Abstract
Objective
To assess the relationship between cortisol slope, a biologic marker of stress, and postpartum weight retention.
Methods
We included 696 women in a secondary analysis from a multi-site study conducted using principles of community-based participatory research to study multi-level sources of stress on pregnancy outcomes. As a stress marker, we included salivary cortisol slope; the rate of cortisol decline across the day. Pre-pregnancy weight and demographic data were obtained from the medical records. At 6 months postpartum, patients were weighed and returned saliva samples. We built stepwise regression models to assess the effect of demographic variables, cortisol slope and cortisol covariates (wake time, tobacco use and breastfeeding) on postpartum weight retention.
Results
45.5 % of participants were African American, 29.2 % White, and 25.3 % Hispanic. Of the Hispanic women 62.5 % were Spanish speaking and 37.5 % were English speaking. In general, participants were young, multiparous, and overweight. Postpartum, almost half (47.6 %) of women studied retained >10 lbs. In multivariable analysis including age, pre-pregnancy BMI and public insurance, cortisol slope was significantly associated with weight retention (β = −1.90, 95 % CI = 0.22–3.58). However, when the model was adjusted for the cortisol covariates, breastfeeding (β = −0.63, 95 % CI = − 1.01 to −0.24) and public insurance (β = 0.62, 95 % CI = 0.20–1.04) were the two strongest correlates of weight retention.
Conclusions for Practice
The association between cortisol slope and postpartum weight retention appears to be influenced breastfeeding status.
Keywords: Postpartum weight retention, Cortisol slope, Breast feeding, Stress
Introduction
Peripartum weight retention can lead to obesity and an increased risk of diabetes, chronic hypertension, and premature death [17]. It is estimated that more than a third of reproductive aged women in the United States are obese [19]. Studies have found high rates (66–70 %) of post-partum weight retention [7, 27] which can lead to lifelong obesity [29]. Variation in weight retention appears to be related to factors including age, race/ethnicity, insurance type, breastfeeding, exercise, pre-pregnancy BMI, smoking, parity, and gestational weight gain [7, 27, 29]. The reasons for peripartum weight retention may also be associated with physiological changes that predispose women to obesity.
Potentially related to weight gain is chronic environmental stress leading to pathological changes in the hypothalamic–pituitary–adrenal (HPA) axis. The interactions of weight gain and psychosocial variables modeled by Bjorntorp and Rosmond [4] and modified by Pasquali [21]. They hypothesize that perinatal programming of the mother, fetus/child, psychosocial/socioeconomic handicaps, depression/anxiety, alcohol/tobacco use and genetic susceptibility provoke a “stress reaction” or arousal of the HPA axis and the sympathetic nervous system. Chronic exposure to the “stress reaction” can lead to hyper-arousal of the HPA axis and consequent aberrations in secretion of its primary byproduct, cortisol. Salivary cortisol has a distinctive secretion pattern throughout the day: waking values are high, a peak occurs approximately 30 min after waking, followed by a steady decline throughout the day [13]. Flatter cortisol slopes (the rate of decline in cortisol from waking to bedtime) have been associated with poorer health outcomes, such as depression, metabolic syndrome, cardiovascular disease, and increased breast cancer mortality [1, 5, 9]. Suboptimal patterns in cortisol secretion and hyper-activation of the sympathetic nervous system have been associated with centralized obesity in both women and men [21].
Although chronic stress and the associated changes in diurnal cortisol patterns have been linked with both the development of obesity and its metabolic consequences, the relationship is complex [4, 21]. Several studies have demonstrated that women with visceral obesity have significantly higher levels of corticotropin (the pituitary hormone that stimulates adrenal cortisol) than normal-weight women [16, 20]. However, these studies were small, involved a homogenous racial/ethnic population and utilized multiple blood draws. Home collection of salivary cortisol presents a practical alternative to study a larger population of participants [1]. In obese women, a single measurement of salivary cortisol can be normal or low, due to increased urinary excretion of circulating cortisol, which further underscores the importance of repeat measures to calculate the slope [4]. Studies have suggested that women with obesity have flatter cortisol slopes (associated with less optimal health outcomes) compared to normal weight controls [15, 24]. However, these studies excluded post-partum women. Arguably, pregnancy and post-partum are the most vulnerable time for weight gain and weight retention [32]. Our objective was to use a previously created large prospective cohort to assess the relationship between cortisol slope and postpartum weight retention.
Methods
The study population was a subsample of a large, prospective study conducted by a group of community organizations and universities partnering with governmental support to gain new insights into the factors contributing to the disparities in maternal health and child development. A 5-year observational study was completed designed on the principles of community-based participatory research to better understand multiple levels of maternal stress, resiliency, and allostatic load (the cumulative biological burden exacted on the body through attempts to adapt to adverse environmental stressors) [21] in the inter-conception period. The methods and philosophy behind this study have been published elsewhere [25]. The study sites included three urban, one mixed urban/suburban, one rural location and a data coordinating center. IRB approval was obtained at each site and the research was conducted in accord with prevailing ethical principles.
Participants were primarily enrolled in the hospital following delivery (except one site which enrolled patients during their preconception care. Women age 18–40 years old with a live birth of greater than or equal to 20 weeks of gestation were approached. Women were excluded if they did not self-identify as Black/African American, White/Caucasian or Hispanic/Latino (the target race/ethnicities), were not either English or Spanish speakers or were unable to give informed consent. In addition, as the primary study objective was to evaluate the inter-conception period, women who were unlikely to become pregnant were excluded. Thus, women who underwent permanent sterilization or had more than four children were excluded. Medical records were reviewed for delivery information. Subjects completed an eligibility interview and were contacted within 1 month postpartum to complete a birth interview. Ninety minute face-to-face interviews were then conducted by dedicated, trained interviewers during home visits at 6 months.
