Abstract
BACKGROUND
The optimal treatment for patients with brain metastases remains controversial as the use of stereotactic radiosurgery (SRS) alone has increased, replacing whole brain radiation therapy (WBRT). We determined the patterns of care at multiple institutions before 2010 and examined whether or not survival was different between patients treated with SRS versus WBRT.
METHODS
We examined the overall survival of patients treated with radiation therapy for brain metastases from non-small cell lung cancer (NSCLC) (initially diagnosed 2007–2009) or breast cancer (initially diagnosed 1997–2009) in five centers. We performed propensity score analyses to adjust for confounding factors, such as the number of metastases, extent of extracranial metastases, and treatment center.
RESULTS
Overall, 27.8% of 400 NSCLC patients and 13.4% of 387 breast cancer patients received SRS alone for treatment of brain metastases. Few patients with > 3 brain metastases or ≥ 4 cm lesions received SRS. Patients with < 4 brain metastases, < 4 cm in size (n = 189 for NSCLC; n = 117 for breast cancer) treated with SRS had longer survival (NSCLC: adjusted hazard ratio [HR], 0.58, 95% Confidence Interval [CI] 0.38 to 0.87, p = 0.01; breast cancer: adjusted HR 0.54, 95% CI 0.33 to 0.91, p = 0.02) than those treated with WBRT.
CONCLUSIONS
Patients treated for < 4 brain metastases from NSCLC or breast cancer with SRS alone had longer survival than those treated with WBRT in this multi-institutional retrospective study, even after adjustment for the propensity to receive SRS.
Keywords: Comparative effectiveness research, radiosurgery, neoplasm metastasis, breast neoplasms, carcinoma, non-small-cell lung
Condensed Abstract
We performed propensity score analyses utilizing data from a multi-institutional longitudinal database of patients treated for brain metastases from non-small cell lung cancer or breast cancer. We found that patients treated for < 4 brain metastases with stereotactic radiosurgery alone had improved survival.
INTRODUCTION
It is estimated that there are approximately 170,000 new patients diagnosed with brain metastases in the United States each year.1 The prognosis of patients with untreated brain metastases is poor, with a median survival of one month.2 In the mid-1900s, the median survival improved to four to six months with the introduction of whole brain radiation therapy (WBRT)3 which quickly became the standard treatment for brain metastases. However with technological advancement, surgical resection and stereotactic radiosurgery (SRS) have become options for the treatment of brain metastases. SRS is a specialized radiation technique that delivers highly collimated radiation to a precisely defined target while minimizing the dose received by surrounding tissue.
While WBRT targets known brain metastases as well as microscopic disease throughout the brain, SRS targets only the brain metastases detected on imaging. Advantages of WBRT include decreased likelihood of development of additional brain metastases, lower cost, and greater availability. Advantages of SRS alone include avoidance of neurocognitive side effects associated with WBRT4 and a shorter treatment course.
Multiple randomized trials have shown that for patients with one to four brain metastases treated with SRS, the addition of WBRT does not add survival benefit.4–6 Use of SRS for brain metastases is variable across regions and centers in the United States7 and overall management remains controversial with no randomized trials comparing SRS alone versus WBRT alone.
In this study, we used the National Comprehensive Cancer Network (NCCN) Oncology Outcomes Database for non-small cell lung cancer (NSCLC) and breast cancer first to determine the treatment patterns for brain metastases at multiple centers. We then compared the overall survival following the two most frequent strategies for patients with < 4 brain metastases, SRS versus WBRT.
METHODS
Data source
The NCCN Oncology Outcomes Database collected comprehensive clinical, histopathologic, treatment, and disease-status information on all patients treated at participating NCCN institutions for NSCLC between 2007–2009, and for breast cancer between 1997–2009.8, 9 Patients included in this study received their primary cancer care at one of five institutions: Arthur G. James Cancer Hospital at the Ohio State University (Columbus, OH), City of Hope Comprehensive Cancer Center (Duarte, CA), Dana Farber Cancer Institute (Boston, MA), University of Texas M.D. Anderson Cancer Center (Houston, TX) and Roswell Park Cancer Institute (Buffalo, NY).
The patients' individual medical records were abstracted by trained data managers at each site. Data were abstracted for breast cancer patients at first visit; 4, 9, and 18 months; and then annually. For NSCLC patients, data were abstracted at first visit; 6, 12, 18, and 24 months; and then annually. Reliability and validity of the abstracted data were assessed through several processes, including real-time logic checks, monthly training teleconferences, quarterly quality assurance testing, biannual in-person training, and annual data audits wherein abstracted data are compared with source documentation. The study data collection process, data transmission methods, and data storage protocols were approved by the institutional review boards at each institution. At City of Hope and Dana Farber Cancer Institute, the institutional review board required signed informed consent for data collection and only patients who provided written consent were included.
Data collected included patient characteristics, disease characteristics at initial cancer diagnosis, socioeconomic characteristics, and treatments. The date of diagnosis with brain metastasis was determined by the date of the imaging providing the diagnosis. Number of brain metastases and size of the largest lesion was determined by radiology report. Number of body sites with extracranial metastases was calculated as a proxy for systemic disease burden. Comorbidity at presentation to the center was assigned using either the Charlson Index10 determined by chart review or the modified version of this index using a patient survey developed by Katz.11 The zip code of the patient’s residence was linked to 2000 Census data to estimate the median household income (U.S. Census 2000, Summary File 3).
Cohorts
From 2,880 potentially eligible NSCLC patients, we identified 672 patients with diagnosis of brain metastases without leptomeningeal disease. Patients were excluded if a radiology report confirming the diagnosis of brain metastases was not available or there was < 60 days of follow-up. Patients who underwent surgical resection were excluded to avoid selection bias since during the study time period, SRS to the tumor bed was uncommon. This resulted in 413 patients who had radiation therapy as initial treatment, defined as within 60 days of brain metastasis diagnosis. This was chosen as a reasonable time period for receiving initial treatment for brain metastases. Since few patients received both WBRT and SRS within 60 days, these 13 patients were excluded. (Supplemental Figure). For diagnosis, 365/400 patients (91.3%) underwent brain MRI. If a patient did not have a brain MRI, a head CT was the source of diagnosis.
From 24,295 potentially eligible breast cancer patients, we identified 614 (2.5%) patients with brain metastases without leptomeningeal disease. Patients were excluded if the radiology report was not available, there was < 60 days of follow-up, or they had undergone surgical resection initially. This resulted in 419 patients who had radiation therapy as initial treatment. For 21 (5.0%) of these patients, the type of radiation therapy was not known. Eleven patients (2.8%) were excluded as they received both WBRT and SRS (Supplemental Figure). For diagnosis, 279 (72.1%) out of 387 patients underwent brain MRI.
Statistical analysis
Univariate determination of factors associated with receiving SRS versus WBRT was performed with chi-square analyses. Based on these results and a priori clinical hypothesis that candidates for SRS during this time period were patients with < 4 brain metastases that were < 4 cm, we performed further analyses within this subgroup. We used a propensity-score approach to account for differences in patient characteristics to estimate the effect of receiving SRS on overall survival versus WBRT.
