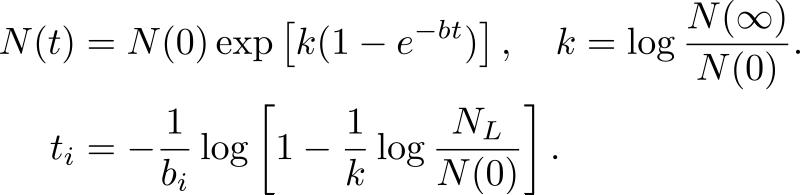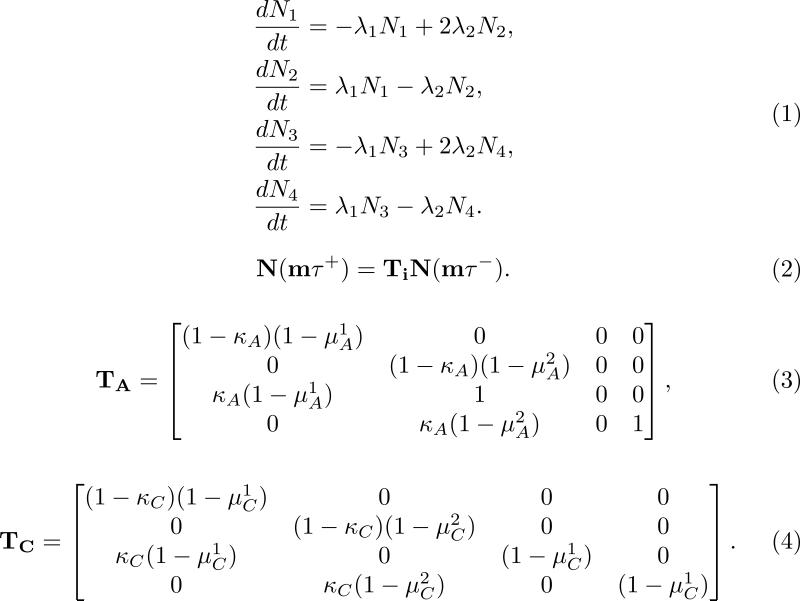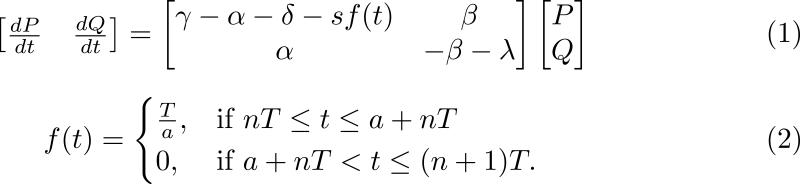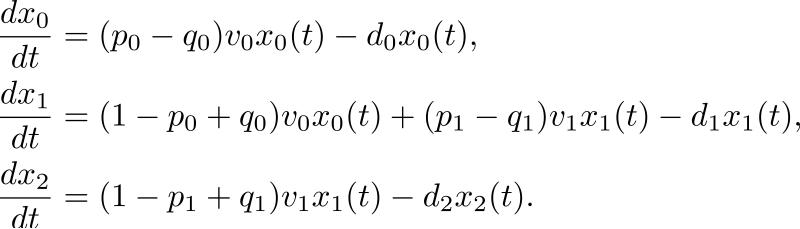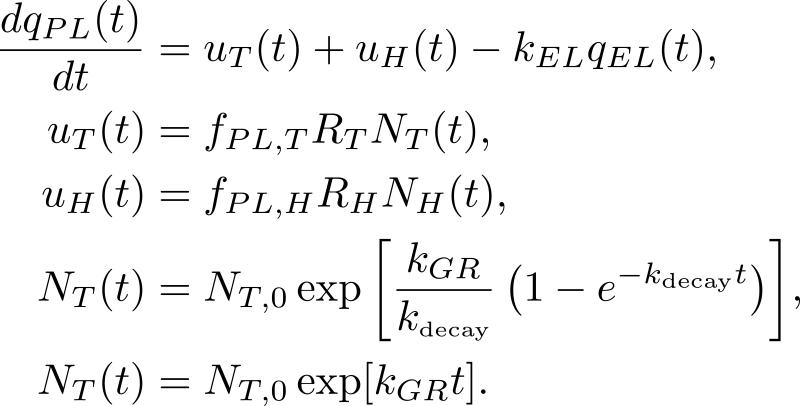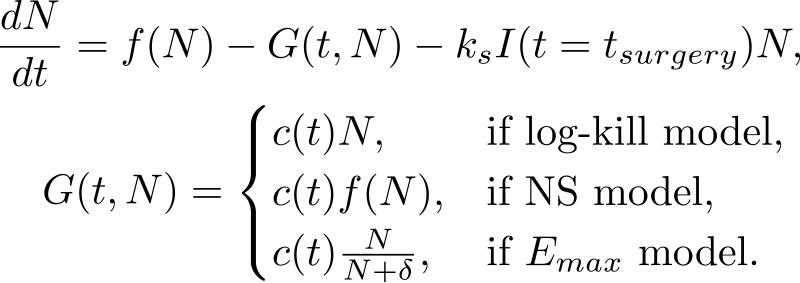Abstract
Women constitute the majority of the aging United States (US) population, and this has substantial implications on cancer population patterns and management practices. Breast cancer is the most common women's malignancy, while ovarian cancer is the most fatal gynecological malignancy in the US. In this review we focus on these subsets of women's cancers, seen more commonly in postmenopausal and elderly women.
In order to systematically investigate the complexity of cancer progression and response to treatment in breast and ovarian malignancies, we assert that integrated mathematical modeling frameworks viewed from a systems biology perspective are needed. Such integrated frameworks could offer innovative contributions to the clinical women's cancers community, since answers to clinical questions cannot always be reached with contemporary clinical and experimental tools.
Here, we recapitulate clinically known data regarding the progression and treatment of the breast and ovarian cancers. We compare and contrast the two malignancies whenever possible, in order to emphasize areas where substantial contributions could be made by clinically inspired and validated mathematical modeling. We show how current paradigms in the mathematical oncology community focusing on the two malignancies do not make comprehensive use of, nor substantially reflect existing clinical data, and we highlight the modeling areas in most critical need of clinical data integration. We emphasize that the primary goal of any mathematical study of women's cancers should be to address clinically relevant questions.
Keywords: ovarian cancer, breast cancer, mathematical modeling, systems biology
INTRODUCTION
Women constitute the majority of the aging United States (US) population, as, on average, they outlive men by 5 years1. According to the US Bureau of the Census in 2010, the life expectancy of a female at birth was 81.1 years versus 76.2 years for a male. In this latest census, women accounted for about 60% of the population aged 70 years or older. Moreover, based on these demographic data, the computed ratio of number of postmenopausal women to women of reproductive age was 2:3. This has substantial implications on existing clinical practices, as an increased proportion of aging women is associated with new disease patterns, such as differential incidence or prevalence rates.
In this review we focus on two subsets of women's cancers, specifically breast and ovarian cancers, which are diseases seen more commonly in postmenopausal and elderly women2. Herein, we assume that the transition to menopause represents the defining threshold between pre- and postmenopausal stage, or between reproductive and non-reproductive age. For a definition of menopause and subsequent terminology used throughout the review, we refer to the attached Sidebars 1, and 2. We seek to emphasize the complex degrees of heterogeneity of breast and ovarian cancers from a clinical perspective, and subsequently highlight the pressing need for developing mathematical models that accurately reflect emerging clinical data.
Breast cancer is the most common cancer type affecting women, representing 29% of all new cancer cases in US women2. Nonetheless, it has a good prognosis, with an approximately 89% five-year overall survival rate3. Breast cancer is most frequently diagnosed among women aged 55-64, with a median age of 61 years4. Ovarian cancer is a relatively rare women's cancer, representing 2.6% of all new cancer cases in US women2. However, it is the most fatal gynecologic cancer type, with an approximately 45% five-year overall survival rate5. Ovarian cancer is most frequently diagnosed among women aged 55-64, with a median age of 63 years2.
Herein, we provide a summary of the clinical progression, and current detection and therapeutic strategies for these two malignancies. We begin by reviewing the latest clinical information and statistics regarding the progression and treatment of breast and ovarian cancers. To this end, we focus our attention on various malignancy-specific aspects such as target organ biology, existing prevention and early detection strategies, current systemic and targeted therapies, and proposed drug resistance mechanisms. In doing so, we highlight the open clinical questions we believe a substantial contribution could be potentially made to by mathematical modeling. Next, we systematically review and comment upon the existing mathematical models aimed at describing various aspects of the malignancies’ natural history and progression. We chose to compare and contrast disease etiologies whenever possible, in order to highlight areas of (di)similarity in view of developing relevant systems biology-oriented mathematical frameworks. In doing so, we note the scarcity of existing inferences aimed at modeling the spatiotemporal growth, progression, and therapeutic targeting of the breast and ovarian malignancies. We also comment on the limitations of the existing modeling frameworks in the context of the mathematical approaches utilized, and point out the areas where the modeling efforts could be further expended.
Our purpose here is two-fold. First, we show how current paradigms in the mathematical oncology community focusing on the two malignancies do not make comprehensive use nor substantially reflect existing clinical knowledge, and we highlight the modeling areas in most critical need of clinical data integration. We seek to emphasize the pressing need for truly integrative mathematical-oncology collaborations that reflect and make use of the clinically known aspects of malignancy heterogeneity, progression, detection and therapeutic options. Second, we argue in favor of the potential of mathematical modeling to integrate clinically derived data and to suggest novel future directions for clinical research, given validated in silico mathematical models of the breast and ovarian malignancies.
Lastly, we note that the focus of the present review is on mathematical models of cancer dynamics, and not on statistical modeling (such as absolute risk prediction models, or multiple regression analyses assessing epidemiologic risk factors), or specific algorithmic approaches (such as machine-learning approaches to specifying rules for genetic and molecular stratification of individual patients). Additionally, we differentiate between mathematical modeling of cancer dynamics or drug resistance and other related topics such as cancer imaging or detection algorithms, in which mathematics has historically played an instrumental role6-10. We consider the latter topics to be beyond the scope of the present review.
Human breast cancers
In 2016, it is estimated that 246,660 new cases of breast cancer with an estimated 40,450 deaths related to the disease will occur in the US, for a calculated 4:25 death to incidence ratio11. The high 5-year survival rate following diagnosis has been attributed to the higher rates of screening for breast cancer in the general risk population, patient presentation during early stages when prognosis is good, and to the effectiveness of surgery, radiation and systemic therapies, such as chemo- or hormonal therapies. Women with localized disease may have no symptoms and be identified by screening or they may present with specific disease symptoms such as a breast mass, skin changes or irritations, nipple discharge other than breast milk, or lumps in the underarm area12. These symptoms however, are not exclusively indicative of a malignant disease, and can also be signs of less serious conditions, such as infection, cysts or other benign masses in the breast.
The target organ
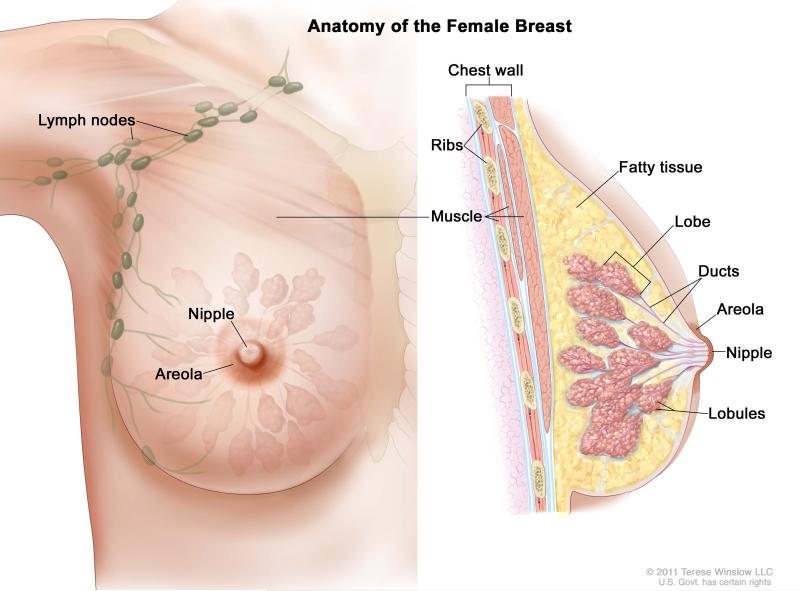
Breast cancers represent a collection of malignancies that arise in the epithelial cells of the breast (Figure 1). Histologic subtypes include the ductal (70-80% of diagnosed cases), lobular (10-15% of diagnosed cases), or medular13 (3-5% of diagnosed cases). About 65% of breast cancers are either estrogen receptor (ER) or progesterone receptor (PR) positive14; these hormone receptor-positive cancers tend to have a higher 5-year survival rate compared to other subtypes15. Other subtypes include HER2-positive breast cancer, characterized by HER2 protein overexpression or HER2 gene amplification, and triple negative breast cancer (TNBC), which lacks ER/PR expression and HER2 amplification or overexpression.
Figure 1.
“Breast Anatomy Female”: For the National Cancer Institute © 2011 Terese Winslow LLC, U.S. Govt. has certain rights.
Morphomolecular analyses have revealed further novel ways of classifying breast cancers16,17. Based on comprehensive genomic classifications17, breast cancers have been divided into four groups: i) the luminal A subtype, which is ER and/or PR positive and HER2-negative, and has a low Ki67 proliferative index; ii) the luminal B subtype, which is ER and/or PR positive and may be HER2 positive, has a high Ki67 index and has a poorer prognosis than the luminal A subtype18; iii) the HER2-enriched subtype, which displays extra copies of the HER2 gene and lacks ER or PR expression, and is more aggressive than the luminal subtypes; and iv) the basal-like subtype, which is largely comprised of TNBC. The latter is a high-grade cancer that grows quickly and has the worst prognosis compared to all other subtypes15. The triple-negative subtype tends to occur more often in younger, premenopausal women19, and is thought to be more prevalent in some high-genetic risk patients (as defined by the National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology20), specifically in germline BRCA1 mutation carriers21. These high-genetic risk patients also have an increased risk of developing ovarian cancer at some point in their life22.
Prophylactic and early detection strategies
The goal of any screening test for breast cancer is early detection and decrease in cancer-related mortality. So far, existing non-invasive tools for screening and detecting early stage breast cancers of all subtypes in general risk women include imaging procedures, such as mammograms and magnetic resonance imaging (MRI), and clinical breast and self-breast examinations. Screening imaging procedures can be used to view and evaluate breast tissue changes, but do not always demonstrate adequate sensitivity or specificity23.
In 2016, the US Preventive Services Task Force (USPSTF) updated their recommendations for mammography screening24. Their most recent recommendation stratifies screening by age, recommending against routine screening in general risk women aged 40-49 years and for biannual screening mammography in asymptomatic women without known genetic mutations aged 50-74 years. In the case of high-risk women, USPSTF recommends that these women be considered for an annual MRI in addition to an annual screening mammogram. Additionally, some high-genetic risk women voluntarily choose to undergo prophylactic mastectomies, which have been proven to reduce breast cancer incidence in this subset of women by approximately 90%25.
For the time being, there is evidence that screening for breast cancer with mammography and MRI reduces breast cancer mortality, with the greatest benefit of screening occurring in women aged 60-69 years24. However, debate surrounding the issues of frequency of screening (i.e. annual versus biannual), low specificity of screening methods and overdiagnosing of non-invasive ductal carcinoma in situ in younger, reproductive age women remains26-29.
