Abstract
Current approaches to adoptive T cell therapy are limited by the difficulty of obtaining sufficient numbers of T cells against targeted antigens with useful in vivo characteristics. Theoretically, this limitation could be overcome by using induced pluripotent stem cells (iPSCs) that could provide an unlimited source of autologous T cells. However, the therapeutic efficacy of iPSC-derived regenerated T cells remains to be demonstrated. Here we report the first successful reprogramming of T-cell receptor (TCR) transgenic CD8+ T cells into pluripotency. As part of the work, we established a syngeneic mouse model for evaluating in vitro and in vivo antitumor reactivity of regenerated T cells from iPSCs bearing a rearranged TCR of known antigen specificity. Stably TCR retained T cell-derived iPSCs differentiated into CD4+CD8+ T cells that expressed CD3 and the desired TCR in vitro. Stimulation of iPSC-derived CD4+CD8+ T cells with the cognate antigen in the presence of IL-7 and IL-15 followed by expansion with IL-2, IL-7 and IL-15 generated large numbers of less-differentiated CD8+ T cells with antigen-specific potent cytokine production and cytolytic capacity. Furthermore, adoptively transferred iPSC-derived CD8+ T cells escaped immune rejection, mediated effective regression of large tumors, improved survival, and established antigen-specific immunological memory. Our findings illustrate the translational potential of iPSCs to provide an unlimited number of phenotypically defined, functional, and expandable autologous antigen-specific T cells with the characteristics needed to enable in vivo effectiveness.
Keywords: stem cells, reprogramming, T cells, melanoma, immunotherapy
Introduction
Adoptive transfer of ex vivo-expanded autologous tumor-infiltrating lymphocytes (TILs) or peripheral blood T cells genetically modified to express a T-cell receptor (TCR) or a chimeric antigen receptor (CAR) specific for an antigen expressed on tumor cells can induce durable clinical responses in significant numbers of patients with various cancers (1–3). Much progress has been made over the past decade in defining the subsets of T cells mediating effective tumor regression following adoptive T cell therapy (1,4–6). Preclinical data have demonstrated that infusion of CD62Lhigh less-differentiated T cells results in great expansion and persistence of T cells (7) and tumor destruction (4–6) compared with CD62Llow more-differentiated T cells. Clinical data have shown that long-term engraftment and persistence of infused T cells have been highly associated with objective tumor responses (8,9). However, TILs harvested for adoptive cell therapy are effector T cells that are functionally impaired and exhausted (10,11), and further stimulation of TILs results in terminally differentiated cells that have poor survival, and reduced proliferative capacity and antitumor efficacy.
This limitation of adoptive T cell therapy can be overcome by using induced pluripotent stem cells (iPSCs) that self-renew, maintain pluripotency (12–14), and can provide an unlimited source of autologous T cells for immunotherapy. Remarkable advances made in reprogramming technology over the past few years have facilitated the generation of human iPSCs from differentiated cells such as T cells (15–18). These advances now allow us to develop strategies for the use of reprogramming technology for autologous cell therapy in the future. Recent studies have shown that mature human T cells can be reprogrammed into iPSCs that can redifferentiate into functional T lymphocytes in vitro (19–22). These iPSC-derived T cells exhibit TCR gene rearrangement patterns identical to the parental T cells and harbor antigen-specific effector functions in vitro (19–21). Moreover, T-cell derived iPSCs genetically engineered to express a CAR differentiate to CAR-expressing T cells that display antitumor immunity in a xenograft model of lymphoma (22). These studies suggest that iPSC-derived regenerated T cells can be potentially utilized for cancer immunotherapy. However, issues including whether or not T cell-derived iPSCs can generate less-differentiated T-cell subsets that can escape immune rejection, mediate effective regression of large established tumors through their endogenous TCRs, and establish immunological memory are not known due to the lack of syngeneic mouse models.
Here, we reprogrammed TCR transgenic CD8+ T cells into iPSCs, and established a preclinical model to determine therapeutic efficacy of iPSC-derived regenerated T cells in a mouse model for melanoma. Our studies demonstrated that adoptive transfer of less-differentiated iPSC-derived regenerated CD8+ T cells mediated potent in vivo antitumor reactivity, and established antigen-specific immunological memory.
Materials and Methods
Mice
C57BL/6 mice and NOD/SCID mice were purchased from Harlan Laboratories and the Jackson Laboratories, respectively. Pmel-1 TCR-transgenic mice (B6.Cg Thy1a-Tg(TcraTcrb)8Rest/J) were purchased from the Jackson Laboratories, and were bred in-house. All mice were 7 to 10 weeks old at the beginning of each experiment, and were housed in the Unit for Laboratory Animal Medicine at the University of Michigan in compliance with the Institutional Animal Care and Use Committee regulations.
Cell lines
The mouse embryonic stem cell (mESC) line R1 and MC38 murine colon adenocarcinoma cell line were gifts from Drs. Sue O’Shea and Weiping Zou (University of Michigan), respectively. SNL and B16F10 cells were obtained from Cell Biolabs, Inc and ATCC, respectively. OP9 and OP9-DL1 cells were kindly provided by Dr. Juan Carlos Zúñiga-Pflücker (University of Toronto, Toronto, Canada). Cells were authenticated by morphology, phenotype and growth, and routinely screened for Mycoplasma, and were maintained at 37°C in a humidified 5% CO2 atmosphere.
Generation of mouse iPSCs from Pmel-1 TCR transgenic CD8+ T cells
Pmel-1 CD8+ T cells were isolated from a single cell suspension of splenocytes using anti-CD8 beads and MACS columns (Miltenyi Biotec). The sorted 1 × 105 Pmel-1 CD8+ T cells were pulsed with 10μM of human (h)gp10025-33 peptide, KVPRNQDWL (GenScript) in the presence of mitomycin-C treated 5 × 104 splenocytes from B6 mice in T-cell reprogramming medium (23). After 24 hours of culture, the solution which contained SeV vectors that individually carried each of Oct3/4, Sox2, Klf4 and c-Myc was added to wells at multiplicities of infection (MOI) 20. After 24 hours of infection, the cells were collected and transferred to a 10-cm dish that contained mitomycin C-inactivated SNL feeder cells in iPSC medium. The iPSC medium was changed every other day until the colonies were picked.
Generation of T cells from iPSCs in vitro
Pmel-1 iPSCs and mESCs were cultured using OP9 and OP9-DL1 stromal cell co-cultured system (24). In brief, 5 × 104 cells were plated on a 10-cm dish containing confluent OP9 cell monolayer in OP9 medium. On day 5 of culture, cells were trypsinized off, and 5 × 105 cells were transferred to a fresh culture 10-cm dish containing confluent OP9 cell monolayer in OP9 medium with the addition of hFlt3 ligand (5 ng/ml; R&D systems). On day 8 of culture and every 4 days thereafter, semiadherent stem cell-derived hematopoietic cells were collected, filtered through a 40μm nylon mesh, and were transferred onto fresh OP9-DL1 monolayers with the addition of hFlt3 ligand and mIL-7 (1 ng/ml; Peprotech). Semiadherent cells obtained from day 16 to 28 were used for further experiments.
In vitro activation of Pmel-1 iPSC-derived T cells, Pmel-1 splenocytes, and Pmel-1 thymocytes
Semiadherent Pmel-1 iPSC-derived cells on OP9-DL1 cells were harvested and filtered through a 40μm nylon mesh. CD8 expressing cells including CD4 CD8 double positive (DP) T cells and CD8 single positive (SP) T cells in Pmel-1 iPSC-derived cells, Pmel-1 splenocytes and thymocytes were isolated using anti-CD8 beads and MACS columns to eliminate OP9-DL1 cells during T cell activation. These cells (2 × 106 cells) were cultured with mIL-7 (10 ng/ml) and mIL-15 (10 ng/ml; Peprotech) for 2 days in the presence of 1μM of hgp100 peptide and mitomycin-C treated splenocytes from B6 mice (5 × 105 cells). These activated cells were cultured with IL-7 and IL-15 or IL-7, IL-15 and IL-2 from day 3, and used for further experiments on day 6–8.