The propensity score model included all available variables potentially associated with either survival or treatment received.12 A stepwise variable selection procedure was used to determine potential associations with the criterion of exclusion of p > 0.20. We grouped the patients into 5 strata, determined by the propensity score. We then performed stratified Cox regression analyses to estimate the effect of SRS on survival. We also applied a standardized mortality ratio (SMR) propensity-score weight that equaled 1 for WBRT patients and the propensity odds [p ÷ (1 − p)] for SRS patients. The SMR method estimates the average improvement in survival with SRS across the WBRT population.
Sensitivity Analyses
Because propensity score analyses can control only for observed characteristics, we examined the robustness of estimated SRS improvement to unobserved confounders.13 We considered the potential unmeasured confounder of poor performance status at diagnosis with brain metastases. Though performance status was available for patients at the initial diagnosis with primary cancer, it was not at diagnosis with brain metastasis. We estimated that the number of patients with Karnofsky Performance Status (KPS) < 70 was two to three times greater in the WBRT cohort compared to the SRS cohort based on a subset analysis of 230 NSCLC patients with synchronous diagnosis of brain metastasis with known performance status where patients treated with WBRT were 2.3 times more likely to have ECOG performance status ≥ 2. We estimated that the hazard ratio for overall survival associated with KPS < 70 was 2.13 based on the Radiation Therapy Oncology Group (RTOG) recursive partitioning analysis classification14,15.
RESULTS
Predictors of receiving SRS in the full cohort
Overall, 111/400 (27.8%) patients with metastatic NSCLC (2007–2009) and 52/ 387 (13.4%) patients with metastatic breast cancer (1997–2009) received SRS. Patients who received SRS were significantly more likely to have fewer brain metastases, size of the largest metastasis < 4 cm, and fewer sites of extracranial metastases compared to those who received WBRT. Among NSCLC patients, the specific NCCN institution was significantly associated with SRS use, ranging from 10.7% to 40.2%. Among breast cancer patients, SRS was more common in the later years compared to the earlier years (Table 1).
Table 1.
Significant predictors of receiving stereotactic radiosurgery(SRS) versus whole brain radiation therapy(WBRT) from univariate analysis on entire study cohort.
| Non small cell lung cancer N=400 |
Breast cancer N=387 |
|||||
|---|---|---|---|---|---|---|
| Demographic or clinical characteristic |
SRS N(%) |
WBRT N(%) |
p- value |
SRS N(%) |
WBRT N(%) |
p- value |
| N | 111 | 289 | 52 | 335 | ||
| Clinical characteristics: brain metastases | ||||||
| Number of brain metastases |
<0.001 | <0.001 | ||||
| 1 | 69(62.2) | 54(19.0) | 23(46.0) | 46(14.6) | ||
| 2 – 3 | 35(31.5) | 68(23.9) | 24(48.0) | 72(22.9) | ||
| ≥ 4 | 7(6.3) | 162(57.0) | 3(6.0) | 196(62.4) | ||
| Unknown | 0 | 5 | 2 | 21 | ||
| Size of largest brain metastases |
<0.001 | 0.007 | ||||
| < 4 cm | 96(86.5) | 220(76.1) | 44(84.6) | 211(63.0) | ||
| ≥ 4 cm | 1(0.9) | 14(4.8) | 1(1.9) | 37(11.0) | ||
| Unknown | 14(12.6) | 55(19.0) | 7(13.5) | 87(26.0) | ||
| Number of sites of extracranial metastases |
0.02 | 0.03 | ||||
| 0 | 51(45.9) | 93(32.2) | 11(21.2) | 42(12.5) | ||
| 1 | 27(24.3) | 76(26.3) | 15(28.8) | 63(18.8) | ||
| 2 | 22(19.8) | 57(19.7) | 13(25.0) | 80(23.9) | ||
| ≥ 3 | 11(9.9) | 63(21.8) | 13(25.0) | 150(44.8) | ||
| Institutional characteristics | ||||||
| Year of diagnosis with brain metastases (lung) |
0.26 | |||||
| 2006–2007 | 35(31.5) | 110(38.1) | ||||
| 2008 | 51(45.9) | 132(45.7) | ||||
| 2009–2010 | 25(22.5) | 47(16.3) | ||||
| Year of diagnosis with brain metastases (breast) |
0.02 | |||||
| 1998–2003 | 10(19.2) | 130(38.8) | ||||
| 2004–2006 | 23(44.2) | 125(37.3) | ||||
| 2007–2009 | 19(36.5) | 80(23.9) | ||||
| Institution | <0.001 | 0.56 | ||||
| 1 | 12(10.8) | 20(6.9) | 3(5.8) | 26(7.8) | ||
| 2 | 8(7.2) | 67(23.2) | 11(21.2) | 74(22.1) | ||
| 3 | 36(32.4) | 103(35.6) | 24(46.2) | 138(41.2) | ||
| 4 | 47(42.3) | 70(24.2) | 8(15.4) | 34(10.1) | ||
| 5 | 8(7.2) | 29(10.0) | 6(11.5) | 63(18.8) | ||
Age, gender, performance status at initial diagnosis with brain metastases, comorbidity score at initial diagnosis, previous chemotherapy, health insurance status, median household income in zip code of residence, race, stage at initial cancer diagnosis, brain metastases at initial cancer diagnosis, and gender were not significant predictors.
Characteristics of the subset population used in comparative effectiveness analysis
Among the subset of 189 NSCLC patients with <4 brain metastases that were < 4 cm, 90 (47.6%) received SRS as opposed to WBRT. Only the specific NCCN institution and fewer brain metastases remained significant predictors for receiving SRS in this subgroup. Among the subset of 117 breast cancer patients with < 4 brain metastases that were < 4 cm, 42 (35.9%) received SRS as opposed to WBRT. Only smaller size of largest brain metastasis remained a significant predictor for receiving SRS in this subgroup (Table 2).
Table 2.
Demographic and clinic characteristics for cohorts with < 4 brain metastases and size of largest brain metastasis < 4 cm.