Additional inclusion criteria for this secondary analysis required that morning and evening salivary samples be returned (n = 1166 women). Women were excluded if there were errors in collection (n = 81), current pregnancy (n = 20), current steroid use (n = 5), lack of documented pre-pregnancy or postpartum weights (n = 349). The maximum weight on the scales used to measure women was 350 lbs. Given this limitation, all women with a weight of 350 lbs or greater were also excluded from analysis (n = 1).
Biomarker Collection
At the 6-month postpartum visit, participants were given salivary sampling kits to collect over a single day at home on the day after the interview. Consistent with previously published protocols, [1] they self-collected saliva at wakeup, 30 min after wakeup and bedtime by expelling saliva through straws into sterile cryogenic vials. For wakeup and bedtime samples, a morning and bedtime diary, respectively, were completed by participants. All completed samples and diaries were mailed to the academic site. Samples were immediately logged and frozen at −80 °F. They were then shipped to ZRT Laboratories (Beaverton, Oregon) where they were assayed. The reportable cortisol range was 0.3–14 ng/ml, with a limit of detection of 0.3 ng/ml. Inter-assay coefficients of variation were 7 % (11.8 ng/ml) for high range, 20 % (3.0 ng/ml) for mid-range, and 14 % (0.3 ng/ml) for low range (ZRT Laboratories, personal communication, July 30, 2012). Cortisol slope was calculated by the equation: (cortisolbedtime – cortisolwakeup)/(total hours awake). A steeper cortisol slope indicated more diurnal decline across the day, is typically negative in sign and is associated with better health outcomes [1, 5, 9]. Our primary independent variable was the diurnal slope of cortisol.
Independent Variables of Interest
In addition to diurnal cortisol slope, independent variables of interest related to weight retention and changes in patterns of cortisol secretion included age, race/ethnicity, insurance status, sleep patterns, pre-pregnancy BMI and gestational weight gain [2, 3, 5–8, 14, 18, 22, 27–30]. Information prior to the 6 month visit was collected from a chart review including: pre-pregnancy BMI and gestational weight gain. Pregnancy weight gain was calculated by subtracting pre-pregnancy weight from weight at delivery, and was evaluated with respect to the IOM guidelines [11]. At the 6 month visit, participants self reported age, race/ethnicity, insurance status, (public or private). Public insurance was a mix of non-private insurance including Medicaid, self-pay and other publically funded insurance or sliding scale programs. We used the patient’s insurance status as a surrogate marker for socio-economic status as insurance status is both related to socio-economic status and other health disparities [12]. Maternal weight was measured at 6 months postpartum using scales brought by the interviewer that allowed a maximal weight of 350 lbs. Maternal height was also measured by the interviewer during one of the visits. BMI was categorized by the Institute of Medicine (IOM) criteria of normal 18.5–24.9 kg/m2, overweight 25.0–29.9 kg/m2, and obese ≥30.0 kg/m2 [11].
Dependent Variables
The main dependent variable was postpartum weight retention at 6-months postpartum, and was evaluated as both a categorical variable (<10, 10–20, >20 lbs) and a continuous variable. The weight retention categories (<10, 10–20, >20 lbs) were chosen based on our prior research on this data set [7] and another recent study looking at postpartum weight retention [26].
Cortisol Covariates
Cortisol covariates collected at 6 months included tobacco use, contraceptive use, wake time, and breastfeeding. These covariates were included in the statistical models. Mothers were considered positive for breastfeeding if they were still breastfeeding at the 6-month interview which included both partial and exclusive breastfeeding. As this was a cross-sectional study, changes in of the above covariates were not assessed.
Statistical Analysis
Analysis proceeded as follows: (a) descriptive statistics including ANOVA, Fisher’s exact test, Student’s t test with Tukey post hoc testing as indicated (b) Pearson correlations were assessed between cortisol variables, postpartum weight retention variables and all other independent variables and covariates (c) and stepwise regression models, both ordinary least squares (OLS) and ordinal (for categorical ordered outcome variables). The initial regression models included pre-pregnancy BMI, age and cortisol slope then insurance status, race/ethnicity or both were added. In subsequent models, relevant cortisol covariates (tobacco, contraceptives, wake time, and breastfeeding at 6 months) were included to predict postpartum weight retention. Of note, pre-pregnancy BMI was included in modeling, but gestational weight gain was not due to missing data [7].
Natural logarithmic transformation was used to transform cortisol slope measures to a normal distribution. Interactions based on a priori literature including those between cortisol and both insurance and race/ethnicity, and interactions with breastfeeding by cortisol [1], were assessed using interaction terms in the regression analysis. A p value of<0.05 was considered statistically significant. Analysis was conducted using SPSS Version 22.0 (IBM Corp. Armonk, NY: IBM Corp.)
Results
Inclusion criteria for this secondary analysis were met by 696 women. Most women in the sample were young, multiparous, non-smoking African-Americans with public insurance (Table 1). Breastfeeding rates at 6 months significantly varied by race/ethnicity; White women had the highest rates (39.9 %, n = 81) followed by Hispanic-Spanish speaking women (33.6 %, n = 37), Hispanic-English speaking women (27.3 %, n = 18) and African American women (12.3 %, n = 39) (p <0.01). Less than half of the participants included in our study started pregnancy with a normal BMI (42.7 %, n = 297).
Table 1.
