Abstract
Objective
Since HIV impairs gut barriers to pathogens, HIV-infected adults may be vulnerable to Minimal Hepatic Encephalopathy (MHE) in the absence of cirrhosis.
Background
Cognitive disorders persist in up to one-half of people living with HIV despite access to combination antiretroviral therapy (cART). MHE occurs in cirrhotic patients with or without HIV infection and may be associated with inflammation.
Design/Methods
A cross-sectional investigation of liver fibrosis severity using the aspartate aminotransferase to platelet ratio index (APRI) and neuropsychological testing performance among women from the Women’s Interagency HIV Study (WIHS). A subset underwent liver transient elastography (FibroScan®, n=303).
Results
We evaluated 1479 women (mean (SD) age of 46 (9.3) years): 770 (52%) only HIV-infected, 73 (5%) only HCV-infected, 235 (16%) HIV/HCV co-infected, and 401 (27%) uninfected. Of these, 1221 (83%) exhibited APRI ≤0.5 (no or only mild fibrosis), 206 (14%) exhibited APRI >0.5 and ≤1.5 (moderate fibrosis), and 52 (3%) exhibited APRI >1.5 (severe fibrosis). Having moderate or severe fibrosis (APRI >0.5) was associated with worse performance in learning, executive function, memory, psychomotor speed, fluency, and fine motor skills. In these models that adjusted for fibrosis, smaller associations were found for HIV (learning and memory) and HCV (executive functioning and attention). The severity of fibrosis, measured by FibroScan®, was associated with worse performance in attention, executive functioning, and fluency.
Conclusions
Liver fibrosis had a contribution to cognitive performance independent of HCV and HIV; however, the pattern of neuropsychological deficit associated with fibrosis was not typical of MHE.
Keywords: HIV, Liver diseases, AIDS Dementia Complex, Cognition, Cirrhosis, Minimal Hepatic Encephalopathy
INTRODUCTION
Hepatic encephalopathy (HE) due to cirrhosis is characterized by broad cognitive deficits including psychomotor retardation, impairment of memory, and deficits in concentration.1 The pathogenesis of HE is thought to arise from astrocyte dysfunction due to excess levels of ammonia and other toxins, with added contributions from inflammation.2 Since astrocytes are involved in ammonia clearance through conversion of glutamine to glutamate, it is postulated that glutamine build-up leads to osmotic imbalance, cellular swelling, and astrocyte dysfunction.
An entity of “subclinical” hepatic encephalopathy was first defined in 1978, occurring in cirrhotic patients who appeared normal but had measurable neuropsychological test abnormalities.3 This condition, later termed Minimal Hepatic Encephalopathy (MHE), occurs intermittently in up to two-thirds of patients with cirrhosis.4 In these patients who lack obvious encephalopathy, subtle cognitive dysfunction may intermittently impact daily activities including driving.5,6 Commonly observed deficits in neuropsychological testing involve psychomotor speed, information processing, and attention or vigilance.7,8 It is typically a diagnosis of exclusion among individuals with chronic liver disease as there are no diagnostic tests to confirm MHE.
Precipitating factors for MHE include those that burden liver processing such as gastrointestinal bleeding, alcohol use, infection, portal vein thrombosis, dehydration, and renal failure.9 These triggers putatively add inflammation and toxins or decrease clearance of toxins that can impact the central nervous system (CNS). The pattern of neuropsychological deficits seen with MHE overlaps with that described among patients living with chronic HIV infection with both conditions characterized by fluctuation and including impaired executive function.10,11 Together, this information provides a rationale for evaluating the independent contributions of liver fibrosis in the setting of HIV. The Women’s Interagency HIV Study (WIHS) offers a unique opportunity to test this hypothesis in a population with substantial hepatitis C virus (HCV) co-infection.
We hypothesized that both the inflammation associated with chronic HIV infection and the HIV-associated loss of intestinal gut protective barriers resulting in gut microbial translocation could add risk for MHE in patients with chronic HIV infection.12 If so, liver fibrosis, even in the absence of cirrhosis, would correlate with neuropsychological test performance, independent of HIV or HCV. Such a finding would add further support to shifting paradigms related to cognition in HIV where greater etiologic heterogeneity is emerging.13 These analyses are timely since neuropsychological testing impairment remains prevalent despite access to effective combination antiretroviral therapy (cART).
METHODS
Participant selection
The WIHS is a multicenter longitudinal observational cohort of HIV-infected and uninfected women enrolled from one of six U.S. sites: New York (Bronx and Brooklyn), California (Los Angeles and San Francisco), Washington DC, and Chicago.14 At the time of enrollment into WIHS, participants were free of known dementia and able to attend an outpatient study visit. All signed Institutional Review Board-approved consent forms.
An extended neuropsychological testing battery was added to the WIHS exam in 2009 with all testing completed by April 2011. Of the active English-speaking participants (n=1,908), 1,595 (84%) completed the test battery of whom 1547 had concurrent blood work to calculate the fibrosis marker and the necessary key covariates. We included 1479 participants (1005 HIV-infected) in the analysis after excluding 68 participants meeting one or more of the following exclusion criteria: a) conditions that limit test validity (e.g., hearing loss, impaired vision, immediate influence of illicit substances, n=11); b) history of stroke (n=10); and c) self-reported use of antipsychotic medication in the previous 6 months (n=47).
