Abstract
Background
Many young adult female cancer survivors (YAFCS) are at risk for premature menopause. This study characterized YAFCS’ post-treatment fertility information needs, reproductive concerns, and decisional conflict about future options for post-treatment fertility preservation (FP).
Methods
Participants completed a web-based, anonymous survey between February and March 2015. The survey included investigator-designed questions of perceived information needs, the Reproductive Concerns after Cancer Scale (RCACS), and the Decisional Conflict Scale (DCS). Analyses included Pearson’s correlations, t-tests, and multiple regression.
Results
Participants (N=346) averaged 29.9 years old (SD=4.1) and were 4.9 years post-treatment (SD=5.4; range, 0-27). Main analyses focused on a subgroup of YAFCS with uncertain fertility status who had not previously undergone/attempted FP and either wanted future children or were unsure (n=179). Across fertility information topics, 43-62% reported unmet information needs. The greatest reproductive concerns related to fertility potential and health of future offspring. The regression model controlled for a priori covariates including current age, age at treatment completion, income, relationship status, nulliparity, and prior fertility evaluation. Greater unmet information needs related to greater decisional conflict (β=0.43, p<.001); greater reproductive concerns were associated at the trend level (β=0.14, p=.08; F[8,118]=6.42, p<.001).
Conclusions
YAFCS with limited awareness or knowledge of their risk for premature menopause and FP options report higher levels of decisional conflict about future FP. Post-treatment survivorship care should include comprehensive reproductive health counseling, including post-treatment FP options and family-building alternatives.
Keywords: cancer survivorship, reproductive health, fertility, fertility preservation, decision-making
An estimated 1 in 47 women will be diagnosed with invasive cancer as a young adult.1 The gonadotoxic effects of many cancer treatments are well established, and providers are increasingly addressing fertility issues with their patients.2,3 However, most women do not pursue fertility preservation (FP) before treatment, despite wanting biological children in the future.3,4 Reasons include not being aware of fertility risks or FP options, time pressures, emotional distress, financial costs, and discomfort with the idea of using donor sperm for embryo freezing, in the period before egg freezing was available.5,6 Health care providers are also less likely to discuss fertility risks and FP with children and adolescents because of their focus on survival and the lack of non-experimental options prior to pubertal onset.7
For young adult female survivors not ready to start a family, post-treatment FP may be an option, but it is an underutilized service.8 Many survivors will indeed maintain reproductive potential after treatment but remain at risk for premature ovarian failure (early menopause), with a narrowed window of reproductive opportunity. Despite this, young adult survivors often do not receive recommended follow-up care regarding their reproductive health.9 Clinicians also self-report as feeling inadequately informed about cancer-related fertility issues and may underestimate patients’ concerns or fail to recognize fertility as a priority.7,10 Fertility information is one of the most cited unmet needs among young adult survivors in pre- and post-treatment care.11,12 Lack of awareness of post-treatment fertility status and uncertainty about reproductive potential has been linked to reduced mental health and quality of life.13–15
Research suggests variability in how female cancer survivors cope with uncertainty and distress related to potential fertility problems. Some women report that fertility concerns dominate their thoughts in survivorship and describe a preoccupation with the “missed opportunity” to have preserved their fertility prior to treatment along with anxiety and regret.16,17 Others minimize or avoid fertility-related thoughts in an effort to prioritize normality and reduce anxiety and grief.18–20 Based on research highlighting distress associated with confirmed infertility, survivors may be negatively affected if they experience premature menopause unexpectedly, without having the chance to consider their FP options.
For many women, the option to preserve fertility may be feasible and medically appropriate, making the decision “preference-sensitive,” based on personal values.21,22 These types of decisions often invoke decisional conflict, particularly when the likelihood of expected outcomes is not definite.23 Retrospectively, most female cancer survivors report clinically significant levels of decisional conflict about pre-treatment FP decisions.5,24,25 The experiences of post-treatment survivors considering future FP, however, are not well understood.
A better understanding of how young women understand their reproductive health and make decisions about the FP options available to them after treatment is needed. This will help to promote informed, values-based decision-making; enabling survivors to take advantage of available reproductive technology when desired and appropriate, and avoid potential future distress. The goals of this study were to describe survivors’ unmet information needs about fertility topics, their reproductive concerns, and the degree of decisional conflict they experienced when prompted to consider the decision to pursue FP in the future. In order to identify potential factors contributing to decision-making distress, the extent to which unmet information needs and reproductive concerns related to decisional conflict about future FP was also evaluated.
Methods
Design
Cross-sectional, internet-based survey designed to measure the fertility-related experiences of young adult female cancer survivors who had completed therapy. Surveys were administered between February – March, 2015. This study was approved as exempt research by the Memorial Sloan Kettering Cancer Center (MSK) Institutional Review Board.
Participants
Eligibility criteria included female survivors between the ages of 18 to 35 years old with a prior cancer diagnosis, who had successfully completed treatment at least one year prior, and were disease-free.
