, most pronounced among those with KRAS-mutated/BRAF-wildtype colon cancer (Supplemental Figure 1).
Figure 3.
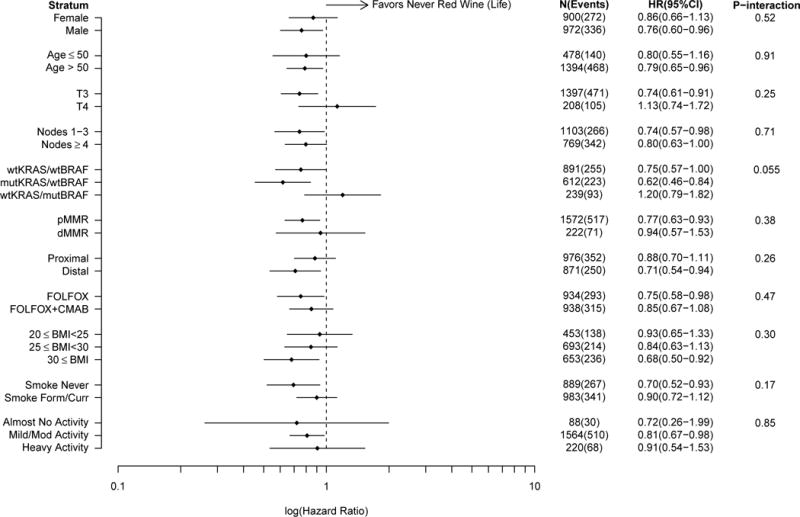
Comparison of disease-free survival in ever versus never consumers (lifetime) of red wine according to patient and tumor characteristics. Analyses adjusted for age, treatment, sex, smoking history, performance score, physical activity, and BMI. Disease-free survival is defined as the time from randomization to the first documented cancer recurrence or death from any cause, whichever came first. Ever consumers of red wine include those who reported that they had consumed red wine on a regular basis (≥1 serving/month) at any time prior to study enrollment. Abbreviations: wt, wildtype; mut, mutated; pMMR, proficient mismatch repair; dMMR, deficient mismatch repair; BMI, body mass index; HR, hazard ratio; CI, confidence interval.
With respect to patterns of recent alcohol consumption, there was no evidence of an association between beer or liquor consumption and outcomes, regardless of the typical amount consumed (Table 3). In analyses of recent wine consumption, there was some suggestion of more favorable outcomes in those who consumed >30 glasses/month relative to never consumers; however, this association did not attain statistical significance with respect to DFS or TTR. No distinction was made between red and white wine in the brief food questionnaire for recent alcohol consumption.
TABLE 3.
Alcohol consumption in the year prior to diagnosis and outcomes in patients with stage III colon cancer
| N (column%) | Disease-Free Survival | Overall Survival | Time-to-Recurrence | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| HR (95% CI)* | Wald P | P† | HR (95% CI)* | Wald P | P† | HR (95% CI)* | Wald P | P† | ||
| Wine servings/month | ||||||||||
| Never | 871 (47%) | 1.0 (ref) | 0.27 | 1.0 (ref) | 0.019 | 1.0 (ref) | 0.32 | |||
| 1–10 | 784 (43%) | 0.98 (0.84–1.16) | 0.80 | 0.87 (0.72–1.04) | 0.13 | 1.04 (0.87–1.24) | 0.69 | |||
| 11–30 | 79 (4%) | 0.87 (0.58–1.30) | 0.49 | 0.76 (0.47–1.21) | 0.24 | 0.89 (0.58–1.38) | 0.62 | |||
| >30 | 100 (6%) | 0.68 (0.45–1.04) | 0.073 | 0.50 (0.30–0.84) | 0.0090 | 0.70 (0.45–1.10) | 0.12 | |||
| Beer servings/month | ||||||||||
| Never | 895 (49%) | 1.0 (ref) | 0.33 | 1.0 (ref) | 0.19 | 1.0 (ref) | 0.51 | |||
| 1–10 | 661 (36%) | 0.99 (0.83–1.19) | 0.94 | 1.07 (0.88–1.30) | 0.52 | 0.97 (0.81–1.18) | 0.79 | |||
| 11–30 | 87 (5%) | 0.77 (0.51–1.17) | 0.23 | 0.68 (0.42–1.13) | 0.13 | 0.85 (0.56–1.31) | 0.46 | |||
| >30 | 191 (10%) | 0.81 (0.60–1.09) | 0.16 | 0.86 (0.62–1.19) | 0.35 | 0.80 (0.58–1.10) | 0.17 | |||
| Liquor servings/month | ||||||||||
| Never | 900 (49%) | 1.0 (ref) | 0.82 | 1.0 (ref) | 0.12 | 1.0 (ref) | 0.94 | |||
| 1–10 | 795 (43%) | 0.93 (0.78–1.10) | 0.40 | 0.86 (0.71–1.03) | 0.11 | 0.95 (0.79–1.14) | 0.55 | |||
| 11–30 | 70 (4%) | 0.88 (0.58–1.36) | 0.57 | 0.62 (0.36–1.07) | 0.085 | 0.97 (0.63–1.51) | 0.90 | |||
| >30 | 71 (4%) | 1.00 (0.66–1.52) | 0.99 | 1.09 (0.72–1.67) | 0.68 | 1.03 (0.66–1.59) | 0.91 | |||
Adjusted for treatment, sex, BMI, smoke, physical activity, performance score, race.