Cognitive characterization
The neuropsychological battery was designed to capture domains impacted by HIV infection.10 A global score of all cognitive tests served as one primary outcome. To minimize the effect of multiple comparisons, we looked at domain scores first and explored individual tests within domains chiefly when the domain score was significant. We also identified primary and secondary outcomes a priori. Individual tests allowed us to evaluate seven cognitive domains, including four also used as primary outcomes based on existing data linking them to MHE: (1) Verbal Learning [Hopkins Verbal Learning Test (HVLT) Trial 1 (single trial learning) and HVLT Total Learning (total words recalled across each of three learning trials)]; (2) Attention and Concentration [Stroop Trials 1 and 2 (average time to complete each trial), Trail Making Test Part A (time to complete), and control condition from Letter Number Sequencing (LNS) (total correct)]; and (3) Executive Function [Stroop Trial 3 (time to completion), Trail Making Test Part B (time to complete), and working memory condition of LNS (total correct)]. Secondary outcomes were those not describe to be linked to MHE and include: (1) Verbal Memory [HVLT delayed recall (total words recalled after 25-minute delay) and percent retention (delayed recall/maximum score on Trial 2 or 3)]; (2) Psychomotor Speed [Symbol Digit Modalities Test (total number correct within 90 seconds)]; (3) Verbal Fluency [Letter Fluency (total words generated in response to the letters F, A, and S) and a Category Fluency Task (total words generated in response to the category of animals)]; and (4) Fine Motor Skills [Grooved Pegboard Test (average time to complete dominant and non-dominant hands)]. All testers were internally certified through structured training and quality assurance. We used the Wide Range Achievement Test (WRAT-3) to estimate education quality.
Clinical variables
We confirmed HIV status with FDA-approved enzyme-linked immunosorbent assays and re-confirmed with western blot assays when immunosorbent assays were positive. Chronic hepatitis C infection was defined using HCV antibody testing from each woman’s enrollment visit into the WIHS and with subsequent confirmatory HCV RNA testing, when available. Women were classified as HCV-uninfected if the HCV antibody was negative or if the HCV RNA level was undetectable among those with positive HCV antibody. Participants who were HCV antibody positive with detectable HCV RNA were coded as HCV-infected. Those with untested HCV RNA (n=30) were also coded as HCV infected, since spontaneous clearance of HCV is uncommon in the setting of HIV infection and since HCV treatment and cure was uncommon during this calendar time frame. Other variables included in the analysis and measured concurrently with the cognitive testing included heavy alcohol use, defined as reporting >7 drinks per week or more than 4 drinks in one sitting, and hypertension, defined as having a systolic blood pressure >140 mmHg, diastolic blood pressure > 90 mmHg, or self-reported use of anti-hypertensive medications.
Definition of Liver Fibrosis
The aspartate aminotransferase to platelet ratio index (APRI) is an indirect serum fibrosis marker combining standard laboratory tests ((AST/upper limit of normal AST)*100)/platelet count). APRI has been studied in large cohorts of HIV-mono-infected, HCV-mono-infected, and HIV/HCV-co-infected patients and can be used to distinguish mild fibrosis from significant fibrosis and cirrhosis.15,16 We categorized APRI using validated cutoffs: ≤0.5 (mild or no fibrosis); >0.5 to ≤1.5 (moderate fibrosis); and >1.5 (severe fibrosis). For our analyses, moderate and severe fibroses were combined as significant fibrosis (i.e. at least moderate fibrosis) since the sample size for severe fibrosis was small (n=52).
In a separate study embedded from three sites in the WIHS (Chicago, San Francisco and DC), 381 women underwent transient elastography (FibroScan®, Echosens, Paris, France) to estimate liver fibrosis by measuring liver stiffness by ultrasound, of which 315 had valid readings within 6 months of neuropsychological testing.17 We again excluded participants for one or more of the following criteria: a) conditions that limit test validity (e.g. hearing loss, impaired vision, or immediate influence of illicit substances, n=1); b) history of stroke (n=11); and c) self-reported use of antipsychotic medication in the past 6 months (n=1) resulting in 303 evaluable FibroScan®-neuropsychological testing pairs. Due to FibroScan® timing, many cases were completed concurrently with the second (n=140; 46%) and third (n=22; 7%) time a participant underwent comprehensive neuropsychological testing.
Statistical analyses
Given the absence of published cognitive norms for low-income minority women, we followed Heaton et al. (1991) and prior work in the WIHS, using a regression-based approach to create demographically corrected normative standards (T-scores) based on a larger sample of HIV-uninfected WIHS women (n=502).18–23 Simply, we used a regression approach to estimate premorbid levels of function for the total sample based on scores of the comparison group (HIV women). Each outcome was first regressed on age, years of education, Reading Recognition subtest from the Wide Range Achievement Test-Revised (WRAT-R), a proxy for educational quality, and race/ethnicity.22 The resulting unstandardized beta weights, constants, and standard errors were used to calculate predicted scores for each test that were then subtracted from each woman’s actual score and transformed to scores (using means of 50 and standard deviations of 10) that could be more easily compared across all cognitive outcomes. The Trail Making, Stroop, and Grooved Pegboard scores were skewed and therefore log-transformed prior to the creation of the T-scores.
We examined demographic characteristics by fibrosis status using t-tests for continuous variables and Chi-square tests for categorical variables. The Breslow Day test was used to compare whether the proportion of women with significant fibrosis differed as a function of both HIV and HCV status. We used multivariable linear regression to determine if liver fibrosis was associated with neuropsychological test performance (i.e. global performance and domain specific performance), independent of HIV and chronic HCV. In order to minimize false discoveries, we defined primary outcomes (see above) and considered associations of individual test scores (T-scores) within these domains as more exploratory. Additional multivariable linear regression models were conducted, as needed, to determine the stability for specific subsets of women (e.g. HIV mono-infected).
In the FibroScan® sub-analysis, we investigated each neurocognitive test since this analysis was conceived of as a confirmatory analysis of our primary APRI analysis. The models adjusted for important potential confounders including marijuana use, crack/cocaine and/or heroin use, smoking, heavy alcohol use, antidepressant use, annual household income, body mass index, hypertension, diabetes, study site, and number of previous cognitive test exposures. The FibroScan® readings were log transformed. Significance was defined as p<0.05 (two-sided). Analyses were performed using SAS PROC GENMOD (version 9.4, SAS Institute Inc., Cary, NC).
RESULTS
The final sample included 1,005 HIV-infected and 474 HIV-uninfected women with mean age of 47 and 44 years (p<0.001), respectively; 64% were African American. Women with significant fibrosis had a higher BMI (p<0.001) and other metabolic risk factors such as hypertension and diabetes (Table 1). They also had worse HIV parameters, such as having a lower proximal and nadir CD4 count and detectable plasma HIV RNA.
