SUMMARY
During the lifespans of most animals, reproductive maturity and mating activity are highly coordinated. In Drosophila melanogaster, for instance, male fertility increases with age and older males are known to have a copulation advantage over young ones. The molecular and neural basis of this age-related disparity in mating behavior is unknown. Here we show that the Or47b odorant receptor is required for the copulation advantage of older males. Notably, the sensitivity of Or47b neurons to a stimulatory pheromone, palmitoleic acid, is low in young males but high in older ones, which accounts for older males’ higher courtship intensity. Mechanistically, this age-related sensitization of Or47b neurons requires a reproductive hormone, juvenile hormone, as well as its binding protein Methoprene-tolerant in Or47b neurons. Together, our study identifies a direct neural substrate for juvenile hormone that permits coordination of courtship activity with reproductive maturity to maximize male reproductive fitness.
INTRODUCTION
Life-history evolutionary theory predicts that for an individual to maximize reproductive fitness, mating activity and reproductive maturity need to be precisely timed and coordinated (Roff, 1992; Stearns, 1992). Animals usually display the highest degree of mating activity when they are most fertile. Mechanisms that underlie such coordination are better understood in females. For example, ovulating female mice release pheromones to attract males (Haga-Yamanaka et al., 2014), and juvenile females lacking mature gametes release a peptide pheromone to inhibit male courtship behavior (Ferrero et al., 2013). In addition, the ability of female mice to detect attractive male pheromones depends on the ovulation status signaled by a sex hormone (Dey et al., 2015). Similarly, in Drosophila melanogaster only sexually mature females produce 7,11-heptacosadiene (Arienti et al., 2010; Bilen et al., 2013), an aphrodisiac pheromone that promotes male courtship (Antony et al., 1985; Billeter et al., 2009). As for mature males, little is known about how courtship activity is regulated to match fertility.
To address this question, we turned to Drosophila melanogaster, a genetically amenable model organism for which male fertility has been shown to gradually increase with age (Kvelland, 1965; Long et al., 1980; Markow and O’Grady, 2008) and male courtship activity peaks later in life at about 7 days (Kvelland, 1965). Two days post eclosion, most males possess mature sperm but young males are less fertile than older adults; they sire a smaller number of offspring per mating than their older counterparts (Long et al., 1980). Interestingly, young males appear less inclined to court females compared to older males (Long et al., 1980). These observations suggest that mating behavior and fertility are also coordinated in males. Across insect species, juvenile hormone, a sesquiterpenoid lipid-like hormone secreted by the corpora allata, has been postulated as a pleiotropic hormone that coordinates all life-history traits, such as body size, timing of sexual maturity, mating behavior and lifespan (Flatt et al., 2005; Wilson et al., 2003). It is therefore possible that male courtship behavior and fertility are coordinated by juvenile hormone in Drosophila. Consistent with this notion, Methoprene-tolerant, a juvenile hormone binding protein and a putative receptor, is critical for both normal male fertility and courtship behavior (Wilson et al, 2003). However, the molecular and cellular mechanism by which juvenile hormone regulates the neural circuits that underlie courtship behavior remains unknown.
The mating behavior of Drosophila consists of multiple discrete steps, the regulation of which involves multisensory inputs. Among them, pheromone cues have a strong influence on mating decision (For reviews, see Dickson, 2008; Ihara et al., 2013; Yamamoto and Koganezawa, 2013). In the past few years, remarkable advances have been made in the identification of chemosensory neurons that detect pheromones, including multiple gustatory receptor neurons (GRNs) (Bray and Amrein, 2003; Fan et al., 2013; Grillet et al., 2006; Koh et al., 2014; Lacaille et al., 2007; Liu et al., 2012; Miyamoto and Amrein, 2008; Moon et al., 2009; Shankar et al., 2015; Starostina et al., 2012; Thistle et al., 2012; Toda et al., 2012; Watanabe et al., 2011) and olfactory receptor neurons (Or67d, Or65a, Ir84a and Or47b ORNs) (Dweck et al., 2015; Ejima et al., 2007; van der Goes van Naters and Carlson, 2007; Grosjean et al., 2011; Kurtovic et al., 2007). However, mutations of individual receptor types in the pheromone sensory neurons impair but do not abolish courtship behavior, suggesting that courtship decision is determined by integrating inputs from multiple parallel, functionally complementary pheromone circuits (Kohl et al., 2015; Clowney et al., 2015). It is therefore important to identify the biological context in which a pheromone circuit contributes to courtship decision.
Here we investigate the molecular basis of the copulation advantage held by older males and identify a direct neural substrate by which juvenile hormone regulates courtship activity. We report that the copulation advantage of older males requires the Or47b odorant receptor. The sensitivity of Or47b neurons to a stimulatory fly pheromone is low in young males but high in older ones, which allows older males to court females more vigorously. Mechanistically, the age-dependent sensitization of the pheromone sensory neurons is mediated by juvenile hormone, which is both necessary and sufficient for the Or47b-dependent copulation advantage. At the molecular level, the increase in Or47b pheromone sensitivity requires the expression of the juvenile hormone binding protein Methoprene-tolerant in Or47b neurons. By modulating Or47b neuronal sensitivity, juvenile hormone regulates mating behavior to match fertility and thereby maximize male reproductive fitness. Together, our study reveals a population of pheromone sensory neurons that functions as an interface between neural circuits for courtship behavior and hormonal signals regulating reproductive maturation.
RESULTS
Or47b receptor is necessary for the copulation advantage of older males
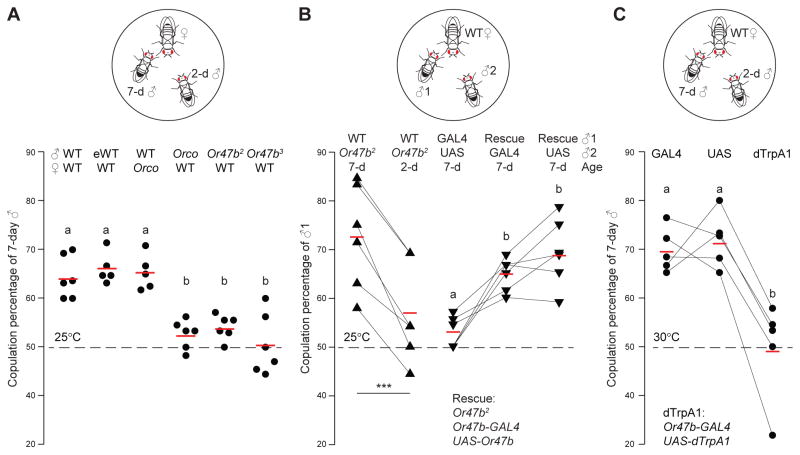
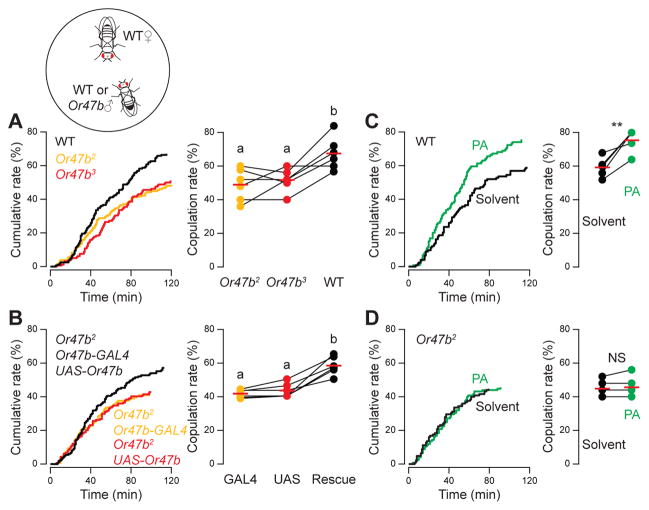
First we devised a courtship competition assay to measure the age-dependent difference in courtship success. We introduced two naive males to one virgin female in the mating chamber. We then recorded which male copulated with the female and when copulation first occurred in the 2-hr observation period under 660-nm illumination which is not visible to flies (Figs. 1A and S1A). In general, older males (7-day old) are more successful than young ones (2-day old); in 64% of the trials in which copulation occurred, the females copulated with the older males (Fig. 1A). We also observed a similar age-dependent copulation advantage in sexually experienced males that had mated one day prior to the competition assay (Fig. 1A). This result indicates that it is age, not the duration of abstinence, that underlies the copulation advantage of older males.
Fig. 1. Or47b neuronal activity is critical for copulation advantage of older males.
