Introduction
African-American (AA) women in the U.S. experience the lowest breast cancer survival rates among all ethnic groups compared to European Americans. 1,2 Breast cancer (BrCA) is the second leading cause of cancer death in South Carolina regardless of race. 3 Additionally, South Carolina has an overall BrCA mortality rate that is greater than the national average, driven exclusively by the high BrCA mortality rates seen in AAs. 4 The state ranks 7th in diabetes prevalence in the United States, affecting approximately 375,000 people. 5 Because diabetes may promote the proliferation of cancer cells and metastasis 6 the increasing prevalence of diabetes raises important questions about the possible relationship between diabetes and BrCA.
Recent meta-analytic studies suggest that type 2 diabetes (T2DM) can have incongruous effects depending on the anatomic site of cancer; e.g., diabetes may show a protective effect in prostate cancer and a detrimental effect in BrCA. 6 The population of SC is an ideal environment to examine the ethnic differences in T2DM and BrCA due to the large percentage of AAs (28%) residing in SC 7 and the excellent quality of available cancer incidence and mortality data. 1,4 Also, T2DM prevalence has increased 51% over the past 10 years in SC and now affects 1 in 8 AAs. 5
For this analysis, we sought to link information from the South Carolina Central Cancer Registry (SCCCR) 4 and Medicaid records, as we previously had done with colorectal cancer,8 to examine the association between incident T2DM and BrCA stage at diagnosis and mortality due to the disease. The rising disparities between EAs and minorities in BrCA patterns of incidence and mortality has been well-documented in the US.1,9 These racial disparities are evident in age at diagnosis, disease virulence, and prognosis; and, ultimately, survival and death. Reasons for the differences are unclear; however, they may be attributed to actual biological differences by race in the nature of the disease; comorbidities; and variable access to, and willingness to use, health care services. In this study, we examine the association of T2DM and BrCA stage and survival rates in both AA and EA women. In addition to understanding the relationship between T2DM and BrCA, we also will compare the association by race to consider potential differences that might be necessary to include in future implementations of race-specific health interventions related to diabetes and BrCA.
Methods
For the use of de-identified data collected for other purposes, the University of South Carolina Office of Research’ Institutional Review Board granted an exemption.
Study participants
This retrospective cohort study was conducted using linked data drawn from the Medicaid records between 1993 and 2002 and the South Carolina Central Cancer Registry (SCCCR) between 1996 and 2001. Both data sets consist of administrative claims data from AA and EA women. Only participants with a BrCA diagnosis in both the SCCCR ICD-O and Medicaid ICD-9 designations were classified as having BrCA (n=1,462). Women with a previous BrCA diagnosis were excluded from analyses.
The SCCCR, which is based in the SC Department of Health & Environmental Control (DHEC), is supported through a cooperative agreement with the Centers for Disease Control and Prevention (CDC). The SCCCR collects data on cancer incidence, changes in diagnosis or treatment, and survival rate 5 and has a gold rating for timeliness and data completion rates.
The study sample consisted of a total of 3835 EA women and 3475 AA women. A retrospective cohort study design was utilized to estimate the BRCA prevalence among women with and without a diagnosis of T2DM and to determine the risk of developing cancer based on diabetes status for the two races. Among women in our study with BrCA approximately half are EA (n=737) and half are AA (n=725). All modeling results are based upon the cohort of women diagnosed with BrCA.
