Abstract
Background
Women with advanced bladder cancer have inferior survival compared to men. However, women treated on clinical trials do not have a survival disadvantage. Less frequent administration of systemic chemotherapy in women with advanced bladder cancer may contribute to their inferior survival.
Methods
We identified patients with stage IV bladder cancer from 1998–2010 using the National Cancer Data Base (NCDB), a national outcomes database that includes 70% of all newly diagnosed cancer cases in the United States. Gender differences in demographics, systemic chemotherapy administration, and overall survival (OS) were compared.
Results
23,981 patients were identified (35% female). Compared with men, women were older, more likely to be black, and less likely to be insured (p<0.01 for all). Charlson Deyo comorbidity score did not differ between men and women. Women were less likely to receive systemic chemotherapy than men (45% vs 52%, adjusted RR 0.91 [95% CI 0.88–0.94]). Women had a lower median OS compared to men (8.0 months (95% CI 7.7–8.3) vs 9.8 months (95% CI 9.5–10.0), p<0.001). OS remained lower for women on multivariable analysis, even after adjusting for administration of systemic chemotherapy (HR for death 1.11 [95% CI 1.08–1.15].
Conclusions
Women are less likely than men to receive systemic chemotherapy for advanced bladder cancer and this difference may partially account for poorer OS in women. However, OS remains lower in women independent of chemotherapy use, and may be related to unmeasured comorbidities, functional status, or tumor biology.
Keywords: bladder cancer, sex, treatment outcomes, chemotherapy, delivery of health care
Introduction
Overall, after a diagnosis of cancer, women have improved survival compared to men.1 However, this is not true with a diagnosis of bladder cancer. Although men are nearly three times more likely to develop bladder cancer than women in the United States,2 women are more likely to die of the disease.1 This survival disadvantage among women occurs across all stages of disease but is more pronounced in advanced stages with an estimated 12% difference in 5-year survival between men and women with stage IV disease.3–5
The cause of the survival disparity between genders is not clear. Interestingly, women with advanced bladder cancer treated with chemotherapy on clinical trials do not appear to have a survival disadvantage compared to men.6 Although this may mean that women in clinical trials are not representative of the general population, it also suggests that less frequent administration of standard chemotherapy could contribute to the survival disadvantage seen in women with advanced disease. It is not known if women with advanced bladder cancer are less likely than men to receive systemic chemotherapy.
The current study uses a large national database to examine rates of chemotherapy use and survival among women and men with advanced bladder cancer. We hypothesize that women with advanced bladder cancer are less likely to receive chemotherapy than men, and that the difference in frequency of chemotherapy use explains the survival disadvantage seen in women.
Methods
1.1 Data Source
The data source for this study was the National Cancer Data Base (NCDB), a nationwide oncology outcomes database established in 1989 that includes approximately 70% of all newly diagnosed cancer cases in the United States annually. The NCDB is a collaboration of the Commission on Cancer (CoC) of the American College of Surgeons and the American Cancer Society in which all types of cancer are included. Data are collected using national standardized data item and coding definitions as specified by the CoC’s Faculty Oncology Registry Data Standards and include patient and facility characteristics, cancer stage, treatment, and outcomes.7 The American College of Surgeons has executed a Business Associate Agreement that includes a data use agreement with each of its Commission on Cancer accredited hospitals.
1.2 Patient Population
The NCDB was queried for all patients with stage IV bladder cancer diagnosed between 1998 and 2010. Staging was defined by the NCDB Analytic Stage Group, which reports the American Joint Committee on Cancer (AJCC) TNM pathologic stage if available, or clinical stage if pathologic stage is not available. 2010 was the latest year with 3-year survival data available and therefore was the last year included in this analysis. Patients were excluded if they did not have information on systemic treatment or were lost to follow-up within one month.
1.3 Data Items
We collected patient demographics, facility information, tumor characteristics, treatment, and outcomes. Demographic variables included gender, age, race, education, household income, insurance status, region of the country, and comorbidity score. Race was classified as white, black, or other. Education was defined as the percentage of adults in the patient’s zip code who did not graduate from high school and was categorized as ≥29%, 20–28.9%, 14–19.9%, and less than 14%. Household income was defined as the quartile of median household income for each patient’s area of residence based on zip code. Insurance status was dichotomized to insured or not. Region of the country was categorized as south, northeast, west, and midwest. Comorbidities were estimated using the Charlson-Deyo comorbidity index with score of 0 (no comorbid conditions), 1, or ≥2 per NCDB standards.8
Facility-level information included facility type (community-based practice or cancer programs, academic or research programs, and other) and distance to the diagnosing facility. The distance from the patient’s residence to the diagnosing facility was estimated by the NCDB using the patient’s and facility’s zip codes. Tumor characteristics included histology and presence of nodal or distant metastases. Stage IV patients were identified using the NCDB analytic stage, which uses pathologic stage if reported, and clinical stage if pathologic stage was not reported. Receipt of chemotherapy was defined as receipt of any cancer-directed systemic therapy. Receipt of cystectomy was also recorded. Outcomes included median overall survival (OS), 1-year, 3-year, and 5-year survival. Survival was defined as the time between the date of diagnosis and the date of death.