| Non-small cell lung cancer N=189 |
Breast cancer N=117 |
|||||
|---|---|---|---|---|---|---|
| Demographic or clinical characteristic |
SRS N(%) |
WBRT N(%) |
p- value |
SRS N(%) |
WBRT N(%) |
p- value |
| N | 90 | 99 | 42 | 75 | ||
| Patient characteristics | ||||||
| Age (NSCLC) | 0.32 | N/A | ||||
| < 50 years | 10(11.1) | 19(19.2) | ||||
| 50–59 years | 28(31.1) | 34(34.3) | ||||
| 60–69 years | 26(28.9) | 25(25.3) | ||||
| ≥ 70 years | 26(28.9) | 21(21.2) | ||||
| Age (breast) | N/A | 0.94 | ||||
| < 40 | 5(11.9) | 9(12.0) | ||||
| 40–49 | 8(19.0) | 16(21.3) | ||||
| 50–59 | 15(35.7) | 29(38.7) | ||||
| ≥60 | 14(33.3) | 21(28.0) | ||||
| Gender | 0.77 | N/A | ||||
| Female | 43(47.8) | 45(45.5) | ||||
| Male | 47(52.2) | 54(54.5) | ||||
| Eastern Cooperative Oncology Group performance status at initial cancer diagnosis |
0.12 | 0.74 | ||||
| 0 | 31(34.4) | 27(27.3) | 25(59.5) | 50(66.7) | ||
| ≥1 | 46(51.1) | 46(46.5) | 12(28.6) | 18(24.0) | ||
| Unknown | 13(14.4) | 26(26.3) | 5(11.9) | 7(9.3) | ||
| Comorbidity score at initial cancer diagnosis |
0.33 | 0.41 | ||||
| 0 | 53(58.9) | 68(68.7) | 34(81.0) | 62(82.7) | ||
| 1 | 24(26.7) | 22(22.2) | 6(14.3) | 6(8.0) | ||
| ≥2 | 13(14.4) | 9(9.1) | 2(4.8) | 7(9.3) | ||
| Health insurance status | 0.80 | 0.59 | ||||
| Medicare | 40(44.4) | 41(41.8) | 8(19.0) | 11(14.7) | ||
| Managed | 46(51.1) | 54(55.1) | 28(66.7) | 48(64.0) | ||
| Other | 4(4.4) | 3(3.1) | 6(14.3) | 16(21.3) | ||
| Unknown | 0 | 1 | 0 | 0 | ||
| Employment status | N/A | 0.35 | ||||
| Employed/student | 18(42.9) | 32(42.7) | ||||
| Homemaker | 3(7.1) | 12(16.0) | ||||
| Others/unknown | 21(50.0) | 31(41.3) | ||||
| Median household income in zip code of residence |
0.28 | 0.77 | ||||
| < $30,000 | 15(17.4) | 11(11.6) | 4(10.0) | 12(16.4) | ||
| $30,000–$44,999 | 37(43.0) | 37(38.9) | 13(32.5) | 19(26.0) | ||
| $45,000–$59,999 | 20(23.3) | 34(35.8) | 12(30.0) | 22(30.1) | ||
| ≥ $60,000 | 14(16.3) | 13(13.7) | 11(27.5) | 20(27.4) | ||
| Unknown | 4 | 4 | 2 | 2 | ||
| Race | 1.00 | 0.39 | ||||
| Non-white | 16(17.8) | 18(18.2) | 9(21.4) | 23(30.7) | ||
| White | 74(82.2) | 81(81.8) | 33(78.6) | 52(69.3) | ||
| Current or past smoker | 0.85 | N/A | ||||
| No | 14(15.6) | 17(17.2) | ||||
| Yes | 76(84.4) | 82(82.8) | ||||
| Clinical characteristics: primary cancer | ||||||
| Stage at initial diagnosis | 1.00 | 0.31 | ||||
| I | 1(1.1) | 1(1.0) | 4(9.5) | 5(6.8) | ||
| II | 3(3.3) | 3(3.0) | 10(23.8) | 28(38.4) | ||
| III | 12(13.3) | 14(14.1) | 18(42.9) | 21(28.8) | ||
| IV | 74(82.2) | 81(81.8) | 10(23.8) | 19(26.0) | ||
| Unknown | 0 | 0 | 0 | 2 | ||
| Triggering event for initial diagnosis with cancer |
0.10 | N/A | ||||
| Signs or symptoms of disease |
73(81.1) | 89(89.9) | ||||
| Incidental finding | 17(18.9) | 10(10.1) | ||||
| Histologic grade | 0.38 | N/A | ||||
| 1–2 | 29(32.2) | 41(41.4) | ||||
| 3–4 | 16(17.8) | 13(13.1) | ||||
| Unknown | 45(50.0) | 45(45.5) | ||||
| Histology | 0.51 | N/A | ||||
