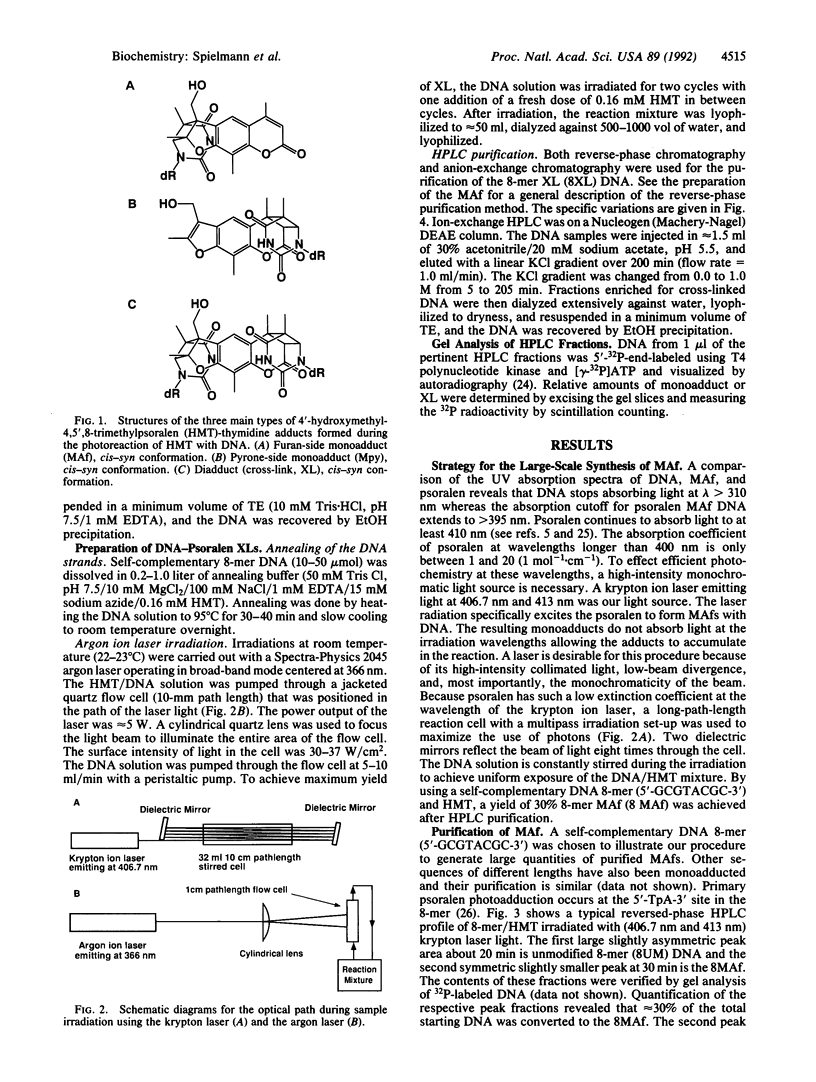
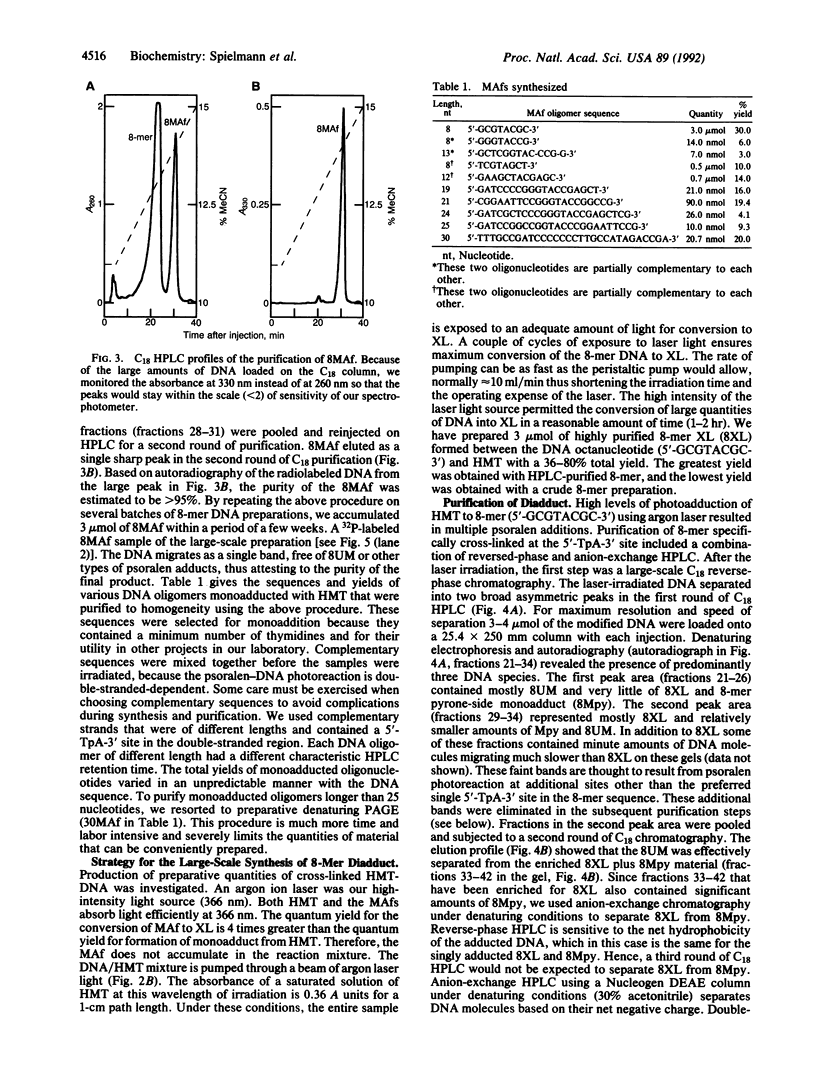
Abstract
We report methods for the preparation of multimicromole quantities of extremely pure uniquely photo-adducted psoralen-DNA furan-side monoadducts and diadducts (cross-links). The methods use high-intensity krypton and argon ion lasers in the photoreactions and HPLC methods to purify the required oligonucleotides containing the photoadducts. With these methods we have synthesized 2-3 mumol of 8-mer psoralen furan-side monoadduct and diadduct. These methods allow one to generate large amounts of psoralenated DNA oligonucleotides and facilitate their study by NMR and x-ray crystallography.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alter H. J., Creagan R. P., Morel P. A., Wiesehahn G. P., Dorman B. P., Corash L., Smith G. C., Popper H., Eichberg J. W. Photochemical decontamination of blood components containing hepatitis B and non-A, non-B virus. Lancet. 1988 Dec 24;2(8626-8627):1446–1450. doi: 10.1016/s0140-6736(88)90931-2. [DOI] [PubMed] [Google Scholar]
- Cheng S., Sancar A., Hearst J. E. RecA-dependent incision of psoralen-crosslinked DNA by (A)BC excinuclease. Nucleic Acids Res. 1991 Feb 11;19(3):657–663. doi: 10.1093/nar/19.3.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng S., Van Houten B., Gamper H. B., Sancar A., Hearst J. E. Use of psoralen-modified oligonucleotides to trap three-stranded RecA-DNA complexes and repair of these cross-linked complexes by ABC excinuclease. J Biol Chem. 1988 Oct 15;263(29):15110–15117. [PubMed] [Google Scholar]
- Cimino G. D., Gamper H. B., Isaacs S. T., Hearst J. E. Psoralens as photoactive probes of nucleic acid structure and function: organic chemistry, photochemistry, and biochemistry. Annu Rev Biochem. 1985;54:1151–1193. doi: 10.1146/annurev.bi.54.070185.005443. [DOI] [PubMed] [Google Scholar]
- Cimino G. D., Shi Y. B., Hearst J. E. Wavelength dependence for the photoreversal of a psoralen-DNA cross-link. Biochemistry. 1986 May 20;25(10):3013–3020. doi: 10.1021/bi00358a042. [DOI] [PubMed] [Google Scholar]
