Abstract
Clostridium difficile infection (CDI) is a major source of morbidity and mortality for the US healthcare system, and frequently complicates the course of inflammatory bowel disease (IBD). Patients with IBD are more likely to be colonized with C. difficile and develop active infection than the general population. They are also more likely to have severe CDI and develop subsequent complications such as IBD flare, colectomy, or death. Even after successful initial treatment and recovery, recurrent CDI is common. Management of CDI in IBD is fraught with diagnostic and therapeutic challenges, since the clinical presentations of CDI and IBD flare have considerable overlap. Fecal microbiota transplantation can be successful in curing recurrent CDI when other treatments have failed, but may also trigger IBD flare and this warrants caution. New, experimental treatments including vaccines, monoclonal antibodies, and non-toxigenic strains of C. difficile offer promise but are not yet available for clinicians. A better understanding of the complex relationship between the gut microbiota, CDI, and IBD is needed.
Keywords: Ulcerative colitis, Crohn’s disease, inflammatory bowel disease, Clostridium difficile, microbiota
Introduction
Clostridium difficile is a Gram-positive, spore-forming, obligate anaerobe and bacillus that was first identified in the 1970s as the cause for the then-rare condition of pseudomembranous colitis (1). Since then, C. difficile infection (CDI) has become one of the most frequent causes of nosocomial diarrhea and colitis and its incidence has been increasing over the past decade. C. difficile is now responsible for nearly 450,000 cases and 35,000 deaths annually in the US (2). This increased incidence and morbidity is due, in part, to the emergence of epidemic strains, including some with increased virulence (3–5).
CDI has become particularly problematic for patients with inflammatory bowel disease (IBD). The prevalence of IBD in the US is estimated at 238 per 100,000 population for UC and at 201 per 100,000 for Crohn’s, which amounts to over 1 million affected individuals (6). There is a higher prevalence of asymptomatic C. difficile carriage in patients with IBD (7, 8), the incidence of symptomatic CDI is higher in this population and has been increasing (9), and patients with IBD are at increased risk for adverse outcomes from CDI, which can also impact the management of the underlying IBD (10). These factors complicate both the diagnosis and management of CDI in patients with IBD and, thus, are the focus of this review.
Pathogenesis of CDI
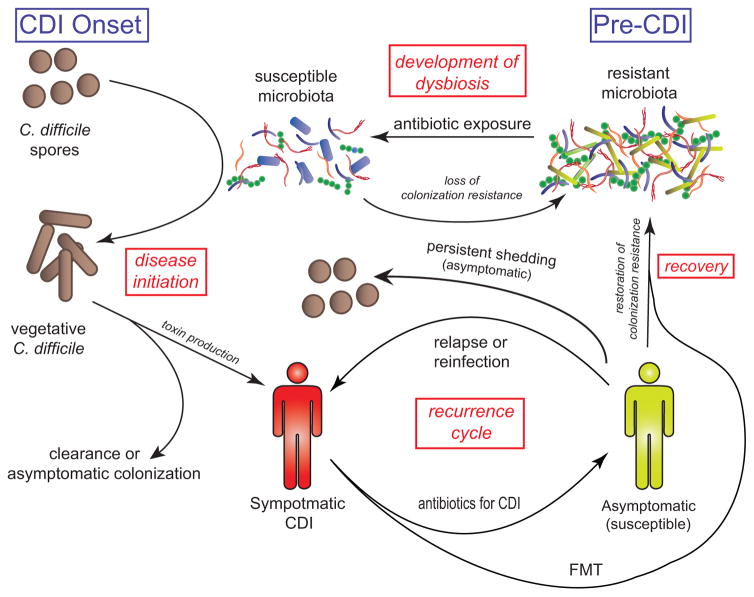
The pathogenesis of CDI was previously covered in detail in an Inflammatory Bowel Diseases review by Monaghan et al. (11) and, therefore, will only be summarized briefly here. Although the complete pathophysiological mechanisms underlying CDI have not been elucidated, it is known that toxin production by C. difficile is required and, consequently, non-toxigenic strains are not associated with clinical disease (12, 13). The basic paradigm of infection, shown in Figure 1, first involves loss of colonization resistance and establishment of susceptibility (14, 15). This is usually mediated by exposure to antimicrobials, which alter the gut microbiota to a susceptible state, discussed further below. Ingested C. difficile spores can then germinate, establish themselves in the colon, and produce toxins that inactivate the Rho family of proteins through glycosylation, which leads to disruption of the gut endothelial cells’ cytoskeletons and cell death(16). One or both of two principal toxins, TcdA and TcdB, are found in most pathogenic strains (16). Some strains, most notably including the epidemic strains known by their various classification schemata as ribotype 027, North American pulsed field gel electrophoresis pattern 1, or restriction endonuclease pattern BI, also produce the binary toxin, CDT (17). Although the precise pathophysiologic role of binary toxin is not known, its presence is associated with increased severity of CDI and mortality (18–20).
Figure 1. Life cycle of Clostridium difficile infection (CDI).
CDI first requires a susceptible host and microbiota, which often occurs following antibiotic exposure that decreases the diversity and composition of the gut’s microbial community. Some patients never develop disease, either clearing the infection or becoming asymptomatically colonized. Many patients shed spores asymptomatically following CDI. Even after successful treatment, some patients remain susceptible and can have recurrences. Fecal microbiota transplantation can help restore colonization resistance and break the cycle of recurrence
In addition to the pathogen’s virulence factors, host characteristics also play a role in the susceptibility to CDI. The humoral immune response is important, as patients without antibodies to C. difficile toxins are at increased risk of primary CDI and recurrence (21, 22). Relevant to IBD, immunocompromised patients are also at higher risk of CDI and subsequent adverse outcomes (23–26) In other populations, host and treatment-related features, such as healthcare exposure, increased comorbid disease, exposure to proton pump inhibitors, advanced age, and decreased functional status, all impact the risk of CDI and/or the subsequent clinical course (26–30). Although immunosuppression is common, patients with IBD have additional, distinct risk characteristics from these traditional risk factors, including younger age, outpatient care, and lack of antibiotic exposure immediately preceding CDI onset, yet still experience a high incidence of CDI (31).
One putative explanation for the high incidence of CDI in the IBD population, even without presence of classic risk factors for CDI, lies with the gut microbiota. The relationship between the gut microbiota, the disruption caused by antimicrobials (termed dysbiosis), and increased susceptibility to CDI has been established and includes features such as decreased diversity and alterations in the gut microbial taxa (14, 15, 32–41). In IBD, dysbiosis can occur independently of antimicrobial exposure, and C. difficile carriage can occur in this setting, suggesting a mechanism by which IBD itself can predispose to colonization by C. difficile (42–46).
Clinical presentation and natural history of CDI
The classic presentation of mild, symptomatic CDI includes diarrhea without systemic signs of infection (47). On endoscopy, pseudomembranes may also be present (Figure 2), though this is uncommon in IBD patients (48). Severe CDI occurs when systemic signs of infection such as fever, leukocytosis, and acute kidney injury are present (49). Complicated CDI results when hypotension, shock, ileus, and/or toxic megacolon are present (49). Although mild CDI may be self-limited, improving upon withdrawal of the inciting antimicrobials alone (50–52), symptomatic improvement often requires treatment as outlined below. Severe and complicated cases of CDI can carry significant morbidity, requiring admission to an intensive care unit and even colectomy. Despite treatment, 8–53% (median 19%) of patients with CDI will die within 30 days of initial disease (26).
Figure 2. Pseudomembranous colitis seen on a pathological colon specimen.
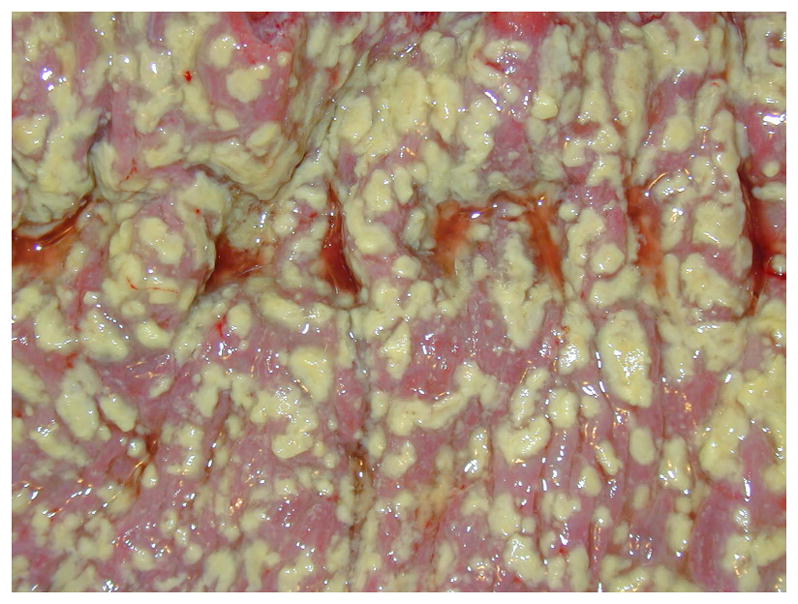
Footnote. Used with permission under an open content license from James Heilman, MD; source: https://commons.wikimedia.org/wiki/File:Pseudomembranous_colitis.JPG.
Even among those that successfully recover from CDI, recurrence within eight weeks is common and occurs in up to 25% of the general population (26). This incidence of recurrent CDI is considerably higher in older adults (26), and happens in up to 34% of patients with IBD (10, 53). Of those with a first recurrence, 30–45% will experience a second recurrence, with 45–60% of those experiencing a third recurrence, and ≤5% of patients entering a chronic cycle of seemingly endless recurrences (Figure 1) (54).