Survey
The survey was designed by an interdisciplinary team with input from young adult female survivors. The survey was anonymous and protected health information was not collected. Standard questions were used to assess sociodemographic, medical, and fertility-related information. Reasons for not pursuing FP before treatment were assessed using items derived from Kim et al.5 Participants responded yes/no to a list of factors shown to be important in the FP decision-making process with the option to select more than one (e.g., time constraints, emotional distress, and cost).5 The survey was administered online using a commercially available website with SSL encryption. Participants were recruited through MSK and 17 young adult cancer survivor advocacy groups, using social media and email listservs. These procedures are consistent with recommended use of social media in young adult oncology research26–28 and similar to previously published studies with this population.16,29 Respondents were required to answer screener items to confirm eligibility.
Unmet information needs
Investigator-designed questions (5 items) assessed unmet information needs about fertility topics. Participants indicated (yes/no) whether they had as much information as they wanted about risk of infertility, risk of early menopause, options to assess their fertility status, options to preserve their fertility, and options for alternative family-building. A total score was calculated by summing the items (yes=0, no=1; range 0-5) such that higher scores indicate greater unmet information needs (Cronbach’s alpha=.81).
Reproductive concerns
The Reproductive Concerns after Cancer Scale (RCACS) is an 18-item, validated measure that includes six subscales (3 items each): Fertility Potential, Partner Disclosure, Child’s Health, Personal Health, Acceptance (reverse coded), and Becoming Pregnant.30 Responses are on a 5-point Likert scale from “Strongly disagree” to “Strongly agree” with total scores ranging from 18 to 90. Mean total and subscale scores were calculated with higher scores indicating greater reproductive concerns (Cronbach’s alpha=.83).
Decisional conflict about future FP
The “low health literacy” version of the Decisional Conflict Scale (DCS) was used to assess four domains of personal uncertainty in making a healthcare decision. This version of the DCS was chosen as a precaution given the online format and anonymity of the survey prevented formal assessment of participants’ reading skills. Subscales include: feeling uninformed, unclear about values, unsupported in decision-making, and feeling uncertain about which option to choose.31 The DCS is valid and reliable,23 and the most widely used measure of decision-making quality.32 The current study included 8 items of the 10-item scale and good internal reliability was demonstrated (Cronbach’s alpha=.84). Total possible scores range from 0 to 100 with higher scores indicating greater decisional conflict.
Statistical Analyses
Descriptive statistics characterized the sample and reasons for not undergoing pre-treatment FP. Differences among the most common diagnoses were examined using ANOVAs and chi-square. Main analyses excluded participants who reported infertility or inability to carry a pregnancy, did not want future children, or had previously attempted or undergone egg or embryo cryopreservation or ovarian transposition. Independent samples t-tests and Pearson’s correlations evaluated bivariate relations among unmet information needs, reproductive concerns, and decisional conflict about future FP in the remaining subgroup. A regression model examined how unmet information needs and reproductive concerns contributed to decisional conflict about future FP. A priori covariates included current age, age at treatment completion, income, relationship status, nulliparity, and prior fertility evaluation. Missing data was not replaced. Percentages that are not based on the total sample of N=346 are specified.
Results
Among 714 respondents who accessed the survey, 359 (50%) met eligibility criteria and 346 of eligible respondents (97%) completed the survey. Participants primarily resided in the United States (84%) and were from suburban (49%), urban (32%), and rural (10%) areas. Average age at survey completion was 29.9 years old (SD=4.1). Participants were a mean of 23.6 years (SD=7.5) at the time of treatment completion; 35 (10%) were less than 15 years of age at diagnosis. The most common diagnoses were lymphoma (23%), breast (20%), gynecologic (14%), and leukemia (13%) with lymphoma and leukemia patients being younger with a longer time since treatment than other disease groups. Sociodemographic and clinical/fertility information are provided in Tables 1 and 2, respectively.
Table 1.
Sociodemographic characteristics of the total sample (N=346) and subgroup (n=179).
| Total Sample | Subgroup1 | |||||
|---|---|---|---|---|---|---|
| Sociodemographics | M (SD), range | n | % | M (SD), range | n | % |
| Age at survey completion (years) | 29.9 (4.1), 18–35 | 29.5 (4.2), 18–35 | ||||
| Age at diagnosis (years) | 23.6 (7.5), 0–35 | 23.4 (7.1), 0–34 | ||||
| < 15 years at diagnosis | 35 | 10 | 19 | 11 | ||
| Race | ||||||
| White | 279 | 81 | 140 | 78 | ||
| Other | 29 | 8 | 13 | 7 | ||
| Hispanic ethnicity | 25 | 7 | 10 | 6 | ||
| Education | ||||||
| < College degree | 76 | 22 | 35 | 20 | ||
| ≥ College degree | 241 | 69 | 121 | 68 | ||
| Enrolled student, full or part-time | 74 | 21 | 37 | 21 | ||
| Employed, full or part-time | 249 | 72 | 125 | 70 | ||
| Household income (annual; U.S. dollars) |
||||||
| <50,000 | 103 | 30 | 52 | 29 | ||
| 50,000 – 100,000 | 116 | 34 | 59 | 33 | ||
| >100,000 | 77 | 22 | 34 | 19 | ||
| Married or living with a partner | 208 | 60 | 106 | 59 | ||
| Had at least 1 child | 109 | 31 | 50 | 28 | ||
Subgroup that had not been told they were infertile or unable to carry a pregnancy, who had not previously undergone egg/embryo cryopreservation or ovarian transposition, and who wanted future children or were unsure.
