Abstract
Enteric dysbiosis is a characteristic feature of progressive human immunodeficiency virus type 1 (HIV-1) infection but has not been observed in simian immunodeficiency virus (SIVmac)-infected macaques, including in animals with end-stage disease. This has raised questions concerning the mechanisms underlying the HIV-1 associated enteropathy, with factors other than virus infection, such as lifestyle and antibiotic use, implicated as playing possible causal roles. Simian immunodeficiency virus of chimpanzees (SIVcpz) is also associated with increased mortality in wild-living communities, and like HIV-1 and SIVmac, can cause CD4+ T cell depletion and immunodeficiency in infected individuals. Given the central role of the intestinal microbiome in mammalian health, we asked whether gut microbial constituents could be identified that are indicative of SIVcpz status and/or disease progression. Here, we characterized the gut microbiome of SIVcpz-infected and -uninfected chimpanzees in Gombe National Park, Tanzania. Subjecting a small number of fecal samples (N = 9) to metagenomic (shotgun) sequencing, we found bacteria of the family Prevotellaceae to be enriched in SIVcpz-infected chimpanzees. However, 16S rRNA gene sequencing of a larger number of samples (N = 123) failed to show significant differences in both the composition and diversity (alpha and beta) of gut bacterial communities between infected (N = 24) and uninfected (N = 26) chimpanzees. Similarly, chimpanzee stool-associated circular virus (Chi-SCV) and chimpanzee adenovirus (ChAdV) identified by metagenomic sequencing were neither more prevalent nor more abundant in SIVcpz-infected individuals. However, fecal samples collected from SIVcpz-infected chimpanzees within 5 months before their AIDS-related death exhibited significant compositional changes in their gut bacteriome. These data indicate that SIVcpz-infected chimpanzees retain a stable gut microbiome throughout much of their natural infection course, with a significant destabilization of bacterial (but not viral) communities observed only in individuals with known immunodeficiency within the last several months before their death.
Keywords: SIVcpz, gut microbiome, dysbiosis, fecal virome, AIDS enteropathy, chimpanzees
INTRODUCTION
Simian immunodeficiency virus (SIVcpz) of chimpanzees (Pan troglodytes) is the progenitor of human immunodeficiency virus type 1 (HIV-1), the causative agent of the human AIDS pandemic [Sharp & Hahn, 2011]. Although only the central (P. t. troglodytes) and eastern (P. t. schweinfurthii) subspecies are naturally infected, SIVcpz is widespread throughout their habitats in west-central and eastern Africa, with overall prevalence rates of 6% and 14%, respectively [Keele et al., 2006; Li et al., 2012; Rudicell et al., 2011; Santiago et al., 2002, 2003; Van Heuverswyn et al., 2007]. Conducting non-invasive natural history studies in Gombe National Park, Tanzania, we previously showed that SIVcpz-infected chimpanzees have a significantly (10–16-fold) increased risk of death compared to uninfected chimpanzees, that infected females are less likely to give birth and have a much higher infant mortality rate than uninfected females, and that SIVcpz can cause CD4+ T lymphocyte depletion and histopathological findings consistent with end-stage AIDS [Keele et al., 2009; Terio et al., 2011]. Moreover, one Gombe community, which exhibited the highest SIVcpz prevalence rate (40–50%), suffered a catastrophic population decline [Rudicell et al., 2010]. These data indicated that SIVcpz, like HIV-1, is capable of causing substantial morbidity and mortality in infected populations. In HIV-1-infected humans, high plasma viral loads, reduced CD4+ T cell counts, and general systemic immune activation are markers of disease progression [Brenchley et al., 2006; Hazenberg et al., 2003; Mellors et al., 1997]. These parameters cannot be measured in wild-living chimpanzees, since even in habituated communities, such as Gombe, it is neither ethical nor practical to repeatedly tranquilize chimpanzees for blood collection.
Many chronic diseases are characterized by changes in the gut microbiota [Cho & Blaser, 2012; Claesson et al., 2012]. In HIV-1 infection, the gastrointestinal tract is a primary site of virus replication, with CD4+ T cell depletion, loss of intestinal immune barriers, translocation of microbial products, and chronic immune activation representing key features of progressive disease [Brenchley & Douek, 2007]. It is thus not surprising that chronic HIV-1 infection has been linked to changes of the gut bacteriome, although results have not always been uniform, most likely because of differences in the types of samples collected, the sequencing and analysis methods used, and the disease and treatment status of the sampled individuals [Dillon et al., 2014; Dinh et al., 2015; Lozupone et al., 2013; McHardy et al., 2013; Mutlu et al., 2014; Vazquez-Castellanos et al., 2015; Vujkovic-Cvijin et al., 2013]. For example, several groups reported an association between HIV-1 infection and decreased alpha diversity, which describes the variety of microbiota (richness) and their relative abundances (evenness) within a sample [McHardy et al., 2013; Mutlu et al., 2014; Vazquez-Castellanos et al., 2015], whereas others reported an increase [Lozupone et al., 2013], or no effect at all [Dillon et al., 2014; Dinh et al., 2015; Vujkovic-Cvijin et al., 2013]. Similarly, different bacterial taxa have been reported to either be enriched or depleted (i.e., present at a significantly higher or lower frequency relative to the total microbial content) in the gut of HIV-1-infected patients, although several groups found an increase in the relative abundance of Prevotella [Dillon et al., 2014; Lozupone et al., 2013; Mutlu et al., 2014; Vazquez-Castellanos et al., 2015] and Proteobacteria [Dillon et al., 2014; Dinh et al., 2015; Vujkovic-Cvijin et al., 2013], and a decrease in the relative abundance of Bacteroides [Dillon et al., 2014; Lozupone et al., 2013; Mutlu et al., 2014; Vazquez-Castellanos et al., 2015; Vujkovic-Cvijin et al., 2013] and Ruminococcus [Dillon et al., 2014; McHardy et al., 2013; Mutlu et al., 2014; Vazquez-Castellanos et al., 2015]. Despite these differences, there is general agreement that compositional changes in gut microbial communities between individuals (beta diversity) are associated with progressive HIV-1 infection [Lozupone et al., 2014; Nwosu et al., 2014].
SIVmac infection of macaques recapitulates many of the clinical sequelae of HIV-1 infection, including the characteristic gut pathology, and represents an important animal model to study HIV-1 pathogenesis [Estes et al., 2010; Li et al., 2005]. It thus came as a surprise when macaques with progressive SIVmac infection were found to have a highly stable gut bacteriome [Handley et al., 2012; Klase et al., 2015; McKenna et al., 2008], although an expansion of their enteric virome was observed [Handley et al., 2012]. These findings raised questions concerning the mechanisms underlying the changes in intestinal microbiota associated with HIV-1 infection, with factors such as geographic location [Yatsunenko et al., 2012], antibiotic use [Jernberg et al., 2007], and antiretroviral treatment [Klase et al., 2015] implicated as playing potential causal roles. Wild-living chimpanzees are not exposed to drug therapy and maintain relatively stable geographic locations, and thus may represent a more relevant model to examine the impact of a pathogenic primate lentivirus infection on gut microbial diversity.
Previous studies of wild chimpanzees showed that their gut microbiota are very similar to those of humans, both with respect to the types of bacterial constituents present [Moeller et al., 2012] and their modulation by environmental factors such as diet and geographic location [Degnan et al., 2012; Moeller et al., 2013a]. We thus reasoned that analyses of the microbiota in chimpanzee fecal samples might yield insight into the natural history and pathogenesis of SIVcpz infection. Studying a small number (N = 6) of Gombe chimpanzees before and after they became infected with SIVcpz, we previously found greater fluctuations in bacterial composition as well as a higher abundance of disease-associated bacterial genera in fecal samples collected after SIVcpz acquisition [Moeller et al., 2013b]. However, an analysis of the gut bacteriome of SIVgor-infected and -uninfected western gorillas (Gorilla gorilla) failed to identify such differences [Moeller et al., 2015], despite the fact that SIVgor originated from the cross-species transfer of SIVcpz and may share some of the same pathogenic properties [Takehisa et al., 2009; Van Heuverswyn et al., 2007]. Here, we used both metagenomic and bacterial 16S rRNA gene sequencing to study a much larger number of SIVcpz-infected and -uninfected chimpanzees in Gombe National Park, Tanzania. Our results indicate a surprising robustness of the chimpanzee gut micro-biome throughout much of the duration of the SIVcpz infection course, with significant compositional changes of bacterial, but not viral, constituents observed only in individuals with known (or suspected) immunodeficiency within the last several months before their death.
METHODS
Chimpanzee Fecal Samples
Fecal samples were collected from wild-living chimpanzees in Gombe National Park, Tanzania, including members of habituated (Mitumba and Kasekela) and non-habituated (Kalande) communities (for a map of Gombe National Park and the ranges of its three communities, see [Lonsdorf EV, Gillespie TR, Wolf TM, et al. 2016. Socioecological correlates of clinical signs in two communities of wild chimpanzees (Pan troglodytes) at Gombe National Park, Tanzania. American Journal of Primatology, submitted.]). The Kasekela and Mitumba chimpanzees have been under direct observation since the 1960s and 1980s, respectively [Pusey et al., 2007; van Lawick-Goodall, 1968], with prospective fecal sampling and SIVcpz diagnostics beginning in 1999 [Santiago et al., 2002]. In Mitumba and Kasekela, stool samples were collected from individually known chimpanzees under direct observation. In Kalande, samples were collected opportunistically. For individual identification, all samples were subjected to mitochondrial, sex, and microsatellite analysis as described elsewhere [Keele et al., 2009; Rudicell et al., 2010]. Fecal material (20–50 g) was placed in conical tubes containing 20 ml of RNAlater (Ambion), a high salt solution that preserves fecal nucleic acids and allows storage and transport at room temperature. The SIVcpz infection status was determined by screening fecal samples for the presence of virus-specific antibodies by Western blot analysis and/or virion RNA by nested reverse transcriptase polymerase chain reaction (RT-PCR) analysis [Santiago et al., 2003]. AIDS-like immunopathology was detected in recovered bodies of a subset of SIVcpz-infected individuals as previously described [Terio et al., 2011]. All fieldwork was approved by the Tanzania Commission for Science and Technology, the Tanzania Wildlife Research Institute, and the Tanzania National Parks, and adhered to the American Society of Primatologists’ Principles for Ethical Treatment of Nonhuman Primates.