Adoptive cell transfer, vaccination and cytokine administration
Female C57BL/6 mice were injected s.c. with 3 × 105 B16F10 cells. Mice (n=5 for all groups) were treated 7–11 days later with i.v. adoptive transfer of in vitro-activated 3 × 106 Pmel-1 iPSC-derived T cells or splenic T cells. We injected 20,000 IU rhIL-2 intraperitoneally once on the day of vaccination and twice a day on the two following days. In some experiments mice were vaccinated with 100 μl of saline containing 100 μg of hgp100 peptide, 50 μg of CD40-specific mAb (clone FGK4.5, BioXcell), 50 μg of poly (I:C) (Invivogen) s.c., and 50 mg of imiquimod cream 5% (Aldara, Fougetra) applied on the vaccination sites after adoptive transfer. Tumor volumes were calculated by determining the length of short (l) and long (L) diameters (volume = l2 × L/2). Experimental end points were reached when tumors exceeded 20 mm in diameter or when mice became moribund and showed signs of lateral recumbency, cachexia, lack of response to noxious stimuli, or observable weight loss.
Statistics
Data sets were compared using 2-tailed unpaired Student’s t test. Survival was analyzed with the Kaplan-Meier method using GraphPad Prism 5.0 (GraphPad Software Inc.), and groups were compared using log-rank test. P <0.05 was considered statistically significant. Data are presented as mean ± SEM.
Results
Generation of iPSCs from TCR transgenic CD8+ T cells
To establish a syngeneic mouse model to evaluate in vitro and in vivo antitumor immunity of iPSC-derived antigen-specific T cells, we chose to reprogram Pmel-1 TCR transgenic CD8+ T cells that were developed as a system to model treatment of melanoma using adoptive T cell therapy (25). The target antigen, pmel-17, is an ortholog of the melanocyte differentiation antigen gp100, which is often overexpressed in human melanomas (26). Adoptive transfer of in vitro-activated Pmel-1 CD8+ T cells expressing the anti-gp100 TCR effectively mediates the regression of large established B16 melanomas in combination with systemic administration of common γ-chain cytokine and antigen-specific vaccination with an altered peptide ligand (4,5,25,27).
Reprogramming of murine lymphocytes into iPSCs to date has been successful for B cells (28) and for natural killer T cells (29). However, mature T cells are known to be among the most difficult cells to reprogram, and reprogramming of differentiated murine T cells thus far has required a p53 knock-out or the addition of a doxycycline-inducible gene expression system for successful iPSC generation (30,31). In the current study, we chose the Sendai virus (SeV) vector reprogramming system (15,19–21,32) to generate iPSCs from Pmel-1 CD8+ T cells because SeV can transduce and express foreign genes efficiently into activated murine and human T cells (33), and have high reprogramming efficiency of human peripheral blood T cells (15).
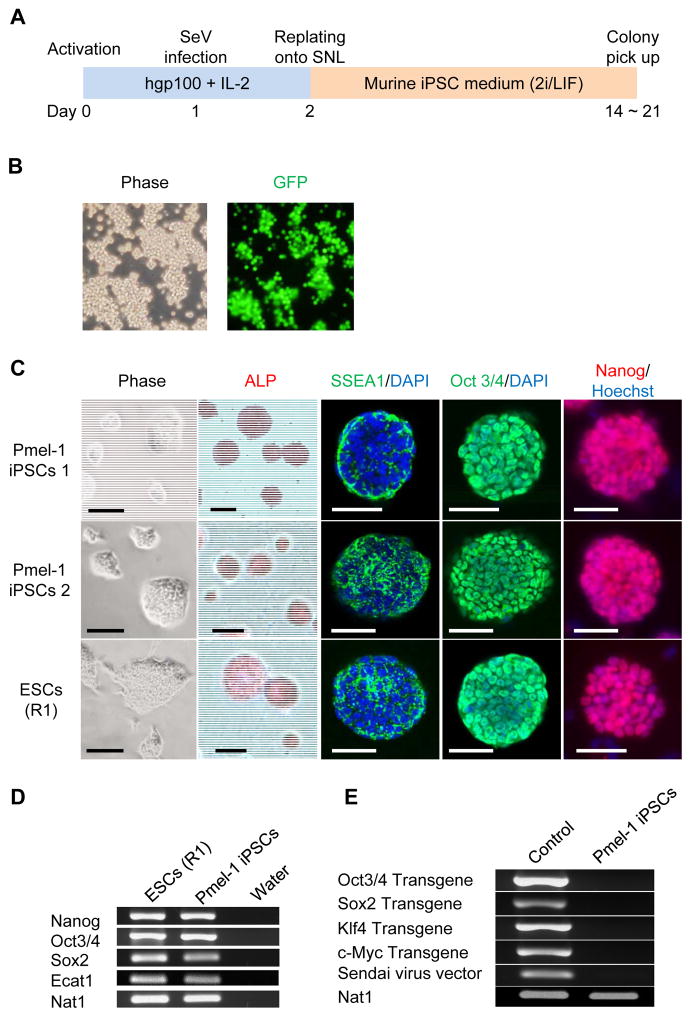
First, Pmel-1 CD8+ T cells were stimulated with hgp100- peptide and IL-2 for 24 hr before SeV infection (Fig. 1A). Efficient expression of an exogenous gene in Pmel-1 CD8+ T cells was confirmed by following the expression of green fluorescent protein (GFP)-carrying SeV (Fig. 1B). To generate iPSCs, we used SeV that individually expressed Oct3/4, Sox2, Klf4 and c-Myc. Twenty-four hours after gene introduction, the cells were replated onto feeder layers of SNL cells with mESC medium containing the self-renewal cytokine, LIF (34). We observed the appearance of mESC-like colonies at 14–21 days after infection with the SeV vectors. However, the colonies isolated and subcultured in conventional mESC medium quickly differentiated and lost typical mESC morphology.
Figure 1. Generation of iPSCs from Pmel-1 TCR transgenic CD8+ T cells.
(A) Schematic illustration showing the generation of iPSCs from Pmel-1 TCR transgenic CD8+ T cells. (B) Efficient GFP introduction by Sendai virus vector in Pmel-1 CD8+ T cells transfected at an MOI of 20. (C) Morphology, alkaline phosphatase (ALP) activity and expression of pluripotency and surface markers (SSEA1, Oct3/4 and Nanog) in Pmel-1 iPSCs 1 and 2, and mESCs. Nuclei were counterstained with DAPI or HOECHST. Scale bar: 200 μm. (D) RT-PCR analysis for the ESC marker genes, Nanog, Oct3/4, Sox2, and Ecat1 in Pmel-1 iPSCs and mESCs. (E) RT-PCR analysis for the transgenes, Oct3/4 Sox2, Klf4, c-Myc and Sendai virus in Pmel-1 iPSCs.
A previous report found that a combination of small molecule inhibitors, CHIR99021, a GSK-3 inhibitor that can activate the Wnt signaling pathway, and PD0325901, a MEK inhibitor, could promote partially reprogrammed cells to full pluripotency (35). Therefore, we investigated whether combining dual inhibition (2i) of MEK and GSK-3, LIF, and overexpression of the pluripotency transcription factors could generate iPSCs from Pmel-1 CD8+ T cells. Genetic transduction using the SeV system and 2i/LIF-based iPSC medium allowed us to establish two colonies with a mESC-like morphology (Fig. 1C) (an efficiency of 0.004%). These iPSCs 1 and 2 were positive for alkaline phosphatase and mESC markers, SSEA1,Oct3/4 and Nanog (Fig. 1C). To further verify expression of pluripotency genes, we performed RT-PCR analysis by primer sets that distinguish endogenous from viral transcripts (Supplementary Table S1), and found that the iPSCs expressed mESC transcripts for Nanog, Oct3/4, Sox2 and Ecat1 (Fig. 1D). Transgenes, Oct3/4, Sox2, Klf4 and c-Myc, and SeV in the iPSCs were lost after several passages (Fig. 1E).
