Abstract
Objective
In the United States (US) sepsis is a major public health problem accounting for over 200,000 annual deaths. The aims of this study were to identify US counties with high sepsis mortality and to assess the community characteristics associated with sepsis mortality.
Design
We performed a descriptive analysis of 2003 through 2012 Compressed Mortality File (CMF) data summarized at the county level. We defined sepsis deaths as deaths associated with an infection as the CMF is derived from death certificates and classified according to the International Classification of Diseases, Version 10 (ICD-10).
Measurements
We identified county-level sepsis clustering groups: strongly clustered, moderately clustered, and non-clustered. We approximated the mean crude, age-adjusted, and community-adjusted sepsis mortality rates nationally and for clustering groups. We contrasted demographic and community characteristics between clustering groups. We performed logistic regression for the association between strongly clustered counties and community characteristics.
Main Results
Among 3,108 US counties, the age-adjusted sepsis mortality rate was 59.6 deaths per 100,000 persons (95% CI: 58.9 – 60.4). Among 161 (5.2%) counties categorized as strongly clustered, the age-adjusted sepsis mortality rate was 93.1 deaths per 100,000 persons (95% CI: 90.5 – 95.7). Strongly clustered sepsis counties were more likely to be located in the South (92.6%, p <0.001), had lower education, larger population in poverty, without medical insurance, and higher unemployment rates (p < 0.001).
Conclusion
Sepsis mortality clustering is prevalent in Southern US, with three definitive sepsis clusters: “Mississippi Valley”, “Middle Georgia”, and “Central Appalachia,” characterized by lower education, income, employment and insurance coverage.
Keywords: Sepsis, Geographic locations, Spatial Autocorrelation, Disease Clustering, mortality
Introduction
Sepsis is a clinical syndrome characterized by an overwhelming inflammatory response that develops following severe infection. Sepsis is associated with a high mortality rate and poses an overwhelming burden of healthcare utilization in the United States.1-4 In the United States sepsis is a major public health problem responsible for over 750,000 hospitalizations and 215,000 deaths annually.1 Past studies have investigated the epidemiology of sepsis.1,3-6 However, less is known about the factors influencing regional disparities in sepsis, thus it is imperative that we determine and target the specific areas within the United States that are devastated by this syndrome with healthcare interventions.
Prior research indicates geographic variations in sepsis and infections.4,7 Burton et al. (2010) found that people living in impoverished census tracts were at an increased risk of developing infections.7 Wang et al. (2010) found that there was variation of sepsis mortality across the United States, and observed a high sepsis mortality cluster that ranged from the southeast to mid-Atlantic United States.4 However, in the latter study, regional differences in sepsis mortality were measured at the state level, limiting the ability to find community-level factors that influenced sepsis disparities. Thus, it may be more feasible to examine community characteristics at a more granular level.
Identifying spatial patterns of sepsis cases may provide insights into the underlying causes of disease.8 Many questions remained unanswered about the demographic and socioeconomic differences that may explain sepsis mortality clusters. The objectives of the current study were to identify US counties with high sepsis mortality and to assess the county-level characteristics associated with sepsis mortality. We hypothesized that there are regional differences in highly clustered sepsis counties within the United States, and that community-level characteristics may explain the observed differences.
Methods
Ethics Statement
This study was considered exempt by the institutional review board of the University of Alabama at Birmingham, as we used existing secondary data that are publicly available and non-identifiable.
Study Design & Data Source
We analyzed 2003 through 2012 Compressed Mortality File (CMF) data from the National Center for Health Statistics (NCHS) summarized at the county level.9 Mortality data in the CMF are derived from death certificates and include a record for every death of a U.S. resident recorded in the United States.9 We complemented the county-level sepsis mortality data with county-level community characteristics obtained from the 2010 American Community Survey (ACS) through the National Historical Geographic Information System (NHGIS).9,10 We obtained demographic data for each county using the 2010 ACS 5-year data for years 2006-2010.
Identification of County-Level Sepsis Mortality
We determined county-level sepsis-related deaths using methods by Wang et al. (2010) as deaths associated with an infection (Appendix A).4 We included sepsis-related deaths for all persons ≥ 15 years of age during the period 2003-2012. We included deaths in individuals aged 15-19 years because the CMF uses a single reference standard population for ages 15-24, and thus the inclusion of the 15-19 year groups is necessary for the estimation of age-adjusted rates.4 The CMF includes data on the age, race, sex, year and causes of all U.S. deaths.9
County-Level Demographic Characteristics
We obtained county-level demographic statistics (e.g., age, sex, and race) for the years 2006 through 2010 from the ACS. We categorized race into five groups: White (non-Hispanic), Black (non-Hispanic), Asian/Pacific Islander (non-Hispanic), Hispanic, and Other (including Native Americans).
County-Level Community Characteristics
We obtained county-level community variables from the ACS that included median household income, median home value in dollars, percentage of the population that completed college, percentage of the population below the poverty line, percentage of the population that is urban, population density, percentage of population without medical insurance coverage (in 2010), and unemployment rate. We also included the county-level ratio per 100,000 persons for number of hospitals, hospital beds, medical doctors, and primary care physician (PCP). We estimated the ratio per 100,000 by dividing each characteristic by the county population and then multiplied the quotient by 100,000 to obtain the ratio per 100,000 persons (all measured for year 2010). Geographic region was defined using the Census definition (i.e., Midwest, Northeast, South, and West).11 We did not include Alaska, Hawaii, and other US Territories in this analysis.