Metagenomic (Shotgun) Sequencing and Analysis
The fecal samples selected for metagenomic sequencing are listed in Table I. Nucleic acids were extracted as described [Minot et al., 2013], with modifications to accommodate RNAlater preservation and ensure maximal virus nucleic acid recovery. RNAlater preserved fecal material (1 ml) was vortexed (1 min) and then added to 20 ml phosphate-buffered saline (PBS) solution, containing 1% bovine serum albumin (BSA), which increased mammalian virus nucleic acid recovery (not shown). Samples were again vortexed at maximum speed (1 min) and then centrifuged at 1,800g (5 min) to pellet debris. The supernatant was passed through a 0.45 μm filter (Millipore, Billerica, MA) to remove bacterial cells and subsequently concentrated by passing through a 100 kDa ultracentrifugal unit (Millipore, Billerica, MA). Following treatment with 10U RQ1 DNase (Promega, Madison, WI), fecal nucleic acids were extracted using the AllPrep DNA/RNA Mini kit (Qiagen, Valencia, CA), and fecal DNA was quantified using a Qubit 2.0 fluorometer (Invitrogen, Carlsbad, CA). Filtering and DNase treatment served to remove bacterial DNA, while at the same time enriching for particle associated (viral) nucleic acids. Both procedures were previously shown not to alter the composition of the fecal bacteriome [Handley et al., 2012]. Fecal DNA was prepared for metagenomic sequencing using the Nextera XT DNA Library Prep Kit (Illumina, San Diego, CA). Libraries were quality controlled using the Agilent 2200 TapeStation and sequenced on an Illumina MiSeq, using a 500-cycle MiSeq Reagent Kit v2 according to the manufacturer’s instructions. Paired-end sequences over 50 base pairs in length were assigned to taxa using a nucleotide BLAST search in combination with a customized nucleotide sequence database that included all RefSeq bacterial and viral reference genomes (http://www.ncbi.nlm.nih.gov/refseq/) as well as all GenBank virus sequence entries. Reads that mapped to mammalian viruses were subsequently blasted against the complete NCBI nonredundant (nr) database (http://blast.ncbi.nlm.nih.gov/) using BLASTx, and those that mapped to non-viral references were removed from the analysis. Tables of bacterial and viral read counts were generated and used for abundance analysis. Bacterial phylogeny was determined using the MEGAN version 4.70.4 software [Huson & Mitra, 2012].
TABLE I.
Metagenomic Sequencing of Microbial Communities in Chimpanzee Fecal Samples
| Sample | Chimpanzee | Sex | Communitya | Sample date | SIVcpz statusb | Minimum number of years infected |
|---|---|---|---|---|---|---|
| GM1586 | Ch-64 | F | KL | June 25, 2009 | Pos | 13 |
| GM2984 | Ch-48 | M | MT | May 31, 2012 | Pos | 13 |
| GM3018 | Ch-22 | F | KK | June 28, 2012 | Pos | 12 |
| GM2423 | Ch-80 | F | KK | April 11, 2011 | Pos | 10 |
| GM3149 | Ch-52 | M | KK | September 7, 2012 | Pos | 12 |
| GM2522 | Ch-88 | F | KL | June 11, 2011 | Neg | – |
| GM2856 | Ch-59 | M | MT | March 6, 2012 | Neg | – |
| GM3028 | Ch-09 | F | KK | July 3, 2012 | Neg | – |
| GM2954 | Ch-15 | F | KK | May 13, 2012 | Neg | – |
KK, Kasekela; MT, Mitumba; KL, Kalande.
Pos, positive; Neg, negative
Bacterial 16S rRNA Gene Sequencing and Analysis
The fecal samples selected for bacterial 16S rRNA gene sequencing are listed in Table II. DNA was extracted from 0.5 ml of RNAlater preserved fecal material using the QIAamp DNA Stool Mini Kit (Qiagen, Valencia, CA) and the QIAcube system. 16S rRNA gene amplification was performed as described [Caporaso et al., 2011; Song et al., 2013], using 100 ng of fecal DNA, the Accuprime Taq DNA Polymerase System (Invitrogen, Carlsbad, CA), and V1V2 region directed primers containing Illumina adapters, barcode, and linker regions [Song et al., 2013]. Each fecal sample was amplified on four independent occasions, with the products pooled and purified using AMPure XP beads (Beckman Coulter, Brea, CA) before sequencing on the Illumina MiSeq. 16S sequence data were analyzed using QIIME v1.7.0 [Caporaso et al., 2010] and default QIIME parameters unless specified. Sequences were separated by barcode, quality filtered, and operational taxonomic units (OTUs) formed using a cutoff of 97% identity and assigned using the Greengenes database. Samples with less than 50,000 sequences per sample were removed from the analysis. After taxonomic assignment, within-sample (alpha) diversity was calculated using observed species, Chao1 [Chao, 1984], Shannon Index [Shannon, 2001] and Simpson’s Diversity measures [Simpson, 1949]. Chao1 is a nonparametric richness calculator, and Shannon and Simpson indices measure diversity both in terms of richness and evenness. Between-sample (beta) diversity was calculated using Bray–Curtis dissimilarity [Bray & Curtis, 1957], which measures compositional dissimilarity, as well as Euclidean and weighted UniFrac distances [Lozupone & Knight, 2005], which measure the compositional distance between microbial communities. Euclidean and weighted UniFrac distances were also used for principal coordinates analysis.
TABLE II.
16S rRNA Gene Sequencing of Bacterial Communities in Chimpanzee Fecal Samples
| Samplea | Chimpanzeeb | Sexc | Communityd | Sample date | SIVcpz statuse | Observable signs of ill health near the time of samplingf |
|---|---|---|---|---|---|---|
| GM2541 | 39 | F | MT | June 18, 2011 | Pos | None |
| GM2935 | 39 | F | MT | April 21, 2012 | Pos | None |
| GM3193 | 39 | F | MT | September 24, 2012 | Pos | None |
| GM1425 | 106 | F | KL | November 5, 2007 | Pos | n/a |
| GM1542 | 106 | F | KL | January 19, 2009 | Pos | n/a |
| GM2321 | 106 | F | KK | Jan 11, 2011 | Pos | n/a |
| GM889 | 107 | F | KL | August 13, 2005 | Pos | n/a (presumed dead) |
| GM3256 | 137 | F | KL | October 9, 2012 | Pos | n/a |
| GM3483 | 137 | F | KK | April 15, 2013 | Pos | n/a |
| GM715 | 99 | F | KK | May 11, 2005 | Pos | None |
| GM783 | 99 | F | KK | July 12, 2005 | Pos | None |
| GM958* | 99 | F | KK | March 15, 2006 | Pos | None (DOD November 30, 2006) |
| GM3401 | 119 | M | KK | January 12, 2013 | Pos | None |
| GM3427 | 119 | M | KK | March 16, 2013 | Pos | n/a |
| GM3521* | 119 | M | KK | June 8, 2013 | Pos | None (DLS August 15, 2013) |
| GM3186 | 4 | M | KK | September 20, 2012 | Pos | None |
| GM3429 | 4 | M | KK | March 21, 2013 | Pos | None |
| GM3551 | 4 | M | KK | July 9, 2013 | Pos | n/a |
| GM329 | 6 | M | KK | August 20, 2003 | Pos | n/a |
| GM414 | 6 | M | KK | July 16, 2004 | Pos | None |
| GM981 | 6 | M | KK | November 5, 2005 | Pos | None (DLS January 23, 2007) |
| GM944 | 89 | F | KL | April 26, 2006 | Pos | n/a |
| GM1277 | 89 | F | KL | August 6, 2007 | Pos | n/a |
| GM3496 | 71 | F | KK | April 25, 2013 | Pos | None |
| GM2556 | 133 | M | KK | July 3, 2011 | Pos | None |
| GM3188 | 133 | M | KK | September 21, 2012 | Pos | None |
| GM3492 | 133 | M | KK | April 25, 2013 | Pos | None |
| GM1163 | 21 | F | KK | February 11, 2007 | Pos | Diarrhea |
| GM1471 | 21 | F | KK | October 23, 2008 | Pos | None |
| GM1602* | 21 | F | KK | August 12, 2009 | Pos | None (DOD January 5, 2010) |
| GM1598 | 103 | F | KK | August 25, 2009 | Pos | None |
| GM2437 | 103 | F | KK | April 18, 2011 | Pos | None |
| GM2736* | 103 | F | KK | October 7, 2011 | Pos | n/a (DLS December 7, 11) |
| GM3251 | 22 | F | KK | October 24, 2012 | Pos | n/a |
| GM3505 | 22 | F | KK | April 20, 2013 | Pos | None |
| GM3595 | 22 | F | KK | August 11, 2013 | Pos | n/a |
| GM3392 | 100 | M | KL | December 25, 2012 | Pos | n/a |
| GM3477 | 100 | M | KL | April 15, 2013 | Pos | n/a |
| GM3543 | 100 | M | KL | July 2, 2013 | Pos | n/a |
| GM1376 | 64 | F | KL | June 9, 2008 | Pos | n/a |
| GM1532 | 64 | F | KL | September 17, 2008 | Pos | n/a |
| GM1618 | 64 | F | KL | August 24, 2009 | Pos | n/a (presumed dead) |
| GM3066 | 86 | F | KL | July 19, 2012 | Pos | n/a |
| GM3328 | 86 | F | KL | November 29, 2012 | Pos | n/a |
| GM3590 | 86 | F | KL | July 29, 2013 | Pos | n/a |
| GM2678 | 48 | M | MT | September 5, 2011 | Pos | None |
| GM3217 | 48 | M | MT | October 10, 2012 | Pos | None |
| GM3453* | 48 | M | MT | June 7, 2013 | Pos | None (DLS November 1, 2013) |
| GM84 | 30 | F | KK | December 13, 2001 | Pos | n/a |
| GM145 | 30 | F | KK | July 2, 2002 | Pos | n/a |
| GM337* | 30 | F | KK | August 7, 2003 | Pos | n/a (DLS December 8, 2003) |
| GM1030 | 33 | F | KK | February 23, 2006 | Pos | None |
| GM1320* | 33 | F | KK | August 9, 2007 | Pos | None (DLS March 2, 2008) |
| GM3075 | 80 | F | KK | July 30, 2012 | Pos | Weight loss |
| GM3326 | 80 | F | KK | November 29, 2012 | Pos | n/a |
| GM3530 | 80 | F | KL | June 11, 2013 | Pos | n/a |
| GM350 | 45 | M | MT | September 21, 2003 | Pos | n/a |
| GM704* | 45 | M | MT | November 27, 2004 | Pos | Weight loss, diarrhea (DOD December 22, 2004) |
| GM1037 | 36 | F | KK | March 15, 2006 | Pos | None |
| GM1325* | 36 | F | KK | June 28, 2007 | Pos | None (DOD November 7, 2007) |
| GM2675 | 52 | M | KK | September 10, 2011 | Pos | None |
| GM3167 | 52 | M | KK | September 14, 2012 | Pos | Diarrhea |