| Squamous cell carcinoma | 12(13.3) | 14(14.1) | ||||
| Adenocarcinoma | 57(63.3) | 55(55.6) | ||||
| Other | 21(23.3) | 30(30.3) | ||||
| Hormone receptor | N/A | 0.28 | ||||
| Negative | 18(45.0) | 42(57.5) | ||||
| Positive | 22(55.0) | 31(42.5) | ||||
| Unknown | 2 | 2 | ||||
| HER2neu | N/A | 0.75 | ||||
| Negative | 20(47.6) | 41(54.7) | ||||
| Positive | 16(38.1) | 24(32.0) | ||||
| Unknown | 6(14.3) | 10(13.3) | ||||
| Clinical characteristics: brain metastases | ||||||
| Number of brain metastases | 0.001 | 0.11 | ||||
| 1 | 62(68.9) | 45(45.5) | 20(47.6) | 24(32.0) | ||
| 2–3 | 28(31.1) | 54(54.5) | 22(52.4) | 51(68.0) | ||
| Size of largest brain metastasis (cm) |
0.09 | 0.02 | ||||
| Median (Q1, Q3) | 1.2 (0.9,1.6) |
1.5 (0.7,2.2) |
1.2 (0.9,2.0) |
1.9 (1.1,2.5) |
||
| Brain metastases at initial diagnosis with cancer |
0.42 | 1.00 | ||||
| No | 23(25.6) | 31(31.3) | 41(97.6) | 73(97.3) | ||
| Yes | 67(74.4) | 68(68.7) | 1(2.4) | 2(2.7) | ||
| Number of sites of extracranial metastases |
0.10 | 0.55 | ||||
| 0 | 45(50.0) | 34(34.3) | 8(19.0) | 16(21.3) | ||
| 1 | 21(23.3) | 25(25.3) | 14(33.3) | 16(21.3) | ||
| 2 | 16(17.8) | 22(22.2) | 11(26.2) | 22(29.3) | ||
| ≥3 | 8(8.9) | 18(18.2) | 9(21.4) | 21(28.0) | ||
| Previous chemotherapy | 0.39 | 0.85 | ||||
| Yes | 72(80.0) | 75(73.7) | 17(40.5) | 32(42.7) | ||
| No | 18(20.0) | 26(26.3) | 25(59.5) | 43(57.3) | ||
| Institutional characteristics | ||||||
| Year of diagnosis with brain metastases (lung) |
0.64 | N/A | ||||
| 2006–2007 | 28(31.1) | 35(35.4) | 0.64 | |||
| 2008 | 43(47.8) | 48(48.5) | ||||
| 2009–2010 | 19(32.2) | 16(16.2) | ||||
| Year of diagnosis with brain metastases (breast) |
N/A | 0.09 | ||||
| 1998–2003 | 9(21.4) | 30(40.0) | ||||
| 2004–2006 | 19(45.2) | 22(29.3) | ||||
| 2007–2009 | 14(33.3) | 23(30.7) | ||||
| Institution | <0.001 | 0.05 | ||||
| 1 | 8(8.9) | 4(4.0) | 2(4.8) | 6(8.0) | ||
| 2 | 6(6.7) | 37(37.4) | 9(21.4) | 23(30.7) | ||
| 3 | 34(37.8) | 29(29.3) | 21(50.0) | 20(26.7) | ||
| 4 | 35(38.9) | 17(17.2) | 6(14.3) | 7(9.3) | ||
| 5 | 7(7.8) | 12(12.1) | 4(9.5) | 19(25.3) | ||
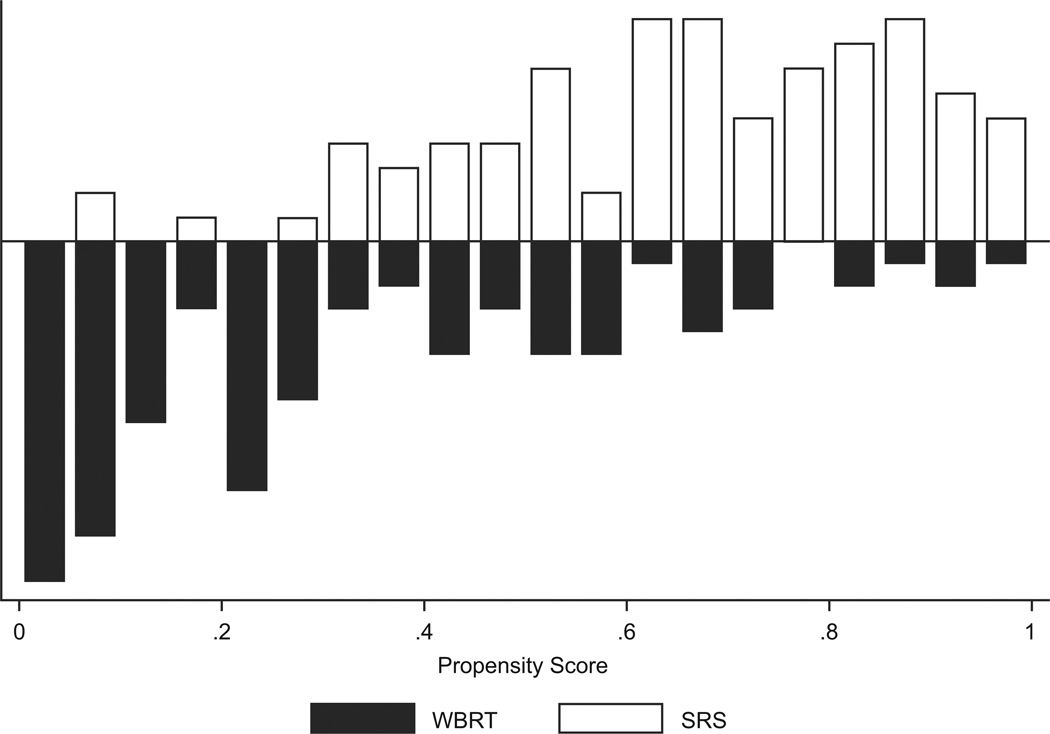
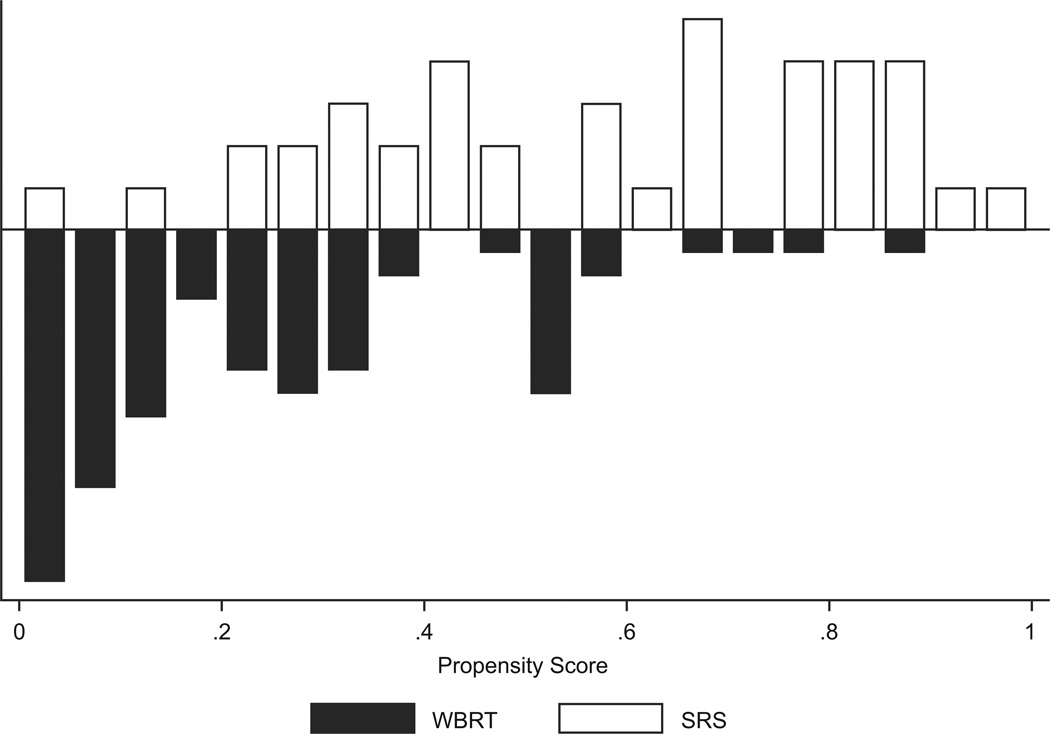
As expected, patients in the WBRT group had a lower propensity for receiving SRS than those in the SRS group. Figure 1 displays the distribution among the cohorts of the propensity score, which is the probability given baseline variables that any patient in either group would be selected for SRS.
Figure 1.
Distribution of propensity score by treatment, whole brain radiation therapy (WBRT) versus stereotactic radiosurgery (SRS) in patients with brain metastases from (a) non-small cell lung cancer (n = 180) and (b) breast cancer (n = 112). The black bars represent the number of WBRT patients and the grey bars represent the number of SRS patients. The propensity score for SRS is the probability given baseline variables that any patient in either group would be selected for SRS.