Among those that do not recur, asymptomatic shedding of C. difficile spores can continue for up to six weeks, complicating the diagnosis of recurrent CDI (55). Furthermore, up to 35% of patients will experience symptoms of a functional bowel disorder in the first two weeks after resolution of CDI (56). Although this is transient and rarely persists, approximately 4% of patients can develop a post-infectious irritable bowel syndrome lasting >3 months from CDI onset (56). Distinguishing a functional bowel disorder with shedding of spores from recurrent CDI remains a clinical challenge without a clear solution.
Standard diagnostic algorithms for CDI
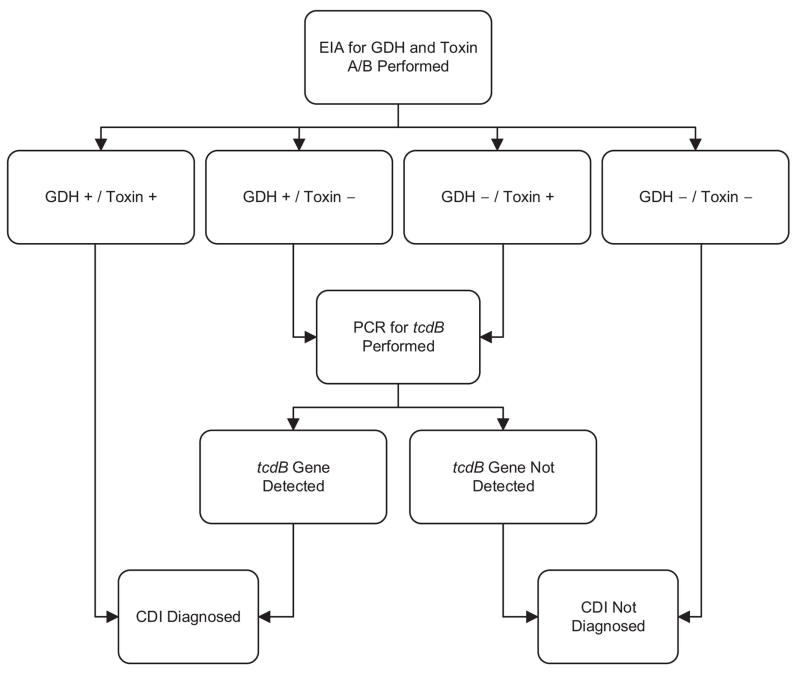
Diagnosis of CDI requires 1) presence of diarrhea (≥ 3 unformed stools in a 24 hour period) or radiographic evidence of ileus/toxic megacolon and 2) a positive stool test result indicating the presence of toxigenic C. difficile or colonoscopic/histopathologic findings demonstrating pseudomembranous colitis (47). There are a number of techniques used to identify the presence of toxigenic C. difficile, but the most common utilize either an enzyme immunoassay (EIA) for presence of C. difficile glutamate dehydrogenase and toxin (TcdA and/or TcdB) or real-time polymerase chain reaction (PCR) for the tcdB gene. Since PCR is quite sensitive for the presence of toxigenic C. difficile, it may increase detection of asymptomatic colonization and shedding (47). Thus, most experts recommend testing only diarrheal stool with laboratory-based rejection of formed specimens (57). Furthermore, there are proponents of multistep testing algorithms that start with EIA-based tests with a reflex to the PCR test for discordant EIA results (Figure 3) (47, 58).
Figure 3. Sample multistep testing algorithm for diagnosis of Clostridium difficile infection.
Footnote. Adapted from Rao et al., 2014 (145). Used with permission under the Creative Commons licence.
Abbreviations. CDI, Clostridium difficile infection; EIA, enzyme immunoassay; GDH, glutamate dehydrogenase; PCR, polymerase chain reaction.
Diagnostic dilemmas surrounding CDI in IBD
As noted above, some individuals can carry toxigenic C. difficile asymptomatically. This leads to one of the major challenges in diagnosing CDI in the IBD population, since there is a high incidence of asymptomatic colonization. Clayton et al. evaluated outpatients with IBD who were in clinical remission and had no recent exposure to antimicrobials, corticosteroids, immunomodulatory agents, or hospitalizations (7). These patients had toxigenic C. difficile carriage rates of 8.2%, compared to only 1% of the healthy outpatient population. Furthermore, there was a higher prevalence of C. difficile carriage in UC patients (9.4%) than in CD patients (6.9%). Although there are reference standards such as cytotoxicity assay and toxigenic culture that definitively establish the presence of toxigenic C. difficile in stool (47), there is no gold standard for diagnosis of symptomatic CDI short of positive testing for the organism plus a histopathologic diagnosis of pseudomembranous colitis. However, pseudomembranes are only present in up to 13% of CDI cases with underlying IBD (48).
Further compounding this dilemma is the considerable overlap seen in the clinical syndromes of CDI and IBD flare (10, 59). It is often impossible to distinguish the two on clinical grounds alone. In addition, the two conditions are not mutually exclusive as they can co-exist temporally, and CDI can even trigger an IBD flare and complicate the subsequent IBD course (53, 59). Strategies for navigating these issues and deciding on the optimal treatment course are discussed below.
Treatment of CDI, IBD, or both?
In patients with IBD, given the clinical conundrum introduced above that often precludes definitively identifying the source of a patient’s diarrhea when toxigenic C. difficile is present in stool, there are multiple approaches that can be entertained. A survey of American and Canadian gastroenterologists on the topic found them split on the issue, with 46% electing to use antibiotics alone to treat the CDI and 54% electing to use both antibiotics and immunomodulators together (60). One might speculate that “hedging your bets” and treating for both conditions would be the safest approach, however a retrospective, multicenter study in Europe noted an increased risk of mortality in patients treated with both antibiotics and immunomodulators (defined as prednisone >20 mg/day, azathioprine, 6-mercaptopurine, methotrexate, biologics, cyclosporine, or tacrolimus)(61). Although this study had several limitations, including a small sample size, lack of randomization, and non-standardized antibiotics for CDI, this warrants caution in starting or escalating immunosuppression without appropriate antimicrobials against CDI.
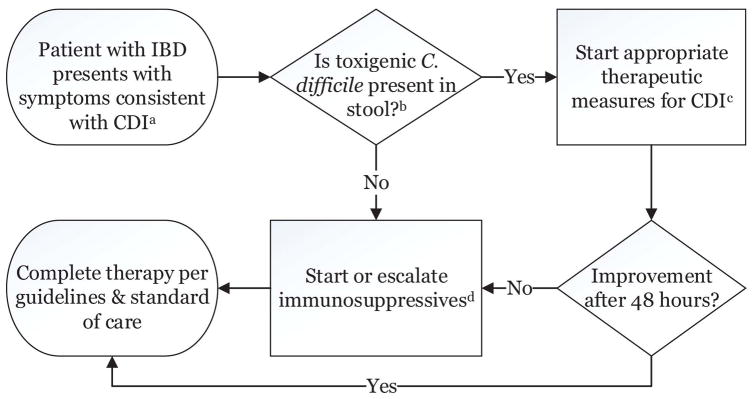
Given this uncertainty and lack of guidance from the literature, we have proposed an algorithm to approach treatment of patients with IBD presenting with diarrhea and toxigenic C. difficile in their stool that balances the uncertainty and risk in this population (Figure 4). We suggest that patients who present with a clinical syndrome consistent with CDI be evaluated with appropriate stool testing. If they test negative, immunosuppressive therapy should be initiated (62). If they test positive, appropriate treatment for CDI should begin, as detailed below. If there is no response to the treatment for CDI after 48 hours, then concurrent immunologic therapy can be started/escalated.
Figure 4. Suggested algorithm for management of patients with IBD presenting with symptoms consistent with CDI.
Footnotes:
aSee text for details on symptoms consistent with CDI.
bWhile awaiting test results, empiric treatment for CDI and/or IBD flare may be appropriate with a severe presentation
cSee table 1 for details.
dThe management of IBD flare is beyond the scope of this review; see Kornbluth et al. 2010 (62) for suggestions on how to proceed.
Abbreviations: CDI, Clostridium difficile infection; IBD, inflammatory bowel disease.
Treatment of CDI: initial measures
Asymptomatic carriers of toxigenic C. difficile should not be treated as this does not impact the risk of subsequent disease, and may even promote shedding and increase transmission to other individuals (63, 64). Intriguingly, carriers may actually be at lower risk of subsequent CDI. Shim et al. followed asymptomatically colonized and non-colonized patients weekly and noted symptomatic CDI occurred in 1% and 3.6%, respectively (65).
Since there are no prospective clinical trials on treating CDI in IBD, evidence from the general population is used to guide management. Initial measures that have been recommended include cessation of other concomitant antimicrobials, antimotility agents, and proton pump inhibitors (47). Though any antibiotic can adversely impact the gut microbiota, penicillins, cephalosporins, clindamycin, and fluoroquinolones are particularly associated with CDI (66–68). Historically, withdrawal of offending antibiotics as monotherapy was sufficient to achieve cure of CDI in up to 15% of patients (50, 51, 69). Both antimotility agents such as loperamide and proton pump inhibitors have been associated with complicated CDI and/or recurrence (70, 71); thus, it is prudent to stop these agents when possible. Cholestyramine has been tested for its role in binding toxins produced by C. difficile (discussed below), however it can also bind antibiotics used to treat CDI such as vancomycin and should be stopped prior to starting such therapy (49).
Treatment of CDI: pharmacologic management
Primary CDI mild and severe CDI
The typical management of CDI involves first assigning a severity category and determining if criteria for a recurrent episode are met, followed by selection of the appropriate antimicrobial agent and course (Table 1)(47). Metronidazole has been promoted by numerous guidelines and reviews as the first line agent for mild, uncomplicated CDI, and vancomycin is only recommended for severe CDI and/or recurrence (47, 49, 72, 73). Prior literature has suggested that there is no difference in cure between vancomycin and metronidazole for mild disease (74). However, in 2014 Johnson et al. conducted a pooled analysis of two clinical trials and found metronidazole to be inferior to vancomycin (odds ratio for clinical success 1.58, 95% confidence interval 1.04–2.4, P = .034), even after adjustment for disease severity (75). Thus, given the higher likelihood of adverse events from CDI in IBD, many experts have argued for the use of vancomycin first-line in the IBD population, though no prospective data demonstrating a benefit over metronidazole exist (76, 77).
