Abstract
PURPOSE
Obesity promotes pancreatic and breast cancer progression via mechanisms that are poorly understood. Although obesity is associated with increased systemic levels of placental growth factor (PlGF), the role of PlGF in obesity-induced tumor progression is not known. PlGF and its receptor vascular endothelial growth factor receptor-1 (VEGFR-1) have been shown to modulate tumor angiogenesis and promote tumor-associated macrophage (TAM) recruitment and activity. Here, we hypothesized that increased activity of PlGF/VEGFR-1 signaling mediates obesity-induced tumor progression by augmenting tumor angiogenesis and TAM recruitment/activity.
EXPERIMENTAL DESIGN
We established diet-induced obese mouse models of wild type C57BL/6, VEGFR-1 tyrosine kinase (TK)-null or PlGF null mice, and evaluated the role of PlGF/VEGFR-1 signaling in pancreatic and breast cancer mouse models and in human samples.
RESULTS
We found that obesity increased TAM infiltration, tumor growth and metastasis in pancreatic cancers, without affecting vessel density. Ablation of VEGFR-1 signaling prevented obesity-induced tumor progression and shifted the tumor immune environment towards an anti-tumor phenotype. Similar findings were observed in a breast cancer model. Obesity was associated with increased systemic PlGF, but not VEGF-A or VEGF-B, in pancreatic and breast cancer patients and in various mouse models of these cancers. Ablation of PlGF phenocopied the effects of VEGFR-1-TK deletion on tumors in obese mice. PlGF/VEGFR-1-TK deletion prevented weight gain in mice fed a high-fat diet, but exacerbated hyperinsulinemia. Addition of metformin not only normalized insulin levels but also enhanced anti-tumor immunity.
CONCLUSIONS
Targeting PlGF/VEGFR-1 signaling reprograms the tumor immune microenvironment and inhibits obesity-induced acceleration of tumor progression.
Keywords: Obesity, PlGF/VEGFR-1, metformin, pancreatic cancer, immunomodulation
Introduction
Excess body weight has become a major public health problem worldwide (1). Furthermore, a number of large-scale studies have demonstrated that obesity leads to an increase in cancer-related mortality in multiple cancer types including pancreatic and breast (2–4). However, the mechanisms underlying obesity-induced tumor progression are not completely understood (5). An excessive gain in adipose tissue in obesity is associated with angiogenesis, inflammation, and immune cell infiltration (6–9). Similarly, obesity may affect the vasculature and immune cell infiltration in cancer to facilitate tumor progression (10, 11).
We and others have shown that vascular endothelial growth factor receptor 1 (VEGFR-1) is expressed on endothelial cells (ECs) and macrophages. Binding of VEGF and placental growth factor (PlGF) to VEGFR-1 promotes tumor angiogenesis, and stimulates the recruitment and/or activation (e.g., cytokine production) of tumor-associated macrophages (TAMs) (12–17). However, the role of VEGFR-1 in tumor progression remains controversial, with some, but not all studies showing an effect of VEGFR-1 in promoting tumor growth and systemic metastasis in mouse models (12, 14, 18, 19). A recent study, in particular, demonstrated a specific effect of VEGFR-1 in metastasis-associated macrophages (12). In addition, VEGFR-1 signaling may regulate energy metabolism, as mice genetically deficient for PlGF gain less weight on a high-fat diet (HFD) than wild-type (WT) animals (20), but develop insulin resistance and hyperinsulinemia (21). These findings indicate that VEGFR-1 activity may have important roles in both tumor and adipose tissue, making it a potential target in cancers specifically in the obesity setting.
In this study, we evaluated whether VEGFR-1 signaling mediates the adipose tissue expansion and accelerated tumor growth in obesity, and whether this occurs via augmented angiogenesis and immune cell recruitment/activation. For this purpose, we used murine pancreatic ductal adenocarcinoma (PDAC) and breast cancer (BC) models orthotopically implanted in syngeneic lean and obese WT or VEGFR-1 tyrosine kinase (TK) knockout (KO) (Flt1TK−/−) mice. Specifically in obese mice, VEGFR-1-TK-deletion led to a reduction in tumor progression associated with a shift in tumor cytokine profile and TAM polarization towards the M1 phenotype. Furthermore, VEGFR-1-TK-deletion prevented body weight (BW) gain in mice fed HFD. However, this was associated with worsened hyperinsulinemia. Metformin not only normalized insulin levels but also further improved the effects of VEGFR-1-TK ablation on tumor progression and the immune environment. In addition, we assessed which ligands — PlGF or VEGFs — are implicated in the effects of VEGFR-1 in tumors. As mentioned above, PlGF has been shown to regulate tumor angiogenesis, immune cell infiltration and tumor progression and, importantly, pre-clinical and clinical evidence suggests a relationship between PlGF and obesity (20, 22–24). We found that plasma PlGF but not VEGF-A or B was associated with obesity in PDAC and BC patient samples as well as multiple mouse models. Consistent with this, PlGF-null mice (Plgf−/−) phenocopied Flt1TK−/− mice on improving the immune environment, systemic metabolism, and reducing tumor growth in the obese setting.
With the majority of pancreatic and breast cancer patients being overweight or obese at diagnosis (4, 25, 26), uncovering potential therapeutic targets within the mechanisms associating obesity with worsening cancer prognoses is the first step towards developing remedies that could disrupt this association and significantly improve patient outcome. This study describes the mechanisms by which PlGF/VEGFR-1 signaling mediates obesity-induced pancreatic and breast cancer progression.
Methods
Flt1TK−/−, Plgf−/−, ob/ob, C57BL/6 and FVB mice were used. At 6/7-weeks of age, all mice except ob/ob switched from standard chow to either low (10%) or high-fat (60%) diet. At 10 weeks we evaluated glucose and insulin tolerance, weighed fat pads. Then, we implanted PAN02, E0771 (C57BL/6) or AK4.4 (FVB) tumors. Diet was maintained. 3 weeks later, tumor burden, immune cell infiltration, inflammatory and metabolic markers in tumors, and plasma levels of insulin, insulin-like growth factor-1 (IGF-1) and angiogenic/inflammatory factors were determined.
Metformin 300 mg/kg (drinking water) was administered to some animals..
Using human samples, a correlation between body mass index (BMI) or visceral adipose tissue (VAT) and systemic levels of PlGF and VEGF-A was determined in 73 pancreatic cancer patients and 61 breast cancer patients.
For statistical methods and detail description of the methods, please see Supplementary Methods.
Results
Obesity alters the tumor immune microenvironment and promotes disease progression
As previously reported (27, 28), we observed that diet-induced obesity promotes tumor progression in PAN02 pancreatic tumors. In this model, the tumor weight (Figure 1A) and metastatic burden (Figure 1B) were approximately twice as high in obese mice than in lean at 3 weeks after tumor implantation. This was associated with increased tumor cell proliferation (Figure 1C) and reduced apoptosis in tumors (Figure 1D). Despite no increase in tumor vessel density in obese mice (Supplementary Figure 1A), obesity was associated with increased infiltration of tumor-associated macrophages (TAMs) (Figure 2A) and expression of pro-tumor cytokines IL-1β, IL-4, and IL-5 (trend for IL-10) in tumors (Figure 3D). We also observed an increase in IL-2, but no difference in the other tumor-associated cytokines measured (IL-6, TNFα, IL-12, INFγ, CXCL-1) (not shown). Taken together, we found that obesity does not affect vessel density in tumors, but is associated with TAM infiltration, increased expression of M2 cytokines, and accelerated tumor progression.
Figure 1. Effect of diet-induced obesity on pancreatic cancer progression.