| GM3333 | 52 | M | KK | December 5, 2012 | Pos | n/a |
| GM1718 | 7 | M | KK | September 7, 2009 | Neg | None |
| GM2469 | 7 | M | KK | May 11, 2011 | Neg | None |
| GM2673 | 7 | M | KK | September 7, 2011 | Neg | n/a |
| GM1389 | 78 | F | KK | June 22, 2008 | Neg | Diarrhea |
| GM1624 | 78 | F | KK | August 5, 2009 | Neg | None |
| GM1348 | 109 | F | KL | March 12, 2008 | Neg | n/a |
| GM1462 | 109 | F | KL | October 14, 2008 | Neg | n/a |
| GM1705 | 109 | F | KL | July 9, 2009 | Neg | n/a |
| GM3096 | 97 | F | KK | August 12, 2012 | Neg | None |
| GM3343 | 97 | F | KK | December 12, 2012 | Neg | n/a |
| GM2619 | 2 | F | KK | August 4, 2011 | Neg | None |
| GM3508 | 2 | F | KK | April 14, 2013 | Neg | None |
| GM295 | 49 | F | MT | October 18, 2003 | Neg | n/a |
| GM622 | 49 | F | MT | December 12, 2004 | Neg | None (DOD June 12, 2010) |
| GM2861 | 68 | M | MT | March 7, 2012 | Neg | None |
| GM3027 | 131 | M | KK | July 3, 2012 | Neg | Respiratory illness |
| GM3351 | 131 | M | KK | December 22, 2012 | Neg | None |
| GM3457 | 131 | M | KK | May 16, 2013 | Neg | None |
| GM195 | 13 | M | KK | June 14, 2002 | Neg | n/a |
| GM1238 | 13 | M | KK | June 28, 2007 | Neg | None |
| GM3146 | 51 | M | KK | September 8, 2012 | Neg | n/a |
| GM3421 | 51 | M | KK | February 26, 2013 | Neg | n/a |
| GM3550 | 51 | M | KK | July 9, 2013 | Neg | n/a |
| GM719 | 14 | M | KK | May 4, 2005 | Neg | None |
| GM858 | 14 | M | KK | October 29, 2005 | Neg | n/a |
| GM975 | 14 | M | KK | March 12, 2006 | Neg | None |
| GM3236 | 15 | F | KK | October 21, 2012 | Neg | None |
| GM3548 | 15 | F | KK | July 6, 2013 | Neg | None |
| GM1104 | 77 | M | KK | July 1, 2006 | Neg | None |
| GM1241 | 77 | M | KK | June 25, 2007 | Neg | None |
| GM3355 | 53 | F | KK | January 2, 2013 | Neg | None |
| GM3475 | 53 | F | KK | May 7, 2013 | Neg | None |
| GM3124 | 17 | F | KK | August 24, 2012 | Neg | None |
| GM3402 | 17 | F | KK | January 15, 2013 | Neg | n/a |
| GM3511 | 17 | F | KK | April 7, 2013 | Neg | None |
| GM87 | 1 | F | KK | December 31, 2001 | Neg | n/a |
| GM196 | 1 | F | KK | April 28, 2002 | Neg | n/a |
| GM327 | 1 | F | KK | August 6, 2003 | Neg | n/a |
| GM1128 | 93 | F | KL | June 6, 2006 | Neg | n/a |
| GM1295 | 93 | F | KL | August 12, 2007 | Neg | n/a |
| GM3065 | 110 | M | KL | July 23, 2012 | Neg | n/a |
| GM3311 | 110 | M | KL | November 20, 2012 | Neg | n/a |
| GM3591 | 110 | M | KL | July 29, 2013 | Neg | n/a |
| GM881 | 108 | F | KL | July 25, 2005 | Neg | n/a |
| GM1000 | 108 | F | KK | March 16, 2006 | Neg | n/a |
| GM2632 | 98 | F | MT | August 14, 2011 | Neg | None |
| GM3162 | 98 | F | MT | September 11, 2012 | Neg | None |
| GM3532 | 98 | F | MT | June 25, 2013 | Neg | None |
| GM3386 | 88 | F | KL | December 20, 2012 | Neg | n/a |
| GM3572 | 88 | F | KL | July 16, 2013 | Neg | n/a |
| GM3620 | 88 | F | KL | May 15, 2013 | Neg | n/a |
| GM1019 | 54 | M | KK | January 25, 2006 | Neg | Respiratory illness |
| GM333 | 25 | F | KK | August 5, 2003 | Neg | n/a |
| GM445 | 25 | F | KK | June 30, 2004 | Neg | None |
| GM1010 | 25 | F | KK | January 19, 2006 | Neg | None |
| GM3292 | 26 | F | KK | November 10, 2012 | Neg | None |
| GM3503 | 26 | F | KK | April 11, 2013 | Neg | None |
| GM1151 | 27 | M | KK | January 30, 2007 | Neg | None |
| GM2363 | 27 | M | KK | March 6, 2011 | Neg | n/a |
| GM168 | 36 | F | KK | June 8, 2002 | Neg | n/a |
n/a, data not available; DOD, date of death; DLS, date last seen.
Asterisks (*) highlight samples that were collected from nine SIVcpz-infected chimpanzees within 8 months of their death.
Chimpanzees Ch-21 and Ch-36 died of AIDS-related causes as determined by necropsy; Ch-45 died of conspecific aggression and Ch-99 died of conditions resulting from spinal cord injury [Keele et al., 2009]; the cause of death of the other chimpanzees was not determined because their bodies were not recovered.
F, female; M, male.
KK, Kasekela; MT, Mitumba; KL, Kalande.
Pos, positive; Neg, negative.
Signs of illness observed within 1 month before or after the date of sample collection [Lonsdorf EV, Gillespie TR, Wolf TM, et al. 2016. Socioecological correlates of clinical signs in two communities of wild chimpanzees (Pan troglodytes) at Gombe National Park, Tanzania. American Journal of Primatology, submitted.].
Detection of Chimpanzee Stool-Associated Circular Virus and Chimpanzee Adenovirus in Fecal Samples
The presence of chimpanzee stool-associated circular virus (Chi-SCV) and chimpanzee (simian) adenovirus (ChAdV) in fecal samples was determined by nested PCR. Primers used to amplify a 214 bp fragment of the Chi-SCV replicase gene included: F1 5′-GGC TTG GTG TTT GTT AGC ACG ATC -3′ and R1 5′-GAG ATG GAA CCA AGA AGG GGC -3′ for the first round, and F2 5′-CKA TAG CCG TGT ATA GCT CGG -3′ and R2 5′-GGC AAC ATG GGC AAA TCG TGG C -3′ for the second round of PCR. First-round PCR amplifications included 35 cycles of denaturation (94°C, 15 sec), annealing (55°C, 30 sec), and elongation (68°C, 1 min) using Platinum Taq DNA Polymerase High Fidelity (Invitrogen, Carlsbad, CA). One microliter of the first-round product was used for the second round PCR, including 45 cycles of denaturation (94°C, 15 sec), annealing (58°C, 30 sec), and elongation (68°C, 1 min). Primers used to amplify a 379 bp fragment of the ChAdV DNA polymerase gene included: F1 5′-TGA TGC GYT TCT TAC CTY KGG TYT CCA TGA G -3′ and R1 5′-GAC AGC GAT SCG GAA GAS AGT G -3′ for the first round, and F2 5′-GTG ACR AAG AGG CTG TCC GTG TCY CCG TA -3′ and R2 5′-TCA CGT GGC MTA CAC YTA CAA GCC AAT CAC -3′ for the second round of PCR. Cycling conditions were the same as above. All amplicons were sequenced without interim cloning.
Quantitation of Chimpanzee Stool-Associated Circular Virus and Chimpanzee Adenovirus
Chi-SCV and ChAdV viral loads in fecal samples (Table II) were determined by real time PCR, using cloned fragments of the Chi-SCV replicase and the ChAdV polymerase as standards, respectively. Fecal DNA (5 μl) was added to TaqMan Fast Advanced master mix (Applied Biosystems, Foster City, CA) and amplified using virus specific forward (Chi-SCV: 5′-CGG AAT GTC GAT GAC TAT GAG G-3′; ChAdV: 5′-CKC GGT CCT CCT CGT AGA G -3′) and reverse (Chi-SCV: 5′-CTA CAT ACC GCC GTA CAT GAC G-3′; ChAdV: 5′-TGG ACA ACG ACC GCT ACC C -3′) primers (750 nM) as well as a virus-specific (Chi-SCV: 5′-FAM-CGC GGT CTT GTA GGA CTA GGC TCG CTA C-BHQ1a -3′; ChAdV: 5′-FAM-CCG GGT CCA GGC CAG CAC GAA GGA AGG-BHQ1a -3′) probe (250 nM). Each sample was tested in triplicate. PCR cycling conditions were chosen according to manufacturer’s instructions on a 7900HT Fast Real-Time PCR System. Sequence Detection Systems version 2.3 software (Applied Biosystems, Foster City, CA) was used to quantify viral copy numbers, which were normalized based on the total amount of DNA in the respective fecal sample.
Statistical Analysis
The abundances of bacterial and viral taxa in fecal samples from SIVcpz-infected and -uninfected chimpanzees were compared using the Mann–Whitney test and Prism version 5.0d software (GraphPad, La Jolla, CA). Bonferroni correction was applied when multiple tests were simultaneously performed using Prism or QIIME software. Statistical analysis of bacterial 16S alpha and beta diversity measures was performed using the default parameters of the QIIME v1.7.0 software.
RESULTS
Study Design
To test for associations between gut microbial communities and SIVcpz infection and/or disease progression, we used both metagenomic (shotgun) and targeted (bacterial) 16 rRNA gene sequencing approaches. Metagenomic sequencing is performed on randomly fragmented DNA and thus provides genetic information for all organisms present in a sample, including bacteria and viruses. However, metagenomic sequencing requires large quantities of fecal material and is computationally intensive. For this reason, we analyzed only a small number of samples (N = 9, Table I) with the goal of identifying potential microbial indicator species, which could then be studied in a larger number of samples from more individuals using real time PCR. As a second approach, we performed bacterial 16S rRNA gene sequencing, which requires only small amounts of starting material and thus allowed us to use the full range of samples collected from the Gombe chimpanzees over the past 15 years. We also took advantage of the well-validated analysis pipeline that exists for 16S rRNA sequence data [Caporaso et al., 2010], which facilitated a direct comparison with results previously obtained for HIV-1-infected humans [Dillon et al., 2014; Dinh et al., 2015; Lozupone et al., 2013; McHardy et al., 2013; Mutlu et al., 2014; Vazquez-Castellanos et al., 2015; Vujkovic-Cvijin et al., 2013] and SIVcpz-infected chimpanzees [Moeller et al., 2013b]. For 16S rRNA gene sequencing, we included up to three fecal samples per individual to control for temporal fluctuations in gut microbial diversity (Table II). The combination of these approaches was intended to ensure unbiased results, while taking full advantage of the contextual knowledge of the sampled chimpanzee hosts.