Global Type-3 Likelihood Ratio
Abbreviations: HR=hazard ratio; CI=confidence interval
DISCUSSION
In this correlative analysis of stage III colon cancer patients participating in a phase III clinical treatment trial, overall alcohol consumption was not associated with disease outcomes, regardless of evaluated patient or tumor characteristics. However, pre-diagnostic wine consumption was suggestively associated with longer OS, DFS, and TTR, especially for those who reported ever having been regular consumers of red wine.
Moderate red wine consumption has long been suggested to confer a health benefit with respect to overall and, in particular, cardiovascular disease mortality.15,21 Several qualities or constituents of red wine may plausibly convey cardiovascular benefits, including polyphenols and resveratrol, which possess anti-oxidant,22 anti-platelet,21 and anti-coagulatory properties.23 Moderate wine consumption has also been shown to result in decreased levels of inflammatory markers.24,25 Thus, although we did not have information on cause of death, it is likely that observed associations with respect to OS reflect, at least in part, some impact of wine consumption on cardiovascular disease mortality.
Associations of wine consumption with DFS and TTR suggest that the impact of wine constituents may extend beyond cardiovascular disease; however, the mechanisms responsible for such associations are yet unclear. Prior studies have found that resveratrol induces apoptosis in several cancer cell lines.26,27 In animal models, resveratrol has also been shown to prevent the formation of colon tumors via down-regulation of genes involved in cell cycle progression genes,28 inhibit the growth of implanted human primary gastric cancer cells,29 and enhance the anti-tumor effect of 5-fluorouracil on H22 murine hepatoma.30 Other studies of red wine extract have suggested that the polyphenolic compounds in red wine induces an antioxidant response and reduces proliferation in colon cancer cell lines31,32 and in mouse models of colon cancer.33 Human studies into the association between wine consumption and cancer outcomes have been limited.11,34–36 However, Slattery et al. recently noted that two of six genes differentially expressed in individuals who consumed high levels of wine versus non-consumers of wine are associated with biological oxidation pathways and response to wound healing.37 Taken together, these findings are consistent with the hypothesis that wine consumption may be favorably associated with colon cancer progression.
Prior studies of colon cancer outcomes and alcohol have indicated no significant association. Most recently, Pelser et al. recently found a non-significant lower risk of colon cancer-specific mortality among moderate drinkers versus non-drinkers [relative risk (RR)=0.86, 95% CI: 0.73–1.01],13 but found that OS was significantly more favorable among moderate versus never drinkers. This latter association was due, at least in part, to a significant association with cardiovascular disease mortality; associations with wine, beer, and liquor consumption were not evaluated separately. In another prior analysis with separate evaluation by alcohol type, Phipps et al. noted no association between wine, beer, or liquor consumption before colorectal cancer diagnosis and subsequent OS or disease-specific survival.11 To our knowledge, only one prior epidemiologic study has reported more favorable outcomes after colorectal cancer diagnosis associated with pre-diagnostic wine consumption.36 In that study, Zell et al. reported significantly more favorable OS among cases who were regular wine consumers (defined as consumption of 1–3 glasses/month) relative to infrequent consumers (<1 glass/month), after adjusting for age, sex, stage, treatment, BMI, and beer and liquor consumption (HR=0.50, 95% CI: 0.25–0.99); however, this association was evident only among those with a family history of colorectal cancer.