1.4 Statistical Analysis
Chi-square tests were used to compare categorical variables between males and females, and t-tests were used for continuous variables. Modeling was used as a tool to evaluate the relationship of gender and outcome, while controlling for important covariates that were specified a priori based on availability in the dataset and clinical expertise.9 Patients with incomplete data (about 15%) were excluded from modeling fitting. Poisson regression was performed, and risk ratios were reported, to evaluate predictors of chemotherapy administration, including gender.10 The Kaplan-Meier method was used to estimate overall survival. Cox proportional hazards modeling was used to evaluate factors associated with overall survival and compare survival between genders with adjustment for potential confounders, after graphically confirming that the proportional hazards assumption held for all covariates. Chemotherapy receipt was treated as a time-varying covariate based on time from diagnosis to the start of treatment with chemotherapy. All statistical tests were two-sided and a p-value of <0.05 was considered to be statistically significant. All analyses were performed using SAS v9.4 (SAS Institute Inc, Cary, NC, USA). Because all patient information was de-identified in the NCDB, this study was approved for exempt status by the University of North Carolina Institutional Review Board.
Results
23,981 patients were identified. Median age was 69. 35% were female, 88% were white, and 96% were insured. 38% had clinical evidence of distant metastatic disease at the time of diagnosis (an additional 8% had distant metastatic disease diagnosed pathologically). 25% of patients had clinically positive lymph nodes (44% of patients were not able to be assessed, and 31% were negative). 40% of patients had pathologically positive lymph nodes (53% were not able to be assessed, and 7% were negative). Patients were categorized as stage IV disease as follows: 6.3% T4N0M0, 44.2% TxN1-3M0, 46.2% TxNxM1, and 3.2% unknown TNM stage.
Differences in demographic variables between genders are summarized in Table 1. Women were older than men at the time of diagnosis (median age 71 vs 68 years, p<0.01). Women were also more likely than men to be black (13% vs 8%, p<0.01) and more likely to be insured (97% vs 96%, p<0.01). Charlson Deyo comorbidity score did not differ between men and women. Women were less likely to have distant metastatic disease (p<0.01) and more likely to undergo cystectomy (p=0.003). Women were more likely to have a higher T stage than men (p<0.01). However, women were less likely to have clinically positive lymph nodes than men (24% vs 26%, p<0.01). Women were also less likely to have pathologically positive lymph nodes than men (39% vs 40%, p=0.03).
Table 1.
Patient demographics in men and women (n=23,981)
| Demographic | Males (n=15,659) |
Females (n=8,322) |
p value |
|---|---|---|---|
| Age, median (range) | 68 (19–90) | 71 (21–90) | <0.001 |
| Race | |||
| White | 90.0% | 84.8% | <0.001 |
| Black | 7.6% | 13.2% | |
| Other | 2.4% | 2.0% | |
| Location/Region | |||
| Northeast | 23.2% | 24.4% | 0.06 |
| South | 25.9% | 26.2% | |
| West | 24.2% | 22.9% | |
| Midwest | 26.7% | 26.6% | |
| Median Household Income | |||
| <$30,000 | 14.6% | 15.5% | 0.32 |
| $30,000–34,999 | 19.5% | 19.3% | |
| $35,000–45,999 | 29.5% | 29.5% | |
| $46,000+ | 36.4% | 35.7% | |
| Education, % of people in patient’s area code who did not graduate high school |
|||
| 29% or more | 17.5% | 17.9% | 0.39 |
| 20–28.9% | 23.5% | 24.3% | |
| 14–19.9% | 26.1% | 25.7% | |
| <14% | 32.8% | 32.1% | |
| Insurance | |||
| Yes | 95.5% | 96.5% | <0.001 |
| No | 4.5% | 3.5% | |
| Distance to treating facility, miles, median (range) |
9.6 (0 – 9516) | 8.4 (0 – 9405) | 0.79 |
| Facility Type | |||
| Community Program | 61.5% | 60.7% | 0.50 |
| Academic/Research Program | 36.9% | 37.6% | |
| Other | 1.6% | 1.7% | |
| Charlson-Deyo Score | |||
| 0 | 46.4% | 45.5% | 0.22 |
| 1 | 13.1% | 12.8% | |
| 2 | 4.9% | 4.7% | |
| Unknown | 35.6% | 37.0% | |
| Histology | <0.001 | ||
| Urothelial | 88.2% | 83.3% | |
| Other | 7.6% | 11.8% | |
| Unknown | 4.1% | 5.0% | |
| Clinical T Stage | |||
| 2 | 34.0% | 31.1% | <0.001 |
| 3 | 9.5% | 10.6% | |
| 4 | 19.4% | 21.7% | |
| Unknown | 37.2% | 36.7% | |
| Clinical N stage | |||
| 0 | 29.7% | 31.9% | <0.001 |
| 1 | 11.4% | 9.8% | |
| 2 | 12.5% | 11.9% | |
| 3 | 2.2% | 1.9% | |
| Unable to be assessed | 44.2% | 44.5% | |
| Presence of distant metastases | 38.8% | 36.3% | <0.001 |
| Radiation Therapy Received | 22.2% | 21.2% | 0.08 |
| Cystectomy Performed | 36.2% | 38.2% | 0.003 |
| Chemotherapy Received | 52.2% | 44.7% | <0.001 |
Women were less likely to receive systemic chemotherapy than men (45% vs 52%, RR 0.86, 95% CI 0.83–0.88). This difference remained significant after adjusting for age, year of diagnosis, race, insurance status, distance to and location of facility, income, education, facility type, comorbidity score, histology, cystectomy, clinical T stage, and presence of nodal and distant metastases (RR 0.91, 95%CI 0.88–0.94). Factors associated with a lower likelihood of receiving chemotherapy on multivariable analysis included older age, black race, lower education level, lack of insurance, less comorbidity, unknown or non-urothelial histology, higher T stage, and presence of distant metastatic disease (table 2).