Table 1.
Demographic characteristics for participants with and without significant fibrosis.
| Background Characteristics (%) | Fibrosis
|
||
|---|---|---|---|
| Significant (n=258) | Not Significant (n=1221) | p-value | |
| Age, mean (SD) | 51.31 (7.25) | 45.11 (9.37) | <0.001 |
| WRAT-3 reading subtest, mean (SD) | 89.91 (16.34) | 92.38 (17.80) | 0.04 |
| Years of education, mean (SD) | 11.80 (2.68) | 12.55 (2.91) | <0.001 |
| HIV status, % | 217 (84) | 788 (64) | <0.001 |
| Race/Ethnicity | 0.04 | ||
| African American, non-Hispanic | 153 (60) | 794 (65) | |
| White, non-Hispanic | 34 (13) | 148 (12) | |
| Hispanic | 65 (25) | 227 (18) | |
| Other | 6 (2) | 52 (4) | |
| Annual household income ≤$12,000/year | 158 (61) | 518 (42) | <0.001 |
| Current depressive symptoms, CES-D ≥16 | 98 (38) | 352 (29) | 0.004 |
| Hepatitis C positive RNA, % | 164 (64) | 144 (12) | <0.001 |
| Recent antidepressant medication use | 46 (18) | 180 (15) | 0.21 |
| Recent alcohol use | 0.10 | ||
| Abstainer | 162 (63) | 679 (56) | |
| Not heavy | 56 (22) | 314 (26) | |
| Heavy | 40 (15) | 228 (18) | |
| Recent marijuana/hash use | 40 (15) | 214 (17) | 0.44 |
| Smoking status | <0.001 | ||
| Never | 36 (14) | 343 (28) | |
| Former | 137 (53) | 501 (41) | |
| Recent | 85 (33) | 377 (31) | |
| Recent Crack, cocaine, and/or heroin use | 22 (8) | 71 (6) | 0.10 |
| Diabetes | 71 (27) | 199 (16) | <0.001 |
| Hypertension | 135 (52) | 476 (39) | <0.001 |
| Body mass index | 28.17 (8.42) | 30.89 (8.31) | <0.001 |
| HIV Disease† | |||
| Nadir CD4 count, median (IQR) | 175.54 (130.77) | 225.51 (164.74) | <0.001 |
| CD4 Count | |||
| > 500 | 75 (34) | 432 (55) | <0.001 |
| ≥ 200 and < 500 | 95 (44) | 270 (34) | |
| < 200 | 47 (22) | 86 (11) | |
| Plasma HIV RNA | <0.001 | ||
| Undetectable | 92 (42) | 437 (55) | |
| < 10,000 | 73 (34) | 259 (33) | |
| ≥ 10,000 | 52 (24) | 92 (12) | |
| cART >95% compliance | 135 (62) | 501 (63) | 0.86 |
| APRI, median (IQR) | 1.34 (0.68) | 0.23 (0.14) | <0.001 |
Significant fibrosis=APRI values ≥0.5. Not Significant Fibrosis APRI values <0.50.
Includes HIV-infected women only. WRAT-3 = Wide Range Achievement Test Standard Score. CES-D Center for Epidemiological Studies Depression scale. “Current” refers to within the past week”; “Recent” refers to within 6 months of the most recent WIHS visit. “Former” refers to any previous use, but not in the past 6 months. cART = combination antiretroviral therapy; Heavy alcohol use = reflects >7 drinks per week or more than 4 drinks in one sitting. Undetectable=<48copies/ml. Diabetes= defined as having a self-reported diagnosis, a fasting glucose of over 125 mg/dL, or being on diabetic medications. Hypertension=defined as any indication of hypertension (systolic blood pressure ≥140, diastolic blood pressure ≥90, self-report, or use of antihypertensive medication.
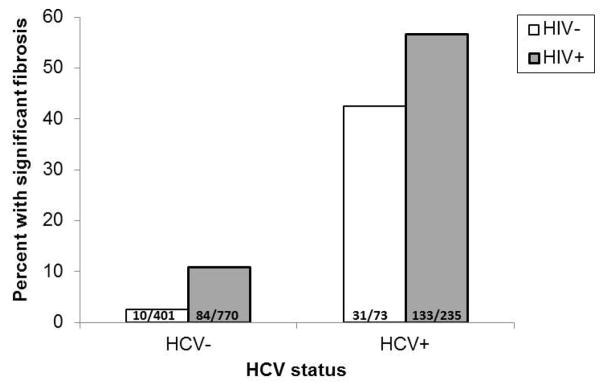
Significant fibrosis was noted in 258 (17%) of which 206 had moderate fibrosis and 52 had severe fibrosis. The proportion with significant fibrosis differed as a function of both HIV and HCV status (Breslow Day Test, p=0.02, Figure 1). Among HCV-infected women, also having HIV increased the odds of significant fibrosis (OR 4.79 95%CI 2.46–9.33, p<0.001). Among HCV-uninfected women, the same pattern was noted but the magnitude of the association was attenuated (OR 1.77 95%CI 1.04–3.00, p=0.03).
Figure 1.
Proportion of women with significant fibrosis as a function of HCV and HIV status.
Note. The proportion of women with significant liver fibrosis differed as a function of both HCV and HIV status (Breslow Day Test, p<0.05).
The association of significant liver fibrosis was demonstrated across numerous primary and secondary outcome measures (Table 2). Specifically, we found independent associations between significant fibrosis and executive functioning (primary outcome) and for verbal memory, psychomotor speed, fluency and fine motor skills (secondary outcomes). Within the executive functioning domain, the primary outcome domain meeting our threshold of significance, only the Stroop trial 3 differed. To explore the degree to which co-infection (HCV and HIV) drove our findings, we ran a series of separate multivariable models including only HIV mono-infected women (n=770). These models identified more limited effects between significant fibrosis and psychomotor speed (B=−2.81, SE=1.10, p=0.01) and a trend for effect on the global neuropsychological test (B=−1.12, SE=0.65, p=0.08).
Table 2.