Measures
T2DM was determined using Medicaid records. Women were considered to have diabetes if there was a diagnosis of T2DM or if a woman filled a prescription for a drug used for treating diabetes. Prescriptions included injectable (Humalog, Humulin, Iletin, Insulatard, Insulin, Lantus, Lente, Novolin, Semilente, Ultralente, and Velosulin) and oral (Acetohexamide, Acarbose, Actos, Amaryl, Avandia, Chlorpropamide, Diabeta, Diabinese, Dymelor, Glimepiride, Glipizide, Glucatrol, Glucophage, Glyburide, Glycron, Glynase, Glyset, Insulase, Metformin, Micronase, Micronized Glyburide, Miglitol, Orinase, Phenazopyridine, Pioglitazone, Prandin, Precose, Rezulin, Repaglinide, Rosiglitazone, Tolazamide, Tolbutamide, Tolinase, Troglitazone) medications. The earliest date was kept when both indicators were present. We stratified AA and EA women into two groups based on T2DM presence at the time of BrCA diagnosis. The outcome variable was BrCA stage-at-diagnosis in our first model and vital status (deceased at follow-up yes/no) in our second. The four 2000 SEER summary stage items of initial diagnosis were categorized as: in situ- 0, localized- 1, regional- 2, and distant- 3. These designations are based on how far the carcinoma has spread from the breast. The initial seven summary stages from SEER 2000 were broken down into these 4 categories, as noted above: in situ includes noninvasive tumors; localized is confined to the breast tissue; regional includes direct extension, involvement of lymph nodes, or a combination of both; and distant spread indicates involvement of other organs. Stage 9 was excluded from the analysis due to unknown nature of the tumor.
We also assessed potential confounding by diabetes medications and menopausal age, which has been associated with both T2DM and BrCA. Menopause was deduced from age obtained from the Medicaid records by categorizing non-menopausal as those women <55 years and menopausal as ≥ 55 years of age. We stratified by race (AA vs. EA), which was self-reported by women in Medicaid. Continuous enrollment after diagnosis was monitored and the date of last follow-up was assigned as the date at which the woman was no longer enrolled in Medicaid or her eligibility status had changed.
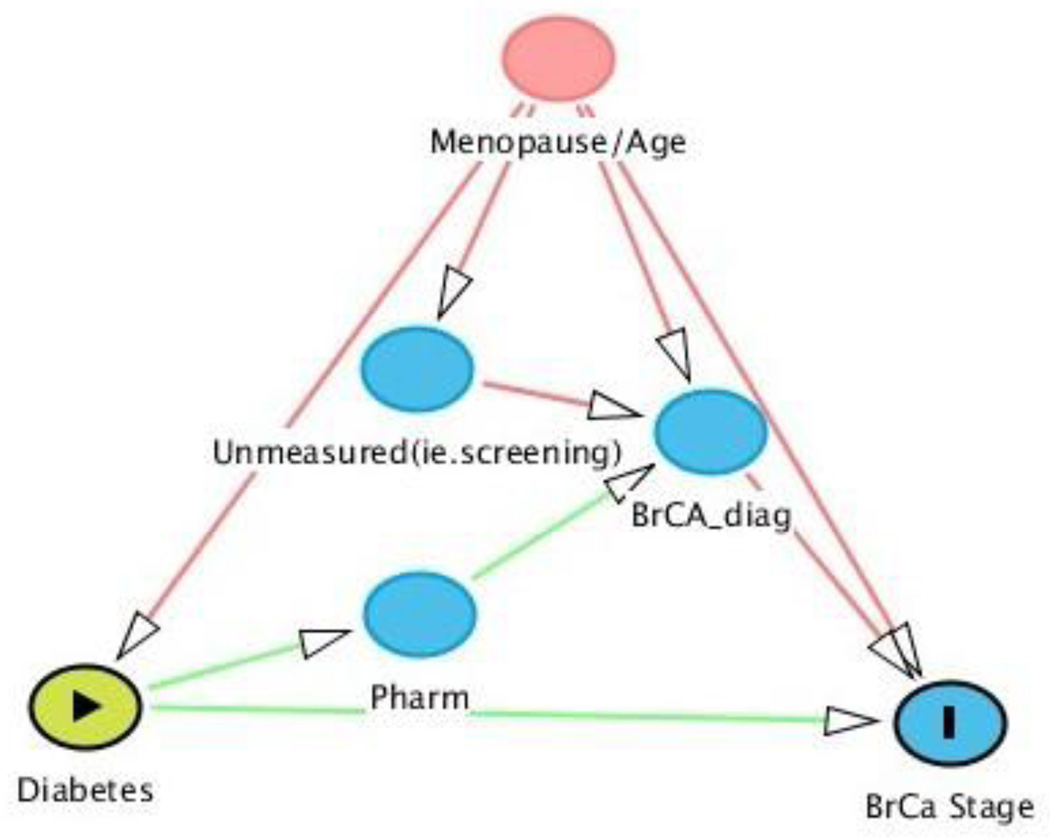
Directed Acyclic Graph (DAG)
To reduce common errors made in confounder selection 10 the analytic approach was based on a causal diagram. The causal diagram in Figure 1 shows the variables as vertices connected by directed edges, or arrows. There are no directed cycles in the figure. These properties are characteristic of a directed acyclic graph (DAG). There is a causal pathway between the exposure (diabetes) and outcome of interest (BrCA stage), which represents a direct effect of T2DM on BrCA stage-at-diagnosis. In observational studies, a common approach to estimating direct effects yields biased information. 11 Using DAG’s, researchers can identify potential confounders and improved understanding of bias that may have occurred. 12Race is an effect modifier and was examined by stratifying analyses by race; i.e., AA and EA women considered separately.
