Abstract
In a subset of patients with non-del(5q) myelodysplastic syndrome (MDS), lenalidomide promotes erythroid lineage competence and effective erythropoiesis. To determine the mechanism by which lenalidomide promotes erythropoiesis, we investigated its action on erythropoietin receptor (EpoR) cellular dynamics. Lenalidomide upregulated expression and stability of JAK2-associated EpoR in UT7 erythroid cells and primary CD71+ erythroid progenitors. The effects of lenalidomide on receptor turnover were Type I cytokine receptor specific, as evidenced by co-regulation of the IL-3β receptor but not c-Kit. To elucidate this mechanism, we investigated the effects of lenalidomide on the E3 ubiquitin ligase RNF41. Lenalidomide promoted EpoR/RNF41 association and inhibited RNF41 auto-ubiquitination, accompanied by a reduction in EpoR ubiquitination. To confirm that RNF41 is the principal target responsible for EpoR stabilization, HEK293T cells were transfected with EpoR and/or RNF41 gene expression vectors. Steady state EpoR expression was reduced in EpoR/RNF41 cells while EpoR upregulation by lenalidomide was abrogated, indicating that cellular RNF41 is a critical determinant of drug-induced receptor modulation. Notably, shRNA suppression of CRBN gene expression failed to alter EpoR upregulation, indicating that drug-induced receptor modulation is independent of cereblon. Immunohistochemical staining showed that RNF41 expression decreased in primary erythroid cells of lenalidomide responding patients, suggesting that cellular RNF41 expression merits investigation as a biomarker for lenalidomide response. Our findings indicate that lenalidomide has E3 ubiquitin ligase inhibitory effects that extend to RNF41, and that inhibition of RNF41 auto-ubiquitination promotes membrane accumulation of signaling competent JAK2/EpoR complexes that augment Epo responsiveness.
Keywords: Myelodysplastic Syndromes, EpoR, Lenalidomide, E3 Ubiquitin Ligases, RNF41
Introduction
Ineffective erythropoiesis manifested as refractory anemia remains the principal management challenge for patients with myelodysplastic syndromes (MDS).(1, 2) Bone marrow progenitors from patients with MDS display diminished STAT5 activation and transcriptional response to erythropoietin (Epo) stimulation despite appropriate Epo receptor (EpoR) membrane display.(3, 4) The precise mechanisms underlying the impairment in cytokine signaling remain unclear.
Lenalidomide, a second-generation immunomodulatory drug (IMiD), has greater potency and a more favorable toxicity profile than its parent, thalidomide.(1, 5) Both lenalidomide and its analogue, pomalidomide, promote erythroid lineage competence and the expansion of primitive erythroid precursors in CD34-enriched hematopoietic progenitors.(6) In transfusion-dependent, lower-risk MDS patients without chromosome 5q deletion, lenalidomide restores effective erythropoiesis and red blood cell transfusion independence in approximately 25% of patients who are unresponsive to treatment with recombinant erythropoietins.(7) Gene expression profiling performed by Ebert et al. showed that lenalidomide responders display inherently lower expression of erythroid-specific genes, and that lenalidomide relieves repression of Epo-induced transcriptional response, suggesting that lenalidomide modulates EpoR signal capacity.(8)
Recent investigations revealed that the IMiDs bind to and inhibit the function of the cereblon E3 ubiquitin ligase complex, which accounts for the teratogenicity of thalidomide and the antiproliferative effects of lenalidomide in multiple myeloma.(9–12) We previously reported that lenalidomide inhibits the function of the E3 ubiquitin ligase, murine double minute-2 protein (MDM2), which stabilizes the protein and fosters its binding to and degradation of p53 in del(5q) MDS.(13) Because EpoR turnover is regulated by ubiquitination and proteasomal degradation, we evaluated the effects of lenalidomide on the E3 ubiquitin ligase, Really Interesting New Gene (RING) finger protein 41 (RNF41, also known as neuroregulin receptor degradation protein-1[Nrdp1]). RNF41 regulates steady state or ligand-independent, JAK2-associated Type I receptor surface expression, signaling, and intracellular sorting by controlling ubiquitin specific peptidase (USP)-8 and the endosomal sorting complexes required for transport (ESCRT)-0 stability.(14, 15) We hypothesized that lenalidomide modulates JAK2/EpoR stability through inhibition of RNF41 to enhance JAK2 competent receptor signaling.
Materials and methods
Reagents and Cells
UT7 cells were acquired from DSMZ and grown in αMEM with 20% FBS, 1% penicillin/streptomycin solution, and 5 ng/mL GM-CSF (Braunschweig, Germany). HEK293T cells acquired from ATCC were maintained in DMEM supplemented with 10% FBS (Manassas, VA). All cell lines were authenticated by DSMZ and ATCC using short tandem repeat (STR) analysis profiling, and passaged for fewer than six months after receipt. Normal bone marrow mononuclear cells (BM-MNC) were purchased from Lonza Walkersville (Walkersville, MD). Lenalidomide was purchased from Fisher Scientific (Pittsburgh, PA). Ubiquitin, IL3-Rα, c-Kit, IFNAR, EpoR (sc-697), and CD71 antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). β-Actin antibody and cycloheximide were purchased from Sigma Aldrich (St. Louis, MO). EpoR (ab10653) and RNF41 antibodies were purchased from Abcam (Cambridge, MA), and USP8 antibody from Bethyl (Montgomery, TX). EpoR and CRBN antibodies were provided by Amgen (Thousand Oaks, CA). Bortezomib and MG132 were purchased from Selleckchem (Houston, TX).