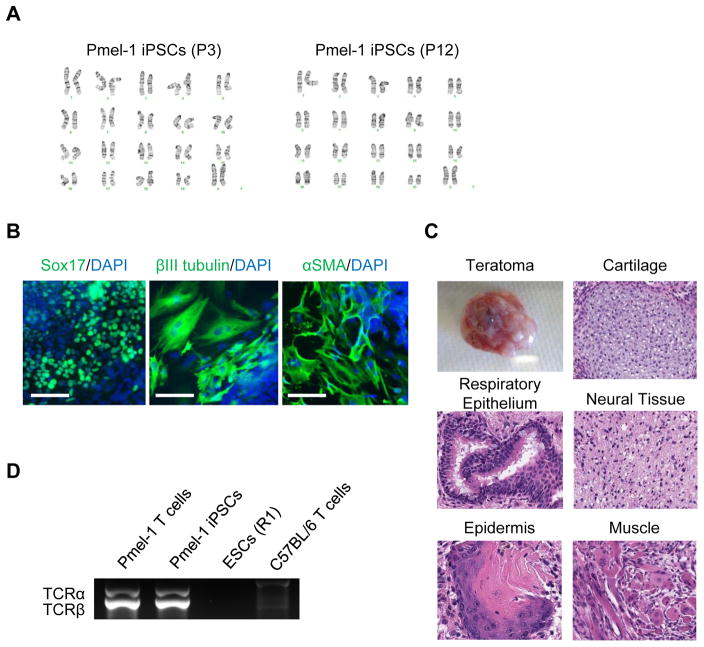
Cytogenetic analysis showed derived iPSCs maintained normal karyotype (Fig. 2A). To evaluate the differentiation potential of the iPSCs, we first performed in vitro differentiation assays. The iPSCs formed embryoid bodies efficiently in vitro, and upregulation of marker genes for all three germ layers was detected by immunostaining (Fig. 2B). Furthermore, we tested the ability of the iPSCs to form teratomas in immune-deficient NOD/SCID mice. These iPSCs differentiated into a variety of cell types from all three germ layers (Fig. 2C). Therefore, those colonies were confirmed as typical murine iPSCs. We then examined the status of TCR gene rearrangement in those iPSCs and confirmed that they retained the same rearranged configuration as the original Pmel-1 T cells (Fig. 2D). These results demonstrated that Pmel-1 CD8+ T cells were successfully reprogrammed to iPSCs. We hereafter referred these established Pmel-1 CD8+ T cell-derived iPSCs as “Pmel-1 iPSCs.”
Figure 2. Detail characterizations of Pmel-1 CD8+ T cell-derived iPSCs.
(A) Normal karyogram of the Pmel-1 iPSCs at passage 3 (left) and 12 (right). (B) Immunostaining for Sox17 (endodermal marker), βIII tubulin (ectodermal marker), and αSMA (mesodermal marker) in Pmel-1 iPSC-derived differentiated cell. Scale bar: 100 μm (C) Gross morphology, hematoxylin and eosin-stained representative teratomas derived from Pmel-1 iPSCs. (D) PCR analysis of rearranged Pmel-1 TCR α- and β-chain genes in Pmel-1 T cells, Pmel-1 iPSCs, mESCs and C57BL/6 mouse T cells.
Generation of less-differentiated CD8+ T cells from iPSCs for adoptive cell therapy
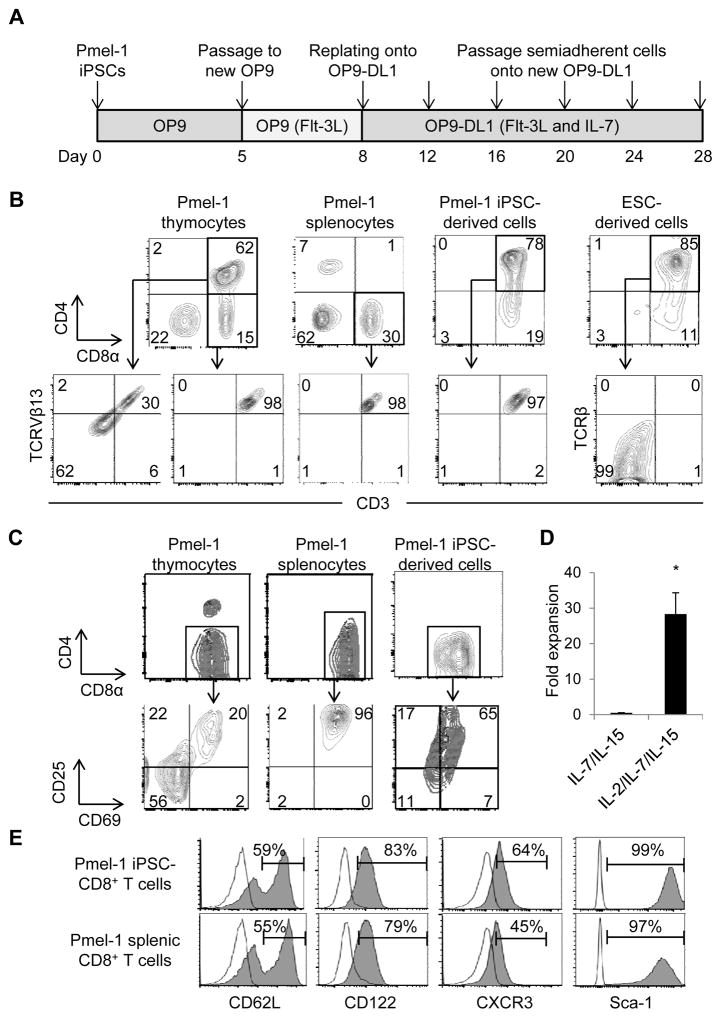
Directed differentiation of mESCs into T lymphocytes required the engagement of Notch receptors to the ligand DL1 expressed on the OP9-DL1 stromal cells (24). mESCs cultured on OP9-DL1 cells follow a thymocyte developmental progression from CD4 CD8 double negative (DN) stage which can be further subdivided into four sequential stages of differentiation based on CD44 and CD25 expression to CD4 CD8 DP T cells (24). To determine whether Pmel-1 iPSCs can differentiate into mature T cells in vitro, we cultured Pmel-1 iPSCs on OP9 cells followed by culture on OP9-DL1 cells (Fig. 3A). On day 8 a population of cells expressing CD45 began to emerge from Pmel-1 iPSCs cultured on OP9 cells (Supplementary Fig. S1A). By day 16 of culture these CD45+ cells exhibited a typical thymocyte developmental progression similar to mESCs on OP9-DL1 cells, and CD4 CD8 DP T cells appeared (Supplementary Fig. S1B). On day 20 Pmel-1 iPSCs cultured on OP9-DL1 cells contained a large population of DP T cells (Fig. 3B), and the majority of DP T cells were CD8αβ T cells that expressed CD5 (Supplementary Fig. S1C). No NK cells or NK-T cells were observed in the Pmel-1 iPSCs on OP9-DL1 cells (Supplementary Fig. S1D). We found very few mESC-derived DP T cells expressed CD3 or TCRβ, and only 30% of natural DP thymocytes expressed CD3 and Pmel-1 TCRβ chain Vβ13. In contrast, virtually all (>95%) of the Pmel-1 iPSC-derived DP T cells expressed CD3 and TCR Vβ13, similar to CD8 SP Pmel-1 thymocytes and splenocytes (Fig. 3B). We further characterized TCR repertoires by high throughput sequencing, and found that a majority of regenerated Pmel-1 iPSC-derived DP and CD8 SP cells bear the same TCRβ chain gene as original Pmel-1 CD8+ T cells (Table 1). These results suggest that iPSCs with rearranged TCR genes efficiently differentiate into TCR-expressing cells at the preselection stage which is consistent with human iPSCs bearing rearranged TCR genes (19,20).
Figure 3. Generation of less-differentiated CD8+ T Cells from Pmel-1 iPSCs for adoptive cell therapy.
(A) Schematic illustration showing the regeneration of T cells from Pmel-1 iPSCs. (B) Phenotypic analysis of Pmel-1 iPSC-derived cells and mESCs on OP9-DL1 cells on day 20. Pmel-1 thymocytes and splenocytes were used for comparison. Viable lymphocytes were analyzed for CD4 and CD8α surface expression (upper), and CD4 CD8 DP or CD8 SP cells were further analyzed for CD3 and TCRVβ13 or TCRβ surface expression (lower). Data are representative of 5 independent experiments. (C) Phenotypic analysis of Pmel-1 iPSC-derived DP T cells, thymocytes and splenocytes 2 days after activation with hgp100 in the presence of IL-7 and IL-15. Viable lymphocytes were gated on CD8α and analyzed for CD25 and CD69 surface expression. Data are representative of 2 independent experiments. Numbers in (B) and (C) indicate the percentage of cells showing in each quadrant. (D) Fold expansion of Pmel-1 iPSC-derived T cells with IL-7 and IL-15 versus IL-2, IL-7 and IL-15 during expansion from day 2 to day 6. (*=P < 0.01 by 2-tailed unpaired Student’s t test) (E) Phenotypic analysis of Pmel-1 iPSC-derived and splenic CD8+ T cells after 2-day activation with hgp100 in the presence of IL-7 and IL-15 followed by 4-day expansion with IL-2, IL-7 and IL-15. Viable lymphocytes were gated on CD8α and analyzed for CD62L, CD122, CXCR3 and Sca-1 surface expression. Open histograms indicate the isotype-control staining. Data are representative of 3 independent experiments.
