Abstract
Pulmonary toxicity studies often use bronchoalveolar lavage (BAL) to investigate potential adverse lung responses to a particulate exposure. The BAL cellular fraction is counted, using automated (i.e. Coulter Counter®), flow cytometry or manual (i.e. hemocytometer) methods, to determine inflammatory cell influx. The goal of the study was to compare the different counting methods to determine which is optimal for examining BAL cell influx after exposure by inhalation or intratracheal instillation (ITI) to different particles with varying inherent pulmonary toxicities in both rat and mouse models. General findings indicate that total BAL cell counts using the automated and manual methods tended to agree after inhalation or ITI exposure to particle samples that are relatively nontoxic or at later time points after exposure to a pneumotoxic particle when the response resolves. However, when the initial lung inflammation and cytotoxicity was high after exposure to a pneumotoxic particle, significant differences were observed when comparing cell counts from the automated, flow cytometry and manual methods. When using total BAL cell count for differential calculations from the automated method, depending on the cell diameter size range cutoff, the data suggest that the number of lung polymorphonuclear leukocytes (PMN) varies. Importantly, the automated counts, regardless of the size cutoff, still indicated a greater number of total lung PMN when compared with the manual method, which agreed more closely with flow cytometry. The results suggest that either the manual method or flow cytometry would be better suited for BAL studies where cytotoxicity is an unknown variable.
Keywords: Bronchoalveolar lavage, carbon nanotubes, cytotoxicity, flow cytometry, hemocytometer, metals, welding fume
Introduction
Pulmonary toxicity studies often use bronchoalveolar lavage (BAL) to investigate the lung response to a particulate. BAL is a procedure where an instillate, usually sterile saline or buffered saline, in a syringe washes the lung through a cannula inserted into the trachea of the animal. Samples are then centrifuged and the cellular fraction is counted to determine total BAL cell number. Common methods used for counting are automated (i.e. Coulter Counter®), flow cytometer and manual (i.e. hemocytometer). We compared these methods to assess the best approach for pulmonary toxicity studies involving BAL and insoluble particulates in rats and mice.
The use of Multisizer Coulter Counter Analyzers is common to a number of pulmonary toxicity studies in rodents and remains a user-friendly, high-throughput method. The analyzer returns data on number, volume, mass and surface area size distributions in a single measurement. The method, known as the Coulter Principle (also referred to as Electrical Sensing Zone Method or ESZ), is based on measurable changes in electrical impedance produced by nonconductive particles suspended in an electrolyte. As the suspended particles pass through the sensing zone, each particle displaces its own volume of electrolyte. Volume displaced is measured as a voltage pulse; the height of each pulse is proportional to the volume of the particle. The results are independent of the particle color, shape, composition or refractive index (www.beckmancoulter.com). When the sample is homogeneous, cell counts are quickly and accurately obtained (Lopez et al., 1986). However, in studies that involve particle exposures inducing inflammation and cytotoxicity, the sample cell population is mixed and accuracy could be affected (Lee et al., 2001).
Flow cytometry is a quantitative, laser-based tool used to count and profile cells in a heterogeneous fluid. In flow cytometry, antibodies tagged with fluorescent dyes and raised against highly specific cell surface antigens are used to segregate specific sub-populations within a live cell suspension. Multiparametric data are rapidly and accurately collected from a cell suspension as thousands of cells, each a distinct event, per second pass by an electronic detection apparatus. Flow cytometry is likely the most expensive of the cell counting methods and requires a greater level of expertise in comparison to the automated analyzer. Once the technical details are mastered, however, this method offers a multitude of potential cell-based assays in addition to being a high-throughput method for cell counting.
A low-cost, manual cell counting method (i.e. hemocytometer) is traditionally used for pulmonary toxicity studies. A small volume of a cell suspension is loaded under a glass cover slip onto a thick glass microscope slide that has rectangular counting chambers containing laser-etched grids of perpendicular lines. After counting the cells in the chamber using a light microscope, the concentration of cells in the original suspension can be calculated, since both the chamber depth and area bound by the grid lines are known. This counting method allows the investigator to visually distinguish debris and dead versus live cells when used with vital stains, such as Trypan Blue. This may be critically important when downstream analyses of the cell sample are planned and accuracy is essential. The manual method, however, requires skill and expertise and may introduce more sources of human error compared to the methods described above. It also has the drawback of being the most time-consuming and, perhaps, tedious of the methods.
Methods
Animals
Specific pathogen-free, male C57BL/6J mice (8–10 weeks) from Jackson Laboratory (Bar Harbor, ME) and male Sprague–Dawley [Hla:(SD) CVF] rats (200–250 g) from Hilltop Lab Animals (Scottdale, PA) were used in this study. Mice and rats were allowed access to a conventional diet (6% Irradiated NIH-31 Diet or Harlan 2918 irradiated Teklad Global 18% rodent chow, respectively, Harlan Laboratories, Inc., Madison, WI) and tap water ad libitum. All animals were housed in an Association for Assessment and Accreditation of Laboratory Animal Care International-approved NIOSH animal facility with a controlled humidity and temperature environment and a 12 h light/dark cycle. Animals were acclimated to the animal facility for a minimum of one week after arrival and all procedures were performed using protocols approved by the National Institute for Occupational Safety and Health Animal Care and Use Committee. The cell counting methods were evaluated as part of ongoing experiments, specifics of the different exposure scenarios are briefly described below (Erdely et al., 2013; Zeidler-Erdely et al., 2014).
Multi-walled carbon nanotube inhalation
Male C57Bl/6J mice were exposed to MWCNT using a computer controlled whole body inhalation exposure system designed and constructed at NIOSH (McKinney et al., 2009). In brief, the inhalation exposure system combines air flow controllers, aerosol particle monitors, data acquisition devices and custom software with automatic feedback control to achieve constant and repeatable exposure chamber temperature, relative humidity, pressure, aerosol concentration and particle size distributions. Mice were exposed to 5 or 0.5 mg/m3 MWCNT (49±13 nm in diameter and a median length of 3.86 μm (GSD 1.94), Hodogaya Chemical Company, Japan) or filtered air (sham controls) for a total of 19 d and sacrificed at 0, 28 and 84 d post-exposure. The MWCNT used in this study, commonly referred to as MWCNT-7, have been extensively characterized (Porter et al., 2010, 2012).