Studies evaluating colon cancer risk have provided more consistent evidence for a modest positive association with alcohol consumption. In a recent meta-analysis, Fedirko et al. reported a statistically significant 1.23-fold elevated risk of colon cancer among modest drinkers (2–3 drinks/day) and 1.43-fold elevated risk among heavy drinkers (≥4 drinks/day) versus non-drinkers.38 Some studies have suggested that alcohol is more strongly associated with risk of colon cancers exhibiting high microsatellite instability;39,40 however, studies evaluating other possible molecular sources of heterogeneity (e.g., KRAS and BRAF mutation status) in the relationship between alcohol consumption and colon cancer risk have indicated no such differential association.41–44 We found no significant heterogeneity in the relationship between colon cancer outcomes and alcohol by tumor attributes, suggesting that any impact of alcohol consumption is likely not specific to particular known molecular pathways of colon cancer development or progression.
The results of this correlative analysis should be interpreted in the context of study limitations. In particular, only pre-diagnostic alcohol consumption data were available. Alcohol consumption patterns during and after colon cancer treatment may impact disease outcomes but could not be evaluated here. We also did not have information on cause of death. Given that our analyses of OS are based on all-cause mortality, it is possible that observed associations could reflect, in part, the effect of alcohol on other causes of death (e.g., cardiovascular disease); however, the fact that we observed similar patterns of association with DFS and TTR suggests that our findings are driven primarily by disease-specific effects of alcohol. Additionally, although we did adjust all analytic models for a number of patient, tumor, and clinical factors, residual confounding by other lifestyle choices and socioeconomic factors associated with alcohol consumption could have contributed, in part, to observed study findings. Lastly, because the present study was conducted within a clinical trial population, our inference is limited to stage III colon cancer patients; generalizability to the broader population of patients with colon cancer is not fully known.
Important strengths of the present study include the availability of information for consumption of specific alcohol types, averaged over the life-course and for the time period immediately preceding colon cancer diagnosis. The availability of information on tumor markers, standardized treatment, and clinical attributes, and the detailed follow-up for recurrence and mortality outcomes also represent critical strengths. Although numerous studies have evaluated the association between alcohol and colon cancer risk, few have considered the possible association of alcohol with colon cancer outcomes.11,13,36 To our knowledge, no prior studies have evaluated the association of alcohol consumption with colon cancer recurrence. Our results are consistent with previous reports, not restricted to colon cancer patients, indicating lower overall mortality associated with red wine consumption. Although more detailed follow-up and replication is merited, our findings suggest that the benefits of wine consumption, particularly red wine, may extend to other clinical outcomes following colon cancer diagnosis.
Supplementary Material
Acknowledgments
Funding Support: This work was supported by the National Cancer Institute, National Institutes of Health (K07CA172298, U10CA180821, U10CA180882, U10CA025224, U10CA180888). The content of this manuscript does not necessarily reflect the views or policies of the National Cancer Institute.
Footnotes
In this analysis of stage III colon cancer patients, alcohol consumption was not associated with disease outcomes overall. However, pre-diagnostic wine consumption was suggestively associated with more favorable outcomes, especially for those who reported ever having been regular consumers of red wine.
Conflict of interest disclosures: Dr. Chan has served on advisory boards for Lilly, Merrimack, Taiho, Amgen, Castle Biosciences, and EMD Serono. The Mayo Clinic has licensed Dr. Limburg’s intellectual property to Exact Sciences and he and Mayo Clinic have contractual rights to receive royalties through this agreement.