Demographic and clinical characteristics of participants
| Participants N = 696 |
|
|---|---|
| Pre-pregnancy BMI (kg/m2) | |
| <25 | 299 (43) |
| 25–30 | 185 (26.6) |
| 30–35 | 116 (16.7) |
| 35–40 | 41 (5.9) |
| >40 | 55 (7.9) |
| Age 6 months postpartum (years) | 26.6 ± 5.9 |
| Nulliparous | 237 (34.1) |
| Race/ethnicity | |
| African American | 317 (45.5) |
| White | 203 (29.2) |
| Hispanic-Spanish speaking | 110 (15.8) |
| Hispanic-English speaking | 66 (9.5) |
| Tobacco use (6 months post partum) | 100 (14.4) |
| Public insurance (6 months postpartum) | 469 (67.4) |
| Not breastfeeding (6 months postpartum) | 521 (74.9) |
All data are presented as n (%) or mean ± standard deviation
Women gained an average of 31.1 ± 16.6 lbs during pregnancy with the majority of women gaining more than the Institute of Medicine guidelines (54.3 %). Most women did not return to their pre-pregnancy weight at 6 months postpartum (n = 524, 75.3 %) with an average weight retention of 11.1 ± 18.9 lbs. There were 331 women (47.6 %) who retained >10 lbs, 191 women (27.4 %) who retained between 10 lbs and 20 lbs, and 174 women (25.0 %) who retained >20 lbs. Of the women with normal pre-pregnancy weight, 69 (23.1 %) became overweight and almost 3 %, became obese. Over a third of overweight women became obese (n = 65, 35.1 %), and only 23 (5.8 %) of overweight or obese women had a normal BMI at 6 months.
We present our Pearson correlations between demographic variables and weight retention in Table 2. Weight retention was associated with younger maternal age, African American race/ethnicity, public insurance, tobacco use, and not breastfeeding at 6 months. Likewise, steeper cortisol slopes were associated with younger maternal age, Hispanic or White race/ethnicity, and breastfeeding at 6 months. A flatter cortisol slope was associated with African American race/ethnicity, public insurance, and tobacco use.
Table 2.
Pearson correlations between weight retention and cortisol slope in women 6 months postpartum
| Weight retentiona |
Cortisol slope |
|||
|---|---|---|---|---|
| Correlation | p value | Correlation | p value | |
| Age | −0.17 | <0.01 | −0.11 | <0.01 |
| Race/ethnicity | ||||
| African American | 0.17 | <0.01 | 0.28 | <0.01 |
| White | −0.14 | <0.01 | −0.14 | <0.01 |
| Hispanic-Spanish speaking | −0.08 | 0.03 | −0.11 | <0.01 |
| Hispanic-English speaking | 0.03 | 0.42 | −0.11 | <0.01 |
| Public insurance | 0.19 | <0.01 | 0.10 | <0.01 |
| Tobacco use | 0.01 | <0.01 | 0.10 | <0.01 |
| Breast feeding | −0.22 | <0.01 | −0.17 | <0.01 |
| Wake time | 0.06 | 0.12 | −0.06 | 0.09 |
| Birth control pill usage | −0.02 | 0.66 | 0.03 | 0.41 |
Weight retention was analyzed as a continuous variable
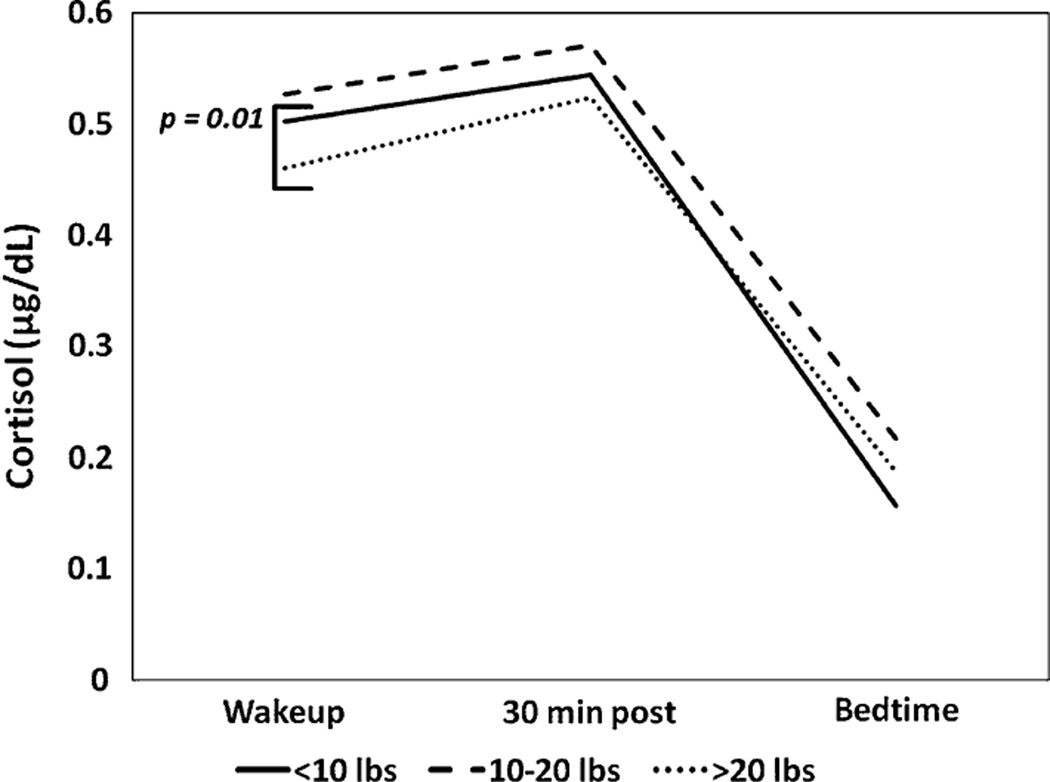
We evaluated the relationship between weight retention and cortisol slope, and found a marginal correlation between weight retention during the postpartum period and cortisol slope (r2 = 0.07, p = 0.06), where women with more weight retention had flatter slopes. Using ordinal categories for weight retention (<10, 10–20, >20 lbs), cortisol slopes differed between the groups [F(2,693) = 4.21, p < 0.05]. Tukey post hoc tests demonstrated that women with the greatest weight retention (>20 lbs) had significantly flatter slopes (−0.09 ± 0.09) compared to women with the least weight retention (<10 lbs) (−0.11 ± 0.08, p = 0.01) (Fig. 1).