Welding fume inhalation
The arc welding fume generation system at NIOSH was previously described (Antonini et al., 2006). Briefly, this system consisted of a welding power source (Power Wave 455, Lincoln Electric, Cleveland, OH), an automated, programmable six-axis robotic arm (Model 100 Bi, Lincoln Electric), a water-cooled arc welding torch (WC 650 amp, Lincoln Electric), a wire feeder and an automatic welding torch cleaner. Gas metal arc (GMA) welding was performed using a stainless steel (SS) electrode (Blue Max E308LSi wire, Lincoln Electric, Cleveland, OH) and a 95% argon and 5% CO2 shielding gas. Welding took place on A36 carbon steel plates for daily exposures of a target concentration of 30 mg/m3 × 3 h/d × 4 d (total inhaled dose = 360 mg delivered over 4 d or 90 mg/d) at 25V and 200 amps. Actual mean daily particle concentrations measured in the exposure chamber was 27.7 mg/m3. The mass median aerodynamic diameter (MMAD) for the welding fume was 0.255 μm. The GMA-SS welding fume was composed of 57% Fe, 20% Cr, 14% Mn and 9% Ni as determined by inductively coupled plasma-atomic emission spectroscopy (ICP-AES) (Antonini et al., 2006). Sham controls were exposed to filtered air. Animals were sacrificed at 1, 7 and 28 d after the last exposure.
The resistance spot welding inhalation system at NIOSH has been previously described (Afshari et al., 2014). Briefly, two rolls that contained strips of low alloy, carbon steel sheet metal or galvanized Zn-coated steel were directed by a set of rollers to copper (class I)-tipped electrodes of the welder, and spot welded at a determined distance of 3/4 in. (20 mm) between each spot weld by an automated, computer-controlled resistance spot welding gun (small new modified “C” style Trans-gun 136 kVa-AC; Milco Manufacturing Company, Warren, MI). The desired aerosol concentration within the animal exposure chamber was dependent on the number of welds made per interval of time. The welding gun “squeezes” the sheet metal strips together by its built-in pneumatic actuator and welds them together by conducting a very high current through copper electrodes. The welding gun parameters (e.g. current, squeezing time, welding and holding time) were set with a computer through the software (BOS6000 version 1.35). Aerosols generated during welding were transported via tubing from the spot welding fume chamber to an animal exposure chamber. Animals were exposed by inhalation to 25 mg/m3 of aerosols during resistance spot welding on mild steel (SWMS; 8 d × 4 h/d) or galvanized steel (SWGS; 8 d × 4 h/d). Sham controls were exposed to filtered air. Animals were sacrificed 1 d after the last exposure.
Welding fume intratracheal instillation (ITI)
Welding fumes were generated and collected onto filters using the NIOSH robotic welding system which includes a six-axis robotic arm, power supply, water-cooled arc welding torch and a wire feeder; as previously detailed. The particles have been fully characterized and had a MMAD of 0.255 μm as determined by SEM (Antonini et al., 1999, 2006; Zeidler- Erdely et al., 2013).
The welding fume was suspended in sterile Ca2+- and Mg2+-free phosphate buffered saline (PBS) and sonicated briefly. Rats were anesthetized by an IP injection of sodium methohexital (Brevital, Eli Lilly, Indianapolis, IN) and rats were instilled with GMA-SS welding fume in 300 μl PBS. Shams were instilled with PBS alone.
The ITI doses were determined from previous SS welding fume inhalation exposure studies. It was found that an exposure to 40 mg/m3 × 3 h/d × 3 d to SS welding fume led to a mean deposited pulmonary dose of 943 μg/g dry left lung weight (Antonini et al., 2011). With a mean dry left lung weight of 0.104 g, 98 μg of SS welding fume (943 μg/g × 0.104 g) deposited in the left lung after the three day exposure. Assuming equal particle distribution in both the right and left lungs and considering that the right lung is 1.75 times larger than the left lung in a rat (Hyde et al., 2004), it was estimated that 269.5 μg of welding fume deposited in the whole lung (98 μg × 1.75 = 171.5 μg right lung deposition; 171.5 μg right lung deposition+98 μg left lung deposition = 270 μg). Rats were instilled with 270 μg, 900 μg or 3000 μg to examine the accuracy of the cell counting methods with different magnitudes of lung cytotoxicity. For the low dose, 1, 7 and 28 d post-ITI time points were examined, while only 1 and 7 d post-ITI were assessed for the middle and high doses.
Rodent bronchoalveolar lavage
Animals were euthanized with an overdose of pentobarbital-based euthanasia solution (100–300 mg/kg IP). The vena cava was then cut to exsanguinate the animal and ensure death. Partial lung lavage was done on the right lung lobes of mice exposed to MWCNT with 0.3mL followed by two subsequent lavages of 0.5mL PBS. Whole lungs from rats exposed to GMA-SS welding fume were lavaged first with 6mL of PBS, kept separate on ice, then 8mL PBS four times. For rats exposed to spot welding fumes, partial lung lavage was done on the right lung lobes with 4mL of PBS, kept separate, then 5mL four times. For consistency, one individual performed the lavages for all exposure scenarios. Both lavage fractions were then centrifuged (500 g × 10 min at 4 ºC) and the acellular supernatant of the first lavage used for evaluation of lactate dehydrogenase (LDH) activity. Finally, the cell pellets of the first and subsequent washes were combined and then suspended in an appropriate final volume to determine total BAL cell number and differentials.
Lung cytotoxicity measured as lactate dehydrogenase activity
LDH activity was determined by measuring the oxidation of lactate to pyruvate coupled with the formation of NADH (nicotinamide adenine dinucleotide) at 340 nm. Measurements were performed with a COBAS c111 analyzer (Roche Diagnostic Systems, Indianapolis, IN).