Results from adjusted analyses of significant fibrosis on cognitive test performance.
| Cognitive Domains | Fibrosis | Multivariable Regression Models B (SE) | |||||
|---|---|---|---|---|---|---|---|
| Significant | Not-Significant | ||||||
|
| |||||||
| n | M (SD) | n | M (SD) | Key covariates | Key covariates+ HIV status | Key covariates+HIV and HCV status | |
| Primary Outcomes | |||||||
|
| |||||||
| Global summary score | 977 | 50.12 (5.40) | 197 | 49.00 (5.30) | −1.21 (0.42)** | −1.11 (0.42)** | −1.22 (0.45)** |
|
| |||||||
| Verbal Learning | 1214 | 49.11 (9.30) | 253 | 48.04 (9.93) | −1.49 (0.66)* | −1.25 (0.66)T | −1.24 (0.73) |
|
| |||||||
| HVLT: Trial 1 | 1214 | 48.91 (9.91) | 253 | 48.67 (10.18) | −0.79 (0.70) | −0.53 (0.70) | −0.57 (0.77) |
| HVLT: Total Trials 1–3 | 1214 | 49.31 (9.80) | 253 | 47.41 (10.78) | −2.18 (0.69)** | −1.96 (0.70)** | −1.90 (0.77)* |
|
| |||||||
| Attention and Concentration | 1070 | 49.52 (7.11) | 220 | 48.71 (8.04) | −0.76 (0.54) | −0.60 (0.55) | −0.41 (0.59) |
|
| |||||||
| Stroop Trial 1&2 | 1202 | 49.08 (10.82) | 254 | 46.42 (12.97) | −1.89 (0.79)* | −1.61 (0.80)* | −0.52 (0.88) |
| Trail Making Test Part A | 1210 | 49.85 (10.18) | 255 | 49.18 (11.11) | −0.83 (0.72) | −0.78 (0.73) | −1.09 (0.80) |
| LNS Attention | 1083 | 48.83 (10.24) | 222 | 48.48 (12.01) | −0.43 (0.78) | −0.17 (0.79) | 0.23 (0.85) |
|
| |||||||
| Executive Functions | 1021 | 50.01 (8.18) | 202 | 48.88 (8.53) | −1.40 (0.64)* | −1.39 (0.64)* | −1.38 (0.69)* |
|
| |||||||
| Stroop Trial 3 | 1165 | 49.74 (12.60) | 242 | 46.96 (13.70) | −2.43 (0.93)** | −2.36 (0.94)* | −2.19 (1.02)* |
| Trail Making Test Part B | 1186 | 49.63 (10.54) | 241 | 48.03 (11.09) | −2.14 (0.75)** | −2.14 (0.76)** | −1.39 (0.83) |
| LNS Working Memory | 1054 | 49.58 (10.76) | 216 | 48.13 (12.33) | −1.51 (0.83) | −1.52 (0.83) | −1.68 (0.90) |
| Secondary Outcomes | |||||||
|
| |||||||
| Verbal Memory | 1214 | 49.20 (9.12) | 253 | 47.18 (10.13) | −2.03 (0.66)** | −1.77 (0.66)** | −2.18 (0.73)** |
|
| |||||||
| HVLT: Delayed free recall | 1214 | 49.14 (9.81) | 253 | 46.55 (10.54) | −2.66 (0.70)*** | −2.83 (0.70)*** | −2.71 (0.77)*** |
| HVLT: Percent Retention | 1214 | 49.27 (9.79) | 253 | 47.81 (10.97) | −1.40 (0.72)T | −1.16 (0.72) | −1.65 (0.79)* |
|
| |||||||
| Psychomotor Speed | 1208 | 49.76 (9.84) | 251 | 47.53 (9.10) | −1.82 (0.69)** | −1.78 (0.69)* | −1.66 (0.77)* |
|
| |||||||
| Fluency | 1204 | 49.93 (8.77) | 254 | 48.44 (8.38) | −1.44 (0.61)* | −1.47 (0.61)* | −1.67 (0.68)* |
|
| |||||||
| Letter | 1206 | 50.11 (10.52) | 254 | 48.19 (10.03) | −1.87 (0.73)* | −1.93 (0.74)** | −2.08 (0.91)* |
| Semantic | 1204 | 49.74 (10.00) | 254 | 48.69 (9.64) | −0.97 (0.70) | −0.98 (0.71) | −1.24(0.78) |
|
| |||||||
| Fine Motor Skills | 1173 | 49.95 (9.53) | 243 | 47.32 (13.34) | −2.02 (0.73)** | −2.05 (0.74)** | −1.92 (0.81)* |
|
| |||||||
| Dominant hand | 1196 | 50.30 (9.83) | 248 | 47.22 (13.26) | −2.39 (0.75)** | −2.45 (0.75)** | −2.20 (0.83)** |
| Non-dominant hand | 1175 | 50.26 (10.26) | 243 | 48.00 (13.71) | −1.57 (0.78)* | −1.55 (0.79) | −1.50 (0.87) |
Note.
p<0.001;
p<0.01;
p<0.05.
HVLT = Hopkins Verbal Learning Test; LNS = Letter-Number Sequence; B = parameter estimates; SE= Standard Errors. All models are adjusted for the following key covariates including site, marijuana use, crack, cocaine, and/or heroin use, smoking, heavy alcohol use, antidepressants, depressive symptoms, body mass index, hypertension, diabetes, income, and number of previous cognitive test exposure.
We also evaluated the independent contributions of HIV and HCV to cognitive functioning in a model that included fibrosis plus key confounding variables (Table 3). As in our primary model, liver fibrosis (B=−1.22, SE=0.45, p=0.007) was independently associated with the omnibus global neuropsychological composite score of all neuropsychological tests where women with significant liver fibrosis had lower scores compared to women without significant fibrosis (Cohen’s d=−0.24, 95% CI −0.39 to −0.09). However, HCV did not have independent associations on any of our primary or secondary cognitive domain scores; although associations were noted for two individuals test scores (Stroop 1 & 2 and Trail Making B). HIV status was associated with both the verbal learning and verbal memory domain score, as previously described.18
Table 3. Independent contributions of HIV and HCV controlling for significant fibrosis by APRI on cognitive test performance.