Defining Sepsis Mortality Clusters
Disease clustering is defined as the spatial aggregation of cases in an identifiable subpopulation.8 Further, cluster investigations provide an opportunity to understand the etiology and causes of disease.8 Currently there is no consensus on the best clustering approach; therefore we adopted novel geospatial autocorrelation methods to define highly clustered sepsis mortality counties. Introduced by Nassel et al. (2014), we combined three separate analytical spatial clustering methods to identify areas that were hot spots for sepsis mortality.12 We categorized county-level sepsis clustering into three groups: strongly clustered, moderately clustered, and non-clustered. We considered a county to be strongly clustered if it was identified as high risk or sepsis hot spot using all three geospatial metrics (5th quartile of Empirical Bayes (EB) smoothed sepsis mortality rates, high-high cluster using Local Moran's I, and sepsis hot spot as defined by Getis-Ord Gi*). In order to be considered as a moderately clustered county, a county had to be identified high-risk using two out of three geospatial metrics. All other U.S. counties were categorized as being non-clustered.
First, we estimated Empirical Bayes (EB) smoothed sepsis mortality rates for the 10-year study period using the total number of sepsis-mortality events in each county divided by the county population, with smoothing performed using the EB tools in GeoDa 1.6.7.9 (http://geodacenter.asu.edu).12 We further categorized the EB smoothed sepsis mortality rates into quintiles, and we defined counties as high-risk if the EB sepsis mortality rates were in the top quintile. Second, we used Local Indicators of Spatial Autocorrelation 13 to measure similarity between counties and calculate values both within and across geographic boundaries, additionally identifying spatial outliers.12,14,15 For each US county, we estimated Local Moran's I Statistic values, using associated z-scores and p-values to assess the magnitude of spatial autocorrelation and statistical significance, respectively.12 Counties with statistically significant positive z-scores indicate areas surrounded by areas with similar sepsis-mortality rates – either similarly high or similarly low (positive spatial autocorrelation).12 Lastly, we used the Gi* statistic to identify areas where sepsis mortality rates with either high or low values clustered within the context of the neighboring county.12,16,17 In contrast to LISA, Gi* does not identify the similarity of values to the surrounding counties. Further details to the geospatial autocorrelation analysis were introduced by Nassel et al. (2014) and are described elsewhere.12
Statistical Analysis
We used mean crude and age-adjusted mortality rates provided by CMF, which uses intercensal (2003-2009), actual (2010), and postcensal (2011-2012) US Census population estimates. All standardization was performed relative to the 2000 US standard population. We conducted a weighted (i.e., by county population) multivariable linear regression to assess the association between each county-level sepsis-clustering group and sepsis mortality rate after adjustment for age and all significant (p≤ 0.05) community-level community characteristics. We additionally calculated the national community-adjusted sepsis mortality rate. We compared regional differences in high-risk sepsis categories (EB, LISA, Gi*) using a Chi-square test of association. We performed the non-parametric Kruskal Wallis test to examine the distribution of community characteristics across county-level clustering groups (e.g., strongly clustered, moderately clustered, and non-clustered).
In a sensitivity analysis, we performed logistic regression to examine the association between strongly clustered sepsis counties and county-level community characteristics. We adjusted the logistic regression model by community characteristics found statistically significant (≤0.05) in crude logistic regression. We examined temporal changes in sepsis mortality using multivariable linear regression for two 5-year periods nested within our study period (i.e., 2003 – 2007 and 2008 – 2012). We used SAS version 9.4, QGIS version 2.8.1-Wien, and GeoDa version 1.6.7.9 for all statistical analyses and geographic mapping. We considered p-values ≤ 0.05 to be statistically significant.
Results
LISA, Gi*, and EB analysis
The LISA analysis identified that 246 of the 352 (69.9%) “high-high” sepsis cluster counties by LISA criteria were located in the South (Appendix B, Table 1). The Gi* analysis identified 516 counties as sepsis “hot spots” (16.6%) [Appendix C, Table 1]. Counties within the South were more likely to be in a sepsis “hot spot” (34.3%) by Gi* criteria (p <0.001). There were 622 (20.0%) counties categorized within the highest quintile for EB smoothed sepsis mortality rate (Appendix D, Table 1). Counties located within the South were more likely to have EB smoothed sepsis mortality rates in the highest quintile (27.4%), corresponding to an EB smoothed sepsis mortality rate of 76.4 deaths per 100,000 persons (95% CI: 58.9 – 60.4) [p <0.001].
Table 1. Comparison of county-level sepsis clustering category by United States region, 2003-2012.
| Midwest Counties† (N = 1,055) | Northeast Counties† (N = 217) | South Counties† (N = 1,422) | West Counties† (N = 414) | p-value§ | |
|---|---|---|---|---|---|
| LISA (%) | |||||
| Non-Significant | 743 (70.4) | 195 (89.9) | 1,073 (75.5) | 193 (46.6) | <0.001 |
| High-High Cluster | 101 (9.6) | 4 (1.8) | 246 (17.3) | 1 (0.2) | |
| High-Low Outlier | 22 (2.1) | 0 (0.0) | 10 (0.7) | 25 (6.0) | |
| Low-High Outlier | 19 (1.8) | 0 (0.0) | 47 (3.3) | 1 (0.2) | |
| Low-Low Cluster | 170 (16.1) | 18 (8.3) | 46 (3.2) | 194 (46.9) | |
| EB (95% CI)* | 68.1 (66.7 – 69.5) | 67.2 (65.2 – 69.2) | 76.4 (75.2 – 77.6) | 52.6 (50.7 – 54.5) | |
| EB (%) | |||||
| 1st quintile | 241 (22.8) | 31 (14.3) | 158 (11.1) | 192 (46.4) | <0.001 |
| 2nd quintile | 239 (22.7) | 45 (20.7) | 220 (15.5) | 117 (28.3) | |
| 3rd quintile | 189 (17.9) | 71 (32.7) | 304 (21.4) | 58 (14.0) | |
| 4th quintile | 185 (17.5) | 51 (23.5) | 350 (24.6) | 35 (8.5) | |
| 5th quintile | 201 (19.1) | 19 (8.8) | 390 (27.4) | 12 (2.9) | |
| Gi* (%) | |||||
| Non-Significant | 674 (63.9) | 199 (91.7) | 905 (63.6) | 247 (59.7) | <0.001 |
| Sepsis Hot Spot | 25 (2.4) | 2 (0.9) | 487 (34.3) | 2 (0.5) | |
| Sepsis Cold Spot | 356 (33.7) | 16 (7.4) | 30 (2.1) | 165 (39.9) |
Significance determined using Chi-Square test.