Following the establishment of diet-induced lean and obesity state in C57BL/6 mice (10 weeks of diet with 60% fat vs. 10% fat diet), PAN02 tumors were implanted orthotopically. Tumors were collected 3 weeks later, and tumor weight, metastases, proliferation and apoptosis were determined. A) The effect of obesity on tumor weight (n = 5–9/group). B) i: Representative images of mesenteric metastases (black arrowheads) in lean and obese mice. ii: Average number of mesenteric metastasis per mouse. C) i: Representative images of Ki67 staining (immunofluorescence) in PAN02 tumors removed from lean and obese mice. Scale bars: 100 µm. ii: Quantification of the expression of Ki67 proliferation marker in tumors from lean and obese mice (n = 3–5/group). D) i: Protein level of cleaved caspase-3 (CC-3) apoptotic marker in tumors from lean and obese mice, determined by western blot. Each band represents an individual tumor. ii: Densitometric analysis normalized to β-actin. * P < 0.05. Error bars represent SEM.
Figure 2. Effect of VEGFR-1 signaling ablation on obesity-induced TAM infiltration and pancreatic cancer progression.
A) i: Flow cytometry was used to determine macrophage infiltration in PAN02 tumors from lean and obese WT and Flt1TK−/− mice. Representative figures of CD11b (myeloid) and F4/80+ double positive cells indicating macrophages. ii: Quantification of CD11b+ F4/80+ cells within total viable cells (n = 3–6/group). B) Tumor weights of PAN02 tumors collected 3 weeks after tumor source implantation in lean and obese WT or Flt1TK−/− mice (n = 8–12 mice/group). C) Average number of mesentery metastasis. Mesenteries collected at the time of primary tumor removal. D) Number of mice affected with mesentery metastasis in obese WT or Flt1TK−/− mice. E) Number of mice that presented with different levels of retroperitoneal wall invasion in obese WT or Flt1TK−/− mice. Animals were given scores of 0 to 3 based on the extent of invasion: 0 = no invasion, 1 = less than 3 metastases, 2 = less than 6 metastases, 3 = more than 6 metastases. F) Body weight loss between tumor implantation and extraction in obese WT or Flt1TK−/− mice. G) Quantification of Ki67 in tumors from obese WT and Flt1TK−/− mice (n = 3–6/group). * P < 0.05, ** P < 0.01. Error bars represent standard error of the mean.
Figure 3. Effect of VEGFR-1 signaling ablation on obesity-altered tumor immune microenvironment.
A) Western blot of VEGFR-1 and VEGFR-2 expression in cell lysates of cultured PAN02, E0771, macrophages (RAW 264.7) and endothelial (HUVECs) cells (samples in duplicate) B) Left panels: Expression of VEGFR-1 and F4/80 in two representative PAN02 tumors obtained by immunofluorescence. Right panels: Enlargement of a region of interest. Scale bar: 100 µm. C) Gene expression of M1/M2 markers comparing tumors from obese Flt1TK−/− to obese WT. Four samples were pooled into each PCR array plate. D) ELISA was used to determine the cytokine expression in PAN02 tumors from lean and obese WT and Flt1TK−/− mice (n = 3–10/group). E) Flow cytometry was performed to determine the expression of the M1 (CD86 and LY6C positive) and M2 (CD186 positive) macrophages within the total leukocyte population in tumors from obese WT and Flt1TK−/− mice (n = 6/group). * P < 0.05. Error bars represent standard error of the mean. Data shown in panel D also presented as part of a more comprehensive table (Supplementary Table 1).
Ablation of VEGFR-1 signaling prevents obesity-induced tumor progression without affecting blood vessels of recruitment of immune cells
Immunosuppressive (M2-like) TAMs have been shown to promote tumor progression (29). We and others have uncovered a role for VEGFR-1 signaling in the recruitment and activity of TAMs (12, 14). Therefore, we evaluated whether ablation of VEGFR-1 signaling could prevent the increased infiltration of TAMs in obese mice and obesity-accelerated tumor progression. For this purpose, we used Flt1TK−/− mice lacking the signaling domain of VEGFR-1 receptor (30). PAN02 tumors grown in Flt1TK−/− lean mice were similar in size to tumors grown in WT lean mice. However, tumors grown in Flt1TK−/− obese mice were significantly smaller compared to those in obese WT mice (Figure 2B). Similarly, ablation of VEGFR-1 signaling reduced the number of mesenteric metastases per animal in obese but not lean mice (Figure 2C). Furthermore, there was a trend towards a reduction in both the incidence of mesenteric metastasis (i.e., number of mice with metastasis) (Figure 2D, p=0.064) and the extent of retroperitoneal abdominal wall invasion (Figure 2E, p=0.09) in obese Flt1TK−/−mice compared to obese WT. The loss of body weight (BW) from the time of implantation until tumor extraction (a measure of health and systemic disease burden — in an extreme case, cachexia) was also significantly improved in obese Flt1TK−/− mice (~4 times less BW loss than obese WT mice) (Figure 2F). Consistent with reduced tumor progression, tumor cell proliferation was reduced in VEGFR-1-TK-deletion in obese mice (Figure 2G), despite no change in apoptosis (Figure 4H). Interestingly, ablation of VEGFR-1 signaling did not affect the number of CD45+ leukocytes, CD11b+F4/80+ TAMs (Figure 2A), NK, CD4+, CD8+ or T regulatory lymphocytes (Figure 4F) in tumors in either lean or obese mice. Considering that the expression of VEGFR-1 in tumor tissues is not restricted to TAMs, but also extends to ECs, and that VEGFR-1 can regulate tumor angiogenesis, we determined if the observed effects on tumor progression could be related to inhibition of angiogenesis. We confirmed that both EC and macrophages express VEGFR-1 in vitro, with tumor cell lines showing comparatively lower levels of expression (Figure 3A). However, VEGFR-1-TK-deletion was not associated with changes in tumor vessel density or hypoxia (Supplementary Figure 1B). Taken together our data reveals a role for VEGFR-1 in obesity-induced tumor progression that does not appear to be via modulation of TAM infiltration or angiogenesis.
Figure 4. Effect of metformin on the metabolic consequences of VEGFR1 signaling ablation and on the tumor microenvironment.

A) Body weight of male C57BL/6 mice after 10 weeks of low fat or high fat diet in WT or Flt1TK−/− background. Number of animals depicted as dots on each column. Low-fat diet generated “lean” mice, and high-fat diet generated “obese” mice. B) Glucose tolerance test in Flt1TK−/− vs. WT obese mice (n = 10/group). C) Levels of plasma Insulin in WT, metformin treated, Flt1TK−/− or combination of Flt1TK−/− + metformin treated obese mice 3 weeks after implantation of PAN02 tumors (n = 6–8/group). D) Weights of PAN02 tumors 3 weeks after chunk implantation in same treatment groups as in C (n = 7–10/group). E) Percentage of vessels (CD31+ expression in total DAPI+ viable area) and perfused vessels (lectin staining in total DAPI+ viable area) in tumors (No less than 10 ROI were analyzed per tumor, n = 3–4 tumors/group). F) i: Representative figures of CD8 and NK cells in tumors. ii: Quantification of immune cells (% of total viable cells) (n = 3–6/group). G) Gene expression of CTLA-4 and PD-L2 in tumors. Data extracted from a PCR array assay, four samples were pooled into each PCR array plate. H) i: Western blot analysis of cleaved caspase-3 (CC3) from PAN02 tumors lysates from panel D. Each band represents an individual tumor. ii: Quantification relative to β-actin in the lower panel. * P < 0.05,** P < 0.01, *** P < 0.005, **** P < 0.001. Error bars represent standard error of the mean.