Fig. 1.
Average cortisol slope by weight retention category (<10, 10–20, >20 lbs) is displayed. There was a significant differences in the slopes of women who retained <10 lbs compared to women who retained >20 lbs (p = 0.01)
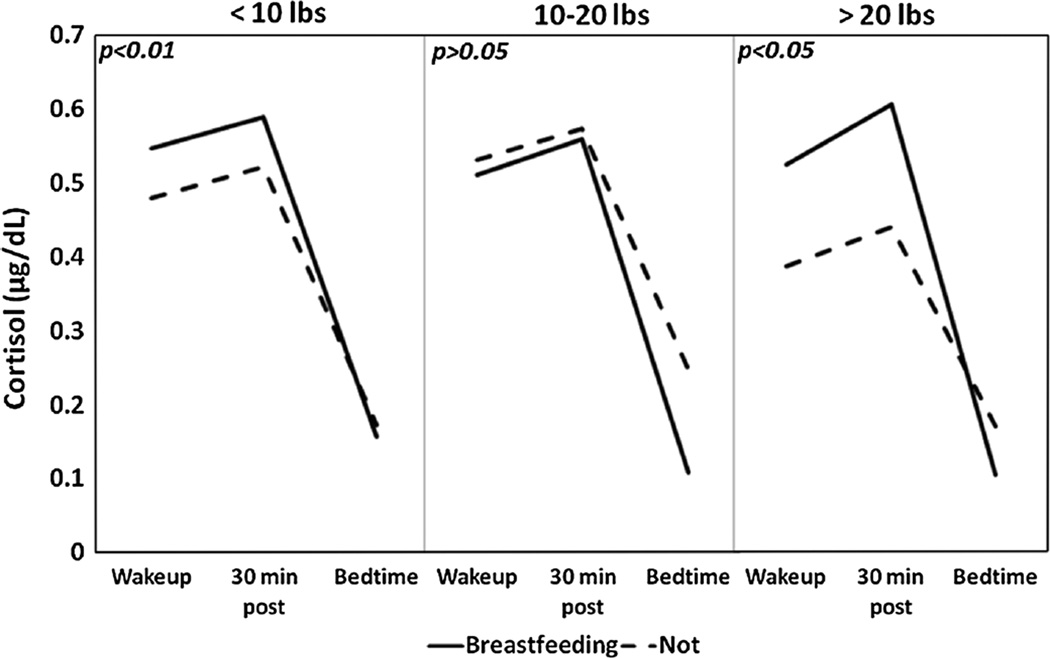
Breastfeeding at 6 months was associated with steeper cortisol slopes [F(1,694) = 20.48, p < 0.01]. When we evaluated the relationship between breastfeeding at 6 months and a steeper cortisol slope by weight retention categories (<10, 10–20, >20 lbs) we found that the relationship was significant in only the <10 lb weight retention category [F(1,363) = 11.72, p < 0.01] and the >20 lbs weight retention category [F(1, 172) = 5.25, p < 0.05)] (Fig. 2). Interaction testing was not significant and thus we did not find effect modification. Of note, African American women had the flattest average slope compared to Hispanic-Spanish speaking women, White women and Hispanic-English speaking women [F(3,692) = 19.92, p < 0.01]. These differences may be explained in part by lower breastfeeding rates in African American women as demonstrated in our descriptive analysis.
Fig. 2.
For each weight retention category (<10, 10–20 and >20 lbs) the average cortisol slope is displayed by breastfeeding status at 6 months. p values are reported in the upper left corner of the respective graphs. Women who breastfed at 6 months in the <10 and >20 lbs categories had significantly steeper slopes than women who were not breastfeeding (p < 0.01 and p < 0.05 respectively)
We analyzed the relationship between race/ethnicity and insurance status and found them to be strongly correlated. Only 31.0 % White women had public insurance compared to 72.7 % Hispanic-English speaking women, 80.4 % African American women, and 93.6 % Hispanic-Spanish speaking women (p <0.01). Therefore, we assessed multiple models including public insurance, race/ethnicity or both public insurance and race/ethnicity in our regression models. The interaction between insurance status and race/ethnicity did not achieve statistical significance in either the ordinary least squares or the ordinal logistic regressions.