Current treatment strategies and targeted therapy
Standard treatments for breast cancer subtypes include surgery (usually a mastectomy or lumpectomy with either sentinel node biopsies or axillary lymph node dissection), adjuvant systemic hormonal and/or chemotherapy, and localized radiation therapy (externally targeted post-surgery, or administered internally during surgery). To exploit the synergistic effects of using multiple drugs, systemic combination therapies are used, through combinations of anthracyclines such as doxorubicin, alkylating agents such as cyclophosphamide, platinum compounds such as cisplatin or carboplatin, or taxanes such as paclitaxel or docetaxel. For example, dose-dense doxorubicin and cyclophosphamide followed by paclitaxel is used in treating early stage breast cancers in the adjuvant or neoadjuvant setting30. Depending on the hormone-receptor status of the cancer upon diagnosis, subtypes of early, localized or metastatic breast cancers may also be treated with hormone-based therapies, such as tamoxifen, which blocks the estrogen receptor, or aromatase inhibitors, which prevent the production of estrogen in post-menopausal women31.
In addition to systemic chemo- or hormone-based therapies, targeted therapies blocking breast cancer cell growth in subtype-specific ways are also used. For example, monoclonal antibodies, such as trastuzumab and pertuzumab, are used in early stage and metastatic HER2 positive cases; lapatinib, a tyrosine kinase inhibitor, is used in metastatic HER2 positive cases, and palbociclib, a cyclin-dependent tyrosine kinase 4/6 inhibitor, is used in treating metastatic ER positive, HER2 negative cases32.
Development of drug resistance: implications for predicting recurrence
Initial response rates to current standard treatments for breast cancer patients are very high (more than 90% of primary breast tumors respond to the first-line therapy5). Yet, with current treatments, more than 20% of the HER2 positive or ER/PR positive cases, and about 40% of the TNBC cases are expected to recur by 10 years after primary tumor diagnosis33. Recurrent breast cancer is often associated with drug resistance, and most patients die with progressive resistant metastatic disease34. Factors that mediate clinical breast cancer recurrence and the timing of drug resistance remain largely unknown, but may be strongly influenced by the presence of dormant breast cancer cells35.
Several mechanisms of cellular resistance to existing breast cancer therapies have been proposed. One possible mechanism of drug resistance is the overexpression of the multi-drug resistance protein 136 (MDR1) , or the breast cancer resistance protein37 (BCRP). Other proposed mechanisms include the overexpression of β1-integrin levels in ER positive metastatic breast cancer patients38, or the acquisition of activating mutations in the ER in hormone resistant metastatic ER positive patients, previously treated with anti-estrogens and estrogen deprivation therapies39.
A further, more recently proposed drug resistance mechanism is the emergence of secondary mutations restoring BRCA function in BRCA1 or BRCA2-deficient patients and enabling cells to survive subsequent drug-induced cellular apoptosis and genomic aberrations40,41. BRCA1 and BRCA2 are tumor suppressor genes considered instrumental in repairing damaged DNA via homologous recombination repair mechanisms42. It is estimated that about 31% of diagnosed TNBCs and 50% of diagnosed high-grade serous ovarian cancers (HGSOCs) initially have some abnormality in their DNA repair mechanisms43. BRCA1 or BRCA2-deficient carcinomas have decreased capacity to repair DNA, and show high responsiveness to platinum agents44. They are also found to be susceptible to synthetic lethality via poly ADP-ribose polymerase inhibitors (PARPis) monotherapy. However, after the emergence of secondary mutations restoring BRCA function, some of the BRCA1 and BRCA2-deficient breast and ovarian cancers are no longer sensitive to PARPi-induced synthetic lethality45-47. The subsequent proliferation of these cells is thought to lead to the lack of response to subsequent treatment and disease recurrence41.
We note that despite the recent advances in early detection and understanding of the molecular bases of breast cancer biology, the precise biological pathways implicated in the development of breast cancer resistance and recurrence remain largely unknown. Great interest in elucidating the molecular mechanisms contributing to clinical drug resistance in breast cancers exists. Exploring and classifying these mechanisms through joint basic, mathematical, and translational research efforts is clinically relevant and crucial for advancing therapeutics.
Human ovarian cancers
In 2016, it is estimated that 22,280 new cases of ovarian cancer with an estimated 14,240 deaths related to this disease will occur in the US, for a computed 2:3 death to incidence ratio11. The high mortality rate following diagnosis has been partly attributed to the approximately two thirds of patients presenting with advanced stage, when recurrence is common, and leads to the incurable disease. Several studies suggest the usefulness of a symptom index tool to identify women who may have ovarian cancer; this includes new (within 1 year) and persistent (more than 12x/month) pelvic/abdominal pain, abdominal size/bloating, difficulty eating/feeling full, and urinary urgency/frequency48,49, which should trigger evaluation by a gynecologic oncologist. The extent and quality of surgical debulking has important prognostic value and is an integral part of the upfront management of ovarian cancer patients. In addition, the absence of accurate early detection screening methods poses additional difficulties in reducing ovarian cancer mortality levels50.
The target organ(s)
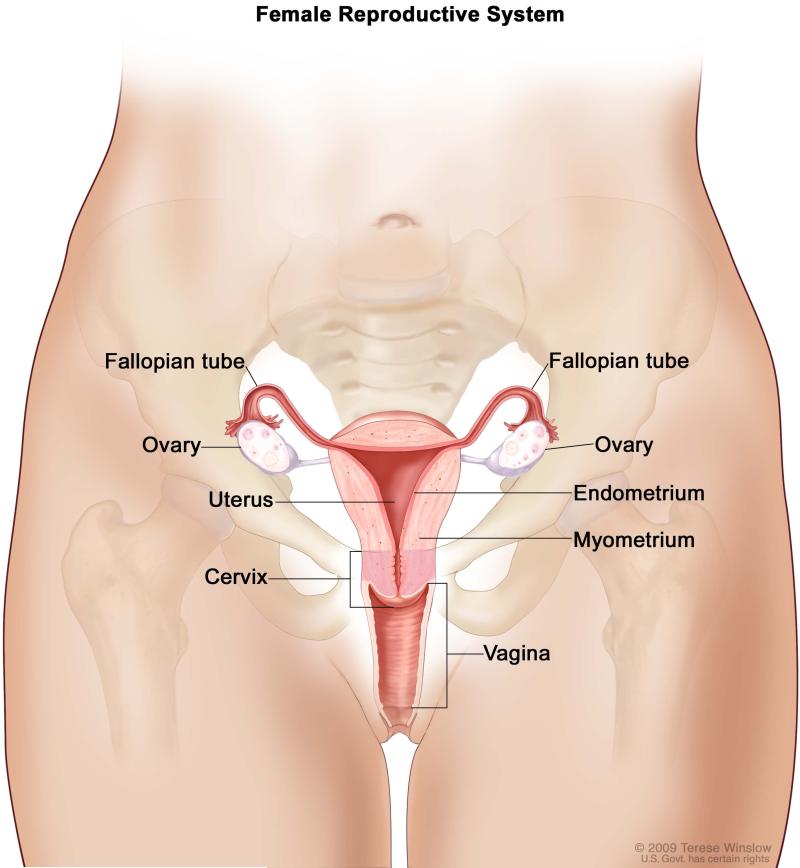
It is becoming increasingly recognized that ovarian cancers do not constitute a single disease, but rather a family of non-uterine tubo-ovarian cancers51-53 (Figure 2). Ovarian tumors may develop from epithelial, stromal or germ cells54. About 10-15% of all ovarian malignant tumors are nonepithelial in origin, are often found at an early stage, and generally have a good prognosis55. Epithelial ovarian cancers constitute about 85-90% of all ovarian cancer cases, with a subset of these epithelial ovarian cancers, HGSOCs, representing nearly 70% of all ovarian cancer cases56. HGSOC is considered to be an aggressive histological subgroup of the ovarian malignancies53. Although the 5-year survival rate for stage I ovarian cancer is greater than 80%, stage I diagnoses represent the exception rather than the rule11. Most patients present with stage III/IV tumors, for which the 5- year survival rate is less than 30%2.
Figure 2.
“Female Reproductive System”: For the National Cancer Institute © 2009 Terese Winslow LLC, U.S. Govt. has certain rights.
One of the obstacles to the detection of early-stage HGSOC, has been the poor understanding of its histopathogenesis. It was initially thought that epithelial ovarian tumors originate from the ovarian surface epithelium, a single cell layer covering the ovaries, coming from the coelomic epithelium57,58. Recent data suggest secretory cells inside the distal fallopian tubes give rise to the earliest precursor lesions in a proportion of HGSOC cases57-60. This finding was first reported in women with germline BRCA1 and BRCA2 mutations undergoing a prophylactic bilateral risk-reducing salpingo-oophorectomy procedure61-63. For the general-risk population however, it remains unclear whether diagnosed ovarian tumors commonly arise with apparently fallopian tube involvement, or constitute truly ovarian-derived diseases.
Prophylactic and early detection strategies
Any proposed screening strategy should be highly sensitive and specific to detecting truly malignant ovarian cancer cases. Transvaginal ultrasound (TVU), serum biomarkers (CA125, HE4) testing, pelvic examinations or simultaneous TVU and CA125 testing have been examined as non-invasive tools for detecting early stage ovarian cancer in general risk women. However, mounting evidence suggests that annual screening with TVU and serum biomarkers, does not reduce mortality64. Furthermore, high false positive rates leading to intervention are associated with potential harm, such as unnecessary surgical intervention and related complications. The lack of utility of such screening tools is possibly due to the absence of adequate TVU sensitivity in detecting small increases in tumor volume and distinguishing between malignant and benign cases65. Furthermore, the addition of serum biomarkers testing (e.g. CA125 levels) does not improve early-stage detection levels either50,66.
The largest ever ovarian cancer screening study of 202,638 general risk women demonstrated that multimodal screening including serial TVU and CA125 level testing yielded a 15% mortality reduction rate compared with a 0% no screening or 11% unimodal TVU-based screening cohort mortality reduction rate over 0-14 follow-up years64,67. However, this study also showed increased CA125 levels can be detected in benign conditions, rendering it inadequate for use in screening asymptomatic women for early-stage ovarian cancer. The lack of adequate early-detection tools might be explained by the fact that any epithelial ovarian cancers diagnosed in stage I might be fundamentally different from those diagnosed in advanced stages, which are preponderantly high and not low-grade cases68.
USPSTF has recently reconfirmed their previous recommendation against screening in asymptomatic women without known genetic risk for ovarian cancer69; existing screening methods are either not recommended as uni- or multimodal prognostic markers for low HGSOC volume detection28,70,71, or have not been shown to confer a mortality benefit in general risk women72. High-genetic risk women should be considered for genetic counseling and offered the option to undergo regular monitoring via a combination of TVU examinations and CA125 tests. Additionally, many high-genetic risk women voluntarily choose to undergo various risk-reducing gynecologic surgeries, such as prophylactic oophorectomies, bilateral salpingo-oophorectomies or hysterectomies28,73-77, which have been proven to reduce mortality from both breast78 and ovarian cancer62,74,79. Collectively, the question of whether ovarian cancer is a valid target for routine screening in general or high genetic-risk women still remains highly controversial67,71,80,81.
Current treatment strategies and targeted therapy
Standard treatments for ovarian cancers include debulking surgery, (neo)adjuvant platinum-based combination chemotherapy, such as cisplatin or carboplatin, and a taxane such as paclitaxel or docetaxel. In addition to systemic chemotherapy, targeted therapies such as small molecule tyrosine kinase inhibitors are also used. For example, platinum-resistant recurrent ovarian cancers have been shown to respond to angiogenesis inhibitors (e.g. bevacizumab) in combination with chemotherapy, which restrict cancer growth by suppressing the development of blood vasculature to the tumor82. Additionally, PARPis have been used in clinical trial settings and the PARPi olaparib (Lynparza) was recently approved by the US Food and Drug Administration (FDA) as a therapy for germline BRCA-mutations associated recurrent ovarian cancer83.
Development of drug resistance: implications for predicting recurrence
Initial response rates to current standard treatments for ovarian cancer patients are high (70%-80%), but the majority of women with advanced disease relapse with within two years5. Recurrent ovarian cancer is not curable, and most women eventually develop platinum-resistant disease.
Several mechanisms of cellular resistance to platinum compounds or PARPis have been described, such as intracellular cisplatin inactivation via augmented glutathione synthesis84-86, or reduced intracellular drug concentration via overexpression of MDR1 protein acting as a drug efflux transporter87. Another possible mechanism of platinum resistance is the development of secondary mutations restoring functions of BRCA and other proteins of homologous recombination repair in BRCA-mutation related ovarian cancers. However, BRCA restored functionality does not explain all cases of cisplatin resistance in these patients40,41, since not all platinum-resistant recurrent ovarian carcinomas exhibit detectable secondary mutations. Norquist et al. showed only 12 of 26 (46%) platinum-resistant recurrent cases had detectable secondary mutations restoring BRCA function45. Further pre-clinical and clinical investigations of mechanisms of platinum or PARPi resistance are thus needed. Larger-scale prospective and retrospective cohort studies are warranted in order to better examine therapy response heterogeneity and adaptability of the ovarian cancer genome under the selective pressure of cytotoxic therapies46,52,88.
MATHEMATICAL MODELS OF WOMEN'S MALIGNANCIES
While considerable mathematical modeling effort has been directed towards modeling carcinogenesis, cancer progression, and cancer treatment6,89-91, relatively few mathematical investigations have been published specifically targeting the breast and ovarian cancers. After a comprehensive literature search, we note that, to best of our knowledge, the models presented below represent the majority of the published breast and ovarian cancer mathematical models. We thus chose to discuss these mathematical models in detail, and highlight areas where novel in silico modeling frameworks could be further developed.
Breast cancer
Modeling disease natural history and the tumor growth law controversy
The pattern of growth of human breast cancers is clinically important, specifically for estimating the duration of pre-diagnosis silent growth and for the design of an optimal post-surgery chemotherapeutic schedule. The theoretical study of breast cancer growth patterns has been the subject of considerable debates and controversy among mathematical oncologists for over two decades. For a more comprehensive recent review and theoretical comparison of the various mathematical formulations used to model tumor growth dynamics, we recommend the interested reader to consult Ribba et al.92.
In 1984, Speer et al. proposed a stochastic numerical model of breast cancer growth wherein all individual tumors are assumed to grow with identical Gompertzian parameters but subsequently develop kinetic heterogeneity by random time-dependent processes93. The basic growth model of Speer et al. is illustrated in Figure 3A. Speer et al. estimated that the average time for the tumor burden to increase from 1 cell to detection was on average 8 years. Their model also derived quantitative relationships between the likelihood of remission versus number of years after mastectomy, and the number of metastatic sites versus number of positive nodes in an in silico cancer-positive cohort. This approach to modeling breast cancer growth kinetics allows for the generation of individual growth curves, rather than averaged, population-based cancer growth dynamics. However, growth dynamics similar to the one quantitatively proposed by Speer et al. are yet to be confirmed in vivo or in vitro, and may involve unnecessary additional parameterization.
Figure 3. The breast cancer growth models proposed by (A) Speer et al.93 and (B) Norton94.
(A) The first equation corresponds to classical Gompertzian growth kinetics, where N(t) is the number of tumor cells measure at time t after the start of tumor growth, A0 is the initial growth rate, and α is the rate of growth decay. In this model, time is incremented in intervals of 5 days. A random number, r1, between 0 and 1 is generated at each time interval, and compared to a predetermined value A4, defined as the probability that α undergoes a change in a 5-day period. If r1>A4, the tumor continues growing at the previous rate. However if r1<A4, α is reduced by an amount depending on A4, r2, another randomly generated number between 0 and 1, and A3, the predefined determinant of the amount of change in α. This process is illustrated in the second equation. Computations of the simulated growth curves are performed until either simulation time runs out, set at 40 years elapsed since the beginning of tumor growth, or until a lethal in silico tumor burden threshold is reached, set at N(t)=1012 cells. The Speer et al. model beings with 1 cell at time 0 and uses predefined values of A0, α, A4, A3. A0 and α are expressed in units of days−1, and A3 and A4 are dimensionless. Simulation time is measured in days. Each computed growth curve is generated using the same baseline parameter set.