Table 1.
Classification and pharmacologic management of CDI.
| Classification | Criteria | Treatment options |
|---|---|---|
| Primary CDI, non-severe | Diarrhea without signs of systemic infection, WBC <15,000 cells/mL, and serum creatinine <1.5 times the premorbid level | metronidazole 500 mg by mouth three times daily for 10–14 days OR vancomycin 125 mg by mouth three times daily for 10–14 days OR fidaxomicin 200 mg by mouth twice daily for 10 daysa |
| Primary CDI, severe | Signs of systemic infection and/or WBC ≥15,000 cells/mL, or serum creatinine ≥1.5 times the premorbid level | vancomycin 125 mg by mouth three times daily for 10–14 days OR fidaxomicin 200 mg by mouth twice daily for 10 daysa |
| Primary CDI, complicated | signs of systemic infection including hypotension, ileus, or megacolon | vancomycin 500 mg by mouth four times daily AND intravenous metronidazole 500 mg three times daily AND (if ileus is present) vancomycin 500 mg by rectum four times daily |
| Recurrent CDI | Return of symptoms with positive C. difficile testing within 8 weeks of onset, but after initial symptoms resolved with treatment |
First recurrence: same as initial treatment, based on severity. Second recurrence: Start treatment based on severity, followed by a vancomycin pulsed and/or tapered regimen over six or more weeks |
Footnotes.
fidaxomicin is not currently represented in US guidelines (49) but is FDA-approved for the treatment of CDI.
Abbreviations: CDI, Clostridium difficile infection; WBC, white blood cell count.
Fidaxomicin is the newest antimicrobial approved for treatment of primary and first recurrence of CDI (78). Fidaxomicin is minimally absorbed from the gut, leading to little systemic exposure and has a very narrow spectrum of activity, leading to little disruption of the microbiota with use (78). In a meta-analysis of the two phase 3 clinical trials that led to approval of fidaxomicin by the US Food and Drug Administration (FDA) for treatment of CDI, it was found to have a lower incidence of recurrence of CDI when compared to vancomycin (78). The FDA granted a superiority designation for this indication. Despite these encouraging data, widespread use of fidaxomicin for primary CDI and first recurrence of CDI has been hampered by its cost and lack of incorporation into national guidelines. Several studies have examined the cost effectiveness of fidaxomicin and have reached contradictory conclusions (79–81). Furthermore, the efficacy of fidaxomicin in the IBD population has not been well-established.
Complicated CDI
Although there is a paucity of high quality data on the treatment of complicated CDI, expert opinion and guidelines favor higher doses of oral vancomycin along with intravenous (IV) metronidazole (Table 1) (47, 49, 72, 73). There are also case reports suggesting that rectally administered vancomycin may be of benefit when ileus is present and this is thus also recommended (Table 1) (82, 83). Both IV metronidazole and rectally administered vancomycin should be used in combination with oral vancomycin and not in lieu of it, as rectal vancomycin does not reach the entire colon and treatment failures are reported with IV metronidazole monotherapy. (51, 83, 84)
Recurrent CDI
The treatment of recurrent CDI beyond the first recurrence is often initiated through tapering and/or pulsed doses of vancomycin (Table 1). The specific construction of the tapering schedule has not been optimized through any prospective studies, however experts generally suggest reducing the dosing frequency by one administration per day per week, ending with three times weekly dosing (pulsing) (47). The pulsed-dosing strategy may have higher efficacy than tapering alone, based on a secondary analysis of two clinical trials with a 31% incidence of recurrence with a tapering course versus 14.3% with pulsed dosing (85). Given the known significant disruption of the gut microbiota from oral vancomycin, some experts recommend use of a “chaser” with a narrower spectrum of activity following vancomycin which still prevents CDI from relapsing while allowing the microbiota to recover (47). Two studied agents for this indication are rifaximin and fidaxomicin, though neither has high quality evidence in support of their use in the “chaser” role (86–88).
Treatments not recommended for CDI
There are several treatments that have been tested over the years for CDI that are currently not recommended. These include intravenous immunoglobulin and toxin binders such as tolevamer, colestipol, and cholestyramine (47, 75, 89), none of which have demonstrated efficacy in high quality studies, and the latter may actually bind antibiotics used to treat CDI (90). Tolevamer has actually been shown to be inferior to both metronidazole and vancomycin in a randomized, controlled clinical trial (RCT) (75). Tigecycline has been efficacious in case reports for severe CDI but its role remains unclear and, thus, it cannot be recommended at present (91). Nitzoxanide has been studied and does show promise for patients who fail metronidazole, but noninferiority to vancomycin has not been demonstrated (92–94). Therefore, patients who fail metronidazole should still receive vancomycin next (Table 1).
Probiotics
Probiotics are live microorganism preparations that are used to treat dysbiosis in various conditions. The role of probiotics in CDI remains poorly defined, based on a paucity of high quality evidence. Meta-analyses of numerous small studies have suggested an adjunctive role when taken alongside other antimicrobials in prevention of initial episodes and recurrence (95–98). One large RCT was unable to demonstrate a benefit of probiotics in reducing incident CDI, but did observe a trend toward reduction and with only 1% of the study population developing CDI, it was likely underpowered to detect a true difference (99). In response to an outbreak, a hospital in Canada noted a significant reduction in both incidence and severity of CDI after implementation of a probiotic prophylaxis bundle for patients started on antibiotics (100). However, the entire patient population moved to a new facility mid-way through the study, and much of the reduction was seen following this relocation, casting doubt on the veracity of the results. A recent case series suggests that daily administration of kefir, a fermented milk drink, alongside staggered, tapered doses of vancomycin or metronidazole was beneficial for recurrent CDI (101). Although probiotics are generally safe and well-tolerated, some risk for bacteremia and fungemia exists and has been described in immunocompromised and critically ill patients (102).
Fecal Microbiota Transplantation
Recurrent CDI
Fecal microbiota transplantation (FMT) delivers donated stool into the gut of an affected individual in order to correct dysbiosis and restore the microbiota to a healthy state. In the US, without an investigational new drug application, FMT can only be used for the treatment of CDI (103). The preponderance of data on FMT for CDI is in the treatment of CDI recurrence. Although initially consisting of only case reports and case series, there are now several high quality RCTs demonstrating efficacy (104–108). Although the optimal parameters for FMT administration have not been rigorously tested, the route, stool preparation methods, amount of stool infusate, and donor characteristics all vary considerably between studies and FMT is up to 92% effective irrespective of these variations (54). One recent trial compared fresh to frozen stool and both were highly efficacious, which is encouraging given the convenience of frozen stool banks that obviate the need for individual clinicians to select and screen donors (107). The recent evidence of the effectiveness of frozen, capsulized stool preparations promises to further reduce the logistical barriers to FMT (109). A popular stool bank, OpenBiome (openbiome.org, Medford, MA), initially made available frozen stool capsules for use by investigators in clinical trials, and recently made this treatment option available to all clinicians based on encouraging preliminary data (110).
Primary CDI
There are few studies examining the use of FMT for primary, non-recurrent CDI aside from case reports (54, 111). However, a mathematical model where FMT was used for primary CDI in an intensive care unit suggested a decrease in the mean incidence of recurrent CDI in patients compared to antimicrobial therapy alone (112). This suggests that despite the increased cost and logistical difficulties, there may be benefit to using FMT in lieu of conventional therapy in a population at high risk of recurrence. A major limitation of this study, in addition to being a mathematical model, was the lack of incorporation of known risk factors for CDI. A non-randomized, open label, before/after prospective study of FMT for primary CDI was conducted by Lagier et al. (113) They compared a period utilizing only conventional therapy with one promoting early FMT via nasogastric infusion. This shift in treatment strategies occurred at their institution due to an outbreak of CDI from a ribotype 027 strain that had a 50.8% mortality rate, prompting clinicians to try non-conventional measures to combat the disease. Mortality in the FMT group was 18.8% versus 64.4% (P <.01). Since this was an older cohort with a mean age of 84 and during an epidemic, the findings may not be generalizable to treatment of primary CDI in other settings.
Severe and Complicated CDI
FMT for severe CDI (both primary and recurrent) similarly has little evidence, though the published case reports show efficacy (114–117). Furthermore, although the study discussed above by Lagier et al. does not provide data on severity classification, the high mortality rate suggests some of the patients who benefited from FMT met the severity definition (113). There was one death reported following FMT for complicated CDI discussed further below (118), and this warrants caution before widespread use can be recommended for this indication.
Safety of FMT with IBD
FMT for CDI in the general population appears safe in the short term with serious adverse events being uncommon (54). Symptoms of an irritable bowel (cramping and bloating) shortly after FMT are reported but usually last less than 48 hours. However, there may be additional risk incurred in the IBD population. A recent case series of immunocompromised patients with CDI treated by FMT did not identify many adverse events overall (119). However, 14% of the subgroup of patients with IBD experienced adverse events including IBD flare, requiring hospitalization in some instances. Other studies have also reported an increased risk of IBD flare, fever, and elevated inflammatory markers after FMT, including one case of sepsis that developed after a home self-FMT (120). Currently, there are no tools to risk stratify patients in terms of these adverse outcomes and/or select donor stool based on microbiota characteristics. In contrast with the published cases, a recent RCT for patients with ulcerative colitis (without concurrent CDI) treated with FMT did not find any significant adverse events, highlighting the complexity of the interaction between IBD and the microbiota that can result in inter-study variability (121).