Table 2.
Unadjusted and adjusted relative risk of receiving chemotherapy based on demographic factors
| Demographic | RR of receiving chemotherapy (95% CI), univariate |
RR of receiving chemotherapy (95% CI), multivariable* |
|---|---|---|
| Sex | ||
| Male | 1.00 | 1.00 |
| Female | 0.86 (0.83–0.88) | 0.91 (0.88–0.94) |
| Age | ||
| <70 | 1.00 | 1.00 |
| >= 70 | 0.59 (0.57–0.60) | 0.59 (0.58–0.61) |
| Race | ||
| White | 1.00 | 1.00 |
| Black | 0.86 (0.82–0.90) | 0.89 (0.84–0.94) |
| Other | 1.03 (0.95–1.12) | 1.05 (0.96–1.14) |
| Location/Region | ||
| South | 1.00 | 1.00 |
| Northeast | 1.01 (0.98–1.05) | 1.00 (0.96–1.04) |
| West | 1.00 (0.96–1.03) | 0.95 (0.91–0.99) |
| Midwest | 1.06 (1.02–1.10) | 1.03 (0.99–1.07) |
| Median Household Income | ||
| $46,000+ | 1.00 | 1.00 |
| $35,000–45,999 | 0.96 (0.93–0.99) | 0.98 (0.94–1.01) |
| $30,000–34,999 | 0.93 (0.90–0.97) | 0.97 (0.92–1.01) |
| <$30,000 | 0.87 (0.83–0.90) | 0.94 (0.89–1.00) |
| Education, % of people in patient’s area graduate high school |
||
| <14% | 1.00 | 1.00 |
| 14–19.9% | 0.97 (0.94–1.01) | 0.99 (0.95–1.02) |
| 20–28.9% | 0.94 (0.91–0.97) | 0.95 (0.91–0.99) |
| 29% or more | 0.86 (0.82–0.89) | 0.88 (0.84–0.93) |
| Insurance | ||
| Yes | 1.00 | 1.00 |
| No | 1.11 (1.05–1.18) | 0.92 (0.86–0.98) |
| Facility Type | ||
| Community Program | 1.00 | 1.00 |
| Academic/Research Program | 1.06 (1.03–1.09) | 0.98 (0.95–1.01) |
| Other | 0.92 (0.83–1.03) | 0.93 (0.82–1.04) |
| Charlson-Deyo Score | ||
| 0 | 1.00 | 1.00 |
| 1 | 0.84 (0.80–0.87) | 0.89 (0.85–0.92) |
| 2 | 0.70 (0.65–0.75) | 0.74 (0.68–0.80) |
| Unknown | 0.87 (0.84–0.89) | 0.86 (0.80–0.92) |
| Histology | ||
| Urothelial | 1.00 | 1.00 |
| Other | 0.84 (0.80–0.89) | 0.84 (0.80–0.89) |
| Unknown | 0.61 (0.56–0.67) | 0.70 (0.64–0.77) |
| Clinical T stage | ||
| 2 | 1.00 | 1.00 |
| 3 | 1.04 (0.99–1.08) | 0.99 (0.94–1.03) |
| 4 | 0.92 (0.89–0.95) | 0.96 (0.92–0.99) |
| Other/Unknown | 0.88 (0.85–0.90) | 0.93 (0.89–0.97) |
| Clinical N stage | ||
| 0 | 1.00 | 1.00 |
| 1 | 1.24 (1.19–1.29) | 1.22 (1.17–1.28) |
| 2 | 1.27 (1.22–1.32) | 1.23 (1.18–1.28) |
| 3 | 1.19 (1.10–1.29) | 1.20 (1.10–1.30) |
| Unable to be assessed | 0.97 (0.94–1.00) | 1.00 (0.96–1.04) |
| Presence of distant metastases | ||
| No | 1.00 | 1.00 |
| Yes | 0.89 (0.86–0.91) | 0.92 (0.89–0.95) |
| Cystectomy Performed | ||
| No | 1.00 | 1.00 |
| Yes | 1.09 (1.06–1.12) | 0.98 (0.95–1.01) |
Adjusted for sex, age, year of diagnosis, race, insurance status, distance to facility, location of facility, income, education, facility type, comorbidity score, histology, cystectomy, clinical T stage, and presence of nodal and distant metastases. N=20,571 included in the model, with complete data for all covariates.
RR = relative risk, CI = confidence interval
In the patients that underwent cystectomy (37% of the total population), women remained less likely to receive chemotherapy than men (49% vs 54%, RR 0.90, 95% CI 0.86–0.94). This difference was no longer significant in the multivariable model (RR 0.96, 95% CI 0.91–1.00). Women also remained less likely to receive chemotherapy than men when the analysis was restricted to patients that had distant metastatic disease (41% vs 48%, RR 0.84, 95% CI 0.80–0.89) and the difference remained significant after adjustment for covariates (RR 0.88, 95%CI 0.84–0.93).