Metagenomic Sequencing Fails to Identify Significant Compositional Differences Between the Gut Bacteriome of SIVcpz-Infected and -Uninfected Chimpanzees
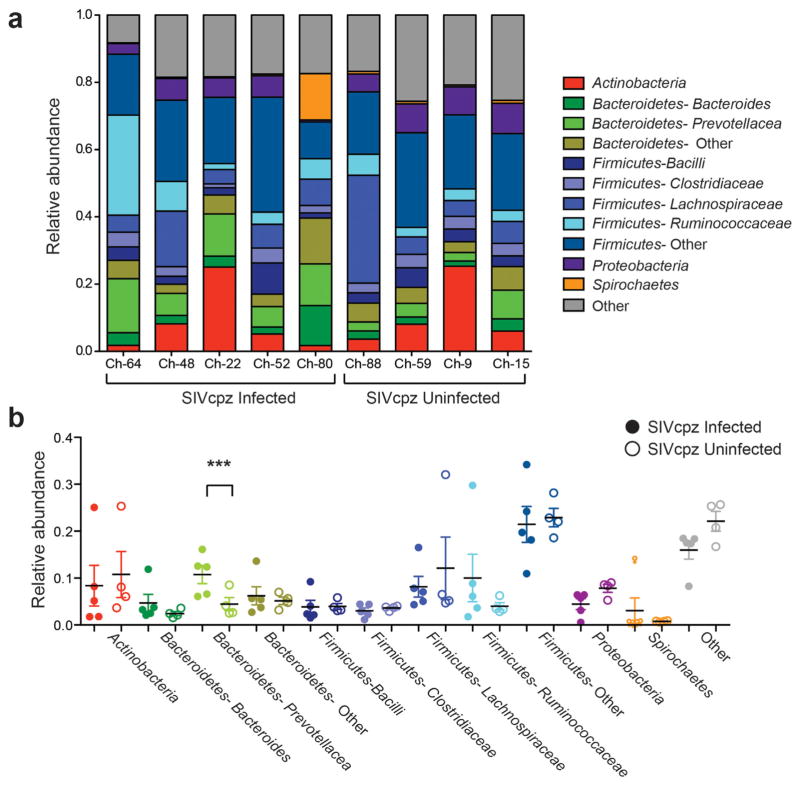
Metagenomic sequencing was performed on fecal samples from four uninfected and five infected Gombe chimpanzees (Table I). To detect both viral (particle-associated) and bacterial nucleic acids, each fecal sample was filtered and DNase treated prior to analysis. Although these procedures resulted in an overall reduction of bacterial DNA, a previous study demonstrated that this decrease was proportional and did not skew the composition of bacterial constituents in the sample [Handley et al., 2012]. The nine fecal samples yielded 15,900,990 high quality paired-end reads, with an average of 1,766,777 reads per sample. Sequences were assigned to bacterial taxa by performing a BLAST search of a database that included all bacterial reference genomes. Consistent with previous results [Degnan et al., 2012], we found that the bacterial phylum Firmicutes comprised the largest proportion of the fecal bacteriome in all nine chimpanzees, with Bacteroidetes, Actinobacteria, Proteobacteria, and Spirochaetes constituting the majority of the remaining bacterial phyla (Fig. 1a). Interestingly, fecal samples from the five SIVcpz-infected chimpanzees were enriched for members of the family Prevotellaceae (P = 0.04), although this result was not statistically significant when corrected for multiple tests (Fig. 1b). Nonetheless, this trend was consistent with results from previous studies that had shown an increased abundance of members of the genus Prevotella (of the Prevotellaceae family) in the intestinal microbiome of HIV-1-infected individuals [Dillon et al., 2014; Lozupone et al., 2013; Mutlu et al., 2014; Vazquez-Castellanos et al., 2015]. None of the other bacterial taxa differed in their relative abundance between SIVcpz-infected and -uninfected chimpanzees (Fig. 1b).
Fig. 1.
Composition of the fecal bacteriome in nine Gombe chimpanzees as determined by metagenomic sequencing. (a) Taxonomy bar chart indicating the relative abundance of major bacterial phyla in fecal samples from SIVcpz-infected (Ch-64, Ch-48, Ch-22, Ch-52, Ch-80) and -uninfected (Ch-88, Ch-59, Ch-9, Ch-15) chimpanzees (see Table I for more information). Only phyla comprising more than 1% of the fecal bacteriome are included and further classified into major families as indicated by different colors (minor Bacterioidetes and Firmicutes families are combined into “other” categories). A gray box (“other”) combines bacteria that could not be classified at the phylum level. (b) Relative abundance of major bacterial taxa in fecal samples from SIVcpz-infected (closed circles) and -uninfected (open circles) chimpanzees. Values are shown as a fraction of the total bacteria detected within each individual. Color-coding is as in (a). Black bars indicate the mean and colored bars the standard error of the mean (SEM). A statistical difference (***) was observed when the relative abundance of the bacterial family Prevotellaceae was compared between infected and uninfected chimpanzees using the Mann–Whitney U-test (P = 0.04); however, this value did not reach statistical significance (P <0.05) after Bonferroni correction for multiple tests.
Chronic SIVcpz Infection Is Not Characterized by a Distinct Gut Bacteriome
Since previous 16S rRNA sequencing was performed on samples from only six Gombe chimpanzees [Moeller et al., 2013b], we designed a follow-up study that included a larger number of samples and individuals. Targeting the V1V2 region of the 16S rRNA gene, we characterized the composition and diversity of gut bacterial communities in 123 fecal samples from 24 SIVcpz infected and 26 uninfected chimpanzees (Table II). Whenever possible, we included two or three samples per individual to control for intra-individual diversity. We also matched each virus positive fecal sample with a virus negative sample from the same community, gender, and collection month to control for environmental, social, and dietary differences [Degnan et al., 2012]. For SIVcpz-infected chimpanzees, the last available sample was included to cover as much of the natural disease course as possible. Very few chimpanzees had signs of ill health at or near the time of sampling (Table II) as determined by observational health surveys [Lonsdorf EV, Gillespie TR, Wolf TM, et al. 2016. Socioecological correlates of clinical signs in two communities of wild chimpanzees (Pan troglodytes) at Gombe National Park, Tanzania. American Journal of Primatology, submitted.]. However, nine SIVcpz-infected chimpanzees, including Malaika (Ch-21), Skosha (Ch-30), Titania (Ch-33), Yolanda (Ch-36), Vincent (Ch-45), Rudi (Ch-48), Echo (Ch-99), Mambo (Ch-103), and Eriki (Ch-119) died within 8 months of the last sample collection. Previous necropsy studies showed that both Yolanda and Malaika exhibited histopathological findings consistent with full-blown AIDS, whereas Vincent and Echo died of conspecific aggression and spinal cord injury, respectively [Keele et al., 2009; Terio et al., 2011]. The cause of death for the other five infected chimpanzees as well as one uninfected ape (Ch-49), who died 6 years after the last sample collection, could not be determined because their bodies were not recovered, although Rudi (Ch-48) is suspected to have also died of conspecific aggression (Table II).
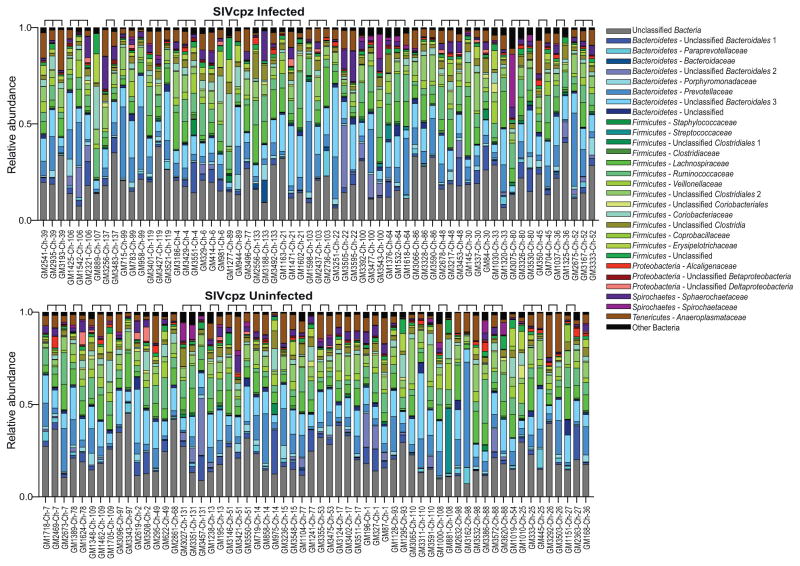
Similar to the results obtained from metagenomic sequencing (Fig. 1a), 16S rRNA gene sequencing revealed a predominance of Bacteroidetes, Firmicutes, Proteobacteria, and Spirochaetes in fecal samples from both SIVcpz-infected and -uninfected chimpanzees (Fig. 2). However, there also was a high degree of compositional variability when longitudinal samples from the same individual were compared. For example, bacteria of the family Spirochaetacea comprised 27% of the fecal bacteriome when chimpanzee Ch-80 was sampled in July 2012 (GM3075), but represented only 0.4% and 3% of bacterial communities when the same chimpanzee was sampled again four (GM3326) and 10 (GM3530) months later, respectively. When analyzed in a phylogenetic tree constructed from weighted UniFrac distances, the three samples from chimpanzee Ch-80 did not cluster together (not shown), and this was also true for longitudinal samples from other chimpanzees. In general, weighted UniFrac distances were not significantly different between longitudinal samples from the same individual and temporally matched samples from different individuals (not shown). Thus, the fecal microbiome of Gombe chimpanzees sampled at different times exhibited as much compositional diversity as did the microbiomes of different chimpanzees sampled at the same time.
Fig. 2.
Composition of the fecal bacteriome in 50 Gombe chimpanzees as determined by 16S rRNA gene sequencing. The relative abundance of major bacterial families (color coded) comprising more than 1% of the fecal bacteriome in at least half of the fecal samples (GM numbers) is shown for SIVcpz-infected (top part) and -uninfected (bottom part) chimpanzees (Ch numbers). Operational taxonomic units (OTUs) are labeled with the highest taxonomic rank as determined by the QIIME software, with those that could not be further classified identified. Brackets denote fecal samples from the same individual (see Table II for more information). Minor bacterial taxa that comprised less than 1% of the microbiome in more than half of the fecal samples are grouped into “other bacteria.”
To investigate whether Prevotellaceae enrichment was a common phenotype of SIVcpz infection, we compared the relative abundance of this family in samples from infected and uninfected individuals. Although the mean Prevotellaceae abundance was higher in SIVcpz-infected chimpanzees, the difference was not statistically significant (P = 0.79). Moreover, a broad search failed to identify bacterial families that were specifically associated with SIVcpz infection status. Thus, there was no significant enrichment or depletion of any bacterial phylum associated with chronic SIVcpz infection.