Outcomes of subset population used in comparative effectiveness analysis
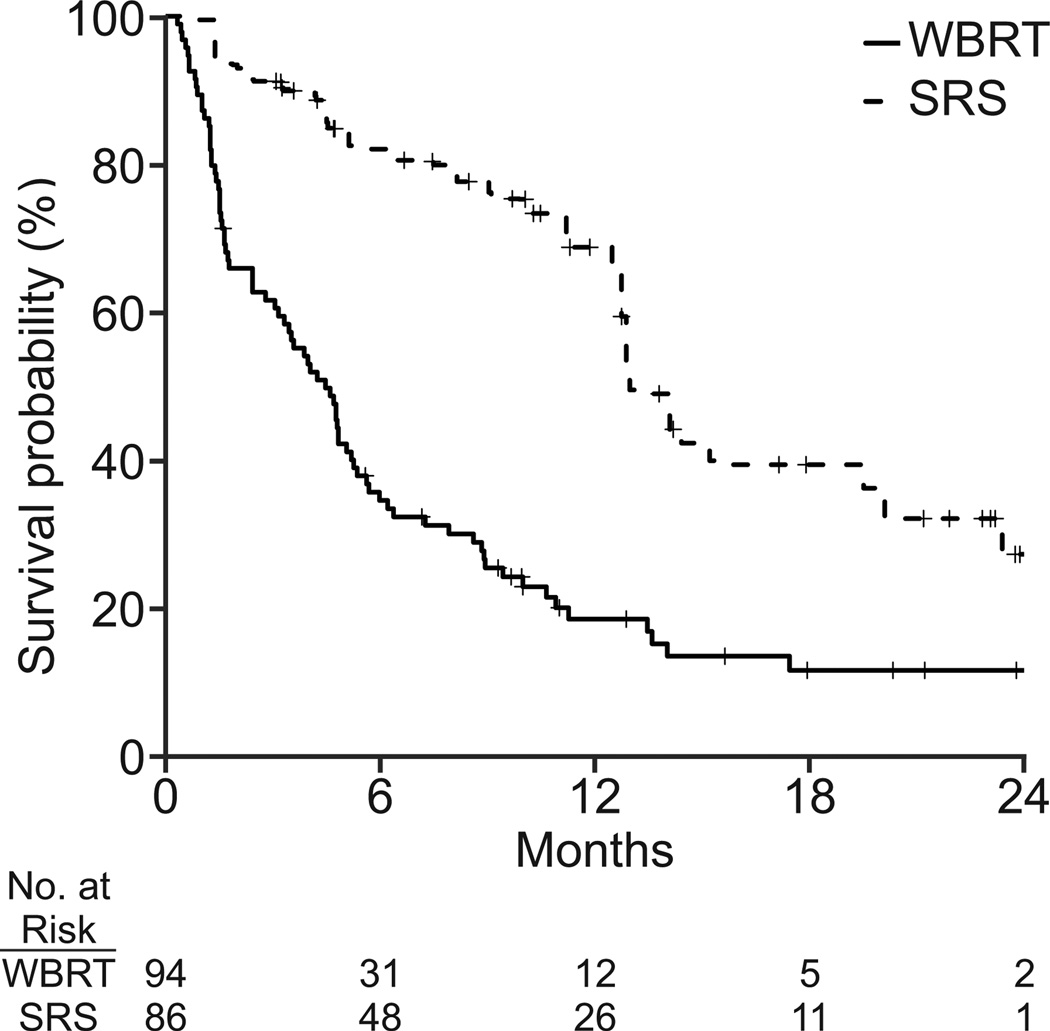
NSCLC and breast cancer patients with < 4 brain metastases that were < 4 cm, had a median overall survival of 5.8 and 8.8 months, respectively. At last follow up, 140/189 (74%) NSCLC patients and 115/117 (98%) breast cancer patients had died.
Patients with < 4 brain metastases < 4 cm in size, who were treated with SRS, had better survival than those treated with WBRT in both the NSCLC (adjusted HR, 0.58; 95% CI, 0.38 to 0.87; p = 0.01) and breast cancer (adjusted HR, 0.54; 95% CI, 0.33 to 0.91; p=0.02) cohorts (Table 3). In the NSCLC cohort, age, brain metastasis at initial diagnosis, prior chemotherapy, performance status at initial diagnosis, smoking history and extracranial disease extent were significantly associated with survival. In the breast cancer cohort, number of brain metastases, race, insurance type, year of brain metastasis diagnosis, and employment were significantly associated with survival.
Table 3.