(B) The first equation corresponds to classical Gompertzian growth kinetics, where N(t) is the number of tumor cells measure at time t after the start of tumor growth set at t = 0, N(0) is the tumor starting size, b is the rate of growth decay, and N(∞) is the limiting size. To generate the probability distribution function of b, the proportion, PL(t) of patients who have died by time t since the onset of symptoms after having reached the lethal tumor size, NL, is generated from the 250 breast cancer survival curve data set in (Bloom et al.). Re-arranging the Gompertz, Equation (2) is obtained, where PL(ti) represents the proportion of the 250 cancers with growth decay rate b<bi. The model is initialized with a set of initial values for N(0), NL, and N(∞), and a least-squares based numerical algorithm is used to determine the mean and standard deviation of b. A randomly generated initial value for bi is then chosen from the computed distribution to calculate ti to ensure N(ti) = NL, and the process described in the second equation is repeated until the value of bi that provides the best fit to PL(ti) is found.
In contrast to the Speer et al. model, Norton et al. demonstrated in 1988 that the deterministic Gompertz equation provided the best fit when used to relate clinical breast cancer tumor sizes and rates of regression post-therapy94. Using a data set comprising of 250 women with untreated diagnosed breast cancer, during the years 1805 to 1933 in the UK95, Norton generated a hypothetical survival curve fitting the classical Gompertzian growth model to the percentage of surviving patients per year after diagnosis (see Figure 3B). He estimated the probability distribution function of the growth decay parameter to be log-normal and dependent on the current number of tumor cells, in contrast to the stochastic nature of the equivalent parameter used by Speer et al93,96. While the model proposed by Norton fits clinical data on untreated breast cancer, it is unclear whether Gompertzian kinetics (or a variant of it) also applies to the disease progression during or post therapy.
Untreated breast cancer growth rates prior to diagnosis were also calculated by Spratt et al. in 199397. They used data derived from mammographic breast cancer tumor measurements and conducted a least squares regression analysis to prove that a generalized logistic equation provided the best fit to the observed data. The mathematical analysis performed excluded data from patients whose tumors were clinically detected between two consecutive mammogram screenings, or whose tumors showed no change in size during the period of clinical observation. Using their growth model, Spratt et al. generated probability distributed functions of tumor doubling time at mammographic detection and untreated tumor size increase after 1 and 2 years after detection. While the model quantitatively underscores the significant natural variability in untreated human breast cancer growth rates, it is unclear from this approach how histological and morphomolecular characteristics influence modeling results. Moreover, the reported mathematical results might have been selectively biased towards reflecting the progression of slower growing tumors, as these tumors are more representative of clinical cases amenable to detection via regular mammographic screening.
In a different attempt at modeling breast cancer natural history, Koscielny et al. considered in 1985 two patterns of growth, exponential and Gompertzian, in order to assess the timing of initiation of distant metastases using observed data in breast cancer patients98. For both growth patterns, Koscielny et al. estimated that the median metastasis growth duration is about 3.8 years, and that a 30% reduction in metastases incidence is predicted if the primary tumors are treated 12 months earlier. In order to assess the time at which metastases are initiated, Koscielny et al. assume a linear relationship between doubling times of primary breast tumors and of their metastases. However, the validity of such a limiting assumption is empirically questionable as, to best of our knowledge, a deterministic relationship between the two has not been established in any published in vitro or in vivo investigations.
We note that none of the models discussed above, specifically focuses on a breast cancer subtype. It is however empirically known that different subtypes exhibit differential growth rates and clinical progressions99. For example, luminal ER positive breast cancers are thought to have a slower growth progression than the TNBC subtypes; moreover, interval breast cancers, defined as cancers detected between two consecutive screening examinations, are more likely to be ER and/or PR negative than screen-detected breast cancers100. Additionally, distinct morphomolecular tumor subtypes can be concomitantly reported in the same breast cancer patient101, underscoring the importance of prospectively assessing spatial intrapatient tumor heterogeneity prior to treatment initiation102. Arguably, considerable biological realism and any translational/clinical relevance of such modeling results are lost if existing mathematical models aggregate the various breast cancer subtypes into one singular disease. Moreover, the existing models use population-based cohort studies in order to predict aggregate clinical statistics of untreated breast cancer growth and model heterogeneous timing of cancer progression in the in silico cohorts. Yet, the extent to which such models contribute to understanding subsequent therapeutic outcomes remains unclear. We point out that hardly any clinically diagnosed breast cancer is nowadays left untreated and the impact of existing therapeutic strategies on growth rate dynamics remains an open clinical question that cannot be quantitatively determined using the existing mathematical models.
We thus argue the need for mathematical models that better integrate current clinical and morphomolecular data, and aim at modeling spatiotemporal breast cancer growth dynamics and heterogeneity.
Modeling sporadic and hereditary breast carcinogenesis
In a more recent attempt in modeling breast cancel natural history, De Vargas Roditi and Michor considered a mathematical model for the evolutionary dynamics of BRCA1 mutations in order to explain the differential role of BRCA1 mutations in sporadic and hereditary cancers103. Initially, the populations of cells within the breast tissue was assumed to be proliferating according to a stochastic Moran process. A linear ODE approximation of the stochastic process was then used to model the different evolutionary trajectories involving spontaneous or hereditary mutations in the BRCA1 and a different tumor suppressor gene. Tumorigenesis was assumed to initiate once sufficiently many mutations in these genes accumulated. The model was calibrated based on published statistics regarding the role of BRCA1 mutations in sporadic and hereditary breast cancers: sporadic BRCA1 mutation incidence, the prevalence of germline BRCA1 mutation carriers, and the differential probability of developing breast cancer in these two subsets of patients. The main results predict that the loss of one BRCA1 allele in combination with the homozygous loss of a different tumor suppressor gene confers a fitness disadvantage compared to a single allele alteration in both genes. Moreover, a cell with two mutated BRCA1 alleles and one mutate tumor suppressor gene allele has a fitness advantage compared with a cell with alterations only in the BRCA gene.
We note that the total cancer population size considered susceptible to genetic alterations in the model was kept constant at very low levels. It would be useful to extend the mathematical framework to more realistic tumor growth models and population size, to validate whether the effect of the heterozygous BRCA1 phenotype and its differential involvement in sporadic and hereditary tumorigenesis are maintained in human tumors.
Modeling therapeutic targeting and treatment
Mathematical models for the growth and invasion of breast cancer tumors into the surrounding tissue, together with modeling frameworks for surgery and radiotherapy were first developed by Anderson et al.104,105. To simulate breast cancer growth prior to diagnosis and quantify the effects of conventional fractionated radiotherapy versus more targeted irradiation, Anderson et al. initially developed a continuum model of ordinary (ODEs) and partial differential equations (PDEs)106. Numerical schemes were subsequently adapted to model the results of simulating radiotherapy treatment in the two scenarios107. A basic description of the ODE-PDE model incorporating the effects of radiotherapy is illustrated in Figure 4. Enderling et al. found that high doses of targeted internal radiotherapy administered immediately post-surgery are more likely to eliminate residual occult sources of cancer recurrence, rather than fractionated external radiotherapy doses. In the latter case, their model simulations showed that a fraction of the remaining tumor cells would be able to repair radiation-induced DNA damage and favor the development of a recurrent tumor.
Figure 4. The ODE-PDE framework used to model breast cancer development, treatment and recurrence105, subsequently used to model radiotherapeutic strategies107.
The system of equations describing the interactions of tumor cells (n), extracellular matrix (f), and matrix-degrading enzymes (m) is illustrated in the first equation. Therein, the terms in the first equation correspond to cellular proliferation, random motility and haptotaxis, defined in the model as the movement of tumor cells according to gradients of chemicals in the tumor environment. The second equation corresponds to the extracellular matrix degradation by existing tumor cells. Lastly, the terms in the third equation correspond to the diffusion of matrix-degrading enzymes secreted by the tumor cells, the production of new enzymes and natural decay.
The fourth equation represents the biologically effective dose 102, where n is the number of radiotherapeutic fractions administered, d is the dose delivered per fraction, α is the coefficient of single-hit DNA double-strand breaks, and β is the number of DNA single-strand break pairs that combine into forming double-strand breaks. d is measured in Gray. The surviving probability S, i.e. the proportion of cells that survive the radiation-induced damaged is modeled in the fifth equation.
It is important to note that like in many other mathematical models, some of the modeling assumptions could potentially influence the qualitative nature of the results. First, the radiotherapeutic doses were assumed to be uniformly administered throughout the simulation domains, which has not been validated in practice108. Second, tumors do not generally grow in a radially symmetric fashion, and thus spatial cellular heterogeneity could be an important factor when predicting disease progression or therapeutic outcomes. Third, a recently published phase III, randomized clinical trial performed on patients with early invasive and in-situ breast cancer showed no difference between intraoperative and external beam radiotherapy with respect to local recurrence, disease-free survival, and overall survival, in contrast to the predicted modeling results109. Lastly, it is well known that the heterogeneous nature of breast cancers mediates differential response outcomes among women treated with radiotherapy110. Hence, the models of Enderling et al. could be potentially improved by including the appropriate disease subtype, using corresponding experimental parameters and subsequently calibrating against the relevant clinical trial data.
In a different attempt to model breast cancer treatment, Roe-Dale et al. used an ODE model to incorporate cell cycle specificity and resistance to study why differential scheduling of CMF and doxorubicin doses used in breast cancer patients yield different clinical outcomes111. In their model, they simulated alternating and sequential regimens assumed to be delivered instantaneously to the tumor cell population and to kill a constant fraction of the cell each dose. Figure 5A illustrates the basic modeling framework used in this mathematical investigation. Roe-Dale et al. assumed cellular resistance induced by increased MDR1 expression, and modelled resistance following drug administration by instantaneously converting a specified fraction of the cells from the sensitive to the resistant compartments. To investigate differential treatment outcomes due to cellular resistance, Roe-Dale et al. used parameters derived from in silico uniform distributions, and performed sensitivity analyses for specific parameter combinations to determine the parameter spaces that yielded the greatest regression (or alternatively the lowest progression) of the in silico tumor cell burdens. Their computational results indicated that the sequential CMF plus doxorubicin treatment results in a 62% survival rate, compared with a 50% survival rate for the alternating regimen. Roe-Dale et al. also found that the two regimens resulted in differential outcomes at a statistically significant level in their simulated in silico patient cohort, and concluded that the sequential treatment would be preferred to the alternating one.
Figure 5. (A) The four compartment cell cycle and resistance framework modeled by Roe-Dale et al.111. (B) The two compartment cell cycle framework modeled by Panetta115.
(A) In the system of equations illustrated in (1), cells are separated by cell cycle status in two states, and drug sensitivity status in two other states, for a total of four possible states: either G1 or S sensitive (N1) or resistant cells (N3) and G2 or M sensitive (N2) or resistant cells (N4). In this model, resistant cells are defined as cells that express the activated MDR1 gene. The terms in each equation correspond to cell-specific constant transitions rates between the four compartments.
The treatment equation for the Roe-Dale et al. model is illustrated by (2), where N represents the matrix-vector notation for the four different compartments whose temporal dynamics is modeled by (1), Ti is the corresponding treatment matrix for drug i, and m is the number of administered treatments with drug i at time intervals of τ hours. The fraction of cells surviving treatment with doxorubicin (TA in the model) and with CMF (TC in the model) are described in Equations (3) and (4), respectively.
(B) In the system of equations illustrated in (1), P is the number of proliferating tumors cells, and Q is the number of quiescent tumor cells. Additional parameters include γ, the growth rate of proliferating cells, α, the transition rate from the proliferating to the quiescent compartment, δ, the natural proliferating cell death rate, β, the transition rate from the quiescent to the proliferating compartment, and λ, the natural quiescent cell death rate. All parameters in the model are assumed to be positive, and constant. Herein, the system outlined in (1) represents a linear system of ODEs modeling the dynamics of the proliferating and quiescent cell compartments. The function f(t) described in Equation (2) represents a step function describing the effects of the chemotherapeutic treatment, e.g. paclitaxel. The periodic function modeling the paclitaxel effects is assumed to target only the proliferating cell compartment. In its functional representation, s is the strength of the drug, a is the active drug time, T is the period of paclitaxel administration and n stands for the nth administered drug dose.
It is important to note that the constant cellular survival fractions illustrated in Equations (3) and (4) of Figure 5A and cellular interactions are not substantiated by current pharmacodynamic and kinetic models of CMF and doxorubicin in breast cancer patients112. It thus remains difficult to quantitatively extrapolate from the proposed model whether MDR1 resistance or rather cell cycle specificity is responsible for the observed superiority of the sequential treatment regimen versus the alternative one in breast cancer patient cohorts113. In addition, the value of the combination therapy mathematically studied in Roe-Dale et al. (i.e. CMF plus doxorubicin) with respect to recurrence and mortality rates has only been clinically established in high-risk early stage cancer patients, but does not otherwise represent current standard therapy protocols for all breast cancer subtypes or stages114. Finally, choosing to model the dynamics as a system of linear ODEs enables Roe-Dale et al. to derive analytical, closed form solutions. We note that such an approach is somewhat limited and could be mathematically adjusted to reflect non-constant dynamics, since clinical transition rates between the modeled compartments are most likely temporally varying.
Another attempt at incorporating cell-cycle specific drug kinetics and interactions is represented by the two-compartment linear ODE model proposed by Panetta115 and illustrated in Figure 5B. Panetta's model takes into account the proliferating cell population, which is assumed to be responsive to cell-cycle specific drug therapies, and the quiescent cell population, which is assumed to be non-responsive to paclitaxel treatment. Using published breast and ovarian cancer data116,117, parameters such as cell-cycle length, proliferative fraction, doubling time, transfer rates between the two compartments, and time spent in either resting or cycling phase were estimated for both the formulated breast and ovarian cancer models. Using the framework exemplified in Figure 5B, Panetta derived analytic, closed form solutions for the proliferating and quiescent compartments. A parameter sensitivity analysis with respect to active drug phase and drug administration times was performed. Using the model, Panetta studied the numerical range of the associated characteristic multipliers for which the simulated total cancer cell mass at the end of treatment period is substantially reduced. Considering a treatment period of 21 days, and a dose strength of 3 units, Panetta observed that for active phases smaller than 8.5 days, the maximum characteristic multiplier was greater than 1, which would translate in a clinical context into breast/ovarian cancer cell growth. For values of the active phase greater than 8.5 days however, the maximum characteristic multiplier was less than 1, which implied that cancer cell decay was ongoing. In the context of the duration of the treatment period (e.g. every 21 days), Panetta was able to conclude that longer active drug phase times with respect to the length of the treatment period lead to shrinking tumors, while shorter active phase times and fixed lengths of treatment periods lead to cancer growth in both the formulated breast and ovarian progression models.
While these modeling results correspond to current breast cancer clinical recommendations118, several of the modeling constraints used in the Panetta model could be relaxed in future work, in order to better integrate the related clinical knowledge. For example, the constant modeling parameters used in the current model could be modified to reflect the action of the drug. A further extension of the model could include a study of non-constant or stochastic growth parameters calibrated against human breast tumor growth data. Moreover, different functional forms could be used to represent the drug action term, or expanded to reflect multi-drug based therapies under study in current clinical trials.