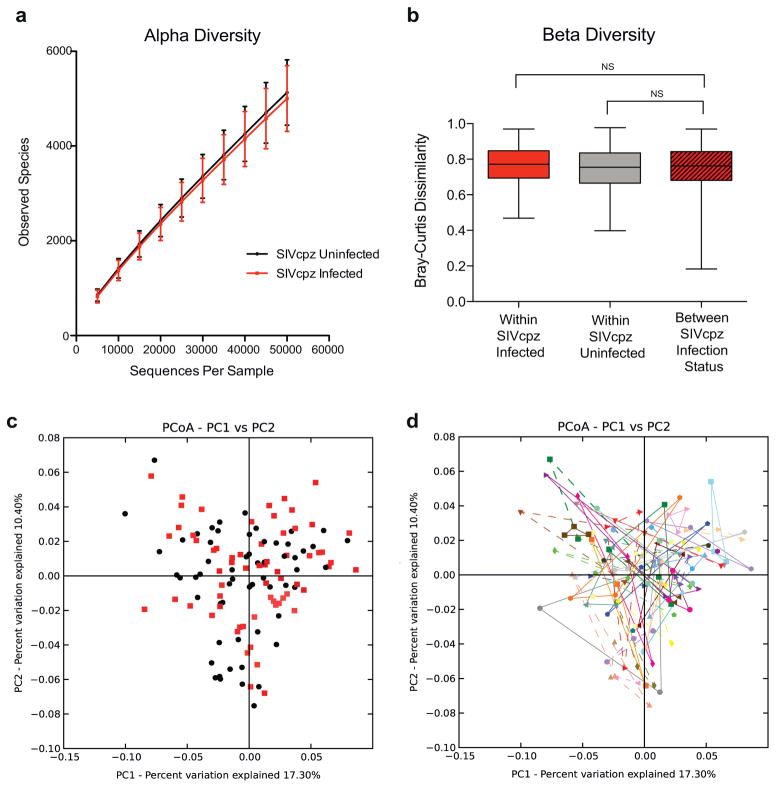
In addition to changes in bacterial abundances, changes in microbiome alpha and beta diversity have been reported to be associated with HIV-1 infection [Dillon et al., 2014; Dinh et al., 2015; Lozupone et al., 2013; McHardy et al., 2013; Mutlu et al., 2014; Vazquez-Castellanos et al., 2015; Vujkovic-Cvijin et al., 2013]. Alpha diversity is an indicator of both the number (richness) and distribution (evenness) of bacterial taxa within a single sample [Morgan & Huttenhower, 2012]. Using Shannon or Simpson indices to summarize alpha diversity, we found no difference between samples of SIVcpz-infected and -uninfected chimpanzees (not shown). Similarly, there was no difference in richness (observed species, P = 0.17) (Fig. 3a) or population size inferred using the Chao1 estimator (not shown). In addition, there was no significant difference in beta diversity, which measures the extent of similarity between microbiota from different samples [Morgan & Huttenhower, 2012]. Comparing Bray–Curtis dissimilarity values (Fig. 3b), we found no significant differences between infected and uninfected groups (P = 0.17 and 0.07 for SIVcpz-infected and -uninfected groups, respectively). This was also apparent in a principal coordinates analysis (PCoA) of weighted UniFrac distances, which revealed no specific grouping of samples from SIVcpz-infected versus -uninfected chimpanzees (Fig. 3c). Rather, the intra-individual diversity dominated the beta diversity, with consecutive samples from the same individual spanning the entire PCoA plot (Fig. 3d). Thus, in contrast to HIV-1 infected humans, the fecal microbiota of SIVcpz-infected and -uninfected chimpanzees were indistinguishable by these measures.
Fig. 3.
SIVcpz-infected and -uninfected chimpanzees are not characterized by a distinct fecal bacteriome. (a) Absence of differences in alpha diversity. The number of observed species of rarefied (sampled at an even depth) OTUs was calculated from 16S rRNA sequencing data for fecal samples from SIVcpz-infected (red) and -uninfected (black) chimpanzees and plotted using QIIME software. Error bars indicate standard deviation. (b) Absence of differences in beta diversity. Bray–Curtis dissimilarity distances were calculated using the QIIME software for all samples from SIVcpz-infected (red) and -uninfected chimpanzees (gray), as well as between samples from SIVcpz-infected and -uninfected individuals (striped). Box plots show the median, upper, and lower quartile ranges for each comparison (indicated by brackets), with whiskers indicating minimum (top) and maximum (bottom) dissimilarities. Statistical analyses were performed using the Mann–Whitney U-test and corrected for multiple comparisons. NS, not significant. (c) Principal coordinates analysis plot (PCoA) constructed from weighted UniFrac distances indicating a lack of primary clustering of bacteriomes from SIVcpz-infected (red) and -uninfected (black) chimpanzees. (d) Identical PCoA plot as shown in (c), with fecal bacteriomes from the same chimpanzee identified by connecting colored solid (SIVcpz infected) or broken (uninfected) lines.
Destabilization of the Fecal Bacteriome Marks the End-Stage of SIVcpz Infection
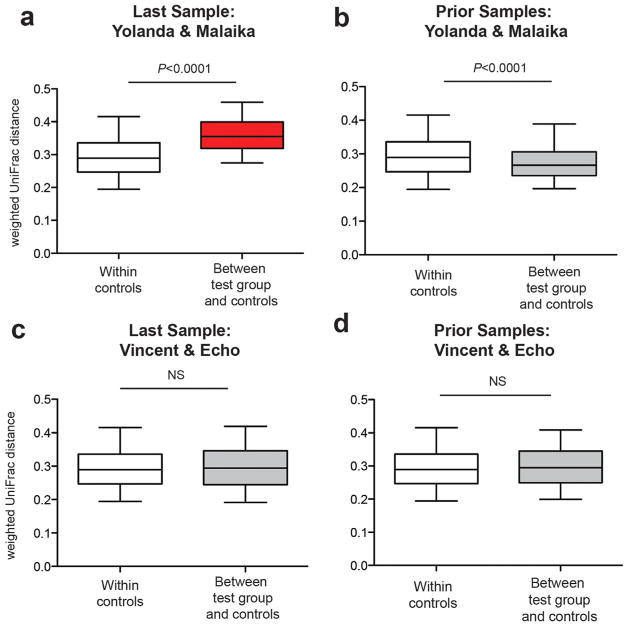
In HIV-1-infected humans, the destabilization of the intestinal microbiome is believed to be caused by a progressive loss of gut immune function [Lozupone et al., 2014], with a normal gut microbiome observed early [Lozupone et al., 2013], but not late [Vujkovic-Cvijin et al., 2013] in infection. Since most of the fecal material subjected to 16S rRNA sequencing was derived from seemingly healthy chimpanzees (Table II), we asked whether samples obtained closer to the time of death might exhibit signs of gut microbiome destabilization. To test this, we calculated all pairwise weighted UniFrac distances between fecal samples from Yolanda (GM1325) and Malaika (GM1602) collected 5 months before their AIDS-related deaths and fecal samples from all other (infected and uninfected) chimpanzees (N = 98) collected more than 8 months before their death (Table II). This analysis revealed a significant compositional change in each of the two immunodeficient chimpanzees relative to the controls, regardless whether samples were analyzed individually (not shown) or in combination (Fig. 4a). However, this same compositional shift was not observed when earlier samples from Yolanda (GM1037) and Malaika (GM1163, GM1471) were compared to the same controls (Fig. 4b). Moreover, fecal samples from Vincent and Echo, both of whom died of trauma-related deaths [Terio et al., 2011], did not exhibit destabilization of their gut community composition, regardless of whether they were collected before death (GM704, GM958) (Fig. 4c) (P = 0.62), or much earlier during their infection (GM715, GM783, GM350) (Fig. 4d) (P = 0.21). Using Euclidean and unweighted UniFrac distances and Bray–Curtis dissimilarity values to measure beta diversity yielded identical results (not shown). Although the number of confirmed AIDS cases in Gombe is small, these data suggest that pronounced compositional changes in the fecal bacteriome portends rapid disease progression and death.
Fig. 4.
Pronounced compositional shifts in the gut bacterial communities of SIVcpz infected chimpanzees shortly before their AIDS-related death. (a) Pairwise weighted UniFrac distances of the last fecal samples from Yolanda and Malaika (test group), who died of (necropsy confirmed) AIDS-like disease within 5 months of sampling, and fecal samples (N = 98) from all chimpanzees who were sampled more than 8 months before their death (control group). Box plots show the median, upper, and lower quartile ranges with whiskers indicating 95% confidence intervals (CI). Statistical analyses were performed using the Mann–Whitney U-test. Red indicates significantly increased beta diversity. (b) Analysis as in (a), but comparing pairwise weighted UniFrac distances from earlier fecal samples (collected more than 8 months before death) from Yolanda and Malaika (test group) to the same controls. (c) Analysis as in (a), but comparing pairwise weighted UniFrac distances from the last two fecal samples of two SIVcpz infected chimpanzees (Vincent and Echo) who died of trauma-related causes (test group) to the same controls. NS, not significant. (d) Analysis as in (c), but comparing pairwise weighted UniFrac distances from earlier fecal samples (collected more than 8 months before death) from Vincent and Echo (test group) to the same controls. See text and Table II for more details.
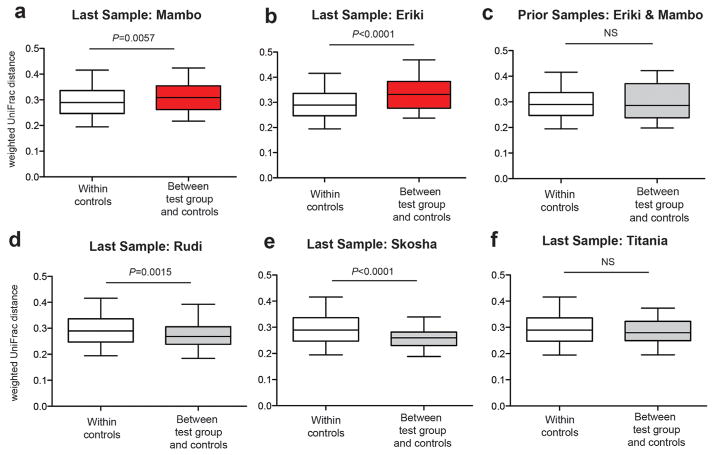
Five additional SIVcpz-infected chimpanzees, including Rudi, Skosha, Titania, Mambo and Eriki, disappeared within 7 months of their last fecal sampling (Table II). To examine whether they also experienced a destabilization of their gut bacteriome, we compared the weighted UniFrac distances of their last fecal samples to those of the same controls. Interestingly, fecal samples from Mambo (GM2736) and Eriki (GM3521), both of which were collected 2 months before their disappearance, also exhibited a significant compositional shift of their bacterial communities (Fig. 5a and b), which was not observed when prior samples from these same two individuals (GM3401, GM1598, GM2437) were analyzed (Fig. 5c) (P = 0.46). Given these findings, it is possible that Mambo and Eriki also died of AIDS, since this could not be confirmed because their bodies were not recovered. In contrast, no significant compositional changes (P = 0.20) were seen for the last fecal samples from Rudi (GM3453), Skosha (GM337), and Titania (GM1320) (Fig. 5d–f). Although these three chimpanzees did not disappear until 4–7 months after their last fecal collection (Table II), they may not have been sampled sufficiently close to the time of their death to see compositional changes in their gut bacteriome. Alternatively, they may have died of causes unrelated to their SIVcpz infection. In fact, Rudi was attacked by another chimpanzee shortly before his disappearance and may have died of the infiicted injuries, although this was not confirmed by necropsy. The same results were obtained when Euclidean distances, unweighted UniFrac distances, and Bray–Curtis dissimilarity values were used to calculate beta diversity, except for the last sample from Eriki, which did not show significantly increased unweighted UniFrac distances, and the last sample from Skosha, which did show significantly increased unweighted UniFrac distances. Although unweighted UniFrac distances indicate the presence and absence of bacterial OTUs, without accounting for changes in their relative abundances, these data indicate that the last sample from Eriki likely contained similar microbial taxa as the control chimpanzees, but exhibited significant differences in their relative abundances. In contrast, the last sample from Skosha exhibited differences in some microbial taxa, but the overall composition of her fecal microbiome remained stable.