Ablation of VEGFR-1 signaling reverts the obesity-associated abnormal tumor immune environment and reprograms TAMs
Although the number of TAMs did not appreciably change in with VEGFR-1-TK-deletion in obese mice, we found that VEGFR-1 appeared to be predominantly associated with TAMs in vivo, with approximately 50% of these cells expressing VEGFR-1 in PAN02 tumors (Figure 3B, Supplementary Figure 3A). This suggests that VEGFR-1 might play a role in TAM activity. In fact, it was recently shown that VEGFR-1 regulates TAM inflammatory response without affecting infiltration of these cells in tumors (12). Thus we determined if the TAM phenotype and the immune environment was altered in Flt1TK−/− mice. We found that VEGFR-1-TK-deletion in obese animals was associated with upregulation of M1- and concomitant downregulation of M2-genes including IL-1β and IL-10 in PAN02 tumors (Figure 3C). Subsequently, at the protein level we confirmed a reduction in pro-tumor M2 cytokines IL-10, IL-4, IL-5 and of IL-1β in tumor tissue (Figure 3D), and of IL-1β and IL-2 (trend for IL-10, p=0.13) in plasma (Supplementary Table 1) in obese Flt1TK−/− mice. IL-1β, in particular, which showed the most dramatic change (50% decrease in obese Flt1TK−/− vs. obese WT mice), was robustly expressed by 65–80% of TAMs and colocalized with the expression of VEGFR-1 in these cells (Supplementary Figures 3A, 3B and Supplementary Table 2): about 75–90% of VEGFR-1-positive TAMs co-expressed IL-1β, while about 55–70% of IL-1β-positive TAMs co-expressed VEGFR-1. This leads to a total of 40–50 % of all TAMs being positive for both VEGFR-1 and IL-1β. In addition, obese WT animals tended to have increased expression of IL-1β in TAMs compared to their lean counterparts, but this difference was abrogated in Flt1TK−/− mice (Supplementary Figure 3A and Supplementary Table 2). Consistent with the expression of M1/M2 cytokines, in tumors from obese Flt1TK−/− mice we observed an enrichment of M1-TAMs (F4/80+CD86+ and F4/80+Ly6C+) within the leukocyte (CD45+) population compared to tumors in obese WT mice (Figure 3E), indicating a shift in TAM phenotype. Finally, we also found a decrease in the expression of immune checkpoint receptors/ligands such as CTLA-4 (9-fold) and PD-L2 (4-fold) in obese Flt1TK−/− mice, suggesting alleviation of T-cell exhaustion (31) (Figure 4G). Collectively, these findings show that ablation of VEGFR-1 signaling in obese mice shifts TAMs towards an M1 phenotype, and promotes anti-tumor immunity.
Ablation of VEGFR-1 signaling prevents weight gain, but the effect on tumor progression is not dependent on its metabolic effects
Blockade of the VEGFR-1 ligand PlGF has been shown to affect adipose tissue expansion (20). Since prevention of obesity itself could also indirectly affect tumor progression, we determined if VEGFR-1-TK-deletion attenuates BW gain. Indeed, we found that Flt1TK−/− mice on a high-fat diet (but not on a low-fat diet) gained significantly less BW than WT mice (BW at the time of tumor implantation in Figure 4A, BW gain kinetics in Supplementary Figure 4A). The reduction in weight gain was associated with reduced perigonadal fat and adipocyte size (Supplementary Figures 4B–C). Remarkably, these effects in adipose tissue occurred without a reduction in the number of leukocytes, macrophages, macrophage-rich crown-like structures or vessel density (Supplementary Figure 4D–J), similarly to what was observed in the tumor setting. To rule out the possibility that the effect of VEGFR-1 signaling on tumor progression could be solely due to changes in body weight, we performed body-weigh matched (Flt1TK−/− mice with the same body weight as obese WT at the time of tumor implantation) subset group analysis. We still found significantly decreased tumor growth in BW-matched Flt1TK−/− mice obese mice compared to WT obese mice (Supplementary Figure 5A). Similar findings were observed for mesenteric and wall metastases (Supplementary Figures 5B,C), and weight loss (cachexia) (Supplementary Figure 5D). In fact, the BWs of tumor-bearing obese WT and Flt1TK−/− mice were similar at the end of the experiment, since WT mice lost BW while Flt1TK−/− mice maintained it (Supplementary Figure 5E). These results indicate that blockade of VEGFR-1 signaling induces BW-independent effects on the tumor microenvironment.
Ablation of VEGFR-1 signaling aggravates glucose metabolism
We found that despite preventing weight gain, VEGFR-1-TK-deletion in obese mice (but not in lean) led to elevated fasting glucose level and impaired glucose tolerance test (GTT) (Figure 4B, Supplementary Figure 6A). Interestingly, insulin tolerance remained similar between the two genotypes in obese mice (Supplementary Figure 6B). Of note, these changes in glucose metabolism were not due to macrophage infiltration, altered pancreatic β-cell, insulin production or an overall change in pancreatic tissue mass (Supplementary Figure 6C–G). We then investigated if the altered systemic metabolism observed in obese Flt1TK−/− mice also affects tumor metabolism upon tumor inoculation. In PAN02-bearing mice, we observed that Flt1TK−/− mice presented with increased plasma insulin (Figure 4C) compared to WT animals. The elevation of insulin was associated with increased phosphorylation of insulin receptor (p-IR) and IGF-1 receptor (p-IGF-1R) in PAN02 tumors (Figure Supplementary 7A). These findings were associated with decreased expression of gluconeogenic genes and decreased autophagy in tumors (Supplementary Figure 8A–B), but with no major changes in genes involved in glycolysis or lipid/protein metabolism (not shown). Collectively, these results indicate that ablation of VEGFR-1 signaling induces adverse effects on systemic metabolism in obese mice, which may have an impact on local tumor metabolism.
Metformin alleviates hyperinsulinemia in Flt1TK−/− obese mice and further improves the immune tumor microenvironment
In order to ameliorate the metabolic aberrations of VEGFR-1-TK-deletion in PAN02-bearing obese mice, we administered the anti-diabetic drug metformin. As anticipated, metformin prevented the increase in insulin levels observed with VEGFR-1-TK-deletion (Figure 4C). In addition, we and others have shown that metformin can also affect tumor progression (32–34). Combined VEGFR-1-TK-deletion and metformin led to the lowest PAN02 tumor weight in obese mice (Figure 4D) and these tumors displayed increased perfusion, despite unaltered vessel density in either tumor (Figure 4E) or adipose tissue (Supplementary Figure 8C). Similar to what was observed in our previous study with anti-VEGFR2-antibody (35), the increased vessel perfusion in the combination group was associated with increased recruitment of cytotoxic CD8+ T cells (trend for NK cells) (Figure 4F). In addition, downregulation of the immune checkpoint markers CTLA-4 and PD-L2 by VEGFR-1-TK deletion was maintained in the combination group (Figure 4G). These effects of the combination therapy on the immune environment were associated with increased tumor cell apoptosis compared to VEGFR-1-TK-deletion alone (Figure 4H). Interestingly, metformin restored the expression of major gluconeogenic genes that were downregulated by VEGFR-1-TK-deletion in obese tumors (Supplementary Figure 8A), although no changes were seen in AMPK/ACC activation (Supplementary Figure 8D) or IR/IGF-1 signaling (not shown). Taken together, our findings indicate that in addition to normalizing systemic metabolism, metformin can be helpful in restoring the abnormal tumor vasculature and immunity in obese Flt1TK−/− mice and in inhibiting tumor progression.
Ablation of VEGFR-1 signaling exerts similar effects in breast cancer
Next, we determined if the effects of VEGFR-1 blockade in obesity could be seen in another tumor type. Similar to PAN02, VEGFR-1-TK-deletion prevented E0771 BC progression in obese, but not lean animals. Lung metastases were significantly decreased by ablation of VEGFR-1 signaling in obese animals (Figure 5A, 5B), even though there was no effect on primary tumor growth (Supplementary Figure 9A). BW loss was also significantly reduced in obese Flt1TK−/− mice (Figure 5C). Again, similar to PAN02, we observed that VEGFR-1-TK-deletion produced no effect on vessel density, hypoxia markers, or immune cell infiltration in tumors of obese mice (Supplementary Figures 9B–C). We found no change in tumor levels of IL-4, IL-5 or IL-10 but instead observed an effect on other pro-tumor M2 markers — IL-6 and matrix metalloproteinase-9 (MMP-9) (36) (Supplementary Figure 9D–E): obesity promoted IL-6 and MMP-9 expression in tumors, and VEGFR-1-TK-deletion decreased expression of these markers in obese but not lean mice. In addition, similar to PAN02 model, ablation of VEGFR-1 signaling prevented body weight gain in obese but not lean animals (Figure 5D), and was associated with increased systemic insulin levels (Figure 5E) and increased activation of p-IGF-1R at the tumor level (Supplementary Figure 7B). Taken together, we observed similar effects of VEGFR-1-TK-deletion in the obese setting in two different cancer types.