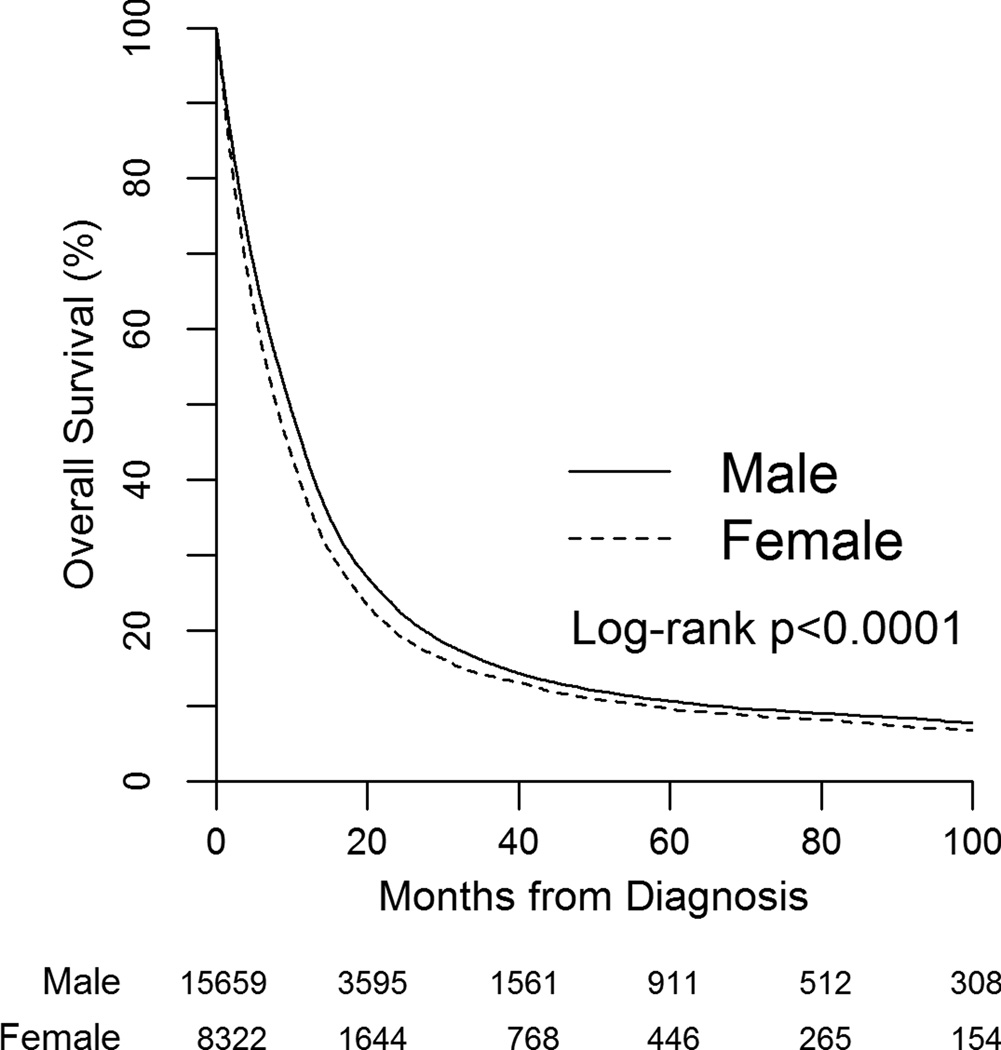
Women had a lower median OS compared to men (8.0 mos (95% CI 7.7–8.3 mos) vs 9.8 mos (95% CI 9.5–10.0 mos), p<0.001) with a HR for death of 1.12, 95% CI 1.09–1.15 (figure 1 and table 3). OS remained lower for women on multivariable analysis after adjusting for age, year of diagnosis, race, insurance status, distance to facility, location of facility, income, education, facility type, comorbidity score, histology, cystectomy, clinical T stage, presence of nodal and distant metastases, and receipt of chemotherapy as a time-varying covariate (HR 1.11, 95% CI 1.08–1.15). Other factors associated with improved survival in the adjusted model included younger age, white race, higher household income, presence of insurance, treatment at a community program, lower comorbidity score, urothelial histology, lower T stage, and absence of nodal or distant metastases (table 4). Receipt of chemotherapy was also associated with improved survival. In patients that underwent cystectomy, the adjusted HR for death of women compared to men was 1.05 (95% CI 1.00–1.11). In patients with distant metastatic disease, the adjusted HR was 1.10 (95%CI 1.05–1.16). OS rates are listed by gender in table 3, with a median follow-up of 24 months (range 1–168 months) for the survivors.
Figure 1.
Kaplan-Meier plot of overall survival by gender
Table 3.
Survival rates in males and females
| Overall Survival | ||
|---|---|---|
| Males | Females | |
| All Stage IV patients | ||
| Median OS, months (95% CI) | 9.76 (9.53–9.99) | 7.95 (7.66–8.28) |
| 1 year OS | 42.9% | 37.3% |
| 3 year OS | 15.8% | 14.0% |
| 5 year OS | 10.7% | 9.7% |
| Patients that had cystectomy | ||
| Median OS, months (95% CI) | 16.79 (16.20–17.35) | 14.46 (13.77–15.21) |
| 1 year OS | 63.4% | 57.0% |
| 3 year OS | 26.9% | 24.8% |
| 5 year OS | 18.6% | 17.6% |
| Patients with distant metastases | ||
| Median OS, months (95% CI) | 4.83 (4.63–5.09) | 3.88 (3.58–4.11) |
| 1 year OS | 23.7% | 19.8% |
| 3 year OS | 5.5% | 5.1% |
| 5 year OS | 3.2% | 2.9% |
OS = overall survival, CI = confidence interval
Table 4.