US regions as determined by the U.S. Census Bureau.
Mean Empirical Bayes smoothed sepsis mortality rate and 95% confidence interval.
Denotes column percentages.
Demographic Characteristics
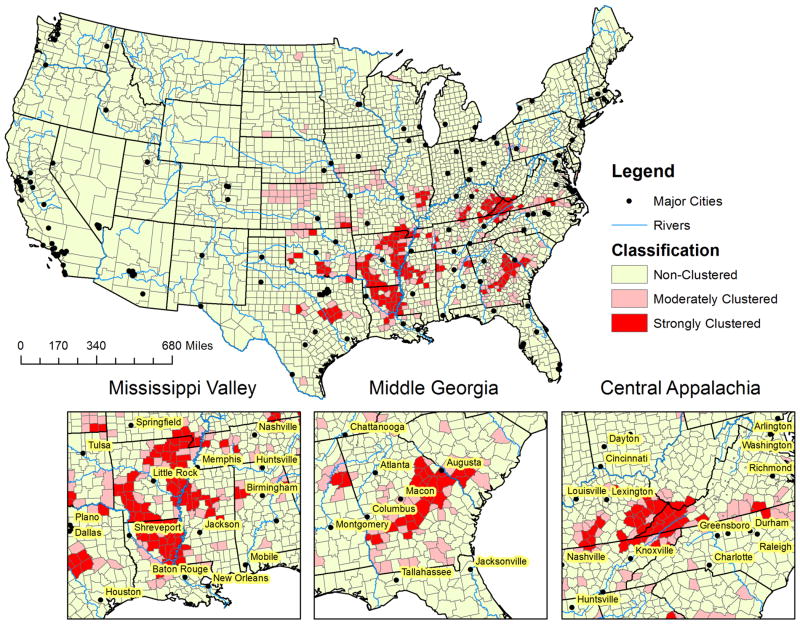
Among 3,108 counties included in the analysis, 161 (5.2%) were categorized as strongly clustered counties and 2,714 (87.3%) counties were categorized as non-clustered counties (Figure 1). Strongly and moderately clustered counties were more likely to have a larger proportion of older adults than non-clustered counties (Table 2). Strongly clustered counties had the largest black population (13.4% vs. 3.7% and 1.7%) and the lowest white population (76.8% vs. 87.5% and 86.5%) compared to moderately and non-clustered counties, respectively. Non-clustered counties had the largest Hispanic population (3.3% vs. 1.4% and 1.8%) and other population (3.0% vs. 2.4% and 2.0%) compared to moderately and strongly clustered counties, respectively.
Figure 1. Moderately and strongly clustered sepsis mortality groups for county-level regional variation in sepsis, United States, 2003-2012. Excludes Alaska and Hawaii.
Table 2. Comparison of county demographic characteristics and community characteristics and region by county-level sepsis clustering categories, 2003-2012.
| Strongly Clustereda Counties (N = 161) | Moderately Clusteredb Counties (N = 233) | Non-Clustered Counties (N = 2,714) | p-value* | |
|---|---|---|---|---|
| Age (%)† | ||||
| Under 18 | 23.4 (22.2 – 25.0) | 23.4 (21.7 – 24.9) | 23.7 (21.8 – 25.5) | 0.05 |
| 18-29 | 14.2 (13.2 – 15.6) | 13.9 (12.4 – 15.4) | 14.0 (12.2 – 16.1) | 0.1 |
| 30-44 | 18.5 (17.4 – 19.6) | 18.1 (16.7 – 19.2) | 18.5 (16.9 – 19.9) | 0.006 |
| 45-64 | 27.2 (26.1 – 28.7) | 27.5 (26.3 – 29.2) | 27.6 (25.9 – 29.2) | 0.4 |
| 65-79 | 11.7 (10.5 – 12.9) | 11.9 (10.6 – 13.2) | 10.9 (9.2 – 12.7) | <0.001 |
| 80+ | 4.2 (3.7 – 4.7) | 4.5 (3.6 – 5.7) | 4.0 (3.2 – 5.0) | <0.001 |
| Sex (%)† | ||||
| Male | 49.0 (48.1 – 50.0) | 49.0 (48.3 – 49.8) | 49.5 (48.8 – 50.4) | <0.001 |
| Female | 51.0 (50.0 – 51.9) | 51.0 (50.2 – 51.7) | 50.5 (49.6 – 51.2) | |
| Race (%)† | ||||
| Black (non-Hispanic) | 13.4 (1.4 – 37.7) | 3.7 (0.7 – 30.7) | 1.7 (0.4 – 8.5) | <0.001 |
| White (non-Hispanic) | 76.8 (56.8 – 94.9) | 87.5 (63.1 – 95.2) | 86.5 (69.6 – 94.3) | 0.02 |
| Asian/ Pacific Islander | 0.3 (0.1 – 0.5) | 0.4 (0.2 – 0.6) | 0.6 (0.2 – 1.2) | <0.001 |
| Hispanic | 1.4 (0.8 – 2.8) | 1.8 (1.1 – 3.5) | 3.3 (1.6 – 8.6) | <0.001 |
| Other | 2.0 (1.2 – 2.8) | 2.4 (1.6 – 3.4) | 3.0 (2.0 – 4.4) | <0.001 |
| Community Characteristics | ||||
| Household Income† | 37,061 (32,342 – 43,635) | 39,574 (35.185 – 45,162) | 42,882 (37,372 – 49,383) | <0.001 |
| Value of housing units† | 85,900 (72,100 – 110,700) | 87,700 (74,400 – 123,500) | 108,850 (81,650 – 155,900) | <0.001 |
| % Completed college† | 12.8 (11.0 – 17.2) | 15.9 (11.6 – 20.2) | 17.2 (13.5 – 23.1) | <0.001 |
| % Below poverty line† | 18.4 (14.9 – 22.1) | 16.1 (11.9 – 20.3) | 13.9 (10.7 – 17.9) | <0.001 |
| % Urban† | 32.2 (12.2 – 51.2) | 35.6 (0.0 – 58.5) | 41.6 (14.4 – 67.8) | <0.001 |
| Population density†** | 43.3 (29.0 – 84.1) | 44.2 (22.6 – 97.7) | 45.7 (16.3 – 120.5) | 0.7 |
| PCP۠ | 5.5 (2.3Р14.9) | 6.2 (2.0 Р24.2) | 5.2 (1.4 Р19.8) | 0.3 |