(A) Courtship competition assay with one virgin female and two males (2-day and 7-day old). Copulation percentages of 7-day old males are shown. Fly genotypes are indicated above. Significant differences between conditions (p<0.05) are denoted by different letters (a, b), ANOVA followed by Tukey’s test. Chi-square test was used to determine whether older males had a copulation advantage over young males. The six competition combinations: (1) WT ♂, WT ♀, p=0.0033, n=104; (2) sexually experienced WT (eWT) ♂, WT ♀, p=0.0034, n=85; (3) WT ♂, Orco ♀, p=0.00085, n=123; (4) Orco ♂, WT ♀, p=0.69, n=98; (5) Or47b2 ♂, WT ♀, p=0.46, n=89; (6) Or47b3 ♂, WT ♀, p=0.91, n=85. Red bars denote average copulation percentage, and the dashed line indicates chance level. (B) Competition between two males of different genotypes for a WT female. Copulation percentages of “genotype 1” males are shown. Lines connect results from parallel experiments. Only older WT males had a significant copulation advantage over Or47b2 males (***, p<0.001, paired t-test). Comparisons between competitions (GAL4 vs. UAS, Rescue vs. GAL4, Rescue vs. UAS) were made using ANOVA followed by Tukey’s test. GAL4: Or47b2, Or47b-GAL4; UAS: Or47b2, UAS-Or47b; Rescue: Or47b2, Or47b-GAL4, UAS-Or47b. Chi-square test was used to determine significance above chance: 7-day old WT vs. Or47b2, p=8.2E-6, n=111; 2-day old WT vs. Or47b2, p=0.08, n=106; GAL4 vs. UAS, p=0.55, n=100; Rescue vs. GAL4, p=0.0023, n=103; Rescue vs. UAS, p=0.00011, n=118. (C) Same as (A), except that the competition assays were conducted at 30°C. The three competition combinations: (1) Or47b-GAL4 ♂, WT ♀, p=0.0001, n=95; (2) UAS-dTrpA1 ♂, WT ♀, p=3.142E-05, n=97; (3) Or47b-GAL4, UAS-dTrpA1 ♂, WT ♀, p=0.8398, n=98. Each data point denotes average copulation percentage of a given experiment, which consisted of 17–44 matches. For each condition, 5–6 independent experiments were performed.
We then asked whether detection of fly odors, either by males or females, gives older males the copulation advantage. This could be a female choice in which females prefer the odors enriched in older males. Alternatively, older males might be more sensitive to female odors than young males and are therefore more responsive to the presence of a female. To discriminate between these two possibilities, we tested flies with the orco mutation. Orco is a co-receptor necessary for the normal function of many ORNs (Larsson et al., 2004), including the ORNs that respond to fly odors (van der Goes van Naters and Carlson, 2007). When competing for an orco mutant female, older wild-type males still displayed an overall 65:35 advantage over young males. In contrast, older males no longer displayed any copulation advantage when both competing males were orco mutants (Fig. 1A). These results suggest that the copulation advantage of older males is determined by differential olfactory functions in males, not by female olfactory preferences.
To determine the neuronal basis of the differential olfactory function in males, we focused on the Or47b neurons, an ORN type that expresses the transcription factor Fruitless, which orchestrates the neural circuits for Drosophila male courtship behavior (Hall, 1994; Kimura et al., 2005; Manoli et al., 2005; Ryner et al., 1996; Stockinger et al., 2005). We hypothesized that the copulation advantage of older males requires Or47b because the receptor has been shown to detect volatile fly odors (Dweck et al., 2015; van der Goes van Naters and Carlson, 2007; Masuyama et al., 2012). In addition, genetic perturbation of the Or47b neural circuit delays copulation onset (Root et al., 2008) and impairs male courtship behavior (Dweck et al., 2015; Lone et al., 2015). Indeed, when both competing males were Or47b mutants, older males no longer exhibited any copulation advantage (Fig. 1A). We tested two independent knockout lines, Or47b2 and Or47b3 (Wang et al., 2011), which are identical to each other across the Or47b locus (sequencing data not shown), and found no difference between these two mutant lines in the courtship assay (Fig. 1A).
To verify that Or47b is required for the copulation advantage of older males, we performed courtship competition assays pitting Or47b mutant males against wild-type males (Fig. 1B). Indeed, wild-type 7-day old males had a 71:29 advantage over the Or47b mutant counterparts (Figs. 1B and S1E), in line with the result of a recent report (Dweck et al., 2015). However, we did not observe this wild-type advantage in young males (Figs. 1B and S1F), suggesting that Or47b neuronal output is not yet significant in young males. As controls, we pitted Or47b mutants against genetically rescued flies (both 7-day old) and observed a similar age-dependent copulation advantage in the Or47b rescue flies (Figs. 1B and S1E). Together, these results demonstrate that Or47b is necessary for the copulation advantage of older males.
Or47b neuronal activity is critical for age-dependent copulation advantage
Finally, we investigated whether differential Or47b neuronal activity underlies the competitive advantage of older males. To test this, we expressed the warmth-activated cation channel Drosophila TRPA1 (dTRPA1) (Viswanath et al., 2003; Hamada et al., 2008) in Or47b neurons. Thermogenetic activation of Or47b neurons in both 2-day and 7-day old males could override the effect age might have on Or47b neuronal activity. Indeed, at a temperature that permits activation of dTRPA1 (30°C), we no longer observed the age-related copulation advantage in Or47b>dTrpA1 males (Fig. 1C). The effect was specific to the thermogenetic manipulation because the copulation advantage of older males persisted in the control flies (Fig. 1C). These results suggest that older males likely have elevated Or47b neuronal activity, which may confer upon them the copulation advantage over young males.
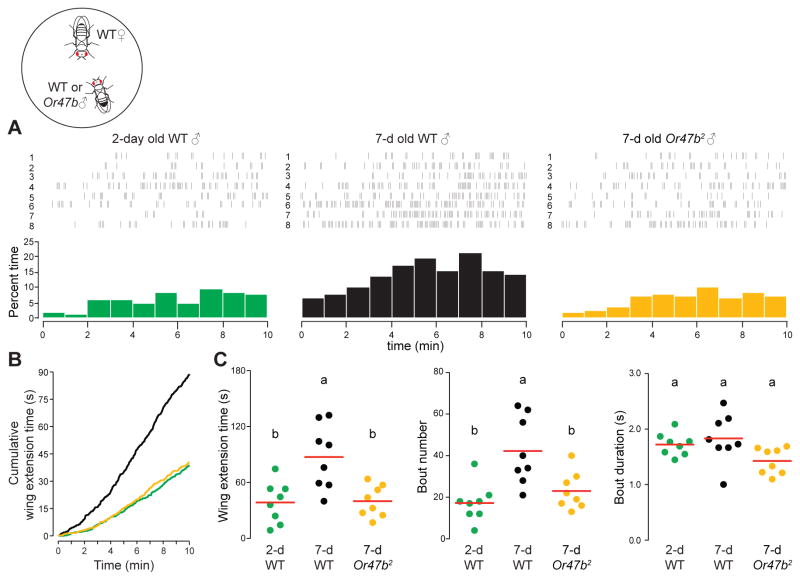
Older males court more vigorously
To determine the behavioral basis that underlies the copulation advantage of older males, we investigated an early-stage courtship behavior using a single-pair assay (one male and one female, Fig. 2) under 660-nm illumination. We focused on wing extension behavior because it produces the courtship song that is an early and measurable readout of male flies’ decision to court (Dickson, 2008), and thus is likely indicative of male mating drive. Compared to 2-day old wild-type or Or47b mutant males, 7-day old wild-type males extended their wings more frequently in the 10-min courtship assay (Fig. 2A). In all, older males spent more time engaging in wing extension (Figs. 2B–C), which is primarily due to elevated bout number (Fig. 2C). We did not find any significant difference in bout duration among the tested groups (Fig. 2C). As a control, we examined the body size of the experimental flies and found no significant difference between 2-day and 7-day old wild-type males (Fig. S2). Importantly, this elevated courtship intensity requires a functional Or47b receptor (Fig. 2B). Taken together, these results suggest that older males have a higher mating drive, which may confer a significant copulation advantage upon them.
Fig. 2. Older males display a higher courtship intensity.
Analysis of wing extension from single-pair courtship assays with one 2-day or 7-day old naïve male and one 2-day old virgin female. (A) Raster plot (top) and percent time spent on wing extension (1-min bins, bottom). Results are from parallel experiments. (B) Cumulative plot of the time spent on wing extension. (C) Overall time spent on wing extension, bout number and average bout duration of wing extension in males. Each data point indicates the result from a single fly. Significant differences (p<0.05) are denoted by different letters, ANOVA followed by Tukey’s test (n=8).