Immunoblotting
Cells were treated as indicated then harvested and lysed in 1× RIPA buffer with 250 µM NaOV4, 2 µg/mL aprotinin, 2 µg/mL leupeptin, 0.2 µg/mL pepstatin A, and 500 µM PMSF. For detection of USP8, cells were lysed in 2× SDS gel loading buffer (62.5 mM Tris-HCl pH 6.8, 3% SDS, 10% glycerol, 5% β-mercaptoethanol, and 0.01% Bromophenol Blue sodium salt sonicated), then sonicated using the Bioruptor Plus (Diagenode, Seraing, Belgium). Proteins were resolved by SDS-PAGE and transferred to PVDF membranes. Membranes were blocked in 5% dry milk PBST solution (PBS with 0.1% Tween 20) and incubated with the indicated antibody. Membranes were washed and developed using ECL or ECL+ according to manufacturer’s protocol (Thermo Scientific, Waltham, MA).
Quantitative PCR
RNA was isolated from UT7 cells using the RNeasy Mini Kit (Qiagen, Valencia, CA). cDNA was generated using High Capacity cDNA Reverse Transcription Kit per protocol (Life Technology, Applied Biosystems, Foster City, CA). ActB, 18S and GAPDH RNA were used as endogenous controls. EpoR and cereblon mRNA were detected using TaqMan® Gene Expression Assays (Life Technology, Applied Biosystems). Real time quantitative PCR (qPCR) was carried out on an ABI PRISM 7900HT Sequence Detection System with triplicate samples using TaqMan® Universal PCR Master Mix with 2 min incubation at 50°C, followed by activation of AmpliTaq Gold for 10 min at 95°C, then 40 cycles of 15 s at 95°C and 1 min at 60°C. Data were analyzed using SDS software (v2.3).
Immunoprecipitation
Two hundred micrograms of protein from total cell lysates were incubated with 2 µg of indicated antibody for 2 hours on ice. Fifty microliters of Protein G Agarose beads (EMD Millipore, Billerica, MA) were added and incubated overnight on a rotator at 4°C. Bead-lysate slurries were washed three times in lysis buffer. Sample buffer was then added, and beads were dissociated at 95°C for 5 min. Proteins were separated by SDS-PAGE and immunoblotted with the indicated antibodies.
Immunofluorescence
Bone marrow mononuclear cells (BM-MNC) were isolated using Ficoll-Hypaque Plus gradient centrifugation and were treated with 1 µM lenalidomide for 1 hour (GE Healthcare, Pittsburgh, PA). The cells were cytospun for 5 min at 450 rpm. Slides were fixed in BD cytofix for 10 min at 37°C, washed with PBS, then blocked in 2% BSA/PBS for 5 min at room temperature (RT). Cells were incubated with primary antibody (1:50 for EpoR and 1:200 for CD71) for 1 hour at RT, washed, and incubated in secondary antibody (1:1000) for 1 hour at RT. Cells were washed again, DAPI and coverslip were added. Micrographs were taken using a Leica TCS SP5 AOBS Laser Scanning Confocal microscope (Leica Microsystems, Germany). Data were analyzed on pooled cells using Image Pro Plus version 6.2 (Media Cybernetics, Inc., Silver Springs, Maryland).
Transfections
HEK293T cells were transfected with pMET7/EpoR and pMET7/RNF41 expression vectors kindly provided by Dr. Jan Tavernier. Briefly, cells were transfected with 2 µg DNA by calcium phosphate method. Three hours after transfection, medium was changed. Cells were either harvested for expression detection or treated after 48 hours. Scrambled and shCRBN-specific shRNA (TG305228) constructs were purchased from Origene Technologies, Inc (Rockville, MD). Six million UT7 cells were suspended in 100 µL of electroporation solution (Ingenio® Solution, Mirus Bio LLC., CITY) and 5 µg of plasmid was added. Cells were electroporated using Amaxa Nucleofector® program T-20 and resuspended in 20 mL of αMEM with 5 ng/mL GM-CSF. Functional assays were performed 72 hours after transfection.
Luciferase reporter assays
HEK293T cells were transfected with 2 µg of RNF41 construct, together with a STAT3-dependent pXP2d2-rPAPI luciferase reporter plasmid (200 ng) and a β-gal reporter construct (200 ng) to correct for transfection efficiency. HEK293T cells were washed 24 hours post-transfection, transferred to a 96-well plate and left untreated or co-treated for at least 24 hours with human LIF (10 ng/mL) (EMD Millipore, Billerica, MA) and the indicated amount of lenalidomide. Luciferase activity from triplicate samples was measured by chemiluminescence in a TopCount luminometer (PerkinElmer, Waltham, MA) and expressed as relative light units normalized for transfection efficiency. All luciferase data shown are based on at least three independent experiments (n=3).
