Acute myelogenous leukemia (AML) is a deadly disease characterized by high relapse rates despite the ability of patients to initially enter complete remission1. While new therapeutics are urgently needed, standard chemotherapy for AML has remained largely unchanged during the last four decades and therapeutic outcomes remain dismal. Relapse in AML is thought to be driven by leukemia stem cells (LSCs), a chemoresistant subpopulation capable of initiating and reestablishing disease2. Studies correlating LSC proportion within individual patients to relapse-free survival have established that higher proportions of LSCs at diagnosis are associated with inferior relapse-free survival3. Thus, as LSCs resist standard therapy, devising new therapeutic strategies that ablate LSCs are likely to improve outcomes. We have shown previously that LSC survival is extensively dependent on constitutively active NF-κB4. Indeed, pre-clinical agents such as parthenolide (PTL) that potently suppress NF-κB can eliminate LSCs in vitro while preserving normal hematopoietic stem cell (HSC) function5. Despite its in vitro efficacy, PTL exhibits poor solubility, high reactivity with serum, and poor pharmacokinetics6 that make it insufficiently bioavailable, limiting its in vivo use. Furthermore, LSCs preferentially reside in the bone marrow (BM) niche, a microenvironment that simultaneously supports LSC survival and provides chemoprotection7. To overcome this protective effect, in this study, we evaluated whether encapsulation of PTL into nanoparticles and using a BM-directed multistage vector (MSV) system (MSV-PTL) would deliver active PTL at sufficiently levels to ablate LSCs in vivo.
Our previous studies have sought to optimize PTL using medicinal chemistry, producing the more bioavailable and soluble derivative dimethylaminoparthenolide (DMAPT), which required a 3x a day oral dosing in a daily schedule to be used in animals (Figure 1a, top panel)8. As an alternative approach to optimize PTL delivery we developed a multistage vector (MSV) system (MSV-PTL) in which PTL is first incorporated into mPEG-PLA micelles, encapsulated in a protective degradable porous silicon (pSi) particles, and coated with E-selectin thioaptamer (ESTA) to direct the particles to the BM9 (Supplemental Figure 1a). The pSi-ESTA conjugate binds to E-selectin with nanomolar affinity (KD = 47 nM) and with minimal cross-reactivity to other selectin family members, enabling delivery of PTL to the BM10, 11 as E-selectin is expressed on the BM endothelium12.
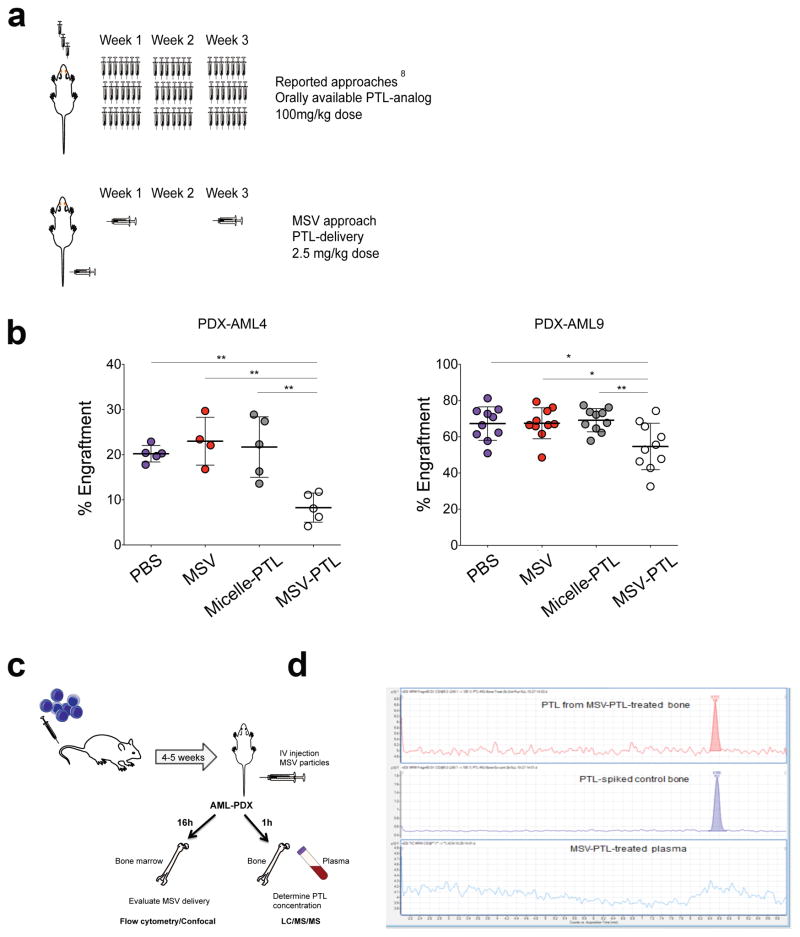
Figure 1. MSV system delivers PTL to the bone marrow of primary human AML xenografts (AML-PDX).