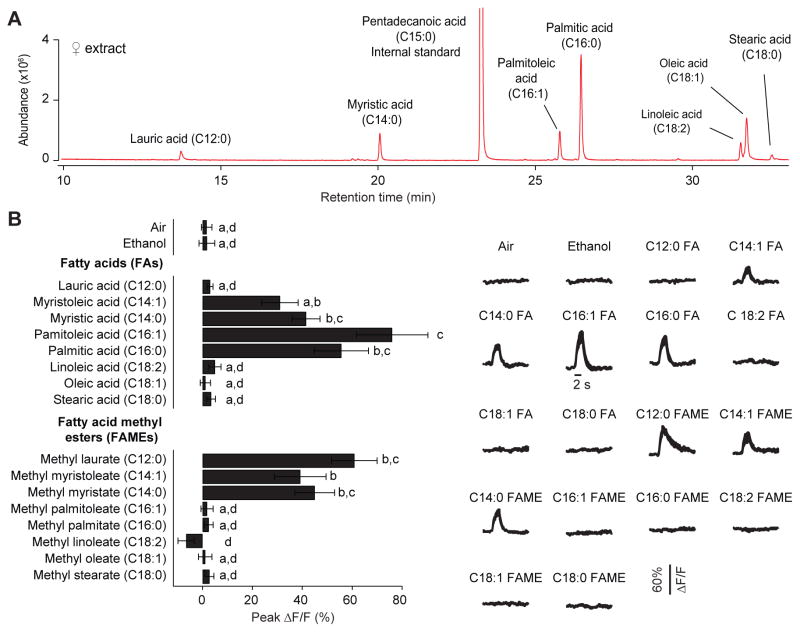
Or47b neurons respond to multiple cuticular compounds
To understand the age-related, differential Or47b neuronal function in males, we sought to identify pheromone ligands specific for Or47b ORNs by combining chemical analysis with in vivo calcium imaging. To this end, we extracted cuticular compounds from virgin females via 20-min hexane extraction at 25°C and fractionated the extract by thin layer chromatography (Fig. S3A). We then presented those fractions as odor stimuli to test which fraction activated Or47b ORNs. Using two-photon calcium imaging in the antennal lobe, we found that Or47b ORNs responded most strongly to volatile compounds in the fatty-acid fraction (Fig. S3B). Among the seven cuticular fatty acids identified in our analysis (Fig. 3A), three elicited significant responses in the Or47b ORNs (C14:0, C16:1, and C16:0, Fig. 3B). Of note, a recent independent investigation identified a fatty acid methyl ester (methyl laurate, C12:0) as a ligand for Or47b based on chemical analyses of cuticular compounds extracted with either high temperature (200°C) or prolonged methanol incubation (24 hr) (Dweck et al., 2015). Interestingly, using pure compounds, we found that Or47b ORNs responded not only to methyl laurate but also to two other fatty acid methyl esters (C14:0 and C14:1, Fig. 3B). In conclusion, our chemical analysis and calcium imaging results indicate that Or47b ORNs respond to multiple fatty acids and fatty acid methyl esters of 12–16 carbon-chain length.
Fig. 3. Or47b neurons respond to multiple cuticular compounds.
(A) Representative GC-MS chromatogram showing esterified fatty acids in the cuticular hexane extracts from 15 virgin females. Identified fatty acids are indicated on the chromatogram. Pentadecanoic acid (C15:0) was added as a standard for quantification. (B) Calcium responses evoked by individual FAs and FAMEs at the axon terminals of Or47b ORNs. Pure compounds were loaded on a filter disc and used as odor stimuli. Left: peak ΔF/F; Right: corresponding traces. Significant differences (p<0.05) are denoted by different letters; ANOVA followed by Tukey’s test. Error bars indicate s.e.m. (n=8–9).
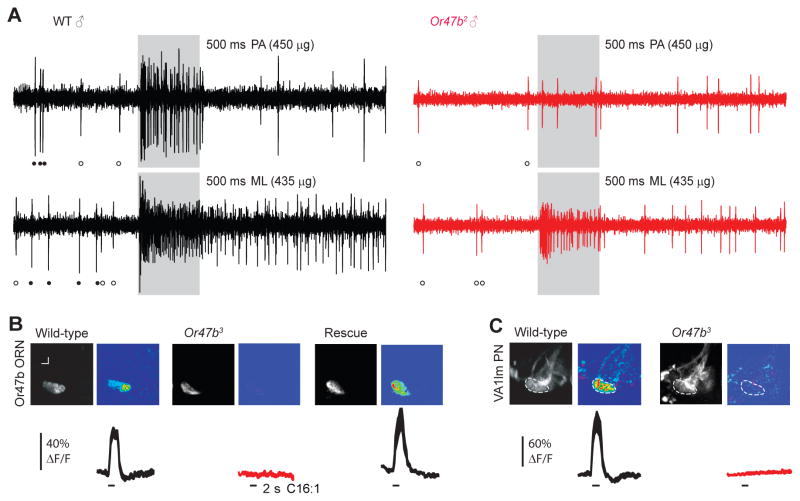
To test whether Or47b is necessary for the response to fatty acid ligands, we performed loss-of-function and genetic rescue experiments. We focused on palmitoleic acid because it elicited the strongest response in our initial analysis (Fig. 3B). Using single-sensillum recording in wild-type and Or47b mutant males, we confirmed that palmitoleic acid activates Or47b ORNs (at4A) and the activation requires functional Or47b receptors (Fig. 4A). Of note, we presented palmitoleic acid at close range (~4 mm from the antenna) in order to elicit significant responses in Or47b ORNs (Fig. S4). For pheromone odorants with low volatility, it has been shown that such close-range stimulation is critical for successful activation of target ORNs (van der Goes van Naters and Carlson, 2007; Gomez-Diaz et al., 2013). The difference in odor delivery method likely accounts for the discrepancy between our study and an earlier study where the authors did not observe at4A responses to fatty acid ligands (Dweck et al., 2015). As a control, we also tested methyl laurate, an odorant which has been shown to activate both Or47b (at4A) and Or88a (at4C) ORNs (Dweck et al., 2015), and observed robust responses in both neuronal types (Fig. 4A). In contrast, palmitoleic acid does not activate other pheromone ORNs: at4B (Or65a/Or65b/Or65c), at4C (Or88a) (Fig. 4A) or at1 ORNs (Or67d) (data not shown). Using calcium imaging, we further verified that the Or47b neuronal signals (Fig. 4B) are propagated to the postsynaptic projection neurons (PNs) in the antennal lobe (Fig. 4C). These responses are Or47b-dependent; we observed palmitoleic acid-evoked responses in the Or47b genetic rescue flies but not in the Or47b mutants, consistent with earlier results using single-sensillum recording (Fig. 4).
Fig. 4. Activation of at4A olfactory circuit by palmitoleic acid requires Or47b receptor.
(A) Sample traces of single-unit recordings of at4 sensillum in WT and Or47b mutant 7-day old males. Filled circles indicate large ORN spikes from at4A (Or47b); empty circles indicate smaller ORN spikes from at4C (Or88a). at4A spike activity was absent in the Or47b mutants. PA: palmitoleic acid; ML: methyl laurate. (B) Calcium activity at the axon terminals of Or47b ORNs in WT, Or47b3 mutant and genetic rescue males expressing GCaMP3 in the Or47b ORNs. (C) Calcium activity at the dendrites of Or47b projection neurons (PNs) in WT and Or47b3 mutant males. Gray-scale images show the Or47b/VA1lm glomerulus and pseudocolored images show representative odor-evoked responses. Mean ΔF/F is plotted against time, with line width indicating s.e.m. (n=5–9).
We next asked whether these Or47b ligands convey sex-specific information. We performed cuticular chemical analysis using both male and female flies and found that fatty acids do not constitute a female-specific odor; gas chromatography-mass spectrotometry (GC-MS) analysis revealed a similar fatty-acid profile between males and females (Fig. S3C), in agreement with earlier reports that Or47b ORNs respond to both male and female odors (Dweck et al., 2015; van der Goes van Naters and Carlson, 2007). We then asked whether Or47b ORNs signal species-specific information. We performed single-sensillum recordings from two other Drosophila species (D. simulans and D. willistoni) and found that at4A ORNs from both species responded robustly to palmitoleic acid (data not shown), implying that Or47b ligands have a similar function across Drosophila species. Collectively, our results suggest that Or47b ligands do not signal sex- or species-specific information.
Palmitoleic acid is a pheromone that promotes male courtship
Does palmitoleic acid modulate male courtship behavior? In a single-pair courtship assay conducted under 660-nm illumination, ~67% of wild-type males successfully copulated within 2 hrs (Fig. 5A). In contrast, only 50% of the Or47b mutant males copulated. Genetic rescue experiments confirmed that this copulation phenotype was caused by the Or47b mutations (Fig. 5B). We then perfumed the mating chamber with palmitoleic acid, which significantly increased the copulation rate of wild-type males by ~17% (Figs. 5C and S5B) but had no effect on the Or47b mutant males (Figs. 5D and S5C). As a control, we perfumed the chamber with linoleic acid, a cuticular fatty acid that does not activate Or47b ORNs (Fig. 3B), and found that the compound had no effect on copulation rate (Fig. S5A). These results indicate that palmitoleic acid is a stimulatory pheromone that promotes male courtship behavior via the Or47b olfactory circuit. Given that palmitoleic acid does not activate other pheromone ORNs (Fig. 4A) and its stimulatory effect entirely depends on functional Or47b receptors (Fig. 5), it is likely a more specific odorant than methyl laurate and thus provides a better opportunity to investigate the function of the Or47b olfactory circuit in courtship behavior.
Fig. 5. Palmitoleic acid is a stimulatory pheromone that promotes male courtship.
Single-pair courtship assays with a 7-day old naïve male and a 2-day old virgin female. Cumulative copulation rates are plotted against time (left panels). Final copulation rates are shown with lines connecting results from parallel experiments (right panels). (A) Copulation rates of Or47b mutant males were significantly lower than that of WT controls. (B) Genetic rescue of Or47b mutation restored the copulation rate. Data were from 6 experiments, 13 to 30 pairs of flies per experiment, 149–164 pairs per genotype. Significant differences (p<0.05) are denoted by different letters, ANOVA followed by Tukey’s test. (C–D) Perfuming the mating chamber with palmitoleic acid (PA) increased the copulation rate of WT (C) but not Or47b2 mutant males (D). Solvent control: ethanol. Results are from 5 parallel experiments, 19–25 pairs of flies per experiment, 119–125 pairs per condition. Paired t-test, **, p<0.01.