Table 1.
The sequence of rearranged TCRβ chain genes and frequency in Pmel-1 iPSC-derived DP, CD8 SP on OP9-DL1 cells and splenic CD8+ T cells.
| Cell type | Productive rearrangement | Productive frequency | Frequency | Amino acid |
|---|---|---|---|---|
| Pmel-1 iPSC-CD8+ T cells | TCRBV14-01*01 TCRBD01-01*01 TCRBJ01-06*01 |
97.8% | 93.2% | CASSFHRDYNSPLYF |
| Pmel-1 splenic CD8+ T cells | TCRBV14-01*01 TCRBD01-01*01 TCRBJ01-06*01 |
97.8% | 95.0% | CASSFHRDYNSPLYF |
| Cell type | Nucleotide |
|---|---|
| Pmel-1 iPSC- CD8+ T cells | CTCAAGATCCAGTCTGCAAAGCAGGGCGACACAGCCACCTATC TCTGTGCCAGCAGTTTCCACAGGGACTATAATTCGCCCCTCTAC |
| Pmel-1 splenic CD8+ T cells |
CTCAAGATCCAGTCTGCAAAGCAGGGCGACACAGCCACCTATC TCTGTGCCAGCAGTTTCCACAGGGACTATAATTCGCCCCTCTAC |
Next, we investigated whether we could differentiate Pmel-1 iPSC-derived DP T cells into CD8 SP T cells, and thereby obtain large numbers of less-differentiated CD8+ T cells suitable for adoptive cell therapy (1,4–6). Signaling via TCRαβ induces DP thymocytes to differentiate into mature SP T cells (36). Therefore, we stimulated Pmel-1 iPSC-derived DP T cells with their peptide epitope, hgp10025-33 in the presence of IL-7 and IL-15, two cytokines that are responsible for cytotoxic lineage specification (37) as well as for generation of long-lived memory T cells (38,39). After 2 days of activation, DP T cells disappeared with a simultaneous increase in CD8 SP T cells (Fig. 3C). Phenotypic analysis of the CD8 SP T cells revealed that expression of IL-2 receptor α chain (CD25) and CD69 in Pmel-1 iPSC-derived CD8 SP T cells was higher than thymocytes but lower than splenocytes after 2 days of activation. Based on increased expression of CD25, we tested if the addition of IL-2 to the culture could increase the expansion of Pmel-1 iPSC-derived CD8 SP T cells. We observed a marked increase in the number of T cells after culture with IL-2, IL-7 and IL-15 from day 3 as compared with IL-7 and IL-15 (Fig. 3D). Since activation of T cells may be accompanied by differentiation to effector T cells and the loss of memory potential (1), we evaluated the phenotype of iPSC-derived CD8+ T cells after activation. Despite increased expansion, in vitro-activated iPSC-derived CD8+ T cells were comprised of more than 50% of CD62Lhigh less-differentiated T cells, retained expression of the memory T cell markers, CD122, chemokine (C-X-C motif) receptor 3 (CXCR3), and stem cell antigen-1 (Sca-1) (1,4), and acquired similar phenotype as in vitro-activated pmel-1 splenic CD8+ T cells (Fig. 3E).
iPSC-derived CD8+ T cells exhibit antigen-specific cytokine production and cytolysis
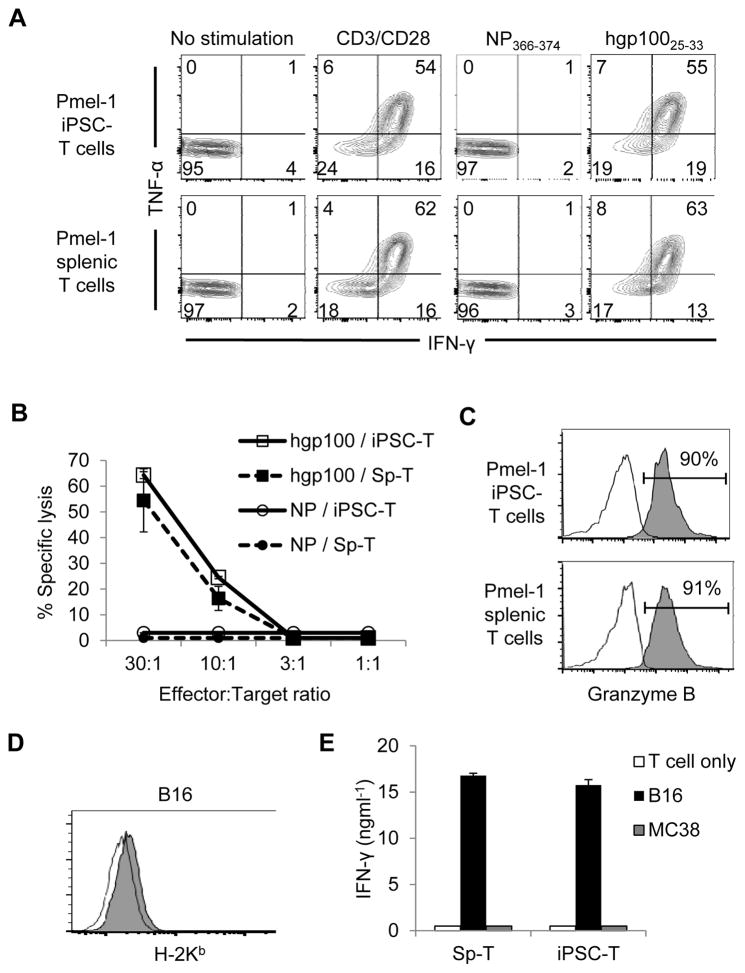
To characterize the effector function of Pmel-1 iPSC-derived CD8+ T cells, we examined intracellular interferon-γ (IFN-γ) and tumor necrosis factor-α (TNF-α) levels after activation using anti-CD3/CD28 monoclonal antibodies (mAbs), or with hgp100 peptide. Influenza nucleoprotein epitope NP366-374 was used as an irrelevant control peptide. A high frequency of cytokine-producing Pmel-1 iPSC-derived CD8+ T cells was documented after stimulation with anti-CD3/CD28 mAbs or with the hgp10025-33 antigen-. Importantly, cytokine production occurred in response to cognate antigen, as evidenced by the failure to detect cytokine producing T cells in response to the NP366-374 antigen (Fig. 4A).
Figure 4. iPSC-derived CD8+ T Cells exhibit antigen-specific cytokine production and cytolysis in vitro.
(A) Intracellular production of IFN-γ and/or TNF-α by Pmel-1 iPSC-derived and splenic CD8+ T cells upon stimulation with or without anti-CD3/CD28 mAb, or against the hgp10025-33 or NP366-374 antigens. CD8-gated populations were shown. Numbers denote percent positive cells. (B) Cytolytic function of Pmel-1 iPSC-derived and splenic T cells against the hgp10025-33 or NP366-374 antigens is shown. (C) Intracellular production of granzyme B induced by stimulation of Pmel-1iPSC-derived and splenic CD8+ T cells with hgp100 pulsed MC38 tumor cells. (D) MHC-I (H-2Kb) expression on B16 melanoma tumors. Open histograms in (C) and (D) indicate the isotype-control staining. (E) Production of IFN-γ by Pmel-1 iPSC-derived and splenic T cells against B16 melanomas and MC38 colon adenocarcinomas. Data shown in (A) – (E) are representative of 2 independent experiments.
We next evaluated the cytolytic activity of Pmel-1 iPSC-derived T cells and Pmel-1 splenic T cells using peptide-pulsed tumor targets. As measured using an LDH release cytotoxicity assay, Pmel-1 iPSC-derived T cells showed antigen-specific cytolytic activity in vitro that was similar to Pmel-1 splenic T cells (Fig. 4B). Consistent with cytolytic activity, granzyme B was significantly enriched in Pmel-1 iPSC-derived T cells (Fig. 4C). Next, Pmel-1 iPSC-derived T cells and Pmel-1 splenic T cells were compared for their abilities to release cytokine against bona fide tumor targets, namely the poorly immunogenic B16 melanomas. MC38 colon adenocarcinoma cells were used as irrelevant targets. Despite very low levels of MHC class I molecules in B16 tumors (Fig. 4D), Pmel-1 iPSC-derived T cells were able to produce abundant IFN-γ to B16 tumors but not to MC38 tumors, which was equivalent to IFN-γ production using Pmel-1 splenic T cells (Fig. 4E). Collectively, these results indicate that Pmel-1 iPSCs are capable of generating less-differentiated antigen-specific T cells with potent effector function suitable for adoptive cell therapy.