We created regression models with weight retention as a three-categorical variable (<10, 10–20, or>20 lbs) shown in Table 3. First maternal demographic variables (pre-pregnancy BMI and age) and cortisol slope were regressed on maternal weight retention using ordinal logistic regression (Model 1). All three variables appeared significant and cortisol slope had the largest effect (β = 1.96, 95 % CI = 0.29–3.63). In Model 2, public health insurance was added and all variables remained significantly associated with weight retention. However, the effect of both age and cortisol slope appeared to be reduced suggesting that there may be an un-measured relationship between age, insurance status and cortisol slope. In Model 3, public health insurance was removed and substituted with race/ethnicity. Now, pre-pregnancy BMI, young age and African American race were significantly associated with postpartum weight retention. Of note, in this model, cortisol slope was no longer significantly correlated with weight retention. This finding suggests that race/ethnicity has a stronger correlation to postpartum weight retention than the cortisol slope and that the changes in the cortisol slope related to weight retention in Models 1 and 2 may be partially influenced by race/ethnicity. When both public health insurance and race/ethnicity were included (Model 4), public health insurance and African American race were significantly associated with weight retention, while cortisol slope was not. In Model 5, cortisol covariates were added in addition to the variables from Model 2 (Pre-pregnancy BMI, age, insurance status and cortisol slope). While age and public health insurance remained significantly associated with weight retention, cortisol slope was now marginally correlated, and age was no longer significant. Also, breastfeeding was significantly correlated with less weight retention. This model suggests that some of the correlation we had previously noted (Models 1 and 2) between flattened cortisol slope and weight retention may be influenced by breastfeeding rates. All variables were included in Model 6 which revealed significant associations between both public health insurance and breastfeeding with weight retention, and marginal correlations between pre-pregnancy BMI and African American race and weight retention. This final model suggests that breastfeeding (β = −0.63, 95 % CI = −1.01 to −0.24) and public insurance (β = 0.62, 95 % CI = 0.20–1.04) were the two strongest correlates of weight retention. Interestingly, these variable appear to be in almost direct opposition to each-other. When models were replicated using weight retention as a continuous variable, only breastfeeding remained significant. (β = −3.29, 95 % CI = −4.88 to −1.76).
Table 3.
Ordinal logistic regression models predicting postpartum weight retention in women at 6 months postpartum
| Model 1 |
Model 2 |
Model 3 |
Model 4 |
Model 5 |
Model 6 |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OR | 95 % CI | OR | 95 % CI | OR | 95 % CI | OR | 95 % CI | OR | 95 % CI | OR | 95 % CI | |
| Pre-pregnancy BMI |
1.02a | 1.01–1.10 | 1.02a | 1.00–1.04 | 1.02a | 1.00–1.04 | 1.02 | 1.00–1.03 | 1.02a | 1.00–1.04 | 1.01 | 1.00–1.03 |
| Age | 0.94b | 0.92–0.97 | 0.97a | 0.94–1.00 | 0.96b | 0.93–0.99 | 0.99 | 0.96–1.02 | 0.98 | 0.95–1.01 | 0.99 | 0.96–1.03 |
| Public insurance | 1.88b | 1.29–2.74 | 2.11b | 1.40–3.18 | 1.68b | 1.15–2.46 | 1.86b | 1.22–2.82 | ||||
| Hispanic- Spanishc |
0.90 | 0.55–1.45 | 0.63 | 0.38–1.07 | 0.71 | 0.42–1.20 | ||||||
| Hispanic- Englishc |
1.70 | 0.98–2.98 | 1.50 | 0.85–2.65 | 1.51 | 0.85–2.69 | ||||||
| African- American |
1.84b | 1.24–2.72 | 1.54a | 1.03–1.09 | 1.47 | 0.97–2.21 | ||||||
| Cortisol slope | 7.10a | 1.34–37.80 | 6.69a | 1.25–35.77 | 3.62 | 0.63–20.64 | 3.47 | 0.60–20.00 | 4.34 | 1.29–24.29 | 2.63 | 0.44–5.75 |
| Wake time | 1.04 | 0.94–1.15 | 1.01 | 0.92–1.12 | ||||||||
| Smoking | 1.30 | 0.86–1.95 | 1.25 | 0.82–1.89 | ||||||||
| Breastfeeding | 0.49b | 0.33–0.72 | 0.53b | 0.36–0.79 | ||||||||
|
Cox & Snell Pseudo-R2 |
0.05 | 0.07 | 0.07 | 0.09 | 0.09 | 0.11 | ||||||
| −2 log Likelihood |
1385.69 | 1374.82 | 1370.55 | 1357.71 | 1356.57 | 1345.10 | ||||||
p ≤ 0.05
p ≤ 0.01
Hispanic patients were categorized as primarily Spanish-speaking or English-speaking
Interactions were assessed and there were no significant interactions among independent variables and covariates in any of the models.
Comment
The relationship between changes in the cortisol slope and postpartum weight retention appears complex. Flatter cortisol slopes (associated with less optimal health outcomes) were seen in women who gained >20 lbs compared to those who gained <10 lbs. In the multivariate regression model which included BMI, age, and public insurance status, we found a significant correlation between flatter cortisol slope and weight retention. However, the effect of both age and cortisol slope appeared to be reduced suggesting that there may be an un-measured relationship between age, insurance status and cortisol slope. When race/ethnicity was included into the models, the correlation disappeared. This finding suggests that cortisol slope is not a strong marker for weight retention and is likely subject to racial/ethnic variation.
Our findings were similar to other studies which evaluated postpartum weight retention and studies which evaluated racial/ethnic variations in cortisol slope. In our study, women who retained weight were more likely to be young, African American women with public health [10, 27]. Our findings were also consistent with other studies with regard to racial/ethnic variations in cortisol slope which may be partially explained by socio-economic status [5, 6]. Cohen and colleagues found that lower socioeconomic status (SES) (defined either by income or education level) was associated with flatter cortisol slopes in young, overweight women. In addition, they hypothesized that these relationships may be due to either allelic variation in glucocorticoid receptors, possible unmeasured stress or depression, or other unmeasured factors. Likewise, Desantis and colleagues found that African American and Hispanic teenagers had flatter slopes when compared to White teenagers. In her study, socio-environmental factors studied did not explain the observed racial/ethnic differences in cortisol slope. Taken together, these findings highlight that young, women from racial minorities may be particularly vulnerable to both peripartum weight retention and abnormalities in cortisol slope.