Age-dependent and male-specific sensitization of Or47b neurons
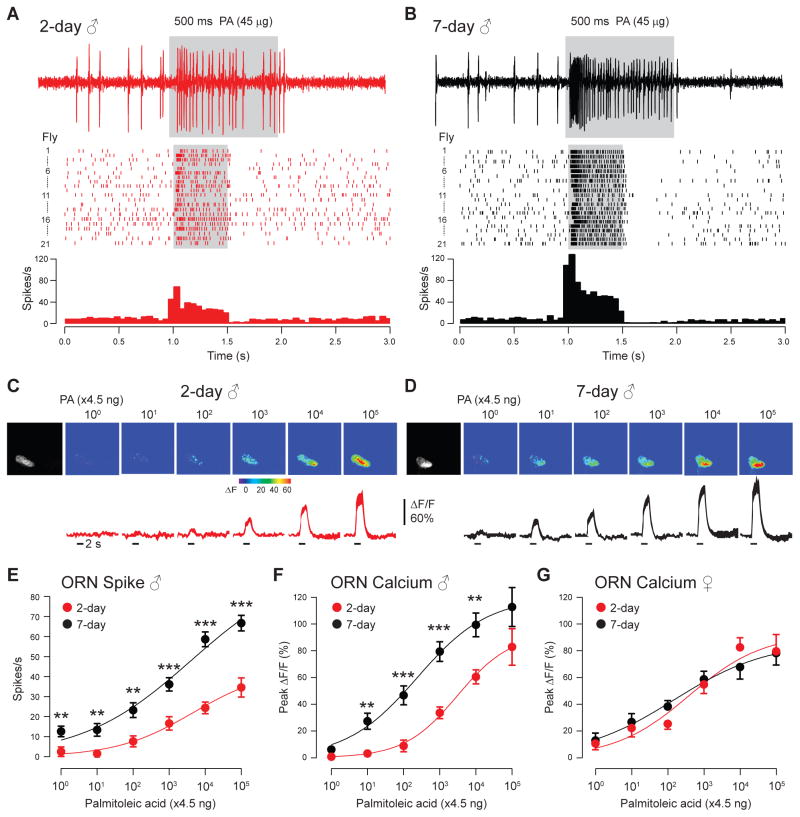
Given that Or47b is required for the copulation advantage of older males (Fig. 1A) and that palmitoleic acid promotes male courtship behavior (Fig. 5), we hypothesized that the sensitivity of Or47b ORNs in male flies increases with age. To test this hypothesis, we measured the spike responses of Or47b ORNs to palmitoleic acid with single-sensillum recording. In addition, we performed calcium imaging in the antennal lobe to measure ORN outputs. Results from both measurements support the occurrence of age-dependent sensitization of Or47b ORNs: the olfactory responses of Or47b ORNs to palmitoleic acid across concentrations are significantly higher in older males than those in young males (Figs. 6A–F). In contrast, female flies did not exhibit any age-dependent change in Or47b olfactory responses (Fig. 6G). These results indicate that the enhanced Or47b olfactory responses are male-specific. Of note, we observed similar elevated Or47b responses in older males to another cuticular fatty acid (Fig. S6), suggesting that the age-dependent sensitization is not odorant-specific. Furthermore, we did not observe any age-dependent sensitization in males in the Or67d pheromone ORNs (Figs. S7A–C). These findings indicate that the elevated Or47b output in the older males likely originates from the sensitization of Or47b ORNs to their pheromone ligands in the periphery.
Fig. 6. Sensitivity of male Or47b ORNs increases with age.
(A–B) Single-sensillum recording from the at4A ORNs that express Or47b. Palmitoleic acid-evoked spike responses in 2-day old (A) and 7-day old (B) males. Corresponding spike rasters (middle) and peri-stimulus time histogram (bottom, binned at 50 ms) are shown below the sample traces (n=21). (C–D) Palmitoleic acid-evoked calcium response at the Or47b axon terminals in 2-day old (C) and 7-day old (D) males. Dose-response curves comparing Or47b ORN spike responses (E) or calcium responses at ORN axonal terminals (F) between 2-day and 7-day old males (*, p<0.05; **, p<0.01; ***, p<0.001, t-test). (G) As in (F), imaging from female flies. Mean± s.e.m. (n=7–9 for imaging). Dose response comparisons between 2-day and 7-day old flies are from parallel experiments (palmitoleic acid: 4.5 ng to 450 μg).
Juvenile hormone mediates age-dependent sensitization of Or47b neurons
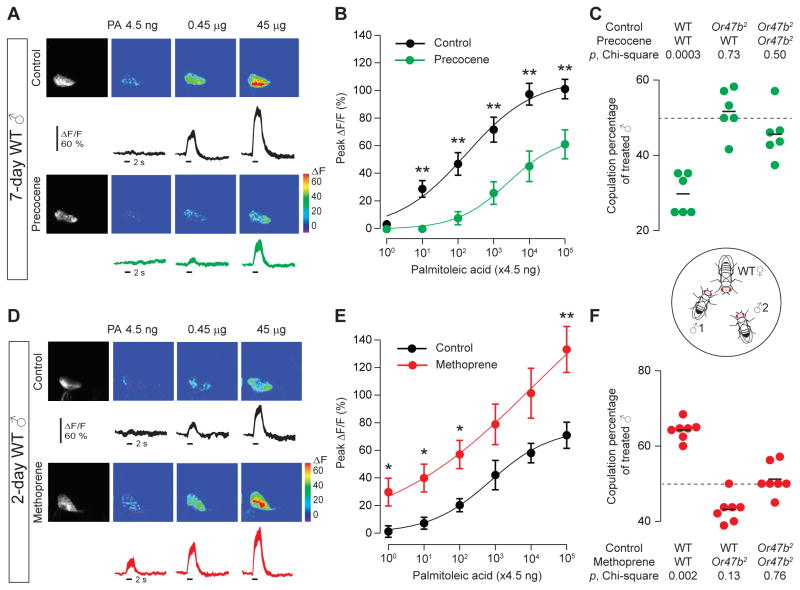
Next we sought to determine the molecular mechanism that underlies the age-dependent sensitization of Or47b ORNs. For many insect species, pheromone sensitivity and onset of mating activity are regulated by juvenile hormone (Gadenne et al., 1993; Anton and Gadenne, 1999; Bownes and Rembold, 1987). We thus hypothesized that Or47b ORNs are a target of juvenile hormone, which may modulate Or47b neuronal sensitivity to regulate courtship activity. We first employed a pharmacological ablation approach. We treated newly eclosed males with precocene, which leads to selective cell death in the corpora allata, thereby inhibiting the production of juvenile hormone (Pratt et al., 1980). In the precocene-treated flies, we observed a significant reduction in the sensitivity of Or47b ORNs, compared to solvent-treated controls (Figs. 7A–B). In addition, the sensitivity of Or47b ORNs in the treated 7-day old males (Fig. 7B) was reduced to a level similar to that of the 2-day old controls (Fig. 7E), suggesting that juvenile hormone is necessary for the elevated Or47b ORN sensitivity in older males.
Fig. 7. Juvenile hormone regulates Or47b neuronal sensitivity to confer copulation advantage.
(A) Palmitoleic acid-evoked calcium responses at the Or47b axon terminals in 7-day old males treated with solvent (ethanol) or precocene. (B) Dose-response curves of Or47b ORN in response to palmitoleic acid. Precocene significantly decreased the response magnitude of Or47b ORNs, mean± s.e.m. (n=8–9). (C) Copulation percentage of precocene-treated 7-day old males. (D–F) Similar to (A–C), with 2-day old males treated with methoprene. (D–E) Methoprene markedly enhanced the response magnitude of the Or47b ORNs to palmitoleic acid, compared to control, mean± s.e.m. (n=8–9). (F) Methoprene conferred a copulation advantage on WT but not Or47b2 mutant males. For imaging experiments, statistical significance was determined by t-test (*, p<0.05; **, p<0.01). For behavioral experiments, Chi-square test was used to determine significance above chance (dashed lines in C and F). Results are from five parallel experiments, 20–22 matches per experiment.
We then asked whether increasing juvenile hormone signaling in young males would elevate their Or47b responses to palmitoleic acid. To this end, we treated newly eclosed males with methoprene, a synthetic juvenile hormone mimic (Jindra et al., 2013). Indeed, methoprene treatment markedly enhanced Or47b responses to palmitoleic acid in young males (Figs. 7D and E) without affecting the sensitivity of Or67d ORNs to cVA (Figs. S7D–E), consistent with our finding that the Or67d ORNs do not exhibit any age-dependent sensitization (Figs. S7A–C). On the other hand, ecdysone, another insect reproductive hormone (Belles and Piulachs, 2014; Hentze et al., 2013), had no effect on Or47b ORN sensitivity (not shown). Taken together, our loss- and gain-of-function experiments indicate that juvenile hormone is both necessary and sufficient for the elevated Or47b ORN sensitivity in males.