Automated cell counter
Cells were counted using a Coulter Multisizer III and AccuComp software (Coulter Electronics, Hialeah, FL). A 10 μl cell sample was added to 20mL of electrolyte solution with a 500 μl analytical volume sampled by the instrument from the sample vial. Each vial was inverted five times before placement on the instrument. Two different diameter ranges routinely used in the laboratory, 6–20 μm and 9–20 μm, were recorded for the GMA-SS welding fume samples, but not samples from the MWCNT and spot welding mild steel exposures. For a total BAL cell count, the 6–20 μm diameter range includes lymphocytes, PMN, and, macrophages and excludes red blood cell contamination in the BAL, if present.
Manual cell counts
A Bright Line Counting Chamber (Hausser Scientific, Horsham, PA) was used and calculations were done according to the manufacturer’s instructions. Briefly, the BAL cell suspensions were thoroughly mixed; then a 1:20 and 1:1 dilution with Trypan Blue was used for the rat and mouse cells, respectively. Both sides of the hemocytometer chamber were loaded while not exceeding the recommended capacity. The cells were then allowed to settle briefly. The four corner squares were counted for viable cells. A different individual counted the cells for each exposure scenario, and the most experienced technician spot-checked samples throughout each experiment. In addition, each sample was counted a minimum of two times.
Flow cytometry for mouse bronchoalveolar lavage cells
Mouse BAL cell differentiation was done according to Stevens et al. (2007) with minor modifications. The BAL cells were re-suspended in 500 μl PBS and 200 μl was added into a 12 × 75mm polystyrene tube with 100 μl of 10% rat serum in FACS buffer for 10 min. Then 50 μl of pre-mixed antibodies in FACS buffer was added and cells were stained for 30 min at room temperature on a shaker. The mixture contained the final concentration of 5 μg/mL of the following antibodies: CD16/32 block, Ly6G-FITC, Siglec-F-PE, CD45- PerCp and CD11c-APC. All the antibodies were purchased from PharMingen (Becton Dickinson, San Diego, CA). The Caltag counting beads (PCB-100, Invitrogen, Carlsbad, CA) were added for cell enumeration prior to analysis in FACSCalibur (BD Biosciences, San Jose, CA). Samples were acquired through a live gate without compensation. After collecting 4000 counting beads, the data of all cells were exported to the analysis software, FlowJo (Treestar, Costa Mesa, CA). The leukocytes were identified by cells expressing CD45+. Neutrophils were defined as cells expressing CD45+Ly6G+. Eosinophils were defined as cells expressing CD45+Siglec-F+ and macrophages were defined as cells expressed CD45+CD11c+. Total leukocyte number was calculated from the number of positive leukocytes/beads registered on the flow cytometer multiplied by the known number of beads per μl. This provided the leukocytes per μl which was multiplied by the volume in the flow tube (200 μl BAL cells+100 μl serum+50 μl antibodies+ 25 μl beads = 375 μl). This number, i.e. number of leukocytes in the flow tube, was then multiplied by the dilution factor from the initial BAL cell pellet (2.5 or 500 μl/ 200 μl) for the final cell count.
Cellular differentials and diameter measurements
Cytospin slides for BAL cell differentials were prepared as described previously (Zeidler-Erdely et al., 2011). Briefly, BAL cells were spun onto glass slides using a Ctyospin 3 centrifuge (Shandon Life Sciences International, Cheshire, England) set at 800 rpm for 5 min. Slides were stained with a Wright-Giemsa stain using a three step protocol (Fisher Scientific, Pittsburgh, PA) then coverslipped. A minimum of 300 cells were identified and counted under light microscopy.
Cell diameter was measured with an Olympus AX70 microscope with a 20X air objective (Olympus America Inc., Lake Success, NY). Cell images were captured with an Olympus DP73 camera. Once the cells were in focus, the slide was moved to the upper left to take the first image. Successive images were taken by moving the slide to the right, making sure previously imaged cells were off the screen. When the slide was all the way to the right, it was moved down and the imaging progressed to the left. When the slide was all the way to the left, it was moved down and the imaging progressed to the right. This sequence was repeated until the entire slide was imaged.
Macrophage and neutrophil cell diameter measurements from the captured images were done with CellSens Dimensions software. Briefly, the “line” icon was chosen then was placed on the left side of the cell membrane approximately in the center of the nucleus. The line was dragged to the right side of the cell membrane and dropped, and a measurement value was recorded. Three slides from the GMA-SS welding fume and MWCNT-exposed inhalation groups were evaluated, and 20 each of macrophages and PMN were imaged and measured for cell diameter.
Statistics
Data from the mouse inhalation studies were log-transformed, if needed, prior to analysis to meet the assumptions of the statistical tests. roc Mix was used to run a three-way factorial analysis of variance. Significant three-way interactions were examined by utilizing two-way ANOVA’s stratified by time and dose. Pairwise comparisons were performed using Fishers Least Significant Difference test. Rat inhalation and ITI outcome variables were analyzed using a Student’s t-test. For all studies, differences between experimental groups were considered significant with a p value less than 0.05.
Results
Inhalation of MWCNT in mice
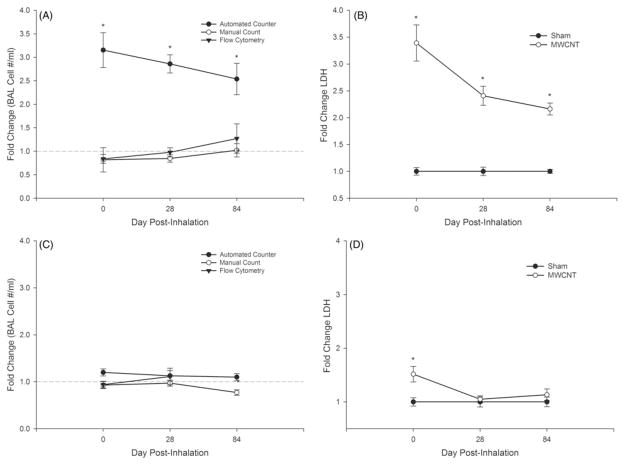
Comparison of cell counting methods (Figure 1, panels A and C) after MWCNT inhalation exposure and lung cytotoxicity, as measured by BAL fluid LDH (Figure 1, panels B and D), in mice. High dose MWCNT inhalation resulted in a 3.5-fold increase in LDH 1 d post-inhalation (Figure 1B). BAL cell counts by the automated counter indicated a 3.2-fold increase (Figure 1A). Conversely, the manual method showed a slight decrease in total cells (0.84) compared to air-exposed (sham) mice, which agreed with flow cytometry results (0.81 decrease). Similar results were observed at 28 and 84 d post-exposure in conjunction with significantly increased LDH levels (2.5 and 2.0-fold, respectively). At the lower dose, LDH was increased minimally (1.4 fold) only at 0 d and the three methods generally agreed through 84 d (Figure 1, panels C and D).