- Courey A. J., Plon S. E., Wang J. C. The use of psoralen-modified DNA to probe the mechanism of enhancer action. Cell. 1986 May 23;45(4):567–574. doi: 10.1016/0092-8674(86)90288-6. [DOI] [PubMed] [Google Scholar]
- Gamper H., Piette J., Hearst J. E. Efficient formation of a crosslinkable HMT monoadduct at the Kpn I recognition site. Photochem Photobiol. 1984 Jul;40(1):29–34. doi: 10.1111/j.1751-1097.1984.tb04549.x. [DOI] [PubMed] [Google Scholar]
- Hearst J. E. A photochemical investigation of the dynamics of oligonucleotide hybridization. Annu Rev Phys Chem. 1988;39:291–315. doi: 10.1146/annurev.pc.39.100188.001451. [DOI] [PubMed] [Google Scholar]
- Hearst J. E. Psoralen photochemistry. Annu Rev Biophys Bioeng. 1981;10:69–86. doi: 10.1146/annurev.bb.10.060181.000441. [DOI] [PubMed] [Google Scholar]
- Kodadek T., Gamper H. Efficient synthesis of a supercoiled M13 DNA molecule containing a site specifically placed psoralen adduct and its use as a substrate for DNA replication. Biochemistry. 1988 May 3;27(9):3210–3215. doi: 10.1021/bi00409a013. [DOI] [PubMed] [Google Scholar]
- Lin L., Wiesehahn G. P., Morel P. A., Corash L. Use of 8-methoxypsoralen and long-wavelength ultraviolet radiation for decontamination of platelet concentrates. Blood. 1989 Jul;74(1):517–525. [PubMed] [Google Scholar]
- Peckler S., Graves B., Kanne D., Rapoport H., Hearst J. E., Kim S. H. Structure of a psoralen-thymine monoadduct formed in photoreaction with DNA. J Mol Biol. 1982 Nov 25;162(1):157–172. doi: 10.1016/0022-2836(82)90166-8. [DOI] [PubMed] [Google Scholar]
- Piette J., Gamper H. B., van de Vorst A., Hearst J. E. Mutagenesis induced by site specifically placed 4'-hydroxymethyl-4,5',8-trimethylpsoralen adducts. Nucleic Acids Res. 1988 Nov 11;16(21):9961–9977. doi: 10.1093/nar/16.21.9961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poppe W., Grossweiner L. I. Photodynamic sensitization by 8-methoxypsoralen via the singlet oxygen mechanism. Photochem Photobiol. 1975 Nov;22(5):217–219. doi: 10.1111/j.1751-1097.1975.tb06739.x. [DOI] [PubMed] [Google Scholar]