Table 2.
Clinical and fertility information of the total sample (N=346) and subgroup (n=179).
| Total Sample | Subgroup | |||
|---|---|---|---|---|
| Clinical information | n | % | n | % |
| Cancer diagnosis | ||||
| Lymphoma | 79 | 23 | 50 | 28 |
| Breast | 68 | 20 | 31 | 17 |
| Gynecologic2 | 50 | 14 | 20 | 11 |
| Leukemia | 45 | 13 | 15 | 8 |
| Colorectal | 27 | 8 | 10 | 6 |
| Sarcoma | 23 | 7 | 11 | 6 |
| Brain | 13 | 4 | 7 | 4 |
| Other | 54 | 16 | 35 | 20 |
| Gonadotoxic treatment | ||||
| Pelvic radiation | 59 | 17 | 13 | 7 |
| Chemotherapy | 285 | 82 | 141 | 79 |
| Surgery | 36 | 10 | 5 | 3 |
| Bone marrow transplant | 35 | 10 | 4 | 2 |
| Time since treatment ended (years) | ||||
| < 2 | 113 | 33 | 56 | 32 |
| 2 – 5 | 133 | 38 | 72 | 40 |
| > 5 | 98 | 29 | 50 | 28 |
| Fertility information |
Before Treatment |
|||
| n | % | |||
| Fertility preservation history3 | ||||
| Egg or embryo cryopreservation | 35 | 10 | ||
| Ovarian tissue cryopreservation | 4 | 1 | ||
| Ovarian transposition | 3 | 1 | ||
| Ovarian suppression | 16 | 5 | ||
| Other type of FP | 4 | 1 | ||
| Total | 56 | 16 | ||
Subgroup that had not been told they were infertile or unable to carry a pregnancy, who had not previously undergone egg/embryo cryopreservation or ovarian transposition, and who wanted future children or were unsure.
Includes ovarian, cervical, and uterine cancers.
Fertility preservation options were not mutually exclusive.
Fifty-six women (16%) underwent FP pre-treatment; four underwent FP post-treatment; and three attempted FP post-treatment but were unsuccessful. Women reported multiple reasons for not having pursued pre-treatment FP. Most common reasons were not knowing about FP (30%), feeling too distressed or overwhelmed (29%), and/or cost (27%).
At the time of survey completion, 106 (31%) participants had been told that they will not be able to get pregnant or carry a pregnancy due to treatment effects; 21 (20%) of those women had preserved their fertility before treatment. Notably, 92% of this subgroup wanted children in the future. Gynecologic and leukemia survivors were more likely to be infertile, but differences in relationship status, nulliparity, or prior fertility evaluation were not significant across disease groups.
Subgroup analyses
The primary subgroup of interest was women who wanted children in the future or were unsure, had not been told they were infertile, had not undergone ovarian transposition, and had not previously attempted or undergone egg or embryo cryopreservation. The following analyses were conducted in this subgroup (n=179; see Tables 1 and 2 for descriptive data).
Regarding unmet information needs, most respondents felt they did not have enough information on infertility risk (58%), early menopause risk (60%), options to assess their fertility (62%), options to preserve their fertility (51%), and options for alternative family-building (43%). The greatest reproductive concerns (M=3.20, SD=0.65) were related to concerns about potential fertility problems and health of a future child. For example, 64% were concerned they may not be able to have (more) children, 41% reported it was stressful to think about getting pregnant, and 59% were worried about passing on a genetic risk for cancer. Potential interpersonal difficulties were also indicated; 53% of women were concerned their partner or a future partner would be disappointed if they were unable to have children.
When prompted to consider the option of pursuing FP in the future, participants indicated high levels of decisional conflict (M=61.09, SD=24.88). Only 13% felt informed about their FP options and 74% were unclear about their personal values related to the decision. Notably, 70% felt they did not have enough advice, and 35% felt they did not have enough support to make a decision.