Much of the safety data, however, are garnered from studies of FMT for recurrent, non-severe CDI. Use of FMT in severe and complicated CDI has not been well studied in terms of efficacy, and thus there is a paucity of safety data to accompany these indications. In one study, a patient developed complicated CDI including an associated septic shock and was treated with standard of care (Table 1) but still had loose stools after 18 days of therapy (118). He did have other milder markers of severity at that time, including pancolitis on visualized on computed tomography (CT) and elevated creatinine. He underwent FMT via gastrojejunostomy tube with stool donated by his wife, the procedure was uncomplicated, and antimicrobials were stopped with clinical stability for the first 48 hours. He subsequently developed septic shock and bacteremia from Pseudomonas aeruginosa, Escherichia coli, and Lactobacillus casei. A repeat CT revealed toxic megacolon and, despite resection, he died on the fourth day following FMT. The authors speculate that withdrawal of antibiotics active against CDI may have contributed to his clinical decline. Nevertheless, this case raises concerns over the safety of FMT in severe and complicated CDI.
Unlike the short-term effects, concerns about the long-term safety of FMT have been raised even in the general, immunocompetent non-IBD population (109). There are demonstrated associations between the gut microbiota and other conditions such as diabetes, obesity, and cancer (122). Animal models of colitis and obesity have even been able to “transmit” the disease phenotype through FMT (122–124). Thus, it is plausible that although effective and safe in the short run for treatment of CDI, FMT may be adversely altering the long-term risk profiles of various other diseases. Data on long-term follow-up of patients following FMT is lacking, but one study that followed 77 patients for 3–68 months (mean 17 months) observed the development of several new medical conditions including ovarian cancer and autoimmune disease (125). Although causality cannot be inferred from these data, the findings do highlight the need for additional studies on long-term outcomes in patients that underwent FMT. A systematic review and meta-analysis of any studies with >90 days of follow-up demonstrated an overall low incidence of late adverse events, and only a 1.7% cumulative incidence of late recurrence (126).
Experimental therapies and future directions
FMT
There are several clinical trials underway that may shed light on the safety and efficacy of FMT for primary CDI, severe CDI, and as prophylaxis against CDI for high risk patients on antimicrobials (127–131). Until the data from these studies are available, given the risk of IBD flare and at least one death following FMT seen in some studies and case reports on severe CDI, it is prudent to reserve FMT for recurrent, non-complicated CDI at this time.
There has also been an interest in the use of FMT to correct the underlying dysbiosis of IBD and induce clinical remission. Initial case reports on use of FMT for IBD outside the context of recurrent CDI were encouraging for treatment of UC (132–135). While patients without IBD have fecal microbial diversity that stably mimics the donor stool following FMT, by six months post-FMT, patients with IBD have stool microbiota that returns to the pre-FMT level of diversity, suggesting host-related mechanisms that modify the microbiota (136). Subsequent reports have raised some concerns, however. In one prospective case series of five patients, none achieved clinical remission after FMT and only one had improvement in symptoms (137). There may also have been harm as all the patients had transient fever and elevation in serum C-reactive protein levels. Another study of six UC patients did note transient clinical improvement in all of them following FMT, but none achieved remission (138).
Two RCTs have been published on the use of FMT for UC and both were stopped early due to futility, though enrolled patients were allowed to complete the studies (121, 139). Intriguingly, in one of these studies, of the nine patients that did achieve UC remission seven received their stool from the same donor (121). This suggests a donor- and microbiota-specific beneficial effect that has yet to be elucidated by basic research.
The data on use of FMT for CD are even sparser than for UC. One patient in a case report demonstrated clinical improvement only to relapse after 18 months (133). Another report noted clinical, endoscopic, and histologic remission after a single infusion of feces (140). Again, unlike FMT for CDI in the general population, where it appears to be generally effective irrespective of many other clinical and microbiological characteristics, there appears to be underlying characteristics for IBD in the donor, microbiota, and/or recipient that interact in a complex manner to produce the clinical outcome, and the details of this have yet to be determined.
Immunotherapy
There is a C. difficile vaccine in development for treatment of primary and recurrent CDI, but the outcomes data have not yet been released (141). Prior data had suggested monoclonal antibodies against C. difficile toxins may be efficacious in preventing recurrence (142). Recently, two phase 3 studies, MODIFY I AND MODIFY II evaluated the efficacy of two monoclonal antibodies, actoxumab (ACT) and bezlotoxumab (BEZ), with activity against TcdA and TcdB, respectively (143). The study arm with ACT alone was stopped early due to lack of efficacy in an interim analysis. The pooled analysis of the 2327 patients that received either ACT + BEZ or BEZ alone observed recurrent CDI in 15.4% and 16.5%, respectively, versus 26.6% in the placebo arm (P <.001). The safety profiles in the interventional arms were similar to the placebo arm. Although patients with IBD were not specifically included, analysis of other subgroups at high risk of recurrence, such as age ≥65 years, history of CDI, ribotype 027 infection, and severity, showed similar reductions in recurrence compared to placebo.
Non-toxigenic C. difficile
Another strategy to prevent recurrent CDI involves administration of spores derived from non-toxigenic strains of C. difficile. A phase 2 RCT studying strain VP20261 administered to patients with primary CDI or first recurrence of CDI found a dose-dependent reduction in recurrent CDI (144). Recurrent CDI overall occurred in 11% of patients receiving spores, compared to 30% in the placebo arm (P = .003). However, only the subgroups receiving 107 spores/day demonstrated a significant benefit, while those receiving 104 spores/day did not. Furthermore, colonization with the non-toxigenic strain was transient, as all study arms had negative fecal cultures by week 26. Since TcdA and TcdB are not produced by this strain, the mechanism of action for protection is not well-understood, but it may be transient given the lack of persistent fecal colonization. Possible non-immunologic mechanisms for resistance to recurrent CDI conferred by non-toxigenic strains include alterations in the microbiota or niche exclusion through physical occupation or competition for nutrients.
Summary and Conclusions
The management of CDI in patients with IBD presents both diagnostic and therapeutic challenges. Balancing the risk of certain treatment strategies is important given that CDI can present with severe features when IBD is present and can affect the subsequent course of IBD. Although FMT shows promise, the role of the microbiota in IBD is complex and initial studies warrant a cautious approach. A better understanding is needed of the specific features of the microbiota that promote IBD remission with or without concomitant CDI. New treatments on the horizon including vaccines, monoclonal antibodies, and spores of non-toxigenic C. difficile may soon give patients multiple additional options for management of what can be a difficult, recalcitrant disease.
Acknowledgments
This work was supported in part by grants from the National Institute of Diabetes and Digestive and Kidney Diseases at the National Institutes of Health [grant number R01-GM097117], the Crohn’s and Colitis Foundation of America [grant number 253590], and the Claude D. Pepper Older Americans Independence Center [grant number AG-024824]. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Bartlett JG, Chang TW, Gurwith M, et al. Antibiotic-associated pseudomembranous colitis due to toxin-producing Clostridia. N Engl J Med. 1978;298:531–534. doi: 10.1056/NEJM197803092981003. [DOI] [PubMed] [Google Scholar]
- 2.Lessa FC, Mu Y, Bamberg WM, et al. Burden of Clostridium difficile infection in the United States. N Engl J Med. 2015;372:825–834. doi: 10.1056/NEJMoa1408913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McDonald LC, Killgore GE, Thompson A, et al. An epidemic, toxin gene-variant strain of Clostridium difficile. N Engl J Med. 2005;353:2433–2441. doi: 10.1056/NEJMoa051590. [DOI] [PubMed] [Google Scholar]