Modeling in vitro invasive cancer cell kinetics
To simulate carcinogenesis and account for the role of angiogenesis in tumor recurrences, Gatenby et al. developed an individual-based, hybrid cellular automaton model in which mutant cells are initially separated from the blood supply by an intact basement membrane119. Their working hypothesis was that a local variation in substrate and glucose metabolite concentrations in ductal carcinoma in situ cells would initiate cellular adaptations required for the emergence of invasive breast cancers120. To test this hypothesis, Gatenby et al. used a two-dimensional cellular automata model, with rules governing the cellular evolution of normal and tumor cells, oxygen, glucose, and H+ fields satisfying reaction-diffusion equations. Each automaton was assumed to be representative of a single cell. The parameters were set to match quantitative data derived from experiments performed on MCF-7/HER2 breast cancer cells growing in spheroids. Simulations of the model demonstrate that malignant cells may evolve towards a phenotype that exhibits constitutive upregulation of glycolysis and resistance to acid-induced toxicity via increased H+ concentrations. Gatenby et al. predicted that the distinctive pattern of nodular growth and cellular evolutionary sequence was representative of late carcinogenesis, in which malignant cells must breech the basement membrane in order to transition to an invasive cancer type.
We point out that it is unclear whether upregulation of the hypoxic and glycolytic metabolic pathways can be considered a universal, constitutive emergent cellular phenomena in the clinical evolution of human breast cancers. Further clinical investigations are warranted to elucidate the validity of these quantitative modeling results applied to patient cases.
Modeling in vivo invasive cancer cell kinetics in mouse xenografts
Marusyk et al.121 developed a mathematical model of an initially heterogeneous tumor growth incorporating clonality interference. This model was developed to investigate the long-term impact of sub-clonal heterogeneity on tumor phenotypes and the possibility of competitive expansion of individual tumor sub-clones, formed after orthotopic transplantation into the mammary fat pads of immunodeficient mice. Two scenarios were considered: sub-clones either compete against parental cells (monoclonal primary tumors or compete against each other (polyclonal primary tumors). The paper concludes that an exponential growth pattern best explains the dynamics of the growth of the monoclonal tumors. In addition, nested ODE models of exponentially growing competing sub-clones were used to study the impact of sub-clonal heterogeneity on polyclonal tumor growth. The modeling results of Marusyk et al. suggest that in the absence of treatment, tumor heterogeneity is predicted to eventually vanish, in the presence of sub-clones with high proliferation rates that outcompete the less fit competitors. However, after treatment with doxorubicin, differences in competitive fitness between sub-clones are amplified. Additional clinical investigations are warranted to extend these conclusions to pre- and post-treatment patient samples.
Modeling stem cell dynamics
Liu et al. developed a mathematical model to explore the growth kinetics of breast cancer stem cells both in vitro (using cell culture experiments of MCF-7/HER2 cells) and in vivo122 (using tumor cells derived from primary MMTV-Her2 transgenic mice tumors). A system of ODEs was used to describe the population dynamics of cancer stem, progenitor and terminally differentiated cells; the model also included a negative feedback regulation on the division probabilities to produce differentiated or stem daughter cells. The Liu et al. model is illustrated in Figure 6. Simulation of the model predicted that the majority of tumor spheres grown in their experiments were derived from progenitor cells. They also showed that the frequency of tumor sphere formation increased with time while the frequency of cancer stem cells decreased over time. According to Liu et al., the proportion of cancer stem cells in any tumor sphere culture would be determined by the self-renewal frequency of stem cells during tumor sphere formation and the ratio of tumor sphere derived from the primary stem or progenitor cells. The results of Liu et al. also suggest that a therapy aimed at targeting the breast cancer stem cell population would be less effective in immediately shrinking tumor sizes, but more effective in the long-term suppression of tumor growth and prevention of tumor relapse.
Figure 6. The Liu el al. breast cancer stem cell model122.
The population dynamics of the cell types considered is illustrated in the above equations. Therein, xi(t) is the number of cells measured at time t of type i, where i=0 is the cancer stem cell phenotype, i=1 is the progenitor cell phenotype, and i=2 is the terminally differentiated cell phenotype. p0(p1) is the probability that a cancer stem cell (progenitor cell) divides into 2 cancer stem cells (progenitor cells), q0(q1) is the probability that a cancer stem cell (progenitor cell) divides into 2 progenitor cells (terminally differentiated cells), v0 and v1 are the synthesis rates representing the transition rates from the cancer stem to the progenitor cell compartment and respectively, from the progenitor cell to the terminally differentiated cell compartment. Lastly, di, where i = 0, 1 or 2, represents the death rates of cells of type i.
We point out that the mathematical model proposed by Liu et al. does support the empirical observations derived from the adjacent in vitro and in vivo MMTV-Her2 transgenic mouse experiments performed in this investigation. However, it is unclear whether the modeling inferences can be translated to human breast cancer recommendations, since the presence and effect of human breast cancer stem cells on therapeutic outcomes are highly debated in the literature13,123,124.
Ovarian cancer
Modeling disease natural history and early detection strategies
Focusing exclusively on unimodal TVU screening, Danesh et al. developed a mathematical model to study the frequency at which ovarian cancer screening should be done in order to be effective125. To model ovarian cancer growth and progression, Danesh et al. developed a multi-type branching processes model, where the different types represent stages in the serous epithelial ovarian cancer progression, but without differentiating between the low and high-grade subtypes. In their model, type 0 cells are present in the primary tumor in the ovary or fallopian tube, type 1 cells are floating in the abdominal cavity, and type 2 cells are attached to the peritoneum. Type 2 cells are assumed to infiltrate the cellular matrix and eventually metastasize to distant organs, so that when they are present in significant (and clinically detectable) numbers, the cancer would be classified as stage III. In addition to giving birth to nonmutant offspring or dying, cells may transition from type 0 to type 1 and from type 1 to type 2 at differential rates. These rates were then used as transition rates in a continuous time Markov chain. In the model, transitions between types are assumed to involve migration of cells rather than genetic mutations. Results for the multi-type branching process that enables Danesh et al. to quantitatively estimate the behavior of all three cell types are illustrated in Theorems 1-3125.
In order to address their motivating question, Danesh et al. introduced the concept of a “window of opportunity” for screening, defined as the difference between the time when cells of type 2 reach 109 cells (corresponding to a late-stage tumor) and cells of type 0 reach 6.5×107 cells (corresponding to the detection threshold). Using data on tumor growth from Brown and Palmer126, they concluded that the window of opportunity corresponds to 2.9 years, with most of the distribution concentrated between 2.5-3 years. According to their model, TVU-based ovarian cancer screening should occur biannually.
The underlying assumption in the Danesh et al. modeling framework is that metastatic ovarian cancer cells growth at a significantly higher rate than primary tumor cells. This assumption has yet to be validated clinically. In contrast to the dynamics portrayed in this work, it is generally believed that ovarian cancers that present clinically during early stages do not necessarily represent precursors to cancers that, if left undetected, would otherwise present at advanced stages60,127. Moreover, the theoretical results derived assume exponential stage residence times and an infinite time period when determining cancer growth and progression. Using non-exponential growth kinetics and a finite observation time (as done empirically) could alter the modeling outcomes. Finally, in contrast to the biannual TVU-based ovarian screening recommendation based on the Danesh et al. modeling inferences, latest data from ovarian cancer screening randomized controlled trials demonstrate the failure of annual, unimodal TVU examinations to improve ovarian cancer detectability and overall survival rates64.
In another attempt to model the preclinical natural history of serous epithelial ovarian cancer as a function of tumor size, stage and its implications for TVU-based screening126, Brown and Palmer identified and analyzed available reports on occult ovarian cancers, and used a comprehensive meta-analyzed of published data to model the growth, progression and TVU-based detection of ovarian cancer62,74,79. Data were collected from published studies of healthy germline BRCA1 and BRCA2 mutation carriers25,63, who had their ovaries and fallopian tubes removed prophylactically. In some of these women, unsuspected ovarian cancers were discovered upon surgery61. Brown and Palmer performed a Monte Carlo simulation of tumor life histories to fit an exponential in silico model for tumor growth using parameters derived from their meta-analysis, with separate growth rate parameters for early and advanced stage cancers. A basic description of the theoretical tumor growth model proposed by Brown and Palmer is demonstrated in Figure 7. In this model, the “window of opportunity” was defined as the time duration for early detection, i.e., the time during which the tumor is expected to remain early stage (localized or regional). They estimated the window of opportunity for TVU detection of early stage cancers to be around 4.3 years, and predicted that most detected advanced stage serous cancers would have become advanced a median of 0.8 years prior to detection.
Figure 7. The tumor growth model proposed by Brown and Palmer126.
Tumors in both early and advanced stages were assumed to grow exponentially. Specifically, the early stage tumor growth model is illustrated in Equation (1). Therein, a is the size at which a particular tumor is detectable by histopathology, b is the (exponential) growth rate constant and t1 is the time since the tumor became detectable by histopathology. Detection thresholds for each individually simulated growth curve were set to match the corresponding value found in the collected tumor data set.
The advanced stage tumor growth model is illustrated in Equation (2). Therein, c is the log value of the tumor size at disease progression from early to advanced stage (estimated from the Monte Carlo simulation of tumor life histories), d is the difference between the log values of the tumor size at empirical diagnosis obtained from the collected tumor data set and the log value of the size at progression from the generated simulation, and t2 is the in silico measured time since progression.
Similar to the Danesh et al. model, the underlying assumption in the Brown and Palmer quantitative analysis is that ovarian cancers that are clinically present during early stages are precursors to cancers that if left undetected, would otherwise present at an advanced stage. We point out that a timely detection of low volume ovarian cancer, which does not necessarily represent early stage disease, should be the goal of any screening studies as well as of any mathematical modeling framework aimed at exploring screening scheduling. Moreover, while prophylactic risk-reducing bilateral salpingo-oophorectomies or hysterectomies are predominantly performed in high-genetic risk women who exhibit an increased risk of developing HGSOC, the data used by Brown and Palmer to calibrate the estimated tumor growth rate were collected from studies where proper histological classification was not performed. Some of the reported clinical cases were non-HGSOC, and ovarian cancer subtypes were aggregated into one singular disease. Failing to recognize such confounding factors could potentially underestimate quantitative data generated by the mathematical model.
To assess dynamic plasma biomarker kinetics in relation to the genesis of cancer, Hori and Gambhir incorporated tumor growth into a linear one-compartment biomarker secretion model, beginning with a single parental tumor cell128. The model is shown in Figure 8. Hori and Gambhir aimed to quantify the time required for a growing malignant tumor cell population to reach a sufficient size so that its shed blood biomarker levels were high enough to be detectable by current clinical blood biomarker assays. The model was then used to calculate changes in the detection capability based on log-order perturbations in the parameters fundamental to biomarker shedding. Tumor cell growth was represented by either the exponential or Gompertzian model, while the healthy cell population was assumed constant. Hori and Gambhir aimed to identify the biomarker-related parameters that would most greatly impact blood-based early cancer detection, and quantify how far each baseline parameter value would need to change in order to achieve earlier, sub-millimeter tumor detection. It was found that tumors in the mm diameter range could only be detected under ideal conditions of extremely high rates of biomarker secretion by tumor-associated cells and zero background level from healthy cells.
Figure 8. The plasma biomarker temporal dynamics model of Hori and Gambhir128.
The change in the mass of the plasma biomarker with respect to time is equal to the difference between the influx of plasma biomarker shed by the tumor cells, uT(t), healthy cells, uH(t) and the outflux of biomarker from the plasma, qEL(t), are as illustrated in the first equation. The rate of biomarker entry into the plasma is the sum of the input from tumor cells (as modeled in the second equation) and from healthy cells (as modeled in the third equation). Tumor cell growth is represented here by either the Gompertzian growth model (the fourth equation) or the exponential growth model (the fifth equation). The healthy cell population is assumed to remain constant throughout simulation time, and is set at NH(t) = NH,0.
Hori and Gambhir concluded that clinical implementation of a CA125 biomarker-based early serous epithelial ovarian cancer detection would be extremely difficult for the time being. Several limiting assumptions were made in the current model that could potentially be addressed by future mathematical framework modeling fluctuating biomarker levels in relationship with tumor progression. Specifically, Hori and Gambhir assumed that CA125 is shed only from malignant cells and not benign or healthy cells. The number of healthy cells considered in the model was assumed to be constant and all tumor cells were assumed to shed the biomarker. These modeling constraints could be relaxed in future models for a more realistic description of the dynamic biomarker blood levels and disease detection times. While the relationship between tumor sizes and CA125 biomarker levels is not entirely understood72 and serial CA125 measurements are not common practice64,129, further modeling efforts could potentially drive further cohort screening studies.
Modeling therapeutic targeting and treatment
A key question related to optimal sequencing and scheduling of chemotherapy and surgery is whether the optimal therapeutic strategy would be to maximally debulk a cancerous tumor followed by chemotherapy or vice versa. Kohandel et al. considered130 one population of tumor cells, a non-cell cycle phase specific drug, and various growth/cell-kill laws in order to compare two approaches for therapy: a) chemotherapy followed by surgery, or b) surgery followed by chemotherapy. Kohandel et al. combined Gompertzian and generalized logistic growth models with different cell-kill hypotheses, and assumed that surgery instantaneously kills a fraction of the tumor cells at the time of the treatment. The model is illustrated in Figure 9. For both the Gompertzian and generalized growth models, chemotherapy followed by surgery proved to be the optimal approach.
Figure 9. The one-compartment ODE model of Kohandel et al.130. Modeled are tumor growth, and surgical and chemotherapeutic treatments.
Kohandel et al. considered one population of tumor cells, a non-cell cycle specific drug and various growth and cell-kill laws formulated in the following manner. The dynamics of the number of tumor cells at time t, N(t), is described by differential functional forms for the growth law, where f(N) is the tumor cell growth dynamics (e.g. f(N) = aN for the exponential growth law, where a is the constant proliferation rate), G(t,N) describes the effects of the drug on the system , and I(t = tsurgery) is an indicator function (equal to 1, if t = tsurgery, and 0 otherwise). Differential functional forms chosen for G(t,N) are provided in the second equation. Surgery is assumed to be instantaneous, and to remove a fixed fraction of exp(−ks) of tumor cells, where ks is the fraction of removed cells during surgery.
We note that the constant parameters and the functional forms proposed in the model of Kohandel et al. can be generalizable to any solid cancer, and bear no affinity to ovarian cancer specifically. The results presented are general and independent of a particular choice of parameters or drug administration protocols. Furthermore, in contrast to the mathematical modeling results, recently performed meta-analyses of randomized trials comparing chemotherapy versus surgery for initial treatment in advanced ovarian epithelial cancer131 as well as locally advanced breast cancer132 showed no difference between the two clinical scenarios in terms of survival or overall disease progression. We note that a key assumption of the Kohandel et al. model is that chemotherapy administered prior to surgery solely alters primary tumor growth, rather than also affect the formation and growth of (micro)metastatic tumor foci, which could be clinically occult at the time of surgery. We point out, however, that the clinical rationale for adjuvant chemotherapy (i.e. after surgical resection) is to eliminate any systemic, distant microscopic disease that would most likely already be present pre- or post-surgical resection. A future mathematical investigation based on the Kohandel et al. model could, for example, address the open clinical question of how to reliably identify the subsets of patients without any microscopic, residual disease, who would not benefit from adjuvant chemotherapy. Lastly, while the choice of the appropriate tumor growth law for modeling disease kinetics is discussed at length130 and its impact on therapeutic sequencing outcomes is theoretically derived through mathematical analysis, ovarian cancer primary tumor growth dynamics and clinical progression prior to or after therapy are most likely more complex than the basic growth and progression dynamics considered in this work.