- Sastry S. S., Hearst J. E. Studies on the interaction of T7 RNA polymerase with a DNA template containing a site-specifically placed psoralen cross-link. I. Characterization of elongation complexes. J Mol Biol. 1991 Oct 20;221(4):1091–1110. [PubMed] [Google Scholar]
- Sastry S. S., Hearst J. E. Studies on the interaction of T7 RNA polymerase with a DNA template containing a site-specifically placed psoralen cross-link. II. Stability and some properties of elongation complexes. J Mol Biol. 1991 Oct 20;221(4):1111–1125. [PubMed] [Google Scholar]
- Shi Y. B., Gamper H., Hearst J. E. Interaction of T7 RNA polymerase with DNA in an elongation complex arrested at a specific psoralen adduct site. J Biol Chem. 1988 Jan 5;263(1):527–534. [PubMed] [Google Scholar]
- Shi Y. B., Gamper H., Van Houten B., Hearst J. E. Interaction of Escherichia coli RNA polymerase with DNA in an elongation complex arrested at a specific psoralen crosslink site. J Mol Biol. 1988 Jan 20;199(2):277–293. doi: 10.1016/0022-2836(88)90314-2. [DOI] [PubMed] [Google Scholar]
- Shi Y. B., Hearst J. E. Wavelength dependence for the photoreactions of DNA-psoralen monoadducts. 2. Photo-cross-linking of monoadducts. Biochemistry. 1987 Jun 30;26(13):3792–3798. doi: 10.1021/bi00387a009. [DOI] [PubMed] [Google Scholar]
- Shi Y. B., Spielmann H. P., Hearst J. E. Base-catalyzed reversal of a psoralen-DNA cross-link. Biochemistry. 1988 Jul 12;27(14):5174–5178. doi: 10.1021/bi00414a034. [DOI] [PubMed] [Google Scholar]
- Sinden R. R., Carlson J. O., Pettijohn D. E. Torsional tension in the DNA double helix measured with trimethylpsoralen in living E. coli cells: analogous measurements in insect and human cells. Cell. 1980 Oct;21(3):773–783. doi: 10.1016/0092-8674(80)90440-7. [DOI] [PubMed] [Google Scholar]
- Sinden R. R., Hagerman P. J. Interstrand psoralen cross-links do not introduce appreciable bends in DNA. Biochemistry. 1984 Dec 18;23(26):6299–6303. doi: 10.1021/bi00321a002. [DOI] [PubMed] [Google Scholar]