In addition, we tested whether modulation of Or47b ORN sensitivity by manipulating juvenile hormone levels affects the outcome of courtship competition. Indeed, down regulation of Or47b responses by precocene (Fig. 7B) rendered the treated flies less successful than the controls in the courtship competition assays (Fig. 7C). Conversely, methoprene-treated young males gained a significant copulation advantage over the control counterparts (Fig. 7F). Importantly, the effect of precocene or methoprene on the outcome of courtship competition depends on Or47b; precocene-treated wild-type males did not suffer any copulation disadvantage when pitted against Or47b mutants (Fig. 7C). In addition, methoprene-treated Or47b mutant young males did not gain any copulation advantage over the wild-type counterparts (Fig. 7F). Similarly, treating the Or47b mutant males with either precocene or methoprene did not impact the outcome of courtship competition (Figs. 7C and F). These results provide evidence that juvenile hormone signaling underlies the courtship advantage of older males. Specifically, juvenile hormone promotes courtship success in older males by elevating the pheromone sensitivity of Or47b ORNs. Notably, we did not find any significant difference in the Or47b transcript level in the antennae between 2-day and 7-day old wild-type males (antennal qPCR, data not shown), suggesting that juvenile hormone signaling regulates Or47b ORN sensitivity by other means.
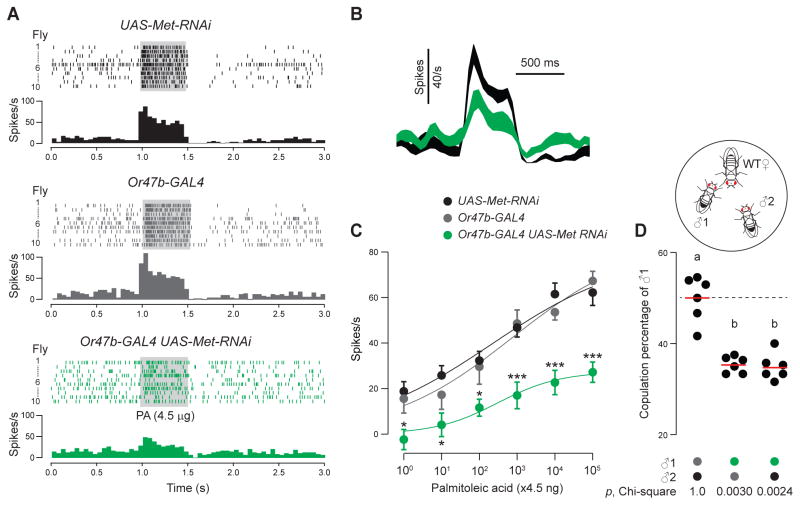
Finally we investigated whether Or47b ORNs are a direct target of juvenile hormone. Among the proteins that bind juvenile hormone, Methoprene-tolerant (Met), a bHLH-PAS protein, has been implicated as a juvenile hormone receptor in Drosophila (Jindra et al., 2013; Wilson and Fabian, 1986) and is expressed in the antenna (RT-PCR data not shown). We therefore hypothesize that juvenile hormone acts on Met to mediate the age-dependent sensitization of Or47b ORNs. In 7-day old Met mutant males (Wilson and Fabian, 1986), the responses of Or47b ORNs to palmitoleic acid were significantly reduced compared to the age-matched controls (Fig. S8). Furthermore, knocking down Met expression specifically in the Or47b ORNs reduced their response to palmitoleic acid in a similar manner (Figs. 8A–C). These results suggest that Met is required in a cell-autonomous manner for juvenile hormone to modulate Or47b sensitivity. Finally, consistent with our results with precocene to suppress juvenile hormone signaling (Fig. 7C), genetic knock-down of Met expression also abolished the copulation advantage of older males (Fig. 8D). Together, these results indicate that Or47b ORNs are a direct neural substrate for juvenile hormone.
Figure 8. Methoprene-tolerant (Met) is required for age-dependent sensitization of Or47b ORNs.
(A) Raster plots and peri-stimulus time histograms of Or47b spike responses of controls (UAS-Met-RNAi or Or47b-GAL4) and Met RNAi knock-down males (Or47b-GAL4 UAS-Met-RNAi). (B) Comparison of the average spike responses to palmitoleic acid (4.5 μg) between 7-day old control (UAS-Met-RNAi) and Met RNAi knock-down males. Traces are smoothed and binned at 50 ms; line width represents s.e.m. (C) Dose-response curves, mean± s.e.m. (n=10). Results are from parallel experiments. *, p<0.05; ***, p<0.001, ANOVA test. (D) Courtship competition assay between two 7-day old males of indicated genotypes. Chi-square test was used to determine significance above chance (dashed line). Results are from six parallel experiments, 11–21 successful matches per experiment. Significant differences (p<0.05) are denoted by different letters, ANOVA followed by Tukey’s test.
DISCUSSION
Here our findings reveal that sensitization of Or47b ORNs by juvenile hormone underlies the copulation advantage of older males. In addition, we establish “age” as an important biological context in which the Or47b pheromone circuit selectively enhances mating drive in males at the peak of their fertility. From fly cuticular extracts, we identified palmitoleic acid, among several ligands for Or47b neurons, as a stimulatory pheromone that promotes male courtship. This allows older males to court females more promptly and vigorously and may confer upon them a copulation advantage over young males. Mechanistically, we found that the age-dependent sensitization of Or47b ORNs requires Met in a cell-autonomous manner, supporting the idea that Or47b ORNs are a direct neural substrate for juvenile hormone. Our results reveal that Or47b ORNs serve as an important interface between neural circuits for courtship behavior and the juvenile hormone signaling that regulates reproductive maturation and other life-history traits.
For a sensory circuit that promotes male courtship behavior, we are intrigued by the observation that the Or47b circuit does not appear to convey sex- or species-specific information. What then is the biological meaning of the pheromones that activate the Or47b circuit? Most fatty acids in fly cuticles are synthesized from nutrients in food (de Renobales and Blomquist, 1984), and some, such as palmitoleic acid, are the precursors of all Drosophila cuticular hydrocarbons, many of which play roles as pheromones (Jallon, 1984; Wicker-Thomas and Chertemps, 2010). It is therefore possible that these pheromones collectively signal the nutritional state of a fly, and the Or47b olfactory circuit may thus allow male flies to favor well-nourished females. Moreover, given that we could markedly enhance male copulation rate by perfuming the mating chamber with palmitoleic acid (Figs. 5 and S5), it is likely that male flies will court more vigorously when more females are nearby. In comparision to the Ir84a olfactory circuit, which signals food abundance to regulate male courtship (Grosjean et al., 2011), the Or47b pheromone circuit may also communicate mate availability to modulate male mating drive. Finally, we note the positive effect of palmitoleic acid on the copulation rate of 2-day old males despite their low Or47b neuronal sensitivity (Fig. S5). This result suggests that regardless of age, male flies will be more inclined to court when the probability of mating success is high.
Although the fatty acid ligands are present at a similar level in both males and females (Fig. S3) and in other insect species (Thompson, 1973), futile courtship behavior could be prevented by contextual inhibitory pheromone cues. For example, males produce cis-vaccenyl acetate (cVA) (Bartelt et al., 1985), which inhibits male courtship behavior towards other males or mated females (Ejima et al., 2007; Jallon, 1984; Kurtovic et al., 2007). In addition, specific gustatory receptor neurons in the male legs detect male and/or heterospecific cuticular hydrocarbons (e.g. CH503, 7-tricosene and 7-pentacosene) which function as anti-aphrodisiacs to further deter futile courtship (Fan et al., 2013; Thistle et al., 2012). Thus, while palmitoleic acid functions as an aphrodisiac, multiple anti-aphrodisiac pheromones could suppress male-male or interspecific courtship. Of note, male-male courtship has been observed in mutant flies which do not produce cuticular hydrocarbons (Billeter et al., 2009; Wang et al., 2011), and this attraction requires a functional Or47b receptor (Wang et al., 2011) that likely detects fatty acids from male cuticles.
Our findings also address a longstanding mystery of the effect of age on courtship behavior. Here we show that the courtship advantage is afforded by elevated mating drive in older males and is less likely a result of female olfactory choice, contrary to the dominant view of sexual selection in which “males compete, females choose” (Bateman, 1948). As courtship decision and intensity is a form of mate choice (Edward and Chapman, 2011), the age-related copulation disparity is in fact a result of male choice. In light of the considerable reproductive cost for male flies (Partridge and Farquhar, 1981), they need to be judicious about mating if they have not yet reached the peak of their fertility (Byrne and Rice, 2006). In addition, we show that boosting mating drive does not necessarily require a multiplex of physiological changes but can instead be achieved by elevating the output of a pheromone sensory neuron. Therefore, our study identifies a simple and elegant neural mechanism which underlies how age impacts male courtship success in Drosophila melanogaster.
In mammals, sex hormones are essential for the development and organization of the sexually dimorphic neural circuits in the brain. In adulthood, sex hormones are required for the activation of those circuits to elicit sex-specific behaviors (for review, Yang and Shah, 2014). In addition to central neurons in the brain, sex hormones exert profound influence on sexual behavior by gating pheromone detection in the periphery (Dey et al., 2015). Although insects do not produce sex-specific hormones like mammals, our study in Drosophila melanogaster suggests a common principle by which reproductive hormones regulate mating behavior. In both flies and mice, pheromone communication is regulated not only by the production of pheromones but also by the detection of those compounds. Moreover, in both species, reproductive status and sexual behavior can be precisely coordinated by a reproductive hormone that targets pheromone sensory neurons in the periphery.