Figure 1. Directed Acyclic Graph (DAG) for diabetes and breast cancer stage.
*(Textor, Hardt, and Knüppel)
Abbreviation: BrCA = Breast Cancer; Pharm: Pharmacological Medicine/ Diabetes Drugs; BrCA_diag: Breast Cancer Diagnosis
For our DAG, we note a few assumptions:
Menopause/Age affects diabetes and BrCA diagnosis, unmeasured confounders (i.e., screening), and BrCA stage
Diabetes diagnosis affects diabetes prescription use (pharm). Diabetes medications are causally related to BrCA stage because the medications may mask symptoms of BrCA and delay BrCA diagnosis. 13
Early menopause is associated with variations in type 2 diabetes. The drop in estrogen after menopause may contribute to detrimental effects of diabetes 14 and there may be underlying factors connecting menopause and the most common histological types BrCA. 15
Statistical Methods
All analyses were performed using SAS® version 9.3. Preliminary analyses were undertaken and results examined for the entire cohort using all demographic data by race with chi-square tests. Conditional logistic regression models including data only from those women diagnosed with BrCA were used to calculate odds ratios with 95% confidence intervals (CI). Non-modifiable risk factors were fit in the model to reduce the effect of confounding. Age was categorized into a dichotomous variable based on our estimate of menopausal age (<55 years of age and ≥55 years). Conditional logistic regression was conducted to model the relationship between T2DM and BrCA stage (using multinomial logistic regression) or death among women diagnosed with BrCA after adjusting for the same covariates for each race. Analyses were conducted by race for stratum-specific effects. All p-values were two-tailed and the significance level was assessed at α= .05 Type I error rate.
Results
Table 1 shows study population characteristics by race. The sample consisted of 7,310 eligible participants. The median ages of diabetes for AA and EA were 61 and 63 years, respectively. However, a much higher percentage of AA women (46%) than EA women (25%) were under 55 years of age (our cut point for assuming women were premenopausal). A higher proportion of AA women were single (53%) compared to EA women (28%). Furthermore, 20% of AAs were using diabetes medication compared to 10% of EAs. The presence of diabetes differed significantly by race (p<0.01). AA women had twice the prevalence of diabetes in this BrCA sample compared to EAs.
Table 1.