- Song P. S., Tapley K. J., Jr Photochemistry and photobiology of psoralens. Photochem Photobiol. 1979 Jun;29(6):1177–1197. doi: 10.1111/j.1751-1097.1979.tb07838.x. [DOI] [PubMed] [Google Scholar]
- Takasugi M., Guendouz A., Chassignol M., Decout J. L., Lhomme J., Thuong N. T., Hélène C. Sequence-specific photo-induced cross-linking of the two strands of double-helical DNA by a psoralen covalently linked to a triple helix-forming oligonucleotide. Proc Natl Acad Sci U S A. 1991 Jul 1;88(13):5602–5606. doi: 10.1073/pnas.88.13.5602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tessman J. W., Isaacs S. T., Hearst J. E. Photochemistry of the furan-side 8-methoxypsoralen-thymidine monoadduct inside the DNA helix. Conversion to diadduct and to pyrone-side monoadduct. Biochemistry. 1985 Mar 26;24(7):1669–1676. doi: 10.1021/bi00328a015. [DOI] [PubMed] [Google Scholar]
- Tomic M. T., Wemmer D. E., Kim S. H. Structure of a psoralen cross-linked DNA in solution by nuclear magnetic resonance. Science. 1987 Dec 18;238(4834):1722–1725. doi: 10.1126/science.3686011. [DOI] [PubMed] [Google Scholar]
- Umlauf S. W., Cox M. M., Inman R. B. Triple-helical DNA pairing intermediates formed by recA protein. J Biol Chem. 1990 Oct 5;265(28):16898–16912. [PubMed] [Google Scholar]
- Van Houten B., Gamper H., Hearst J. E., Sancar A. Construction of DNA substrates modified with psoralen at a unique site and study of the action mechanism of ABC excinuclease on these uniformly modified substrates. J Biol Chem. 1986 Oct 25;261(30):14135–14141. [PubMed] [Google Scholar]
- Van Houten B., Gamper H., Holbrook S. R., Hearst J. E., Sancar A. Action mechanism of ABC excision nuclease on a DNA substrate containing a psoralen crosslink at a defined position. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8077–8081. doi: 10.1073/pnas.83.21.8077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung A. T., Dinehart W. J., Jones B. K. Alkali reversal of psoralen cross-link for the targeted delivery of psoralen monoadduct lesion. Biochemistry. 1988 Aug 23;27(17):6332–6338. doi: 10.1021/bi00417a020. [DOI] [PubMed] [Google Scholar]