EXPERIMENTAL PROCEDURES
Behavioral assays
Flies were raised on standard fly food containing molasses at 25°C in a 12:12 light-dark cycle. Two independent Or47b mutant alleles, Or47b2 and Or47b3, were backcrossed to Berlin (w−) for 6 generations. Wild-type Berlin (w+) males were used as controls. All experimental flies are either w+ or have a mini-white marker. Or47b mutation/expression was verified by antennal qRT-PCR. Or47b-GAL4 (Fishilevich and Vosshall, 2005) and UAS-Or47b (Hallem and Carlson, 2006; Hallem et al., 2004) were also backcrossed to Berlin (w−). Flies were collected at eclosion, separated by sex, and raised in groups of ten. All the experimental flies were group-housed. For each experiment, typically, 25 trials were set up and only trials in which copulation occurred within 2 hrs were included in the analysis.
For competition assay with sexually experienced males, we first collected mated males. Briefly, one 1-day or 6-day old naïve male was paired with one virgin female. Mating was visually confirmed and mated male flies were transferred into new food vials and group-housed for courtship competition experiments the next day.
The mating chamber is positioned atop a petri-dish containing fly food to mimic the natural environment where flies mate on food (Fig. S1). The base of the chamber is made of a piece of gauze to allow food odors to permeate the chamber. Courtship experiments were conducted under 660-nm red light with a larger mating chamber (2 cm in diameter and 1 cm in height, 3.14 cm3) than conventional ones (0.4 cm3, Siegel and Hall, 1979). Fly behavior was observed for 120 min, 50% relative humidity at 25°C, except for the thermogenetic experiments, which were conducted at 30°C.
For the single-pair courtship assay, one 2-day or 7-day old naïve male of a given genotype and one 2-day old virgin Canton-S female were tested. For perfuming experiments, a piece of filter paper (1.4 x 1.4 cm) containing 0.45 mg palmitoleic acid, 0.45 mg linoleic acid or 10 μl ethanol (solvent control) was placed underneath the mating chamber. Flies did not have any direct contact with the compounds. Ethanol was allowed to evaporate for at least 60 min in a fume hood prior to experiments. For the wing extension analysis shown in Fig. 2, single-pair courtship assays were recorded with a video camera under 660-nm red light for 10 min at 3 frames/sec. As a control to estimate basal wing extension frequency in males, similar analyses were conducted in the absence of female flies. Spontaneous wing extensions were observed and they usually lasted less than two consecutive frames (<1 sec). Wing extensions that lasted for more than three frames were marked for subsequent analysis.
For the courtship competition assay, two naïve males of given genotypes/ages and one 2-day old virgin Canton-S female were tested. The copulated males and time of copulation were recorded during the 2-hr observation period. The genotypes of competing males were determined by their eye color at the end of the experiment: Fig. 1B, WT (red) vs Or47b2 (orange); 6C and F, WT (red) vs Or47b2 (orange). In cases that genotypes could not be determined by eye color, one of the two males was dusted with a fluorescent dye (UVXPBR, LDP LLC, Carlstadt, NJ) 48 hrs prior to the experiment. Dye application was alternated between the two genotypes to minimize any possible dye-induced behavioral bias.
Single-sensillum recording
Single-sensillum recording was performed as previously described (Su et al., 2012). A sharp electrode filled with AHL saline (Wang et al., 2003) was inserted into the recorded sensillum. Odorants, including palmitoleic acid (Sigma, 76169), methyl laurate (Sigma, 234591), palmitic acid (Sigma, P0500) and cVA (Cayman Chemical, 10010101), were freshly diluted in ethanol (v/v) and 5 μl of the odorant was applied to filter paper inserted inside a truncated 200-μl pipette tip. Ethanol was allowed to evaporate for at least 30 min in a fume hood prior to experiments. The odor cartridge was positioned around 4 mm away from the antenna. Odor stimulus was delivered via a 500-ms pulse of air (500 ml/min) directly at the antenna in the presence of humidified air flow at 2 L/min from a different direction.
Two-photon calcium imaging
Fly dissection was performed in chilled calcium-free AHL saline as published (Wang et al., 2003). Prior to experiments, calcium-free AHL was replaced by regular AHL saline. Odor stimuli were delivered via Teflon tubing containing filter paper blotted with 5 μl cuticular hexane extracts (Fig. S3B) or 5 μl fatty acids and fatty acid methyl esters (1 μg/μl in ethanol, Fig. 3B). For experiments shown in Figs. 4B–C, 1 μl palmitoleic acid was applied via a 2-s pulse of air (25 ml/min) with a main air flow at 1000 ml/min (2.5% of saturated vapor). For other imaging experiments (Figs. 6 and 7), odor was diluted in ethanol as described in single-sensillum recording and applied via a 2-s pulse of air (50 ml/min) with a main air flow at 1000 ml/min. Images were taken at 4 frames/s for 20 s at a resolution of 128X128 pixels using a custom two-photon microscope (Wang et al., 2003). At the end of each experiment, image stacks were collected at a high resolution (512X512 pixels) for glomerulus identification. Imaging data were analyzed and plotted in Igor Pro 6.2 (Wavemetrics).
Pharmacological manipulations of juvenile hormone or ecdysone
Flies were raised on food with 10 μl methoprene (0.25%, v/v in ethanol, Sigma 33375), precocene I (0.1%, v/v in ethanol, Sigma 195855), 20-hydroxyecdysone (1 mM, Sigma H5142) or solvent (ethanol) applied to the surface of medium. Solvent was evaporated for one hour in a fume hood. Naïve male flies were collected soon after eclosion and transferred to a vial with drug- or solvent-treated food. Flies were transferred to fresh vials every other day.
Thin layer chromatography
1500 virgin Canton-S female flies, aged 2–7 days were immersed in hexane for 20 mins. The solvent was evaporated completely under a stream of N2. Cuticular extracts were separated using glass-backed silica gel plates (20x10 cm, coated with 0.21–0.27 mm of silica gel 60; EMD Millipore) with a mixture of hexane/diethyl ether/acetic acid (90:9:1, v/v). Standard compounds (fatty acids, fatty acid methyl esters, and glycerol trioleate) were analyzed in parallel. The plate was stained with primuline (0.1% w/v in 20% acetone) and imaged under 366-nm UV light.
Gas chromatography mass spectrometry (GC-MS)
30 virgin male or female flies (2-day old) were immersed in 240 μl hexane containing 100 nM pentadecanoic acid (C15:0) and 10 μg/ml hexacosane at room temperature for 20 min. Hexane in the extracts was evaporated completely under a stream of N2. Half of the extract was trans-esterified by adding 100 μl of 0.5 N methanolic HCl (Sigma-Aldrich) and incubating at 60°C for 1 hour with occasional vortexing. The other half was used as a control without esterification. After incubation, the solvent was evaporated under N2 and re-dissolved in 120 μl of hexane prior to analysis. GC-MS analysis was performed using a GC-MS 7820A system coupled to a 5975 mass selective detector (Agilent) with a 5% diphenyl-dimethylpolysiloxane column (Rtx-5ms, 30 m, 0.25 mm ID, 0.25 μm df, Restek). The initial oven temperature was 60°C, increased at a rate of 15°C/min to 120°C and held there for 1 min, and then increased at a rate of 3°C/min to a final temperature of 240°C and held at the temperature for 10 min. Column injection was performed in splitless mode. Flow rate was 1.19 ml/min. The inlet temperature was set at 200°C and the column transfer temperature was set at 280°C. Mass spectra was acquired in EI mode from 50 to 550 m/z. Pentadecanoic acid (C15:0) and hexacosane were used as internal standards. Peaks corresponding to fatty acid methyl esters (FAMEs) were identified based on retention times and electron ionization spectra of known standards. Absolute quantities from extract were calculated based on standard response curves established for each FAME and normalized to the internal standards to account for sample loss during preparation.
Supplementary Material
Acknowledgments
We thank Steve Wasserman, Susy Kim, Kausik Si, Ron Yu, and James Nieh for comments on the manuscript; Kenta Asahina for help with wing extension analysis; Leslie Vosshall for providing the Or47b2 and Or47b3 fly stocks; Maxi Richmond for discussion on Drosophila species. We thank UCSD Glycotechnology Core for GC-MS services. This work was supported by NIH and NSF grants (R01DC009597, R01DK092640, 0920668) to J.W.W., UCSD Start-up Fund and a Hellman Fellowship to C.Y.S. and a Singapore National Research Foundation grant to J.Y.Y. (NRF2010-06).
Footnotes
Author Contributions H.H.L., D.S.C., S.S., J.Y.Y., C.Y.S. and J.W.W. designed the experiments and wrote the paper. H.H.L. performed behavioral experiments with assistance from C.A.N. and single-sensillum recording with assistance from C.Y.S. and S.S (data analysis). D.S.C. performed two-photon imaging. S.S., J.S.R.C. and J.Y.Y. performed chemical analysis. Z.Z. and J.W.W. designed the behavioral assay. T.C. performed preliminary antennal calcium imaging. A.K.S. performed qRT-PCR and edited the manuscript. All authors read and commented on the manuscript.