Figure 5. Effect of VEGFR-1 signaling ablation on a breast cancer model.
A) Lungs collected 3 weeks after implantation of E0771 tumors in lean and obese WT or Flt1TK−/− mice (n = 6–16/group). i: Representative image of metastases in the lungs (black arrows). ii: Average number of lung metastases per mice. B) Incidence (number of mice affected with lungs metastasis) in obese WT or Flt1TK−/− mice. C) Body weight loss from implantation until tumor extraction in obese WT or Flt1TK−/− mice. D) Body weight of female C57BL/6 mice after 10 weeks of high fat or low fat diet in WT or Flt1TK−/− group. Number of animals depicted as dots on each column. E) Levels of plasma Insulin in WT vs. Flt1TK−/− obese mice 3 weeks after implantation of E0771 tumors (n = 7–10/group). * P < 0.05, ** P < 0.01, *** P < 0.005, **** P < 0.001. Error bars represent standard error of the mean.
Obesity is associated with increased systemic PlGF levels in pancreatic and breast cancer patients as well as in pre-clinical mouse models
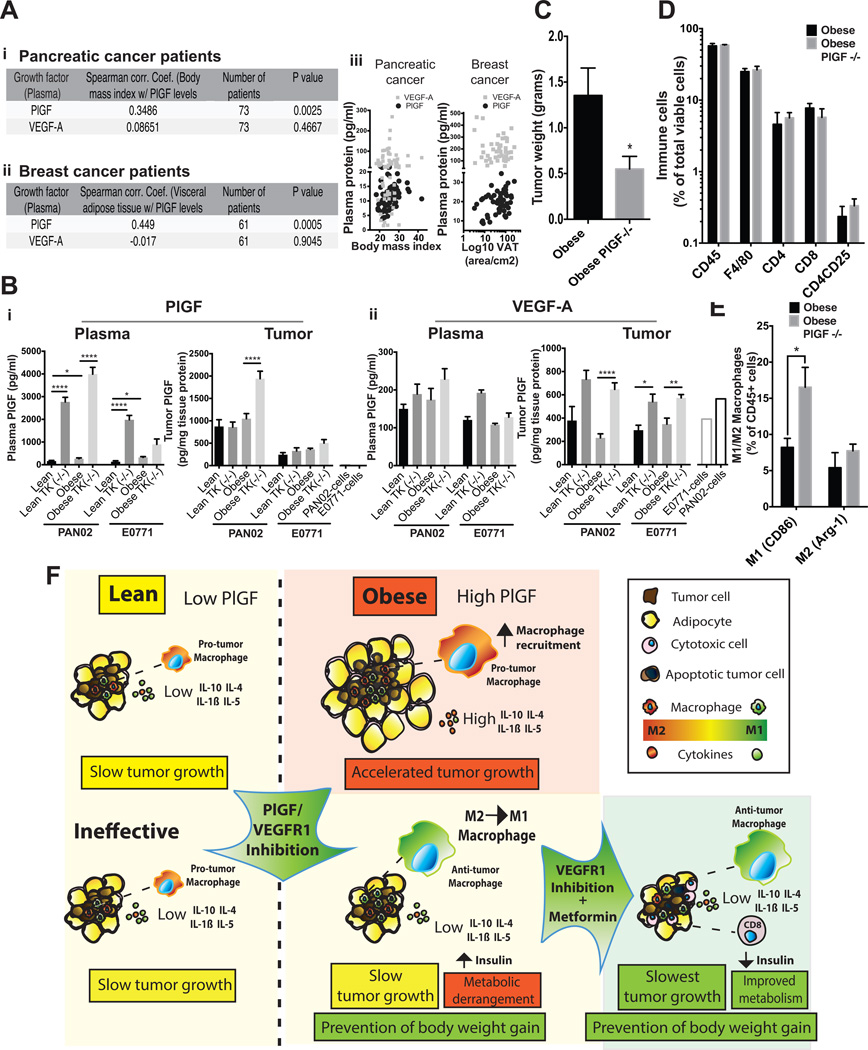
PlGF and VEGF-A are ligands of VEGFR-1 with a role in tumor progression (37). Plasma levels of PlGF and VEGF-A were measured in PDAC (n=73) and BC (n=61) patients and were correlated with body-mass-index (BMI) or, in the case of BC, with visceral adipose tissue (VAT). We found that PlGF (r=0.35 and 0.45 for BMI and VAT), but not VEGF-A (r=0.09 and −0.02 for BMI and VAT), correlated with adiposity in both PDAC and BC patients (Figure 6A). We then confirmed that plasma PlGF was also elevated in WT obese mice compared to lean mice in the PAN02 and E0771 tumor models, as well as in an additional PDAC tumor model (AK4.4) (Figure 6Bi, Supplementary Figure 10A). Of note, we observed a similar elevation in plasma PlGF in both diet-induced obesity and genetically obese leptin deficient mice (ob/ob) (Supplementary Figure 10A). Consistent with human data, VEGF-A was not increased in plasma (systemic expression) in WT obese mice compared to lean mice in both PAN02 and E0771 models (Figure 6Bii). On the other hand, the expression of VEGF-A in tumors (local expression) (Figure 6Bii) significantly increased in Flt1TK−/− mice as compared to corresponding WT mice in both tumor models. However, the increase in VEGF-A levels was essentially the same in lean and obese mice. Conversely, another VEGFR-1 ligand — VEGF-B — was increased in circulation in obese mice in the PAN02 model, but not in the E0771 model (Supplementary Figure 10B). Most importantly, in Flt1TK−/− mice, plasma PlGF – but not VEGF-A or VEGF-B – was dramatically increased compared to WT in both lean and obese settings (more than 10-fold increase in obese PAN02 bearing mice) (Figure 6B and Supplementary Figure 10B). This indicates that signaling via VEGFR-1 is particularly associated with PlGF rather than VEGFs in the models used. Taken together, our data shows that PlGF is increased in obese mice and patients, and may mediate the obesity-induced VEGFR-1 dependent effects in tumors.
Figure 6. Effect of PlGF inhibition on obesity-induced tumor progression.
A) i-ii: Correlation between plasma levels of PlGF and VEGF and visceral adipose tissue (VAT) in (i) pancreatic cancer patients (n=61) or body mass index (BMI) in (ii) breast cancer patients (n = 73) that have received no prior treatment (for more information, see Supplementary Methods). B) Levels of plasma and tumor PlGF (i) and VEGF-A (ii) in lean and obese WT or Flt1TK−/− mice 3 weeks after implantation of PAN02 and E0771 tumors (n = 3–20/group). C) Tumor weights of PAN02 tumors collected 3 weeks after tumor source implantation in obese WT or Plgf−/− mice (n = 3–6/group). D) Flow cytometry was used to determine immune cell infiltration in PAN02 tumors from obese WT and Plgf−/− mice (n = 3–6/group). E) Flow cytometry was performed to determine the expression of M1 (CD86 positive) and M2 (Arg1 positive) macrophages within the total leukocyte population in tumors from obese WT and Plgf−/− mice (n = 3–6/group). F) Graphical abstract. Increased levels of plasma PlGF in obese mice associate with increased recruitment of TAMs, M2-like cytokine profile, and accelerated tumor progression compared to lean setting. While ineffective in the lean mice, in obese setting PlGF/VEGFR-1 signaling ablation did not affect TAM recruitment or vessel density, but shifted TAM polarization and the immune environment towards an M1 phenotype, and prevented the obesity-induced tumor progression. This occurred with an aggravation of the systemic metabolism and increase in plasma Insulin levels. Metformin not only normalized the systemic metabolism, but also promoted increased tumor vascular perfusion, cytotoxic cell recruitment, tumor cell apoptosis, and reduced tumor growth. * P < 0.05, ** P < 0.01, **** P < 0.001. Error bars represent standard error of the mean.