Characteristics of the Study Population according to Medicaid and South Carolina Central Cancer Registry (SCCCR), 1996–2001
| Race | ||
|---|---|---|
| African-American, N (%) | European-American, N (%) | |
| No. of patients | 3429 (47) | 3790 (53) |
| Menopause | ||
| No | 310 (46) | 172 (25) |
| Yes | 369 (54) | 520 (75) |
| Breast Cancer Stage | ||
| 0 (in situ) | 98 (14) | 74 (11) |
| I | 292 (43) | 382 (55) |
| II | 243 (36) | 193 (28) |
| III | 46 (7) | 43 (6) |
| Marital Status | ||
| Divorced | 118 (4) | 268 (8) |
| Married | 653 (21) | 874 (26) |
| Single | 1652 (53) | 978 (28) |
| Widowed | 664 (22) | 1301 (38) |
| Breast Cancer | ||
| Present | 679 (20) | 692 (18) |
| Absent | 2750 (80) | 3098 (82) |
| Diabetes Medication | ||
| Diabetes | 687 (20) | 365 (10) |
| Present | ||
| Absent | 834 (24) | 459 (12) |
| Mean age of diabetes diagnosis (SD) |
2595 (76) | 3331(88) |
| Mean age of breast | 61 (12) | 63 (13) |
| cancer diagnosis | ||
| (SD) | 57 (15) | 65 (15) |
| Mean death age (SD) | 75 (15) | 80 (11) |
Table 2 shows the crude measures of BrCA stage. For AA women with BrCA, there is a significant (protective) association between BrCA and menopause. The correlation between menopause and decreased risk of late-stage BrCA also is evident in EAs despite non-significant point estimates. When adjusting for these covariates (Table 3) by race for the BrCA stage outcome, menopause remained significant in AA women with BrCA with the same lower odds of having BrCA diagnosed at more advanced stages. The other variables, T2DM and diabetes medication were not significant for either race.
Table 2.
Univariable associations with breast cancer stage
| Stage 0/ In-situ* |
No. | Stage I ** OR ‡ (CI) § |
No. | Stage II ** OR ‡ (CI) § |
No. | Stage III ** OR ‡ (CI) § |
|
|---|---|---|---|---|---|---|---|
| African- American (n=679) |
|||||||
| Menopause^ | |||||||
| Yes | 64 | 151 | 0.57 (0.35,0.92) | 119 | 0.51 (0.31, 0.83) | 20 | 0.41 (0.20,0.84) |
| No | 34 | 141 | 1.00 | 124 | 1.00 | 26 | 1.00 |
| Diabetes | |||||||
| Yes | 29 | 73 | 0.79 (0.48, 1.32) | 59 | 0.76 (0.45, 1.29) | 9 | 0.58 (0.25, 1.35) |
| No | 69 | 219 | 1.00 | 184 | 1.00 | 37 | 1.00 |
| Diabetes Medication | |||||||
| Yes | 26 | 62 | 0.75 (0.44, 1.27) | 49 | 0.70 (0.41, 1.21) | 7 | 0.50 (0.20, 1.25) |
| No | 72 | 230 | 1.00 | 194 | 1.00 | 39 | 1.00 |
| European- American (n=692) |
|||||||
| Menopause | |||||||
| Yes | 52 | 299 | 1.52 (0.88, 2.65) | 127 | 0.81 (0.46, 1.46) | 29 | 0. 88 (0.39, 1.97) |
| No | 22 | 83 | 1.00 | 66 | 1.00 | 14 | 1.00 |
| Diabetes | |||||||
| Yes | 11 | 47 | 0.80 (0.40, 1.63) | 14 | 0.45 (0.19, 1.04) | 1 | 0.14 (0.02,1.04) |
| No | 63 | 335 | 1.00 | 179 | 1.00 | 42 | 1.00 |
| Diabetes Medication | |||||||
| Yes | 7 | 40 | 1.12 (0.48, 2.61) | 11 | 0.58 (0.22, 1.55) | 1 | 0.23 (0.03, 1.92) |
| No | 67 | 342 | 1.00 | 182 | 1.00 | 42 | 1.00 |
Menopause is estimated based on age (cut at age 55)
Controls- Stage 0- In situ
Stage I- Localized; Stage II- Regional; Stage III- Distant
Odds Ratio
95% Confidence Interval
Table 3.