In a different attempt to model combination therapy, Jain et al. developed a mathematical model of ovarian cancer xenograft growth to study the effect of carboplatin, and ABT-737, a small-molecule inhibitor of anti-apoptotic proteins Bcl-2 and Bcl-xL, on tumor growth inhibition133. Their model of ovarian cancer growth and treatment was based on a coupled system of ODEs and PDEs134, representing the temporal dynamics of proliferating and arrested cancer cells, and concentrations of the two drugs inside the peritoneum, plasma and tissue. This combination therapy model carefully accounted for the pharmacodynamics and pharmacokinetics of both drugs, and was calibrated against in vivo experimental data collected from xenografted mice treated with carboplatin and/or ABT-737 on a fixed period schedule135. Their goal was to study dosage/timing combinations of the two drugs leading to the fastest time to minimal residual disease, or to the minimization of total drug lead in order to achieve a predetermined tumor growth inhibition. Simulations of the Jain et al. model suggest that when combined with ABT-737, the infusion time of carboplatin doses together with the AUC of the drug were the most important predictors of the tumor long-term response to the combination therapy. While the model is validated by in vitro ovarian cancer cell lines xenografted from patient ascitic tumor cells, further clinical investigations are needed to explore and examine the synergy between carboplatin and Bcl-2 family inhibitors.
Lastly, the previously discussed two-compartment linear ODE model proposed by Panetta115 attempted to mathematically derive optimal treatment strategies in the sense of promoting the biggest tumor cell burden reduction. Using clinical variables such as treatment period, drug-infusion time, and proliferative fraction of ovarian cancerous cells, Panetta quantitatively demonstrate that for short periods (i.e. close to the active phase time) more drug is required to notice a decay in the ovarian cancer cell population. The results were qualitatively similar to those obtained when simulating breast cancer cells. It is likely, however, that a careful calibration of the model may alter the results. For instance, the work of Panetta involved combining growth rates/doubling times from ovarian tumors xenografted in nude mice116 with cell cycle kinetics derived from ovarian cancer cell lines117.
Modeling in vitro invasive cancer cell kinetics
To simulate in vitro cancer-cell kinetics after cisplatin administration, Montalenti et al. developed a linear model of cell-cycle phase transitions based on experiments using IGROV-1 ovarian cancer cell line136. They used flow cytometry variables (derived experimentally) and implemented them as the input to a quantitative description of the action of cisplatin on the carcinoma cells. The aim was to specifically model the intermitotic time of cell-cycle phases, delays and block-effects, with consequent repair or cell mortality following the exit from the blocks. It was assumed that drug administration forces cells to leave asynchronous growth, with the main effects being a) cell death; b) cell-cycle phase delays; or c) cell-cycle blocks. At the end of each cell-cycle phase, cells were assumed to progress to the next phase only if they passed an internal molecular checkpoint. Montalenti et al. considered various levels of complexity in their cell-cycle simulation: (i) inter-cell differences in phase duration (modeled via a two-parameter probability distribution of the likelihood of a cell of a certain phase age leaving the specific phase); (ii) the probability that a cell can either bypass the quiescent phase or enter it for an indefinite period of time; and (iii) the probability that cells can be killed by cisplatin at a distinct rate in every phase, blocked but then repair damage and recycle, or frozen in a specific age compartment, inhibiting age maturation.
While a certain amount of information could be obtained by visual inspection of the raw experimental data performed and used, the task of the parameter-fitting simulation was to consider all experimental data together with a number of drug doses and recovery times, in order to derive a coherent kinetic scenario. Once the input baseline set of kinetic parameters was determined, the data were simulated on the cisplatin-treated IGROV-1 cells using a trial-and-error-procedure to find biologically appropriate estimates of the cisplatin-induced delays, block effects and cell mortality induced at every cell-cycle phase. The Montalenti et al. modeling results yielded a very detailed kinetic description of the IGROV-1 cell cycle dynamics treated with cisplatin. It remains unclear whether their modeling inferences can be easily translatable to human ovarian cancer tumor-drug interactions. No such description of in vivo cancer cell kinetics has been described to date, and the clinical relevance of ovarian cancer cell lines remains an issue subject to further exploration137.
Modeling stem cell dynamics
To estimate stem cell self-renewal probabilities in serous epithelial ovarian carcinomas, Ciampi et al. proposed a method for estimating the probability of self-renewal of serous epithelial ovarian tumor stem cells starting from experimentally derived distributions of clonal colonies obtained in cell culture138. The model was based on tumor cell populations being composed of (i) tumor stem cells, capable of self-renewal and subsequent tumor self-regeneration; (ii) transitional cells, not capable of self-renewal but characterized by a limited potential for further proliferation (i.e. de-differentiation); and (iii) end-stage cells, incapable of further proliferation, and thus considered terminally differentiated. In this model, the proliferation of the cancer cell population was treated as a multi-type Galton-Watson process in which stochastic fluctuations lead to probability distributions in the number of each cell type, under the assumption that cell division does not necessarily occur at the same time for same-age cells. Using a Nelder-Mead algorithm and equating the theoretical moments of the distribution of cell types with the observed experimental colony size mean and variance, Ciampi et al. derived parameter estimates for the probability of self-renewal of a tumor stem cell, and the clonal expansion number expressed in the generations, i.e. the number of de-differentiated states.
We note that a key assumption of this model is that ovarian cell populations are organized in a hierarchy of decreasing proliferation potential and increasing degrees of cellular differentiation. However, it is unclear if stem cells are responsible for the initiation and progression of clinical ovarian cancers139. Moreover, the lack of unique markers that are able to identify stem cells in the context of ovarian cancers make it difficult to characterize the proliferative landscape of such cells in vitro or in vivo.
FUTURE PERSPECTIVES
In this review, we survey the existing mathematical oncology literature, focusing on two major subsets of women's cancers, specifically the breast and ovarian malignancies. First, we summarize the existing clinical data regarding malignancy progression and breast/ovarian cancer patients’ specific therapy response. From a systems biology perspective, we demonstrate that few mathematical modeling inferences have been concerned with any of breast cancer subtypes, and even fewer with ovarian cancer subtypes. Whenever possible, we compare and contrast the known clinical information about the families of breast and ovarian cancers, and complement the existing clinical paradigms with a description and discussion of the corresponding mathematical modeling attempts. In doing so, we show how current mathematical models that focus on the two women's malignancies do not make comprehensive use nor substantially reflect existing clinical data. We then highlight the modeling areas in most critical need of clinical data integration. Lastly, we argue that the existing mathematical models reviewed and discussed in this paper are not adapted to reflect the complex, heterogeneous behaviors of women's malignancies.
With respect to main themes addressed by current breast and ovarian cancer mathematical modeling, the models discussed in this review generally address primary tumor growth, optimal biomarker characteristics, and therapy sequencing, omitting to varying degrees disease-specific characteristics, such as histological classification, or specific drug-body pharmacodynamics and pharmacokinetics. We note, however, that considerable biological realism and any translational/clinical relevance of such modeling results are lost if the existing mathematical models aggregate the various women's cancers subtypes into one singular disease. Moreover, existing mathematical models tend to either completely ignore current standard therapeutic approaches and existing combination treatments, or model any multidrug therapeutic combinations as single drugs, without reflecting the differential pharmacodynamic and pharmacokinetic behavior of the various drugs.
From a mathematical point of view, the quantitative tools employed in these models range from probabilistic techniques (e.g. branching processes, bootstrap resampling, time-homogeneous Markov chains), through differential equation-based approaches (single or multiple compartment ODE, PDE or coupled ODE-PDE models). There are, however, several underlying, limiting assumptions with using such modeling approaches. Most such models rely on the “perfect mixing” assumption that cellular populations are spatially homogeneous, and are only able to quantify average cellular behavior, rather than heterogeneous genetic and phenotypic cellular profiles. In doing so, existing models are only able to generate aggregate statistics or outcomes similar to results derived from population-based cohort studies. The extent to which such modeling results would contribute to understanding patient-specific cancer progression or subsequent therapeutic outcomes is thus unclear. Mathematical modelers of women's cancers should also devote careful attention to parameterization details and model calibration against experimental data, as more often than not, parameter estimations for a singular model are derived from various cancer cell lines, mouse models, and/or xenografted tumor data. The applicability of such modeling attempts to clinical scenarios thus remains tenuous at its best.
Outlook for the future
Studying the natural history, growth, progression or dynamic response to treatment of breast and ovarian cancers in an integrated systems biology/mathematical framework offers an innovative contribution and a complementary tool at the disposal of the women's cancers clinical community. If accurately and realistically applied to existing clinical data, such frameworks could represent a substantial step forward, and can be performed in a relatively inexpensive manner, that relies only on computing power. Mathematical modeling can provide insights about the disease dynamics that reach beyond contemporary clinical and experimental tools and are impossible to obtain even in large-scale cohort studies. Dynamic spatiotemporal mathematical models of women's cancers and their evolution can provide a quantitative understanding of the likelihood of occurrence of specific clinical scenarios in response to detection, treatment and the when and the why of emergence of resistance.
This is precisely why we believe mathematical modeling could potentially make a substantial contribution to cancer research and therapy. To that end, current and future modeling attempts should be properly integrated with clinical data, and thus parameterized to reflect disease heterogeneity in progression and therapeutic responses. We point, however, that this issue is double-sided.
First, we believe that an adaptation of the modeling attempts discussed in the present review to current clinical data does not require any revolutionary or novel mathematical modeling framework. Existing mathematical approaches and techniques utilized in modeling the progression and therapeutics of one malignancy can be similarly translated to another malignancy, despite the complex degrees of heterogeneity of different malignancies from a clinical perspective. Instead, we argue that all that is needed are an increased awareness of the mathematical modeling community to the limitations of current models in providing an accurate representation of the contemporary clinical knowledge, and more sustained efforts at understanding disease specifics before attempting any modeling implementation. To that end, we believe that mathematical oncologists focused on modeling the breast and ovarian malignancies would greatly benefit from extensive collaboration with specialists outside their primary domain of expertise, in order to refine the aims of their modeling efforts, calibrate and mechanistically validate their modeling results in the context of clinical applications. We stress that augmented and sustained efforts at bringing together researchers with complimentary expertise (e.g. mathematicians, cancer biologists, and clinicians/experimentalists) should be initiated to introduce a new research paradigm to women's cancers studies. There is a pressing urgency to bring in quantitative, truly integrated modeling to preclinical and clinical development of novel breast and ovarian cancer therapeutics.
Second, to successfully calibrate and validate modeling frameworks, it is crucial to point that more often than not, data collected by clinicians during therapeutic interventions or follow-up of patients in trials is not reported in a format that is suitable for mathematical modeling. This may severely limit the translational value of any mathematical model. Mathematical modeling could substantially benefit from a more comprehensive data collection and reporting. Single values (such as tumor size at diagnosis, categorical thresholds such surgical debulking cytoreduction values, tumor shrinkage response and related endpoints, or single serum biomarker measurements), should be replaced by reporting dynamical information, such as the levels of change. Longitudinally measured markers, molecular and imaging data performed and measured over the course of a patient's care would provide excellent discriminatory and predictive power to mathematical models.
Several recent randomized controlled trials have begun incorporating serial measurements of relevant clinical parameters, and the inclusion of such temporal data yielded substantially improved predictive power when analysis was performed within tumor subsets stratified by patient characteristics. Examples include the I-SPY 1 TRIAL - a cohort of 221 patients with histologically confirmed invasive breast cancer whose pathologic complete response to therapeutic care and recurrence-free survival rates were temporally evaluated140, and the UK Collaborative Trial of Ovarian Cancer Screening that randomly allocated 202,638 post-menopausal UK women to different temporal multimodal screening algorithms in order to establish the effect early detection by screening on ovarian cancer mortality64. Herein, serial CA125 serum levels and TVU examinations were collected and used to inform their risk of ovarian cancer prediction algorithm.
Open challenges
More recently, the increasing availability of high throughput genomic datasets to identify the molecular basis of a number of cancers has heralded an emerging approach to cancer treatment, specifically precision medicine. The long term goal of such an approach is to identify and subsequently use the observed changes and patterns in a patient's individual cancer to better inform therapeutic outcomes and yield treatment options tailored to an individual's specific clinical parameters.
A significant amount of clinically motivated efforts has been aimed in producing dynamic molecular datasets. High depth sequencing is used to study the functional role of genetic heterogeneity during a cancer's evolutionary progression and to better disseminate between individual tumors of different subtypes based on their molecular profiling. Novel clinical trial designs are attempting to identify the rules for the dissemination of individual patients according to a comprehensive set of clinical characteristics and match them to therapeutic outcomes52,141-143. Such designs play a key role in providing data for mathematical models, and the relatively few modeling efforts informed by such datasets have yielded promising results, leading to the inference of novel mechanistic relationships and possible links to therapeutic outcomes in chronic myelogenous144 and chronic lymphocytic leukemias145, Barrett's esophagus146, or glioblastomas147, to name a few.
With regards to breast and ovarian cancers, such high throughput dynamic molecular datasets are yet not widely available, and enhanced serial data collection is not routinely performed. As such, numerous open questions remain, many of which cannot be feasibly or exclusively addressed experimentally. A list summarizing questions presently of great interest to the clinical community, that could potentially be addressed using mathematical models, is given in Table 1.
Table 1.
List of clinically relevant open questions regarding the breast and ovarian malignancies.
| 1. Cancer initiation | Can we predict whether subtypes arise from distinct lineages or from a common precursor in both malignancies? Can we predict who is more likely to get breast or ovarian cancer in high-genetic risk cases? Can we integrate the knowledge about causative disease factors to identify more effective prevention and treatments? |
| 2. Cancer progression | Can we develop a quantitative metric to predict which ductal carcinomas in situ are more likely to progress to invasive cancer? Similarly, can we develop a quantitative metric to predict which in situ fallopian tube lesions would progress to invasive high-grade serous cancer? Can we predict which invasive breast/ovarian detected carcinomas are more likely to have become metastatic prior to diagnosis? Which specific women's cancer would be more likely to benefit from systemic therapy? Can we quantitatively stratify the risk of relapse and/or need for chemotherapy without being able to or having to conduct large scale prospective cohort studies? |
| 3. Cancer dormancy | Can we quantitatively predict why dormant breast cancer tumor cells start to grow and invade, as observed anecdotally? How do dormant residual cells switch to a proliferating stage and regenerate tumors? Can we determine what the qualitative mechanism behind dormancy activation is? Specifically, are dormant cancer cells already present within the tumor or are they induce by therapy? |
| 4. Treatment of metastatic and/or resistant disease | Is it feasible to develop mechanistic models of drug penetrability and/or biological function in order to better study drug sequencing and emergence of resistance? Can we use mathematical analysis to guide the selection of drugs to be used for each patient? Specifically, can we mathematically quantify the spatio-temporal propagation of resistance in order to better predict treatment outcomes? Can we use generated in silico cumulative distributions of disease-free survival times prior to recurrence to guide subsequent therapeutic scheduling? |
Conclusions
In this review, we seek to emphasize the pressing need for truly integrative mathematical-oncology collaborations that reflect and make use of the clinically known aspects of malignancy heterogeneity, progression, detection and therapeutic options. We believe that the primary goal of any mathematical study of women's cancers should ultimately be to address current clinical open questions. In order to achieve this goal, the design of any future mathematical models will have to closely follow women's cancers biology, be driven by it, and be closely supported and informed by enhanced clinical and experimental data.
SIDEBARS.
Sidebar 1. Clinical terminology used throughout the review listed by order of reference.