We found that cortisol slope was significantly associated with both weight retention and breastfeeding in the lowest (<10 lbs) and highest (>20 lbs) weight retention categories. These findings suggest a U-shaped relationship between weight retention and cortisol slope. A similar pattern of U-shaped relationship between BMI and cortisol slope was noted by Kumari et al. [15] where both participants with abnormally low and abnormally high BMIs had flatter slopes. In addition, our findings of a flattened cortisol slope in women with >20 lbs retention is similar to other studies that correlated postpartum weight retention and other biomarkers of poorer health including an atherogenic lipid profile [23].
Perhaps our most interesting findings involve the role of breastfeeding and its relationship with weight retention and cortisol slope. Breastfeeding at 6 months was correlated with a more favorable cortisol slope in women with the greatest weight retention. In addition, breastfeeding was significantly associated with less weight retention in all models where it was assessed. Part of this relationship may be related to varying breastfeeding rates based on race/ethnicity. These findings also correspond to emerging evidence that breastfeeding may prevent the development of obesity and cardio-metabolic disorders [31]. Wiklund and colleagues theorized that pregnancy induced fat deposition may be partially reversed due to the “fluctuating web of hormones” related to breastfeeding through increased prolactin which leads to (1) decreased estrogen and mobilization of adipose tissue, (2) inhibition of lipo-genesis, and (3) decreased uptake of glucose in adipose tissue.
Our study has several limitations. Like other epidemiologic studies that evaluate salivary cortisol levels, there may be unmeasured variables or other covariates that underlie the relationships noted [1]. In addition, we do not have detailed information regarding exercise or dietary habits of the participants as these were not included in the original study. Also, the salivary samples for this report were only collected on 1 day at the 6 month postpartum time point and do not reflect longitudinal changes. Finally, breastfeeding was not limited to women who were exclusively breastfeeding which may have influenced the strength of the association between cortisol slope, breastfeeding and weight retention [28].
There were several strengths to our study. These data were drawn from a large, diverse, multi-site prospective cohort. The data collection was designed on the principles of community-based participatory research with an over-sampling of ethnic minority and low socioeconomic status women. This strategic sampling allows for more robust data and controls for common confounders. Unlike many retrospective studies, in our study postpartum weight was objectively measured rather than subjectively reported.
Our study adds to the growing body of literature that suggests certain groups of women, particularly young, African American women with public insurance, are at high risk for postpartum weight retention and adverse health outcomes. Our study also underscores the complex relationship between race/ethnicity, weight retention, breastfeeding, and cortisol slope. However, cross-sectional studies, like ours, may not fully capture chronic or longstanding alterations on the HPA axis. As such, a longitudinal prospective study to evaluate postpartum weight retention, across racial-ethnic groups and the changes in the HPA axis would greatly enhance our knowledge in these critical elements of women’s health care.
Significance.
Diurnal cortisol slope is a marker for stress and aberrations in cortisol slope have been associated with obesity. Postpartum weight retention is also linked to both ante- and post-partum obesity. In this study, we explore the complex relationship between post-partum weight retention and aberrations in diurnal cortisol slope. The associations between altered cortisol slope and weight retention appear to be influenced by breastfeeding. Interaction testing was not significant between these factors. Interestingly, breastfeeding has been linked to both improved psychological well-being and post-partum weight loss and may serve as a potentially modifiable factor to improve health outcomes for post-partum women.
Acknowledgments
The Community Child Health Network is supported through cooperative agreements with the Eunice Kennedy Shriver National Institute of Child Health and Human Development (U HD44207, U HD44219, U HD44226, U HD44245, U HD44253, U HD54791, U HD54019, U HD44226-05S1, U HD44245-06S1, R03 HD59584) and the National Institute for Nursing Research (U NR008929).
Appendix
This paper is designated a Core Paper of the Child Community Health Network (CCHN), because it reflects major ideas and work considered central to our network. Accordingly, the last designated author is the network itself preceded by the names of those on the writing team who directly prepared this paper in the order that best reflects their relative contributions. Members of each site are listed below.
Baltimore, MD: Baltimore City Healthy Start, Johns Hopkins University
Community PI: M. Vance
Academic PI: C. S. Minkovitz; Co-Invs: P. O’Campo, P. Schafer
Project Coordinators: N. Sankofa, K. Walton
Lake County, IL: Lake County Health Department and Community Health Center, the North Shore University Health System
Community PI: K. Wagenaar
Academic PI: M. Shalowitz
Co-Invs: E. Adam, G. Duncan*, A. Schoua-Glusberg, C. McKinney, T. McDade, C. Simon
Project Coordinator: E. Clark-Kauffman
Los Angeles, CA: Healthy African American Families, Cedars-Sinai Medical Center, University of California, Los Angeles
Community PI: L. Jones
Academic PI: C. Hobel; Co-PIs: C. Dunkel Schetter, M. C. Lu; Co-I: B. Chung
Project Coordinators: F. Jones, D. Serafin, D. Young
North Carolina: East Carolina University, NC Division of Public Health, NC Eastern Baby Love Plus Consortium, University of North Carolina, Chapel Hill
Community PIs: S. Evans, J. Ruffin, R. Woolard
Academic PI: J. Thorp; Co-Is J. DeClerque, C. Dolbier, C. Lorenz
Project Coordinators L. S. Sahadeo, K. Salisbury
Washington, DC: Virginia Tech Carilion Research Institute, Virginia Tech, Washington Hospital Center, Developing Families Center
Community PI: L. Patchen
Academic PI: S. L. Ramey; Academic Co-PI R.Gaines Lanzi
Co-Invs: L. V. Klerman, M. Miodovnik, C. T. Ramey, L. Randolph
Project Coordinator: N. Timraz
Community Coordinator: R. German
Data Coordination and Analysis Center DCAC (Pennsylvania State University)
PI: V. M. Chinchilli
Co-Invs: R, Belue, G. Brown Faulkner*, M, Hillemeier, I. Paul, M. L. Shaffer
Project Coordinator: G. Snyder
Biostatisticians: E. Lehman, C. Stetter
Data Managers: J. Schmidt, K. Cerullo, S. Whisler
Programmers: J. Fisher, J, Boyer, M. Payton
NIH Program Scientists: V. J. Evans and T. N.K. Raju, Eunice Kennedy Shriver National Institute of Child Health and Human Development; L. Weglicki, National Institute of Nursing Research, Program Officers: M. Spittel* and M. Willinger, NICHD; Y. Bryan,* NINR.