(a) Schematic representation of the reported drug delivery for DMAPT8, a water-soluble analog of the anti-LSC agent PTL, and for the novel multi-stage vector (MSV). (b) Percent human engraftment in established AML-PDX mice. Animals were treated with either PBS (blue), MSV-empty (red), PTL formulated in micelles (micelle-PTL; gray), or MSV-PTL (white) once every two weeks for a total period of four weeks. Each circle represents an individual mouse. Horizontal bar represents the mean. Error bars represent S.E.M. * p<0.05, **p<0.01. (c) Schematic representation of the generation of AML-PDX mice and the experimental design to evaluate nanoparticle delivery to the BM (5–10 animals per cohort) (d) Chromatograms of PTL from PTL in bone sample from MSV-PTL-treated mice at 1 h (top), untreated AML-PDX bones spiked with PTL (middle), and plasma sample from MSV-PTL-treated mice showing no PTL at 1h (bottom) n=4.
Primary AML cells were obtained with informed consent and IRB approval from Weill Cornell Medical College-New York Presbyterian Hospital. Primary cryopreserved AML samples were thawed and prepared for xenotransplants as described previously14. NOD/SCID were then injected via the tail (5–10 animals per cohort). Treatment with MSV or MSV-PTL (one billion particles) was started five weeks after transplantation and mice were treated once every two weeks, for four weeks. The presence of human cells was evaluated by flow cytometry. For the secondary transplants, equal numbers of human cells were injected (5 animals per cohort). The percentage of human AML cells was determined by staining the cells with antibodies for PE-Cy5 rat anti-mouse CD45 (eBiosciences) and APC-H7 anti-human CD45 (BD Biosciences). For viability, Annexin V-FITC (BD Biosciences) and 7-aminoactinomycin (7-AAD; Molecular Probes-Invitrogen) were used. For intracellular assays, cells were fixed with 4% formaldehyde and permeabilized with methanol. Analyses and graphs were performed using GraphPad Prism software to evaluate significance. The specific test utilized is indicated in the figure legends *p<0.05, **p<0.01. (Additional methods can be found in the supplementary information).
To evaluate the therapeutic efficacy of MSV-PTL, we established patient-derived AML xenografts (AML-PDXs). The AML-PDXs were treated with either: (i) PBS; (ii) empty-MSV; (iii) PTL-loaded micelles (micelle-PTL); and (iv) MSV-PTL. Empty-MSV and MSV-PTL particles (1 billion/mouse) were administered intravenously once every two weeks for four weeks (Figure 1a, bottom panel) for a total of two doses per animal. We found that treatment with two doses containing 50μg of PTL delivered via MSV-PTL (approximately 2.5mg/kg) resulted in a significant decrease in AML tumor burden (20% decrease AML9-PDX to 60% decrease AML4-PDX) when compared to PBS, MSV-empty, or micelle-PTL treated mice (no significant change among these groups in both AML-PDX tested) (Figure 1b). Therefore, the MSV system was highly effective for enhancing small molecule delivery. Importantly, we found that mice receiving two treatments MSV-PTL containing 50μg of PTL nanoparticles spaced two weeks apart, resulted in effective killing of leukemia cells in vivo, using approximately 40-fold lower dosage and 20-fold lower frequency that the chemical analog of PTL (DMAPT; used at minimal activity doses)8.