PlGF deletion reproduces the findings of VEGFR-1-TK-deletion in obese mice
To confirm that PlGF is responsible for the observed metabolic and tumor effects of VEGFR-1 signaling, we implanted PAN02 tumors in obese WT and Plgf−/− mice. Similar to the findings in the Flt1TK−/− background, tumors grown in Plgf−/− obese mice were significantly smaller compared to those grafted in obese WT mice (Figure 6C). The number of TAMs, CD4+, CD8+, and T-regulatory lymphocytes were not altered by PlGF ablation in obese mice (Figure 6D), but instead we observed an increased enrichment of M1-TAMs (F4/80+CD86+) in obese Plgf−/− mice, similar to that in obese Flt1TK−/− mice (Figure 6E). Of note, PAN02 and E0771 cells cultured in vitro had no detectable production of PlGF, while secreted VEGF-A and B (Figure 6B and Supplementary Figure 10B, right columns.), suggesting that the stroma has a substantial contribution to PlGF expression in vivo compared with other VEGFR-1 ligands. Finally, and again similar to what we observed in obese Flt1TK−/− mice, weight gain in high-fat fed (but not low-fat fed), and consequently body weight at the time of tumor implantation were both reduced in Plgf−/− mice (Supplementary Figure 11C), and plasma insulin was elevated compared to obese WT mice (Supplementary Figure 11D). Taken together, these data indicate that PlGF blockade reproduces the effects of VEGFR-1-TK-deletion and reduces the obesity-induced alterations in metabolism, immune microenvironment and tumor progression.
Discussion
We demonstrated here for the first time that obesity is associated with increased systemic levels of PlGF in breast and pancreatic cancer patients as well as in obese tumor mouse models. Furthermore, we revealed that PlGF/VEGFR-1 signaling mediates obesity-induced tumor progression (Figure 6F).
In a PDAC model, at the tumor level obesity was associated with increased TAM infiltration, expression of M2 cytokines, tumor growth, and metastasis. VEGFR-1 was abundantly expressed in TAMs, and blockade of VEGFR-1 signaling in obese but not lean mice led to a shift in pro-tumor cytokine production (e.g. IL-1β) and TAM polarization from an M2 pro-tumor to an M1 anti-tumor phenotype, ultimately reducing obesity-induced tumor progression. Furthermore, PlGF blockade in obese mice reproduced the effects of VEGFR-1-TK-deletion on the tumor immune environment and tumor growth. Rolny et al. reported an M1 shift in TAM polarization after inhibition of PlGF in a non-obese setting (38). Furthermore, we and others have shown that PlGF/VEGFR-1 signaling promotes secretion of IL-1β by monocytes/macrophages (14, 39). However, this is the first study to demonstrate a specific role for VEGFR-1 in TAM polarization. Similar to previous studies, inhibition of VEGFR-1 signaling did not alter immune cell infiltration in tumors (12, 40–42). However, this effect has been shown to be highly tumor- and context-dependent (14, 15, 18, 43), and we show here that PlGF/VEGFR-1 signaling affects the function rather than the number of infiltrating immune cells. Of note, despite that differences in TAM polarization markers between lean and obese settings were not clear (Supplementary Figure 2), PlGF/VEGFR-1 inhibition functionally promoted an M1 shift only in the obese setting. Hence this pathway is clearly involved in immunosupression in this metabolic setting. Importantly, plasma PlGF – but not VEGF-A or VEGF-B – was robustly associated with obesity across all models/human samples. In addition, unlike VEGFs, PlGF was not produced by these tumor cells, further supporting that PlGF levels can be more affected by the obese-altered host tumor microenvironment. It is therefore not surprising that stromal deletion of PlGF replicated the effects of VEGFR-1-deletion on metabolism and tumors in obese mice. This is also consistent with our previous study using Flt1TK−/−mice, where we found that VEGFR-1-TK deletion affects primary tumor growth only in the presence of PlGF overexpression, but not of VEGF overexpression (18). Of note, we found that VEGF-A in tumors (local expression) did increase in Flt1TK−/− mice in both the lean and obese setting. The fact that it increased in both settings further validates the lack of any association between VEGF-A with obesity in this study. On the other hand, we have shown before that VEGF-A was decreased in peritoneal macrophages derived from Flt1TK−/− mice compared with those from WT mice (44). This indicates that the effect of VEGFR-1-TK-deletion on the expression levels of VEGF in specific cells of the tumor microenvironment may vary, despite an overall increase.
Importantly, similar effects of VEGFR-1-TK-deletion on systemic metabolism, tumor progression, vasculature, immune cell infiltration and TAM polarization were observed not only in PAN02 but also in a BC model (E0771). This is consistent with the elevated systemic levels of PlGF in the obese setting also in this model. Despite inducing a smaller impact on the tumor immune environment compared to PAN02 tumors, we found that VEGFR-1-TK-deletion reduced the expression of IL-6 and MMP-9 in the obese setting. This may explain the effect of VEGFR-1-TK-deletion on metastasis in this model, in line with previous findings that IL-6 and VEGFR-1 dependent MMP-9 expression in the stroma (TAMs in particular) can affect systemic metastasis (14, 45). On the other hand, VEGFR-1-TK-deletion did not affect primary tumor growth in obese mice in the E0771 model, contrary to results in the PAN02 model which is consistent with the smaller effects on the tumor immune microenvironment in the former model. We and others have shown that the growth rate of tumors implanted in Flt1TK−/− mice compared to WT may be affected differently depending on the tumor model (12, 15). In addition, as mentioned above VEGFR-1-TK-deletion appears to affect primary tumor growth only in the presence of PlGF overexpression (15, 18). Similarly, overexpression of PlGF in colorectal cancer cell lines increased the migration and invasion of tumor cells in a VEGFR-1-dependent manner (46). In fact, the lack of effect on primary tumor growth (and smaller effect on the immune environment) by VEGFR-1-TK-deletion in the E0771 tumor model could have been due to the lower levels of PlGF in tumors (local levels) compared to PAN02 tumors, which might have contributed to the lower sensitivity to VEGFR-1-TK-deletion. Conversely, the effect of VEGFR-1-TK-deletion on metastases may be more dependent on the levels of systemic PlGF, which was increased in obese mice in both PAN02 and E0771 tumor models. Our results on systemic metastasis are in line with our previous work in normal weight Flt1TK−/− mice (that have relatively low-levels of plasma PlGF), where we showed no effect of VEGFR-1 genetic or pharmacological inhibition on spontaneous metastases formation (41, 42, 47).
Despite that VEGFR-1 can regulate tumor angiogenesis in a context dependent manner (14, 37, 48), here we found that obesity did not promote tumor vessel density, and VEGFR-1 signaling ablation did not modify vessel density in either tumors or adipose tissue. Carmeliet et al. have shown that the angiogenic role of VEGFR-1 is in part due to the recruitment of TAMs that secrete pro-angiogenic factors in the tumor microenvironment (37). Hence, the lack of the effect on TAM recruitment observed here may explain at least in part the absence of an effect on angiogenesis in our models.