Figure 1.
Comparison of bronchoalveolar lavage (BAL) cell counting methods after multi-walled carbon nanotube (MWCNT) exposure in mice at 0, 28 and 84 d post-inhalation. Cell counts for the high dose (5 mg/m3) and low dose (0.5 mg/m3) are shown in panels A and C, respectively, via an automated (Coulter Counter®), manual (hemocytometer) and flow cytometry method. Lactate dehydrogenase (LDH) activity (U/L), an indicator of lung cytotoxicity, for sham and MWCNT-exposed mice is shown in panels B and D for the high and low dose, respectively. Data are expressed as fold change from sham (means ±SE; *p≤0.05).
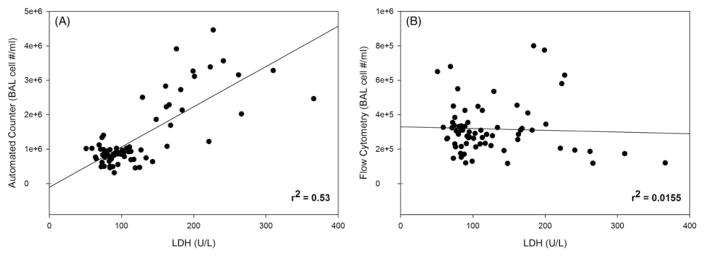
Linear regression analysis showed a general correlation (r2 = 0.53; p<0.0001) between lung cytotoxicity (LDH) and the automated cell counting method numbers (Figure 2A). However, no correlation (Figure 2B) between the flow cytometry method and LDH was found (r2 = 0.0155; p = 0.31).
Figure 2.
Linear regression analysis between lung cytotoxicity, measured as LDH activity (U/L) in the bronchoalveolar lavage fluid, and the automated (panel A; Coulter Counter®) or flow cytometry (panel B) cell counting methods after MWCNT inhalation exposure in mice.
Inhalation of stainless steel welding aerosols in rats
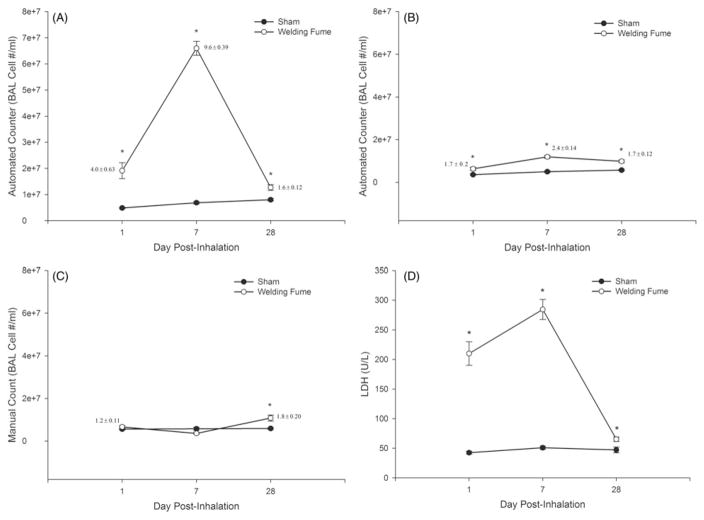
Comparison of cell counts from the automated cell counter using diameter ranges of 6–20 μm (Figure 3, panel A) and 9–20 μm (Figure 3, panel B) and from the manual method (Figure 3, panel C). A significant and marked cytotoxic (>5- fold increased LDH; Figure 3D) response following GMA-SS welding fume exposure was associated with a 4.0±0.6-fold (numbers shown next to each point on graph) and 1.2±0.1- fold increase in BAL cell counts compared to sham for the automated (6–20 μm; Figure 3A) and manual method (Figure 3C), respectively, at 1 d post-inhalation. When the counter was adjusted to a range of 9–20 μm the fold change discrepancy was less between the two methods (1.7±0.2- fold (automated); Figure 3, panels B and C). Similar results were also found at 7 d post-inhalation. By 28 d, when lung cytotoxicity was near control levels (approximately 1.4-fold increased), the two methods yielded similar results, with fold changes of 1.6±0.1 (6–20 μm) and 1.8±0.2 for the automated and manual methods, respectively (Figure 3, panels A and C). Of note, cell differential analysis showed the percentage of PMN in the BAL was only significantly increased at 7 d (0.5±0.1-sham; 3.1± 0.5-GMA-SS fume) and not 1 d (0.5±0.2) or 28 d (1.8±0.7).
Figure 3.