Steering Committee Chairs: M. Phillippe (University of Vermont) and E. Fuentes-Afflick* (University of California - San Francisco School of Medicine)
*Indicates those who participated in only the planning phase of the CCHN.
Footnotes
This study was presented in part at the 34th Annual Society for Maternal Fetal Medicine meeting Feb. 3–8, 2014 New Orleans, LA.
The members at each site of the Child Community Health Network are listed in the “Appendix”.
Compliance with Ethical Standards
Conflict of interest The authors have no conflict of interest to report.
References
- 1.Adam EK, Kumari M. Assessing salivary cortisol in large-scale, epidemiological research. Psychoneuroendocrinology. 2009;34(10):1423–1436. doi: 10.1016/j.psyneuen.2009.06.011. [DOI] [PubMed] [Google Scholar]
- 2.Aldabal L, Bahammam AS. Metabolic, endocrine and immune consequences of sleep deprivation. The Open Respiratory Medicine Journal. 2011;5:31–43. doi: 10.2174/1874306401105010031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Badrick E, Kirschbaum C, Kumari M. The relationship between smoking status and cortisol secretion. The Journal of Clinical Endocrinology & Metabolism. 2007;92:819–824. doi: 10.1210/jc.2006-2155. [DOI] [PubMed] [Google Scholar]
- 4.Bjorntorp P, Rosmond R. Obesity and cortisol. Nutrition. 2000;16:924–936. doi: 10.1016/s0899-9007(00)00422-6. [DOI] [PubMed] [Google Scholar]
- 5.Cohen S, Schwartz JE, Epel E, Kirschbaum C, Sidney S, Seeman T. Socioeconomic status, race and diurnal cortisol decline in the coronary artery risk development in young adults (CARDIA) study. Psychosomatic Medicine. 2006;68:41–50. doi: 10.1097/01.psy.0000195967.51768.ea. [DOI] [PubMed] [Google Scholar]
- 6.DeSantis AS, Adam EK, Doane LD, Mineka S, Zinbarg RE, Craske MG. Racial/ethnic differences in cor-tisol diurnal rhythms in a community sample of adolescents. Journal of Adolescent Health. 2007;41:3–13. doi: 10.1016/j.jadohealth.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 7.Endres LK, Straub H, McKinney C, Plunkett BA, Min-kovitz CS, Schetter CD, et al. Postpartum weight retention risk factors and relationship to obesity at 1 year. Obstetrics and Gynecology. 2015;125(1):144–152. doi: 10.1097/AOG.0000000000000565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ferrari E, Cravello L, Muzzoni B, Casarotti D, Paltro M, Solerte S, et al. Age-related changes of the hypothala-mic-pituitary-adrenal axis: Pathophysiological correlates. European Journal of Endocrinology. 2001;144:319–329. doi: 10.1530/eje.0.1440319. [DOI] [PubMed] [Google Scholar]
- 9.Gunnar MR, Vazquez D. Low cortisol and a flattening of the expected daytime rhythm: Potential indices of risk in human development. Development and Psychopathology. 2001;13:515–538. doi: 10.1017/s0954579401003066. [DOI] [PubMed] [Google Scholar]
- 10.Headen IE, Davis EM, Mujahid MS, Abrams B. Racial-ethnic difference in pregnancy-related weight. Advances in Nutrition. 2012;3(1):83–94. doi: 10.3945/an.111.000984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Institute of Medicine (US) and National Research Council (US) Committee to Reexamine IOM Pregnancy Weight Guidelines. Institute of Medicine (Committee to Reexamine IOM Pregnancy Weight Guidelines, Food and Nutrition Board and Board on Children, Youth, and Families) In: Rasmussen KM, Yaktine AL, editors. Weight gain during pregnancy: Reexamining the guidelines. Washington, DC: National Academy Press; 2009. [Google Scholar]
- 12.Issar NM, Sethi MK. Health disparities. In: Sethi MK, Frist WH, editors. An introduction to health policy: A primer for physicians and medical students. New York, NY: Springer; 2013. pp. 126–127. [Google Scholar]
- 13.Kirschbaum C, Hellhammer DH. Salivary cortisol in psychobiological research: An overview. Neuropsychobiology. 1989;22(3):150–169. doi: 10.1159/000118611. [DOI] [PubMed] [Google Scholar]
- 14.Kudielka BM, Kirschbaum C. Awakening cortisol responses are influenced by health status and awakening time but not by menstrual cycle phase. Psychoneuroendocrinology. 2003;28:35–47. doi: 10.1016/s0306-4530(02)00008-2. [DOI] [PubMed] [Google Scholar]
- 15.Kumari M, Candola T, Brunner E, Kivimaki M. A non-linear relationship of generalized and central obesity with diurnal cortisol secretion in the Whitehall II study. Journal of Clinical Endocrinology and Metabolism. 2010;95(9):4415–4423. doi: 10.1210/jc.2009-2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marin P, Darin N, Amemiya T, Andersson B, Jern S, Bjorntorp P. Cortisol secretion in relation to body fat distribution in obese premenopausal women. Metabolism. 1992;41:882–886. doi: 10.1016/0026-0495(92)90171-6. [DOI] [PubMed] [Google Scholar]
- 17.National Institutes of Health. National Institutes of Health Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults—The evidence report. Obesity Research. 1998;6:51S–209S. [PubMed] [Google Scholar]
- 18.Neville CE, McKinley MC, Holmes VA, Spence D, Woodside JV. The relationship between breastfeeding and postpartum weight change—A systematic review and critical evaluation. Journal of Obesity. 2014;38(4):577–590. doi: 10.1038/ijo.2013.132. [DOI] [PubMed] [Google Scholar]
- 19.Ogden CL, Carroll MD, Kit BK, Flegal KM. [Accessed April 21, 2013];Prevalence of obesity in the United States; 2009–2010. 2010 NCHS data brief no. 82. http://www.cdc.gov/nchs/data/databriefs/db82.pdf.