Beyond the effects on tumors, we showed that PlGF and VEGFR-1 deficiency also reduced weight gain in mice maintained on HFD. This is consistent with previous findings from Lijnen et al. in PlGF-deficient mice (20), although previous work with pharmacological inhibition of PlGF or VEGFR-1 did not demonstrate the metabolic derangement observed here (20, 49). The transient effects of pharmaceutical agents on metabolic parameters may not always be the same as phenotypes caused by life-long genetic deficiencies. Imperfect delivery/efficacy of pharmaceutical agents may also cause differences in phenotypes compared to complete genetic blockade of the same molecular pathways. The reduction of body weight gain with VEGFR-1-TK-deletion was not associated with reduced vasculature, as in Lijnen et al (20), or immune cell infiltration. However, the shift to M1-macrophages and M1 cytokine profile with VEGFR-1-TK-deletion is known to be associated with insulin resistance (50) and promote adipocyte cell death (51), which could explain the impaired glucose metabolism, increased levels of insulin and reduced weight gain in Flt1TK−/− and Plgf−/− obese mice compared to WT. This is consistent with findings from Hemmeryckx et al., who showed that PlGF deficiency in mice fed with a HFD promoted insulin resistance and hyperinsulinemia, presumably via reduced fraction of brown adipocytes and stimulation of white adipocyte hypertrophy (21). Interestingly, however, we observed that VEGFR-1-TK-deletion actually reduced adipocyte size. In addition, various pancreatic morphological parameters including β-cells and insulin production, as well as macrophage infiltration in the pancreas, which can also influence insulin production (52), were unaltered with VEGFR-1-TK-deletion. This indicates that the lack of VEGFR-1 signaling did not alter pancreatic insulin production, likely affecting peripheral resistance to this hormone. In fact, inhibition of Pi3K-eNOS signaling by VEGFR-1 inhibition may have been involved, as previously observed in a model of diabetic nephropathy (53). Importantly, previous studies have demonstrated similar glucose metabolism impairments in mice with genetic VEGF deficiencies in the whole pancreas (54), or specifically in β-cells (55) where an impairment is thought to be caused by defective transport of insulin across vascular ECs. Of note, we found that the effects observed on tumor progression were not due to the protective effects on BW. In fact, in BW-matched mice - mice that present with same BW at the time of tumor implantation -, VEGFR-1-TK-deletion still significantly decreased tumor progression, allowing us to conclude that the bulk of the mechanism of action is via direct impact on the tumor microenvironment. Nevertheless, considering the physical and psychologic beneficial effects of preventing weight gain in BC patients (56, 57), long term VEGFR-1 inhibition may further improve the quality of life.
The aggravated diabetes-like systemic metabolism in obese Flt1TK−/− compared to obese WT mice was associated with increased plasma insulin and activation of insulin/IGF-1 signaling in tumors. This was associated with decreased expression of gluconeogenic genes and autophagy in tumors, but no major changes in other metabolic pathways were observed. Whilst activation of Insulin/IGF-1 in tumors may be detrimental (5), recent evidence indicate that inhibition of gluconeogenesis may lead to prevention of tumor growth (58). Further studies are necessary to explore the role of VEGFR-1 in local tumor metabolism. Besides normalizing the plasma levels of insulin in obese Flt1TK−/−, remarkably metformin also normalized pancreatic tumor vasculature and immune microenvironment—by increasing perfusion and recruitment of CD8+ T cells—that were associated with increased cell death and reduced tumor growth. Since VEGFR-1-TK-deletion decreased expression levels of CTLA-4 and PDL-2, those findings suggest a synergy between enhanced T cell function by VEGFR-1-TK-deletion (hence acting as an immune checkpoint inhibitor) and increased infiltration of T/NK cells by metformin. Of note, the effect of metformin on perfusion is consistent with the ability of this drug to reduce tumor desmoplasia, which we have recently described (32). Contrary to previous studies, we did not find a significant change in activating of major metabolic pathways—including IGF-1 and AMPK—after metformin treatment (33, 34), indicating that the major effects of metformin seem to be on the stromal-vascular-immune tumor microenvironment and not on local metabolism. This is consistent with our previous work showing that metformin can modulate metabolic pathways specifically in TAMs but not at the whole tumor level (32). The positive impact of metformin on tumors are consistent with reports in preclinical models of obesity from our laboratory and others (32–34) and in diabetic PDAC patients (59), and multiple trials are ongoing to validate its beneficial effects (59).
Collectively, our findings reveal that PlGF/VEGFR-1 signaling contributes to weight gain and to a pro-tumor immune microenvironment in the obese but not lean setting, which promotes accelerated tumor progression. We found this effect in both PDAC and BC models, suggesting that this may be a common mechanism of tumor induction by obesity that could apply to other cancer types. Furthermore, the increased plasma levels of PlGF observed in obese PDAC and BC patients strongly support the findings of this study, and suggest the PlGF/VEGFR-1 pathway as a potential target for obese cancer patients. Since most PDAC and BC patients have excess weight at diagnosis, stratifying these patients by BW for treatment may enhance the efficacy of anti-VEGFR-1 agents such as multi-kinase inhibitors, which have failed to show efficacy in unselected populations (60). In addition, this study suggests that PlGF/VEGFR-1 may be a valid target particularly in combination with therapies that control systemic metabolism, such as metformin.
Supplementary Material
Statement of translational relevance.
With the current worldwide obesity epidemic, the majority of pancreatic and breast cancer patients are either overweight or obese at diagnosis. Importantly, obesity is associated with poor prognosis in these patients. Hence, uncovering the cellular mechanisms underlying this poor outcome is imperative. We revealed a significant correlation between plasma PlGF and adiposity in pancreatic and breast cancer patients and described how PlGF/VEGFR-1 signaling contributes to obesity-induced tumor progression using clinically relevant mouse models. We found that blocking the PlGF/VEGFR-1 signaling axis is effective in obese but not in lean condition. Therefore, anti-VEGFR-1 agents may prove therapeutically beneficial in pancreatic and breast cancer patient populations stratified by body mass index. On the other hand, ablation of PlGF/VEGFR-1 signaling worsened the impaired systemic glucose metabolism associated with obesity, which could be reverted by the anti-diabetic drug metformin. Controlling the metabolic status of patients may enhance the clinical translation of an anti-PlGF/VEGFR-1 strategy.
Acknowledgments
We would like to thank Eleftheria Maratos-Flier, Michael Badman (Division of Endocrinology, Beth Israel Deaconess Medical Center) and Brian Seed (Department of Genetics, Massachusetts General Hospital) for valuable advice. We would also like to thank Peigen Huang for re-derivation and generation of gnotobiotic Flt1TK−/− mice; Sylvie Roberge and Julia Kahn for assistance with tumor implantation, Penny Wuo for assistance with animal husbandry; Emanuelle di Tomaso, Johanna Lahdenranta and Carolyn Smith for assistance with immunohistochemical studies.
Financial Support: This study was supported in part by the National Institutes of Health (http://nih.gov/; R35-CA197743, CA080124, CA085140, CA096915, CA115767, CA126642 to RKJ and DF), the Lustgarten Foundation (http://www.lustgarten.org/; research grant to RKJ), the US Department of Defense Breast Cancer Research Innovator Award W81XWH-10-1-0016 (research grant to RKJ), the Warshaw Institute for Pancreatic Cancer Research (http://www.massgeneral.org/warshawinstitute/about/; research grant to DF), and the Foundation for Science and Technology (http://www.fct.pt/; Portugal, POPH/FSE funding program, fellowship and research grant to JI). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Disclosure: RKJ received consultant fees from Ophthotech, SPARC, SynDevRx and XTuit. RKJ owns equity in Enlight, Ophthotech, SynDevRx, and XTuit and serves on the Board of Directors of XTuit and the Boards of Trustees of Tekla Healthcare Investors, Tekla Life Sciences Investors, Tekla Healthcare Opportunities Fund and Tekla World Healthcare Fund. No reagents or funding from these companies was used in these studies.