Comparison of BAL cell count methods after gas metal arc-stainless steel welding fume exposure in rats at 1, 7 and 28 d post-inhalation. Rats were exposed at an aerosol concentration of ~30 mg/m3 × 3 h/d × 4 d. Cell counts (number per mL) are shown for the automated (Coulter Counter®) at two investigator selected cell diameter ranges of 6–20 μm (panel A) and 9–20 μm (panel B) and the manual (hemocytometer) method (panel C). LDH activity (U/L), an indicator of lung cytotoxicity, for air- and welding fume-exposed rats is shown in panel D. Data are expressed as mean±SE and fold changes are also shown (panels A–C); n = 7/group; *p.≤0.05 versus sham
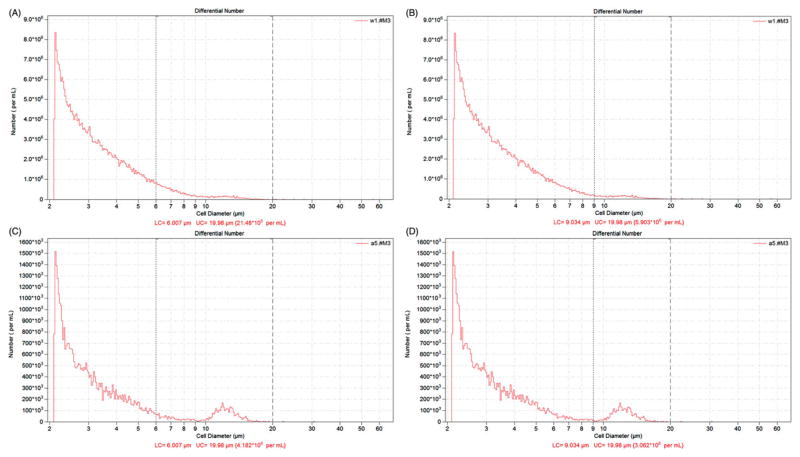
Shown in Figure 4 are representative histograms from the automated counter of a GMA-SS inhalation-exposed rat BAL sample. Total cells numbers were 21.5 × 106 cells/mL and 5.9 × 106 cells/mL for the 6–20 and 9–20 μm diameter ranges, respectively (Figure 4, panels A and B). The manual count for this particular sample was 5.7 × 106 cells/mL. A sham (air-exposed) rat BAL sample (Figure 4, panels C and D), typically a homogenous population of cells (>98% alveolar macrophages), is shown for comparison. The macrophage peak is easily distinguishable in this representative control histogram.
Figure 4.
Representative histograms generated by the automated cell counting method (i.e. Coulter Counter®) after inhalation exposure to gas metal arc-stainless steel welding fume in rats. Rats were exposed at an aerosol concentration of ~30 mg/m3 × 3 h/d × 4 d and bronchoalveolar lavage was done at 1 d post-inhalation. Cell counts (number per mL) are shown for the automated (at two investigator selected cell diameter ranges of 6–20 μm (panel A) and 9–20 μm (panel B). A sham (air-exposed) BAL sample is shown for comparison in panels C and D.
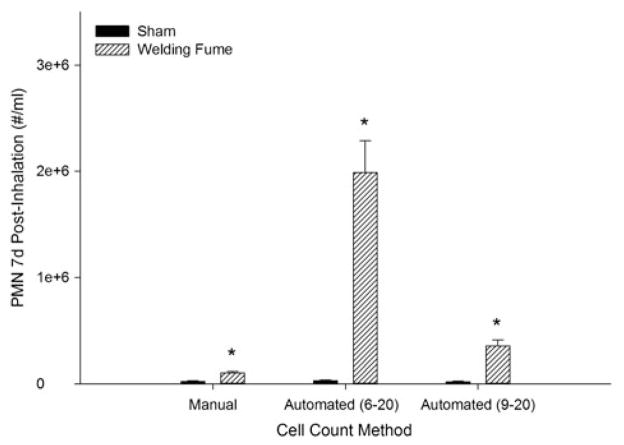
Comparison of PMN cell numbers derived from the manual and automated (Coulter Counter®, 6–20 μm and 9–20 μm diameter ranges) after GMA-SS welding fume inhalation in rats is shown in Figure 5. Data from the 7 d post-inhalation time point are shown. These data suggest a variance in PMN number occurs when using the total cell count for differential calculations from the automated method, particularly at the lower diameter range cutoff of 6 μm. The automated measurements, irrespective of the cutoff, still indicated a greater number of BAL total PMN versus the manual approach.
Figure 5.
Comparison of polymorphonuclear leukocyte (PMN) numbers derived from two cell-counting methods after gas metal arc-stainless steel welding fume inhalation in rats. Manual, automated (Coulter Counter®, 6–20 μm diameter range) and automated (9–20 μm diameter range) cell number per mL were calculated from the total cell number from each method after a minimum of 300 cells were differentiated. Cell numbers are from the 7 d post-inhalation time point. Data are expressed as mean±SE; *p≤0.05 versus sham.
Automated and manual cell counting methods were compared in rats after 8 d of inhalation exposure to SWMS (non-cytotoxic) or SWGS (cytotoxic). Automated cell counts (1.2±0.1 fold of control) were comparable to manual cell counts (1.4±0.2 fold of control) at 1 d after the final SWMS exposure (Figure 6A). Further, a 3 d SWMS exposure resulted in fold changes of 1.1±0.1 for both the automated and manual methods at 1 d post-inhalation. BAL LDH activity confirmed that SWMS had negligible cytotoxicity (sham LDH: 93.5± 9.6 U/L; SWMS LDH: 85.3±9.04 U/L). In contrast, a significant increase in total cells was found for the automated method (3.2±0.2 fold-increase; cutoff diameter was 6–20 μm) and not the manual method at 1 d post-inhalation SWGS (Figure 6B). BAL LDH confirmed the cytotoxicity of SWGS (LDH>2.3±0.2 fold-increase over sham). Of note, both the SWMS and SWGS sham control total cell counts (cell #/mL) were similar between the methods and the manual counts were done by a different technician to verify the other experimental findings.
Figure 6.
Comparison of BAL cell counting methods after resistance spot welding on mild steel (SWMS; panel A) or galvanized steel (SWGS; panel B) at 1 d post-inhalation. Rats were exposed at an aerosol concentration of 25 mg/m3 × 4 h/d × 8 d. Cell counts (number per mL) are shown for the automated (Coulter Counter®) at an investigator selected cell diameter range of 6–20 μm and the manual (hemocytometer) method. Data are expressed as mean±SE and fold changes are also shown; n = 4–6/group; *p ≤ 0.05 versus sham.