We corroborated that the system can effectively deliver PTL to the mouse BM, using MSV loaded with non-specific-siRNA labeled with Alexa555. AML-PDX animals were treated with the MSV particles with one bolus dose as shown in the schema (Figure 1c). We found MSV-delivery to human cells by the detection of Ax555-positive human AML cells obtained from the mouse BM 16 h after injection with MSV-siRNA-Ax555 compared to empty particles or MSV-PTL controls (Supplemental Figure 1b). Furthermore, we observed inhibition of NF-κB and activation of γ-H2AX in the human cells obtained from the mouse BM, which are known activities of PTL (Supplemental Figure 1c)5, 13 as soon as 16 h of treatment with MSV-PTL, further demonstrating that the MSV system delivered active PTL to the murine BM. Together, these data suggest that the MSV system can effectively release drugs to mouse BM, thus enabling an otherwise low-bioavailability drug to kill AML cells.
Importantly, we evaluated the concentration of PTL in plasma and bones from the AML-PDX mice 1 h after administration of nanoparticles (Figure 1c). Strikingly, PTL was not detected in the plasma at the 1 h time point thus indicating that there was likely no degradation of the MSV-PTL nanoparticles to release PTL in plasma. PTL was only detected in bone samples (Figure 1d). The average concentration of PTL was 375.0 ± 14.7 nM (n=4). Our data demonstrate that PTL was delivered in chemically intact form to the bone tissue and not released into the plasma.
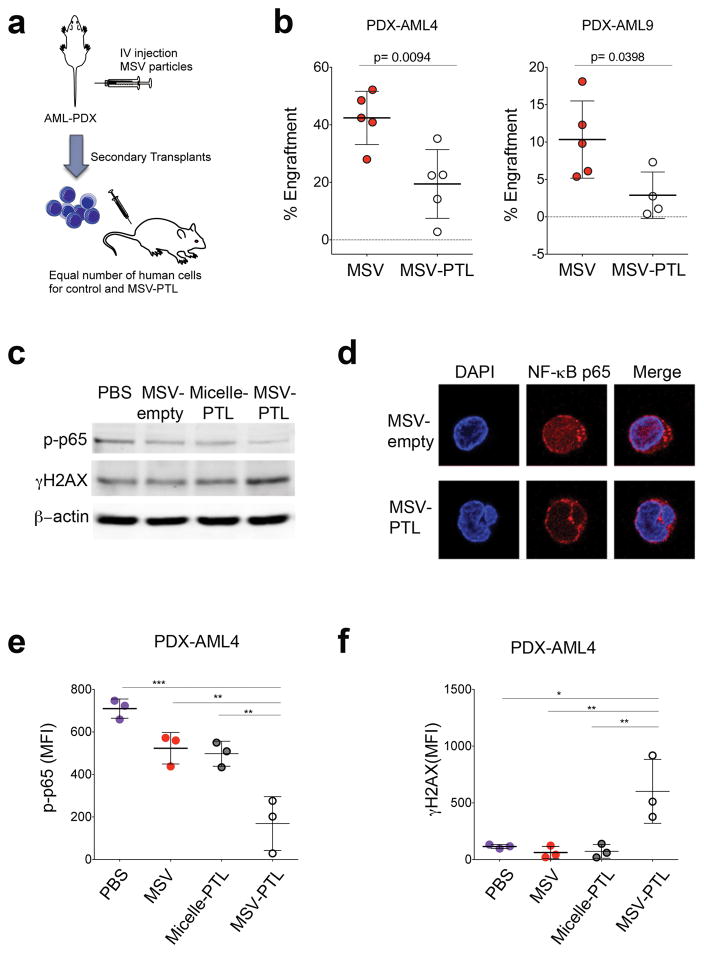
Because PTL is known to be an anti-LSC agent, we evaluated the impact of the in vivo treatment on LSC function by performing secondary xenotransplants, injecting equal numbers of human AML cells from the mice treated mice (Figure 2a). Treatment with MSV-PTL resulted in a 3.7-fold and 2.2-fold reduction in secondary AML engraftment, compared to the MSV control group (p<0.05) for cells obtained from AML9-PDX and AML4-PDX, respectively (Figure 2b). The reduced level of secondary engraftment demonstrated that directed delivery of PTL to the BM using the MSV system resulted in killing of LSCs.