All authors declare that they do not have any competing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anton S, Gadenne C. Effect of juvenile hormone on the central nervous processing of sex pheromone in an insect. Proc Natl Acad Sci U S A. 1999;96:5764–5767. doi: 10.1073/pnas.96.10.5764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antony C, Davis TL, Carlson DA, Pechine JM, Jallon JM. Compared behavioral responses of male Drosophila melanogaster (Canton S) to natural and synthetic aphrodisiacs. J Chem Ecol. 1985;11:1617–1629. doi: 10.1007/BF01012116. [DOI] [PubMed] [Google Scholar]
- Arienti M, Antony C, Wicker-Thomas C, Delbecque JP, Jallon JM. Ontogeny of Drosophila melanogaster female sex-appeal and cuticular hydrocarbons. Integr Zool. 2010;5:272–282. doi: 10.1111/j.1749-4877.2010.00213.x. [DOI] [PubMed] [Google Scholar]
- Bartelt RJ, Schaner AM, Jackson LL. cis-Vaccenyl acetate as an aggregation pheromone in Drosophila melanogaster. J Chem Ecol. 1985;11:1747–1756. doi: 10.1007/BF01012124. [DOI] [PubMed] [Google Scholar]
- Bateman AJ. Intra-sexual selection in Drosophila. Heredity (Edinb) 1948;2:349–368. doi: 10.1038/hdy.1948.21. [DOI] [PubMed] [Google Scholar]
- Belles X, Piulachs MD. Ecdysone signalling and ovarian development in insects: from stem cells to ovarian follicle formation. Biochim Biophys Acta - Gene Regul Mech. 2014;1849:181–186. doi: 10.1016/j.bbagrm.2014.05.025. [DOI] [PubMed] [Google Scholar]
- Bilen J, Atallah J, Azanchi R, Levine JD, Riddiford LM. Regulation of onset of female mating and sex pheromone production by juvenile hormone in Drosophila melanogaster. Proc Natl Acad Sci U S A. 2013;110:18321–18326. doi: 10.1073/pnas.1318119110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billeter JC, Atallah J, Krupp JJ, Millar JG, Levine JD. Specialized cells tag sexual and species identity in Drosophila melanogaster. Nature. 2009;461:987–991. doi: 10.1038/nature08495. [DOI] [PubMed] [Google Scholar]
- Bownes M, Rembold H. The titre of juvenile hormone during the pupal and adult stages of the life cycle of Drosophila melanogaster. Eur J Biochem. 1987;164:709–712. doi: 10.1111/j.1432-1033.1987.tb11184.x. [DOI] [PubMed] [Google Scholar]
- Bray S, Amrein H. A putative Drosophila pheromone receptor expressed in male-specific taste neurons is required for efficient courtship. Neuron. 2003;39:1019–1029. doi: 10.1016/s0896-6273(03)00542-7. [DOI] [PubMed] [Google Scholar]
- Byrne PG, Rice WR. Evidence for adaptive male mate choice in the fruit fly Drosophila melanogaster. Proc Biol Sci. 2006;273:917–922. doi: 10.1098/rspb.2005.3372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clowney EJ, Iguchi S, Bussell JJ, Scheer E, Ruta V. Multimodal chemosensory circuits controlling male courtship in Drosophila. Neuron. 2015;87:1036–1049. doi: 10.1016/j.neuron.2015.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dey S, Chamero P, Pru JK, Chien MS, Ibarra-Soria X, Spencer KR, Logan DW, Matsunami H, Peluso JJ, Stowers L. Cyclic Regulation of Sensory Perception by a Female Hormone Alters Behavior. Cell. 2015;161:1334–1344. doi: 10.1016/j.cell.2015.04.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson BJ. Wired for sex: the neurobiology of Drosophila mating decisions. Science. 2008;322:904–909. doi: 10.1126/science.1159276. [DOI] [PubMed] [Google Scholar]
- Dweck HKM, Ebrahim SAM, Thoma M, Mohamed AAM, Keesey IW, Trona F, Lavista-Llanos S, Svatoš A, Sachse S, Knaden M, et al. Pheromones mediating copulation and attraction in Drosophila. Proc Natl Acad Sci. 2015;112:E2829–E2835. doi: 10.1073/pnas.1504527112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edward DA, Chapman T. The evolution and significance of male mate choice. Trends Ecol Evol. 2011;26:647–654. doi: 10.1016/j.tree.2011.07.012. [DOI] [PubMed] [Google Scholar]
- Ejima A, Smith BPC, Lucas C, van der Goes van Naters W, Miller CJ, Carlson JR, Levine JD, Griffith LC. Generalization of Courtship Learning in Drosophila Is Mediated by cis-Vaccenyl Acetate. Curr Biol. 2007;17:599–605. doi: 10.1016/j.cub.2007.01.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan P, Manoli DS, Ahmed OM, Chen Y, Agarwal N, Kwong S, Cai AG, Neitz J, Renslo A, Baker BS, et al. Genetic and neural mechanisms that inhibit Drosophila from mating with other species. Cell. 2013;154:89–102. doi: 10.1016/j.cell.2013.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrero DM, Moeller LM, Osakada T, Horio N, Li Q, Roy DS, Cichy A, Spehr M, Touhara K, Liberles SD. A juvenile mouse pheromone inhibits sexual behaviour through the vomeronasal system. Nature. 2013;502:368–371. doi: 10.1038/nature12579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishilevich E, Vosshall LB. Genetic and functional subdivision of the Drosophila antennal lobe. Curr Biol. 2005;15:1548–1553. doi: 10.1016/j.cub.2005.07.066. [DOI] [PubMed] [Google Scholar]
- Flatt T, Tu MP, Tatar M. Hormonal pleiotropy and the juvenile hormone regulation of Drosophila development and life history. Bioessays. 2005;27:999–1010. doi: 10.1002/bies.20290. [DOI] [PubMed] [Google Scholar]
- Gadenne C, Renou M, Sreng L. Hormonal control of pheromone responsiveness in the male black cutworm Agrotis ipsilon. Experentia. 1993;49:721–724. [Google Scholar]
- Van der Goes van Naters W, Carlson JR. Receptors and neurons for fly odors in Drosophila. Curr Biol. 2007;17:606–612. doi: 10.1016/j.cub.2007.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Diaz C, Reina JH, Cambillau C, Benton R. Ligands for pheromone-sensing neurons are not conformationally activated odorant binding proteins. PLoS Biol. 2013;11:e1001546. doi: 10.1371/journal.pbio.1001546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillet M, Dartevelle L, Ferveur JF. A Drosophila male pheromone affects female sexual receptivity. Proc Biol Sci. 2006;273:315–323. doi: 10.1098/rspb.2005.3332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosjean Y, Rytz R, Farine JP, Abuin L, Cortot J, Jefferis GSXE, Benton R. An olfactory receptor for food-derived odours promotes male courtship in Drosophila. Nature. 2011;478:236–240. doi: 10.1038/nature10428. [DOI] [PubMed] [Google Scholar]
- Haga-Yamanaka S, Ma L, He J, Qiu Q, Lavis LD, Looger LL, Yu CR. Integrated action of pheromone signals in promoting courtship behavior in male mice. Elife. 2014;2014–3:e03025. doi: 10.7554/eLife.03025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall JC. The mating of a fly. Science. 1994;264:1702–1714. doi: 10.1126/science.8209251. [DOI] [PubMed] [Google Scholar]
- Hallem EA, Carlson JR. Coding of Odors by a Receptor Repertoire. Cell. 2006;125:143–160. doi: 10.1016/j.cell.2006.01.050. [DOI] [PubMed] [Google Scholar]
- Hallem EA, Ho MG, Carlson JR. The molecular basis of odor coding in the Drosophila antenna. Cell. 2004;117:965–979. doi: 10.1016/j.cell.2004.05.012. [DOI] [PubMed] [Google Scholar]
- Hamada FN, Rosenzweig M, Kang K, Pulver SR, Ghezzi A, Jegla TJ, Garrity Pa. An internal thermal sensor controlling temperature preference in Drosophila. Nature. 2008;454:217–220. doi: 10.1038/nature07001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hentze JL, Moeller ME, Jørgensen AF, Bengtsson MS, Bordoy AM, Warren JT, Gilbert LI, Andersen O, Rewitz KF. Accessory Gland as a Site for Prothoracicotropic Hormone Controlled Ecdysone Synthesis in Adult Male Insects. PLoS One. 2013;8:e55131. doi: 10.1371/journal.pone.0055131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ihara S, Yoshikawa K, Touhara K. Chemosensory signals and their receptors in the olfactory neural system. Neuroscience. 2013;254:45–60. doi: 10.1016/j.neuroscience.2013.08.063. [DOI] [PubMed] [Google Scholar]
- Jallon JM. A few chemical words exchanged by Drosophila during courtship and mating. Behav Genet. 1984;14:441–478. doi: 10.1007/BF01065444. [DOI] [PubMed] [Google Scholar]
- Jindra M, Palli SR, Riddiford LM. The juvenile hormone signaling pathway in insect development. Annu Rev Entomol. 2013;58:181–204. doi: 10.1146/annurev-ento-120811-153700. [DOI] [PubMed] [Google Scholar]
- Kimura KI, Ote M, Tazawa T, Yamamoto D. Fruitless specifies sexually dimorphic neural circuitry in the Drosophila brain. Nature. 2005;438:229–233. doi: 10.1038/nature04229. [DOI] [PubMed] [Google Scholar]