Unadjusted and adjusted hazard ratios for death based on demographic factors
| Demographic | HR for death (univariate) |
HR for death (multivariable*) |
|---|---|---|
| Sex | ||
| Male | 1.00 | 1.00 |
| Female | 1.12 (1.09 – 1.15) | 1.11 (1.07–1.14) |
| Age | ||
| <70 | 1.00 | 1.00 |
| >= 70 | 1.60 (1.56–1.65) | 1.39 (1.34–1.43) |
| Race | ||
| White | 1.00 | 1.00 |
| Black | 1.19 (1.14–1.25) | 1.12 (1.06–1.18) |
| Other | 0.94 (0.85–1.03) | 0.97 (0.88–1.08) |
| Location/Region | ||
| South | 1.00 | 1.00 |
| Northeast | 0.99 (0.96–1.03) | 1.01 (0.97–1.05) |
| West | 0.94 (0.90–0.98) | 0.98 (0.93–1.02) |
| Midwest | 0.98 (0.94–1.02) | 1.03 (0.99–1.07) |
| Median Household Income | ||
| $46,000+ | 1.00 | 1.00 |
| $35,000–45,999 | 1.11 (1.07–1.15) | 1.10 (1.06–1.15) |
| $30,000–34,999 | 1.11 (1.06–1.15) | 1.08 (1.03–1.14) |
| <$30,000 | 1.17 (1.12–1.22) | 1.10 (1.03–1.16) |
| Education, % of people in patient’s area graduate high school |
||
| <14% | 1.00 | 1.00 |
| 14–19.9% | 1.08 (1.04–1.12) | 1.01 (0.96–1.05) |
| 20–28.9% | 1.11 (1.07–1.15) | 1.01 (0.96–1.06) |
| 29% or more | 1.13 (1.09–1.18) | 1.02 (0.96–1.08) |
| Insurance | ||
| Yes | 1.00 | 1.00 |
| No | 1.06 (0.98–1.13) | 1.20 (1.11–1.29) |
| Facility Type | ||
| Community Program | 1.00 | 1.00 |
| Academic/Research Program | 0.79 (0.77–0.82) | 0.89 (0.86–0.92) |
| Other | 1.04 (0.93–1.17) | 0.98 (0.86–1.11) |
| Charlson-Deyo Score | ||
| 0 | 1.00 | 1.00 |
| 1 | 1.22 (1.17–1.28) | 1.18 (1.13–1.24) |
| 2 | 1.63 (1.53–1.74) | 1.45 (1.36–1.56) |
| Unknown | 1.10 (1.07–1.13) | 0.94 (0.87–1.03) |
| Histology | ||
| Urothelial | 1.00 | 1.00 |
| Other | 1.46 (1.39–1.53) | 1.39 (1.32–1.46) |
| Unknown | 2.14 (2.01–2.29) | 1.57 (1.47–1.69) |
| Clinical T stage | ||
| 2 | 1.00 | 1.00 |
| 3 | 1.08 (1.03–1.14) | 1.13 (1.07–1.19) |
| 4 | 1.47 (1.41–1.52) | 1.31 (1.26–1.37) |
| Other/Unknown | 1.07 (1.04–1.11) | 1.16 (1.11–1.22) |
| Clinical N stage | ||
| 0 | 1.00 | 1.00 |
| 1 | 0.96 (0.91–1.01) | 0.94 (0.89–0.99) |
| 2 | 1.10 (1.05–1.15) | 1.04 (0.99–1.09) |
| 3 | 1.37 (1.24–1.51) | 1.22 (1.10–1.36) |
| Unable to be assessed | 1.03 (1.00–1.07) | 1.11 (1.06–1.16) |
| Presence of distant metastases | ||
| No | 1.00 | 1.00 |
| Yes | 2.25 (2.19–2.32) | 1.74 (1.68–1.81) |
| Cystectomy Received | ||
| No | 1.00 | 1.00 |
| Yes | 0.47 (0.45–0.48) | 0.62 (0.60–0.65) |
| Chemotherapy Received | ||
| No | 1.00 | 1.00 |
| Yes | 0.79 (0.77–0.82) | 0.74 (0.72–0.77) |
Adjusted for age, year of diagnosis, race, insurance status, distance to facility, location of facility, income, education, facility type, comorbidity score, histology, clinical T stage, presence of nodal and distant metastases, cystectomy, and chemotherapy use. N=20,571 included in the model, with complete data for all covariates.
HR = hazard ratio
Discussion
In the current analysis using the NCDB, women were less likely than men to receive systemic chemotherapy for advanced bladder cancer. Although this difference may partially account for the survival disadvantage in women compared to men, OS remains lower in women independent of chemotherapy use.