References
- 1.World Health Organization. Obesity and overweight. [cited 2014 20 May];2014 Available from: http://www.who.int/mediacentre/factsheets/fs311/en/ [Google Scholar]
- 2.Chan DS, Vieira AR, Aune D, Bandera EV, Greenwood DC, McTiernan A, et al. Body mass index and survival in women with breast cancer-systematic literature review and meta-analysis of 82 follow-up studies. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 2014;25:1901–1914. doi: 10.1093/annonc/mdu042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Majumder K, Gupta A, Arora N, Singh PP, Singh S. Premorbid Obesity and Mortality in Patients With Pancreatic Cancer: A Systematic Review and Meta-analysis. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2015 doi: 10.1016/j.cgh.2015.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li D, Morris JS, Liu J, Hassan MM, Day RS, Bondy ML, et al. Body mass index and risk, age of onset, and survival in patients with pancreatic cancer. JAMA. 2009;301:2553–2562. doi: 10.1001/jama.2009.886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hursting SD, Dunlap SM. Obesity, metabolic dysregulation, and cancer: a growing concern and an inflammatory (and microenvironmental) issue. Annals of the New York Academy of Sciences. 2012;1271:82–87. doi: 10.1111/j.1749-6632.2012.06737.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Silverman KJ, Lund DP, Zetter BR, Lainey LL, Shahood JA, Freiman DG, et al. Angiogenic activity of adipose tissue. Biochemical and biophysical research communications. 1988;153:347–352. doi: 10.1016/s0006-291x(88)81229-4. [DOI] [PubMed] [Google Scholar]
- 7.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Costa C, Incio J, Soares R. Angiogenesis and chronic inflammation: cause or consequence? Angiogenesis. 2007;10:149–166. doi: 10.1007/s10456-007-9074-0. [DOI] [PubMed] [Google Scholar]
- 9.Incio J, Soares R. Obesity, Diabetes and Metabolic Syndrome Impact on TUMOR ANGIOGENESIS. In: Ruben R, Gonzalez-Perez BRR, editors. Tumor Angiogenesis Regulators. CRC Press; 2013. [Google Scholar]
- 10.Okwan-Duodu D, Umpierrez GE, Brawley OW, Diaz R. Obesity-driven inflammation and cancer risk: role of myeloid derived suppressor cells and alternately activated macrophages. American journal of cancer research. 2013;3:21–33. [PMC free article] [PubMed] [Google Scholar]
- 11.Chen CT, Du Y, Yamaguchi H, Hsu JM, Kuo HP, Hortobagyi GN, et al. Targeting the IKKbeta/mTOR/VEGF signaling pathway as a potential therapeutic strategy for obesity-related breast cancer. Molecular cancer therapeutics. 2012;11:2212–2221. doi: 10.1158/1535-7163.MCT-12-0180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Qian BZ, Zhang H, Li J, He T, Yeo EJ, Soong DY, et al. FLT1 signaling in metastasis-associated macrophages activates an inflammatory signature that promotes breast cancer metastasis. The Journal of experimental medicine. 2015;212:1433–1448. doi: 10.1084/jem.20141555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shibuya M. Involvement of Flt-1 (VEGF receptor-1) in cancer and preeclampsia. Proceedings of the Japan Academy Series B, Physical and biological sciences. 2011;87:167–178. doi: 10.2183/pjab.87.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hiratsuka S, Duda DG, Huang Y, Goel S, Sugiyama T, Nagasawa T, et al. C-X-C receptor type 4 promotes metastasis by activating p38 mitogen-activated protein kinase in myeloid differentiation antigen (Gr-1)-positive cells. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:302–307. doi: 10.1073/pnas.1016917108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Muramatsu M, Yamamoto S, Osawa T, Shibuya M. Vascular endothelial growth factor receptor-1 signaling promotes mobilization of macrophage lineage cells from bone marrow and stimulates solid tumor growth. Cancer Res. 2010;70:8211–8221. doi: 10.1158/0008-5472.CAN-10-0202. [DOI] [PubMed] [Google Scholar]
- 16.Marcellini M, De Luca N, Riccioni T, Ciucci A, Orecchia A, Lacal PM, et al. Increased melanoma growth and metastasis spreading in mice overexpressing placenta growth factor. Am J Pathol. 2006;169:643–654. doi: 10.2353/ajpath.2006.051041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dewerchin M, Carmeliet P. PlGF: a multitasking cytokine with disease-restricted activity. Cold Spring Harbor perspectives in medicine. 2012:2. doi: 10.1101/cshperspect.a011056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hiratsuka S, Maru Y, Okada A, Seiki M, Noda T, Shibuya M. Involvement of Flt-1 tyrosine kinase (vascular endothelial growth factor receptor-1) in pathological angiogenesis. Cancer Res. 2001;61:1207–1213. [PubMed] [Google Scholar]
- 19.Bais C, Wu X, Yao J, Yang S, Crawford Y, McCutcheon K, et al. PlGF blockade does not inhibit angiogenesis during primary tumor growth. Cell. 2010;141:166–177. doi: 10.1016/j.cell.2010.01.033. [DOI] [PubMed] [Google Scholar]
- 20.Lijnen HR, Christiaens V, Scroyen I, Voros G, Tjwa M, Carmeliet P, et al. Impaired adipose tissue development in mice with inactivation of placental growth factor function. Diabetes. 2006;55:2698–2704. doi: 10.2337/db06-0526. [DOI] [PubMed] [Google Scholar]
- 21.Hemmeryckx B, van Bree R, Van Hoef B, Vercruysse L, Lijnen HR, Verhaeghe J. Adverse adipose phenotype and hyperinsulinemia in gravid mice deficient in placental growth factor. Endocrinology. 2008;149:2176–2183. doi: 10.1210/en.2007-1272. [DOI] [PubMed] [Google Scholar]
- 22.Voros G, Maquoi E, Demeulemeester D, Clerx N, Collen D, Lijnen HR. Modulation of angiogenesis during adipose tissue development in murine models of obesity. Endocrinology. 2005;146:4545–4554. doi: 10.1210/en.2005-0532. [DOI] [PubMed] [Google Scholar]
- 23.Pervanidou P, Chouliaras G, Akalestos A, Bastaki D, Apostolakou F, Papassotiriou I, et al. Increased placental growth factor (PlGF) concentrations in children and adolescents with obesity and the metabolic syndrome. Hormones. 2014;13:369–374. doi: 10.14310/horm.2002.1491. [DOI] [PubMed] [Google Scholar]
- 24.Lappas M. Markers of endothelial cell dysfunction are increased in human omental adipose tissue from women with pre-existing maternal obesity and gestational diabetes. Metabolism: clinical and experimental. 2014;63:860–873. doi: 10.1016/j.metabol.2014.03.007. [DOI] [PubMed] [Google Scholar]
- 25.Sparano JA, Strickler HD. Breast cancer patients who are obese at diagnosis: alea iacta est? or "is the die cast"? Oncology. 2011;25 1002, 4, 7. [PubMed] [Google Scholar]
- 26.McWilliams RR, Matsumoto ME, Burch PA, Kim GP, Halfdanarson TR, de Andrade M, et al. Obesity adversely affects survival in pancreatic cancer patients. Cancer. 2010;116:5054–5062. doi: 10.1002/cncr.25465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zyromski NJ, Mathur A, Pitt HA, Wade TE, Wang S, Nakshatri P, et al. Obesity potentiates the growth and dissemination of pancreatic cancer. Surgery. 2009;146:258–263. doi: 10.1016/j.surg.2009.02.024. [DOI] [PubMed] [Google Scholar]
- 28.Cifarelli V, Lashinger LM, Devlin KL, Dunlap SM, Huang J, Kaaks R, et al. Metformin and Rapamycin Reduce Pancreatic Cancer Growth in Obese Prediabetic Mice by Distinct MicroRNA-Regulated Mechanisms. Diabetes. 2015 doi: 10.2337/db14-1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ruffell B, Coussens LM. Macrophages and therapeutic resistance in cancer. Cancer Cell. 2015;27:462–472. doi: 10.1016/j.ccell.2015.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hiratsuka S, Minowa O, Kuno J, Noda T, Shibuya M. Flt-1 lacking the tyrosine kinase domain is sufficient for normal development and angiogenesis in mice. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:9349–9354. doi: 10.1073/pnas.95.16.9349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Topalian SL, Drake CG, Pardoll DM. Immune checkpoint blockade: a common denominator approach to cancer therapy. Cancer Cell. 2015;27:450–461. doi: 10.1016/j.ccell.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Incio J, Suboj P, Chin SM, Vardam-Kaur T, Liu H, Hato T, et al. Metformin Reduces Desmoplasia in Pancreatic Cancer by Reprogramming Stellate Cells and Tumor-Associated Macrophages. PloS one. 2015;10:e0141392. doi: 10.1371/journal.pone.0141392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goodwin PJ, Stambolic V. Obesity and insulin resistance in breast cancer - Chemoprevention strategies with a focus on metformin. Breast. 20(Suppl 3):S31–S35. doi: 10.1016/S0960-9776(11)70291-0. [DOI] [PubMed] [Google Scholar]