- 4.Loo VG, Poirier L, Miller MA, et al. A Predominantly Clonal Multi-Institutional Outbreak of Clostridium difficile–Associated Diarrhea with High Morbidity and Mortality. N Engl J Med. 2005;353:2442–2449. doi: 10.1056/NEJMoa051639. [DOI] [PubMed] [Google Scholar]
- 5.Warny M, Pepin J, Fang A, et al. Toxin production by an emerging strain of Clostridium difficile associated with outbreaks of severe disease in North America and Europe. Lancet. 2005;366:1079–1084. doi: 10.1016/S0140-6736(05)67420-X. [DOI] [PubMed] [Google Scholar]
- 6.Kappelman MD, Rifas-Shiman SL, Kleinman K, et al. The prevalence and geographic distribution of Crohn’s disease and ulcerative colitis in the United States. Clin Gastroenterol Hepatol. 2007;5:1424–1429. doi: 10.1016/j.cgh.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 7.Clayton EM, Rea MC, Shanahan F, et al. The Vexed Relationship Between Clostridium difficile and Inflammatory Bowel Disease: An Assessment of Carriage in an Outpatient Setting Among Patients in Remission. Am J Gastroenterol. 2009;104:1162–1169. doi: 10.1038/ajg.2009.4. [DOI] [PubMed] [Google Scholar]
- 8.Hourigan SK, Chirumamilla SR, Ross T, et al. Clostridium difficile Carriage and Serum Antitoxin Responses in Children with Inflammatory Bowel Disease. Inflamm Bowel Dis. 2013;19:2744–2752. doi: 10.1097/01.MIB.0000435434.53871.36. [DOI] [PubMed] [Google Scholar]
- 9.Bossuyt P, Verhaegen J, Van Assche G, et al. Increasing incidence of Clostridium difficile-associated diarrhea in inflammatory bowel disease. Journal of Crohn’s and Colitis. 2009;3:4–7. doi: 10.1016/j.crohns.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 10.Goodhand JR, Alazawi W, Rampton DS. Systematic review: Clostridium difficile and inflammatory bowel disease. Aliment Pharmacol Ther. 2011;33:428–441. doi: 10.1111/j.1365-2036.2010.04548.x. [DOI] [PubMed] [Google Scholar]
- 11.Monaghan TM, Cockayne A, Mahida YR. Pathogenesis of Clostridium difficile Infection and Its Potential Role in Inflammatory Bowel Disease. Inflamm Bowel Dis. 2015;21:1957–1966. doi: 10.1097/MIB.0000000000000461. [DOI] [PubMed] [Google Scholar]
- 12.Kuijper EJ, Coignard B, Tüll P. Emergence of Clostridium difficile-associated disease in North America and Europe. Clin Microbiol Infect. 2006;12:2–18. doi: 10.1111/j.1469-0691.2006.01580.x. [DOI] [PubMed] [Google Scholar]
- 13.Natarajan M, Walk ST, Young VB, et al. A clinical and epidemiological review of non-toxigenic Clostridium difficile. Anaerobe. 2013;22:1–5. doi: 10.1016/j.anaerobe.2013.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reeves AE, Theriot CM, Bergin IL, et al. The interplay between microbiome dynamics and pathogen dynamics in a murine model of Clostridium difficile Infection. Gut Microbes. 2011;2:145–158. doi: 10.4161/gmic.2.3.16333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Buffie CG, Jarchum I, Equinda M, et al. Profound alterations of intestinal microbiota following a single dose of clindamycin results in sustained susceptibility to Clostridium difficile-induced colitis. Infect Immun. 2012;80:62–73. doi: 10.1128/IAI.05496-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pothoulakis C, Lamont JT. Microbes and Microbial Toxins: Paradigms for Microbial- Mucosal Interactions II. The integrated response of the intestine to Clostridium difficile toxins. Am J Physiol Gastrointest Liver Physiol. 2001;280:G178–G183. doi: 10.1152/ajpgi.2001.280.2.G178. [DOI] [PubMed] [Google Scholar]
- 17.Gerding DN, Johnson S, Rupnik M, et al. Clostridium difficile binary toxin CDT: Mechanism, epidemiology, and potential clinical importance. Gut Microbes. 2014:5. doi: 10.4161/gmic.26854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goldenberg SD, French GL. Lack of association of tcdC type and binary toxin status with disease severity and outcome in toxigenic Clostridium difficile. J Infect. 2011;62:355–362. doi: 10.1016/j.jinf.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 19.Bacci S, Mølbak K, Kjeldsen MK, et al. Binary Toxin and Death after Clostridium difficile Infection. Emerg Infect Dis. 2011;17:976–982. doi: 10.3201/eid1706.101483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou Y, Burnham CA, Hink T, et al. Phenotypic and genotypic analysis of Clostridium difficile isolates: a single center study. J Clin Microbiol. 2014 doi: 10.1128/JCM.02115-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kyne L, Warny M, Qamar A, et al. Asymptomatic Carriage of Clostridium difficile and Serum Levels of IgG Antibody against Toxin A. N Engl J Med. 2000;342:390–397. doi: 10.1056/NEJM200002103420604. [DOI] [PubMed] [Google Scholar]
- 22.Kyne L, Warny M, Qamar A, et al. Association between antibody response to toxin A and protection against recurrent Clostridium difficile diarrhoea. Lancet. 2001;357:189–193. doi: 10.1016/S0140-6736(00)03592-3. [DOI] [PubMed] [Google Scholar]
- 23.Das R, Feuerstadt P, Brandt LJ. Glucocorticoids are associated with increased risk of short-term mortality in hospitalized patients with Clostridium difficile-associated disease. Am J Gastroenterol. 2010;105:2040–2049. doi: 10.1038/ajg.2010.142. [DOI] [PubMed] [Google Scholar]
- 24.Haines CF, Moore RD, Bartlett JG, et al. Clostridium difficile in a HIV-infected cohort: incidence, risk factors, and clinical outcomes. AIDS. 2013;27:2799–2807. doi: 10.1097/01.aids.0000432450.37863.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jain T, Croswell C, Urday-Cornejo V, et al. Clostridium difficile colonization in hematopoietic stem cell transplant recipients: A prospective study of the epidemiology and outcomes involving toxigenic and non-toxigenic strains. Biol Blood Marrow Transplant. 2015 doi: 10.1016/j.bbmt.2015.07.020. [DOI] [PubMed] [Google Scholar]
- 26.Abou Chakra CN, Pepin J, Sirard S, et al. Risk Factors for Recurrence, Complications and Mortality in Clostridium difficile Infection: A Systematic Review. PLoS ONE. 2014;9:e98400. doi: 10.1371/journal.pone.0098400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rao K, Micic D, Chenoweth E, et al. Poor Functional Status as a Risk Factor for Severe Clostridium difficile Infection in Hospitalized Older Adults. J Am Geriatr Soc. 2013;61:1738–1742. doi: 10.1111/jgs.12442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Welfare MR, Lalayiannis LC, Martin KE, et al. Co-morbidities as predictors of mortality in Clostridium difficile infection and derivation of the ARC predictive score. J Hosp Infect. 2011;79:359–363. doi: 10.1016/j.jhin.2011.08.015. [DOI] [PubMed] [Google Scholar]
- 29.Kyne L, Sougioultzis S, McFarland LV, et al. Underlying disease severity as a major risk factor for nosocomial Clostridium difficile diarrhea. Infect Control Hosp Epidemiol. 2002;23:653–659. doi: 10.1086/501989. [DOI] [PubMed] [Google Scholar]
- 30.Dubberke ER, Yan Y, Reske KA, et al. Development and validation of a Clostridium difficile infection risk prediction model. Infect Control Hosp Epidemiol. 2011;32:360–366. doi: 10.1086/658944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Epple HJ. Therapy- and Non-Therapy-Dependent Infectious Complications in Inflammatory Bowel Disease. Dig Dis. 2009;27:555–559. doi: 10.1159/000233297. [DOI] [PubMed] [Google Scholar]
- 32.HOPKINS MJ, MACFARLANE GT. Changes in predominant bacterial populations in human faeces with age and with Clostridium difficile infection. J Med Microbiol. 2002;51:448–454. doi: 10.1099/0022-1317-51-5-448. [DOI] [PubMed] [Google Scholar]
- 33.Buffie CG, Pamer EG. Microbiota-mediated colonization resistance against intestinal pathogens. Nat Rev Immunol. 2013;13:790–801. doi: 10.1038/nri3535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dethlefsen L, Huse S, Sogin ML, et al. The Pervasive Effects of an Antibiotic on the Human Gut Microbiota, as Revealed by Deep 16S rRNA Sequencing. PLoS Biol. 2008;6:e280. doi: 10.1371/journal.pbio.0060280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Antonopoulos DA, Huse SM, Morrison HG, et al. Reproducible Community Dynamics of the Gastrointestinal Microbiota following Antibiotic Perturbation. Infect Immun. 2009;77:2367–2375. doi: 10.1128/IAI.01520-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Borriello SP, Barclay FE. An in-vitro model of colonisation resistance to Clostridium difficile infection. J Med Microbiol. 1986;21:299–309. doi: 10.1099/00222615-21-4-299. [DOI] [PubMed] [Google Scholar]
- 37.Sullivan Å, Edlund C, Nord CE. Effect of antimicrobial agents on the ecological balance of human microflora. Lancet Infect Dis. 2001;1:101–114. doi: 10.1016/S1473-3099(01)00066-4. [DOI] [PubMed] [Google Scholar]
- 38.Jernberg C, Lofmark S, Edlund C, et al. Long-term ecological impacts of antibiotic administration on the human intestinal microbiota. ISME J. (1) :56–66. doi: 10.1038/ismej.2007.3. [DOI] [PubMed] [Google Scholar]
- 39.Chang JY, Antonopoulos DA, Kalra A, et al. Decreased Diversity of the Fecal Microbiome in Recurrent Clostridium difficile—Associated Diarrhea. J Infect Dis. 2008;197:435–438. doi: 10.1086/525047. [DOI] [PubMed] [Google Scholar]