Fig. 5.
Pronounced compositional shifts in the gut bacterial communities of two SIVcpz-infected chimpanzees shortly before their disappearance. (a) Pairwise weighted UniFrac distances from the last fecal sample of Mambo (test group), an SIVcpz-infected chimpanzee who disappeared two months after sample collection, were compared to pairwise weighted UniFrac distances of fecal samples (N = 98) from all chimpanzees who were sampled more than 8 months before their death (control group). Box plots show the median, upper, and lower quartile ranges with whiskers indicating 95% confidence intervals (CI). Statistical analyses were performed using the Mann–Whitney U-test. Red indicates significantly increased beta diversity. (b) Analysis as in (a), but comparing pairwise weighted UniFrac distances from the last fecal sample of Eriki (test group), an SIVcpz-infected chimpanzee who also disappeared 2 months after sample collection, to the same controls. (c) Analysis as in (a), but comparing weighted UniFrac distances from earlier fecal samples (collected more than 8 months before death) from Mambo and Eriki (test group) to the same controls. (d, e, and f) Analysis as in (a), but comparing pairwise weighted UniFrac distances from the last fecal sample of Rudi, Skosha, and Titania (test groups), SIVcpz-infected chimpanzees who disappeared 5, 4, and 7 months after sample collection, respectively, to the same controls.
Absence of an Expanded Gut Virome in SIVcpz-Infected Chimpanzees
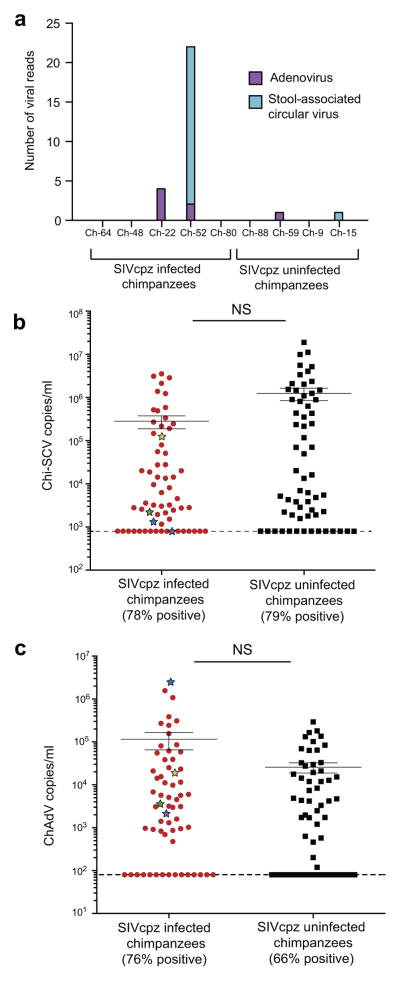
Previous studies of SIVmac-infected macaques showed that clinical immunodeficiency can also be manifested by an expansion of the fecal virome [Handley et al., 2012]. To determine whether a similar expansion was occurring in SIVcpz-infected chimpanzees, we searched our metagenomic sequences for reads that mapped to mammalian viruses. This analysis identified only a very small number of paired reads (N = 29), which either mapped to chimpanzee stool associated circular virus (Chi-SCV) (N = 22) or chimpanzee adenovirus (ChAdV) (N = 7). Both Chi-SCV [Blinkova et al., 2010] and ChAdV [Cross, 2013; Lonsdorf et al., 2014] infections have previously been documented in Gombe chimpanzees. Moreover, most of the virus-specific reads (93%) were recovered from fecal samples of SIVcpz-infected chimpanzees (Fig. 6a), which is consistent with data from SIVmac-infected macaques, which also had a greater abundance of simian adenoviruses in their shotgun sequenced fecal samples [Handley et al., 2012]. However, the paucity of mammalian virus reads in the metagenomic sequencing data came as a surprise. Although the reasons for this are not entirely clear, partial virus degradation likely played a role, since in contrast to the macaque study Gombe fecal samples were not flash frozen and were shipped (in RNAlater) at room temperature. Moreover, most of the SIVmac-infected macaques were sampled within days of death, whereas this was clearly not the case for the SIVcpz-infected chimpanzees. Reads from plant viruses and bacteriophages were detected, but not further analyzed with respect to SIVcpz infection.
Fig. 6.
Absence of an expanded virome in fecal samples from SIVcpz-infected chimpanzees. (a) Detection of chimpanzee adenovirus (ChAdV) and chimpanzee stool associated circular virus (Chi-SCV) viral reads in fecal samples by metagenomic sequencing, with the number of paired-end viral reads (color coded) indicated for each individual. Virus infection was independently confirmed by nested PCR amplification and sequencing of ChAdV and Chi-SCV sequences from fecal DNA. (b and c) Chi-SCV (b) and ChAdV (c) viral loads were determined for all fecal samples listed in Table II using a virus-specific real time PCR and expressed as copies per ml of fecal sample (normalized by total sample DNA). The percentage of positive samples in each group is indicated, with the limit of detection denoted by a broken line. For each group, the median and standard error of the mean (SEM) are shown. Fecal samples from four chimpanzees that exhibited a compositional shift in their fecal bacteriome (Figs. 4 and 5) are highlighted (Eriki, blue; Mambo, purple; Malaika, green; Yolanda, orange). Statistical analyses were performed using the Mann–Whitney U-test. NS, not significant.
To quantify Chi-SCV and ChAdV infection rates and fecal viral loads in a larger number of SIVcpz-infected and -uninfected chimpanzees, we developed virus-specific nested and real-time PCR assays. Testing all fecal samples (N = 123) listed in Table II, we found a high prevalence of Chi-SCV in both SIVcpz-infected and -uninfected chimpanzees, but no significant difference in fecal viral loads in either group (Fig. 6b). Although both ChAdV infection rates and fecal viral loads were slightly higher in SIVcpz-infected than -uninfected chimpanzees (Fig. 6c), these differences were not significant (P = 0.07 and 0.31 for Chi-SCV and ChAdV, respectively), including when values from the known and/or suspected immunodeficient chimpanzees were compared (colored in Fig. 6c). Thus, unlike in SIVmac-infected macaques, we did not observe an expansion of the fecal virome in SIVcpz-infected chimpanzees, including in the last samples from individuals who died of AIDS-related illness.
DISCUSSION
Enteric dysbiosis is recognized as a characteristic feature of progressive HIV-1 infection [Lozupone et al., 2014; Nwosu et al., 2014], but the underlying mechanisms have been the subject of debate [Brenchley 2013; Nwosu et al., 2014]. In particular, the absence of an altered gut bacteriome in macaques with progressive SIVmac infection seemed to argue against lentiviral infection as the main driver of HIV-1 associated enteropathy [Handley et al., 2012; McKenna et al., 2008]. However, SIVmac infection is not an ideal model to assess primate lentivirus induced alterations of host-bacterial interactions since its disease course is greatly accelerated [Staprans et al., 1999]. Moreover, captive macaques differ in lifestyle and diet from naturally infected primates, and are prone to enteritis even in the absence of SIVmac infection [McKenna et al., 2008]. Reasoning that SIVcpz infection constitutes a physiologically more relevant “pathogenic SIV model,” we examined the composition of the fecal microbiome in infected (N = 24) and uninfected (N = 26) chimpanzees in Gombe National Park. Using both metagenomic and 16S rRNA gene sequencing, we failed to identify significant differences in the abundance, alpha diversity and beta diversity of bacterial communities between SIVcpz-infected and -uninfected groups (Figs. 1, 2, and 3). However, fecal samples from two chimpanzees, who died of an AIDS-like illness, exhibited significant compositional changes 5 months before their death (Fig. 4). Since earlier samples from these same individuals failed to exhibit the same compositional shifts, it seems likely that effective immune control prevented the destabilization of their gut bacteriome until shortly before their death. These findings are consistent with observational health data that failed to find an association between signs of illness and SIVcpz infection in these same communities [Lonsdorf EV, Gillespie TR, Wolf TM, et al. 2016. Socioecological correlates of clinical signs in two communities of wild chimpanzees (Pan troglodytes) at Gombe National Park, Tanzania. American Journal of Primatology, submitted.; Wolf TM, Singer RS, Lonsdorf EV, et al. 2016. Epidemiology of syndromic respiratory disease in chimpanzees of Gombe Stream National Park, Tanzania, 2004–2012. American Journal of Primatology, submitted.]. These findings are also consistent with the absence of gut bacteriome alterations in SIVgor-infected western gorillas, the great majority of whom would not have been sampled shortly before their death [Moeller et al., 2015]. The new data thus provide a plausible explanation for previous discrepant gut microbiome results. It now seems clear that SIVcpz-infected chimpanzees are able to maintain a stable gut microbiome for many years, but seem to die relatively rapidly (within only a few months) after the compositional divergence of their gut microbiome marks the loss of effective immune control. In contrast, HIV-1-infected humans seem to tolerate a more prolonged course of declining immune functions, most likely because of effective medical interventions, which independently contribute to alterations of gut bacterial communities [Jernberg et al., 2007; Klase et al., 2015]. Thus, both HIV-1 and SIVcpz infection are capable of causing a disruption of gut microbial homeostasis, but the timing and circumstances of this disruption and the associated health consequences differ between humans and apes.