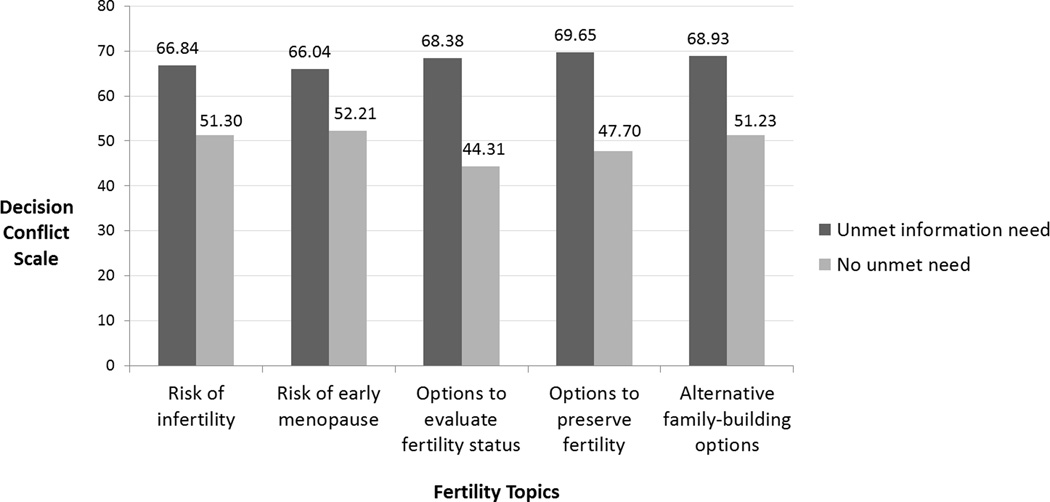
In bivariate analysis, greater decisional conflict was associated with having greater unmet information needs (Information Needs total, r=.47, p<.001) and reproductive concerns (RCACS total, r=.26, p=.001). Across all information topics, women who indicated that they had unmet information needs reported higher levels of decisional conflict (Figure 1; p <.01).
Figure 1. Unmet information needs about fertility topics is associated with greater decisional conflict about future fertility preservation.
Participants who reported unmet information needs about fertility topics reported significantly higher levels of decisional conflict about future fertility preservation (independent samples t-tests; all p’s<.01). Mean levels of decisional conflict for subgroups with and without unmet information needs are depicted.
In multiple regression analysis controlling for current age, age at treatment completion, income, relationship status, nulliparity, and prior fertility evaluation, the relation between greater unmet information needs (β=0.43, p<.001) and higher levels of decisional conflict about future FP remained significant; greater reproductive concerns were associated with greater conflict at the trend level (β=0.14, p=.08; F[8,118]=6.42, p<.001). Having undergone a fertility evaluation post-treatment related to lower decisional conflict (β=-0.19, p=.02). Unmet information needs and reproductive concerns accounted for 22% of the variance in decisional conflict (Fchange[2,118]=18.79, p<.001; R2 total=.30). See Table 3.
Table 3.
Regression model predicting decisional conflict about future fertility preservation (n=179).1
Greater decisional conflict about future fertility preservation was associated with greater unmet information needs and, at a trend level, greater reproductive concerns. Unmet information needs and reproductive concerns accounted for 22% of the variance in decisional conflict.
| Dependent Variable: Decisional Conflict Scale (F[8,118]=6.42, p<.001) | |||||||
|---|---|---|---|---|---|---|---|
| Step | Variable2 | R2 | B | SE | β | t | p |
| 1 | Constant | .08 | 27.19 | 16.84 | |||
| Current age | −.30 | .61 | −.05 | −.49 | .62 | ||
| Age at treatment completion | .46 | .38 | .12 | 1.20 | .23 | ||
| Relationship status (0=single) | −4.87 | 4.71 | −.10 | −1.03 | .30 | ||
| Nulliparity (0=no children) | 4.90 | 4.97 | .09 | .99 | .33 | ||
| Annual income (0=less than 50k) | .45 | 4.38 | .01 | .10 | .92 | ||
| Fertility Evaluation (0=no evaluation) | −15.29 | 6.31 | −.19 | −2.42 | .02 | ||
| 2 | Unmet Information Needs | 5.45 | 1.05 | .43 | 5.19 | <.001 | |
| Reproductive concerns | .30 | 5.49 | 3.15 | .14 | 1.74 | .08 | |
Subgroup that had not been told they were infertile or unable to carry a pregnancy, who had not previously undergone egg/embryo cryopreservation or ovarian transposition, and who wanted future children or were unsure.
The dichotomized variables include: relationship status (0=single; 1=partnered/married), nulliparity (0=no children; 1=at least one child), annual income (0=less than $50,000, 1=greater than/equal to $50,000), and fertility evaluation (0=no evaluation since treatment completion, 1=had an evaluation post-treatment).
Discussion
Many young adult female cancer survivors report a desire for biological children in the future but, for a variety of reasons, are unable to undergo FP prior to treatment. Those who maintain reproductive capacity after treatment, but are at risk for premature menopause, may have a second opportunity to pursue FP. This study is the first to our knowledge to examine the decisional conflict of young female survivors’ when prompted to consider post-treatment FP. For those who hope to have children in the future, failure to provide information and address concerns with respect to fertility-related decisions may have lasting consequences for their future family-building options.