Immunohistochemistry
Paraffin embedded bone marrow trephine biopsies were deparaffinized using EZ prep solution (Ventana Medical System, Inc., Oro Valley, AZ). Slides were stained sequentially, first with pre-diluted spectrin (Cell Marque, Rocklin, CA) for 16 min followed by secondary for 8 min, and was detected with red chromagen. RNF41 secondary antibody (Abcam) was added (1:400) for 1 hour, with secondary incubation of 16 min, and detection by 3,3’ Diaminobenzidine (DAB) chromagen. Retrieval was done with cell conditioning 1 (Ventana Medical Systems, Inc.). Slides were dehydrated and cover-slipped for analysis. Slides were scanned using Aperio™ (Vista, CA) ScanScope XT with a 200x/0.8NA objective lens via tri-liner-array. Three regions from each slide were manually selected by the study pathologist and extracted without compression into Definiens Tissue Studio v3.0 software suite for quantitative analysis. These regional images were segmented using Tissue Composer to classify co-localized regions of interest using the red spectrin staining as the initial nuclear detection marker. The cells of interest were spectrin positive erythroid cells which also displayed RNF41 staining. Therefore, each nucleus within the regions of interest was identified with a hematoxylin threshold of 0.16 and an IHC threshold of 1. Cytoplasmic border was identified outside the nuclear border. The training algorithm was closely monitored by the study pathologists and applied to all images representative of the patients’ slides.
Results
Lenalidomide upregulates EpoR protein expression
To determine the effect of lenalidomide on EpoR expression, UT7 erythroid progenitor cells were treated with increasing concentrations of lenalidomide for 1 hour. We previously reported that both recombinant erythropoietin stimulation and treatment with lenalidomide induces lipid raft formation and aggregation in UT7 and MDS cells, accompanied by recruitment of EpoR, JAK2, and STAT5 into raft fractions. (16, 17) Immunoblot analyses showed that lenalidomide treatment of UT7 cells increased EpoR protein expression in a concentration-dependent manner at physiologically relevant concentrations ranging from 0.1 to 10 µM (Figure 1A–B). We next treated UT7 cells up to 24 hours and found that upregulation of EpoR by lenalidomide increased 2-fold within 1 hour of treatment, and increased thereafter through 8 hours of incubation with no significant decay until 24 hours of drug exposure (Figure 1C–D). Of note, certain EpoR antibodies have been reported to detect doublets in UT7 cells, corresponding to suspected maturation isoforms of the receptor.(18) Specificity of EpoR antibodies used in these studies was confirmed using additional EpoR antibodies, including one provided by Amgen. To determine whether EpoR upregulation was transcriptionally mediated, EpoR gene expression was assessed by real time quantitative PCR after 1 µM lenalidomide exposure. There was no change in EpoR mRNA expression after drug treatment, indicating that receptor protein upregulation is mediated by a post-transcriptional mechanism (Figure 1E). To investigate whether lenalidomide had similar receptor modulating effects in primary erythroid progenitors, bone marrow mononuclear cells (BM-MNC) were isolated from three normal donors and changes in EpoR expression were assessed by fluorescence microscopy in erythroid precursors identified by CD71 expression (Figure 2A). Lenalidomide induced a significant increase in EpoR expression in normal, primary erythroid progenitors after 1 hour of drug exposure (Figure 2B). Mean fluorescence intensity (MFI) of pooled untreated erythroid progenitors was 1043.5 ± 32.5 (SE) compared to 1216.6 ± 51.7 following 1 µM lenalidomide 1 h exposure (p=0.003).
Figure 1. Lenalidomide increases EpoR expression.
(A) Western blot of UT7 cells treated with increasing concentrations of lenalidomide for 1 hour showing a concentration-dependent increase in EpoR expression. (B) Densitometry analysis. (C) Western blot of UT7 cells treated with 1 µM lenalidomide over the indicated time intervals showing an increase in EpoR through 8 hours after treatment. (D) Densitometry analysis. (E) Relative expression of UT7 EpoR mRNA detected by qPCR showing no change in transcription following treatment with 1 µM lenalidomide, indicating that lenalidomide increases EpoR expression by a post-transriptional mechanism. Western blots are representative of at least two independent experiments.
Figure 2. Lenalidomide induces EpoR expression in primary erythroid progenitors.
BM-MNC were isolated from three primary normal donors, pooled and treated with 1 µM lenalidomide for 1 hour. (A) Representative immunofluorescence micrographs (20 µm scale) of erythroid progenitors, identified as CD71+ (green). Dapi (blue), EpoR (red), and merged image. (B) Mean fluorescence intensites (MFI) ± standard error showing an increase in EpoR expression in erythroid progenitors after lenalidomide treatment (p=0.003).
Lenalidomide increases EpoR stability and Type I cytokine receptor expression
The findings that lenalidomide yielded a sustained increase in cellular EpoR expression suggested that lenalidomide may influence receptor turnover. To determine whether lenalidomide increased the stability of EpoR protein, we first treated cells with cycloheximide (CHX) to inhibit new protein synthesis. UT7 cells were treated with 1 µM CHX for 24 hours either with or without lenalidomide (co-treated after 24 hours CHX pre-treatment), and lysates were collected at the indicated time points over a 24 hour period. Western blot was performed to assess levels of EpoR at each time point. Addition of lenalidomide markedly extended the half-life of EpoR to beyond 24 hours (Figure 3A–B). These data demonstrate that lenalidomide stabilizes the EpoR protein, which increases cellular density of signaling competent receptors. Furthermore, to determine if the effects of lenalidomide on receptor turnover involve only Type I or both Type I and Type II cytokine receptors, we examined the effects of lenalidomide on cellular expression of interleukin-3 receptor (IL3-Rα; Type I) and c-Kit (Type II). Lenalidomide upregulated IL3-Rα expression in a concentration-dependent manner, whereas c-Kit expression was unchanged, confirming Type I receptor specificity (Figure 3C–D).