Menopause is defined as occurring 12 months after a woman's last menstrual period and marks the end of menstrual cycles. It implies the permanent cessation of ovulation and menses without any obvious pathological cause.
Early detection refers to the discovery and diagnosis of a malignancy prior to the occurrence of any clinical symptoms.
Systemic cancer therapy refers to a type therapy that uses substances which travel throughout the body via the bloodstream, and affects cancer cells wherever they may be located.
Targeted therapy refers to a type of therapy that uses drugs or other substances to identify and attack specific pathways in cancer cells that are necessary for the cancer cell growth, with limited toxicity to normal cells.
General risk women are defined as asymptomatic women without any known genetic mutations or known family history of breast and/or ovarian cancer.
High-genetic risk women are defined as individuals from families with a known deleterious BRCA1 or BRCA2 mutation or women with a family history of breast or ovarian cancer, such as a first- or second-degree blood relative with a personal history of breast or invasive ovarian cancer.
Screening refers to monitoring of asymptomatic, apparently healthy patients with the intent of achieving an earlier diagnosis, if disease is found.
Tumor dormancy refers to clinically undetectable microscopic metastases that remain in a dormant state for prolonged periods of time, and that eventually progress to detectable cancer sometime during a patient's lifetime.
Sidebar 2. Mathematical modeling terminology used throughout the review listed by order of reference.
Temporal refers to the progression in time of a clinical variable, e.g. tumor size, biomarker levels, effects of a drug on the body.
In silico refers to a numerical simulation performed on a computer.
ACKNOWLEDGEMENTS
Funding: The work was supported by the Intramural Research Program of the National Institutes of Health, Center for Cancer Research, National Cancer Institute as part of a seed grant from the UMD-NCI Partnership for Cancer Technology. The work of DL was supported in part by the John Simon Guggenheim Memorial Foundation. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Competing interests: The authors have declared that no competing interests exist.
REFERENCES
- 1.US Census Bureau Age and Sex Composition: 2010. 2010 [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65(1):5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 3.DeSantis C, Ma J, Bryan L, Jemal A. Breast cancer statistics, 2013. CA Cancer J Clin. 2014;64(1):52–62. doi: 10.3322/caac.21203. [DOI] [PubMed] [Google Scholar]
- 4.2015 Breast Cancer Facts & Figures 2015-2016. American Cancer Society, Inc.; 2015. [Google Scholar]
- 5.DeSantis CE, Lin CC, Mariotto AB, Siegel RL, Stein KD, Kramer JL, Alteri R, Robbins AS, Jemal A. Cancer treatment and survivorship statistics, 2014. CA Cancer J Clin. 2014;64(4):252–71. doi: 10.3322/caac.21235. [DOI] [PubMed] [Google Scholar]
- 6.Maini PK, Gatenby RA. Some mathematical modelling challenges and approaches in cancer. In: Nagl S, editor. Cancer Bioinformatics: From Therapy Design to Treatment. John Wiley & Sons, Ltd; 2006. pp. 95–107. [Google Scholar]
- 7.Cristini V, Lowengrub J. Multiscale Modeling of Cancer: An Integrated Experimental and Mathematical Modeling Approach. Cambridge University Press; 2010. p. 298. [Google Scholar]
- 8.Wodarz D, Komarova NL. Dynamics of Cancer: Mathematical Foundations of Oncology. World Scientific Publishing Company; 2014. p. 532. [Google Scholar]
- 9.Epstein C. Introduction to the Mathematics of Medical Imaging, Second Edition. 2nd edition. Society for Industrial & Applied Mathematics; Sep 28, 2007. p. 795. 2007. [Google Scholar]
- 10.Yankeelov TE, Pickens DR, Price RR. 1 edition. CRC Press; Sep 13, 2011. Quantitative MRI in Cancer (Imaging in Medical Diagnosis and Therapy). p. 338. 2011. [Google Scholar]
- 11.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66(1):7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 12.Key TJ, Verkasalo PK, Banks E. Epidemiology of breast cancer. Lancet Oncol. 2001;2(3):133–40. doi: 10.1016/S1470-2045(00)00254-0. [DOI] [PubMed] [Google Scholar]
- 13.Smalley M, Ashworth A. Stem cells and breast cancer: A field in transit. Nat Rev Cancer. 2003;3(11):832–44. doi: 10.1038/nrc1212. [DOI] [PubMed] [Google Scholar]
- 14.Sørlie T, Perou CM, Tibshirani R, Aas T, Geisler S, Johnsen H, Hastie T, Eisen MB, van de Rijn M, Jeffrey SS. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci U S A. 2001;98(19):10869–74. doi: 10.1073/pnas.191367098. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Parise CA, Caggiano V. Breast Cancer Survival Defined by the ER/PR/HER2 Subtypes and a Surrogate Classification according to Tumor Grade and Immunohistochemical Biomarkers. J Cancer Epidemiol. 2014;2014:469251. doi: 10.1155/2014/469251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lehmann BD, Bauer JA, Chen X, Sanders ME, Chakravarthy AB, Shyr Y, Pietenpol JA. Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J Clin Invest. 2011;121(7):2750–67. doi: 10.1172/JCI45014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sorlie T, Tibshirani R, Parker J, Hastie T, Marron JS, Nobel A, Deng S, Johnsen H, Pesich R, Geisler S. Repeated observation of breast tumor subtypes in independent gene expression data sets. Proc Natl Acad Sci U S A. 2003;100(14):8418–23. doi: 10.1073/pnas.0932692100. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Network CGA. Comprehensive molecular portraits of human breast tumours. Nature. 2012;490(7418):61–70. doi: 10.1038/nature11412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Criscitiello C, Azim HA, Schouten PC, Linn SC, Sotiriou C. Understanding the biology of triple- negative breast cancer. Ann Oncol. 2012;23(Suppl 6):vi13–8. doi: 10.1093/annonc/mds188. [DOI] [PubMed] [Google Scholar]
- 20.Oncology NCCNCPGi Clinical Practice Guidelines in Oncology: Genetic/Familial High-Risk Assessment: Breast and Ovarian. 2015 www.nccn.org.
- 21.Wong-Brown MW, Meldrum CJ, Carpenter JE, Clarke CL, Narod SA, Jakubowska A, Rudnicka H, Lubinski J, Scott RJ. Prevalence of BRCA1 and BRCA2 germline mutations in patients with triple-negative breast cancer. Breast Cancer Res Treat. 2015;150(1):71–80. doi: 10.1007/s10549-015-3293-7. [DOI] [PubMed] [Google Scholar]
- 22.Petrucelli N, Daly MB, Feldman GL. Hereditary breast and ovarian cancer due to mutations in BRCA1 and BRCA2. Genet Med. 2010;12(5):245–59. doi: 10.1097/GIM.0b013e3181d38f2f. [DOI] [PubMed] [Google Scholar]
- 23.Humphrey LL, Helfand M, Chan BK, Woolf SH. Breast cancer screening: a summary of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med. 2002;137(5 Part 1):347–60. doi: 10.7326/0003-4819-137-5_part_1-200209030-00012. [DOI] [PubMed] [Google Scholar]
- 24.Nelson HD, Fu R, Cantor A, Pappas M, Daeges M, Humphrey L. Effectiveness of Breast Cancer Screening: Systematic Review and Meta-analysis to Update the 2009 U.S. Preventive Services Task Force Recommendation. Ann Intern Med. 2016 doi: 10.7326/M15-0969. [DOI] [PubMed] [Google Scholar]
- 25.Rebbeck TR, Friebel T, Lynch HT, Neuhausen SL, van 't Veer L, Garber JE, Evans GR, Narod SA, Isaacs C, Matloff E. Bilateral prophylactic mastectomy reduces breast cancer risk in BRCA1 and BRCA2 mutation carriers: the PROSE Study Group. J Clin Oncol. 2004;22(6):1055–62. doi: 10.1200/JCO.2004.04.188. others. [DOI] [PubMed] [Google Scholar]
- 26.Esserman LJ, Shieh Y, Rutgers EJ, Knauer M, Retèl VP, Mook S, Glas AM, Moore DH, Linn S, van Leeuwen FE. Impact of mammographic screening on the detection of good and poor prognosis breast cancers. Breast Cancer Res Treat. 2011;130(3):725–34. doi: 10.1007/s10549-011-1748-z. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Force UPST Screening for breast cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2009;151(10):716–26. W-236. doi: 10.7326/0003-4819-151-10-200911170-00008. [DOI] [PubMed] [Google Scholar]
- 28.Singer CF, Tea MK, Pristauz G, Hubalek M, Rappaport C, Riedl CC, Helbich TH. Clinical Practice Guideline for the prevention and early detection of breast and ovarian cancer in women from HBOC (hereditary breast and ovarian cancer) families. Wien Klin Wochenschr. 2015;127(23-24):981–6. doi: 10.1007/s00508-015-0880-x. [DOI] [PubMed] [Google Scholar]
- 29.Morrell S, Barratt A, Irwig L, Howard K, Biesheuvel C, Armstrong B. Estimates of overdiagnosis of invasive breast cancer associated with screening mammography. Cancer Causes Control. 2010;21(2):275–82. doi: 10.1007/s10552-009-9459-z. [DOI] [PubMed] [Google Scholar]
- 30.Shulman LN, Berry DA, Cirrincione CT, Becker HP, Perez EA, O'Regan R, Martino S, Shapiro CL, Schneider CJ, Kimmick G. Comparison of doxorubicin and cyclophosphamide versus single-agent paclitaxel as adjuvant therapy for breast cancer in women with 0 to 3 positive axillary nodes: CALGB 40101 (Alliance). J Clin Oncol. 2014;32(22):2311–7. doi: 10.1200/JCO.2013.53.7142. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Winer EP, Hudis C, Burstein HJ, Chlebowski RT, Ingle JN, Edge SB, Mamounas EP, Gralow J, Goldstein LJ, Pritchard KI. American Society of Clinical Oncology technology assessment on the use of aromatase inhibitors as adjuvant therapy for women with hormone receptor-positive breast cancer: status report 2002. J Clin Oncol. 2002;20(15):3317–27. doi: 10.1200/JCO.2002.06.020. others. [DOI] [PubMed] [Google Scholar]
- 32.DeMichele A, Clark AS, Tan KS, Heitjan DF, Gramlich K, Gallagher M, Lal P, Feldman M, Zhang P, Colameco C. CDK 4/6 inhibitor palbociclib (PD0332991) in Rb+ advanced breast cancer: phase II activity, safety, and predictive biomarker assessment. Clin Cancer Res. 2015;21(5):995–1001. doi: 10.1158/1078-0432.CCR-14-2258. others. [DOI] [PubMed] [Google Scholar]
- 33.Esserman LJ, Moore DH, Tsing PJ, Chu PW, Yau C, Ozanne E, Chung RE, Tandon VJ, Park JW, Baehner FL. Biologic markers determine both the risk and the timing of recurrence in breast cancer. Breast Cancer Res Treat. 2011;129(2):607–16. doi: 10.1007/s10549-011-1564-5. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pagani O, Senkus E, Wood W, Colleoni M, Cufer T, Kyriakides S, Costa A, Winer EP, Cardoso F, Force E-MT. International guidelines for management of metastatic breast cancer: can metastatic breast cancer be cured? J Natl Cancer Inst. 2010;102(7):456–63. doi: 10.1093/jnci/djq029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wikman H, Vessella R, Pantel K. Cancer micrometastasis and tumour dormancy. APMIS. 2008;116(7- 8):754–70. doi: 10.1111/j.1600-0463.2008.01033.x. [DOI] [PubMed] [Google Scholar]
- 36.Nooter K, Brutel de la Riviere G, Look MP, van Wingerden KE, Henzen-Logmans SC, Scheper RJ, Flens MJ, Klijn JG, Stoter G, Foekens JA. The prognostic significance of expression of the multidrug resistance-associated protein (MRP) in primary breast cancer. Br J Cancer. 1997;76(4):486–93. doi: 10.1038/bjc.1997.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gottesman MM, Fojo T, Bates SE. Multidrug resistance in cancer: role of ATP-dependent transporters. Nat Rev Cancer. 2002;2(1):48–58. doi: 10.1038/nrc706. [DOI] [PubMed] [Google Scholar]
- 38.Lesniak D, Xu Y, Deschenes J, Lai R, Thoms J, Murray D, Gosh S, Mackey JR, Sabri S, Abdulkarim B. Beta1-integrin circumvents the antiproliferative effects of trastuzumab in human epidermal growth factor receptor-2-positive breast cancer. Cancer Res. 2009;69(22):8620–8. doi: 10.1158/0008-5472.CAN-09-1591. [DOI] [PubMed] [Google Scholar]
- 39.Robinson DR, Wu YM, Vats P, Su F, Lonigro RJ, Cao X, Kalyana-Sundaram S, Wang R, Ning Y, Hodges L. Activating ESR1 mutations in hormone-resistant metastatic breast cancer. Nat Genet. 2013;45(12):1446–51. doi: 10.1038/ng.2823. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sakai W, Swisher EM, Karlan BY, Agarwal MK, Higgins J, Friedman C, Villegas E, Jacquemont C, Farrugia DJ, Couch FJ. Secondary mutations as a mechanism of cisplatin resistance in BRCA2-mutated cancers. Nature. 2008;451(7182):1116–20. doi: 10.1038/nature06633. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Swisher EM, Sakai W, Karlan BY, Wurz K, Urban N, Taniguchi T. Secondary BRCA1 mutations in BRCA1-mutated ovarian carcinomas with platinum resistance. Cancer Res. 2008;68(8):2581–6. doi: 10.1158/0008-5472.CAN-08-0088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sakai W, Swisher EM, Jacquemont C, Chandramohan KV, Couch FJ, Langdon SP, Wurz K, Higgins J, Villegas E, Taniguchi T. Functional restoration of BRCA2 protein by secondary BRCA2 mutations in BRCA2-mutated ovarian carcinoma. Cancer Res. 2009;69(16):6381–6. doi: 10.1158/0008-5472.CAN-09-1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Greenup R, Buchanan A, Lorizio W, Rhoads K, Chan S, Leedom T, King R, McLennan J, Crawford B, Kelly Marcom P. Prevalence of BRCA mutations among women with triple-negative breast cancer (TNBC) in a genetic counseling cohort. Ann Surg Oncol. 2013;20(10):3254–8. doi: 10.1245/s10434-013-3205-1. others. [DOI] [PubMed] [Google Scholar]
- 44.Byrski T, Gronwald J, Huzarski T, Grzybowska E, Budryk M, Stawicka M, Mierzwa T, Szwiec M, Wisniowski R, Siolek M. Pathologic complete response rates in young women with BRCA1-positive breast cancers after neoadjuvant chemotherapy. J Clin Oncol. 2010;28(3):375–9. doi: 10.1200/JCO.2008.20.7019. others. [DOI] [PubMed] [Google Scholar]