- 40.Antharam VC, Li EC, Ishmael A, et al. Intestinal Dysbiosis and Depletion of Butyrogenic Bacteria in Clostridium difficile Infection and Nosocomial Diarrhea. J Clin Microbiol. 2013;51:2884–2892. doi: 10.1128/JCM.00845-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Theriot CM, Koenigsknecht MJ, Carlson PE, Jr, et al. Antibiotic-induced shifts in the mouse gut microbiome and metabolome increase susceptibility to Clostridium difficile infection. Nat Commun. 2014;5:3114. doi: 10.1038/ncomms4114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kostic AD, Xavier RJ, Gevers D. The Microbiome in Inflammatory Bowel Disease: Current Status and the Future Ahead. Gastroenterol. 2014;146:1489–1499. doi: 10.1053/j.gastro.2014.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Frank DN, St Amand AL, Feldman RA, et al. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. PNAS. 2007;104:13780–13785. doi: 10.1073/pnas.0706625104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Manichanh C, Rigottier-Gois L, Bonnaud E, et al. Reduced diversity of faecal microbiota in Crohn’s disease revealed by a metagenomic approach. Gut. 2006;55:205–211. doi: 10.1136/gut.2005.073817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sokol H, Pigneur B, Watterlot L, et al. Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patients. PNAS. 2008;105:16731–16736. doi: 10.1073/pnas.0804812105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Machiels K, Joossens M, Sabino J, et al. A decrease of the butyrate-producing species Roseburia hominis and Faecalibacterium prausnitzii defines dysbiosis in patients with ulcerative colitis. Gut. 2013 doi: 10.1136/gutjnl-2013-304833. [DOI] [PubMed] [Google Scholar]
- 47.Bagdasarian N, Rao K, Malani PN. Diagnosis and Treatment of Clostridium difficile in Adults: A Systematic Review. JAMA. 2015;313:398–408. doi: 10.1001/jama.2014.17103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ben-Horin S, Margalit M, Bossuyt P, et al. Prevalence and clinical impact of endoscopic pseudomembranes in patients with inflammatory bowel disease and Clostridium difficile infection. Journal of Crohn’s and Colitis. 2010;4:194–198. doi: 10.1016/j.crohns.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 49.Cohen SH, Gerding DN, Johnson S, et al. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the society for healthcare epidemiology of America (SHEA) and the infectious diseases society of America (IDSA) Infect Control Hosp Epidemiol. 2010;31:431–455. doi: 10.1086/651706. [DOI] [PubMed] [Google Scholar]
- 50.Bartlett JG. Treatment of antibiotic-associated pseudomembranous colitis. Rev Infect Dis. 1984;6(Suppl 1):S235–241. doi: 10.1093/clinids/6.supplement_1.s235. [DOI] [PubMed] [Google Scholar]
- 51.Olson MM, Shanholtzer CJ, Lee JT, Jr, et al. Ten years of prospective Clostridium difficile-associated disease surveillance and treatment at the Minneapolis VA Medical Center, 1982–1991. Infect Control Hosp Epidemiol. 1994;15:371–381. doi: 10.1086/646934. [DOI] [PubMed] [Google Scholar]
- 52.MDSM, Gollamudi S, Friedenberg F. Continuation of Antibiotics Is Associated With Failure of Metronidazole for Clostridium difficile-Associated Diarrhea. J Clin Gastroenterol. 2006;40:49–54. doi: 10.1097/01.mcg.0000190761.80615.0f. [DOI] [PubMed] [Google Scholar]
- 53.Kelsen JR, Kim J, Latta D, et al. Recurrence rate of Clostridium difficile infection in hospitalized pediatric patients with inflammatory bowel disease. Inflamm Bowel Dis. 2011;17:50–55. doi: 10.1002/ibd.21421. [DOI] [PubMed] [Google Scholar]
- 54.Gough E, Shaikh H, Manges AR. Systematic Review of Intestinal Microbiota Transplantation (Fecal Bacteriotherapy) for Recurrent Clostridium difficile Infection. Clin Infect Dis. 2011;53:994–1002. doi: 10.1093/cid/cir632. [DOI] [PubMed] [Google Scholar]
- 55.Fekety R, Silva J, Kauffman C, et al. Treatment of antibiotic-associated Clostridium difficile colitis with oral vancomycin: Comparison of two dosage regimens. Am J Med. 1989;86:15–19. doi: 10.1016/0002-9343(89)90223-4. [DOI] [PubMed] [Google Scholar]
- 56.Piche T, Vanbiervliet G, Pipau FG, et al. Low risk of irritable bowel syndrome after Clostridium difficile infection. Can J Gastroenterol. 2007;21:727–731. doi: 10.1155/2007/262478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Brecher SM, Novak-Weekley SM, Nagy E. Laboratory diagnosis of Clostridium difficile infections: there is light at the end of the colon. Clin Infect Dis. 2013;57:1175–1181. doi: 10.1093/cid/cit424. [DOI] [PubMed] [Google Scholar]
- 58.Wilcox M. Overcoming barriers to effective recognition and diagnosis of Clostridium difficile infection. Clin Microbiol Infect. 2012;18:13–20. doi: 10.1111/1469-0691.12057. [DOI] [PubMed] [Google Scholar]
- 59.Berg AM, Kelly CP, Farraye FA. Clostridium difficile infection in the inflammatory bowel disease patient. Inflamm Bowel Dis. 2012 doi: 10.1002/ibd.22964. [DOI] [PubMed] [Google Scholar]
- 60.Yanai H, Nguyen GC, Yun L, et al. Practice of gastroenterologists in treating flaring inflammatory bowel disease patients with Clostridium difficile: antibiotics alone or combined antibiotics/immunomodulators? Inflamm Bowel Dis. 2011;17:1540–1546. doi: 10.1002/ibd.21514. [DOI] [PubMed] [Google Scholar]
- 61.Ben-Horin S, Margalit M, Bossuyt P, et al. Combination immunomodulator and antibiotic treatment in patients with inflammatory bowel disease and Clostridium difficile infection. Clin Gastroenterol Hepatol. 2009;7:981–987. doi: 10.1016/j.cgh.2009.05.031. [DOI] [PubMed] [Google Scholar]
- 62.Kornbluth A, Sachar DB. Ulcerative Colitis Practice Guidelines in Adults: American College of Gastroenterology, Practice Parameters Committee. Am J Gastroenterol. 2010;105:501–523. doi: 10.1038/ajg.2009.727. [DOI] [PubMed] [Google Scholar]
- 63.Johnson S, Homann SR, Bettin KM, et al. Treatment of Asymptomatic Clostridium difficile Carriers (Fecal Excretors) with Vancomycin or Metronidazole: A Randomized, Placebo-controlled Trial. Ann Intern Med. 1992;117:297–302. doi: 10.7326/0003-4819-117-4-297. [DOI] [PubMed] [Google Scholar]
- 64.Johnson S, Clabots CR, Linn FV, et al. Nosocomial Clostridium difficile colonisation and disease. The Lancet. 1990;336:97–100. doi: 10.1016/0140-6736(90)91605-a. [DOI] [PubMed] [Google Scholar]
- 65.Shim JK, Johnson S, Samore MH, et al. Primary symptomless colonisation by Clostridium difficile and decreased risk of subsequent diarrhoea. The Lancet. 1998;351:633–636. doi: 10.1016/S0140-6736(97)08062-8. [DOI] [PubMed] [Google Scholar]
- 66.Slimings C, Riley TV. Antibiotics and hospital-acquired Clostridium difficile infection: update of systematic review and meta-analysis. J Antimicrob Chemother. 2014;69:881–891. doi: 10.1093/jac/dkt477. [DOI] [PubMed] [Google Scholar]
- 67.Thomas C, Stevenson M, Riley TV. Antibiotics and hospital-acquired Clostridium difficile-associated diarrhoea: a systematic review. J Antimicrob Chemother. 2003;51:1339–1350. doi: 10.1093/jac/dkg254. [DOI] [PubMed] [Google Scholar]
- 68.Vardakas KZ, Konstantelias AA, Loizidis G, et al. Risk factors for development of Clostridium difficile infection due to BI/NAP1/027 strain: a meta-analysis. Int J Infect Dis. 2012;16:e768–e773. doi: 10.1016/j.ijid.2012.07.010. [DOI] [PubMed] [Google Scholar]
- 69.Modena S, Gollamudi S, Friedenberg F. Continuation of Antibiotics Is Associated With Failure of Metronidazole for Clostridium difficile-Associated Diarrhea. J Clin Gastroenterol. 2006;40:49–54. doi: 10.1097/01.mcg.0000190761.80615.0f. [DOI] [PubMed] [Google Scholar]
- 70.Koo HL, Koo DC, Musher DM, et al. Antimotility Agents for the Treatment of Clostridium difficile Diarrhea and Colitis. Clin Infect Dis. 2009;48:598–605. doi: 10.1086/596711. [DOI] [PubMed] [Google Scholar]
- 71.Rao K, Micic D, Natarajan M, et al. Clostridium difficile Ribotype 027: Relationship to Age, Detectability of Toxins A or B in Stool with Rapid Testing, Severe Infection, and Mortality. Clin Infect Dis. 2015;61:233–241. doi: 10.1093/cid/civ254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Surawicz CM, Brandt LJ, Binion DG, et al. Guidelines for Diagnosis, Treatment, and Prevention of Clostridium difficile Infections. Am J Gastroenterol. 2013;108:478–498. doi: 10.1038/ajg.2013.4. [DOI] [PubMed] [Google Scholar]
- 73.Debast SB, Bauer MP, Kuijper EJ. European Society of Clinical Microbiology and Infectious Diseases: update of the treatment guidance document for Clostridium difficile infection. Clin Microbiol Infect. 2014;20(Suppl 2):1–26. doi: 10.1111/1469-0691.12418. [DOI] [PubMed] [Google Scholar]
- 74.Drekonja DM, Butler M, MacDonald R, et al. Comparative Effectiveness of Clostridium difficile Treatments: A Systematic Review. Ann Intern Med. 2011;155:839–847. doi: 10.7326/0003-4819-155-12-201112200-00007. [DOI] [PubMed] [Google Scholar]
- 75.Johnson S, Louie TJ, Gerding DN, et al. Vancomycin, Metronidazole, or Tolevamer for Clostridium difficile Infection: Results From Two Multinational, Randomized, Controlled Trials. Clin Infect Dis. 2014;59:345–354. doi: 10.1093/cid/ciu313. [DOI] [PubMed] [Google Scholar]