Comparing 16S rRNA gene sequences, we noted that the gut bacterial communities from both infected and uninfected chimpanzees exhibited extensive compositional variability when compared over time (Figs. 2 and 3). Although this may seem surprising, these results are consistent with a recent study that identified social interactions as the main driver of chimpanzee gut microbiome species richness and compositional fiuctuation [Moeller et al., 2016]. This study showed that the community memberships of individual chimpanzee gut micro-biomes were more similar and more species-rich during seasons of high social interaction [Moeller et al., 2016]. Thus, our failure to identify particular bacterial taxa and/or combinations of taxa to be associated with SIVcpz infection is not unexpected. Although metagenomic sequencing identified an increased frequency of the bacterial family Prevotellaceae in a small number of number of SIVcpz-infected chimpanzees, this association did not reach significance when 16S rRNA gene sequences from a larger sample set were compared. Similarly, we previously reported increased bacterial diversity after SIVcpz acquisition in six Gombe chimpanzees, all of whom were also included in the current study [Moeller et al., 2013b]. Again, samples from a small number of individuals allowed detection of micro-biome compositional changes, while these same samples (except the very last ones collected before death) failed to show an increased compositional diversity when compared to a much larger number of controls (Figs. 3 and 4). Thus, the extensive compositional variability of the fecal bacteriome in the same individuals over time precludes the identification of subtle differences between infected and uninfected chimpanzees.
In addition to the gut bacteriome, we also investigated the gut virome of SIVcpz-infected and -uninfected chimpanzees. Metagenomic sequencing identified chimpanzee adenovirus (ChAdV) and chimpanzee stool-associated circular virus (Chi-SCV) in fecal samples from four Gombe chimpanzees (Fig. 6a). Suppression of the human immune system is frequently associated with an outgrowth of both pathogenic and non-pathogenic viruses [De Vlaminck et al., 2013; Handley et al., 2012; Li et al., 2013; Young et al., 2015]. For example, human torque teno (TT) virus, a small nonpathogenic DNA virus similar to Chi-SCV, is more prevalent in HIV-1-infected humans and replicates to higher titers in the blood of immunosuppressed individuals [Christensen et al., 2000; Shibayama et al., 2001]. Similarly, anelloviruses (a viral family that includes TT viruses) were more abundant in blood samples from immunosuppressed transplant patients, with an increase in viral titers determined by metagenomic sequencing [De Vlaminck et al., 2013; Young et al., 2015]. Finally, SIVmac-infected macaques exhibited higher fecal viral titers of circoviruses, which are related to Chi-SCV, and simian adenoviruses [Handley et al., 2012]. In light of these data, the absence of significant differences in fecal Chi-SCV and ChAdV abundance in SIVcpz-infected and -uninfected chimpanzees was unexpected (Fig. 6b), especially when this was also the case for the last fecal samples of the four chimpanzees (Yolanda, Malaika, Mambo, Eriki) who exhibited increased bacterial diversity (Figs. 4 and 5). Although there was a trend toward increased titers of adenovirus in fecal samples from SIVcpz-infected chimpanzees (Fig. 6c), this finding was nowhere close to the fecal virome expansion observed in SIVmac-infected macaques, where mammalian viruses constituted up to 90% of fecal metagenomic reads [Handley et al., 2012]. Unlike the SIVcpz-infected chimpanzees studied here, the SIVmac-infected macaques were moribund at the time of sampling, with many requiring euthanasia before the completion of the study, which may have influenced the metagenomic results. Nonetheless, adenovirus infection of the intestine is associated with diarrhea, and may therefore contribute to the higher levels of diarrhea observed in SIVcpz-infected female chimpanzees [Lonsdorf EV, Gillespie TR, Wolf TM, et al. 2016. Socioecological correlates of clinical signs in two communities of wild chimpanzees (Pan troglodytes) at Gombe National Park, Tanzania. American Journal of Primatology, submitted.].
Noninvasive studies of habituated chimpanzees have revealed much of the natural history of the precursor of the human AIDS virus. While the effects of SIVcpz infection on chimpanzee longevity and population dynamics can be readily monitored, disease progression is difficult to assess without frequent access to blood and tissue samples. The aim of this study was to explore whether characteristic gut microbiome changes could be found that might be suitable to noninvasively monitor disease progression. Although we failed to identify particular bacterial taxa and/or microbiome compositional changes that correlated with SIVcpz infection status, we found an increased beta diversity to mark the end-stage of SIVcpz infection in a small number of apes with necropsy confirmed immunodeficiency. The practical importance of this finding for the management of chimpanzee communities with endemic SIVcpz infection remains to be determined. Fecal microbiome analyses seem of little value in non-habituated communities where longitudinal sampling and observational studies of known individuals are not possible. In contrast, monitoring the fecal microbiome may prove useful in habituated communities, such as in Gombe, where a spike in beta diversity may foreshadow rapid disease progression and death. This could prompt intensified observational studies to more closely monitor signs of ill health, more focused sample collection to identify additional markers of disease progression, and enhanced surveillance to increase the likelihood of recovering tissues for necropsy. Monitoring disease progression in habituated chimpanzees could also be used to identify individuals that might benefit from therapy, provided that the intended intervention is truly necessary, practical in wild settings, and known to be efficacious in chimpanzees [Barbian et al., 2015]. As ape populations continue to dwindle in the wild, such an approach may become increasingly important to ensure their survival [Ryan & Walsh, 2011; Walsh et al., 2003; Warfield et al., 2014].
Acknowledgments
Contract grant sponsor: National Institutes of Health; contract grant numbers: R01 AI50529, R01 AI58715, P30 AI045008, T32 AI 055400; contract grant sponsor: National Science Foundation; contract grant number: IOS-LTREB-1052693; contract grant sponsor: Jane Goodall Institute.
We thank the Jane Goodall Institute field staff at the Gombe Stream Research Centre for collecting behavioral and observational health data as well as fecal samples from wild-living chimpanzees; Jacque Young, Christel Chehoud, and Paul Sharp for expert advice; Andrew Smith, Andrew Caffro, Eric Ruff, and Aubrey Bailey for technical assistance; Shivani Sethi for artwork and manuscript preparation; the Tanzania Commission for Science and Technology (COSTECH), the Tanzania Wildlife Research Institute (TAWIRI), and the Tanzania National Parks Association (TANAPA) for their support and permission to conduct research in Gombe. This work was supported by grants from the National Institutes of Health (R01 AI50529, R01 AI58715; P30 AI 045008), the National Science Foundation (IOS-LTREB-1052693), and the Jane Goodall Institute. HJB was funded by a training grant in Emerging Infectious Diseases (T32 AI 055400).
Footnotes
Conflicts of interest: The authors have no conflicts of interest to disclose.
References
- Barbian HJ, Decker JM, Bibollet-Ruche F, et al. Neutralization properties ofsimian immunodeficiency viruses infecting chimpanzees and gorillas. MBio. 2015;6:e00296–15. doi: 10.1128/mBio.00296-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blinkova O, Victoria J, Li Y, et al. Novel circular DNA viruses in stool samples of wild-living chimpanzees. Journal of General Virology. 2010;91:74–86. doi: 10.1099/vir.0.015446-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray JR, Curtis JT. An ordination of the upland forest communities of Southern Wisconsin. Ecological Monographs. 1957;27:326–349. [Google Scholar]
- Brenchley JM, Douek DC. HIV infection and the gastrointestinal immune system. Mucosal Immunology. 2007;1:23–30. doi: 10.1038/mi.2007.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenchley JM, Price DA, Schacker TW, et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nature Medicine. 2006;12:1365–1371. doi: 10.1038/nm1511. [DOI] [PubMed] [Google Scholar]
- Brenchley JM. Mucosal immunity in human and simian immunodeficiency lentivirus infections. Mucosal Immunology. 2013;6:657–665. doi: 10.1038/mi.2013.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caporaso JG, Kuczynski J, Stombaugh J, et al. QIIME allows analysis of high-throughput community sequencing data. Nature Methods. 2010;7:335–336. doi: 10.1038/nmeth.f.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caporaso JG, Lauber CL, Walters WA, et al. Global patterns of 16S rRNA diversity at a depth of millions of sequences per sample. Proceedings of the National Academy of Sciences. 2011;108:4516–4522. doi: 10.1073/pnas.1000080107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao A. Nonparametric estimation of the number of classes in a population. Scandinavian Journal of Statistics. 1984;11:265–270. [Google Scholar]
- Cho I, Blaser MJ. The human microbiome: at the interface of health and disease. Nature Reviews Genetics. 2012;13:260–270. doi: 10.1038/nrg3182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen JK, Eugen-Olsen J, Sørensen M, et al. Prevalence and prognostic significance of infection with TT virus in patients infected with human immunodeficiency virus. Journal of Infectious Diseases. 2000;181:1796–1799. doi: 10.1086/315440. [DOI] [PubMed] [Google Scholar]
- Claesson MJ, Jeffery IB, Conde S, et al. Gut microbiota composition correlates with diet and health in the elderly. Nature. 2012;488:178–184. doi: 10.1038/nature11319. [DOI] [PubMed] [Google Scholar]
- Cross KE. Retrieved from the Emory Electronic Theses and Dissertations Repository. 2013. Factors affecting enteric adenovirus infection dynamics in wild chimpanzees (Pan troglodytes) in Gombe National Park, Tanzania: Implications for global health and biodiversity conservation (Honors thesis) [Google Scholar]