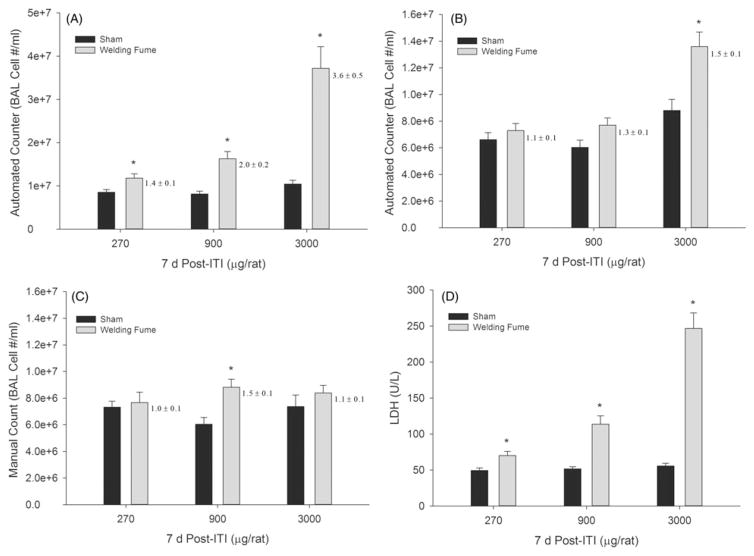
To further examine the accuracy of the cell counting methods with different magnitudes of lung cytotoxicity and to assess another commonly used pulmonary exposure technique, 270 μg, 900 μg or 3000 μg of GMA-SS welding fume were instilled in rats. At 7 d post-ITI, BAL cells were counted using a diameter range of 6–20 μm and an automated counter (Figure 7A), a diameter range of 9–20 μm and an automated counter (Figure 7B), and the manual method (Figure 7C). BAL LDH significantly increased at all exposures (Figure 7D), confirming the cytotoxicity of these exposures. The lower diameter range cutoff (6–20 μm; Figure 7, panel A) further confirms that the level of cytotoxicity correlates to the total cell counts reported by the automated method, as was observed with the evaluated inhalation exposures. As with the GMA-SS inhalation exposure, the fold changes and total cell numbers, generated by each method, better agreed when the diameter range was 9–20 μm (Figure 7, panel B). As stated above (Figure 5), the resulting PMN number derived from the differential count at the 3000 μg ITI dose, for example, would have been reported as 1.5 × 107 using the automated method (6–20 μm) compared to 6.2 × 106 for the manual method.
Figure 7.
Comparison of BAL cell counting methods after gas metal arc-stainless steel welding fume exposure in rats at 7 d post-exposure. Rats were exposed to 270, 900 or 3000 μg by intratracheal instillation. Cell counts (number per mL) are shown for the automated (Coulter Counter®) at two investigator selected cell diameter ranges of 6–20 μm (panel A) and 9–20 μm (panel B) and the manual (hemocytometer) method (panel C). Fold changes relative for controls are next to each bar. LDH activity (U/L), an indicator of lung cytotoxicity, for sham- and welding fume-exposed rats is shown in panel D. Data are expressed as mean±SE and fold changes are also shown (panels A–C); n = 7/group; *p≤0.05 versus sham.
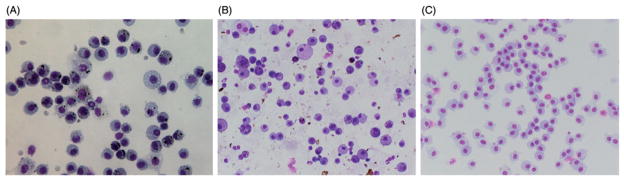
Although changing the automated cell counter diameter cutoff from 6–20 μm to 9–20 μm agreed better with manual counting, the arbitrary change may exclude smaller diameter inflammatory cells. Representative light microscopy images are shown in Figure 8 of a SWMS [non-cytotoxic/non-inflammatory (panel A)], GMA-SS inhalation [cytotoxic/ inflammatory (panel B)], and sham (panel C) rat BAL cell cytospins. The homogenous and heterogeneous nature of the cell populations obtained from BAL in rodent pulmonary toxicity studies was evident. To evaluate the cell diameter ranges ordinarily used for pulmonary toxicity studies, measurements for the two main cell types observed in the cytospins – PMN and macrophages – were taken. Macrophages and PMN measured 19.0±0.4 μm (range 13.5–25.5 μm) and 8.1± 0.1 μm (range 6.0–10.8), respectively, after GMA-SS inhalation exposure. In the mouse, 18.7±0.5 μm (range 13.8– 31.3 μm) and 10.1±0.2 μm (range 7.3–13.5 μm), were the measured diameters for the MWCNT-exposed macrophage and PMN, respectively.
Figure 8.
Representative light microscopy images of non-cytotoxic/non-inflammatory (panel A) and cytotoxic/inflammatory (panel B) BAL cytospins. Animals were exposed by inhalation to aerosols during resistance spot welding on mild steel (25 mg/m3 × 8 d ×4 h/d) or gas metal arc welding on stainless steel (~30 mg/m3 ×3 h/d ×4 d). A cytospin (panel C) from a sham animal is shown in panel C. Images were taken with the 40 × objective.
Discussion
The goal of this study was to evaluate methods for counting BAL cells following exposures which alter cell homogeneity, create cytotoxicity and contain exogenous particles. Three different procedures for cell counting (Coulter Counter®, flow cytometer and hemocytometer) were compared to evaluate total BAL cell numbers recovered from rodents after a pulmonary exposure. Inhalation and ITI exposure scenarios of particles with differing physicochemical characteristics, as well as associated lung toxicities, were examined. These included MWCNT, a cylindrical-shaped particle with a high aspect ratio, and three types of metal-rich particulate matter (i.e. GMA-SS, SWMS and SWGS fumes) that have spherical, chain-like aggregate morphology. The experimental doses induced differing degrees of lung cytotoxicity and variable cellular heterogeneity in the BAL. The samples also were sometimes particle- and/or debris-laden. Results suggest that a pulmonary particle exposure associated with significant lung cytotoxicity is best evaluated by a manual cell counting method or flow cytometry. Automated counting using the Coulter Counter®, although accurate for samples from sham-and low/non-cytotoxic particle-exposed animals, returned a false increased total BAL cell number due to artifact(s) present when using standard cell diameter size ranges.