Figure 3. Lenalidomide increases EpoR stability and expression of Type I cytokine receptors.
(A) Western blot of UT7 cells treated with cycloheximide (CHX), with or without 1 µM lenalidomide treatment. Treatment with lenalidomide increased EpoR stability beyond 24 hours. (B) Densitometry analysis. (C) Western blot of UT7 cells treated with lenalidomide at increasing concentrations. (D) Densitometry analysis. Lenalidomide increased expression of Type I receptors (IL3-Rα and EpoR) specifically, and had no effect on the Type II receptor, c-Kit, confirming Type I receptor specificity. Western blots are representative of at least two independent experiments.
Lenalidomide inhibits the E3 ubiquitin ligase activity of RNF41
Recent investigations have shown that steady state turnover of Type I receptors, such as EpoR, is regulated by the E3 ubiquitin ligase, RNF41.(14, 19) RNF41 increases the ligand-independent poly-ubiquitination of both Epo- and IL3-receptors and controls the intracellular sorting and processing of several Type I cytokine receptors by ubiquitination and suppression of cellular USP8 expression (14, 15) We first confirmed that RNF41 bound to EpoR/JAK2 complexes after lenalidomide treatment by RNF41 immunoprecipitation (IP) followed by EpoR and JAK2 immunoblot (IB). EpoR:RNF41 binding increased in a concentration dependent fashion following lenalidomide exposure (Figure 4A). Additionally, IP of EpoR followed by RNF41 IB showed similar results (data not shown). To investigate the effects of lenalidomide on the E3 ubiquitin ligase function of RNF41, we assessed ubiquitination of several RNF41 substrates after proteasomal inhibition with bortezomib or MG132, followed by lenalidomide treatment. RNF41 IP followed by ubiquitin IB showed that lenalidomide treatment resulted in concentration-dependent stabilization and cellular accumulation of RNF41 (Figure 4B) due to inhibition of RNF41 auto-ubiquitination (Figure 4C). Drug inhibition of RNF41 E3 ubiquitin ligase activity was accompanied by increase in cellular USP8 in HEK293T cells (Figure 4D) and increased RNF41 association with EpoR and JAK2 in UT7 cells (Figure 4A). Moreover, IP of EpoR followed by ubiquitin IB after lenalidomide treatment showed decreased ubiquitination of the receptor (Figure 4E). Similarly, IP of IL3-Rα, whose receptor expression is also modulated by RNF41, and ubiquitin IB following lenalidomide treatment demonstrated a similar concentration-dependent decrease in ubiquitination of the receptor (Figure 4F). Given that bortezomib promotes internalization and the lysosomal degradation of c-Kit, the effects of lenalidomide on Type II receptor ubiquitination were assessed on an alternative receptor, i.e., interferon-α/β receptor (IFNAR). (20) Ubiquitination of IFNAR was unchanged following treatment with lenalidomide, confirming Type I cytokine receptor specificity (Figure 4G). These findings suggest that the E3 ubiquitin ligase inhibitory effects of lenalidomide extend to RNF41.
Figure 4. Lenalidomide inhibits RNF41 ubiquitin ligase activity.
(A) Immunoprecipitation (IP) of RNF41 in UT7 cells treated with lenalidomide at the indicated concentrations for 1 hour. There is a concentration-dependent increase in co-immunoprecipitation of EpoR with JAK2 and RNF41 after lenalidomide treatment. (B) RNF41 protein expression levels increase in total cell lysates of UT7 cells treated with lenalidomide for 1 hour at the indicated concentrations, corresponding to decreased RNF41 ubiquitination. (C) Following treatment with 20 nM bortezomib to block proteasomal degradation, RNF41 was immunoprecipitated, then immunoblotted (IB) for ubiquitin. Lenalidomide decreases the ubiquitination of RNF41 in a concentration-dependent manner. (D) HEK293T cells transiently transfected with the irrelevant construct pMET7-solIL-5Rα (mock) or pMET7-RNF41 were treated with vehicle alone (DMSO) or were incubated overnight with 5 nM MG132, together with increasing concentrations of lenalidomide. Total cell lysates were immunoblotted for USP8. (E) Ubiquitination of EpoR [IP: EpoR, IB: ubiquitin] and (F) IL3-Rα [IP: IL3-Rα, IB: ubiquitin] decreased with lenalidomide treatment via inhibition of RNF41 ligase activity. (G) Ubiquitination of the Type II receptor IFNAR [IP: IFNAR, IB: ubiquitin] remained unchanged following lenalidomide treatment, confirming Type I cytokine receptor specificity. Blots are representative of at least two independent experiments.
RNF41 overexpression abrogates lenalidomide-induced EpoR upregulation and leukemia inhibitor factor (LIF) receptor signaling
To confirm that RNF41 is the principal target of lenalidomide responsible for EpoR stabilization, we transfected HEK293T cells with EpoR and/or RNF41 expression vectors using the calcium phosphate method. Steady state EpoR expression was lower in EpoR/RNF41 cells compared to cells transfected with EpoR alone (Figure 5A–B). Moreover, EpoR upregulation by lenalidomide was abrogated in EpoR/RNF41 cells, indicating that RNF41 is a critical determinant of EpoR upregulation by lenalidomide. Parallel to enhanced EpoR signaling (17), lenalidomide treatment increases the STAT3 reporter activity of HEK293T cells stimulated with leukemia inhibitory factor (LIF) (Figure 5C). Similar to lenalidomide-induced EpoR upregulation, this effect is lost upon ectopic RNF41 overexpression.