- De Vlaminck I, Khush Kiran K, Strehl C, et al. Temporal response of the human virome to immunosuppression and antiviral therapy. Cell. 2013;155:1178–1187. doi: 10.1016/j.cell.2013.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degnan PH, Pusey AE, Lonsdorf EV, et al. Factors associated with the diversification of the gut microbial communities within chimpanzees from Gombe National Park. Proceedings of the National Academy of Sciences. 2012;109:13034–13039. doi: 10.1073/pnas.1110994109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillon SM, Lee EJ, Kotter CV, et al. An altered intestinal mucosal microbiome in HIV-1 infection is associated with mucosal and systemic immune activation and endotoxemia. Mucosal Immunology. 2014;7:983–994. doi: 10.1038/mi.2013.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinh DM, Volpe GE, Duffalo C, et al. Intestinal microbiota, microbial translocation, and systemic inflammation in chronic HIV infection. Journal of Infectious Diseases. 2015;211:19–27. doi: 10.1093/infdis/jiu409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estes JD, Harris LD, Klatt NR, et al. Damaged intestinal epithelial integrity linked to microbial translocation in pathogenic simian immunodeficiency virus infections. PLoS Pathogens. 2010;6:e1001052. doi: 10.1371/journal.ppat.1001052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handley Scott A, Thackray Larissa B, Zhao G, et al. Pathogenic simian immunodeficiency virus infection is associated with expansion of the enteric virome. Cell. 2012;151:253–266. doi: 10.1016/j.cell.2012.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazenberg MD, Otto SA, van Benthem BH, et al. Persistent immune activation in HIV-1 infection is associated with progression to AIDS. AIDS. 2003;17:1881–1888. doi: 10.1097/00002030-200309050-00006. [DOI] [PubMed] [Google Scholar]
- Huson DH, Mitra S. Introduction to the analysis of environmental sequences: metagenomics with MEGAN. In: Anisimova M, editor. Evolutionary genomics: statistical and computational methods. Vol. 2. New York: Humana Press; 2012. pp. 415–429. [DOI] [PubMed] [Google Scholar]
- Jernberg C, Lofmark S, Edlund C, Jansson JK. Long-term ecological impacts of antibiotic administration on the human intestinal microbiota. ISME Journal. 2007;1:56–66. doi: 10.1038/ismej.2007.3. [DOI] [PubMed] [Google Scholar]
- Keele BF, Van Heuverswyn F, Li Y, et al. Chimpanzee reservoirs of pandemic and nonpandemic HIV-1. Science. 2006;313:523–526. doi: 10.1126/science.1126531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keele BF, Jones JH, Terio KA, et al. Increased mortality and AIDS-like immunopathology in wild chimpanzees infected with SIVcpz. Nature. 2009;460:515–519. doi: 10.1038/nature08200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klase Z, Ortiz A, Deleage C, et al. Dysbiotic bacteria translocate in progressive SIV infection. Mucosal Immunology. 2015;8:1009–1020. doi: 10.1038/mi.2014.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Duan L, Estes JD, et al. Peak SIV replication in resting memory CD4+ T cells depletes gut lamina propria CD4+ T cells. Nature. 2005;434:1148–1152. doi: 10.1038/nature03513. [DOI] [PubMed] [Google Scholar]
- Li Y, Ndjango JB, Learn GH, et al. Eastern chimpanzees, but not bonobos, represent a simian immunodeficiency virus reservoir. Journal of Virology. 2012;86:10776–107791. doi: 10.1128/JVI.01498-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Deng X, Linsuwanon P, et al. AIDS alters the commensal plasma virome. Journal of Virology. 2013;87:10912–10915. doi: 10.1128/JVI.01839-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonsdorf E, Travis D, Ssuna R, et al. Field immobilization for treatment of an unknown illness in a wild chimpanzee (Pan troglodytes schweinfurthii) at Gombe National Park, Tanzania: findings, challenges, and lessons learned. Primates. 2014;55:89–99. doi: 10.1007/s10329-013-0372-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozupone C, Knight R. UniFrac: a new phylogenetic method for comparing microbial communities. Applied and Environmental Microbiology. 2005;71:8228–8235. doi: 10.1128/AEM.71.12.8228-8235.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozupone CA, Rhodes ME, Neff CP, et al. HIV-induced alteration in gut microbiota. Gut Microbes. 2014;5:562–570. doi: 10.4161/gmic.32132. [DOI] [PubMed] [Google Scholar]
- Lozupone CA, Li M, Campbell TB, et al. Alterations in the gut microbiota associated with HIV-1 infection. Cell Host Microbe. 2013;14:329–339. doi: 10.1016/j.chom.2013.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHardy IH, Li X, Tong M, et al. HIV Infection is associated with compositional and functional shifts in the rectal mucosal microbiota. Microbiome. 2013;1:26. doi: 10.1186/2049-2618-1-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenna P, Hoffmann C, Minkah N, et al. The macaque gut microbiome in health, lentiviral infection, and chronic enterocolitis. PLoS Pathogens. 2008;4:e20. doi: 10.1371/journal.ppat.0040020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellors JW, Munoz A, Giorgi JV, et al. Plasma viral load and CD4+ lymphocytes as prognostic markers of HIV-1 infection. Annals of Internal Medicine. 1997;126:946–954. doi: 10.7326/0003-4819-126-12-199706150-00003. [DOI] [PubMed] [Google Scholar]
- Minot S, Bryson A, Chehoud C, et al. Rapid evolution of the human gut virome. Proceedings of the National Academy of Sciences. 2013;110:12450–12455. doi: 10.1073/pnas.1300833110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moeller AH, Degnan PH, Pusey AE, et al. Chimpanzees and humans harbour compositionally similar gut enterotypes. Nature Communications. 2012;3:1179. doi: 10.1038/ncomms2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moeller AH, Peeters M, Ndjango JB, et al. Sympatric chimpanzees and gorillas harbor convergent gut microbial communities. Genome Research. 2013a;23:1715–1720. doi: 10.1101/gr.154773.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moeller AH, Shilts M, Li Y, et al. SIV-induced instability of the chimpanzee gut microbiome. Cell Host Microbe. 2013b;14:340–345. doi: 10.1016/j.chom.2013.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moeller AH, Peeters M, Ayouba A, et al. Stability of the gorilla microbiome despite simian immunodeficiency virus infection. Molecular Ecology. 2015;24:690–697. doi: 10.1111/mec.13057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moeller AH, Foerster S, Wilson M, et al. Social behavior shapes the chimpanzee pan-microbiome. Science Advances. 2016 doi: 10.1126/sciadv.1500997. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan XC, Huttenhower C. Chapter 12: Human micro-biome analysis. PLoS Computational Biology. 2012;8:e1002808. doi: 10.1371/journal.pcbi.1002808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutlu EA, Keshavarzian A, Losurdo J, et al. A compositional look at the human gastrointestinal micro-biome and immune activation parameters in HIV infected subjects. PLoS Pathogens. 2014;10:e1003829. doi: 10.1371/journal.ppat.1003829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nwosu FC, Avershina E, Wilson R, Rudi K. Gut microbiota in HIV infection: implication for disease progression and management. Gastroenterology Research and Practice. 2014;2014:803185. doi: 10.1155/2014/803185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pusey AE, Pintea L, Wilson ML, Kamenya S, Goodall J. The contribution of long-term research at Gombe National Park to chimpanzee conservation. Conservation Biology. 2007;21:623–634. doi: 10.1111/j.1523-1739.2007.00704.x. [DOI] [PubMed] [Google Scholar]
- Rudicell RS, Holland Jones J, Wroblewski EE, et al. Impact of simian immunodeficiency virus infection on chimpanzee population dynamics. PLoS Pathogens. 2010;6:e1001116. doi: 10.1371/journal.ppat.1001116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudicell RS, Piel AK, Stewart F, et al. High prevalence of simian immunodeficiency virus infection in a community of savanna chimpanzees. Journal of Virology. 2011;85:9918–9928. doi: 10.1128/JVI.05475-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan SJ, Walsh PD. Consequences of non-intervention for infectious disease in African great apes. PLoS ONE. 2011;6:e29030. doi: 10.1371/journal.pone.0029030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santiago ML, Rodenburg CM, Kamenya S, et al. SIVcpz in wild chimpanzees. Science. 2002;295:465. doi: 10.1126/science.295.5554.465. [DOI] [PubMed] [Google Scholar]
- Santiago ML, Lukasik M, Kamenya S, et al. Foci of endemic simian immunodeficiency virus infection in wild-living eastern chimpanzees (Pan troglodytes schweinfurthii) Journal of Virology. 2003;77:7545–7562. doi: 10.1128/JVI.77.13.7545-7562.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannon CE. A mathematical theory of communication. SIGMOBILE Mobile Computing Communications Review. 2001;5:3–55. [Google Scholar]
- Sharp PM, Hahn BH. Origins of HIV and the AIDS pandemic. Cold Spring Harbor Perspectives Medicine. 2011;1:a006841. doi: 10.1101/cshperspect.a006841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibayama T, Masuda G, Ajisawa A, et al. Inverse relationship between the titre of TT virus DNA and the CD4 cell count in patients infected with HIV. AIDS. 2001;15:563–570. doi: 10.1097/00002030-200103300-00004. [DOI] [PubMed] [Google Scholar]
- Simpson EH. Measurement of diversity. Nature. 1949;163:688. [Google Scholar]
- Song SJ, Lauber C, Costello EK, et al. Cohabiting family members share microbiota with one another and with their dogs. eLife. 2013;2:e00458. doi: 10.7554/eLife.00458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staprans SI, Dailey PJ, Rosenthal A, et al. Simian immunodeficiency virus disease course is predicted by the extent of virus replication during primary infection. Journal of Virology. 1999;73:4829–4839. doi: 10.1128/jvi.73.6.4829-4839.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takehisa J, Kraus MH, Ayouba A, et al. Origin and biology of simian immunodeficiency virus in wild-living western gorillas. Journal of Virology. 2009;83:1635–1648. doi: 10.1128/JVI.02311-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terio KA, Kinsel MJ, Raphael J, et al. Pathologic lesions in chimpanzees (Pan trogylodytes schweinfurthii) from Gombe National Park, Tanzania, 2004–2010. Journal of Zoo and Wildlife Medicine. 2011;42:597–607. doi: 10.1638/2010-0237.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Heuverswyn F, Li Y, Bailes E, et al. Genetic diversity and phylogeographic clustering of SIVcpzPtt in wild chimpanzees in Cameroon. Virology. 2007;368:155–171. doi: 10.1016/j.virol.2007.06.018. [DOI] [PubMed] [Google Scholar]
- van Lawick-Goodall J. The behaviour of free-living chimpanzees in the Gombe Stream Reserve. Animal Behaviour Monographs. 1968;1:161–311. [Google Scholar]
- Vazquez-Castellanos JF, Serrano-Villar S, Latorre A, et al. Altered metabolism of gut microbiota contributes to chronic immune activation in HIV-infected individuals. Mucosal Immunology. 2015;8:760–772. doi: 10.1038/mi.2014.107. [DOI] [PubMed] [Google Scholar]
- Vujkovic-Cvijin I, Dunham RM, Iwai S, et al. Dysbiosis of the gut microbiota is associated with HIV disease progression and tryptophan catabolism. Science Translational Medicine. 2013;5:193ra91. doi: 10.1126/scitranslmed.3006438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh PD, Abernethy KA, Bermejo M, et al. Catastrophic ape decline in western equatorial Africa. Nature. 2003;422:611–614. doi: 10.1038/nature01566. [DOI] [PubMed] [Google Scholar]
- Warfield KL, Goetzmann JE, Biggins JE, et al. Vaccinating captive chimpanzees to save wild chimpanzees. Proceedings of the National Academy of Sciences. 2014;111:8873–8876. doi: 10.1073/pnas.1316902111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yatsunenko T, Rey FE, Manary MJ, et al. Human gut microbiome viewed across age and geography. Nature. 2012;486:222–227. doi: 10.1038/nature11053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JC, Chehoud C, Bittinger K, et al. Viral metagenomics reveal blooms of anelloviruses in the respiratory tract of lung transplant recipients. American Journal of Transplantation. 2015;15:200–209. doi: 10.1111/ajt.13031. [DOI] [PMC free article] [PubMed] [Google Scholar]