Results from multivariable linear regression analyses of primary and secondary outcome measures.
| Cognitive Domains | n | Primary Predictors
|
|
|---|---|---|---|
| HIV-infection B (SE) |
Hepatitis C positive B (SE) |
||
| Primary Outcomes | |||
|
| |||
| Verbal Learning | 1467 | −1.34 (0.53)* | −0.02 (0.71) |
|
| |||
| HVLT: Trial 1 | 1467 | −1.46 (0.56)** | 0.10 (0.75) |
| HVLT: Total Trials 1–3 | 1467 | −1.23 (0.56)* | −0.13 (0.75) |
|
| |||
| Attention and Concentration | 1290 | −0.83 (0.44) | −0.50 (0.59) |
|
| |||
| Stroop Trial 1&2 | 1456 | −1.53 (0.64)* | −2.58 (0.86)** |
| Trail Making Test Part A | 1465 | −0.28 (0.58) | 0.73 (0.78) |
| LNS Attention | 1305 | −1.37 (0.63)* | −1.03 (0.84) |
|
| |||
| Executive Functions | 1223 | −0.06 (0.51) | −0.66 (0.50) |
|
| |||
| Stroop Trial 3 | 1407 | −0.32 (0.75) | −0.48 (0.73) |
| Trail Making Test Part B | 1427 | −0.07 (0.60) | −1.76 (0.81)* |
| LNS Working Memory | 1270 | 0.03 (0.67) | 0.43 (0.89) |
|
| |||
| Secondary Outcomes | |||
|
| |||
| Verbal Memory | 1467 | −1.45 (0.53)** | 0.94 (0.71) |
|
| |||
| HVLT: Delayed free recall | 1467 | −1.57 (0.56)** | 0.78 (0.79) |
| HVLT: Percent Retention | 1467 | −1.33 (0.58)* | 1.13 (0.77) |
|
| |||
| Psychomotor Speed | 1459 | −0.26 (0.56) | −0.28 (0.75) |
|
| |||
| Fluency | 1458 | 0.19 (0.49) | 0.46 (0.66) |
|
| |||
| Letter | 1460 | 0.36 (0.59) | 0.35 (0.79) |
| Semantic | 1458 | 0.07 (0.57) | 0.60 (0.76) |
|
| |||
| Fine Motor Skills | 1416 | 0.18 (0.59) | −0.30 (0.79) |
|
| |||
| Dominant hand | 1444 | 0.37 (0.60) | −0.60 (0.80) |
| Non-dominant hand | 1416 | −0.11 (0.63) | −0.12 (0.84) |
Note.
p<0.001;
p<0.01;
p<0.05.
HVLT = Hopkins Verbal Learning Test; LNS = Letter-Number Sequence; B = parameter estimates; SE= Standard Errors. All models are adjusted for APRI, site, marijuana use, crack, cocaine, and/or heroin use, smoking, heavy alcohol use, antidepressants, depressive symptoms, body mass index, hypertension, diabetes, income, and number of previous cognitive test exposure.
We evaluated liver fibrosis as measured by FibroScan® for 303 women. Fibrosis was associated with two of our secondary domain scores (fluency and fine motor skill, Table 4). Although not associated with the primary domain scores, fibrosis was associated with individual test scores in other primary and secondary domains (attention & concentration, executive functioning, and verbal memory). In models adjusted for HCV and HIV, fibrosis remained associated with the fluency domain and with several tests in the executive functioning and attention & concentration domains.
Table 4.
Results from unadjusted and adjusted analyses of liver stiffness by FibroScan® on cognitive test performance.
| Cognitive Domains | n | Unadjusted Regression Model B (SE) | Multivariable Regression Models B (SE) | ||
|---|---|---|---|---|---|
|
| |||||
| Liver Stiffness | Key covariates | Key covariates+ HIV status | Key covariates+ HIV and HCV status | ||
| Primary Outcomes | |||||
|
| |||||
| Global summary score | 255 | −0.33 (0.76) | 0.13 (0.75) | 0.07 (0.75) | 0.12 (0.80) |
|
| |||||
| Verbal Learning | 297 | −0.95 (1.56) | −1.11 (1.54) | −1.22 (1.55) | −1.34 (1.32) |
|
| |||||
| HVLT: Trial 1 | 297 | 0.84 (1.64) | 0.22 (1.59) | 0.09 (1.59) | 0.50 (1.71) |
| HVLT: Total Trials 1–3 | 297 | −2.16 (1.67) | −2.43 (1.63) | −2.52 (1.64) | −1.88 (1.76) |
|
| |||||
| Attention and Concentration | 276 | −0.46 (1.22) | −0.59 (1.19) | −0.64 (1.20) | −1.74 (1.04)T |
|
| |||||
| Stroop Trial 1&2 | 295 | −4.02 (1.27)** | −4.25 (1.29)** | −4.24 (1.29)** | −3.54 (1.42)* |
| Trail Making Test Part A | 297 | −2.44 (1.12)* | −2.04 (1.13)T | −2.01 (1.14)T | −1.00 (1.24) |
| LNS Attention | 258 | −2.18 (1.25)T | −2.59 (1.24)* | −2.59 (1.25)* | −2.25 (1.36)T |
|
| |||||
| Executive Functions | 265 | −0.84 (1.10) | −0.75 (1.12) | −0.80 (1.13) | −0.99 (1.20) |
|
| |||||
| Stroop Trial 3 | 288 | −4.45 (1.48)** | −4.03 (1.48)** | −3.92 (1.49)** | −3.78 (1.63)* |
| Trail Making Test Part B | 284 | −3.76 (1.22)** | −3.29 (1.27)** | −3.24 (1.28)* | −3.20 (1.40)* |
| LNS Working Memory | 278 | 0.03 (1.47) | 0.10 (1.51) | −0.10 (1.52) | 0.22 (1.64) |
| Secondary Outcomes | |||||
|
| |||||
| Verbal Memory | 297 | −2.23 (1.45) | −1.96 (1.44) | −2.02 (1.44) | −1.37 (1.24) |
|
| |||||
| HVLT: Delayed free recall | 297 | −2.72 (1.16)* | −2.45 (1.20)* | −2.52 (1.20)* | −2.04 (1.32) |
| HVLT: Percent Retention | 297 | −1.26 (1.12) | −0.81 (1.17) | −0.87 (1.18) | −0.72 (1.30) |
|
| |||||
| Psychomotor Speed | 294 | −2.17 (1.51) | −1.50 (1.48) | −1.64 (1.48) | −2.32 (1.26)T |
|
| |||||
| Fluency | 296 | −2.98 (0.99)** | −2.53 (0.99)* | −2.65 (0.99)** | −2.26 (1.09)* |
|
| |||||
| Letter | 297 | −2.38 (1.17)* | −1.69 (1.19) | −1.85 (1.19) | −1.28 (1.31) |
| Semantic | 296 | −3.59 (1.16)** | −3.38 (1.16)** | −3.45 (1.17)** | −3.25 (1.29)* |
|
| |||||
| Fine Motor Skills | 285 | −3.03 (1.13)** | −2.50 (1.16)* | −2.45 (1.16)* | −1.98 (1.27) |
|
| |||||
| Dominant hand | 295 | −2.72 (1.15)* | −2.28 (1.16)* | −2.29 (1.16)* | −1.84 (1.27) |
| Non-dominant hand | 285 | −3.08 (1.15)** | −2.28 (1.18)T | −2.20 (1.18)T | −1.80 (1.29) |
Note.