Figure 2. Encapsulation of PTL in BM targeting MSV impairs LSC in vivo.
(a) Schematic representation of the secondary transplant experimental design (b) Percent human cells in mouse BM from secondary transplants from the indicated treatment groups. Each circle represents an individual mouse. Horizontal bar represents the mean. Error bars represent S.E.M. (c) Immunoblotting using anti-phospho p65, γH2AX and β-actin antibodies. Lysates were obtained from mice from AML-PDX9 (d) Confocal micrograph for NF-κB p65 (green) and DAPI (blue). (e–f) Mean fluorescence intensity (MFI) for phospho-p65 (p-p65, left panel) and gamma-H2AX (γH2AX, right panel) with the indicated treatments. BM cells are from AML-PDX4. Expression of proteins was evaluated by intracellular staining and flow cytometry. Cells were gated for human cells. Each circle represents an individual mouse. Horizontal bar represents the mean. Error bars represent S.E.M. * p<0.05, **p<0.01.
To corroborate the biological activity of PTL, we next analyzed the intracellular changes characteristic of PTL that are indicative of drug target engagement. Human cells from AML-PDX mice were evaluated after the in vivo treatment regimen with MSV-PTL. Two known features that reflect the anti-leukemia activity of PTL are inhibition of NF-κB and activation of γH2AX13. Using immunoblots, confocal microscopy, and flow cytometry, we demonstrated that treatment with MSV-PTL indeed resulted in increased inhibition of NF-κB and elevated activation of γ-H2AX. In contrast, PBS, MSV-empty, and micelle-PTL did not demonstrate these activities (Figure 2c–f).
Translation of in vitro pre-clinical findings to the clinic represents a significant obstacle in improving patient outcomes in AML where novel therapies are urgently needed. Often, a major hurdle is development chemical derivatives with useful drug-like characteristics, which can significantly delay the development of new therapeutics and clinical trials. One such example is the small molecule natural product PTL, which has established potent anti-LSC activity in AML in vitro.
This study demonstrates the efficacy and utility of encapsulating unmodified pre-clinical small molecules such as PTL in a MSV system to facilitate delivery to the tumor niche. LSCs reside within and are protected by the BM niche7. Encapsulation of PTL into the MSV coated with ESTA resulted in rapid delivery of chemically intact PTL to the BM niche. Two doses of MSV-PTL delivered two weeks apart were capable of reducing AML burden and dramatically impairing LSC function in vivo. The poor bioavailability and pharmacokinetic profile of PTL were overcome by encapsulation in the MSV, producing in vivo efficacy against LSCs using AML-PDX mice. Indeed, PTL delivered via this system engaged cellular responses consistent with PTL activity, leading to a decrease in both AML burden and LSC function. Overall, these studies demonstrate that the use of targeted MSV nanoparticles is a novel and promising therapeutic approach in AML by facilitating anti-LSC small molecules such as PTL to the BM niche.
Supplementary Material
Acknowledgments
The authors are supported by Leukemia and Lymphoma Society (M.L.G, G.J.R., H.S). WCMC-TMHRI Pilot grant (M.L.G and H.S), US National Institutes of Health (NIH) through the NIH Director’s New Innovator Award Program, 1 DP2 OD007399-01 (M.L.G), National Cancer Institute (R21 CA158728-01A1; M.L.G.). M.L.G is a V Foundation Scholar.
Footnotes
Disclosure of Potential Conflicts of Interest. The authors declare no competing financial interests.
Author Contributions H.Z, S.S. performed experiments. G.Z., C.M., D.G.G., X.L generated nanoparticles All authors participated in the design and analysis of various experiments. H.Z., G.J.R., H.S. and M.L.G. wrote the paper.