Unadjusted and propensity model-adjusted survival outcomes for stereotactic radiosurgery versus whole brain radiation therapy for cohorts with < 4 brain metastases and size of largest brain metastasis < 4 cm.
| Non-small cell lung cancer | Breast cancer | |||||||
|---|---|---|---|---|---|---|---|---|
| N | Hazard Ratio |
95% CI | p- value |
N | Hazard Ratio |
95% CI | p- value |
|
| Unadjusted | 189 | 0.53 | 0.38,0.74 | <0.001 | 117 | 0.70 | 0.47,1.03 | 0.07 |
| Adjusted by propensity score model |
180 | 0.58 | 0.38,0.87 | 0.01 | 112 | 0.54 | 0.33,0.91 | 0.02 |
| Adjusted by standardized mortality ratio propensity weighting |
180 | 0.26 | 0.20,0.34 | <0.001 | 112 | 0.61 | 0.45,0.81 | <0.00 1 |
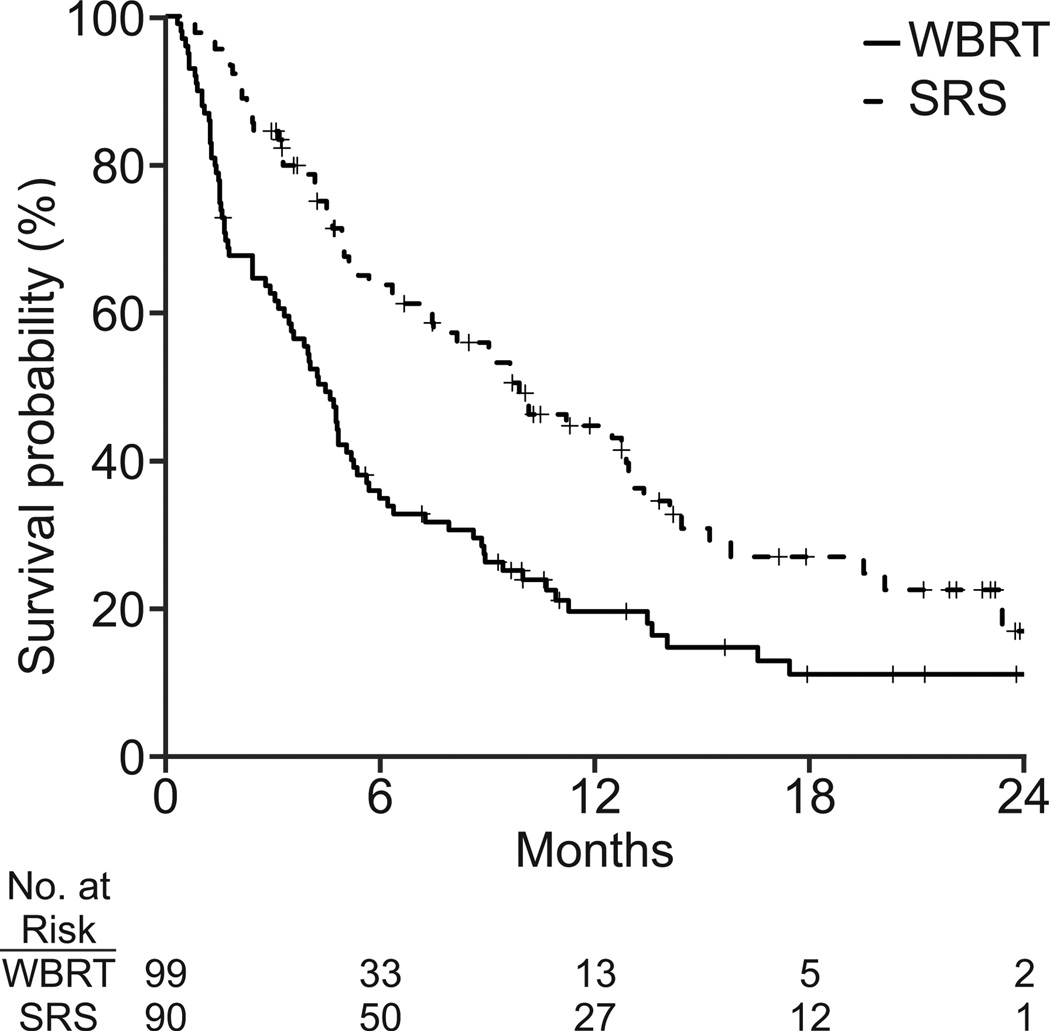
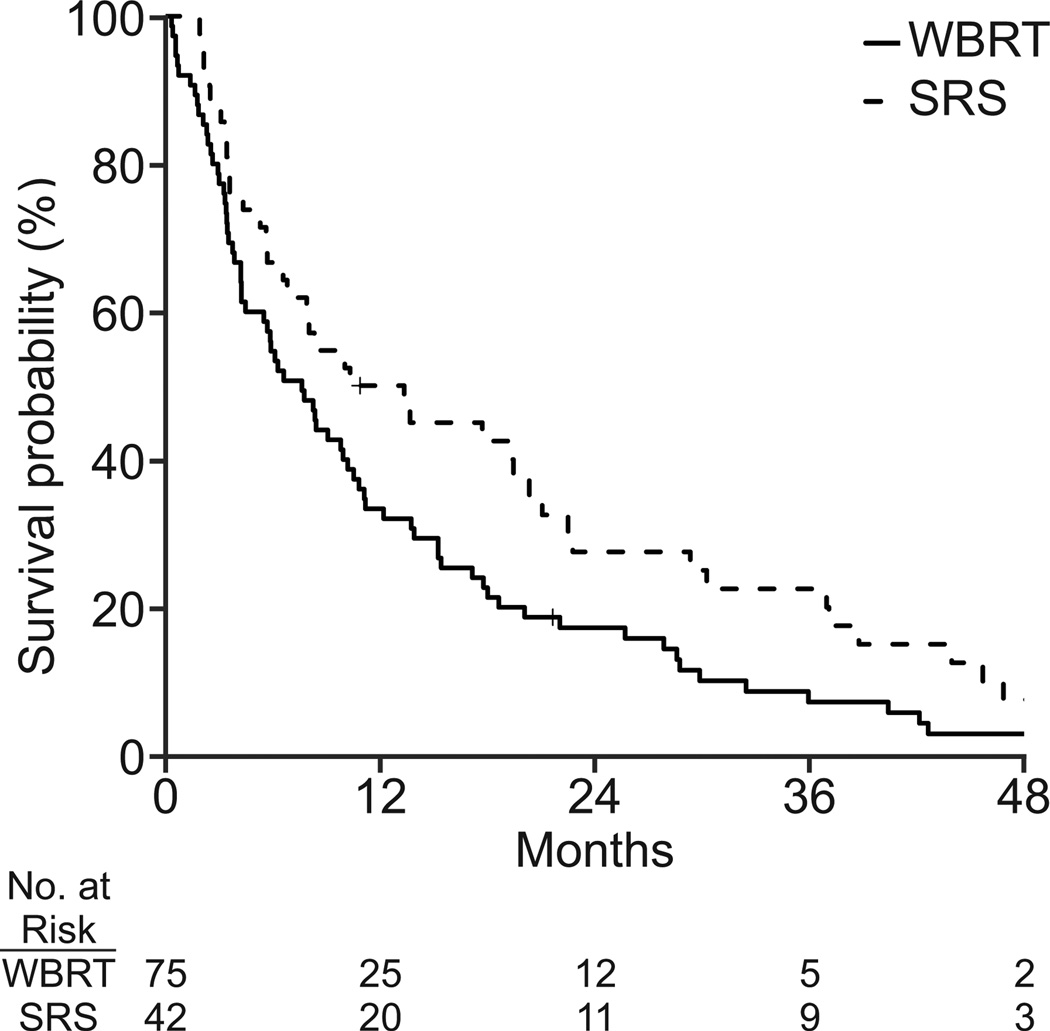
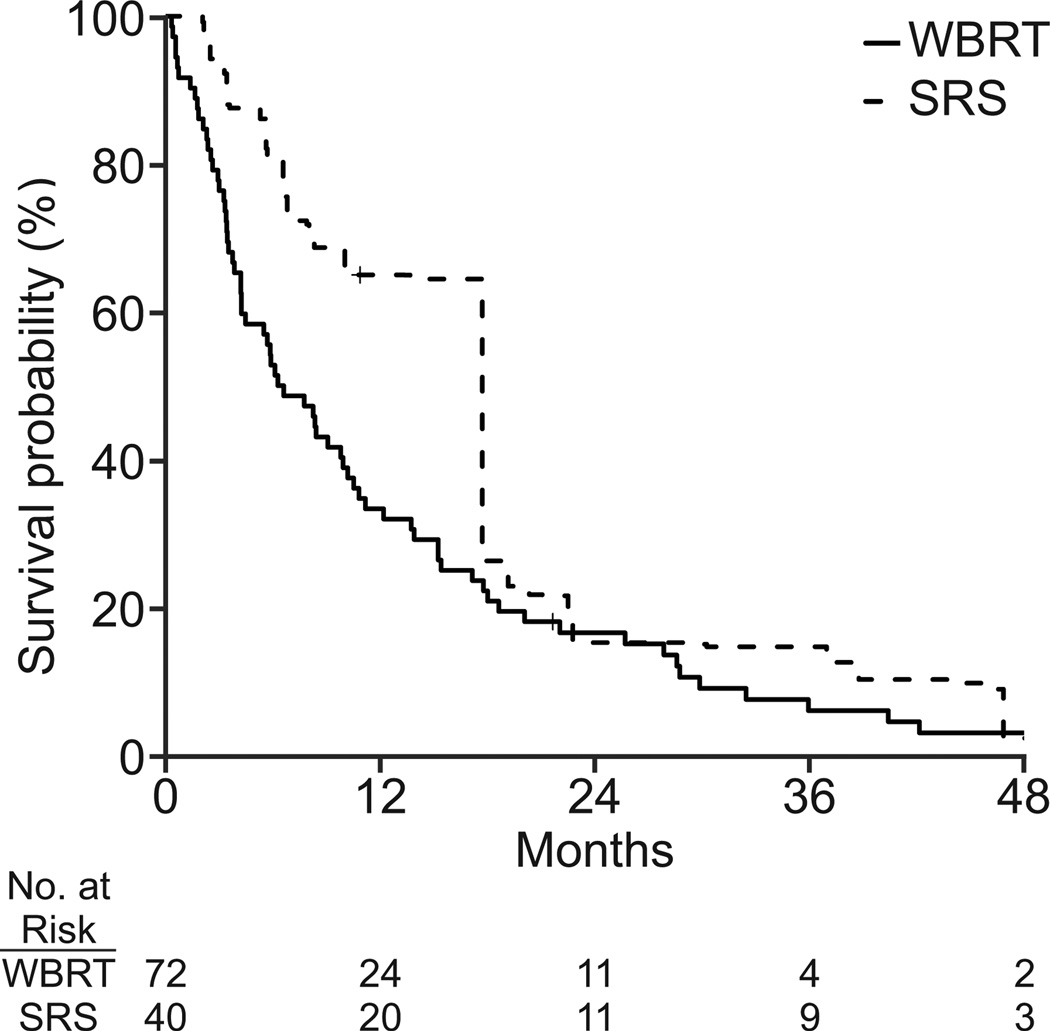
Additional analyses performed using standardized mortality ratios also showed that SRS improved survival for the average WBRT patient (Figure 2). The estimated treatment effect of SRS on overall survival using this technique was found in both the NSCLC (adjusted HR, 0.26; 95% CI: 0.20, 0.34; p<0.001) and breast cancer (adjusted HR, 0.61; 95% CI: 0.45, 0.81; p<0.001) cohorts.