Consistent with existing literature, we report high rates of unmet fertility information needs and reproductive concerns.11,12,33 To best inform clinical practice, we focused on the subgroup of women who had not been told they were infertile, believed they may want children in the future, and had not previously undergone FP. This subgroup of women reported high levels of decisional conflict about future FP. In other healthcare contexts, decisional conflict is associated with greater emotional distress, future decision regret, and greater likelihood of blaming providers.34 Unmet information and support needs increase decisional conflict and the risk for regret and distress.35–38 Women who receive pre-treatment fertility counseling experience less regret and report better quality of life post-treatment.17,39,40 However, fertility counseling alone may still result in low knowledge about fertility issues,41,42 suggesting more comprehensive approaches to providing decision support may be warranted.
Decisional conflict is also associated with an increased likelihood of avoiding or delaying decisions.34 Quinn et al. found that adolescent female survivors used a range of strategies to cope with potential fertility loss, including avoidance and denial of distressing cognitions.19 Notably, all of the adolescents in their sample reported a desire for biologic children, but neither they nor their parents were aware of the adolescents’ fertility status.19 Likewise, survivors have also reported a desire to postpone addressing fertility issues until they were ready to have children.43 Women who delay decision-making about FP may lose the option to take advantage of reproductive technologies and preserve their fertility post-treatment, if desired.
Importantly, decisional conflict may arise from multiple sources, and there may be subgroups of survivors with greater or different types of decision support needs. Providing information and addressing misperceptions about fertility topics is an important first step for all survivors, regardless of medical factors. In this survey, 71% of those queried worried about their family health history affecting future children, independent of a hereditary cancer diagnosis. Young survivors also report reproductive concerns while concurrently acknowledging their oncologists’ reassurance otherwise.43 Periodic assessment of survivors’ level of understanding about reproductive health issues and the nature of their concerns will help guide clinical practice and potentially avoid or ameliorate fertility-related distress. Importantly, ovarian function should be monitored irrespective of survivors’ desire for future children as lack of estrogen resulting from ovarian failure may exacerbate other late effects of cancer treatment such as cardiotoxicity, bone health, and endocrine disorders.44
Limited evidence suggests decision aids and counseling improve patient decision-making about pre-treatment FP and reduce later regret.24,38 However, factors influencing post-treatment FP decisions are different from pre-treatment decisions. Decision support needs may differ as women move beyond the emotional, physical, and financial aftermath of their cancer experience and fertility-related treatment effects are more fully realized.45,46 After treatment, survivors have more time to clarify their options, consider personal values and priorities, access support, and gather financial resources. Further work should determine the types of resources women need and the best approaches to provide those resources. While it is clear that information should be provided, additional efforts may help those who are highly distressed, anxious, or avoidant. A stepped care model of support may best address the varying levels of support needs by providing the opportunity for women to “step up” to increasingly supportive resources as needed (e.g., decision aid plus decision counselor).
It is also critical that reproductive health counseling be comprehensive. In addition to FP, support services should address alternative paths to achieving motherhood. We found that only a minority of the women (20%) who were unable to become pregnant or carry a pregnancy had preserved their fertility, despite 92% wanting future children. Forty-three percent reported unmet information needs regarding alternative family-building options such as adoption. Contraceptive use and counseling around safe sexual practices is equally important.47 Addressing issues related to dating and disclosure may also help survivors navigate interpersonal difficulties.33 Fertility topics affect a range of psychosocial concerns among young survivors and support services are sorely lacking.48
While the use of social media and web-based procedures have been recommended when conducting research with young adult cancer survivors,26,27 a number of limitations must be considered. Although screening items assessed eligibility, participant responses were not externally validated. Recruitment using young adult cancer advocacy groups’ social media outlets may have compromised the generalizability of the findings. Additionally, the cross-sectional design precluded conclusions regarding causality. Analyses were driven by empirically-based decision-making research,34,49 but further work is needed to determine the directionality of relations. Longitudinal studies may identify changes in survivors’ decisional conflict and support needs as they age with shifting priorities and life goals. Despite these limitations, findings fill an important gap in the literature regarding young adult female survivors’ decision-making about FP after treatment. The relatively large sample size and geographic diversity of the sample are important study strengths.
Conclusions
These data underline the importance of addressing fertility issues in post-treatment survivorship care to ensure that women at risk for premature ovarian failure do not miss their narrowed window of reproductive opportunity. There is a critical need to develop resources for survivors and for clinicians to use to support patients in making informed, values-based decisions about their reproductive options. This should be done in parallel to research addressing other sources of survivors’ unmet needs and barriers to clinical implementation of interventions (e.g., providers’ lack of knowledge or prioritization of fertility). Research supporting the use of biomarkers such as anti-Müllerian hormone to predict post-treatment reproductive potential is also growing with great promise of improving patient counseling.50 While patients will certainly benefit from more personalized information, this alone is not likely to eliminate decisional distress as women must still determine how their unique values, preferences, and circumstance weigh against the pros and cons of treatment options.34 Research prioritization strategies may be used to identify the relative value of targeting different approaches to improving patient outcomes, alone or in combination, and to ensure efficient use of research resources.51
Acknowledgments
Support for this research was provided by grants from the Memorial Sloan Kettering Cancer Center Support Grant/Core Grant (P30 CA008748) and National Institutes of Health/National Center for Advancing Translational Sciences grants KL2TR000458 and UL1TR000457-06.