The cause of the disparity in chemotherapy use may be related to a number of factors. Most research in this area has focused on gender differences in tumor characteristics such as histology and stage, and healthcare-related factors such as time to diagnosis and operative mortality.11 Women with bladder cancer tend to be older at diagnosis, although we show that the disparity in chemotherapy use between men and women remains significant even after adjusting for age. Women may be less likely to be offered chemotherapy or more likely to decline it, and we could not differentiate between these possibilities in our study. Using the Charlson-Deyo comorbidity index, the analysis shows that women do not have increased comorbidities compared to men to suggest a potential cause for their lower rates of chemotherapy use. However, women may have poorer performance status compared to men with advanced bladder cancer. Performance status is not captured by the Charlson-Deyo comorbidity index, and could independently contribute to a lower likelihood of chemotherapy receipt and inferior survival among women. It is interesting to note that patients with distant metastatic disease were less likely than those without distant metastases to receive chemotherapy with a RR of 0.92 (95% CI 0.89–0.95), similar to the RR of women compared to men. Patients with distant metastases are generally less healthy than those without, suggesting the possibility that the gender disparity could be related to fitness as well. However, a prior study by Konety et al showed that women with stage IV bladder cancer have the same rates of radical cystectomy and slightly higher rates of radiation therapy compared with men,12 suggesting that fitness for aggressive treatment may not differ between genders. We did find that women were more likely to have higher T stage but less likely to have clinically positive lymph nodes at diagnosis, perhaps related to a greater difficulty in staging with conventional imaging studies, although women also had lower rates of pathologically positive lymph nodes. Data are conflicting with regard to if perioperative complication rates are indeed different between women and men, but gender-specific differences in post-operative recovery could influence rates of adjuvant chemotherapy administration.13–16 Our data indicate that the gender disparity in chemotherapy administration is less pronounced in those who undergo cystectomy, suggesting that differences in perioperative use of chemotherapy and surgical complications are less likely to explain the findings.
Gender disparity in systemic treatment rates has been documented in other diseases, despite the overall resource utilization of healthcare spending being higher for women.17 In particular, women with cardiovascular disease have lower rates of evidence-based medication use such as aspirin and statins compared to men.18–21 Gender disparities in receipt of chemotherapy are less well studied. Our study results align with existing oncologic literature, including a recent analysis of stage IV pancreatic cancer patients in which decreased utilization of systemic therapy was found in women compared to men.22 Other studies have also demonstrated decreased use of chemotherapy in female patients compared to men in lymphoma,23 renal cell carcinoma,24 and colon cancer.25 However, a causative association between lower rates of chemotherapy use and decreased survival among women is unclear.
The survival rates seen in our study are overall in agreement with prior studies of stage IV bladder. Mungan et al found the 5 year OS of stage IV bladder cancer to be 11.7% in men and 9.6% in women in a study cohort from the Netherlands, and 27% in men and 15% in women using SEER data.3 Our study found similar results to the Netherlands cohort with a 5 year OS of 10.7% in men and 9.7% in women. Nearly 40% of patients in our study cohort had distant metastatic disease at the time of diagnosis, which may explain the lower survival compared to the SEER analysis. The survival rates in our study are significantly lower than published rates reported in clinical trials (8–10 mos vs 14–15 mos, respectively), and are likely more reflective of a typical oncology practice.26 We found the median OS of patients with distant metastatic disease was less than 5 months, suggesting many patients die soon after diagnosis and reflecting the limited effective treatment options for this population. In terms of the survival disparity between genders, Micheli et al found an overall estimated 1.3 relative excess risk of death in women with bladder cancer compared to men (95% CI 1.27–1.33)1, similar to our unadjusted HR of 1.12 (95% CI 1.09–1.15). Our finding of an association between treatment at an academic medical center and decreased survival is likely reflective of referral bias with sicker patients being treated at academic centers.
Women are more likely to have a delay in diagnosis27 and more likely to present with muscle-invasive disease at diagnosis,11 although such differences do not explain the large disparity in survival among patients with advanced disease at diagnosis. Prior studies have suggested that women more often present with advanced disease at the time of diagnosis which may contribute to women’s survival disadvantage.28 Since OS was defined starting from the date of diagnosis, it is possible that even among patients with stage IV disease, men are diagnosed earlier and therefore appear to have a survival benefit over women that is in reality just an artifact of diagnostic efficiency. Our study found that women did have a higher T stage, but were less likely to harbor nodal and distant metastases, suggesting that diagnostic delay is not the only contributing factor. Decreased functional status or a high burden of unmeasured comorbidities in women could also be a potential explanation for the survival disparity. Ultimately, the cause of the gender survival disparity in advanced bladder cancer remains unclear.
Recently, distinct molecular subtypes of high-grade bladder cancer have been identified as “basal” and “luminal,” which have clinically meaningful differences in outcome.29, 30 Basal-like tumors are associated with decreased disease-specific and overall survival. Interestingly, women have a significantly higher incidence of basal-like bladder cancer than males.29 In addition, African Americans are also more likely to have the basal-like subtype, and our data shows that women with bladder cancer are more likely to be African-American.31 Therefore, our paper supports the premise that tumor biology may partially be responsible for the inferior survival seen in women. Differences in tumor biology have also been suggested as an explanation for the lower incidence rates of bladder cancer in women, mostly focusing on a potentially deleterious effect of male sex hormones (or lack of female sex hormones).32 However, sex hormones alone are unlikely to explain the survival disparity after diagnosis across ages.