- 45.Norquist B, Wurz KA, Pennil CC, Garcia R, Gross J, Sakai W, Karlan BY, Taniguchi T, Swisher EM. Secondary somatic mutations restoring BRCA1/2 predict chemotherapy resistance in hereditary ovarian carcinomas. J Clin Oncol. 2011;29(22):3008–15. doi: 10.1200/JCO.2010.34.2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pennington KP, Walsh T, Harrell MI, Lee MK, Pennil CC, Rendi MH, Thornton A, Norquist BM, Casadei S, Nord AS. Germline and somatic mutations in homologous recombination genes predict platinum response and survival in ovarian, fallopian tube, and peritoneal carcinomas. Clin Cancer Res. 2014;20(3):764–75. doi: 10.1158/1078-0432.CCR-13-2287. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Barber LJ, Sandhu S, Chen L, Campbell J, Kozarewa I, Fenwick K, Assiotis I, Rodrigues DN, Reis Filho JS, Moreno V. Secondary mutations in BRCA2 associated with clinical resistance to a PARP inhibitor. J Pathol. 2013;229(3):422–9. doi: 10.1002/path.4140. others. [DOI] [PubMed] [Google Scholar]
- 48.Goff BA, Mandel LS, Drescher CW, Urban N, Gough S, Schurman KM, Patras J, Mahony BS, Andersen MR. Development of an ovarian cancer symptom index: possibilities for earlier detection. Cancer. 2007;109(2):221–7. doi: 10.1002/cncr.22371. [DOI] [PubMed] [Google Scholar]
- 49.Lim AW, Mesher D, Gentry-Maharaj A, Balogun N, Jacobs I, Menon U, Sasieni P. Predictive value of symptoms for ovarian cancer: comparison of symptoms reported by questionnaire, interview, and general practitioner notes. J Natl Cancer Inst. 2012;104(2):114–24. doi: 10.1093/jnci/djr486. [DOI] [PubMed] [Google Scholar]
- 50.Buys SS, Partridge E, Black A, Johnson CC, Lamerato L, Isaacs C, Reding DJ, Greenlee RT, Yokochi LA, Kessel B. Effect of screening on ovarian cancer mortality: the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Randomized Controlled Trial. JAMA. 2011;305(22):2295–303. doi: 10.1001/jama.2011.766. others. [DOI] [PubMed] [Google Scholar]
- 51.Kohn EC, Romano S, Lee JM. Clinical implications of using molecular diagnostics for ovarian cancers. Ann Oncol. 2013;24(Suppl 10):x22–26. doi: 10.1093/annonc/mdt464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bashashati A, Ha G, Tone A, Ding J, Prentice LM, Roth A, Rosner J, Shumansky K, Kalloger S, Senz J. Distinct evolutionary trajectories of primary high-grade serous ovarian cancers revealed through spatial mutational profiling. J Pathol. 2013;231(1):21–34. doi: 10.1002/path.4230. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bast RC. Molecular approaches to personalizing management of ovarian cancer. Ann Oncol. 2011;22(Suppl 8):viii5–viii15. doi: 10.1093/annonc/mdr516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chen VW, Ruiz B, Killeen JL, Coté TR, Wu XC, Correa CN. Pathology and classification of ovarian tumors. Cancer. 2003;97(10 Suppl):2631–42. doi: 10.1002/cncr.11345. [DOI] [PubMed] [Google Scholar]
- 55.Roth LM. Recent advances in the pathology and classification of ovarian sex cord-stromal tumors. Int J Gynecol Pathol. 2006;25(3):199–215. doi: 10.1097/01.pgp.0000192271.22289.e6. [DOI] [PubMed] [Google Scholar]
- 56.Bowtell DD. The genesis and evolution of high-grade serous ovarian cancer. Nat Rev Cancer. 2010;10(11):803–8. doi: 10.1038/nrc2946. [DOI] [PubMed] [Google Scholar]
- 57.Piek JM, van Diest PJ, Zweemer RP, Jansen JW, Poort-Keesom RJ, Menko FH, Gille JJ, Jongsma AP, Pals G, Kenemans P. Dysplastic changes in prophylactically removed Fallopian tubes of women predisposed to developing ovarian cancer. J Pathol. 2001;195(4):451–6. doi: 10.1002/path.1000. others. [DOI] [PubMed] [Google Scholar]
- 58.Lee Y, Miron A, Drapkin R, Nucci MR, Medeiros F, Saleemuddin A, Garber J, Birch C, Mou H, Gordon RW. A candidate precursor to serous carcinoma that originates in the distal fallopian tube. J Pathol. 2007;211(1):26–35. doi: 10.1002/path.2091. others. [DOI] [PubMed] [Google Scholar]
- 59.Crum CP, Drapkin R, Miron A, Ince TA, Muto M, Kindelberger DW, Lee Y. The distal fallopian tube: a new model for pelvic serous carcinogenesis. Curr Opin Obstet Gynecol. 2007;19(1):3–9. doi: 10.1097/GCO.0b013e328011a21f. [DOI] [PubMed] [Google Scholar]
- 60.Crum CP, McKeon FD, Xian W. The oviduct and ovarian cancer: causality, clinical implications, and “targeted prevention”. Clin Obstet Gynecol. 2012;55(1):24–35. doi: 10.1097/GRF.0b013e31824b1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Carcangiu ML, Peissel B, Pasini B, Spatti G, Radice P, Manoukian S. Incidental carcinomas in prophylactic specimens in BRCA1 and BRCA2 germ-line mutation carriers, with emphasis on fallopian tube lesions: report of 6 cases and review of the literature. Am J Surg Pathol. 2006;30(10):1222–30. doi: 10.1097/01.pas.0000202161.80739.ac. [DOI] [PubMed] [Google Scholar]
- 62.Kauff ND, Satagopan JM, Robson ME, Scheuer L, Hensley M, Hudis CA, Ellis NA, Boyd J, Borgen PI, Barakat RR. Risk-reducing salpingo-oophorectomy in women with a BRCA1 or BRCA2 mutation. N Engl J Med. 2002;346(21):1609–15. doi: 10.1056/NEJMoa020119. others. [DOI] [PubMed] [Google Scholar]
- 63.Callahan MJ, Crum CP, Medeiros F, Kindelberger DW, Elvin JA, Garber JE, Feltmate CM, Berkowitz RS, Muto MG. Primary fallopian tube malignancies in BRCA-positive women undergoing surgery for ovarian cancer risk reduction. J Clin Oncol. 2007;25(25):3985–90. doi: 10.1200/JCO.2007.12.2622. [DOI] [PubMed] [Google Scholar]
- 64.Jacobs IJ, Menon U, Ryan A, Gentry-Maharaj A, Burnell M, Kalsi JK, Amso NN, Apostolidou S, Benjamin E. Ovarian cancer screening and mortality in the UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS): a randomised controlled trial. Cruickshank D and others. Lancet. 2015 doi: 10.1016/S0140-6736(15)01224-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Havrilesky LJ, Sanders GD, Kulasingam S, Chino JP, Berchuck A, Marks JR, Myers ER. Development of an ovarian cancer screening decision model that incorporates disease heterogeneity: implications for potential mortality reduction. Cancer. 2011;117(3):545–53. doi: 10.1002/cncr.25624. [DOI] [PubMed] [Google Scholar]
- 66.Kobayashi H, Yamada Y, Sado T, Sakata M, Yoshida S, Kawaguchi R, Kanayama S, Shigetomi H, Haruta S, Tsuji Y. A randomized study of screening for ovarian cancer: a multicenter study in Japan. Int J Gynecol Cancer. 2008;18(3):414–20. doi: 10.1111/j.1525-1438.2007.01035.x. others. [DOI] [PubMed] [Google Scholar]
- 67.Jacobs I, Menon U. Can ovarian cancer screening save lives? The question remains unanswered. Obstet Gynecol. 2011;118(6):1209–11. doi: 10.1097/AOG.0b013e31823b49b3. [DOI] [PubMed] [Google Scholar]
- 68.Jones S, Wang TL, Kurman RJ, Nakayama K, Velculescu VE, Vogelstein B, Kinzler KW, Papadopoulos N, Shih IM. Low-grade serous carcinomas of the ovary contain very few point mutations. J Pathol. 2012;226(3):413–20. doi: 10.1002/path.3967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Moyer VA, Force USPST. Screening for ovarian cancer: U.S. Preventive Services Task Force reaffirmation recommendation statement. Ann Intern Med. 2012;157(12):900–4. doi: 10.7326/0003-4819-157-11-201212040-00539. [DOI] [PubMed] [Google Scholar]
- 70.van Nagell JR, Miller RW, DeSimone CP, Ueland FR, Podzielinski I, Goodrich ST, Elder JW, Huang B, Kryscio RJ, Pavlik EJ. Long-term survival of women with epithelial ovarian cancer detected by ultrasonographic screening. Obstet Gynecol. 2011;118(6):1212–21. doi: 10.1097/AOG.0b013e318238d030. [DOI] [PubMed] [Google Scholar]
- 71.van der Velde NM, Mourits MJ, Arts HJ, de Vries J, Leegte BK, Dijkhuis G, Oosterwijk JC, de Bock GH. Time to stop ovarian cancer screening in BRCA1/2 mutation carriers? Int J Cancer. 2009;124(4):919–23. doi: 10.1002/ijc.24038. [DOI] [PubMed] [Google Scholar]
- 72.Menon U, Gentry-Maharaj A, Hallett R, Ryan A, Burnell M, Sharma A, Lewis S, Davies S, Philpott S, Lopes A. Sensitivity and specificity of multimodal and ultrasound screening for ovarian cancer, and stage distribution of detected cancers: results of the prevalence screen of the UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS). Lancet Oncol. 2009;10(4):327–40. doi: 10.1016/S1470-2045(09)70026-9. others. [DOI] [PubMed] [Google Scholar]
- 73.Villella JA, Parmar M, Donohue K, Fahey C, Piver MS, Rodabaugh K. Role of prophylactic hysterectomy in patients at high risk for hereditary cancers. Gynecol Oncol. 2006;102(3):475–9. doi: 10.1016/j.ygyno.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 74.Finch A, Evans G, Narod SA. BRCA carriers, prophylactic salpingo-oophorectomy and menopause: clinical management considerations and recommendations. Womens Health (Lond Engl) 2012;8(5):543–55. doi: 10.2217/whe.12.41. [DOI] [PubMed] [Google Scholar]
- 75.Madalinska JB, van Beurden M, Bleiker EM, Valdimarsdottir HB, Lubsen-Brandsma L, Massuger LF, Mourits MJ, Gaarenstroom KN, van Dorst EB, van der Putten H. Predictors of prophylactic bilateral salpingo-oophorectomy compared with gynecologic screening use in BRCA1/2 mutation carriers. J Clin Oncol. 2007;25(3):301–7. doi: 10.1200/JCO.2006.07.4922. others. [DOI] [PubMed] [Google Scholar]
- 76.Orozco LJ, Tristan M, Vreugdenhil MM, Salazar A. Hysterectomy versus hysterectomy plus oophorectomy for premenopausal women. Cochrane Database Syst Rev. 2014;7:CD005638. doi: 10.1002/14651858.CD005638.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Domchek SM, Friebel TM, Neuhausen SL, Wagner T, Evans G, Isaacs C, Garber JE, Daly MB, Eeles R, Matloff E. Mortality after bilateral salpingo-oophorectomy in BRCA1 and BRCA2 mutation carriers: a prospective cohort study. Lancet Oncol. 2006;7(3):223–9. doi: 10.1016/S1470-2045(06)70585-X. others. [DOI] [PubMed] [Google Scholar]
- 78.Eisen A, Lubinski J, Klijn J, Moller P, Lynch HT, Offit K, Weber B, Rebbeck T, Neuhausen SL, Ghadirian P. Breast cancer risk following bilateral oophorectomy in BRCA1 and BRCA2 mutation carriers: an international case-control study. J Clin Oncol. 2005;23(30):7491–6. doi: 10.1200/JCO.2004.00.7138. others. [DOI] [PubMed] [Google Scholar]
- 79.Kauff ND, Domchek SM, Friebel TM, Robson ME, Lee J, Garber JE, Isaacs C, Evans DG, Lynch H, Eeles RA. Risk-reducing salpingo-oophorectomy for the prevention of BRCA1- and BRCA2-associated breast and gynecologic cancer: a multicenter, prospective study. J Clin Oncol. 2008;26(8):1331–7. doi: 10.1200/JCO.2007.13.9626. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Menon U, Griffin M, Gentry-Maharaj A. Ovarian cancer screening--current status, future directions. Gynecol Oncol. 2014;132(2):490–5. doi: 10.1016/j.ygyno.2013.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bowtell DD, Böhm S, Ahmed AA, Aspuria PJ, Bast RC, Beral V, Berek JS, Birrer MJ, Blagden S, Bookman MA. Rethinking ovarian cancer II: reducing mortality from high-grade serous ovarian cancer. Nat Rev Cancer. 2015;15(11):668–79. doi: 10.1038/nrc4019. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Pujade-Lauraine E, Hilpert F, Weber B, Reuss A, Poveda A, Kristensen G, Sorio R, Vergote I, Witteveen P, Bamias A. Bevacizumab combined with chemotherapy for platinum-resistant recurrent ovarian cancer: The AURELIA open-label randomized phase III trial. J Clin Oncol. 2014;32(13):1302–8. doi: 10.1200/JCO.2013.51.4489. others. [DOI] [PubMed] [Google Scholar]
- 83.Bixel K, Hays JL. Olaparib in the management of ovarian cancer. Pharmgenomics Pers Med. 2015;8:127–35. doi: 10.2147/PGPM.S62809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rodrigues FF, Santos RE, Melo MB, Silva MA, Oliveira AL, Rozenowicz RL, Ulson LB, Aoki T. Correlation of polymorphism C3435T of the MDR-1 gene and the response of primary chemotherapy in women with locally advanced breast cancer. Genet Mol Res. 2008;7(1):177–83. doi: 10.4238/vol7-1gmr400. [DOI] [PubMed] [Google Scholar]
- 85.Sakamoto M, Kondo A, Kawasaki K, Goto T, Sakamoto H, Miyake K, Koyamatsu Y, Akiya T, Iwabuchi H, Muroya T. Analysis of gene expression profiles associated with cisplatin resistance in human ovarian cancer cell lines and tissues using cDNA microarray. Hum Cell. 2001;14(4):305–15. others. [PubMed] [Google Scholar]
- 86.Kelland L. The resurgence of platinum-based cancer chemotherapy. Nat Rev Cancer. 2007;7(8):573–84. doi: 10.1038/nrc2167. [DOI] [PubMed] [Google Scholar]
- 87.Nakayama K, Kanzaki A, Ogawa K, Miyazaki K, Neamati N, Takebayashi Y. Copper-transporting P- type adenosine triphosphatase (ATP7B) as a cisplatin based chemoresistance marker in ovarian carcinoma: comparative analysis with expression of MDR1, MRP1, MRP2, LRP and BCRP. Int J Cancer. 2002;101(5):488–95. doi: 10.1002/ijc.10608. [DOI] [PubMed] [Google Scholar]
- 88.Patch AM, Christie EL, Etemadmoghadam D, Garsed DW, George J, Fereday S, Nones K, Cowin P, Alsop K, Bailey PJ. Whole-genome characterization of chemoresistant ovarian cancer. Nature. 2015;521(7553):489–94. doi: 10.1038/nature14410. others. [DOI] [PubMed] [Google Scholar]
- 89.Byrne HM. Dissecting cancer through mathematics: from the cell to the animal model. Nat Rev Cancer. 2010;10(3):221–30. doi: 10.1038/nrc2808. [DOI] [PubMed] [Google Scholar]
- 90.Schättler H, Ledzewicz U. Optimal Control for Mathematical Models of Cancer Therapies: An Application of Geometric Methods (Interdisciplinary Applied Mathematics) 1st ed. 2015 edition. Springer; Sep 16, 2015. p. 496. 2015. [Google Scholar]
- 91.Eftimie R, Bramson JL, Earn DJ. Interactions between the immune system and cancer: a brief review of non-spatial mathematical models. Bull Math Biol. 2011;73(1):2–32. doi: 10.1007/s11538-010-9526-3. [DOI] [PubMed] [Google Scholar]
- 92.Ribba B, Holford NH, Magni P, Trocóniz I, Gueorguieva I, Girard P, Sarr C, Elishmereni M, Kloft C, Friberg LE. A review of mixed-effects models of tumor growth and effects of anticancer drug treatment used in population analysis. CPT Pharmacometrics Syst Pharmacol. 2014;3:e113. doi: 10.1038/psp.2014.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Speer JF, Petrosky VE, Retsky MW, Wardwell RH. A stochastic numerical model of breast cancer growth that simulates clinical data. Cancer Res. 1984;44(9):4124–30. [PubMed] [Google Scholar]