We would like to thank Samantha Watson for her contribution to survey development and data collection, and to the adolescent and young adult cancer organizations that facilitated data collection.
Footnotes
Conflict of interest: The authors report no conflicts of interest.
Author Contributions: Benedict Catherine: Conceptualization, methodology, formal analysis, investigation, writing – original draft, writing – review and editing, and project administration. Bridgette Thom: Conceptualization, methodology, formal analysis, investigation, writing – review and editing, and project administration. Danielle Friedman: Conceptualization, methodology, and writing – review and editing. Debbie Diotallevi: Conceptualization and writing – review and editing. Elaine Pottenger: Conceptualization and writing – review and editing. Nirupa Raghunathan: Conceptualization and writing – review and editing. Joanne F. Kelvin: Conceptualization, methodology, formal analysis, investigation, writing – review and editing, supervision, and project administration.
Contributor Information
Catherine Benedict, Email: cbenedict@nshs.edu.
Bridgette Thom, Email: thomb@mskcc.org.
Danielle Friedman, Email: friedmad@mskcc.org.
Debbie Diotallevi, Email: diotalld@mskcc.org.
Elaine Pottenger, Email: pottenge@mskcc.org.
Nirupa Raghunathan, Email: raghunan@mskcc.org.
Joanne F. Kelvin, Email: kelvinj@mskcc.org.
References
- 1.American Cancer Society. Cancer facts & figures 2014. Atlanta, GA: American Cancer Society; [Google Scholar]
- 2.Peccatori FA, Azim HA, Jr, Orecchia R, et al. Cancer, pregnancy and fertility: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013;24(Suppl 6):vi160–vi170. doi: 10.1093/annonc/mdt199. [DOI] [PubMed] [Google Scholar]
- 3.Loren AW, Mangu PB, Beck LN, et al. Fertility preservation for patients with cancer: American Society of Cinical Oncology clinical practice guideline update. J Clin Oncol. 2013;31(19):2500–2510. doi: 10.1200/JCO.2013.49.2678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coccia PF, Pappo AS, Altman J, et al. Adolescent and young adult oncology, version 2.2014. J Natl Compr Canc Netw. 2014;12(1):21–32. doi: 10.6004/jnccn.2014.0004. [DOI] [PubMed] [Google Scholar]
- 5.Kim J, Deal AM, Balthazar U, et al. Fertility preservation consultation for women with cancer: Are we helping patients make high-quality decisions? Reprod Biomed Online. 2013;27(1):96–103. doi: 10.1016/j.rbmo.2013.03.004. [DOI] [PubMed] [Google Scholar]
- 6.Blazer KR, Nehoray B, Solomon I, et al. Next-generation testing for cancer risk: Perceptions, experiences, and needs among early adopters in community healthcare settings. Genet Test Mol Biomarkers. 2015;5:5. doi: 10.1089/gtmb.2015.0061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thompson K, Dyson G, Holland L, et al. An exploratory study of oncology specialists' understanding of the preferences of young people living with cancer. Soc Work Health Care. 2013;52(2–3):166–190. doi: 10.1080/00981389.2012.737898. [DOI] [PubMed] [Google Scholar]
- 8.Johnson RH, Kroon L. Optimizing fertility preservation practices for adolescent and young adult cancer patients. J Natl Compr Canc Netw. 2013;11(1):71–77. doi: 10.6004/jnccn.2013.0010. [DOI] [PubMed] [Google Scholar]
- 9.Murphy D, Klosky JL, Reed DR, et al. The importance of assessing priorities of reproductive health concerns among adolescent and young adult patients with cancer. Cancer. 2015;121(15):2529–2536. doi: 10.1002/cncr.29466. [DOI] [PubMed] [Google Scholar]
- 10.Quinn GP, Vadaparampil ST, Gwede CK, et al. Discussion of fertility preservation with newly diagnosed patients: Oncologists' views. J Cancer Surviv. 2007;1(2):146–155. doi: 10.1007/s11764-007-0019-9. [DOI] [PubMed] [Google Scholar]
- 11.McClellan W, Klemp JR, Krebill H, et al. Understanding the functional late effects and informational needs of adult survivors of childhood cancer. Oncol Nurs Forum. 2013;40(3):254–262. doi: 10.1188/13.ONF.254-262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zebrack B. Information and service needs for young adult cancer survivors. Support Care Cancer. 2009;17(4):349–357. doi: 10.1007/s00520-008-0469-2. [DOI] [PubMed] [Google Scholar]
- 13.Kent EE, Arora NK, Rowland JH, et al. Health information needs and health-related quality of life in a diverse population of long-term cancer survivors. Patient Educ Couns. 2012;89(2):345–352. doi: 10.1016/j.pec.2012.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Halliday LE, Boughton MA. Exploring the concept of uncertain fertility, reproduction and motherhood after cancer in young adult women. Nurs Inq. 2011;18(2):135–142. doi: 10.1111/j.1440-1800.2011.00532.x. [DOI] [PubMed] [Google Scholar]