Figure 5. Overexpression of RNF41 blocks lenalidomide-induced upregulation of EpoR expression.
(A) Western blot and corresponding (B) densitometry analysis of HEK293T cells transfected with EpoR (pMET7-EpoR) or EpoR and RNF41 (pMet7-EpoR/RNF41) expression vectors demonstrate a decrease in steady state EpoR in the dual gene transfected cells accompanied by abrogation of lenalidomide-induction of EpoR that is retained only in cells transfected with EpoR alone. (C) STAT3-responsive luciferase assay of HEK293T cells transiently transfected with increasing amounts of pMET7-RNF41. The pMET7-solIL-5Rα construct(15) was used to normalize the total amount of transfected DNA and load of the transcriptional and translational machinery. The next day, cells were split into 96-wells and stimulated overnight with or without LIF, simultaneously with increasing amounts of lenalidomide. Absolute luciferase counts of triplicate measurements normalized for transfection efficiency are represented for three separate experiments.
EpoR upregulation by lenalidomide is independent of cereblon
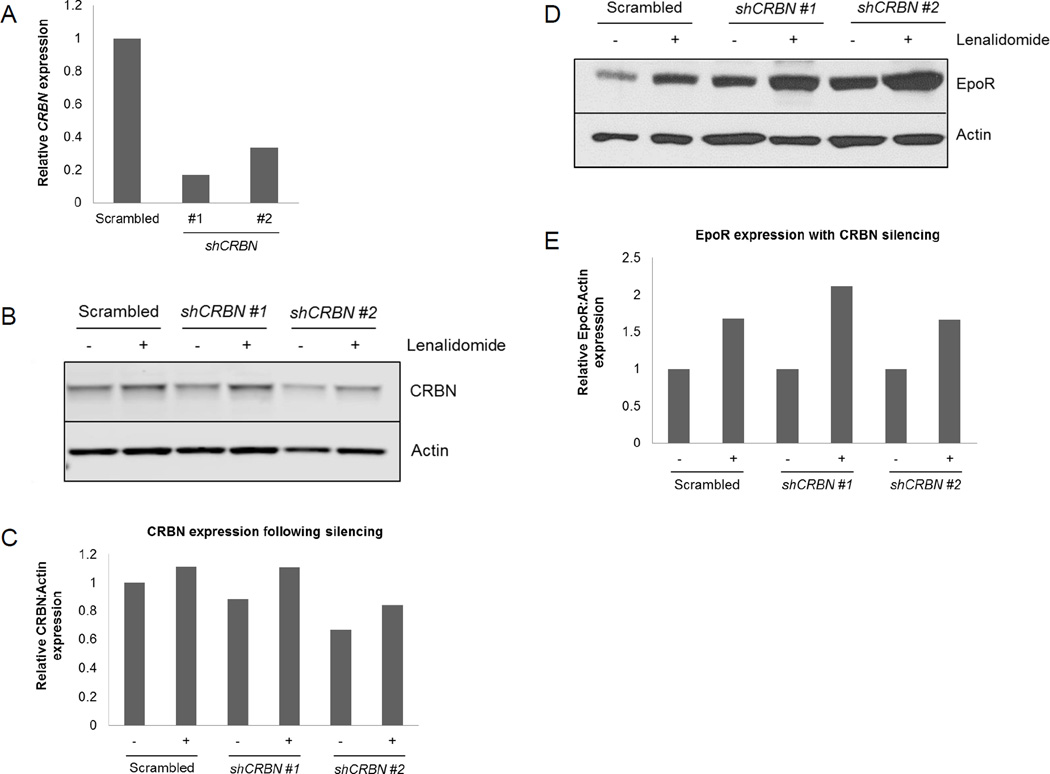
As binding of lenalidomide to the CUL4-RBX1-DDB1-CRBN E3 ubiquitin ligase complex is implicated in many of its biological effects in multiple myeloma and MDS with deletion of chromosome 5q [del(5q)],(21, 22) we investigated the possible role of cereblon in EpoR modulation by lenalidomide. UT7 cells were transfected with shRNAs targeting CRBN (Figure 6A–C) and EpoR modulation was assessed following treatment with 1 µM lenalidomide for 1 hour. Upregulation of EpoR by lenalidomide was preserved after CRBN knockdown (Figure 6D–E), indicating that lenalidomide-induced receptor modulation is independent of cereblon. Interestingly, lenalidomide treatment modestly increased CRBN protein expression (Figure 6B–C), consistent with recent reports that lenalidomide stabilizes CRBN.(23)
Figure 6. EpoR upregulation by lenalidomide is independent of cereblon.
(A) Relative expression of UT7 CRBN mRNA detected by qPCR following transfection with scrambled or CRBN-specific shRNAs. Western blot and corresponding densitometry analysis of CRBN (B–C) and EpoR (D–E) expression, respectively, in UT7 cells treated with 1 µM lenalidomide for 1 hour following transfection with scrambled or CRBN-specific shRNAs. Data are representative of three independent experiments.