| Hospitals۠ | 0.7 (0.1 Р1.4) | 0.7 (0.1 Р1.8) | 0.4 (0.1 Р1.3) | 0.01 |
| Beds۠ | 31.0 (3.3 Р92.9) | 37.8 (5.7 Р118.7) | 23.7 (3.2 Р103.2) | 0.2 |
| Medical Doctors۠ | 9.5 (3.2Р24.7) | 8.8 (2.3 Р46.8) | 8.7 (2.0 Р42.1) | 0.5 |
| % Without insurance coverage† | 20.3 (17.7 – 23.1) | 18.9 (16.3 – 22.1) | 17.9 (14.1 – 21.8) | <0.001 |
| Unemployment rate† | 0.05 (0.04 – 0.06) | 0.05 (0.03 – 0.06) | 0.04 (0.03 – 0.05) | 0.01 |
| Region (%)*** | ||||
| Midwest | 12 (7.5) | 71 (30.5) | 972 (35.8) | <0.001 |
| Northeast | 0 (0.0) | 2 (0.9) | 215 (7.9) | |
| South | 149 (92.6) | 160 (68.7) | 1,113 (41.0) | |
| West | 0 (0.0) | 0 (0.0) | 414 (15.3) |
Defined as counties estimated as high risk by all definitions (LISA, EB, & Gi*)
Defined as counties estimated as high risk by two of three definitions.
Median (IQR)
Ratio per 100,000 persons
Significance determined using Kruskal-Wallis or Chi-Square test.
Persons per square mile.
US regions as determined by the U.S. Census Bureau
Community Characteristics
The clustering groups had similar population density (p = 0.7), PCP per 100,000 persons (p = 0.3), beds per 100,000 persons (p=0.2), and medical doctors per 100,000 persons (p = 0.5) [Table 2]. However, there were differences in all other county-level community characteristics. Strongly clustered and moderately clustered counties had lower median household income, median value of housing units, and were less urban when compared to counties within the non-clustered group. Moreover, strongly clustered sepsis counties had lower education represented by a lower college completion percentage (p<0.001). Strongly clustered sepsis counties had a higher percentage of persons living in poverty, without medical insurance, and higher unemployment rates (p < 0.001). Strongly and moderately clustered counties were more likely to be located in the South (92.6%, p <0.0001).
Sepsis Mortality Rates
During 2003-2012, and among people aged ≥15 years, there were a total of 1,451,986 sepsis-related deaths, corresponding to a national crude mortality rate of 69.9 deaths per 100,000 persons (95% CI: 68.9 – 70.9) [Table 3]. Among 3,108 United States counties included in the analysis, the age-adjusted sepsis mortality rate was 59.6 deaths per 100,000 persons (95% CI: 58.9 – 60.4). The national community-adjusted sepsis mortality rate was 58.0 deaths per 100,000 persons (95% CI: 57.4 – 58.7).
Table 3. Sepsis mortality† in the United States by county-level sepsis clustering category, 2003-2012, excluding Alaska and Hawaii.
| Sepsis Clustering Category | Total | |||
|---|---|---|---|---|
|
| ||||
| Strongly Clustered (N = 161) | Moderately Clustered (N = 233) | Non-Clustered (2,714) | All Counties (N = 3,108) | |
| Sepsis Deaths* | 33,532 | 50,188 | 1,368,266 | 1,451,986 |
| Mean Annual Population** | 199,148 | 228,433 | 852,304 | 771,699 |
| Crude Sepsis Mortality Rate (95% CI) | 108.8 (105.9 – 111.6) | 101.2 (98.1 – 111.6) | 64.9 (64.0 – 65.8) | 69.9 (68.9 – 70.9) |
| Age-Adjusted (95% CI) a | 93.1 (90.5 – 95.7) | 80.2 (77.6 – 82.8) | 55.9 (55.1 – 56.6) | 59.6 (58.9 – 60.4) |
| Community-Adjusted (95% CI) b | 85.7 (82.1 – 89.4) | 74.8 (71.9 – 77.6) | 56.8 (56.2 – 57.4) | 58.0 (57.4 – 58.7) |
Identified using IC10-codes for infection.