- 15.Halliday LE, Boughton MA, Kerridge I. Mothering and self-othering: the impact of uncertain reproductive capability in young women after hematological malignancy. Health Care Women Int. 2013 doi: 10.1080/07399332.2013.770005. [DOI] [PubMed] [Google Scholar]
- 16.Tschudin S, Bunting L, Abraham J, et al. Correlates of fertility issues in an internet survey of cancer survivors. J Psychosom Obstet Gynaecol. 2010;31(3):150–157. doi: 10.3109/0167482X.2010.503910. [DOI] [PubMed] [Google Scholar]
- 17.Benedict C, Thom B, Kelvin J. Young adult female cancer survivors’ decision regret about fertility preservation. J Adolesc Young Adult Oncol. doi: 10.1089/jayao.2015.0002. –Not available-, ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Crawshaw MA, Sloper P. 'Swimming against the tide'--the influence of fertility matters on the transition to adulthood or survivorship following adolescent cancer. Eur J Cancer Care (Engl) 2010;19(5):610–620. doi: 10.1111/j.1365-2354.2009.01118.x. [DOI] [PubMed] [Google Scholar]
- 19.Quinn GP, Murphy D, Knapp CA, et al. Coping styles of female adolescent cancer patients with potential fertility loss. J Adolesc Young Adult Oncol. 2013;2(2):66–71. doi: 10.1089/jayao.2012.0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Armuand GM, Wettergren L, Rodriguez-Wallberg KA, et al. Women more vulnerable than men when facing risk for treatment-induced infertility: a qualitative study of young adults newly diagnosed with cancer. Acta Oncol. 2015;54(2):243–252. doi: 10.3109/0284186X.2014.948573. [DOI] [PubMed] [Google Scholar]
- 21.Stacey D, Legare F, Col NF, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev. 2014;1:CD001431. doi: 10.1002/14651858.CD001431.pub4. [DOI] [PubMed] [Google Scholar]
- 22.Kassirer JP. Incorporating patients' preferences into medical decisions. N Engl J Med. 1994;330(26):1895–1896. doi: 10.1056/NEJM199406303302611. [DOI] [PubMed] [Google Scholar]
- 23.O'Connor AM. Validation of a decisional conflict scale. Med Decis Making. 1995;15(1):25–30. doi: 10.1177/0272989X9501500105. [DOI] [PubMed] [Google Scholar]
- 24.Peate M, Meiser B, Cheah BC, et al. Making hard choices easier: a prospective, multicentre study to assess the efficacy of a fertility-related decision aid in young women with early-stage breast cancer. Br J Cancer. 2012;106(6):1053–1061. doi: 10.1038/bjc.2012.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peate M, Meiser B, Friedlander M, et al. It's now or never: fertility-related knowledge, decision-making preferences, and treatment intentions in young women with breast cancer—an Australian fertility decision aid collaborative group study. J Clin Oncol. 2011;29(13):1670–1677. doi: 10.1200/JCO.2010.31.2462. [DOI] [PubMed] [Google Scholar]
- 26.Gorman JR, Roberts SC, Dominick SA, et al. A diversified recruitment approach incorporating social media leads to research participation among young adult-aged female cancer survivors. J Adolesc Young Adult Oncol. 2014;3(2):59–65. doi: 10.1089/jayao.2013.0031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Knapp CA, Quinn GP, Murphy D. Assessing the reproductive concerns of children and adolescents with cancer: challenges and potential solutions. J Adolesc Young Adult Oncol. 2011;1(1):31–35. doi: 10.1089/jayao.2010.0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brier MJ, Schwartz LA, Kazak AE. Psychosocial, health-promotion, and neurocognitive interventions for survivors of childhood cancer: a systematic review. Health Psychol. 2014 doi: 10.1037/hea0000119. No Pagination Specified. [DOI] [PubMed] [Google Scholar]
- 29.Partridge AH, Gelber S, Peppercorn J, et al. Web-based survey of fertility issues in young women with breast cancer. J Clin Oncol. 2004;22(20):4174–4183. doi: 10.1200/JCO.2004.01.159. [DOI] [PubMed] [Google Scholar]
- 30.Gorman JR, Su HI, Pierce JP, et al. A multidimensional scale to measure the reproductive concerns of young adult female cancer survivors. J Cancer Surviv. 2014;8(2):218–228. doi: 10.1007/s11764-013-0333-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reed D, Block RG, Johnson R. Creating an adolescent and young adult cancer program: Lessons learned from pediatric and adult oncology practice bases. J Natl Compr Canc Netw. 2014;12(10):1409–1415. doi: 10.6004/jnccn.2014.0138. [DOI] [PubMed] [Google Scholar]