- 34.Algire C, Zakikhani M, Blouin MJ, Shuai JH, Pollak M. Metformin attenuates the stimulatory effect of a high-energy diet on in vivo LLC1 carcinoma growth. Endocrine-related cancer. 2008;15:833–839. doi: 10.1677/ERC-08-0038. [DOI] [PubMed] [Google Scholar]
- 35.Huang Y, Yuan J, Righi E, Kamoun WS, Ancukiewicz M, Nezivar J, et al. Vascular normalizing doses of antiangiogenic treatment reprogram the immunosuppressive tumor microenvironment and enhance immunotherapy. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:17561–17566. doi: 10.1073/pnas.1215397109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Karnevi E, Andersson R, Rosendahl AH. Tumour-educated macrophages display a mixed polarisation and enhance pancreatic cancer cell invasion. Immunology and cell biology. 2014;92:543–552. doi: 10.1038/icb.2014.22. [DOI] [PubMed] [Google Scholar]
- 37.Fischer C, Mazzone M, Jonckx B, Carmeliet P. FLT1 and its ligands VEGFB and PlGF: drug targets for anti-angiogenic therapy? Nat Rev Cancer. 2008;8:942–956. doi: 10.1038/nrc2524. [DOI] [PubMed] [Google Scholar]
- 38.Rolny C, Mazzone M, Tugues S, Laoui D, Johansson I, Coulon C, et al. HRG inhibits tumor growth and metastasis by inducing macrophage polarization and vessel normalization through downregulation of PlGF. Cancer Cell. 2011;19:31–44. doi: 10.1016/j.ccr.2010.11.009. [DOI] [PubMed] [Google Scholar]
- 39.Selvaraj SK, Giri RK, Perelman N, Johnson C, Malik P, Kalra VK. Mechanism of monocyte activation and expression of proinflammatory cytochemokines by placenta growth factor. Blood. 2003;102:1515–1524. doi: 10.1182/blood-2002-11-3423. [DOI] [PubMed] [Google Scholar]
- 40.Casanovas O, Hicklin DJ, Bergers G, Hanahan D. Drug resistance by evasion of antiangiogenic targeting of VEGF signaling in late-stage pancreatic islet tumors. Cancer Cell. 2005;8:299–309. doi: 10.1016/j.ccr.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 41.Dawson MR, Duda DG, Fukumura D, Jain RK. VEGFR1-activity-independent metastasis formation. Nature. 2009;461:E4. doi: 10.1038/nature08254. discussion E5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dawson MR, Duda DG, Chae SS, Fukumura D, Jain RK. VEGFR1 activity modulates myeloid cell infiltration in growing lung metastases but is not required for spontaneous metastasis formation. PloS one. 2009;4:e6525. doi: 10.1371/journal.pone.0006525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kerber M, Reiss Y, Wickersheim A, Jugold M, Kiessling F, Heil M, et al. Flt-1 signaling in macrophages promotes glioma growth in vivo. Cancer Res. 2008;68:7342–7351. doi: 10.1158/0008-5472.CAN-07-6241. [DOI] [PubMed] [Google Scholar]
- 44.Murakami M, Iwai S, Hiratsuka S, Yamauchi M, Nakamura K, Iwakura Y, et al. Signaling of vascular endothelial growth factor receptor-1 tyrosine kinase promotes rheumatoid arthritis through activation of monocytes/macrophages. Blood. 2006;108:1849–1856. doi: 10.1182/blood-2006-04-016030. [DOI] [PubMed] [Google Scholar]
- 45.Dirat B, Bochet L, Dabek M, Daviaud D, Dauvillier S, Majed B, et al. Cancer-associated adipocytes exhibit an activated phenotype and contribute to breast cancer invasion. Cancer Res. 2011;71:2455–2465. doi: 10.1158/0008-5472.CAN-10-3323. [DOI] [PubMed] [Google Scholar]
- 46.Wei SC, Tsao PN, Weng MT, Cao Z, Wong JM. Flt-1 in colorectal cancer cells is required for the tumor invasive effect of placental growth factor through a p38-MMP9 pathway. Journal of biomedical science. 2013;20:39. doi: 10.1186/1423-0127-20-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Duda DG, Jain RK. Premetastatic lung "niche": is vascular endothelial growth factor receptor 1 activation required? Cancer Res. 2010;70:5670–5673. doi: 10.1158/0008-5472.CAN-10-0119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shibuya M. Vascular endothelial growth factor and its receptor system: physiological functions in angiogenesis and pathological roles in various diseases. Journal of biochemistry. 2013;153:13–19. doi: 10.1093/jb/mvs136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tam J, Duda DG, Perentes JY, Quadri RS, Fukumura D, Jain RK. Blockade of VEGFR2 and not VEGFR1 can limit diet-induced fat tissue expansion: role of local versus bone marrow-derived endothelial cells. PloS one. 2009;4:e4974. doi: 10.1371/journal.pone.0004974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hong EG, Ko HJ, Cho YR, Kim HJ, Ma Z, Yu TY, et al. Interleukin-10 prevents diet-induced insulin resistance by attenuating macrophage and cytokine response in skeletal muscle. Diabetes. 2009;58:2525–2535. doi: 10.2337/db08-1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mauer J, Chaurasia B, Goldau J, Vogt MC, Ruud J, Nguyen KD, et al. Signaling by IL-6 promotes alternative activation of macrophages to limit endotoxemia and obesity-associated resistance to insulin. Nature immunology. 2014;15:423–430. doi: 10.1038/ni.2865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tessem JS, Jensen JN, Pelli H, Dai XM, Zong XH, Stanley ER, et al. Critical roles for macrophages in islet angiogenesis and maintenance during pancreatic degeneration. Diabetes. 2008;57:1605–1617. doi: 10.2337/db07-1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yang KS, Lim JH, Kim TW, Kim MY, Kim Y, Chung S, et al. Vascular endothelial growth factor-receptor 1 inhibition aggravates diabetic nephropathy through eNOS signaling pathway in db/db mice. PloS one. 2014;9:e94540. doi: 10.1371/journal.pone.0094540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lammert E, Gu G, McLaughlin M, Brown D, Brekken R, Murtaugh LC, et al. Role of VEGF-A in vascularization of pancreatic islets. Curr Biol. 2003;13:1070–1074. doi: 10.1016/s0960-9822(03)00378-6. [DOI] [PubMed] [Google Scholar]
- 55.Brissova M, Shostak A, Shiota M, Wiebe PO, Poffenberger G, Kantz J, et al. Pancreatic islet production of vascular endothelial growth factor--a is essential for islet vascularization, revascularization, and function. Diabetes. 2006;55:2974–2985. doi: 10.2337/db06-0690. [DOI] [PubMed] [Google Scholar]
- 56.Playdon MC, Bracken MB, Sanft TB, Ligibel JA, Harrigan M, Irwin ML. Weight Gain After Breast Cancer Diagnosis and All-Cause Mortality: Systematic Review and Meta-Analysis. J Natl Cancer Inst. 2015;107 doi: 10.1093/jnci/djv275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ligibel JA, Strickler HD. Obesity and its impact on breast cancer: tumor incidence, recurrence, survival, and possible interventions. American Society of Clinical Oncology educational book / ASCO American Society of Clinical Oncology Meeting. 2013:52–59. doi: 10.14694/EdBook_AM.2013.33.52. [DOI] [PubMed] [Google Scholar]
- 58.Leithner K, Hrzenjak A, Olschewski H. Gluconeogenesis in cancer: door wide open. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:E4394. doi: 10.1073/pnas.1415680111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sadeghi N, Abbruzzese JL, Yeung SC, Hassan M, Li D. Metformin use is associated with better survival of diabetic patients with pancreatic cancer. Clin Cancer Res. 2012;18:2905–2912. doi: 10.1158/1078-0432.CCR-11-2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cardin DB, Goff L, Li CI, Shyr Y, Winkler C, DeVore R, et al. Phase II trial of sorafenib and erlotinib in advanced pancreatic cancer. Cancer medicine. 2014;3:572–579. doi: 10.1002/cam4.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.