Potent in vivo antitumor immunity of iPSC-derived CD8+ T cells
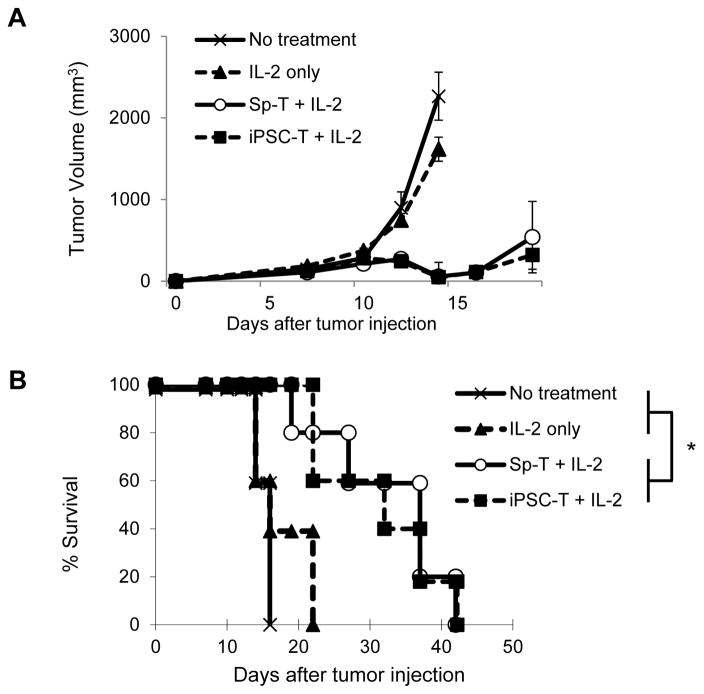
Given the potent in vitro antitumor reactivity against B16 tumors, next we investigated the in vivo antitumor immunity of Pmel-1 iPSC-derived CD8+ T cells using an established subcutaneous B16 tumor model. In vitro-activated Pmel-1 iPSC-derived CD8+ T cells were adoptively transferred into C57BL/6 mice bearing subcutaneous B16 melanomas that had been established for 7 days. Systemic administration of IL-2 was used to enhance antitumor immunity of transferred T cells. In vitro-activated Pmel-1 splenic CD8+ T cells were used as a comparison. B16 tumor growth was unaltered in C57BL/6 mice receiving IL-2 without adoptive cell therapy. In contrast, significant growth delays (Fig. 5A) and prolonged survival (Fig. 5B) were observed when C57BL/6 mice were adoptively transferred with Pmel-1 iPSC-derived CD8+ T cells in combination with IL-2. Consistent with the in vitro functional studies (Fig. 4A-D), the in vivo antitumor immunity of Pmel-1 iPSC-derived CD8+ T cells was comparable to Pmel-1 splenic CD8+ T cells.
Figure 5. Potent in vivo antitumor immunity of iPSC-derived less-differentiated CD8+ T cells.
(A and B) Tumor growth curves (A) and survival curves (B) in C57BL/6 mice bearing B16 melanomas established for 7 days in different treatment groups. Sp-T: Pmel-1 splenic CD8+ T cells. iPSC-T: Pmel-1 iPSC-derived CD8+ T cells. Tumor volume results are the mean of measurements from 5 mice per group. (* = P < 0.0001 using log-rank (Mantel-Cox) test.) Data shown are representative of 2 independent experiments.
Stimulation of adoptively transferred iPSC-derived CD8+ T cells through antigen-specific vaccination and IL-2 causes effective regression of large tumors and establishes immunological memory
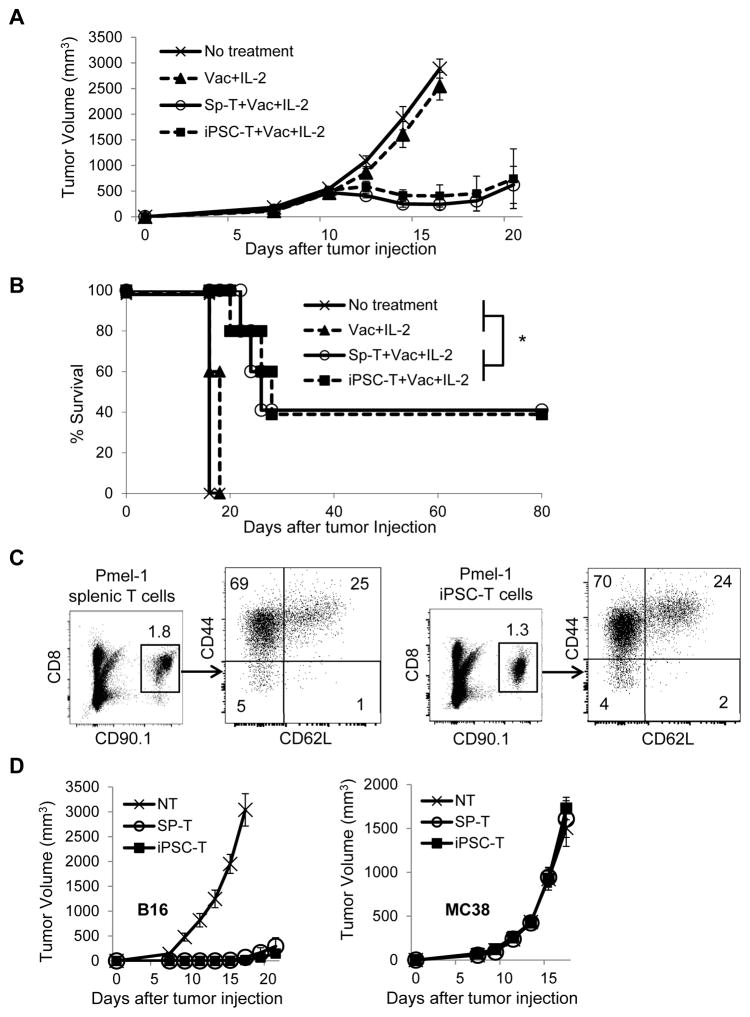
Previous reports showed adoptive transfer of tumor-specific T cells in combination with T cell stimulation through antigen-specific vaccination and co-administration of immunostimulatory molecules and/or T cell growth and activation factors can provide to inducing maximal tumor regression (4,5,25,27). Therefore, we tested if antitumor immunity of adoptively transferred iPSC-derived CD8+ T cells can be further enhanced by subcutaneous vaccination with hgp100, anti-CD40 mAb, and toll-like receptor (TLR) agonists, poly (I:C), and imiquimod cream, agonists for TLR3 and TLR7, respectively, in combination with administration of IL-2 in mice with large subcutaneous B16 melanomas established for 11 days. As shown in Fig. 6A, adoptive transfer of iPSC-derived T cells together with vaccination with cognate antigen, hgp100, immunostimulatory molecles, TLR agonists, and IL-2 resulted in complete responses in ~ 40% of mice bearing large established tumors. These complete responses were found to be durable over an observation period of >80 days (Fig. 6B).
Figure 6. Stimulation of adoptively transferred iPSC-derived CD8+ T cells through antigen-specific vaccination and IL-2 causes effective regression of large tumors and establishes immunological memory.
(A and B) Tumor growth curves (A) and survival curves (B) in C57BL/6 mice bearing B16 melanomas established for 11 days in different treatment groups. Vac: vaccination with the gp100 antigen, anti-CD40 mAb, poly (I:C), and imiquimod cream. Tumor volume results are the mean of measurements from 5 mice per group. (*=P < 0.0001 using log-rank (Mantel-Cox) test.) Data shown are representative of 2 independent experiments. (C) Phenotypic analysis of spleen from surviving C57BL/6 (CD90.2) mice that were treated with adoptive transfer of Pmel-1 iPSC-derived or splenic T cells (CD90.1) in combination with vaccination and IL-2. Numbers denote percent CD8+CD90.1+ cells or indicate the percentage of cells showing in each quadrant. (D) Surviving mice (n=4) after adoptive transfer of Pmel-1 iPSC-derived or splenic T cells, vaccination and IL-2 were rechallenged with B16 cells into the contralateral flank and MC38 cells on back on day 80. Tumor growth curves are depicted in which T=0 corresponds to the time of injection of secondary tumors. As a control, tumor growth was monitored following inoculation of the same tumor cell dose into non-tumor (NT) experienced naive C57BL/6 mice (n=5).