The first exposure scenario examined all three counting methods in BAL from exposed mice. The animals were exposed to cylindrical-shaped MWCNT by inhalation. Overall, the flow cytometry and manual methods produced near identical fold changes to each other, and data were unaffected by significant lung cytotoxicity. These two methods were effective at primarily registering viable cells, as they are dependent upon microscopic evaluation with a vital stain or cell-specific antibodies, and the user’s ability to “gate out” cellular debris. The automated method (i.e. Coulter Counter®) was ineffective at distinguishing debris from viable cells and, therefore, returned elevated total cell numbers and fold changes. In fact, a correlation was found between BAL LDH levels and total cell counts using the automated method; however, none was found with flow cytometry. The manual and flow cytometry methods indicate that in a given snapshot evaluation of the total BAL cells, the number of viable cells is not necessarily drastically increased at any given point. One explanation is likely a result of cell death, as indicated by the marked LDH increases when the methods are in disagreement. Previous reports have noted cell number discrepancies when results are gathered with the Coulter Counter® analyzer compared to other methods, particularly when the automated counter is used with particleor debris-laden cell suspensions. For example, this limitation has been noted with talc particles in mouse pleural lavage fluid, clumped platelets in domestic cat blood and microorganisms in human blood using older models of the automated counter (Berger et al., 1992; Lee et al., 2001; Marshall et al., 1990; Whittington & Comer, 1984).
A similar result to the fiber-like MWCNT exposure was found with inhalation of metal-rich particulate matter. In rats, GMA-SS welding fume produced a>5-fold increase in BAL LDH. For this exposure scenario, an additional parameter was used for the automated counter to assess the effect of a more stringent diameter range (i.e. 9–20 μm) on the total cell count. This was done in an attempt to reduce the apparent debris artifact evident in the histograms (Figure 4) from exposures resulting in significantly elevated LDH activity. It was immediately evident that the 6–20 μm diameter range was likely incorporating an artifact, as a 4-fold increased BAL cell number was measured for the 1 d post-exposure time point. Based on historical data in the laboratory, inflammatory cell influx was not observed at this early stage with this fume (Antonini et al., 2007; Zeidler-Erdely et al., 2011). When the BAL cells were differentiated under light microscopy to yield the percentage of each cell type present, the absolute numbers, when calculated from the automated cell counting method, were erroneously increased compared to numbers calculated using the manual method (as shown in Figure 5).
Unfortunately, if different methods for cell counting are used, as they often are, results will likely be misleading in inter-laboratory comparisons of the PMN response in a BAL study involving cytotoxic particles. This is complicated by the fact that BAL as a procedure in and of itself has inherent variability (i.e. lavage volumes, animal handling and “massage” of the lung, instillate dwell time, recovered BAL fluid amount, temperature and type of instillate, etc.) (Cherniack et al., 1990). Details of the counting method used, including diameter ranges (for the automated method), the BAL protocol, total BAL cell count per mL, and a percentage differential cell count, should be reported. Fold changes and the total cell count number/mL were more comparable in this instance when the 9–20 μm diameter range was applied. However, altering this parameter of the automated analyzer is not ideal because it would “gate out” a proportion of PMN and smaller cell types, specifically lymphocytes. The diameter range of the rodent PMN measured in this study was 6.0– 13.5 μm. Because the cells were sized after cytocentrifugation, which reportedly causes some cell flattening and spreading at least in macrophages (Haley et al., 1991; Krombach et al., 1997), the PMN size range is likely lower than measured.
The association between a cytotoxic lung response and falsely elevated total BAL cell counts arising from an automated cell counting method was further examined using two additional inhalation exposure scenarios (non-cytotoxic SWMS versus cytotoxic SWGS fumes) and ITI of GMA-SS in rats. Bolus exposure methods, such as ITI or oropharyngeal aspiration (in mice), are typically accompanied by an early, acute lung inflammatory cell influx that resolves with time. This contrasts with a more gradual inflammatory response following an inhalation exposure (Erdely et al., 2013; Zeidler-Erdely et al., 2011). Since many pulmonary toxicology studies use a dose–response and time-course design, the potential also exists for bolus exposures to produce artificially increased cell counts when an automated method is used, primarily at early post-exposure times at the highest dose. Fold changes, as well as the total BAL cell number per mL, from the automated and manual methods showed strong agreement with a noncytotoxic SWMS fume exposure. This exposure scenario also excluded the possibility of interference of the welding particles (~3 μm) that were present in the BAL fluid within the selected diameter ranges of the automated analyzer. However, interference has been reported with talc particles similar in diameter to a leukocyte, median diameter of 7.8 μm (Lee et al., 2001). With a cytotoxic response in the lung causing ~2-fold increased LDH, however, the total BAL cell numbers increased more than 3-fold with the automated method but not with the manual method. Further, LDH mirrored the automated cell count number with an increasing dose of welding fume following a dose-dependent ITI exposure. Based on the low-dose exposure at 7 d, the cutoff point when a different counting method would need to be used so as to not generate spurious results would likely be an approximate 1.3-fold elevation of LDH. When the LDH reached a 1.4-fold increase, the automated method erroneously reported a significant increase in cell number.
The present study found the automated counter repeatedly indicated an increased total BAL cell count when significant lung cytotoxicity was present. The manual method when performed by a trained user, although more laborious, did not confirm the results of the automated cell counter but did agree closely with flow cytometry. Therefore, regardless of exposure method, the level of cytotoxicity associated with the particle exposure appears to be a primary indicator as to whether the manual or automated method should be used. Since most pulmonary toxicity studies begin with cytotoxicity as an unknown variable, the ideal method would be flow cytometry or the manual method with differential cell profiles also reported.
Acknowledgments
The authors would like to acknowledge Aliakbar Afshari, Amy Cumpston, Jared L. Cumpston and Donny Leonard for their technical support with the aerosol inhalation exposure systems.
Footnotes
Declaration of interest
The authors report no declarations of interest. “The findings and conclusions in this report are those of the author(s) and do not necessarily represent the views of the National Institute for Occupational Safety and Health”.