RNF41 expression is decreased in lenalidomide responsive MDS primary erythroid cells
To determine the effects of lenalidomide on RNF41 expression in vivo, we performed immunohistochemistry (IHC) staining on 26 (10 lenalidomide responders and 16 non-responders) bone marrow biopsies from non-del(5q) MDS patients. The relationship between cellular RNF41 level in erythroid precursors and clinical erythroid response was assessed following co-staining for RNF41 and the erythroid marker spectrin (Figure 7A). Responding patients demonstrated a reduction in cellular RNF41 expression in erythroids that approached significance (p=0.058), whereas non-responders showed no discernible change (Figure 7B). These results suggest that erythroid RNF41 expression is modulated by lenalidomide in patients, and the ability of lenalidomide to reduce expression in responding patients may be an important biological marker of therapeutic efficacy.
Figure 7. RNF41 expression decreases in primary lenalidomide responders.
(A) Representative immunohistochemical micrograph of MDS bone marrow biopsies depicting RNF41 (brown) and spectrin (red) staining, and the corresponding mean RNF41 intensity in the erythroid population. (B) Quantitation of the mean RNF41 intensity in the spectrin positive cells. Lenalidomide responders (n=10) demonstrate a reduction in cellular RNF41 expression (p=0.058), whereas no significant reduction is observed in non-responders (n=16).
Discussion
Ebert et al. reported that bone marrow progenitors from lenalidomide responsive MDS patients display lower levels of expression of erythroid-specific genes that is restored by lenalidomide treatment. Although these findings have not been validated in a larger cohort, the data suggest that lenalidomide positively modifies EpoR signaling. Indeed, both lenalidomide and pomalidomide promote the expansion of primitive erythroid precursors in response to Epo stimulation.(6) Moreover, we recently reported that lenalidomide treatment of UT7 and primary MDS erythroid progenitors enhances phosphorylation of the Janus kinase (JAK)-2 and Signal Transducer and Activator of Transcription (STAT)-5 in response to Epo stimulation.(17) Ubiquitination and endocytosis of the EpoR governs the amplitude and duration of cytokine signaling by controlling both membrane residence of signal competent receptors and the rapidity of receptor degradation. Our investigations show that lenalidomide stabilizes and upregulates expression of signaling competent JAK2-associated EpoR complexes in a concentration-dependent fashion. This is mediated by post-transcriptional inhibition of the E3 ubiquitin ligase activity of RNF41, which regulates steady state Type I cytokine receptor membrane turnover. In addition to diminishing RNF41 auto-ubiquitination, which results in increased RNF41 expression, lenalidomide treatment increased USP8 expression and basal expression and signaling of EpoR and interleukin-3 receptors, without altering expression of Type II receptors such as c-Kit and IFNAR. Furthermore, forced expression of RNF41 in HEK293T cells completely abrogated lenalidomide’s capacity to upregulate EpoR and LIF receptor expression, confirming that RNF41 is a key determinant of receptor modulation by lenalidomide. Importantly, we demonstrate that drug-induced EpoR upregulation occurs independent of cereblon. Furthermore, immunohistochemical analysis of cellular RNF41 expression in bone marrow erythroid precursors of responding patients demonstrated marked reductions in RNF41 expression following treatment, suggesting that RNF41 expression may be a useful biomarker predictive for response to lenalidomide that merits further investigation in a larger patient cohort.
RNF41 serves as a scaffold protein coordinating ubiquitin transfer from a ubiquitin-conjugating enzyme (E2) recruited by its N-terminal RING domain to substrates that interact with its C-terminal substrate binding domain. Of particular interest, RNF41 was recently found to control Toll-like receptor (TLR)-mediated responses through ubiquitination of the central adaptor MyD88 and the TANK-binding kinase 1 (TBK1).(24) Increasing evidence implicates activation of innate immune signaling in the pathobiology of MDS.(25–27) These findings suggest that lenalidomide, through inhibition of RNF41, may stabilize TLR signal intermediates to augment TLR-induced myeloid proliferation while enhancing erythroid receptor signaling.(28) This notion supports our previous findings that lenalidomide treatment promotes proliferation of immature myeloid precursors at the expense of terminal differentiation, thereby contributing to the myelosuppressive effects of lenalidomide while promoting effective erythropoiesis.(29)
Our data support the recent findings that lenalidomide inhibits E3 ubiquitin ligase complexes, including cereblon and MDM2.(9, 10, 12, 13) Collectively, these data suggest that lenalidomide may act as a much broader RING finger E3 ubiquitin ligase inhibitor than originally appreciated. For instance, Rac GTPases, responsible for actin cytoskeletal reorganization and plasma membrane compartmentalization, are activated by IMiDs by an unknown mechanism. Perhaps inhibition of specific ligases that ubiquitinate these GTPases underlies the drug activating effects. Furthermore, E3 ligases are important for cellular transport, and inhibition by lenalidomide may have a profound effect on the spatial organization of cellular machinery.(30) Additionally, since E3 ubiquitin ligases are important for chromatin remodeling, lenalidomide inhibition of these proteins may have significant effects on gene expression.(31) Recently, the E3 ubiquitin ligase SMURF2 (Smad ubiquitin regulating factor 2) was shown to regulate histone 2B (H2B) ubiquitination, and consequently methylation, through inhibition of the RING finger ligase, RNF20.(31)
Notably, lenalidomide-induced degradation of casein kinase 1A1 (CK1α) and the lymphoid transcription factors Ikaros and Aiolos has been shown to occur through modulation of the CUL4-RBX1-DDB1-CRBN E3 ubiquitin ligase complex, contributing to the therapeutic efficacy of this agent in both MDS with deletion of chromosome 5q [del(5q)] and myeloma, respectively.(21, 22) In these contexts, lenalidomide binds CRBN to induce activity of the E3 ubiquitin ligase. Therefore, it is likely that the effects of lenalidomide are mediated by alteration of multiple cellular regulatory networks via inhibition or enhanced activation of varied ubiquitin ligases, resulting in biological consequences that are largely context-dependent. Further investigations of ubiquitin ligases are warranted, and will provide additional insight into the molecular actions of this immunomodulatory agent.