Per County
Annual Deaths per 100,000 persons.
Adjusted for age.
Additionally adjusted for county level - sex, race, household income, value of housing unit, % completed college, % below poverty line, % urban, number of hospitals, % without insurance coverage, unemployment rate.
During the observation period, the age-adjusted sepsis mortality rate was lowest for non-clustered counties, corresponding to a rate of 55.9 deaths per 100,000 persons (95%CI: 55.1 – 56.6). However, the age-adjusted sepsis mortality rate was highest for strongly clustered counties, with a corresponding rate of 93.1 deaths per 100,000 persons (95% CI: 90.5 – 95.7). The community-adjusted sepsis mortality rate was lowest for non-clustered counties, corresponding to a rate of 56.8 deaths per 100,000 persons (95%CI: 56.2 – 57.4). The community-adjusted sepsis mortality rate was highest for strongly clustered counties, corresponding to a rate of 85.7 deaths per 100,000 persons (95%CI: 82.1 – 89.4).
Sensitivity Analysis
After adjustment for significant county-level community characteristics (i.e., median household income, and median home value) none of the community characteristics remained associated with odds of strongly sepsis clustering (Appendix E). After adjustment for age and county-level community characteristics there were no temporal differences in sepsis mortality (Appendix F).
Discussion
Our results also indicate that sepsis remains a major public health burden responsible for greater than 140,000 deaths annually. These results also suggest that sepsis mortality clustering is prevalent in counties located in the southeastern US with three specific clusters: 1) “Mississippi Valley” - counties bordering the southern Mississippi river in three southern states (Louisianna, Arkansas, and Mississippi); 2) “Middle Georgia” - a belt of counties expanding from southwest Georgia through middle to southeast Georgia; 3) and “Central Appalachia” - a cluster of counties in southeastern Kentucky and southwest Virginia (Figure 1). Over the ten-year observation period, 5.2% (161 of 3,108) of US counties were defined as strongly clustered counties. After adjustment for age and community level characteristics, those living in the strongly clustered counties were about 1.5 times more likely to have a sepsis related death than people living in non-clustering counties. Demographic and socio-economic characteristics associated with sepsis mortality clustering were race, household income, value of housing property, education, rural population, poverty, ratio of hospitals per 100,000 persons, insurance coverage, and higher unemployment rate. The odds of strongly sepsis clustering was non-significant after multivariate adjustment for community characteristics.
This study builds upon previous work by Wang et al (2010)4, and to our knowledge, this is the first study to utilize geospatial analysis to identifiy county-level sepsis clustering groups. Wang et al. identified the first “sepsis cluster” consisting of 11 contiguous states from Southeastern to Mid-Atlantic US.4 Wang et al.'s sepsis cluster had increased sepsis mortality compared other US regions (80.1 vs. 61.9 deaths per 100,000 persons).4 Comparatively, this study further describes that sepsis mortality is prevalent within these same states, with three prominent clusters (e.g., Mississippi Valley, Middle Georgia, and Central Appalachia). Moreover, we further delineate that the counties within these states are part of our strongly clustered counties, corresponding with an increased sepsis mortality compared to other US counties of 93.1 vs. 55.9 deaths per 100,000 persons.
Regional differences in sepsis mortality may be explained by community and socio-demographic characteristics.18-21 Numerous studies have shown that uninsured critically ill patients are less likely to receive life-saving critical care procedures, receive less post acute care during critical illness recovery, and have increased mortality compared to those with insurance.22-27 As seen in our study, strongly clustered counties had greater socioeconomic and access to care disparities. Analogously, Mendu et al. (2012) elucidated that neighborhoods with greater than 20% poverty rates had 32% increased odds of community-acquired infections compared to those with neighborhood poverty rate <5%.18 Additionally, we found that race was a significant determinant in regional disparities of sepsis mortality. Many epidemiolgic studies based on hospital discharge records have reported that black persons have higher rates of sepsis, hospitalization mortality, and are twice as likely to develop sepsis as white persons.20,28-36 In contrast, while using prospective data from the REasons for Geographic and Racial Differences in Stroke (REGARDS) we found that black participants were less likely to develop sepsis when compared to white participants.37 Nevertheless, higher community-level proportion of black race may serves as a proxy to overall lower socio-economic status and access to care, and thus our efforts should focus on establishing healthcare coverage for those currently living in these underserved communities.
With the exception of Arkansas and Kentucky, the identified clustered sepsis counties were in states that did not adopt the Affordable Care Act (ACA).22 Historically, regional disparities in health outcomes have been found in the three geographic regions observed in this study.38-48 Similar to our sepsis cluster found in the Mississippi Valley, studies have shown that the Mississippi Valley also has higher rates for coronary heart disease.43 Howard et al. elucidated that there is a “Stroke-Belt” located in the Southeastern US, which comprises of our “Middle Georgia” and Mississippi Valley sepsis clusters.40,48 Lastly, the Central Appalachia sepsis cluster has been previously described as a very vulnerable population with limited access to health care.49 These geographic regions could generally be representable of overall poor health, increased health risk, and low access to care areas within the US. These geographic areas have consistently faced healthcare disparities and should be specifically targeted for future health care interventions, such as the adoption of the ACA.22,49
Limitations
The results of this study should be interpreted in the light of certain limitations. We used publicly available death records, and as a result, missclassification of the listed causes of death could affect the sepsis mortality rates observed in this study. We did not use the SIRS criteria for identification of sepsis; however we used methods perfomed in previous sepsis invesigations.4 Additionally, the compressed mortality file suppressed sub-national data representing fewer than ten persons for years 1989 and later 9, and as result we were unable to determine differences in infections types by cluster status. We identified sepsis deaths using ICD-10 codes for infections and consequently the rates presented may be underestimates of the true number for sepsis mortality in the US. We used the mean community characteristic estimates for years 2006 through 2010 to approximate the association between sepsis mortality and county-level demographic information; however the population estimates were adequate because they are within median time of the overall observation period. Lastly, this study is an ecological analysis, thus individual level factors associated with sepsis were not attainable.