References
- 1.Dohner H, Estey EH, Amadori S, Appelbaum FR, Buchner T, Burnett AK, et al. Diagnosis and management of acute myeloid leukemia in adults: recommendations from an international expert panel, on behalf of the European LeukemiaNet. Blood. 2010 Jan 21;115(3):453–474. doi: 10.1182/blood-2009-07-235358. [DOI] [PubMed] [Google Scholar]
- 2.Guzman ML, Allan JN. Concise review: Leukemia stem cells in personalized medicine. Stem cells. 2014 Apr;32(4):844–851. doi: 10.1002/stem.1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van Rhenen A, Feller N, Kelder A, Westra AH, Rombouts E, Zweegman S, et al. High stem cell frequency in acute myeloid leukemia at diagnosis predicts high minimal residual disease and poor survival. Clin Cancer Res. 2005 Sep 15;11(18):6520–6527. doi: 10.1158/1078-0432.CCR-05-0468. [DOI] [PubMed] [Google Scholar]
- 4.Guzman ML, Neering SJ, Upchurch D, Grimes B, Howard DS, Rizzieri DA, et al. Nuclear factor-kappaB is constitutively activated in primitive human acute myelogenous leukemia cells. Blood. 2001 Oct 15;98(8):2301–2307. doi: 10.1182/blood.v98.8.2301. [DOI] [PubMed] [Google Scholar]
- 5.Guzman ML, Rossi RM, Karnischky L, Li X, Peterson DR, Howard DS, et al. The sesquiterpene lactone parthenolide induces apoptosis of human acute myelogenous leukemia stem and progenitor cells. Blood. 2005 Jun 1;105(11):4163–4169. doi: 10.1182/blood-2004-10-4135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Curry EA, 3rd, Murry DJ, Yoder C, Fife K, Armstrong V, Nakshatri H, et al. Phase I dose escalation trial of feverfew with standardized doses of parthenolide in patients with cancer. Invest New Drugs. 2004 Aug;22(3):299–305. doi: 10.1023/B:DRUG.0000026256.38560.be. [DOI] [PubMed] [Google Scholar]
- 7.Konopleva MY, Jordan CT. Leukemia stem cells and microenvironment: biology and therapeutic targeting. J Clin Oncol. 2011 Feb 10;29(5):591–599. doi: 10.1200/JCO.2010.31.0904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hassane DC, Sen S, Minhajuddin M, Rossi RM, Corbett CA, Balys M, et al. Chemical genomic screening reveals synergism between parthenolide and inhibitors of the PI-3 kinase and mTOR pathways. Blood. 2010 Dec 23;116(26):5983–5990. doi: 10.1182/blood-2010-04-278044. [DOI] [PubMed] [Google Scholar]
- 9.Mai J, Huang Y, Mu C, Zhang G, Xu R, Guo X, et al. Bone marrow endothelium-targeted therapeutics for metastatic breast cancer. J Control Release. 2014 Aug 10;187:22–29. doi: 10.1016/j.jconrel.2014.04.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mann AP, Somasunderam A, Nieves-Alicea R, Li X, Hu A, Sood AK, et al. Identification of thioaptamer ligand against E-selectin: potential application for inflamed vasculature targeting. PloS one. 2010;5(9) doi: 10.1371/journal.pone.0013050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mann AP, Tanaka T, Somasunderam A, Liu X, Gorenstein DG, Ferrari M. E-selectin-targeted porous silicon particle for nanoparticle delivery to the bone marrow. Adv Mater. 2011 Sep 22;23(36):H278–282. doi: 10.1002/adma.201101541. [DOI] [PubMed] [Google Scholar]
- 12.Schweitzer KM, Drager AM, van der Valk P, Thijsen SF, Zevenbergen A, Theijsmeijer AP, et al. Constitutive expression of E-selectin and vascular cell adhesion molecule-1 on endothelial cells of hematopoietic tissues. The American journal of pathology. 1996 Jan;148(1):165–175. [PMC free article] [PubMed] [Google Scholar]
- 13.Guzman ML, Rossi RM, Neelakantan S, Li X, Corbett CA, Hassane DC, et al. An orally bioavailable parthenolide analog selectively eradicates acute myelogenous leukemia stem and progenitor cells. Blood. 2007 Dec 15;110(13):4427–4435. doi: 10.1182/blood-2007-05-090621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guzman ML, Yang N, Sharma KK, Balys M, Corbett CA, Jordan CT, et al. Selective activity of the histone deacetylase inhibitor AR-42 against leukemia stem cells: a novel potential strategy in acute myelogenous leukemia. Molecular cancer therapeutics. 2014 Aug;13(8):1979–1990. doi: 10.1158/1535-7163.MCT-13-0963. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.