A limitation of our study is the inability to fully account for socioeconomic status and functional status. We were able to classify insurance on a patient-level basis, but education and household income are based on each patient’s zip code. The NCDB also does not report smoking or other environmental exposures that may be important to the pathogenesis and natural history of bladder cancer, which also limits our analysis. However, prior data suggest that population-attributable risk of smoking in the pathogenesis of bladder cancer is similar between men and women.33 In addition, although we were able to control for time from diagnosis to initiation of chemotherapy, we were unable to control for type and duration of chemotherapy received.
Our study shows that women are less likely to receive chemotherapy for stage IV bladder cancer even after controlling for multiple potential confounders. Women also have inferior overall survival as compared to men independent of chemotherapy use, perhaps related to unmeasured comorbidities, functional status, or tumor biology. Future research should focus on the difference in treatment decisions in men and women, as well as the differences in tumor biology between genders.
Acknowledgments
Funding: M.E.N. was supported in part by the American Cancer Society (Grant No. MRSG-13-154-01-CPPB) and the Urology Care Foundation/Astellas. A.B.S. was supported in part by the National Center for Research Resources, the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant No. KL2TR000084, and PCORI.
M.I.M. has received research funding from Pfizer, BIND Therapeutics, Dendreon, Exelixis, Johnson & Johnson, Astellas Pharma, Mirati Therapeutics, Merck, and Cerulean Pharma, Inc.
Footnotes
Conflict of Interest: The authors have no other conflicts of interest to disclose.
References
- 1.Micheli A, Ciampichini R, Oberaigner W, et al. The advantage of women in cancer survival: an analysis of EUROCARE-4 data. Eur J Cancer. 2009;45:1017–1027. doi: 10.1016/j.ejca.2008.11.008. [DOI] [PubMed] [Google Scholar]
- 2.Howlader NNA, Krapcho M, Garshell J, Neyman N, Altekruse SF, Kosary CL, Yu M, Ruhl J, Tatalovich Z, Cho H, Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA, editors. National Cancer Institute. Bethesda, MD: 2013. Apr, [based on November 2012]. SEER Cancer Statistics Review, 1975–2010. SEER data submission, posted to the SEER web site. [Google Scholar]
- 3.Mungan NA, Aben KK, Schoenberg MP, et al. Gender differences in stage-adjusted bladder cancer survival. Urology. 2000;55:876–880. doi: 10.1016/s0090-4295(00)00523-9. [DOI] [PubMed] [Google Scholar]
- 4.Mallin K, David KA, Carroll PR, Milowsky MI, Nanus DM. Transitional cell carcinoma of the bladder: racial and gender disparities in survival (1993 to 2002), stage and grade (1993 to 2007) J Urol. 2011;185:1631–1636. doi: 10.1016/j.juro.2010.12.049. [DOI] [PubMed] [Google Scholar]
- 5.Sant M, Allemani C, Santaquilani M, Knijn A, Marchesi F, Capocaccia R. EUROCARE-4. Survival of cancer patients diagnosed in 1995–1999. Results and commentary. Eur J Cancer. 2009;45:931–991. doi: 10.1016/j.ejca.2008.11.018. [DOI] [PubMed] [Google Scholar]
- 6.Haines L, Bamias A, Krege S, et al. The impact of gender on outcomes in patients with metastatic urothelial carcinoma. Clin Genitourin Cancer. 2013;11:346–352. doi: 10.1016/j.clgc.2013.04.010. [DOI] [PubMed] [Google Scholar]
- 7.Bilimoria KY, Stewart AK, Winchester DP, Ko CY. The National Cancer Data Base: a powerful initiative to improve cancer care in the United States. Ann Surg Oncol. 2008;15:683–690. doi: 10.1245/s10434-007-9747-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 9.Harrel FE. Regression Modeling Strategies. Springer; 2001. [Google Scholar]
- 10.Spiegelman D, Hertzmark E. Easy SAS calculations for risk or prevalence ratios and differences. Am J Epidemiol. 2005;162:199–200. doi: 10.1093/aje/kwi188. [DOI] [PubMed] [Google Scholar]
- 11.Scosyrev E, Noyes K, Feng C, Messing E. Sex and racial differences in bladder cancer presentation and mortality in the US. Cancer. 2009;115:68–74. doi: 10.1002/cncr.23986. [DOI] [PubMed] [Google Scholar]
- 12.Konety BR, Joslyn SA. Factors influencing aggressive therapy for bladder cancer: an analysis of data from the SEER program. J Urol. 2003;170:1765–1771. doi: 10.1097/01.ju.0000091620.86778.2e. [DOI] [PubMed] [Google Scholar]
- 13.Otto W, May M, Fritsche HM, et al. Analysis of sex differences in cancer-specific survival and perioperative mortality following radical cystectomy: results of a large German multicenter study of nearly 2500 patients with urothelial carcinoma of the bladder. Gend Med. 2012;9:481–489. doi: 10.1016/j.genm.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 14.Siegrist T, Savage C, Shabsigh A, Cronin A, Donat SM. Analysis of gender differences in early perioperative complications following radical cystectomy at a tertiary cancer center using a standardized reporting methodology. Urol Oncol. 2010;28:112–117. doi: 10.1016/j.urolonc.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 15.Stimson CJ, Chang SS, Barocas DA, et al. Early and late perioperative outcomes following radical cystectomy: 90-day readmissions, morbidity and mortality in a contemporary series. J Urol. 2010;184:1296–1300. doi: 10.1016/j.juro.2010.06.007. [DOI] [PubMed] [Google Scholar]