Although the data used in this study are from a national representative sample, a future study investigating regional variation in sepsis among a longitudinal cohort may give further details regarding the lifestyle and community factors that explain differences in sepsis mortality. Sepsis mortality is a function of both case-fatality and sepsis incidence4; therefore, a prospective analysis will provide an opportunity to differentiate whether incident sepsis cases or case-fatality are driving these differences in sepsis mortality among the counties observed in this study. We are currently investigating these objectives within the REGARDS cohort, one of the largest ongoing national longitudinal cohorts of community-dwelling adults in the United States.40
Conclusion
Sepsis mortality clustering is prevalent in Southern US counties, with three definitive sepsis clusters: the Mississippi Valley, Middle Georgia, and Central Appalachia. Regional variations in sepsis mortality are important because they allow explanation for susceptibility and case-fatality. This study reiterates that sepsis mortality varies by region, while further illuminating that high risk sepsis counties are differentiated by lower education, income, employment, insurance coverage, and race. As these risk factors are highly related to health care access, enhancements of community-level access to care for the defined areas may contribute to a reduction in sepsis mortality.
Supplementary Material
Appendix B: Local Moran's I county-level regional variation in sepsis, United States, 2003-2012. Excludes Alaska and Hawaii.
Appendix C: Getis-Ord Gi* county-level regional variation in sepsis, United States, 2003-2012. Excludes Alaska and Hawaii.
Appendix D: Empirical Bayes sepsis rates for county-level regional variation in sepsis, United States, 2003-2012. Excludes Alaska and Hawaii.
Acknowledgments
Financial Support: Dr. Safford reports the following potential conflicts of interest: Amgen - salary support to study patterns of statin use in Medicare and other large databases; diaDexus - salary support for a research grant on lipids and CHD outcomes; diaDexus - consulting to help with FDA application; NIH, AHRQ - salary support for research grants. Dr. Wang received grant support from R01-NR012726 from the National Institute for Nursing Research, Bethesda, Maryland. Mr. Donnelly received grant support from grant T32-HS013852 from the Agency for Healthcare Research and Quality, Rockville, Maryland.
Copyright form disclosures: Dr. Donnelly received support for article research from the National Institutes of Health (NIH). His institution received funding from the AHRQ Predoctoral Fellowship in Health Services Research. Dr. Howard received support for article research from the NIH. Dr. Safford received support for article research from the NIH. Her institution received funding from the NIH, PCORI, and Amgen. The remaining authors have disclosed that they do not have any potential conflicts of interests.
Footnotes
Author Contributions: JXM, JPD, RG, GH, MMS, and HEW conceived the study. HEW, MMS, RG, and GH oversaw data collection. JXM, JPD, HEW conducted the analysis. JXM drafted the manuscript and all authors contributed to its critical review. JXM assumes overall responsibility for the paper.
Conflicts of Interest: Mr. Moore, and Drs., Griffin and Howard do not report any related financial support or conflicts of interest.
References
- 1.Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: Analysis of incidence, outcome, and associated costs of care. Critical Care Medicine. 2001;29(7):1303–1310. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Angus DC. The lingering consequences of sepsis: A hidden public health disaster? JAMA. 2010;304(16):1833–1834. doi: 10.1001/jama.2010.1546. [DOI] [PubMed] [Google Scholar]
- 3.Wang HE, Shapiro NI, Angus DC, Yealy DM. National estimates of severe sepsis in United States emergency departments. Critical Care Medicine. 2007;35(8):1928–1936. doi: 10.1097/01.CCM.0000277043.85378.C1. [DOI] [PubMed] [Google Scholar]
- 4.Wang HE, Devereaux RS, Yealy DM, Safford MM, Howard G. National variation in United States sepsis mortality: a descriptive study. International Journal of Health Geographics. 2010;9(9):1–9. doi: 10.1186/1476-072X-9-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martin GS, Mannino DM, Eaton S, Moss M. The epidemiology of sepsis in the United States from 1979 through 2000. New England journal of Medicine. 2003;348:1546–1554. doi: 10.1056/NEJMoa022139. [DOI] [PubMed] [Google Scholar]
- 6.Miday RK, Wilson ER. Toxic shock syndrome: incidence and geographic distribution from a hospital medical records reporting system. American Journal of Public Health. 1988;78(5):578–580. doi: 10.2105/ajph.78.5.578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burton DC, Flannery B, Bennett NM, Farley MM, Gershman K, Harrison LH, Lynfield R, Petit S, Reingold AL, Schaffner W, Thomas A, Plikaytis BD, Rose CE, Whitney CG, Schuchat A. Socioeconomic and racial/ethnic disparities in the incidence of bacteremic pneumonia among U.S. adults. Research and Practice. 2010;100(10):1904–1911. doi: 10.2105/AJPH.2009.181313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wartenberg D. Investigating disease clusters: why, when, and how? JR Statist Soc A. 2001;164(Part 1):13–22. [Google Scholar]
- 9. [March 30, 2015];Wide-Ranging Online Data for Epidemiologic Research (CDC-WONDER) 2015 http://wonder.cdc.gov.