- 32.Sepucha K, Borkhoff C, Lally J, et al. Establishing the effectiveness of patient decision aids: key constructs and measurement instruments. BMC Med Inform Decis Mak. 2013;13(2):1–11. doi: 10.1186/1472-6947-13-S2-S12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Crawshaw M. Psychosocial oncofertility issues faced by adolescents and young adults over their lifetime: a review of the research. Hum Fertil (Camb) 2013;16(1):59–63. doi: 10.3109/14647273.2012.733480. [DOI] [PubMed] [Google Scholar]
- 34.O'Connor AM, Jacobsen MJ, Stacey D. An evidence-based approach to managing women's decisional conflict. J Obstet Gynecol Neonatal Nurs. 2002;31(5):570–581. doi: 10.1111/j.1552-6909.2002.tb00083.x. [DOI] [PubMed] [Google Scholar]
- 35.Tschudin S, Bitzer J. Psychological aspects of fertility preservation in men and women affected by cancer and other life-threatening diseases. Hum Reprod Update. 2009;15(5):587–597. doi: 10.1093/humupd/dmp015. [DOI] [PubMed] [Google Scholar]
- 36.Crawshaw MA, Glaser AW, Hale JP, et al. Male and female experiences of having fertility matters raised alongside a cancer diagnosis during the teenage and young adult years. Eur J Cancer Care (Engl) 2009;18(4):381–390. doi: 10.1111/j.1365-2354.2008.01003.x. [DOI] [PubMed] [Google Scholar]
- 37.Zebrack BJ, Mills J, Weitzman TS. Health and supportive care needs of young adult cancer patients and survivors. J Cancer Surviv. 2007;1(2):137–145. doi: 10.1007/s11764-007-0015-0. [DOI] [PubMed] [Google Scholar]
- 38.Letourneau JM, Ebbel EE, Katz PP, et al. Pretreatment fertility counseling and fertility preservation improve quality of life in reproductive age women with cancer. Cancer. 2012;118(6):1710–1717. doi: 10.1002/cncr.26459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Letourneau JM, Katz PP, Smith JF, et al. The impact of fertility counseling and fertility preservation on long-term psychosocial outcomes in young female cancer survivors. Fertil Steril. 2010;94(4):S65-S. [Google Scholar]
- 40.Balthazar U, Deal AM, Fritz MA, et al. The current fertility preservation consultation model: are we adequately informing cancer patients of their options? Hum Reprod. 2012;27(8):2413–2419. doi: 10.1093/humrep/des188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Garvelink MM, Ter Kuile MM, Bakker RM, et al. Women's experiences with information provision and deciding about fertility preservation in the Netherlands: 'satisfaction in general, but unmet needs'. Health Expect. 2015;18(5):956–968. doi: 10.1111/hex.12068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Benedict C, Ford J. Fertility issues in adolescent and young adult cancer survivors. J Adolesc Young Adult Oncol. doi: 10.1089/jayao.2015.0024. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Murphy D, Orgel E, Termuhlen A, et al. Why healthcare providers should focus on the fertility of aya cancer survivors: It's not too late! Front Oncol. 2013;3:248. doi: 10.3389/fonc.2013.00248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Connell S, Patterson C, Newman B. A qualitative analysis of reproductive issues raised by young australian women with breast cancer. Health Care Women Int. 2006;27(1):94–110. doi: 10.1080/07399330500377580. [DOI] [PubMed] [Google Scholar]
- 45.Goossens J, Delbaere I, Van Lancker A, et al. Cancer patients' and professional caregivers' needs, preferences and factors associated with receiving and providing fertility-related information: a mixed-methods systematic review. Int J Nurs Stud. 2014;51(2):300–319. doi: 10.1016/j.ijnurstu.2013.06.015. [DOI] [PubMed] [Google Scholar]
- 46.Quinn MM, Letourneau JM, Rosen MP. Contraception after cancer treatment: describing methods, counseling, and unintended pregnancy risk. Contraception. 2014;89(5):466–471. doi: 10.1016/j.contraception.2014.01.014. [DOI] [PubMed] [Google Scholar]
- 47.Zebrack B. Information and service needs for young adult cancer survivors. Support Care Cancer. 2009;17(4):349–357. doi: 10.1007/s00520-008-0469-2. [DOI] [PubMed] [Google Scholar]
- 48.O'Connor A. [Accessed December 28, 2015];Ottawa Decision Support Framework to address decisional conflict. 2006 http://decisionaid.ohri.ca/docs/develop/ODSF.pdf.
- 49.Dunlop CE, Anderson RA. Uses of anti-mullerian hormone (amh) measurement before and after cancer treatment in women. Maturitas. 2015;80(3):245–250. doi: 10.1016/j.maturitas.2014.12.005. [DOI] [PubMed] [Google Scholar]
- 50.Myers E, Sanders G, Ravi D, et al. Evaluating the potential use of modeling and value-of-information analysis for future research prioritization within the evidence-based practice center program. Rockville, MD: Agency for Healthcare Research and Quality: Duke Evidence-based Practice Center; 2011. [PubMed] [Google Scholar]