- 20.Pasquali R, Cantobelli S, Casimirri F, Capelli M, Bortoluzzi L, Flamia R. The hypothalamic-pituitary-adrenal axis in obese women with different patterns of body fat distribution. Journal of Clinical Endocrinology and Metabolism. 1993;77:341–346. doi: 10.1210/jcem.77.2.8393881. [DOI] [PubMed] [Google Scholar]
- 21.Pasquali R. The hypothalamic-pituitary-adrenal axis and sex hormones in chronic stress and obesity: Pathophysiological and clinical aspects. Annals of the New York Academy of Sciences. 2012;1264:20–35. doi: 10.1111/j.1749-6632.2012.06569.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Patel SR, Hu FB. Short sleep duration and weight gain: A systematic review. Obesity. 2008;16:643–653. doi: 10.1038/oby.2007.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Puhkala J, Luoto R, Ahotupa M, Raitanen J, Vasankari T. Postpartum weight retention is associated with elevated ratio of oxidized LDL lipids to HDL-cholesterol. Lipids. 2013;48(12):1227–1235. doi: 10.1007/s11745-013-3852-9. [DOI] [PubMed] [Google Scholar]
- 24.Putignano P, Dubini A, Toja P, Invitti C, Bonfanti S, Redaelli G, et al. Salivary cortisol measures in normal-weight, obese and anorexic women: Comparison with plasma cortisol. European Journal of Endocrinology. 2001;145:165–171. doi: 10.1530/eje.0.1450165. [DOI] [PubMed] [Google Scholar]
- 25.Ramey SL, Schafer P, DeClerque JL, Lanzi RG, Hobel C, Schalowitz M, et al. The preconception stress and resiliency pathways model: A multi-level framework on maternal, paternal, and child health disparities derived by community-based participatory research. Maternal and Child Health Journal. 2015;19(4):707–719. doi: 10.1007/s10995-014-1581-1. [DOI] [PubMed] [Google Scholar]
- 26.Rode L, Kjaegaard H, Ottesen B, Damm P, Hegaard HK. Association between gestational weight gain according to body mass index and postpartum weight in a large cohort of Danish women. Maternal and Child Health Journal. 2012;16:406–413. doi: 10.1007/s10995-011-0775-z. [DOI] [PubMed] [Google Scholar]
- 27.Rothberg BEG, Magriples U, Kershaw TS, Rising SS, Ickovics JR. Gestational weight gain and post-partum weight loss among young, low-income, ethnic minority women. American Journal of Obstetrics and Gynecology. 2011;204(1):52.e1, 52.e52. doi: 10.1016/j.ajog.2010.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tu MT, Lupien SJ, Walker CD. Diurnal salivary cortisol levels in postpartum mothers as a function of infant feeding choice and parity. Psychoneuroendocrinology. 2006;31(7):812–824. doi: 10.1016/j.psyneuen.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 29.Turner MJ, Layte R. Obesity level is a national cohort of women 9 months after delivery. American Journal of Obstetrics and Gynecology. 2013;209:124.e1–124.e7. doi: 10.1016/j.ajog.2013.04.020. [DOI] [PubMed] [Google Scholar]
- 30.Vreeburg SA, Kruijtzer BP, van Pelt J, van Dyck R, DeRijk RH, Hoogendijk WK, et al. Associations between sociodemographic, sampling and health factors and various salivary cortisol indicators in a large sample without psychopathology. Psychoneuroendocrinology. 2009;34:1109–1120. doi: 10.1016/j.psyneuen.2009.04.024. [DOI] [PubMed] [Google Scholar]
- 31.Wiklund P, Xu L, Lyytikainen A, Saltevo J, Wang Q, Volgyi E, et al. Prolonged breast-feeding protects mothers from later-life obesity and related cardio-metabolic disorders. Public Health Nutrition. 2012;15(1):67–74. doi: 10.1017/S1368980011002102. [DOI] [PubMed] [Google Scholar]
- 32.Williamson DF, Kahn HS, Remington PL, Anda RF. The 10-year incidence of overweight and major weight gain in US adults. Archives of Internal Medicine. 1990;150(3):665–672. [PubMed] [Google Scholar]