Given our findings of long-term responders after the treatment, we investigated whether adoptively transferred Pmel-1 iPSC-derived T cells also had the capacity to become long-lived memory T cells in surviving mice. Thirty days after adoptive transfer of Pmel-1 iPSC-derived T cells (CD90.1), splenocytes from surviving C57BL/6 mice (CD90.2) still contained transferred T cells, of which more than 20% were CD44highCD62Lhigh central memory T cells similar to surviving mice treated with Pmel-1 splenic T cells (Fig. 6C).
Finally, we evaluated whether treated mice developed antitumor reactivity by rechallenging survivors with implanted B16 tumors in the contralateral flank. MC38 tumors were also injected on the back to determine the antigen-specific systemic immunity by adoptive transfer of Pmel-1 iPSC-derived T cells. Age-matched, untreated non-tumor experienced mice were used as controls. As shown in Fig. 6D, significant growth delays of the B16 tumors but normal growth of the MC38 tumors were observed in surviving C57BL/6 mice that were previously treated with adoptive transfer of Pmel-1 iPSC-derived T cells or Pmel-1 splenic T cells, suggesting development of antigen-specific long-lasting systemic memory.
Discussion
Adoptive transfer of antigen-specific CD8+ T cells has emerged as one of the most effective treatments for variety of cancers (1–3). A major limitation of this approach is poor survival of T cells in vivo following infusion. Although less-differentiated T cells would be an ideal T-cell subset for adoptive T cell therapy (1,4–6), generating large numbers of these “young” T cells is problematic. Human iPSCs hold great promise as unlimited sources of autologous T cells and can potentially generate an infinite number of naïve antigen-specific T cells for adoptive cell therapy. Two approaches have been described to generate iPSCs that can produce antigen-specific T cells (19–22). One is to reprogram T cells with known antigen specificity into iPSCs (19–21). Recent studies have shown that T cell-derived iPSCs retaining rearranged TCR genes from original T cell clone can differentiate into T cells that exhibited antigen-specific cytokine production and cytotoxicity in vitro (19–21). This strategy is especially relevant for TILs because nearly 80% of TILs possess autologous tumor reactivity (40), but exhibit a more differentiated phenotype and functional exhaustion (1,10,11). We have recently described that melanoma TILs can be reprogrammed to human iPSCs, albeit with low efficiency compared to peripheral blood T cells (23). Another approach to generate antigen-specific T cells from iPSCs is to genetically engineer T cell-derived iPSCs to express a CAR specific for the known tumor antigen such as CD19, which is expressed on B-cell leukemias and lymphomas, and differentiate them into CAR-expressing T cells (22). Although iPSC-derived, CAR-expressing T cells recognize the targeted antigen via a CAR not through their endogenous TCRαβ, in line with our findings, they mediate antitumor reactivity in vivo in a xenograft model of lymphoma (22). Of note, the latter approach is useful to target only known tumor antigens while the former approach can be used to target both known and unknown antigens as long as antigen-specificity of the T cell is identified.
Our study has provided insight into the in vivo immunogenicity and antitumor reactivity of CD8+ T cells derived from iPSCs bearing a rearranged TCR of known antigen specificity. A central finding was that iPSC-derived regenerated CD8+ T cells escaped immune rejection, and mediated potent antitumor immunity in vivo comparable to splenic CD8+ T cells from TCR transgenic mice. This in vivo antitumor immunity of iPSC-derived CD8+ T cells was supported by the findings that they not only had antigen-specific cytokine-producing and cytolytic function against the cognate antigen, hgp100 but also had the ability to produce cytokine against bona fide tumor targets, poorly immunogenic B16 melanomas. Taken together, these results suggest that T cells not only retain antigen specificity via endogenous TCRαβ but also maintain functional integrity, proliferative capacity and effector function after reprogramming and redifferentiation processes.
The current study demonstrate for the first time that murine T cells can be reprogrammed without using gene knockout mice (30) or drug-inducible gene expression systems (31). We found the SeV reprogramming system that was shown to be highly efficient method for the generation of iPSCs from human T cells (15,19–22), could also be used for reprogramming murine T cells. The reprogramming efficiency, however, was quite low (0.004%) even with a higher MOI of 20. Furthermore, conventional mESC medium containing LIF was not sufficient to generate iPSCs from Pmel-1 T cells. We found that dual inhibition (2i) of both prodifferentiation MEK and GSK-3 pathways that was shown to support the establishment of mouse iPSCs from partially reprogrammed cells (35) was required for reprogramming of Pmel-1 T cells. The low efficiency of iPSC derivation remains one of the major limitations to apply this approach into clinical practice. Of note, we observed Pmel-1 iPSCs can be maintained in conventional mESC medium without 2i once they are established, and they maintain pluripotency and their ability to differentiate in the T lineage.
Persistence of TCR rearrangements is a distinct feature of T cell-derived iPSCs (15–18) as well as plays a pivotal role in differentiating iPSCs to CD8 SP T cells (19,20). Our findings further expand on effector function of iPSC-derived CD8+ T cells in comparison to naive CD8+ TCR transgenic T cells. We demonstrated Pmel-1 iPSC-derived DP T cells in culture with OP9-DL1 expressed high levels of CD3 and TCRVβ13, efficiently upregulated CD25 and CD69 upon stimulation via TCR, and quickly became CD8 SP T cells functionally similar to in vitro-activated Pmel-1 splenic CD8+ T cells. Although we observed a subset of mESC-derived CD8 SP T cells expressing CD3 and TCRβ (data not shown) as reported in a previous study (24), Pmel-1 iPSCs cultured on OP9-DL1 cells differentiated more efficiently than mESCs, and expressed CD3 and TCRVβ13 at the DP stage.
The early expression of prerearranged TCRα and TCRβ genes may cause undesired outcomes. Serwold et al. showed that mice derived from a reprogrammed T cell, developed spontaneous T cell lymphoma, which originated in the thymus (41). Although these mice have prerearranged TCR genes in all cells, and are different from mice receiving adoptive transfer of iPSC-derived T cells in the current studies, potential tumorigenicity of iPSC-derived T cells needs to be determined before this strategy is translated into clinical practice. Notably, we did not find any mice that developed tumors including lymphoma after adoptive transfer of iPSC-derived T cells.
Immunogenicity of autologous iPSC-derived cells is still not well understood. Recent studies have shown that certain iPSC-derived cells such as smooth muscle cells and cardiomyocytes are immunogenic while other cell types such as retinal pigment epithelial, hepatocytes, and neuronal cells exhibit little to no immunogenicity (42–45). Although our study did not evaluate host immune response against infused iPSC-derived T cells, our findings of long-lived adoptively transferred T cells that establish immunological memory suggest iPSC-derived T cells might not cause immune rejection.
T cells with CD62Lhigh less-differentiated phenotype maintain proliferative capacity and produce effector progeny in vivo, resulting in a greater objective response upon adoptive transfer relative to CD62Llow more-differentiated T cells (1,4–6). The strategy of reprogramming and regeneration of antitumor T cells provides a more meaningful rationale for adoptive T cell therapy if we can generate T cell subsets that possess robust proliferative and engraftment capacities. A recent report showed that human T-cell derived iPSCs can differentiate to T cells with a central-memory phenotype (19). We further determined that iPSC-derived less-differentiated T cells expressing memory T-cell markers had long-term persistence and established antigen-specific immunological memory in vivo after adoptive transfer. Interestingly, we found potent in vivo antitumor reactivity of adoptive transfer of iPSC-derived T cells and IL-2 without antigen-specific vaccination which was not seen in the previous study (25). A possible explanation for this finding may reside in the different in vitro activation protocol of T cells where we used IL-2, IL-7 and IL-15 while others used IL-2 only (25). IL-7 and IL-15 play integral role in CD8+ T-cell memory generation and maintenance (38). IL-7 is involved for the survival and homeostatic expansion of naïve CD8+ T cells, and also can contribute to memory CD8+ T cell homeostasis (46,47). IL-15 induces proliferation of naïve and memory CD8+ T cells and is involved in the maintenance of long-lasting antigen-specific memory CD8+ T cells (48–50). We found the majority of Pmel-1 iPSC-derived and splenic T cells after activation with IL-2 were CD62Llow more-differentiated T cells (data not shown) while ones activated with IL-2, IL-7 and IL-15 contained more than 50% of CD62Lhigh less-differentiated T cells (Fig. 3E).