References
- 1.Phipps AI, Scoggins J, Rossing MA, Li CI, Newcomb PA. Temporal trends in incidence and mortality rates for colorectal cancer by tumor location: 1975–2007. Am J Public Health. 2012;102:1791–7. doi: 10.2105/AJPH.2011.300393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.International Agency for Research on Cancer, World Health Organization. Alcohol consumption and ethyl carbamate. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. 2010:96. [PMC free article] [PubMed] [Google Scholar]
- 3.Kune GA, Vitetta L. Alcohol consumption and the etiology of colorectal cancer: a review of the scientific evidence from 1957 to 1991. Nutr Cancer. 1992;18:97–111. doi: 10.1080/01635589209514210. [DOI] [PubMed] [Google Scholar]
- 4.Le Marchand L, Wilkens LR, Kolonel LN, Hankin JH, Lyu LC. Associations of sedentary lifestyle, obesity, smoking, alcohol use, and diabetes with the risk of colorectal cancer. Cancer Res. 1997;57:4787–94. [PubMed] [Google Scholar]
- 5.Longnecker MP, Orza MJ, Adams ME, Vioque J, Chalmers TC. A meta-analysis of alcoholic beverage consumption in relation to risk of colorectal cancer. Cancer Causes Control. 1990;1:59–68. doi: 10.1007/BF00053184. [DOI] [PubMed] [Google Scholar]
- 6.Mizoue T, Inoue M, Wakai K, Nagata C, Shimazu T, Tsuji I, Otani T, Tanaka K, Matsuo K, Tamakoshi A, Sasazuki S, Tsugane S. Alcohol drinking and colorectal cancer in Japanese: a pooled analysis of results from five cohort studies. Am J Epidemiol. 2008;167:1397–406. doi: 10.1093/aje/kwn073. [DOI] [PubMed] [Google Scholar]
- 7.Moskal A, Norat T, Ferrari P, Riboli E. Alcohol intake and colorectal cancer risk: a dose-response meta-analysis of published cohort studies. Int J Cancer. 2007;120:664–71. doi: 10.1002/ijc.22299. [DOI] [PubMed] [Google Scholar]
- 8.Sharpe CR, Siemiatycki J, Rachet B. Effects of alcohol consumption on the risk of colorectal cancer among men by anatomical subsite (Canada) Cancer Causes Control. 2002;13:483–91. doi: 10.1023/a:1015700415808. [DOI] [PubMed] [Google Scholar]
- 9.Maeda M, Nagawa H, Maeda T, Koike H, Kasai H. Alcohol consumption enhances liver metastasis in colorectal carcinoma patients. Cancer. 1998;83:1483–8. doi: 10.1002/(sici)1097-0142(19981015)83:8<1483::aid-cncr2>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 10.Anton-Culver H. Smoking and other risk factors associated with the stage and age of diagnosis of colon and rectum cancers. Cancer Detect Prev. 1991;15:345–50. [PubMed] [Google Scholar]
- 11.Phipps AI, Baron J, Newcomb PA. Prediagnostic smoking history, alcohol consumption, and colorectal cancer survival: The Seattle Colon Cancer Family Registry. Cancer. 2011;117:4948–57. doi: 10.1002/cncr.26114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Park SM, Lim MK, Shin SA, Yun YH. Impact of prediagnosis smoking, alcohol, obesity, and insulin resistance on survival in male cancer patients: National Health Insurance Corporation Study. J Clin Oncol. 2006;24:5017–24. doi: 10.1200/JCO.2006.07.0243. [DOI] [PubMed] [Google Scholar]
- 13.Pelser C, Arem H, Pfeiffer RM, Elena JW, Alfano CM, Hollenbeck AR, Park Y. Prediagnostic lifestyle factors and survival after colon and rectal cancer diagnosis in the National Institutes of Health (NIH)-AARP Diet and Health Study. Cancer. 2014;120:1540–7. doi: 10.1002/cncr.28573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Patel SA, Chen YH, Hornick JL, Catalano P, Nowak JA, Zukerberg LR, Bleday R, Shellito PC, Hong TS, Mamon HJ. Early-stage rectal cancer: clinical and pathologic prognostic markers of time to local recurrence and overall survival after resection. Dis Colon Rectum. 2014;57:449–59. doi: 10.1097/DCR.0b013e3182a70709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Di Castelnuovo A, Costanzo S, Bagnardi V, Donati MB, Iacoviello L, de Gaetano G. Alcohol dosing and total mortality in men and women: an updated meta-analysis of 34 prospective studies. Arch Intern Med. 2006;166:2437–45. doi: 10.1001/archinte.166.22.2437. [DOI] [PubMed] [Google Scholar]