- 76.Issa M, Vijayapal A, Graham MB, et al. Impact of Clostridium difficile on Inflammatory Bowel Disease. Clinical Gastroenterology and Hepatology. 2007;5:345–351. doi: 10.1016/j.cgh.2006.12.028. [DOI] [PubMed] [Google Scholar]
- 77.Musher DM, Aslam S, Logan N, et al. Relatively Poor Outcome after Treatment of Clostridium difficile Colitis with Metronidazole. Clin Infect Dis. 2005;40:1586–1590. doi: 10.1086/430311. [DOI] [PubMed] [Google Scholar]
- 78.Crook DW, Walker AS, Kean Y, et al. Fidaxomicin Versus Vancomycin for Clostridium difficile Infection: Meta-analysis of Pivotal Randomized Controlled Trials. Clin Infect Dis. 2012;55:S93–S103. doi: 10.1093/cid/cis499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Nathwani D, Cornely OA, Van Engen AK, et al. Cost-effectiveness analysis of fidaxomicin versus vancomycin in Clostridium difficile infection. J Antimicrob Chemother. 2014;69:2901–2912. doi: 10.1093/jac/dku257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Stranges PM, Hutton DW, Collins CD. Cost-Effectiveness Analysis Evaluating Fidaxomicin versus Oral Vancomycin for the Treatment of Clostridium difficile Infection in the United States. Value in Health. 2013;16:297–304. doi: 10.1016/j.jval.2012.11.004. [DOI] [PubMed] [Google Scholar]
- 81.Bartsch SM, Umscheid CA, Fishman N, et al. Is Fidaxomicin Worth the Cost? An Economic Analysis. Clin Infect Dis. 2013;57:555–561. doi: 10.1093/cid/cit346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Musgrave CR, Bookstaver PB, Sutton SS, et al. Use of alternative or adjuvant pharmacologic treatment strategies in the prevention and treatment of Clostridium difficile infection. Int J Infect Dis. 2011;15:e438–e448. doi: 10.1016/j.ijid.2011.03.017. [DOI] [PubMed] [Google Scholar]
- 83.Apisarnthanarak A, Razavi B, Mundy LM. Adjunctive Intracolonic Vancomycin for Severe Clostridium difficile Colitis: Case Series and Review of the Literature. Clin Infect Dis. 2002;35:690–696. doi: 10.1086/342334. [DOI] [PubMed] [Google Scholar]
- 84.Bublin JG, Barton TL. Rectal use of vancomycin. Ann Pharmacother. 1994;28:1357–1358. [PubMed] [Google Scholar]
- 85.McFarland LV, Elmer GW, Surawicz CM. Breaking the cycle: treatment strategies for 163 cases of recurrent Clostridium difficile disease. Am J Gastroenterol. 2002;97:1769–1775. doi: 10.1111/j.1572-0241.2002.05839.x. [DOI] [PubMed] [Google Scholar]
- 86.Johnson S, Gerding DN. Fidaxomicin ‘Chaser’ Regimen following Vancomycin for Patients with Multiple C. difficile Recurrences. Clin Infect Dis. 2012;56:309–310. doi: 10.1093/cid/cis833. [DOI] [PubMed] [Google Scholar]
- 87.Garey KW, Ghantoji SS, Shah DN, et al. A randomized, double-blind, placebo-controlled pilot study to assess the ability of rifaximin to prevent recurrent diarrhoea in patients with Clostridium difficile infection. J Antimicrob Chemother. 2011;66:2850–2855. doi: 10.1093/jac/dkr377. [DOI] [PubMed] [Google Scholar]
- 88.Johnson S, Schriever C, Patel U, et al. Rifaximin Redux: Treatment of recurrent Clostridium difficile infections with Rifaximin immediately post-vancomycin treatment. Anaerobe. 2009;15:290–291. doi: 10.1016/j.anaerobe.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 89.O’Horo J, Safdar N. The role of immunoglobulin for the treatment of Clostridium difficile infection: a systematic review. Int J Infect Dis. 2009;13:663–667. doi: 10.1016/j.ijid.2008.11.012. [DOI] [PubMed] [Google Scholar]
- 90.Pantosti A, Luzzi I, Cardines R, et al. Comparison of the in vitro activities of teicoplanin and vancomycin against Clostridium difficile and their interactions with cholestyramine. Antimicrob Agents Chemother. 1985;28:847–848. doi: 10.1128/aac.28.6.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Larson KC, Belliveau PP, Spooner LM. Tigecycline for the Treatment of Severe Clostridium difficile Infection. Ann Pharmacother. 2011;45:1005–1010. doi: 10.1345/aph.1Q080. [DOI] [PubMed] [Google Scholar]
- 92.Musher DM, Logan N, Bressler AM, et al. Nitazoxanide versus Vancomycin in Clostridium difficile Infection: A Randomized, Double-Blind Study. Clin Infect Dis. 2009;48:e41–e46. doi: 10.1086/596552. [DOI] [PubMed] [Google Scholar]
- 93.Musher DM, Logan N, Mehendiratta V, et al. Clostridium difficile colitis that fails conventional metronidazole therapy: response to nitazoxanide. J Antimicrob Chemother. 2007;59:705–710. doi: 10.1093/jac/dkl553. [DOI] [PubMed] [Google Scholar]
- 94.Freeman J, Baines SD, Todhunter SL, et al. Nitazoxanide is active against Clostridium difficile strains with reduced susceptibility to metronidazole. J Antimicrob Chemother. 2011;66:1407–1408. doi: 10.1093/jac/dkr077. [DOI] [PubMed] [Google Scholar]
- 95.Szajewska H, Kolodziej M. Systematic review with meta-analysis: Saccharomyces boulardii in the prevention of antibiotic-associated diarrhoea. Aliment Pharmacol Ther. 2015 doi: 10.1111/apt.13344. [DOI] [PubMed] [Google Scholar]
- 96.Johnston BC, Ma SSY, Goldenberg JZ, et al. Probiotics for the Prevention of Clostridium difficile–Associated Diarrhea: A Systematic Review and Meta-analysis. Ann Intern Med. 2012;157:878–888. doi: 10.7326/0003-4819-157-12-201212180-00563. [DOI] [PubMed] [Google Scholar]
- 97.Hempel S, Newberry SJ, Maher AR, et al. Probiotics for the prevention and treatment of antibiotic-associated diarrhea: A systematic review and meta-analysis. JAMA. 2012;307:1959–1969. doi: 10.1001/jama.2012.3507. [DOI] [PubMed] [Google Scholar]
- 98.McFarland LV. Probiotics for the primary and secondary prevention of C. difficile infections: a meta-analysis and systematic review. Antibiotics. 2015;4:160–178. doi: 10.3390/antibiotics4020160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Allen SJ, Wareham K, Wang D, et al. A high-dose preparation of lactobacilli and bifidobacteria in the prevention of antibiotic-associated and Clostridium difficile diarrhoea in older people admitted to hospital: a multicentre, randomised, double-blind, placebo-controlled, parallel arm trial (PLACIDE) Health Technol Assess. 2013;17:1–140. doi: 10.3310/hta17570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Maziade PJ, Andriessen JA, Pereira P, et al. Impact of adding prophylactic probiotics to a bundle of standard preventative measures for Clostridium difficile infections: enhanced and sustained decrease in the incidence and severity of infection at a community hospital. Curr Med Res Opin. 2013;29:1341–1347. doi: 10.1185/03007995.2013.833501. [DOI] [PubMed] [Google Scholar]
- 101.Bakken JS. Staggered and tapered antibiotic withdrawal (STAW) with administration of Kefir for recurrent Clostridium difficile infection. Clin Infect Dis. 2014 doi: 10.1093/cid/ciu429. [DOI] [PubMed] [Google Scholar]
- 102.Segarra-Newnham M. Probiotics for Clostridium difficile–Associated Diarrhea: Focus on Lactobacillus rhamnosus GG and Saccharomyces boulardii. Ann Pharmacother. 2007;41:1212–1221. doi: 10.1345/aph.1K110. [DOI] [PubMed] [Google Scholar]
- 103.Guidance for Industry: Enforcement Policy Regarding Investigational New Drug Requirements for Use of Fecal Microbiota for Transplantation to Treat Clostridium difficile Infection Not Responsive to Standard Therapies. [Accessed 2014 July 1]; Available at: http://www.fda.gov/biologicsbloodvaccines/guidancecomplianceregulatoryinformation/guidances/vaccines/ucm361379.htm.
- 104.Youngster I, Sauk J, Pindar C, et al. Fecal Microbiota Transplant for Relapsing Clostridium difficile Infection Using a Frozen Inoculum From Unrelated Donors: A Randomized, Open-Label, Controlled Pilot Study. Clin Infect Dis. 2014;58:1515–1522. doi: 10.1093/cid/ciu135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.van Nood E, Vrieze A, Nieuwdorp M, et al. Duodenal Infusion of Donor Feces for Recurrent Clostridium difficile. N Engl J Med. 2013;368:407–415. doi: 10.1056/NEJMoa1205037. [DOI] [PubMed] [Google Scholar]
- 106.Youngster I, Russell GH, Pindar C, et al. Oral, Capsulized, Frozen Fecal Microbiota Transplantation for Relapsing Clostridium difficile Infection. JAMA. 2014 doi: 10.1001/jama.2014.13875. [DOI] [PubMed] [Google Scholar]
- 107.Lee CH, Steiner T, Petrof EO, et al. Frozen vs fresh fecal microbiota transplantation and clinical resolution of diarrhea in patients with recurrent Clostridium difficile infection: A randomized clinical trial. JAMA. 2016;315:142–149. doi: 10.1001/jama.2015.18098. [DOI] [PubMed] [Google Scholar]
- 108.Cammarota G, Masucci L, Ianiro G, et al. Randomised clinical trial: faecal microbiota transplantation by colonoscopy vs. vancomycin for the treatment of recurrent Clostridium difficile infection. Aliment Pharmacol Ther. 2015 doi: 10.1111/apt.13144. [DOI] [PubMed] [Google Scholar]
- 109.Rao K, Malani PN. Expanded Evidence for Frozen Fecal Microbiota Transplantation for Clostridium difficile Infection: A Fresh Take. JAMA. 2016;315:137–138. doi: 10.1001/jama.2015.18100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Fischer M, Allegretti JR, Smith M, et al. A multi-center, cluster randomized dose-finding study of fecal microbiota transplantation capsules for recurrent Clostridium difficile infection. United European Gastroenterology Journal. 2015;3:561–571. [Google Scholar]