In summary, our results demonstrate that adoptive transfer of iPSC-derived regenerated T cells is an effective cancer immunotherapy that improves local tumor control and overall survival in a lethal murine model of melanoma. Importantly, an establishment of antigen-specific immunological memory reveals the feasibility of generation of long-lived tumor-specific T cells via reprogramming to pluripotency and redifferentiation. Applying techniques described herein may provide an unlimited number of phenotypically defined, functional and expandable autologous antigen-specific T cells harboring characteristics necessary for in vivo effectiveness.
Supplementary Material
Acknowledgments
Grant support: This work was supported by institutional funds from the University of Michigan and Roswell Park Cancer Institute, and grants from the Central Surgical Association, the American College of Surgeons, the Melanoma Research Alliance, and the NIH/NCI (K08CA197966) to F. Ito, and by the National Center for Advancing Translational Sciences of the NIH (UL1TR000433).
We acknowledge Dr. Mamoru Hasegawa in DNAVEC Corporation for kindly providing Sendai virus vectors. We thank Drs. Tomonori Hosoya and James Douglas Engel for scientific discussion; Dr. Cindy DeLong for her technical assistance; Dr. Richard Lieberman for histological analyses; Dr. Ren Shimamoto in ETH Zürich and Dr. Noemi Fusaki in Keio University for their technical assistance, and Ms. Elizabeth LaPensee, Ms. Link Hope and Ms. Katherine Wood for administrative assistance.
Footnotes
Competing interests: The authors declare no conflict of interest.
References
- 1.Restifo NP, Dudley ME, Rosenberg SA. Adoptive immunotherapy for cancer: harnessing the T cell response. Nat Rev Immunol. 2012;12(4):269–81. doi: 10.1038/nri3191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rosenberg SA, Yang JC, Sherry RM, Kammula US, Hughes MS, Phan GQ, et al. Durable Complete Responses in Heavily Pretreated Patients with Metastatic Melanoma Using T-Cell Transfer Immunotherapy. Clinical Cancer Research. 2011;17(13):4550–57. doi: 10.1158/1078-0432.CCR-11-0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maus MV, Fraietta JA, Levine BL, Kalos M, Zhao Y, June CH. Adoptive immunotherapy for cancer or viruses. Annu Rev Immunol. 2014;32:189–225. doi: 10.1146/annurev-immunol-032713-120136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gattinoni L, Zhong X-S, Palmer DC, Ji Y, Hinrichs CS, Yu Z, et al. Wnt signaling arrests effector T cell differentiation and generates CD8+ memory stem cells. Nat Med. 2009;15(7):808–13. doi: 10.1038/nm.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Klebanoff CA, Gattinoni L, Torabi-Parizi P, Kerstann K, Cardones AR, Finkelstein SE, et al. Central memory self/tumor-reactive CD8+ T cells confer superior antitumor immunity compared with effector memory T cells. Proc Natl Acad Sci U S A. 2005;102(27):9571–6. doi: 10.1073/pnas.0503726102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gattinoni L, Lugli E, Ji Y, Pos Z, Paulos CM, Quigley MF, et al. A human memory T cell subset with stem cell–like properties. Nature Medicine. 2011;17(10):1290–97. doi: 10.1038/nm.2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang X, Berger C, Wong CW, Forman SJ, Riddell SR, Jensen MC. Engraftment of human central memory-derived effector CD8+ T cells in immunodeficient mice. Blood. 2011;117(6):1888–98. doi: 10.1182/blood-2010-10-310599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kalos M, Levine BL, Porter DL, Katz S, Grupp SA, Bagg A, et al. T cells with chimeric antigen receptors have potent antitumor effects and can establish memory in patients with advanced leukemia. Sci Transl Med. 2011;3(95):95ra73. doi: 10.1126/scitranslmed.3002842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Robbins PF, Dudley ME, Wunderlich J, El-Gamil M, Li YF, Zhou J, et al. Cutting edge: persistence of transferred lymphocyte clonotypes correlates with cancer regression in patients receiving cell transfer therapy. J Immunol. 2004;173(12):7125–30. doi: 10.4049/jimmunol.173.12.7125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baitsch L, Baumgaertner P, Devevre E, Raghav SK, Legat A, Barba L, et al. Exhaustion of tumor-specific CD8(+) T cells in metastases from melanoma patients. J Clin Invest. 2011;121(6):2350–60. doi: 10.1172/JCI46102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ahmadzadeh M, Johnson LA, Heemskerk B, Wunderlich JR, Dudley ME, White DE, et al. Tumor antigen-specific CD8 T cells infiltrating the tumor express high levels of PD-1 and are functionally impaired. Blood. 2009;114(8):1537–44. doi: 10.1182/blood-2008-12-195792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126(4):663–76. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 13.Okita K, Ichisaka T, Yamanaka S. Generation of germline-competent induced pluripotent stem cells. Nature. 2007;448(7151):313–7. doi: 10.1038/nature05934. [DOI] [PubMed] [Google Scholar]
- 14.Wernig M, Meissner A, Foreman R, Brambrink T, Ku M, Hochedlinger K, et al. In vitro reprogramming of fibroblasts into a pluripotent ES-cell-like state. Nature. 2007;448(7151):318–24. doi: 10.1038/nature05944. [DOI] [PubMed] [Google Scholar]
- 15.Seki T, Yuasa S, Oda M, Egashira T, Yae K, Kusumoto D, et al. Generation of induced pluripotent stem cells from human terminally differentiated circulating T cells. Cell Stem Cell. 2010;7(1):11–4. doi: 10.1016/j.stem.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 16.Loh YH, Hartung O, Li H, Guo C, Sahalie JM, Manos PD, et al. Reprogramming of T cells from human peripheral blood. Cell Stem Cell. 2010;7(1):15–9. doi: 10.1016/j.stem.2010.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Staerk J, Dawlaty MM, Gao Q, Maetzel D, Hanna J, Sommer CA, et al. Reprogramming of human peripheral blood cells to induced pluripotent stem cells. Cell Stem Cell. 2010;7(1):20–4. doi: 10.1016/j.stem.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brown ME, Rondon E, Rajesh D, Mack A, Lewis R, Feng X, et al. Derivation of induced pluripotent stem cells from human peripheral blood T lymphocytes. PLoS One. 2010;5(6):e11373. doi: 10.1371/journal.pone.0011373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nishimura T, Kaneko S, Kawana-Tachikawa A, Tajima Y, Goto H, Zhu D, et al. Generation of rejuvenated antigen-specific T cells by reprogramming to pluripotency and redifferentiation. Cell Stem Cell. 2013;12(1):114–26. doi: 10.1016/j.stem.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 20.Vizcardo R, Masuda K, Yamada D, Ikawa T, Shimizu K, Fujii S, et al. Regeneration of Human Tumor Antigen-Specific T Cells from iPSCs Derived from Mature CD8(+) T Cells. Cell Stem Cell. 2013;12(1):31–6. doi: 10.1016/j.stem.2012.12.006. [DOI] [PubMed] [Google Scholar]
- 21.Wakao H, Yoshikiyo K, Koshimizu U, Furukawa T, Enomoto K, Matsunaga T, et al. Expansion of functional human mucosal-associated invariant T cells via reprogramming to pluripotency and redifferentiation. Cell Stem Cell. 2013;12(5):546–58. doi: 10.1016/j.stem.2013.03.001. [DOI] [PubMed] [Google Scholar]
- 22.Themeli M, Kloss CC, Ciriello G, Fedorov VD, Perna F, Gonen M, et al. Generation of tumor-targeted human T lymphocytes from induced pluripotent stem cells for cancer therapy. Nat Biotechnol. 2013 doi: 10.1038/nbt.2678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saito H, Okita K, Fusaki N, Sabel MS, Chang AE, Ito F. Reprogramming of Melanoma Tumor-Infiltrating Lymphocytes to Induced Pluripotent Stem Cells. Stem Cells International. 2016;2016(8394960):11. doi: 10.1155/2016/8394960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schmitt TM, de Pooter RF, Gronski MA, Cho SK, Ohashi PS, Zuniga-Pflucker JC. Induction of T cell development and establishment of T cell competence from embryonic stem cells differentiated in vitro. Nat Immunol. 2004;5(4):410–7. doi: 10.1038/ni1055. [DOI] [PubMed] [Google Scholar]