Figure 2.
Unadjusted survival curves for (a) non-small cell lung cancer (n = 189) and (b) breast cancer (N=117). Survival curves adjusted by standardized mortality ratio for (c) non-small cell lung cancer (N=180) and (d) breast cancer (N=112).
Sensitivity analyses to evaluate whether unmeasured variables such as performance status which could confound survival differences between the two groups revealed our results to be robust. Under the assumption that patients receiving WBRT were three times more likely to have a KPS < 70 and that these patients would have increased hazard of dying of 2.13, we found that treatment with SRS was still associated with a hazard ratio of 0.56 (95% CI: 0.36, 0.88, p =0.01) in the NSCLC cohort, and 0.54 (95% CI: 0.32, 0.92, p =0.02) in the breast cancer cohort.
Salvage treatment
Of the 90 NSCLC patients who received SRS, 12 underwent salvage SRS after a median 4.3 months (range, 1.0–8.4 months) and 11 underwent salvage WBRT after a median 7.5 months (range, 1.8–30.4 months). Of the 12 patients who received salvage SRS, 5 later underwent WBRT. Of the 42 breast cancer patients who received SRS, 9 underwent salvage SRS after a median 7.5 months (range, 1.5–20.0 months) and 15 underwent salvage WBRT after a median 9.0 months (range, 1.7–37.8 months). Of the 9 patients who received salvage SRS, 6 later underwent WBRT.
DISCUSSION
In this study analyzing a multi-institutional, longitudinal database of patients with brain metastases, we found improved survival among patients who had < 4 brain metastases that were < 4 cm and were treated with SRS versus WBRT. This advantage persisted when accounting for the propensity score, the probability that any patient in either group would be selected for SRS based on patient, clinical, and institutional variables. These included significant predictors of receiving SRS, such as the number of brain metastases, size of the largest brain metastasis, extent of extracranial metastases, year of diagnosis with brain metastases and particular NCCN institution.
A strength of this study is the use of a prospectively collected longitudinal database, which evaluates outcomes of actual practice patterns rather than clinical trials that enroll a select group of patients. We first determined the treatment patterns for all patients treated initially with radiation therapy. Surprisingly we found that though RTOG 9508 provided randomized data for improved survival with the addition of SRS to WBRT for patients with a single brain metastasis,16 we found that few patients in NCCN centers received combined treatment. Thus, we concentrated on those who received WBRT or SRS alone.
Several interesting conclusions can be made from our initial patterns of care analysis presented in Table1. Multivariate analysis from RTOG 9508 showed that patients with age≤65, KPS≥70, controlled primary and no extracranial metastases benefited from SRS. Correspondingly, we found that patients were more likely to receive SRS if they had fewer brain metastases, largest brain metastases < 4 cm, and fewer sites of extracranial metastases. However, we did not find that younger age was associated with receiving SRS, supporting the hypothesis that clinicians chose SRS to avoid neurocognitive side effects of WBRT that may be especially impactful for the elderly. We detected an increase in the use of SRS for breast cancer patients after 2004, corresponding with the publication of RTOG 9508. The lack of correlation between year and treatment choice in the NSCLC cohort likely represents the shorter time period this database spanned.
Importantly, one of the main determinants of treatment was the particular NCCN institution for patients in the NSCLC cohort. This factor was not significant in the full breast cohort, but was nearly significant in the subset with < 4 brain metastases < 4 cm (p=0.05). This discrepancy could be explained by the long time period (1998–2009) when practice patterns could change within a given institution given the introduction of technology or new clinicians.
Next, we analyzed the subgroup with < 4 brain metastases < 4 cm in size since few patients outside this group received SRS and thus we concluded were not candidates for either treatment approach during the study period. As shown in table 2, the comparator groups were well-matched for all covariates aside from number of brain metastases, size of largest brain metastasis, and institution. Interestingly, other clinical factors associated with prognosis, such as age, grade and histology (for NSCLC), hormone receptor and Her2neu status (for breast cancer), and number of extracranial metastases were not predictive of the treatment choice, perhaps highlighting that providers consider factors other than prognosis when recommending treatment.
We chose the propensity score method for comparing the two groups for several reasons. First, the diversity in practice patterns we found across institutions allowed for fairly balanced groups in terms of clinical factors. In addition, given the relatively small number of events for the final analysis, the regression approach is inadequate for eliminating selection bias. The propensity score approach does not have limitations regarding the number of confounding factors used for adjusting.
Though a prospective longitudinal database may collect more “real-world” outcomes, it does have limitations regarding the available data. For instance, we were not able to record performance status at brain metastasis diagnosis. Although adjustment with propensity score strata resulted in balanced treatment groups, the potential remains for unmeasured confounders, such as performance status or anatomical location of the brain metastasis, to have influenced the results. Thus, we performed sensitivity analysis and showed that even if those in the WBRT group were three times more likely to have poor performance status, our results remained significant. It is conceivable that a single powerful variable could account for the survival difference, but other confounders could also increase the difference.
Our main finding that SRS is associated with improved survival is consistent with the recently published meta-analysis of individual patient data from phase 3 trials, which showed a survival benefit for patients ≤50 years of age who received SRS alone versus those who received WBRT and SRS.17 However, it should also be noted that we compared slightly different cohorts, SRS alone versus WBRT alone, because these were the most common treatment regimens. Though treatment with SRS and WBRT could be similar to WBRT alone, we cannot be certain. It may be that better local control associated with SRS improves survival or the side effects of WBRT are detrimental. We cannot discern this as we do not have data on local control nor cause of death. Alternatively, treatment with SRS may allow for patients to start chemotherapy earlier, improving survival.
The generalizability of our findings is limited in that all patients were treated at large specialized cancer centers and may not be reflective of clinical practice or patient characteristics in community practices. In addition, propensity score matching analysis excludes many to account for selection bias so that our findings pertain only to patients without surgical resection and with < 4 brain metastases. Finally, it should be noted that while we are interested in overall survival as an outcome, the advantage of an SRS alone approach may also be in avoiding the neurocognitive side effects of WBRT that were not captured in this database.