- 16.Alberts SR, Sargent DJ, Nair S, Mahoney MR, Mooney M, Thibodeau SN, Smyrk TC, Sinicrope FA, Chan E, Gill S, Kahlenberg MS, Shields AF, et al. Effect of oxaliplatin, fluorouracil, and leucovorin with or without cetuximab on survival among patients with resected stage III colon cancer: a randomized trial. JAMA. 2012;307:1383–93. doi: 10.1001/jama.2012.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Domingo E, Laiho P, Ollikainen M, Pinto M, Wang L, French AJ, Westra J, Frebourg T, Espin E, Armengol M, Hamelin R, Yamamoto H, et al. BRAF screening as a low-cost effective strategy for simplifying HNPCC genetic testing. Journal of medical genetics. 2004;41:664–8. doi: 10.1136/jmg.2004.020651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lindor NM, Burgart LJ, Leontovich O, Goldberg RM, Cunningham JM, Sargent DJ, Walsh-Vockley C, Petersen GM, Walsh MD, Leggett BA, Young JP, Barker MA, et al. Immunohistochemistry versus microsatellite instability testing in phenotyping colorectal tumors. J Clin Oncol. 2002;20:1043–8. doi: 10.1200/JCO.2002.20.4.1043. [DOI] [PubMed] [Google Scholar]
- 19.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–81. [Google Scholar]
- 20.Hosmer DW, Jr, Lemeshow S. Applied Survival Analysis: Regression Modeling for Time to Event Dataed. New York: John Wiley & Sons; 1999. [Google Scholar]
- 21.Renaud S, de Lorgeril M. Wine, alcohol, platelets, and the French paradox for coronary heart disease. Lancet. 1992;339:1523–6. doi: 10.1016/0140-6736(92)91277-f. [DOI] [PubMed] [Google Scholar]
- 22.Hung LM, Chen JK, Huang SS, Lee RS, Su MJ. Cardioprotective effect of resveratrol, a natural antioxidant derived from grapes. Cardiovascular research. 2000;47:549–55. doi: 10.1016/s0008-6363(00)00102-4. [DOI] [PubMed] [Google Scholar]
- 23.Mukamal KJ, Jensen MK, Gronbaek M, Stampfer MJ, Manson JE, Pischon T, Rimm EB. Drinking frequency, mediating biomarkers, and risk of myocardial infarction in women and men. Circulation. 2005;112:1406–13. doi: 10.1161/CIRCULATIONAHA.105.537704. [DOI] [PubMed] [Google Scholar]
- 24.Imhof A, Woodward M, Doering A, Helbecque N, Loewel H, Amouyel P, Lowe GD, Koenig W. Overall alcohol intake, beer, wine, and systemic markers of inflammation in western Europe: results from three MONICA samples (Augsburg, Glasgow, Lille) European heart journal. 2004;25:2092–100. doi: 10.1016/j.ehj.2004.09.032. [DOI] [PubMed] [Google Scholar]
- 25.Blanco-Colio LM, Valderrama M, Alvarez-Sala LA, Bustos C, Ortego M, Hernandez-Presa MA, Cancelas P, Gomez-Gerique J, Millan J, Egido J. Red wine intake prevents nuclear factor-kappaB activation in peripheral blood mononuclear cells of healthy volunteers during postprandial lipemia. Circulation. 2000;102:1020–6. doi: 10.1161/01.cir.102.9.1020. [DOI] [PubMed] [Google Scholar]
- 26.Chen Y, Tseng SH, Lai HS, Chen WJ. Resveratrol-induced cellular apoptosis and cell cycle arrest in neuroblastoma cells and antitumor effects on neuroblastoma in mice. Surgery. 2004;136:57–66. doi: 10.1016/j.surg.2004.01.017. [DOI] [PubMed] [Google Scholar]
- 27.Surh YJ, Hurh YJ, Kang JY, Lee E, Kong G, Lee SJ. Resveratrol, an antioxidant present in red wine, induces apoptosis in human promyelocytic leukemia (HL-60) cells. Cancer Lett. 1999;140:1–10. doi: 10.1016/s0304-3835(99)00039-7. [DOI] [PubMed] [Google Scholar]
- 28.Schneider Y, Duranton B, Gosse F, Schleiffer R, Seiler N, Raul F. Resveratrol inhibits intestinal tumorigenesis and modulates host-defense-related gene expression in an animal model of human familial adenomatous polyposis. Nutr Cancer. 2001;39:102–7. doi: 10.1207/S15327914nc391_14. [DOI] [PubMed] [Google Scholar]
- 29.Zhou HB, Chen JJ, Wang WX, Cai JT, Du Q. Anticancer activity of resveratrol on implanted human primary gastric carcinoma cells in nude mice. World J Gastroenterol. 2005;11:280–4. doi: 10.3748/wjg.v11.i2.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu SL, Sun ZJ, Yu L, Meng KW, Qin XL, Pan CE. Effect of resveratrol and in combination with 5-FU on murine liver cancer. World J Gastroenterol. 2004;10:3048–52. doi: 10.3748/wjg.v10.i20.3048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Signorelli P, Fabiani C, Brizzolari A, Paroni R, Casas J, Fabrias G, Rossi D, Ghidoni R, Caretti A. Natural grape extracts regulate colon cancer cells malignancy. Nutr Cancer. 2015;67:494–503. doi: 10.1080/01635581.2015.1004591. [DOI] [PubMed] [Google Scholar]