Acknowledgments
Support for this work was provided by the National Cancer Institute/National Institute of Health (5 R01 CA131076-04) and by grants from the Belgian government (IUAP P6/36) and the Group-ID Multidisciplinary Research Partnership of Ghent University. JT holds an ERC Advanced Grant (N° 340941).
Footnotes
Disclosure of Potential Conflicts of Interest
There are no other conflicts of interest to disclose.
Authors’ Contributions
AAB and LDC designed and performed research, collected, interpreted and analyzed data, and wrote the manuscript. LNG, LZ, JC, and GC performed research and collected data. LS, RK, GR, SW, and JT analyzed and interpreted data. KLM and AFL designed research, analyzed and interpreted data, and wrote the manuscript.
LITERATURE CITED
- 1.List AF, Baker AF, Green S, Bellamy W. Lenalidomide: targeted anemia therapy for myelodysplastic syndromes. Cancer control : journal of the Moffitt Cancer Center. 2006;13(Suppl):4–11. doi: 10.1177/107327480601304s02. [DOI] [PubMed] [Google Scholar]
- 2.Komrokji RS, List AF. Lenalidomide for Treatment of Myelodysplastic Syndromes. Current pharmaceutical design. 2012;18:1–6. doi: 10.2174/1381612811209023198. [DOI] [PubMed] [Google Scholar]
- 3.Hoefsloot LH, van Amelsvoort MP, Broeders LC, van der Plas DC, van Lom K, Hoogerbrugge H, et al. Erythropoietin-induced activation of STAT5 is impaired in the myelodysplastic syndrome. Blood. 1997;89:1690–1700. [PubMed] [Google Scholar]
- 4.Ebert BL, Galili N, Tamayo P, Bosco J, Mak R, Pretz J, et al. An erythroid differentiation signature predicts response to lenalidomide in myelodysplastic syndrome. PLoS medicine. 2008;5:e35. doi: 10.1371/journal.pmed.0050035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.List AF. Lenalidomide--the phoenix rises. The New England journal of medicine. 2007;357:2183–2186. doi: 10.1056/NEJMe078203. [DOI] [PubMed] [Google Scholar]
- 6.Moutouh-de Parseval LA, Verhelle D, Glezer E, Jensen-Pergakes K, Ferguson GD, Corral LG, et al. Pomalidomide and lenalidomide regulate erythropoiesis and fetal hemoglobin production in human CD34+ cells. The Journal of clinical investigation. 2008;118:248–258. doi: 10.1172/JCI32322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Raza A, Reeves JA, Feldman EJ, Dewald GW, Bennett JM, Deeg HJ, et al. Phase 2 study of lenalidomide in transfusion-dependent, low-risk, and intermediate-1 risk myelodysplastic syndromes with karyotypes other than deletion 5q. Blood. 2008;111:86–93. doi: 10.1182/blood-2007-01-068833. [DOI] [PubMed] [Google Scholar]
- 8.Ebert BL, Galili N, Tamayo P, Bosco J, Mak R, Pretz J, et al. An erythroid differentiation signature predicts response to lenalidomide in myelodysplastic syndrome. PLoS Biol. 2008;5:e35. doi: 10.1371/journal.pmed.0050035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ito T, Ando H, Suzuki T, Ogura T, Hotta K, Imamura Y, et al. Identification of a primary target of thalidomide teratogenicity. Science. 2010;327:1345–1350. doi: 10.1126/science.1177319. [DOI] [PubMed] [Google Scholar]
- 10.Ito T, Handa H. Deciphering the mystery of thalidomide teratogenicity. Congenital anomalies. 2012;52:1–7. doi: 10.1111/j.1741-4520.2011.00351.x. [DOI] [PubMed] [Google Scholar]
- 11.Melchert M, List A. The thalidomide saga. The international journal of biochemistry & cell biology. 2007;39:1489–1499. doi: 10.1016/j.biocel.2007.01.022. [DOI] [PubMed] [Google Scholar]
- 12.Chang XB, Stewart AK. What is the functional role of the thalidomide binding protein cereblon? International journal of biochemistry and molecular biology. 2011;2:287–294. [PMC free article] [PubMed] [Google Scholar]
- 13.Wei S, Chen X, McGraw K, Zhang L, Komrokji R, Clark J, et al. Lenalidomide promotes p53 degradation by inhibiting MDM2 auto-ubiquitination in myelodysplastic syndrome with chromosome 5q deletion. Oncogene. 2012 doi: 10.1038/onc.2012.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wauman J, De Ceuninck L, Vanderroost N, Lievens S, Tavernier J. RNF41 (Nrdp1) controls type 1 cytokine receptor degradation and ectodomain shedding. J Cell Science. 2011;124:921–932. doi: 10.1242/jcs.078055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.De Ceuninck L, Wauman J, Masschaele D, Peelman F, Tavernier J. Reciprocal cross-regulation between RNF41 and USP8 controls cytokine receptor sorting and processing. Journal of cell science. 2013;126:3770–3781. doi: 10.1242/jcs.131250. [DOI] [PubMed] [Google Scholar]