- 10.Center MP. 2011 UoM, ed. Minneapolis, MN: 2015. National Historical Geographic Information System: Version 2.0. [Google Scholar]
- 11. [March 31, 2015];Geographic Terms and Concepts. 2015 https://www.census.gov/geo/reference/gtc/gtc_census_divreg.html.
- 12.Nassel AF, Root ED, Haukoos JS, McVaney K, Colwell C, Robinson J, Eigel B, Magid DJ, Sasson C. Multiple cluster analysis for the identification of high-risk census tracts for out-of-hospital cardiac arrest (OHCA) in Denver, Colorado. Resuscitation. 2014;85:1667–1673. doi: 10.1016/j.resuscitation.2014.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hollisaaz MT, Lorgard-Dezfuli-Nezad M, Assari S, Hafezie R, Ebrahiminia M. Medical comorbidities after renal transplantation. Trasplantation Proceedings. 2007;39:1048–1050. doi: 10.1016/j.transproceed.2007.03.061. [DOI] [PubMed] [Google Scholar]
- 14.Anselin L. Local indicators of spatial association - LISA. Geogr Anal. 1995;27:93–115. [Google Scholar]
- 15.Anselin L. Exploring spatial data with GeoDa: a workbook. Urbana-Champaign: University of Illinois; 2004. [Google Scholar]
- 16.Li H, Calder CA, Cressie NAC. Beyond Moran's I: testing for spatial dependence based on the spatial autoregressive model. Geogr Anal. 2007;39:357–375. [Google Scholar]
- 17.Li H, Calder CA, Cressie NAC. One-step estimation of spatial dependence parameters: properties and extensions of the APLE statistic. J Multivariate Anal. 2012;105:68–84. [Google Scholar]
- 18.Mendu ML, Zager S, Gibbons FK, Christopher KB. Relationship between neighborhood poverty rate and bloodstream infections in the critically ill. Critical Care Medicine. 2012;40(5):1427–1436. doi: 10.1097/CCM.0b013e318241e51e. [DOI] [PubMed] [Google Scholar]
- 19.Cohen S. Social status and susceptibility to respiratory infections. Ann NY Acad Sci. 1999;896:246–253. doi: 10.1111/j.1749-6632.1999.tb08119.x. [DOI] [PubMed] [Google Scholar]
- 20.Zajacova A, Dowd JB, Aiello AE. Socioeconomic and race/ethnic patterns in persistent infection burden among U.S . adults. Journal of Gerontology: Medical Sciences. 2009;64A(2):272–279. doi: 10.1093/gerona/gln012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Flory JH, Joffe M, Fishman NO, Edelstein PH, Metlay JP. Socioeconomic risk factors for bacteraemic pneumococcal pneumonia in adults. Epidemiol Infect. 2009;137:717–726. doi: 10.1017/S0950268808001489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lyon SM, Douglas IS, Cooke CR. Medicaid expansion under the Affordable Care Act. Implications for insurance-related disparities in pulmonary, critical care, and sleep. Ann Am Thorac Soc May. 2014;11(4):661–667. doi: 10.1513/AnnalsATS.201402-072PS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lyon SM, Benson NM, Cooke CR, Iwashyna TJ, Ratcliffe SJ, Kahn JM. The effect of insurance status on mortality and procedural use in critically ill patients. Am J Respir Crit Care Med. 2011 Oct 1;184(7):809–815. doi: 10.1164/rccm.201101-0089OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Haas JS, G L. Acutely injured patients with trauma in Massachusetts: differences in care and mortality, by insurance status. Am J Public Health. 1994 Oct;84(10):1605–1608. doi: 10.2105/ajph.84.10.1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rapoport J, Teres D, Steingrub J, Higgins T, McGee W, Lemeshow S. Patient characteristics and ICU organizational factors that influence frequency of pulmonary artery catheterization. JAMA. 2000 May 17;283(19):2559–2567. doi: 10.1001/jama.283.19.2559. [DOI] [PubMed] [Google Scholar]
- 26.Danis M, Linde-Zwirble WT, Astor A, Lidicker JR, Angus DC. How does lack of insurance affect use of intensive care? A population-based study. Crit Care Med. 2006 Aug;34(8):2043–2048. doi: 10.1097/01.CCM.0000227657.75270.C4. [DOI] [PubMed] [Google Scholar]
- 27.Lane-Fall MB, Iwashyna TJ, Cooke CR, Benson NM, Kahn JM. Insurance and racial differences in long-term acute care utilization after critical illness. Crit Care Med. 2012 Apr;40(4):1143–1149. doi: 10.1097/CCM.0b013e318237706b. [DOI] [PubMed] [Google Scholar]
- 28.Mayr FB, Yende S, Linde-Zwirble WT, Peck-Pamler OM, Weissfeld LA, Angus DC. Infection rate and acute organ dysfunction risk as explanations for racial differences in severe sepsis. JAMA. 2010;303(24):2495–2503. doi: 10.1001/jama.2010.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mayr FB, Yende S, Angus DC. Epidemiology of severe sepsis. Virulence. 2014;5(1):4–11. doi: 10.4161/viru.27372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dombrovskiy VY, Martin AA, Sunderram J, Paz HL. Occurrence and outcomes of sepsis: influence of race. Critical Care Medicine. 2007;35(3):763–768. doi: 10.1097/01.CCM.0000256726.80998.BF. [DOI] [PubMed] [Google Scholar]
- 31.Baine W, Yu W, Summe JP. The epidemiology of hospitalization of elderly Americans for septicemia or bacteremia in 1991-1998. Application of Medicare claims data. Ann Epidemiol. 2001;11(2):118–126. doi: 10.1016/s1047-2797(00)00184-8. [DOI] [PubMed] [Google Scholar]