Because patients enrolled in clinical trials are from a highly selected subset of patients, we believe that comparative analysis utilizing longitudinal databases are informative and important despite the inherent biases of a retrospective study. As practice patterns are currently changing to increase use of SRS, it would be difficult to compare the two most commonly used treatment strategies for patients with < 4 brain metastases in a randomized setting. Thus, our findings that patients receiving SRS had better overall survival than those receiving WBRT are important to validating this approach. Given that the radiation therapy costs associated with SRS are estimated to be at least twice that of the cost of WBRT (or more with the added cost of more frequent MRI surveillance associated with an SRS alone approach), comparative effectiveness research examining these treatments is important not only to patients, but also to policy makers.
Supplementary Material
Acknowledgments
Financial support: NIH/NCI grant “Building CER Capacity: Aligning CRN, CMS, and State Resources to Map Cancer Care” (RC2CA148185); NIH Career Development Award (1K07CA118629 to R.S.P.); National Comprehensive Cancer Network funding for data collection.
Footnotes
Previous presentation: Oral presentation at the Annual Meeting of the American Society for Therapeutic Radiation Oncology. September 23, 2013. Atlanta, Georgia.
Conflict of interest: Dr. D'Amico reports a consulting and advisory role for SCANLAN outside the submitted work. Drs. Dexter and Theriault report royalties from UpToDate outside the submitted work.
Author Contributions: Lia M. Halasz: Conceptualization, methodology, software, validation, formal analysis, writing – original draft, writing – review and editing, visualization, and supervision. Hajime Uno: Methodology, software, validation, formal analysis, writing – original draft, writing – review and editing, and visualization. Melissa Hughes: Methodology, software, investigation, resources, data curation, writing – original draft, writing – review and editing, and project administration. Thomas D’Amico: Conceptualization, formal analysis, investigation, writing – review and editing, and supervision. Elisabeth U. Dexter: Resources and writing – review and editing. Stephen B. Edge: Conceptualization, formal analysis, resources, data curation, and writing – review and editing. James A. Hayman: Formal analysis and writing – review and editing. Joyce C. Niland: Conceptualization, resources, data curation, and writing – review and editing. Gregory A. Otterson: Investigation, resources, data curation, and writing – review and editing. Katherine M.W. Pisters: Conceptualization, formal analysis, resources, data curation, and writing – review and editing. Richard Theriault: Conceptualization, formal analysis, resources, data curation, and writing – review and editing. Jane C. Weeks: Conceptualization, methodology, resources, writing – original draft, writing – review and editing, visualization, supervision, and funding acquisition. Rinaa S. Punglia: Conceptualization, methodology, formal analysis, writing – review and editing, supervision, and funding acquisition.
REFERENCES
- 1.Greenberg H, Chandler WF, Sandler HM. Brain tumors. New York: Oxford University Press; 1999. [Google Scholar]
- 2.Zimm S, Wampler GL, Stablein D, Hazra T, Young HF. Intracerebral metastases in solid-tumor patients: natural history and results of treatment. Cancer. 1981;48:384–394. doi: 10.1002/1097-0142(19810715)48:2<384::aid-cncr2820480227>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 3.Borgelt B, Gelber R, Kramer S, et al. The palliation of brain metastases: final results of the first two studies by the Radiation Therapy Oncology Group. Int J Radiat Oncol Biol Phys. 1980;6:1–9. doi: 10.1016/0360-3016(80)90195-9. [DOI] [PubMed] [Google Scholar]
- 4.Chang EL, Wefel JS, Hess KR, et al. Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: a randomised controlled trial. Lancet Oncol. 2009;10:1037–1044. doi: 10.1016/S1470-2045(09)70263-3. [DOI] [PubMed] [Google Scholar]
- 5.Aoyama H, Shirato H, Tago M, et al. Stereotactic radiosurgery plus whole-brain radiation therapy vs stereotactic radiosurgery alone for treatment of brain metastases: a randomized controlled trial. JAMA. 2006;295:2483–2491. doi: 10.1001/jama.295.21.2483. [DOI] [PubMed] [Google Scholar]
- 6.Kocher M, Soffietti R, Abacioglu U, et al. Adjuvant whole-brain radiotherapy versus observation after radiosurgery or surgical resection of one to three cerebral metastases: results of the EORTC 22952–26001 study. J Clin Oncol. 2011;29:134–141. doi: 10.1200/JCO.2010.30.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Halasz LM, Weeks JC, Neville BA, Taback N, Punglia RS. Use of stereotactic radiosurgery for brain metastases from non-small cell lung cancer in the United States. Int J Radiat Oncol Biol Phys. 2013;85:e109–e116. doi: 10.1016/j.ijrobp.2012.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weeks J. Outcomes assessment in the NCCN: 1998 update. National Comprehensive Cancer Network. Oncology (Williston Park) 1999;13:69–71. [PubMed] [Google Scholar]
- 9.Niland JC. NCCN outcomes research database: data collection via the Internet. Oncology (Williston Park) 2000;14:100–103. [PubMed] [Google Scholar]
- 10.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 11.Katz JN, Chang LC, Sangha O, Fossel AH, Bates DW. Can comorbidity be measured by questionnaire rather than medical record review? Med Care. 1996;34:73–84. doi: 10.1097/00005650-199601000-00006. [DOI] [PubMed] [Google Scholar]
- 12.D'Agostino RB., Jr Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med. 1998;17:2265–2281. doi: 10.1002/(sici)1097-0258(19981015)17:19<2265::aid-sim918>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 13.Lin DY, Psaty BM, Kronmal RA. Assessing the sensitivity of regression results to unmeasured confounders in observational studies. Biometrics. 1998;54:948–963. [PubMed] [Google Scholar]
- 14.Sperduto PW, Chao ST, Sneed PK, et al. Diagnosis-specific prognostic factors, indexes, and treatment outcomes for patients with newly diagnosed brain metastases: a multi-institutional analysis of 4,259 patients. Int J Radiat Oncol Biol Phys. 2010;77:655–661. doi: 10.1016/j.ijrobp.2009.08.025. [DOI] [PubMed] [Google Scholar]
- 15.Gaspar L, Scott C, Rotman M, et al. Recursive partitioning analysis (RPA) of prognostic factors in three Radiation Therapy Oncology Group (RTOG) brain metastases trials. Int J Radiat Oncol Biol Phys. 1997;37:745–751. doi: 10.1016/s0360-3016(96)00619-0. [DOI] [PubMed] [Google Scholar]
- 16.Andrews DW, Scott CB, Sperduto PW, et al. Whole brain radiation therapy with or without stereotactic radiosurgery boost for patients with one to three brain metastases: phase III results of the RTOG 9508 randomised trial. Lancet. 2004;363:1665–1672. doi: 10.1016/S0140-6736(04)16250-8. [DOI] [PubMed] [Google Scholar]
- 17.Sahgal A, Aoyama H, Kocher M, et al. Phase 3 trials of stereotactic radiosurgery with or without whole-brain radiation therapy for 1 to 4 brain metastases: individual patient data meta-analysis. Int J Radiat Oncol Biol Phys. 2015;91:710–717. doi: 10.1016/j.ijrobp.2014.10.024. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.