- 16.McGraw KL, Fuhler GM, Johnson JO, Clark JA, Caceres GC, Sokol L, et al. Erythropoietin receptor signaling is membrane raft dependent. PloS one. 2012;7:e34477. doi: 10.1371/journal.pone.0034477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McGraw KL, Basiorka AA, Johnson JO, Clark J, Caceres G, Padron E, et al. Lenalidomide induces lipid raft assembly to enhance erythropoietin receptor signaling in myelodysplastic syndrome progenitors. PloS one. 2014;9:e114249. doi: 10.1371/journal.pone.0114249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Walrafen P, Verdier F, Kadri Z, Chretien S, Lacombe C, Mayeux P. Both proteasomes and lysosomes degrade the activated erythropoietin receptor. Blood. 2005;105:600–608. doi: 10.1182/blood-2004-03-1216. [DOI] [PubMed] [Google Scholar]
- 19.Jing X, Infante J, Nachtman RG, Jurecic R. E3 ligase FLRF (Rnf41) regulates differentiation of hematopoietic progenitors by governing steady-state levels of cytokine and retinoic acid receptors. Experimental hematology. 2008;36:1110–1120. doi: 10.1016/j.exphem.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fang HT, Zhang B, Pan XF, Gao L, Zhen T, Zhao HX, et al. Bortezomib interferes with C-KIT processing and transforms the t(8;21)-generated fusion proteins into tumor-suppressing fragments in leukemia cells. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:2521–2526. doi: 10.1073/pnas.1121341109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kronke J, Fink EC, Hollenbach PW, MacBeth KJ, Hurst SN, Udeshi ND, et al. Lenalidomide induces ubiquitination and degradation of CK1alpha in del(5q) MDS. Nature. 2015;523:183–188. doi: 10.1038/nature14610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kronke J, Udeshi ND, Narla A, Grauman P, Hurst SN, McConkey M, et al. Lenalidomide causes selective degradation of IKZF1 and IKZF3 in multiple myeloma cells. Science. 2014;343:301–305. doi: 10.1126/science.1244851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu Y, Huang X, He X, Zhou Y, Jiang X, Chen-Kiang S, et al. A novel effect of thalidomide and its analogs: suppression of cereblon ubiquitination enhances ubiquitin ligase function. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2015 doi: 10.1096/fj.15-274050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang C, Chen T, Zhang J, Yang M, Li N, Xu X, et al. The E3 ubiquitin ligase Nrdp1 'preferentially' promotes TLR-mediated production of type I interferon. Nature immunology. 2009;10:744–752. doi: 10.1038/ni.1742. [DOI] [PubMed] [Google Scholar]
- 25.Maratheftis CI, Andreakos E, Moutsopoulos HM, Voulgarelis M. Toll-like receptor-4 is up-regulated in hematopoietic progenitor cells and contributes to increased apoptosis in myelodysplastic syndromes. Clinical cancer research : an official journal of the American Association for Cancer Research. 2007;13:1154–1160. doi: 10.1158/1078-0432.CCR-06-2108. [DOI] [PubMed] [Google Scholar]
- 26.Hofmann WK, de Vos S, Komor M, Hoelzer D, Wachsman W, Koeffler HP. Characterization of gene expression of CD34+ cells from normal and myelodysplastic bone marrow. Blood. 2002;100:3553–3560. doi: 10.1182/blood.V100.10.3553. [DOI] [PubMed] [Google Scholar]
- 27.Rhyasen GW, Bolanos L, Fang J, Jerez A, Wunderlich M, Rigolino C, et al. Targeting IRAK1 as a therapeutic approach for myelodysplastic syndrome. Cancer cell. 2013;24:90–104. doi: 10.1016/j.ccr.2013.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.List A, Kurtin S, Roe DJ, Buresh A, Mahadevan D, Fuchs D, et al. Efficacy of lenalidomide in myelodysplastic syndromes. The New England journal of medicine. 2005;352:549–557. doi: 10.1056/NEJMoa041668. [DOI] [PubMed] [Google Scholar]
- 29.Pal R, Monaghan SA, Hassett AC, Mapara MY, Schafer P, Roodman GD, et al. Immunomodulatory derivatives induce PU. 1 down-regulation, myeloid maturation arrest, and neutropenia. Blood. 2010;115:605–614. doi: 10.1182/blood-2009-05-221077. [DOI] [PubMed] [Google Scholar]
- 30.MacGurn JA, Hsu PC, Emr SD. Ubiquitin and membrane protein turnover: from cradle to grave. Annual review of biochemistry. 2012;81:231–259. doi: 10.1146/annurev-biochem-060210-093619. [DOI] [PubMed] [Google Scholar]
- 31.Blank M, Tang Y, Yamashita M, Burkett SS, Cheng SY, Zhang YE. A tumor suppressor function of Smurf2 associated with controlling chromatin landscape and genome stability through RNF20. Nature medicine. 2012;18:227–234. doi: 10.1038/nm.2596. [DOI] [PMC free article] [PubMed] [Google Scholar]