p<0.001;
p<0.01;
p<0.05.
HVLT = Hopkins Verbal Learning Test; LNS = Letter-Number Sequence; B = parameter estimates; SE= Standard Errors. All models are adjusted for the following key covariates including site, marijuana use, crack, cocaine, and/or heroin use, smoking, heavy alcohol use, antidepressants, depressive symptoms, body mass index, hypertension, diabetes, income, and number of previous cognitive test exposure.
In exploratory analyses, we investigated interactions between HIV status and liver fibrosis on cognition finding that liver fibrosis interacted with HIV status to influence fine motor skills (p=0.020). This interaction was in a counterintuitive direction, such that among HIV-uninfected women, women with significant fibrosis performed worse on fine motor skills than did women without significant fibrosis. However, among HIV-infected women, there was no difference in performance between women with and without significant fibrosis. When we explored interaction effects between HCV and fibrosis, we noted an effect on the executive functioning domain where, among HCV+ women, women with significant fibrosis performed worse than women without significant fibrosis (B=−3.00, SE=1.05, p=0.004). Among HCV− women, women with significant and not significant fibrosis performed similar (B=−0.17, SE=0.90, p=0.85). The same significant interaction was seen on both Trail Making Test Part B (p=0.020) and on the Delayed free recall portion of the HVLT (p=0.040). Trends for interactions were noted on LNS working memory (p=0.060) and semantic fluency (p=0.06). These were deemed exploratory since sample size for HCV− women with significant fibrosis was only 94.
DISCUSSIONS
Findings from this study provide evidence for an alternative mechanistic pathway of cognitive dysfunction in patients living with HIV as a chronic illness regardless of access to cART, a finding that is most important for HCV dual-infected individuals since they have the highest burden of fibrosis. We find that significant liver fibrosis, as measured by APRI, is associated with worse neuropsychological testing performance and that these associations are independent of both HIV and HCV. Together, these data buttress the contention that cognitive dysfunction represents a set of factors that converge in chronically infected patients.
Our findings are unlikely due to cirrhosis alone since most cases with “significant” fibrosis had an APRI score < 2 (217/258, 84%) a threshold previously linked to cirrhosis.15 We did not have sufficient cases with APRI >2 to include this as a separate analysis. Although not meeting statistical significance, the beta coefficients for the impact of APRI in both moderate and severe degrees of fibrosis were in the same direction. The identified associations despite less severe fibrosis would support the hypothesis that patients with HIV are at higher risk for MHE; however, the pattern of cognitive impairment identified here is not typical of that described in MHE. This raises suspicion that mechanisms may not fully overlap. The role of inflammation cannot be excluded either since both fibrosis and cognitive impairment have been linked to this common mechanism. Combining measures of gut permeability with that of fibrosis could be informative.
In a sub-sample of participants, our APRI analyses are bolstered by ultrasound measures of fibrosis using FibroScan®, which has an area under the curve (AUC) of 0.81 for predicting significant fibrosis based on biopsies among patients with HCV.24 Although there is incomplete overlap regarding individual cognitive domains when compared to our main analyses, the beta coefficients are in the same direction and differences in significance may reflect smaller sample sizes. We used the APRI rather than fibrosis-4 (Fib-4) index, a second calculation of fibrosis involving serological measures available in this cohort. This strengthens our work since both the Fib-4 index and neuropsychological testing are strongly influenced by age. In contrast, age is not a factor of the APRI calculation.
In the era of cART, cognitive impairment in HIV more frequently involves tests that evaluate more complex cognitive function, including executive dysfunction.11,25 In a separate analysis from the WIHS, women were noted to have more prominent deficits in verbal memory than has typically been described in predominantly male cohorts.18 Not surprisingly, our analyses, which included approximately the same individuals as this prior WIHS study, broadly recapitulated these HIV-associated findings even when adjusted for fibrosis.
While HCV has a substantial impact on morbidity and mortality, the impact on cognition in the setting of HIV is less certain, in part owing to methodological differences, small sample sizes, and insufficient cognitive testing batteries.26–29 Limitations in everyday function associated with deficits in neuropsychological testing performance have been documented in co-infected participants.30 But one recent study (n=1582) including subjects enrolled at US academic centers noted no substantial differences in cognitive performance when comparing HCV infected and uninfected participants living with chronic HIV.31 Similarly, we find only limited effects of HCV in our cohort, but note substantial associations with liver fibrosis instead. This reinforces the idea that the degree of liver fibrosis is a more important predictor of cognitive performance than HCV status.