- 25.Overwijk WW, Theoret MR, Finkelstein SE, Surman DR, de Jong LA, Vyth-Dreese FA, et al. Tumor regression and autoimmunity after reversal of a functionally tolerant state of self-reactive CD8+ T cells. The Journal of experimental medicine. 2003;198(4):569–80. doi: 10.1084/jem.20030590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Overwijk WW, Tsung A, Irvine KR, Parkhurst MR, Goletz TJ, Tsung K, et al. gp100/pmel 17 is a murine tumor rejection antigen: induction of “self”-reactive, tumoricidal T cells using high-affinity, altered peptide ligand. The Journal of experimental medicine. 1998;188(2):277–86. doi: 10.1084/jem.188.2.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hailemichael Y, Dai Z, Jaffarzad N, Ye Y, Medina MA, Huang XF, et al. Persistent antigen at vaccination sites induces tumor-specific CD8(+) T cell sequestration, dysfunction and deletion. Nat Med. 2013;19(4):465–72. doi: 10.1038/nm.3105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hanna J, Markoulaki S, Schorderet P, Carey BW, Beard C, Wernig M, et al. Direct reprogramming of terminally differentiated mature B lymphocytes to pluripotency. Cell. 2008;133(2):250–64. doi: 10.1016/j.cell.2008.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Watarai H, Fujii S, Yamada D, Rybouchkin A, Sakata S, Nagata Y, et al. Murine induced pluripotent stem cells can be derived from and differentiate into natural killer T cells. J Clin Invest. 2010;120(7):2610–8. doi: 10.1172/JCI42027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hong H, Takahashi K, Ichisaka T, Aoi T, Kanagawa O, Nakagawa M, et al. Suppression of induced pluripotent stem cell generation by the p53-p21 pathway. Nature. 2009;460(7259):1132–5. doi: 10.1038/nature08235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Eminli S, Foudi A, Stadtfeld M, Maherali N, Ahfeldt T, Mostoslavsky G, et al. Differentiation stage determines potential of hematopoietic cells for reprogramming into induced pluripotent stem cells. Nat Genet. 2009;41(9):968–76. doi: 10.1038/ng.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fusaki N, Ban H, Nishiyama A, Saeki K, Hasegawa M. Efficient induction of transgene-free human pluripotent stem cells using a vector based on Sendai virus, an RNA virus that does not integrate into the host genome. Proc Jpn Acad Ser B Phys Biol Sci. 2009;85(8):348–62. doi: 10.2183/pjab.85.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Okano S, Yonemitsu Y, Nagata S, Sata S, Onimaru M, Nakagawa K, et al. Recombinant Sendai virus vectors for activated T lymphocytes. Gene therapy. 2003;10(16):1381–91. doi: 10.1038/sj.gt.3301998. [DOI] [PubMed] [Google Scholar]
- 34.Williams RL, Hilton DJ, Pease S, Willson TA, Stewart CL, Gearing DP, et al. Myeloid leukaemia inhibitory factor maintains the developmental potential of embryonic stem cells. Nature. 1988;336(6200):684–7. doi: 10.1038/336684a0. [DOI] [PubMed] [Google Scholar]
- 35.Silva J, Barrandon O, Nichols J, Kawaguchi J, Theunissen TW, Smith A. Promotion of reprogramming to ground state pluripotency by signal inhibition. PLoS biology. 2008;6(10):e253. doi: 10.1371/journal.pbio.0060253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Starr TK, Jameson SC, Hogquist KA. Positive and negative selection of T cells. Annu Rev Immunol. 2003;21:139–76. doi: 10.1146/annurev.immunol.21.120601.141107. [DOI] [PubMed] [Google Scholar]
- 37.McCaughtry TM, Etzensperger R, Alag A, Tai X, Kurtulus S, Park JH, et al. Conditional deletion of cytokine receptor chains reveals that IL-7 and IL-15 specify CD8 cytotoxic lineage fate in the thymus. The Journal of experimental medicine. 2012;209(12):2263–76. doi: 10.1084/jem.20121505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schluns KS, Lefrancois L. Cytokine control of memory T-cell development and survival. Nat Rev Immunol. 2003;3(4):269–79. doi: 10.1038/nri1052. [DOI] [PubMed] [Google Scholar]
- 39.Cieri N, Camisa B, Cocchiarella F, Forcato M, Oliveira G, Provasi E, et al. IL-7 and IL-15 instruct the generation of human memory stem T cells from naive precursors. Blood. 2013;121(4):573–84. doi: 10.1182/blood-2012-05-431718. [DOI] [PubMed] [Google Scholar]
- 40.Dudley ME, Wunderlich JR, Shelton TE, Even J, Rosenberg SA. Generation of tumor-infiltrating lymphocyte cultures for use in adoptive transfer therapy for melanoma patients. J Immunother. 2003;26(4):332–42. doi: 10.1097/00002371-200307000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Serwold T, Hochedlinger K, Swindle J, Hedgpeth J, Jaenisch R, Weissman IL. T-cell receptor-driven lymphomagenesis in mice derived from a reprogrammed T cell. Proc Natl Acad Sci U S A. 2010;107(44):18939–43. doi: 10.1073/pnas.1013230107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhao T, Zhang ZN, Westenskow PD, Todorova D, Hu Z, Lin T, et al. Humanized Mice Reveal Differential Immunogenicity of Cells Derived from Autologous Induced Pluripotent Stem Cells. Cell Stem Cell. 2015;17(3):353–9. doi: 10.1016/j.stem.2015.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.de Almeida PE, Meyer EH, Kooreman NG, Diecke S, Dey D, Sanchez-Freire V, et al. Transplanted terminally differentiated induced pluripotent stem cells are accepted by immune mechanisms similar to self-tolerance. Nature communications. 2014;5:3903. doi: 10.1038/ncomms4903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Araki R, Uda M, Hoki Y, Sunayama M, Nakamura M, Ando S, et al. Negligible immunogenicity of terminally differentiated cells derived from induced pluripotent or embryonic stem cells. Nature. 2013;494(7435):100–4. doi: 10.1038/nature11807. [DOI] [PubMed] [Google Scholar]
- 45.Guha P, Morgan JW, Mostoslavsky G, Rodrigues NP, Boyd AS. Lack of immune response to differentiated cells derived from syngeneic induced pluripotent stem cells. Cell Stem Cell. 2013;12(4):407–12. doi: 10.1016/j.stem.2013.01.006. [DOI] [PubMed] [Google Scholar]
- 46.Schluns KS, Kieper WC, Jameson SC, Lefrancois L. Interleukin-7 mediates the homeostasis of naive and memory CD8 T cells in vivo. Nat Immunol. 2000;1(5):426–32. doi: 10.1038/80868. [DOI] [PubMed] [Google Scholar]
- 47.Kaech SM, Tan JT, Wherry EJ, Konieczny BT, Surh CD, Ahmed R. Selective expression of the interleukin 7 receptor identifies effector CD8 T cells that give rise to long-lived memory cells. Nat Immunol. 2003;4(12):1191–8. doi: 10.1038/ni1009. [DOI] [PubMed] [Google Scholar]
- 48.Schluns KS, Williams K, Ma A, Zheng XX, Lefrancois L. Cutting edge: requirement for IL-15 in the generation of primary and memory antigen-specific CD8 T cells. J Immunol. 2002;168(10):4827–31. doi: 10.4049/jimmunol.168.10.4827. [DOI] [PubMed] [Google Scholar]
- 49.Zhang X, Sun S, Hwang I, Tough DF, Sprent J. Potent and selective stimulation of memory-phenotype CD8+ T cells in vivo by IL-15. Immunity. 1998;8(5):591–9. doi: 10.1016/s1074-7613(00)80564-6. [DOI] [PubMed] [Google Scholar]
- 50.Becker TC, Wherry EJ, Boone D, Murali-Krishna K, Antia R, Ma A, et al. Interleukin 15 is required for proliferative renewal of virus-specific memory CD8 T cells. The Journal of experimental medicine. 2002;195(12):1541–8. doi: 10.1084/jem.20020369. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.