- Koh TW, He Z, Gorur-Shandilya S, Menuz K, Larter N, Stewart S, Carlson J. The Drosophila IR20a Clade of Ionotropic Receptors Are Candidate Taste and Pheromone Receptors. Neuron. 2014;83:850–865. doi: 10.1016/j.neuron.2014.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohl J, Huoviala P, Jefferis GSXE. Pheromone processing in Drosophila. Curr Opin Neurobiol. 2015;34:149–157. doi: 10.1016/j.conb.2015.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurtovic A, Widmer A, Dickson BJ. A single class of olfactory neurons mediates behavioural responses to a Drosophila sex pheromone. Nature. 2007;446:542–546. doi: 10.1038/nature05672. [DOI] [PubMed] [Google Scholar]
- Kvelland I. Some observations on the mating activity and fertility of Drosophila melanogaster males. Hereditas. 1965;53:281–306. doi: 10.1111/j.1601-5223.1965.tb01997.x. [DOI] [PubMed] [Google Scholar]
- Lacaille F, Hiroi M, Twele R, Inoshita T, Umemoto D, Manière G, Marion-Poll F, Ozaki M, Francke W, Cobb M, et al. An inhibitory sex pheromone tastes bitter for Drosophila males. PLoS One. 2007;2:e661. doi: 10.1371/journal.pone.0000661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson MC, Domingos AI, Jones WD, Chiappe ME, Amrein H, Vosshall LB. Or83b encodes a broadly expressed odorant receptor essential for Drosophila olfaction. Neuron. 2004;43:703–714. doi: 10.1016/j.neuron.2004.08.019. [DOI] [PubMed] [Google Scholar]
- Liu T, Starostina E, Vijayan V, Pikielny CW. Two Drosophila DEG/ENaC channel subunits have distinct functions in gustatory neurons that activate male courtship. J Neurosci. 2012;32:11879–11889. doi: 10.1523/JNEUROSCI.1376-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lone SR, Venkataraman A, Srivastava M, Potdar S, Sharma VK. Or47b-neurons promote male-mating success in Drosophila. Biol Lett. 2015;11 doi: 10.1098/rsbl.2015.0292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long CE, Markow TA, Yaeger P. Relative male age, fertility, and competitive mating success in Drosophila melanogaster. Behav Genet. 1980;10:163–170. doi: 10.1007/BF01066266. [DOI] [PubMed] [Google Scholar]
- Manoli DS, Foss M, Villella A, Taylor BJ, Hall JC, Baker BS. Male-specific fruitless specifies the neural substrates of Drosophila courtship behaviour. Nature. 2005;436:395–400. doi: 10.1038/nature03859. [DOI] [PubMed] [Google Scholar]
- Markow TA, O’Grady P. Reproductive ecology of Drosophila. Funct Ecol. 2008;22:747–759. [Google Scholar]
- Masuyama K, Zhang Y, Rao Y, Wang JW. Mapping neural circuits with activity-dependent nuclear import of a transcription factor. J Neurogenet. 2012;26:89–102. doi: 10.3109/01677063.2011.642910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto T, Amrein H. Suppression of male courtship by a Drosophila pheromone receptor. Nat Neurosci. 2008;11:874–876. doi: 10.1038/nn.2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon SJ, Lee Y, Jiao Y, Montell C. A Drosophila gustatory receptor essential for aversive taste and inhibiting male-to-male courtship. Curr Biol. 2009;19:1623–1627. doi: 10.1016/j.cub.2009.07.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partridge L, Farquhar M. Sexual activity reduces lifespan of male fruitflies. Nature. 1981;294:580–582. [Google Scholar]
- Pratt GE, Jennings RC, Hamnett AF, Brooks GT. Lethal metabolism of precocene-I to a reactive epoxide by locust corpora allata. Nature. 1980;284:320–323. [Google Scholar]
- De Renobales M, Blomquist GJ. Biosynthesis of medium chain fatty acids in Drosophila melanogaster. Arch Biochem Biophys. 1984;228:407–414. doi: 10.1016/0003-9861(84)90004-3. [DOI] [PubMed] [Google Scholar]
- Roff DA. The evolution of life histories: theory and analysis. 1992;3 [Google Scholar]
- Root CM, Masuyama K, Green DS, Enell LE, Nassel DR, Lee CH, Wang JW. A presynaptic gain control mechanism fine-tunes olfactory behavior. Neuron. 2008;59:311–321. doi: 10.1016/j.neuron.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryner LC, Goodwin SF, Castrillon DH, Anand A, Villella A, Baker BS, Hall JC, Taylor BJ, Wasserman SA. Control of male sexual behavior and sexual orientation in Drosophila by the fruitless gene. Cell. 1996;87:1079–1089. doi: 10.1016/s0092-8674(00)81802-4. [DOI] [PubMed] [Google Scholar]
- Shankar S, Chua JY, Tan KJ, Calvert MEK, Weng R, Ng WC, Mori K, Yew JY. The neuropeptide tachykinin is essential for pheromone detection in a gustatory neural circuit. Elife. 2015;4:e06914. doi: 10.7554/eLife.06914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel RW, Hall JC. Conditioned responses in courtship behavior of normal and mutant Drosophila. Proc Natl Acad Sci U S A. 1979;76:3430–3434. doi: 10.1073/pnas.76.7.3430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starostina E, Liu T, Vijayan V, Zheng Z, Siwicki KK, Pikielny CW. A Drosophila DEG/ENaC subunit functions specifically in gustatory neurons required for male courtship behavior. J Neurosci. 2012;32:4665–4674. doi: 10.1523/JNEUROSCI.6178-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stearns SC. The evolution of life histories. 1992;1 [Google Scholar]
- Stockinger P, Kvitsiani D, Rotkopf S, Tirián L, Dickson BJ. Neural circuitry that governs Drosophila male courtship behavior. Cell. 2005;121:795–807. doi: 10.1016/j.cell.2005.04.026. [DOI] [PubMed] [Google Scholar]
- Su CY, Menuz K, Reisert J, Carlson JR. Non-synaptic inhibition between grouped neurons in an olfactory circuit. Nature. 2012;492:66–71. doi: 10.1038/nature11712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thistle R, Cameron P, Ghorayshi A, Dennison L, Scott K. Contact chemoreceptors mediate male-male repulsion and male-female attraction during Drosophila courtship. Cell. 2012;149:1140–1151. doi: 10.1016/j.cell.2012.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson SN. A review and comparative characterization of the fatty acid compositions of seven insect orders. Comp Biochem Physiol Part B Comp Biochem. 1973;45:467–482. [Google Scholar]
- Toda H, Zhao X, Dickson BJ. The Drosophila female aphrodisiac pheromone activates ppk23(+) sensory neurons to elicit male courtship behavior. Cell Rep. 2012;1:599–607. doi: 10.1016/j.celrep.2012.05.007. [DOI] [PubMed] [Google Scholar]
- Viswanath V, Story GM, Peier AM, Petrus MJ, Lee VM, Hwang SW, Patapoutian A, Jegla T. Opposite thermosensor in fruitfly and mouse. Nature. 2003;423:822–823. doi: 10.1038/423822a. [DOI] [PubMed] [Google Scholar]
- Wang JW, Wong AM, Flores J, Vosshall LB, Axel R. Two-photon calcium imaging reveals an odor-evoked map of activity in the fly brain. Cell. 2003;112:271–282. doi: 10.1016/s0092-8674(03)00004-7. [DOI] [PubMed] [Google Scholar]
- Wang L, Han X, Mehren J, Hiroi M, Billeter JC, Miyamoto T, Amrein H, Levine JD, Anderson DJ. Hierarchical chemosensory regulation of male-male social interactions in Drosophila. Nat Neurosci. 2011;14:757–762. doi: 10.1038/nn.2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe K, Toba G, Koganezawa M, Yamamoto D. Gr39a, a Highly diversified gustatory receptor in drosophila, has a role in sexual behavior. Behav Genet. 2011;41:746–753. doi: 10.1007/s10519-011-9461-6. [DOI] [PubMed] [Google Scholar]
- Wicker-Thomas C, Chertemps T. Molecular biology and genetics of hydrocarbon production. Insect Hydrocarbons: Biology, Biochemistry and Chemical Ecology. 2010:53–74. [Google Scholar]
- Wilson TG, DeMoor S, Lei J. Juvenile hormone involvement in Drosophila melanogaster male reproduction as suggested by the Methoprene-tolerant27 mutant phenotype. Insect Biochem Mol Biol. 2003;33:1167–1175. doi: 10.1016/j.ibmb.2003.06.007. [DOI] [PubMed] [Google Scholar]
- Wilson TG, Fabian J. A Drosophila melanogaster mutant resistant to a chemical analog of juvenile hormone. Dev Biol. 1986;118:190–201. doi: 10.1016/0012-1606(86)90087-4. [DOI] [PubMed] [Google Scholar]
- Yamamoto D, Koganezawa M. Genes and circuits of courtship behaviour in Drosophila males. Nat Rev Neurosci. 2013;14:681–692. doi: 10.1038/nrn3567. [DOI] [PubMed] [Google Scholar]
- Yang CF, Shah NM. Representing sex in the brain, one module at a time. Neuron. 2014;82:261–278. doi: 10.1016/j.neuron.2014.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.