There have been two previous studies of HCV and cognition in the WIHS, each noting no effects of HCV in properly adjusted models.27,32 One evaluated a substantially smaller sample in which HCV-related findings did not withstand adjustment for age; the other relied upon an abbreviated neuropsychological testing battery. There are mixed reports on the use of plasma HCV RNA levels, with some identifying a predictive role among HCV mono-infected patients, but others noting no association with HCV and HIV co-infected participants.26,33
The correlative nature of our study does not permit us to establish causal relationships, limiting this study. Our study is strengthened by a strict definition of chronic hepatitis C, which involves both antibody testing and RNA confirmation, therefore excluding most participants who cleared their infection. Other strengths include the size of the cohort and the extent of neuropsychological testing.
Several clinical implications emerge from this work. First, our data note that an APRI >0.5 may be a useful independent marker of risk for cognitive impairment, particularly among co-infected patients. Second, we highlight the importance of fibrosis over simple serology as a marker of cognitive performance in HCV. Third, we provide evidence of contributions to cognitive impairment that are not HIV-specific in the current era, broadly expanding our understanding of the multifactorial determinants of cognitive impairment in patients with HIV.
Acknowledgments
Source of Funding: Adaora A. Adimora MD, MPH received a consultant’s fee from Viiv in 2014 that was unrelated to the current study. Dr. Valcour’s work was supported by K24-MH-098759. This work was supported by a Diversity supplement to the UCSF Women’s Interagency HIV Study of Northern California (U01AI034989) from the National Institute of Allergy and Infectious Diseases (NIAID)/National Institutes of Health (NIH). Dr. Rubin’s effort was supported by K01-MH098798 from NIMH. The WIHS is funded by the National Institute of Allergy and Infectious Diseases (UO1-AI-35004, UO1-AI-31834, UO1-AI-34994, UO1-AI-34989, UO1-AI-34993, and UO1-AI-42590) and by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (UO1-HD-32632). The study is co-funded by the National Cancer Institute, the National Institute on Drug Abuse, and the National Institute on Deafness and Other Communication Disorders. Funding is also provided by the National Center for Research Resources (UCSF-CTSI Grant Number UL1 RR024131). The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
We thank Collin Adams for critical review and editing. Data in this manuscript were collected by the Women’s Interagency HIV Study (WIHS) Collaborative Study Group with centers (Principal Investigators) at New York City/Bronx Consortium (Kathryn Anastos); Brooklyn, NY (Howard Minkoff and Deborah Gustafson); Washington, DC, Metropolitan Consortium (Mary Young); The Connie Wofsy Study Consortium of Northern California (Ruth Greenblatt, Bradley Aouizerat, and Phyllis Tien); Los Angeles County/Southern California Consortium (Alexandra Levine and Marek Nowicki); Chicago Consortium (Mardge Cohen); Data Coordinating Center (Stephen Gange and Elizabeth Golub).
Footnotes
Conflicts of Interest
For the remaining authors none were declared.
References
- 1.Garcia-Martinez R, Rovira A, Alonso J, et al. Hepatic encephalopathy is associated with posttransplant cognitive function and brain volume. Liver Transpl. 2011;17(1):38–46. doi: 10.1002/lt.22197. [DOI] [PubMed] [Google Scholar]
- 2.Felipo V, Urios A, Montesinos E, et al. Contribution of hyperammonemia and inflammatory factors to cognitive impairment in minimal hepatic encephalopathy. Metabolic brain disease. 2012;27(1):51–58. doi: 10.1007/s11011-011-9269-3. [DOI] [PubMed] [Google Scholar]
- 3.Rikkers L, Jenko P, Rudman D, et al. Subclinical hepatic encephalopathy: detection, prevalence, and relationship to nitrogen metabolism. Gastroenterology. 1978;75(3):462–469. [PubMed] [Google Scholar]
- 4.Gitlin N, Lewis DC, Hinkley L. The diagnosis and prevalence of subclinical hepatic encephalopathy in apparently healthy, ambulant, non-shunted patients with cirrhosis. J Hepatol. 1986;3(1):75–82. doi: 10.1016/s0168-8278(86)80149-0. [DOI] [PubMed] [Google Scholar]
- 5.Groeneweg M, Quero JC, De Bruijn I, et al. Subclinical hepatic encephalopathy impairs daily functioning. Hepatology. 1998;28(1):45–49. doi: 10.1002/hep.510280108. [DOI] [PubMed] [Google Scholar]
- 6.Kircheis G, Knoche A, Hilger N, et al. Hepatic encephalopathy and fitness to drive. Gastroenterology. 2009;137(5):1706–1715. e1701–1709. doi: 10.1053/j.gastro.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 7.Lauridsen MM, Jepsen P, Vilstrup H. Critical flicker frequency and continuous reaction times for the diagnosis of minimal hepatic encephalopathy: a comparative study of 154 patients with liver disease. Metabolic brain disease. 2011;26(2):135–139. doi: 10.1007/s11011-011-9242-1. [DOI] [PubMed] [Google Scholar]
- 8.McCrea M, Cordoba J, Vessey G, et al. Neuropsychological characterization and detection of subclinical hepatic encephalopathy. Archives of neurology. 1996;53(8):758–763. doi: 10.1001/archneur.1996.00550080076015. [DOI] [PubMed] [Google Scholar]
- 9.Cordoba J. New assessment of hepatic encephalopathy. J Hepatol. 2011;54(5):1030–1040. doi: 10.1016/j.jhep.2010.11.015. [DOI] [PubMed] [Google Scholar]
- 10.Antinori A, Arendt G, Becker JT, et al. Updated research nosology for HIV-associated neurocognitive disorders. Neurology. 2007;69(18):1789–1799. doi: 10.1212/01.WNL.0000287431.88658.8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sacktor N, Skolasky R, Selnes OA, et al. Neuropsychological test profile differences between young and old human immunodeficiency virus-positive individuals. Journal of neurovirology. 2007;13(3):203–209. doi: 10.1080/13550280701258423. [DOI] [PubMed] [Google Scholar]