- 32.Angel-Morales G, Noratto G, Mertens-Talcott S. Red wine polyphenolics reduce the expression of inflammation markers in human colon-derived CCD-18Co myofibroblast cells: potential role of microRNA-126. Food Funct. 2012;3:745–52. doi: 10.1039/c2fo10271d. [DOI] [PubMed] [Google Scholar]
- 33.Mazue F, Delmas D, Murillo G, Saleiro D, Limagne E, Latruffe N. Differential protective effects of red wine polyphenol extracts (RWEs) on colon carcinogenesis. Food Funct. 2014;5:663–70. doi: 10.1039/c3fo60417a. [DOI] [PubMed] [Google Scholar]
- 34.Kwan ML, Kushi LH, Weltzien E, Tam EK, Castillo A, Sweeney C, Caan BJ. Alcohol consumption and breast cancer recurrence and survival among women with early-stage breast cancer: the life after cancer epidemiology study. J Clin Oncol. 2010;28:4410–6. doi: 10.1200/JCO.2010.29.2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Han X, Zheng T, Foss FM, Ma S, Holford TR, Boyle P, Leaderer B, Zhao P, Dai M, Zhang Y. Alcohol consumption and non-Hodgkin lymphoma survival. Journal of cancer survivorship: research and practice. 2010;4:101–9. doi: 10.1007/s11764-009-0111-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zell JA, McEligot AJ, Ziogas A, Holcombe RF, Anton-Culver H. Differential effects of wine consumption on colorectal cancer outcomes based on family history of the disease. Nutr Cancer. 2007;59:36–45. doi: 10.1080/01635580701413926. [DOI] [PubMed] [Google Scholar]
- 37.Slattery ML, Pellatt DF, Mullany LE, Wolff RK. Differential Gene Expression in Colon Tissue Associated With Diet, Lifestyle, and Related Oxidative Stress. PLoS One. 2015;10:e0134406. doi: 10.1371/journal.pone.0134406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fedirko V, Tramacere I, Bagnardi V, Rota M, Scotti L, Islami F, Negri E, Straif K, Romieu I, La Vecchia C, Boffetta P, Jenab M. Alcohol drinking and colorectal cancer risk: an overall and dose-response meta-analysis of published studies. Ann Oncol. 2011;22:1958–72. doi: 10.1093/annonc/mdq653. [DOI] [PubMed] [Google Scholar]
- 39.Diergaarde B, Braam H, van Muijen GN, Ligtenberg MJ, Kok FJ, Kampman E. Dietary factors and microsatellite instability in sporadic colon carcinomas. Cancer Epidemiol Biomarkers Prev. 2003;12:1130–6. [PubMed] [Google Scholar]
- 40.Slattery ML, Curtin K, Anderson K, Ma KN, Ballard L, Edwards S, Schaffer D, Potter J, Leppert M, Samowitz WS. Associations between cigarette smoking, lifestyle factors, and microsatellite instability in colon tumors. J Natl Cancer Inst. 2000;92:1831–6. doi: 10.1093/jnci/92.22.1831. [DOI] [PubMed] [Google Scholar]
- 41.Razzak AA, Oxentenko AS, Vierkant RA, Tillmans LS, Wang AH, Weisenberger DJ, Laird PW, Lynch CF, Anderson KE, French AJ, Haile RW, Harnack LJ, et al. Alcohol intake and colorectal cancer risk by molecularly defined subtypes in a prospective study of older women. Cancer Prev Res (Phila) 2011;4:2035–43. doi: 10.1158/1940-6207.CAPR-11-0276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bongaerts BW, de Goeij AF, de Vogel S, van den Brandt PA, Goldbohm RA, Weijenberg MP. Alcohol consumption and distinct molecular pathways to colorectal cancer. Br J Nutr. 2007;97:430–4. doi: 10.1017/S0007114507381336. [DOI] [PubMed] [Google Scholar]
- 43.Slattery ML, Anderson K, Curtin K, Ma K, Schaffer D, Edwards S, Samowitz W. Lifestyle factors and Ki-ras mutations in colon cancer tumors. Mutat Res. 2001;483:73–81. doi: 10.1016/s0027-5107(01)00228-7. [DOI] [PubMed] [Google Scholar]
- 44.Slattery ML, Curtin K, Sweeney C, Levin TR, Potter J, Wolff RK, Albertsen H, Samowitz WS. Diet and lifestyle factor associations with CpG island methylator phenotype and BRAF mutations in colon cancer. Int J Cancer. 2007;120:656–63. doi: 10.1002/ijc.22342. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