- 32.Barnato AE, Alexander SL, Linde-Zwirble WT, Angus DC. Racial variation in the incidence, care, and outcomes or severe sepsis: analysis of population, patient, and hospital characteristics. Am J Respir Crit Care Med. 2008;177:279–284. doi: 10.1164/rccm.200703-480OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Esper AM, Moss M, Lewis CA, Nisbet R, Mannino DM, Martin GS. The role of infection and comorbidity: Factors that influence disparities in sepsis. Critical Care Medicine. 2006;34(10):2576–2582. doi: 10.1097/01.CCM.0000239114.50519.0E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Martin GS, Mannino DM, Eaton S, Moss M. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. 2003;348(16):1546–1554. doi: 10.1056/NEJMoa022139. [DOI] [PubMed] [Google Scholar]
- 35.McBean M, Rajamani S. Increasing rates of hospitalization due to septicemia in the US elderly population, 1986-1997. J Infect Dis. 2001;183(4):596–603. doi: 10.1086/318526. [DOI] [PubMed] [Google Scholar]
- 36.Richardus JH, Kunst AE. Black-white differences in infectious disease mortality in the United States. Am J Public Health. 2001;91(8):1251–1253. doi: 10.2105/ajph.91.8.1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moore JX, Donnelly JP, Griffin R, Safford MM, Howard G, Baddley J, Wang HE. Black-white racial disparities in sepsis: a prospective analysis of the REasons for Geographic And Racial Differences in Stroke (REGARDS) cohort. Critical Care. 2015;19(279):10. doi: 10.1186/s13054-015-0992-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Howard G, Evans GW, Pearce K, Howard VJ, Bell RA, Mayer EJ, Burke GL. Is the stroke belt disappearing? An analysis of racial, temporal, and age effects. Stroke. 1995;26:1153–1158. doi: 10.1161/01.str.26.7.1153. [DOI] [PubMed] [Google Scholar]
- 39.Howard G. Why Do We Have a Stroke Belt in the Southeastern United States? A Review of Unlikely and Uninvestigated Potential Causes. American Journal of The Medical Sciences. 1999;317:160–167. doi: 10.1097/00000441-199903000-00005. [DOI] [PubMed] [Google Scholar]
- 40.Howard VJ, Cushman M, Pulley L, Gomez CR, Go RC, Prineas RJ, Graham A, Moy CS, Howard G. The Reasons for Geographic and Racial Differences in Stroke Study: Objectives and Design. Neuroepidemiology. 2005;25:135–143. doi: 10.1159/000086678. [DOI] [PubMed] [Google Scholar]
- 41.Le A, Judd SE, Allison DB, Oza-Frank R, Affuso O, Safford MM, Howard VJ, Howard G. The geographic distribution of obesity in the US and the potential regional differences in misreporting of obesity. Obesity (Silver Spring) 2014;22(1):300–306. doi: 10.1002/oby.20451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pickle LW, Mungiole M, Gillum RF. Geographic variation in stroke mortality in blacks and whites in the United States. Stroke. 1997;28:1639–1647. doi: 10.1161/01.str.28.8.1639. [DOI] [PubMed] [Google Scholar]
- 43.Pickle LW, Gillum RF. Geographic variation in cardiovascular disease mortality in US blacks and whites. J Natl Med Assoc. 1999;91:545–556. [PMC free article] [PubMed] [Google Scholar]
- 44.Lanska DJ, Kuller LH. The geography of stroke mortality in the United States and the concept of a stroke belt. Stroke. 1995;26:1145–1149. doi: 10.1161/01.str.26.7.1145. [DOI] [PubMed] [Google Scholar]
- 45.Gillum RF, Ingram DD. Relation between residence in the southeast region of the United States and stroke incidence: The NHANES I epidemiologic followup study. American Journal of Epidemiology. 1996;144(7):665–673. doi: 10.1093/oxfordjournals.aje.a008979. [DOI] [PubMed] [Google Scholar]
- 46.Casper ML, Wing S, Anda RF, Knowles M, Pollard RA. The shifting stroke belt: changes in the geographic pattern of stroke mortality in the United States, 1962 to 1988. Stroke. 1995;26(5):755–760. doi: 10.1161/01.str.26.5.755. [DOI] [PubMed] [Google Scholar]
- 47.Lanska DJ, Peterson PM. Geographic variation in the decline of stroke mortality in the United States. Stroke. 1995;26:1159–1165. doi: 10.1161/01.str.26.7.1159. [DOI] [PubMed] [Google Scholar]
- 48.Howard G, Anderson R, Johnson NJ, Sorlie P, Russell G, Howard VJ. Evaluation of social status as a contributing factor to the stroke belt region of the United States. Stroke. 1997;28:936–940. doi: 10.1161/01.str.28.5.936. [DOI] [PubMed] [Google Scholar]
- 49.Gardner T, Gavaza P, Meade P, Adkins DM. Delivering free healthcare to rural Central Appaclachia population: the case of the Health Wagon. Rural and Remote Health. 2012;12:2035. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix B: Local Moran's I county-level regional variation in sepsis, United States, 2003-2012. Excludes Alaska and Hawaii.
Appendix C: Getis-Ord Gi* county-level regional variation in sepsis, United States, 2003-2012. Excludes Alaska and Hawaii.
Appendix D: Empirical Bayes sepsis rates for county-level regional variation in sepsis, United States, 2003-2012. Excludes Alaska and Hawaii.