- 12.Ananworanich J, Schuetz A, Vandergeeten C, et al. Impact of multi-targeted antiretroviral treatment on gut T cell depletion and HIV reservoir seeding during acute HIV infection. PloS one. 2012;7(3):e33948. doi: 10.1371/journal.pone.0033948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Valcour V, Sithinamsuwan P, Letendre S, et al. Pathogenesis of HIV in the central nervous system. Curr HIV/AIDS Rep. 2011;8(1):54–61. doi: 10.1007/s11904-010-0070-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bacon MC, von Wyl V, Alden C, et al. The Women’s Interagency HIV Study: an observational cohort brings clinical sciences to the bench. Clin Diagn Lab Immunol. 2005;12(9):1013–1019. doi: 10.1128/CDLI.12.9.1013-1019.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wai CT, Greenson JK, Fontana RJ, et al. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology. 2003;38(2):518–526. doi: 10.1053/jhep.2003.50346. [DOI] [PubMed] [Google Scholar]
- 16.Shaheen AA, Myers RP. Diagnostic accuracy of the aspartate aminotransferase-to-platelet ratio index for the prediction of hepatitis C-related fibrosis: a systematic review. Hepatology (Baltimore, Md) 2007;46(3):912–921. doi: 10.1002/hep.21835. [DOI] [PubMed] [Google Scholar]
- 17.Abd El Rihim AY, Omar RF, Fathalah W, et al. Role of fibroscan and APRI in detection of liver fibrosis: a systematic review and meta-analysis. Arab journal of gastroenterology: the official publication of the Pan-Arab Association of Gastroenterology. 2013;14(2):44–50. doi: 10.1016/j.ajg.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 18.Maki PM, Rubin LH, Valcour V, et al. Cognitive function in women with HIV: findings from the Women’s Interagency HIV Study. Neurology. 2015;84(3):231–240. doi: 10.1212/WNL.0000000000001151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rubin LH, Sundermann EE, Cook JA, et al. Investigation of menopausal stage and symptoms on cognition in human immunodeficiency virus-infected women. Menopause (New York, NY) 2014;21(9):997–1006. doi: 10.1097/GME.0000000000000203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rubin LH, Cook JA, Weber KM, et al. The association of perceived stress and verbal memory is greater in HIV-infected versus HIV-uninfected women. Journal of neurovirology. 2015;21(4):422–432. doi: 10.1007/s13365-015-0331-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Valcour V, Rubin LH, Tien P, et al. Human immunodeficiency virus (HIV) modulates the associations between insulin resistance and cognition in the current combination antiretroviral therapy (cART) era: a study of the Women’s Interagency HIV Study (WIHS) Journal of neurovirology. 2015;21(4):415–421. doi: 10.1007/s13365-015-0330-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Manly JJ, Smith C, Crystal HA, et al. Relationship of ethnicity, age, education, and reading level to speed and executive function among HIV+ and HIV− women: the Women’s Interagency HIV Study (WIHS) Neurocognitive Substudy. Journal of clinical and experimental neuropsychology. 2011;33(8):853–863. doi: 10.1080/13803395.2010.547662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Heaton RK, Grant I, Matthews CG. Comprehensive norms for an expanded Halstead-Reitan Battery: demographic corrections, research findings, and clinical applications. Odessa, FL: Psychological Assessment Resources; 1991. [Google Scholar]
- 24.Shaheen AA, Wan AF, Myers RP. FibroTest and FibroScan for the prediction of hepatitis C-related fibrosis: a systematic review of diagnostic test accuracy. The American journal of gastroenterology. 2007;102(11):2589–2600. doi: 10.1111/j.1572-0241.2007.01466.x. [DOI] [PubMed] [Google Scholar]
- 25.Woods SP, Moore DJ, Weber E, et al. Cognitive neuropsychology of HIV-associated neurocognitive disorders. Neuropsychol Rev. 2009;19(2):152–168. doi: 10.1007/s11065-009-9102-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Clifford DB, Smurzynski M, Park LS, et al. Effects of active HCV replication on neurologic status in HIV RNA virally suppressed patients. Neurology. 2009;73(4):309–314. doi: 10.1212/WNL.0b013e3181af7a10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Crystal H, Kleyman I, Anastos K, et al. Effects of hepatitis C and HIV on cognition in women: data from the Women’s Interagency HIV Study. J Acquir Immune Defic Syndr. 2012;59(2):149–154. doi: 10.1097/QAI.0b013e318240566b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sun B, Abadjian L, Rempel H, et al. Differential cognitive impairment in HCV coinfected men with controlled HIV compared to HCV monoinfection. J Acquir Immune Defic Syndr. 2013;62(2):190–196. doi: 10.1097/QAI.0b013e31827b61f1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Limketkai BN, Mehta SH, Sutcliffe CG, et al. Relationship of liver disease stage and antiviral therapy with liver-related events and death in adults coinfected with HIV/HCV. JAMA: the journal of the American Medical Association. 2012;308(4):370–378. doi: 10.1001/jama.2012.7844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vigil O, Posada C, Woods SP, et al. Impairments in fine-motor coordination and speed of information processing predict declines in everyday functioning in hepatitis C infection. Journal of clinical and experimental neuropsychology. 2008;30(7):805–815. doi: 10.1080/13803390701802354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Clifford DB, Vaida F, Kao YT, et al. Absence of neurocognitive effect of hepatitis C infection in HIV-coinfected people. Neurology. 2015;84(3):241–250. doi: 10.1212/WNL.0000000000001156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Richardson MA, Morgan EE, Vielhauer MJ, et al. Utility of the HIV dementia scale in assessing risk for significant HIV-related cognitive-motor deficits in a high-risk urban adult sample. AIDS Care. 2005;17(8):1013–1021. doi: 10.1080/09540120500100858. [DOI] [PubMed] [Google Scholar]
- 33.Sun HY, Chang SY, Yang ZY, et al. Recent hepatitis C virus infections in HIV-infected patients in Taiwan: incidence and risk factors. Journal of clinical microbiology. 2012;50(3):781–787. doi: 10.1128/JCM.06014-11. [DOI] [PMC free article] [PubMed] [Google Scholar]