- 111.Sha S, Liang J, Chen M, et al. Systematic review: faecal microbiota transplantation therapy for digestive and nondigestive disorders in adults and children. Aliment Pharmacol Ther. 2014;39:1003–1032. doi: 10.1111/apt.12699. [DOI] [PubMed] [Google Scholar]
- 112.Lofgren ET, Moehring RW, Anderson DJ, et al. A Mathematical Model to Evaluate the Routine Use of Fecal Microbiota Transplantation to Prevent Incident and Recurrent Clostridium difficile Infection. Infect Control Hosp Epidemiol. 2013;35:18–27. doi: 10.1086/674394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lagier JC, Delord M, Million M, et al. Dramatic reduction in Clostridium difficile ribotype 027-associated mortality with early fecal transplantation by the nasogastric route: a preliminary report. Eur J Clin Microbiol Infect Dis. 2015 doi: 10.1007/s10096-015-2394-x. [DOI] [PubMed] [Google Scholar]
- 114.Neemann K, Eichele DD, Smith PW, et al. Fecal microbiota transplantation for fulminant Clostridium difficile infection in an allogeneic stem cell transplant patient. Transplant Infect Dis. 2012;14:E161–E165. doi: 10.1111/tid.12017. [DOI] [PubMed] [Google Scholar]
- 115.Trubiano JA, Gardiner B, Kwong JC, et al. Faecal microbiota transplantation for severe Clostridium difficile infection in the intensive care unit. Eur J Gastroenterol Hepatol. 2013;25:255–257. doi: 10.1097/MEG.0b013e32835b2da9. [DOI] [PubMed] [Google Scholar]
- 116.Gallegos-Orozco J, Paskvan-Gawryletz C, Gurudu S, et al. Successful colonoscopic fecal transplant for severe acute Clostridium difficile pseudomembranous colitis. Rev Gastroenterol Mex. 2011;77:40–42. [PubMed] [Google Scholar]
- 117.You DM, Franzos MA, Holman RP. Successful Treatment of Fulminant Clostridium difficile Infection with Fecal Bacteriotherapy. Ann Intern Med. 2008;148:632–633. doi: 10.7326/0003-4819-148-8-200804150-00024. [DOI] [PubMed] [Google Scholar]
- 118.Solari PR, Fairchild PG, Noa LJ, et al. Tempered Enthusiasm for Fecal Transplant. Clin Infect Dis. 2014;59:319. doi: 10.1093/cid/ciu278. [DOI] [PubMed] [Google Scholar]
- 119.Kelly CR, Ihunnah C, Fischer M, et al. Fecal Microbiota Transplant for Treatment of Clostridium difficile Infection in Immunocompromised Patients. Am J Gastroenterol. 2014;109:1065–1071. doi: 10.1038/ajg.2014.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Hohmann EL, Ananthakrishnan AN, Deshpande V. Case 25–2014—A 37-Year-Old Man with Ulcerative Colitis and Bloody Diarrhea. N Engl J Med. 2014;371:668–675. doi: 10.1056/NEJMcpc1400842. [DOI] [PubMed] [Google Scholar]
- 121.Moayyedi P, Surette MG, Kim PT, et al. Fecal Microbiota Transplantation Induces Remission in Patients With Active Ulcerative Colitis in a Randomized Controlled Trial. Gastroenterol. 2015;149:102–109. e106. doi: 10.1053/j.gastro.2015.04.001. [DOI] [PubMed] [Google Scholar]
- 122.Sekirov I, Russell SL, Antunes LCM, et al. Gut Microbiota in Health and Disease. Physiol Rev. 2010;90:859–904. doi: 10.1152/physrev.00045.2009. [DOI] [PubMed] [Google Scholar]
- 123.Garrett WS, Lord GM, Punit S, et al. Communicable Ulcerative Colitis Induced by T-bet Deficiency in the Innate Immune System. Cell. 2007;131:33–45. doi: 10.1016/j.cell.2007.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Turnbaugh PJ, Ley RE, Mahowald MA, et al. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444:1027–1131. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- 125.Brandt LJ, Aroniadis OC, Mellow M, et al. Long-Term Follow-Up of Colonoscopic Fecal Microbiota Transplant for Recurrent Clostridium difficile Infection. Am J Gastroenterol. 2012;107:1079–1087. doi: 10.1038/ajg.2012.60. [DOI] [PubMed] [Google Scholar]
- 126.Li YT, Cai HF, Wang ZH, et al. Systematic review with meta-analysis: long-term outcomes of faecal microbiota transplantation for Clostridium difficile infection. Aliment Pharmacol Ther. 2016;43:445–457. doi: 10.1111/apt.13492. [DOI] [PubMed] [Google Scholar]
- 127.Inc. R. ClinicalTrials.gov [Internet] Bethesda (MD): National Library of Medicine (US); 2000. Microbiota Restoration Therapy for Recurrent Clostridium difficile Infection (PUNCH CD 2) [cited 2016 January 29]. Available from: https://clinicaltrials.gov/ct2/show/NCT02299570. NLM Identifier: NCT02299570. [Google Scholar]
- 128.Duke University. ClinicalTrials.gov [Internet] Bethesda (MD): National Library of Medicine (US); 2000. Stool Transplants to Treat RefractoryClostridium difficile Colitis. [cited 2016 January 29]. Available from: http://clinicaltrials.gov/ct2/show/NCT02127398. NLM Identifier: NCT02127398. [Google Scholar]
- 129.University Hospital Tuebingen. ClinicalTrials.gov [Internet] Bethesda (MD): National Library of Medicine (US); 2000. Fecal Microbiota Transplantation in Recurrent or Refractory Clostridium difficile Colitis (TOCSIN) [cited 2016 January 29]. Available from: http://clinicaltrials.gov/ct2/show/NCT01942447. NLM Identifier: NCT01942447. [Google Scholar]
- 130.Hadassah Medical Organization. ClinicalTrials.gov [Internet] Bethesda (MD): National Library of Medicine (US); 2000. Efficacy and Safety of Fecal Microbiota Transplantation for Severe Clostridium difficile Associated Colitis. [cited 2016 January 29]. Available from: http://clinicaltrials.gov/ct2/show/NCT01959048. NLM Identifier: NCT01959048. [Google Scholar]
- 131.Tel-Aviv Sourasky Medical Center. ClinicalTrials.gov [Internet] Bethesda (MD): National Library of Medicine (US); 2000. Transplantation of Fecal Microbiota for Clostridium difficile Infection. [cited 2016 January 29]. Available from: http://clinicaltrials.gov/ct2/show/NCT01958463. NLM Identifier: NCT01958463. [Google Scholar]
- 132.Bennet J, Brinkman M. Treatment of ulcerative colitis by implantation of normal colonic flora. The Lancet. 1989;333:164. doi: 10.1016/s0140-6736(89)91183-5. [DOI] [PubMed] [Google Scholar]
- 133.Borody TJ, George L, Andrews P, et al. Bowel-flora alteration: a potential cure for inflammatory bowel disease and irritable bowel syndrome? Med J Aust. 1989;150:604. doi: 10.5694/j.1326-5377.1989.tb136704.x. [DOI] [PubMed] [Google Scholar]
- 134.Borody TJ, Warren EF, Leis S, et al. Treatment of Ulcerative Colitis Using Fecal Bacteriotherapy. J Clin Gastroenterol. 2003;37:42–47. doi: 10.1097/00004836-200307000-00012. [DOI] [PubMed] [Google Scholar]
- 135.Bowman KA, Broussard EK, Surawicz CM. Fecal microbiota transplantation: current clinical efficacy and future prospects. Clin Exp Gastroenterol. 2015;8:285–291. doi: 10.2147/CEG.S61305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Hourigan SK, Chen LA, Grigoryan Z, et al. Microbiome changes associated with sustained eradication of Clostridium difficile after single faecal microbiota transplantation in children with and without inflammatory bowel disease. Aliment Pharmacol Ther. 2015 doi: 10.1111/apt.13326. [DOI] [PubMed] [Google Scholar]
- 137.Angelberger S, Reinisch W, Makristathis A, et al. Temporal Bacterial Community Dynamics Vary Among Ulcerative Colitis Patients After Fecal Microbiota Transplantation. Am J Gastroenterol. 2013;108:1620–1630. doi: 10.1038/ajg.2013.257. [DOI] [PubMed] [Google Scholar]
- 138.Kump PK, Gröchenig H-P, Lackner S, et al. Alteration of Intestinal Dysbiosis by Fecal Microbiota Transplantation Does not Induce Remission in Patients with Chronic Active Ulcerative Colitis. Inflamm Bowel Dis. 2013;19:2155–2165. doi: 10.1097/MIB.0b013e31829ea325. [DOI] [PubMed] [Google Scholar]
- 139.Rossen NG, Fuentes S, van der Spek MJ, et al. Findings From a Randomized Controlled Trial of Fecal Transplantation for Patients With Ulcerative Colitis. Gastroenterol. 149:110–118.e114. doi: 10.1053/j.gastro.2015.03.045. [DOI] [PubMed] [Google Scholar]
- 140.Kao D, Hotte N, Gillevet P, et al. Fecal Microbiota Transplantation Inducing Remission in Crohn’s Colitis and the Associated Changes in Fecal Microbial Profile. J Clin Gastroenterol. 2014;48:625–628. doi: 10.1097/MCG.0000000000000131. [DOI] [PubMed] [Google Scholar]
- 141.Gerding DN. Clostridium difficile infection prevention: biotherapeutics, immunologics, and vaccines. Discov Med. 2012;13:75–83. [PubMed] [Google Scholar]
- 142.Lowy I, Molrine DC, Leav BA, et al. Treatment with Monoclonal Antibodies against Clostridium difficile Toxins. N Engl J Med. 2010;362:197–205. doi: 10.1056/NEJMoa0907635. [DOI] [PubMed] [Google Scholar]
- 143.Wilcox M, Gerding D, Poxton I, et al. Bezlotoxumab Alone and With Actoxumab for Prevention of Recurrent Clostridium difficile Infection in Patients on Standard of Care Antibiotics: Integrated Results of 2 Phase 3 Studies (MODIFY I and MODIFY II) Open Forum Infect Dis. 2015;2:S3. [Google Scholar]
- 144.Gerding DN, Meyer T, Lee C, et al. Administration of spores of nontoxigenic Clostridium difficile strain m3 for prevention of recurrent C. difficile infection: A randomized clinical trial. JAMA. 2015;313:1719–1727. doi: 10.1001/jama.2015.3725. [DOI] [PubMed] [Google Scholar]
- 145.Rao K, Erb-Downward JR, Walk ST, et al. The Systemic Inflammatory Response to Clostridium difficile Infection. PLoS ONE. 2014;9:e92578. doi: 10.1371/journal.pone.0092578. [DOI] [PMC free article] [PubMed] [Google Scholar]