References
- Afshari A, Zeidler-Erdely PC, McKinney W, et al. Development and characterization of a resistance spot welding aerosol generator and inhalation exposure system. Inhal Toxicol. 2014;26:708–19. doi: 10.3109/08958378.2014.941118. [DOI] [PubMed] [Google Scholar]
- Antonini JM, Afshari AA, Stone S, et al. Design, construction, and characterization of a novel robotic welding fume generator and inhalation exposure system for laboratory animals. J Occup Environ Hyg. 2006;3:194–203. doi: 10.1080/15459620600584352. [DOI] [PubMed] [Google Scholar]
- Antonini JM, Lawryk NJ, Krishna Murthy GG, Brain JD. Effect of welding fume solubility on lung macrophage viability and function in vitro. J Toxicol Environ Health A. 1999;58:343–63. doi: 10.1080/009841099157205. [DOI] [PubMed] [Google Scholar]
- Antonini JM, Roberts JR, Stone S, et al. Persistence of deposited metals in the lungs after stainless steel and mild steel welding fume inhalation in rats. Arch Toxicol. 2011;85:487–98. doi: 10.1007/s00204-010-0601-1. [DOI] [PubMed] [Google Scholar]
- Antonini JM, Stone S, Roberts JR, et al. Effect of short-term stainless steel welding fume inhalation exposure on lung inflammation, injury, and defense responses in rats. Toxicol Appl Pharmacol. 2007;223:234–45. doi: 10.1016/j.taap.2007.06.020. [DOI] [PubMed] [Google Scholar]
- Berger SA, Waitman DA, Baruch R. Coulter counter identifies Cryptococcus neoformans as leukocytes a case of pseudopleocytosis. Am J Clin Pathol. 1992;97:663–4. doi: 10.1093/ajcp/97.5.663. [DOI] [PubMed] [Google Scholar]
- Cherniack RM, Banks DE, Bell DY, et al. Bronchoalveolar lavage constitutents in healthy individuals, idiopathic pulmonary fibrosis, and selected comparison groups. Am Rev Respir Dis. 1990;141:S169–202. doi: 10.1164/ajrccm/141.5_Pt_2.S169. [DOI] [PubMed] [Google Scholar]
- Erdely A, Dahm M, Chen BT, et al. Carbon nanotube dosimetry: from workplace exposure assessment to inhalation toxicology. Part Fibre Toxicol. 2013;10:53. doi: 10.1186/1743-8977-10-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haley PJ, Muggenburg BA, Weissman DN, Bice DE. Comparative morphology and morphometry of alveolar macrophages from six species. Am J Anat. 1991;191:401–7. doi: 10.1002/aja.1001910407. [DOI] [PubMed] [Google Scholar]
- Hyde DM, Tyler NK, Putney LF, et al. Total number and mean size of alveoli in mammalian lung estimated using fractionator sampling and unbiased estimates of the Euler characteristic of alveolar openings. Anat Rec Part A. 2004;274A:216–26. doi: 10.1002/ar.a.20012. [DOI] [PubMed] [Google Scholar]
- Krombach F, Munzing S, Allmeling A-M, et al. Cell size of alveolar macrophages: an interspecies comparison. Environ Health Perspect. 1997;105:1261–3. doi: 10.1289/ehp.97105s51261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YCG, Lane KB, Light RW. Coulter counter registers talc particles as leukocytes. Chest. 2001;119:669–70. doi: 10.1378/chest.119.2.669. [DOI] [PubMed] [Google Scholar]
- Lopez A, Yong S, Sharma A, Bailey D. Effect of sex, age, number of bronchoalveolar lavages and quantitation methods on the bronchoalveolar cell counts in rats. Can J Vet Res. 1986;50:101–5. [PMC free article] [PubMed] [Google Scholar]
- Marshall BA, Theil KS, Brandt JT. Abnormalities of leukocyte histograms resulting from microorganisms. Am J Clin Pathol. 1990;93:526–32. doi: 10.1093/ajcp/93.4.526. [DOI] [PubMed] [Google Scholar]
- McKinney W, Chen B, Frazer D. Computer controlled multi-walled carbon nanotube inhalation exposure system. Inhal Toxicol. 2009;21:1053–61. doi: 10.1080/08958370802712713. [DOI] [PubMed] [Google Scholar]
- Porter DW, Hubbs AF, Mercer RR, et al. Mouse pulmonary dose-and time course responses induced by exposure to multi-walled carbon nanotubes. Toxicology. 2010;269:136–47. doi: 10.1016/j.tox.2009.10.017. [DOI] [PubMed] [Google Scholar]
- Porter DW, Hubbs AF, Chen BT, et al. Acute pulmonary dose-responses to inhaled multi-walled carbon nanotubes. Nanotoxicology. 2012;7:1179–94. doi: 10.3109/17435390.2012.719649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens WW, Kim TS, Pujanauski LM, et al. Detection and quantitation of eosinophils in the murine respiratory tract by flow cytometry. J Immunol Methods. 2007;327:63–74. doi: 10.1016/j.jim.2007.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittington RJ, Comer DAM. Discrepancy between hemocytometer and electronic counts of blood cells. J Wildl Dis. 1984;20:258–60. doi: 10.7589/0090-3558-20.3.258. [DOI] [PubMed] [Google Scholar]
- Zeidler-Erdely PC, Battelli LA, Stone S, et al. Short-term inhalation of stainless steel welding fume causes sustained lung toxicity but no tumorigenesis in lung tumor susceptible A/J mice. Inhal Toxicol. 2011;23:112–20. doi: 10.3109/08958378.2010.548838. [DOI] [PubMed] [Google Scholar]
- Zeidler-Erdely PC, Meighan TG, Erdely A, et al. Lung tumor promotion by chromium-containing welding particulate matter in a mouse model. Part Fibre Toxicol. 2013;10:45. doi: 10.1186/1743-8977-10-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeidler-Erdely PC, Meighan TG, Erdely A, et al. Effects of acute inhalation of aerosols generated during resistance spot welding with mild-steel on pulmonary, vascular and immune responses in rats. Inhal Toxicol. 2014;26:697–707. doi: 10.3109/08958378.2014.944287. [DOI] [PMC free article] [PubMed] [Google Scholar]