- 16.Lee KL, Freiha F, Presti JC, Jr, Gill HS. Gender differences in radical cystectomy: complications and blood loss. Urology. 2004;63:1095–1099. doi: 10.1016/j.urology.2004.01.029. [DOI] [PubMed] [Google Scholar]
- 17.Owens GM. Gender differences in health care expenditures, resource utilization, and quality of care. J Manag Care Pharm. 2008;14:2–6. doi: 10.18553/jmcp.2008.14.S6-A.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johansen ME, Hefner JL, Foraker RE. Antiplatelet and Statin Use in US Patients With Coronary Artery Disease Categorized by Race/Ethnicity and Gender, 2003 to 2012. Am J Cardiol. 2015;115:1507–1512. doi: 10.1016/j.amjcard.2015.02.052. [DOI] [PubMed] [Google Scholar]
- 19.Jneid H, Fonarow GC, Cannon CP, et al. Sex differences in medical care and early death after acute myocardial infarction. Circulation. 2008;118:2803–2810. doi: 10.1161/CIRCULATIONAHA.108.789800. [DOI] [PubMed] [Google Scholar]
- 20.Bugiardini R, Yan AT, Yan RT, et al. Factors influencing underutilization of evidence-based therapies in women. Eur Heart J. 2011;32:1337–1344. doi: 10.1093/eurheartj/ehr027. [DOI] [PubMed] [Google Scholar]
- 21.Virani SS, Woodard LD, Ramsey DJ, et al. Gender disparities in evidence-based statin therapy in patients with cardiovascular disease. Am J Cardiol. 2015;115:21–26. doi: 10.1016/j.amjcard.2014.09.041. [DOI] [PubMed] [Google Scholar]
- 22.Khanal N, Upadhyay S, Dahal S, Bhatt VR, Silberstein PT. Systemic therapy in stage IV pancreatic cancer: a population-based analysis using the National Cancer Data Base. Ther Adv Med Oncol. 2015;7:198–205. doi: 10.1177/1758834015579313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Olszewski AJ, Shrestha R, Castillo JJ. Treatment selection and outcomes in early-stage classical Hodgkin lymphoma: analysis of the National Cancer Data Base. J Clin Oncol. 2015;33:625–633. doi: 10.1200/JCO.2014.58.7543. [DOI] [PubMed] [Google Scholar]
- 24.Smaldone MC, Handorf E, Kim SP, et al. Temporal trends and factors associated with systemic therapy after cytoreductive nephrectomy: an analysis of the National Cancer Database. J Urol. 2015;193:1108–1113. doi: 10.1016/j.juro.2014.10.095. [DOI] [PubMed] [Google Scholar]
- 25.Paulson EC, Wirtalla C, Armstrong K, Mahmoud NN. Gender influences treatment and survival in colorectal cancer surgery. Dis Colon Rectum. 2009;52:1982–1991. doi: 10.1007/DCR.0b013e3181beb42a. [DOI] [PubMed] [Google Scholar]
- 26.von der Maase H, Sengelov L, Roberts JT, et al. Long-term survival results of a randomized trial comparing gemcitabine plus cisplatin, with methotrexate, vinblastine, doxorubicin, plus cisplatin in patients with bladder cancer. J Clin Oncol. 2005;23:4602–4608. doi: 10.1200/JCO.2005.07.757. [DOI] [PubMed] [Google Scholar]
- 27.Cohn JA, Vekhter B, Lyttle C, Steinberg GD, Large MC. Sex disparities in diagnosis of bladder cancer after initial presentation with hematuria: a nationwide claims-based investigation. Cancer. 2014;120:555–561. doi: 10.1002/cncr.28416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mungan NA, Kiemeney LA, van Dijck JA, van der Poel HG, Witjes JA. Gender differences in stage distribution of bladder cancer. Urology. 2000;55:368–371. doi: 10.1016/s0090-4295(99)00481-1. [DOI] [PubMed] [Google Scholar]
- 29.Damrauer JS, Hoadley KA, Chism DD, et al. Intrinsic subtypes of high-grade bladder cancer reflect the hallmarks of breast cancer biology. Proc Natl Acad Sci U S A. 2014;111:3110–3115. doi: 10.1073/pnas.1318376111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Choi W, Porten S, Kim S, et al. Identification of distinct basal and luminal subtypes of muscle-invasive bladder cancer with different sensitivities to frontline chemotherapy. Cancer Cell. 2014;25:152–165. doi: 10.1016/j.ccr.2014.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kardos J, Melquist JJ, Chism DD, et al. Evaluation of basal and luminal subtypes of urothelial carcinoma in African American and non-African American patients. ASCO Meeting Abstracts. 33:305. [Google Scholar]
- 32.Apolo AB, Hoffman V, Kaag MG, et al. Summary of the 8th Annual Bladder Cancer Think Tank: Collaborating to move research forward. Urol Oncol. 2015;33:53–64. doi: 10.1016/j.urolonc.2014.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Freedman ND, Silverman DT, Hollenbeck AR, Schatzkin A, Abnet CC. Association between smoking and risk of bladder cancer among men and women. Jama. 2011;306:737–745. doi: 10.1001/jama.2011.1142. [DOI] [PMC free article] [PubMed] [Google Scholar]