- 94.Norton L. A Gompertzian model of human breast cancer growth. Cancer Res. 1988;48(24 Pt 1):7067–71. [PubMed] [Google Scholar]
- 95.Bloom HJ. The natural history of untreated breast cancer. Ann N Y Acad Sci. 1964;114(2):747–54. [PubMed] [Google Scholar]
- 96.Retsky M, Swartzendruber D, Wardwell R, Bame P, Petrosky V. Re: Larry Norton, a Gompertzian model of human breast cancer growth. Cancer Res. 1989;49(22):6443–4. [PubMed] [Google Scholar]
- 97.Spratt JA, von Fournier D, Spratt JS, Weber EE. Mammographic assessment of human breast cancer growth and duration. Cancer. 1993;71(6):2020–6. doi: 10.1002/1097-0142(19930315)71:6<2020::aid-cncr2820710616>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 98.Koscielny S, Tubiana M, Valleron AJ. A simulation model of the natural history of human breast cancer. Br J Cancer. 1985;52(4):515–24. doi: 10.1038/bjc.1985.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hsiao YH, Chou MC, Fowler C, Mason JT, Man YG. Breast cancer heterogeneity: mechanisms, proofs, and implications. J Cancer. 2010;1:6–13. doi: 10.7150/jca.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lowery JT, Byers T, Kittelson J, Hokanson JE, Mouchawar J, Lewin J, Merrick D, Hines L, Singh M. Differential expression of prognostic biomarkers between interval and screen-detected breast cancers: does age or family history matter? Breast Cancer Res Treat. 2011;129(1):211–9. doi: 10.1007/s10549-011-1448-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Pekar G, Hofmeyer S, Tabár L, Tarján M, Chen TH, Yen AM, Chiu SY, Hellberg D, Gere M, Tot T. Multifocal breast cancer documented in large-format histology sections: long-term follow-up results by molecular phenotypes. Cancer. 2013;119(6):1132–9. doi: 10.1002/cncr.27877. [DOI] [PubMed] [Google Scholar]
- 102.Bedard PL, Hansen AR, Ratain MJ, Siu LL. Tumour heterogeneity in the clinic. Nature. 2013;501(7467):355–64. doi: 10.1038/nature12627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.De Vargas Roditi L, Michor F. Evolutionary dynamics of BRCA1 alterations in breast tumorigenesis. J Theor Biol. 2011;273(1):207–15. doi: 10.1016/j.jtbi.2010.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Baum M, Chaplain MA, Anderson AR, Douek M, Vaidya JS. Does breast cancer exist in a state of chaos? Eur J Cancer. 1999;35(6):886–91. doi: 10.1016/s0959-8049(99)00067-2. [DOI] [PubMed] [Google Scholar]
- 105.Enderling H, Anderson AR, Chaplain MA, Munro AJ, Vaidya JS. Mathematical modelling of radiotherapy strategies for early breast cancer. J Theor Biol. 2006;241(1):158–71. doi: 10.1016/j.jtbi.2005.11.015. [DOI] [PubMed] [Google Scholar]
- 106.Anderson AR, Chaplain MA. Continuous and discrete mathematical models of tumor-induced angiogenesis. Bull Math Biol. 1998;60(5):857–99. doi: 10.1006/bulm.1998.0042. [DOI] [PubMed] [Google Scholar]
- 107.Enderling H, Chaplain MA, Anderson AR, Vaidya JS. A mathematical model of breast cancer development, local treatment and recurrence. J Theor Biol. 2007;246(2):245–59. doi: 10.1016/j.jtbi.2006.12.010. [DOI] [PubMed] [Google Scholar]
- 108.Debeb BG, Smith DL, Li L, Larson R, Xu W, Woodward WA. Differential effect of phosphorylation- defective survivin on radiation response in estrogen receptor-positive and -negative breast cancer. PLoS One. 2015;10(3):e0120719. doi: 10.1371/journal.pone.0120719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Strnad V, Ott OJ, Hildebrandt G, Kauer-Dorner D, Knauerhase H, Major T, Lyczek J, Guinot JL, Dunst J, Miguelez CG. 5-year results of accelerated partial breast irradiation using sole interstitial multicatheter brachytherapy versus whole-breast irradiation with boost after breast-conserving surgery for low-risk invasive and in-situ carcinoma of the female breast: a randomised, phase 3, non-inferiority trial. Lancet. 2015 doi: 10.1016/S0140-6736(15)00471-7. others. [DOI] [PubMed] [Google Scholar]
- 110.Langlands FE, Horgan K, Dodwell DD, Smith L. Breast cancer subtypes: response to radiotherapy and potential radiosensitisation. Br J Radiol. 2013;86(1023):20120601. doi: 10.1259/bjr.20120601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Roe-Dale R, Isaacson D, Kupferschmid M. A mathematical model of breast cancer treatment with CMF and doxorubicin. Bull Math Biol. 2011;73(3):585–608. doi: 10.1007/s11538-010-9549-9. [DOI] [PubMed] [Google Scholar]
- 112.Joerger M, Huitema AD, Richel DJ, Dittrich C, Pavlidis N, Briasoulis E, Vermorken JB, Strocchi E, Martoni A, Sorio R. Population pharmacokinetics and pharmacodynamics of doxorubicin and cyclophosphamide in breast cancer patients: a study by the EORTC-PAMM-NDDG. Clin Pharmacokinet. 2007;46(12):1051–68. doi: 10.2165/00003088-200746120-00005. others. [DOI] [PubMed] [Google Scholar]
- 113.Bonadonna G, Valagussa P, Brambilla C, Moliterni A, Zambetti M, Ferrari L. Adjuvant and neoadjuvant treatment of breast cancer with chemotherapy and/or endocrine therapy. Semin Oncol. 1991;18(6):515–24. [PubMed] [Google Scholar]
- 114.Rampurwala MM, Rocque GB, Burkard ME. Update on adjuvant chemotherapy for early breast cancer. Breast Cancer (Auckl) 2014;8:125–33. doi: 10.4137/BCBCR.S9454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Panetta JC. A mathematical model of breast and ovarian cancer treated with paclitaxel. Math Biosci. 1997;146(2):89–113. doi: 10.1016/s0025-5564(97)00077-1. [DOI] [PubMed] [Google Scholar]
- 116.Riondel J, Jacrot M, Picot F, Beriel H, Mouriquand C, Potier P. Therapeutic response to taxol of six human tumors xenografted into nude mice. Cancer Chemother Pharmacol. 1986;17(2):137–42. doi: 10.1007/BF00306742. [DOI] [PubMed] [Google Scholar]
- 117.Baker FL, Sanger LJ, Rodgers RW, Jabboury K, Mangini OR. Cell proliferation kinetics of normal and tumour tissue in vitro: quiescent reproductive cells and the cycling reproductive fraction. Cell Prolif. 1995;28(1):1–15. doi: 10.1111/j.1365-2184.1995.tb00035.x. [DOI] [PubMed] [Google Scholar]
- 118.Pomahac B, Recht A, May JW, Hergrueter CA, Slavin SA. New trends in breast cancer management: is the era of immediate breast reconstruction changing? Ann Surg. 2006;244(2):282–8. doi: 10.1097/01.sla.0000217626.88430.c3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Gatenby RA, Smallbone K, Maini PK, Rose F, Averill J, Nagle RB, Worrall L, Gillies RJ. Cellular adaptations to hypoxia and acidosis during somatic evolution of breast cancer. Br J Cancer. 2007;97(5):646–53. doi: 10.1038/sj.bjc.6603922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Gatenby RA, Gillies RJ. Why do cancers have high aerobic glycolysis? Nat Rev Cancer. 2004;4(11):891–9. doi: 10.1038/nrc1478. [DOI] [PubMed] [Google Scholar]
- 121.Marusyk A, Tabassum DP, Altrock PM, Almendro V, Michor F, Polyak K. Non-cell-autonomous driving of tumour growth supports sub-clonal heterogeneity. Nature. 2014;514(7520):54–8. doi: 10.1038/nature13556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Liu X, Johnson S, Liu S, Kanojia D, Yue W, Singh UP, Singn U, Wang Q, Nie Q, Chen H. Nonlinear growth kinetics of breast cancer stem cells: implications for cancer stem cell targeted therapy. Sci Rep. 2013;3:2473. doi: 10.1038/srep02473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A. 2003;100(7):3983–8. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Tan BT, Park CY, Ailles LE, Weissman IL. The cancer stem cell hypothesis: a work in progress. Lab Invest. 2006;86(12):1203–7. doi: 10.1038/labinvest.3700488. [DOI] [PubMed] [Google Scholar]
- 125.Danesh K, Durrett R, Havrilesky LJ, Myers E. A branching process model of ovarian cancer. J Theor Biol. 2012;314:10–5. doi: 10.1016/j.jtbi.2012.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Brown PO, Palmer C. The preclinical natural history of serous ovarian cancer: defining the target for early detection. PLoS Med. 2009;6(7):e1000114. doi: 10.1371/journal.pmed.1000114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Köbel M, Kurman RJ, Seidman JD. New Views of Ovarian Carcinoma Types: How Will This Change Practice? In: Ledermann J, Creutzberg CL, Quinn MA, editors. Controversies in the Management of Gynecological Cancers. Springer; London: 2014. pp. 29–38. [Google Scholar]
- 128.Hori SS, Gambhir SS. Mathematical model identifies blood biomarker-based early cancer detection strategies and limitations. Sci Transl Med. 2011;3(109):109ra116. doi: 10.1126/scitranslmed.3003110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Skates SJ. Ovarian cancer screening: development of the risk of ovarian cancer algorithm (ROCA) and ROCA screening trials. Int J Gynecol Cancer. 2012;22(Suppl 1):S24–6. doi: 10.1097/IGC.0b013e318256488a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kohandel M, Sivaloganathan S, Oza A. Mathematical modeling of ovarian cancer treatments: sequencing of surgery and chemotherapy. J Theor Biol. 2006;242(1):62–8. doi: 10.1016/j.jtbi.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 131.Morrison J, Haldar K, Kehoe S, Lawrie TA. Chemotherapy versus surgery for initial treatment in advanced ovarian epithelial cancer. Cochrane Database Syst Rev. 2012;8:CD005343. doi: 10.1002/14651858.CD005343.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Mauri D, Pavlidis N, Ioannidis JP. Neoadjuvant versus adjuvant systemic treatment in breast cancer: a meta-analysis. J Natl Cancer Inst. 2005;97(3):188–94. doi: 10.1093/jnci/dji021. [DOI] [PubMed] [Google Scholar]
- 133.Jain HV, Meyer-Hermann M. The molecular basis of synergism between carboplatin and ABT-737 therapy targeting ovarian carcinomas. Cancer Res. 2011;71(3):705–15. doi: 10.1158/0008-5472.CAN-10-3174. [DOI] [PubMed] [Google Scholar]
- 134.Jain HV, Nör JE, Jackson TL. Quantification of endothelial cell-targeted anti-Bcl-2 therapy and its suppression of tumor growth and vascularization. Mol Cancer Ther. 2009;8(10):2926–36. doi: 10.1158/1535-7163.MCT-08-1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Jain HV, Richardson A, Meyer-Hermann M, Byrne HM. Exploiting the synergy between carboplatin and ABT-737 in the treatment of ovarian carcinomas. PLoS One. 2014;9(1):e81582. doi: 10.1371/journal.pone.0081582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Montalenti FS, Cappella G, Ubezio, P. P. Simulating cancer-cell kinetics after drug treatment: Application to cisplatin on ovarian carcinoma. Physical Review. 1998;57:58–77. Phys. Rev. E 57, 5877. [Google Scholar]
- 137.Beaufort CM, Helmijr JC, Piskorz AM, Hoogstraat M, Ruigrok-Ritstier K, Besselink N, Murtaza M, van IJcken WF, Heine AA, Smid M. Ovarian cancer cell line panel (OCCP): clinical importance of in vitro morphological subtypes. PLoS One. 2014;9(9):e103988. doi: 10.1371/journal.pone.0103988. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Ciampi A, Kates L, Buick R, Kriukov Y, Till JE. Multi-type Galton-Watson process as a model for proliferating human tumour cell populations derived from stem cells: estimation of stem cell self- renewal probabilities in human ovarian carcinomas. Cell Tissue Kinet. 1986;19(2):129–40. doi: 10.1111/j.1365-2184.1986.tb00723.x. [DOI] [PubMed] [Google Scholar]
- 139.Zhan Q, Wang C, Ngai S. Ovarian cancer stem cells: a new target for cancer therapy. Biomed Res Int. 2013;2013:916819. doi: 10.1155/2013/916819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Esserman LJ, Berry DA, DeMichele A, Carey L, Davis SE, Buxton M, Hudis C, Gray JW, Perou C, Yau C. Pathologic complete response predicts recurrence-free survival more effectively by cancer subset: results from the I-SPY 1 TRIAL--CALGB 150007/150012, ACRIN 6657. J Clin Oncol. 2012;30(26):3242–9. doi: 10.1200/JCO.2011.39.2779. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Gerlinger M, Rowan AJ, Horswell S, Larkin J, Endesfelder D, Gronroos E, Martinez P, Matthews N, Stewart A, Tarpey P. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N Engl J Med. 2012;366(10):883–92. doi: 10.1056/NEJMoa1113205. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Cooke SL, Ng CK, Melnyk N, Garcia MJ, Hardcastle T, Temple J, Langdon S, Huntsman D, Brenton JD. Genomic analysis of genetic heterogeneity and evolution in high-grade serous ovarian carcinoma. Oncogene. 2010;29:4905–4913. doi: 10.1038/onc.2010.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Schwarz RF, Ng CK, Cooke SL, Newman S, Temple J, Piskorz AM, Gale D, Sayal K, Murtaza M, Baldwin PJ. Spatial and temporal heterogeneity in high-grade serous ovarian cancer: a phylogenetic analysis. PLoS Med. 2015;12(2):e1001789. doi: 10.1371/journal.pmed.1001789. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Clapp GD, Lepoutre T, El Cheikh R, Bernard S, Ruby J, Labussière-Wallet H, Nicolini FE, Levy D. Implication of the Autologous Immune System in BCR-ABL Transcript Variations in Chronic Myelogenous Leukemia Patients Treated with Imatinib. Cancer Res. 2015;75(19):4053–62. doi: 10.1158/0008-5472.CAN-15-0611. [DOI] [PubMed] [Google Scholar]
- 145.Komarova NL, Burger JA, Wodarz D. Evolution of ibrutinib resistance in chronic lymphocytic leukemia (CLL). Proc Natl Acad Sci U S A. 2014;111(38):13906–11. doi: 10.1073/pnas.1409362111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Kostadinov RL, Kuhner MK, Li X, Sanchez CA, Galipeau PC, Paulson TG, Sather CL, Srivastava A, Odze RD, Blount PL. NSAIDs modulate clonal evolution in Barrett's esophagus. PLoS Genet. 2013;9(6):e1003553. doi: 10.1371/journal.pgen.1003553. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Leder K, Pitter K, Laplant Q, Hambardzumyan D, Ross BD, Chan TA, Holland EC, Michor F. Mathematical modeling of PDGF-driven glioblastoma reveals optimized radiation dosing schedules. Cell. 2014;156(3):603–16. doi: 10.1016/j.cell.2013.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]