Multivariate Adjusted Stratum Specific- measures of association for breast cancer stage
| Stage 0/ In Situ* |
No. | Stage I ** OR ‡ (CI) § |
No. | Stage II ** OR ‡ (CI) § |
No. | Stage III ** OR ‡ (CI) § |
|
|---|---|---|---|---|---|---|---|
| African- American (n=679) |
|||||||
| Menopause | |||||||
| Yes | 64 | 151 | 0.58 (0.36, 0.95) | 119 | 0 .53 (0.32, 0.86) | 20 | 0.44 (0.21, 0.91) |
| No | 34 | 141 | 1.00 | 124 | 1.00 | 26 | 1.00 |
| Diabetes | |||||||
| Yes | 29 | 73 | 1.23 (0.33, 4.54) | 59 | 1.34 (0.36, 5.05) | 9 | 1.36 (0.22, 8.59) |
| No | 69 | 219 | 1.00 | 184 | 1.00 | 37 | 1.00 |
| Diabetes Medication | |||||||
| Yes | 26 | 62 | 0.69 (0.18, 2.68) | 49 | 0.60 (0.15, 2.40) | 7 | 0.44 (0.06, 3.20) |
| No | 72 | 230 | 1.00 | 194 | 1.00 | 39 | 1.00 |
| European- American (n=692) |
|||||||
| Menopause | |||||||
| Yes | 52 | 299 | 1.46 (0.83, 2.56) | 127 | 0.80 (0.44, 1.44) | 29 | 0.86 (0.38, 1.96) |
| No | 22 | 83 | 1.00 | 66 | 1.00 | 14 | 1.00 |
| Diabetes | |||||||
| Yes | 11 | 47 | 0.40 (0.10, 1.28) | 14 | 0.25 (0.05, 1.16) | 1 | N/A*** |
| No | 63 | 335 | 1.00 | 179 | 1.00 | 42 | |
| Diabetes Medication | |||||||
| Yes | 7 | 40 | 2.86 (0.65, 12.6) | 11 | 2.29 (0.38, 13.7) | 1 | N/A*** |
| No | 67 | 342 | 1.00 | 182 | 1.00 | 42 | |
Referent - Stage 0- In situ
Stage I- Localized; Stage II- Regional; Stage III- Distant
Odds Ratio
95% Confidence Interval
N/A: values do not converge due to low cell numbers (point estimate <0001)
Table 4 shows the crude, stratum-specific measures of association for mortality. For AA women with BrCA, menopause, diabetes diagnosis and having a cancer diagnosis at anything but in situ stage doubles the odds of death compared to EAs. For EA, each variable is significant; however, diabetes diagnosis and diabetes medications decrease the odds of dying relative to AAs. Comparing the two races’ odds of death at different stages of diagnosis compared to in situ, AA have twice the probability of death. When adjusting for each variable in the mortality model (Table 5), AA’s odds of death are increased significantly by menopause and BrCA stage. In EA women, menopause, diabetes and BrCA stage increased the odds of death. AA women have approximately twice the odds of not surviving within each BrCA stage compared to EA women with the corresponding BrCA stage in both the crude and adjusted models.
Table 4.
Univariable models with outcome of death (deceased vs. not deceased at date of last follow-up) OR Crude measures of Vital Statistics
| African- Americans (n=679) | European-American (n=692) | |||||
|---|---|---|---|---|---|---|
| No. of controls* |
No. | OR (CI) | No. of controls |
No. | OR (CI) | |
| Menopause | ||||||
| Yes | 244 | 110 | 1.45 (1.03, 2.04) | 359 | 148 | 2.13 (1.38, 3.29) |
| No | 248 | 77 | 1.00 | 155 | 30 | 1.00 |
| Diabetes | ||||||
| Yes | 113 | 57 | 1.47 (1.01, 2.14) | 63 | 10 | 0.43 (0.21, 0.85) |
| No | 379 | 130 | 1.00 | 451 | 168 | 1.00 |
| Diabetes Medication |
||||||
| Yes | 96 | 48 | 1.43 (0.96, 2.12) | 53 | 6 | 0.30 (0.13, 0.72) |
| No | 396 | 139 | 1.00 | 461 | 172 | 1.00 |
| Breast Cancer | ||||||
| Stage III | 8 | 38 | 53.4 (18.7, 152.8) | 8 | 35 | 31.6 (11.2, 89.1) |
| Stage II | 155 | 88 | 6.39 (2.96, 13.8) | 138 | 55 | 2.88 (1.34, 6.18) |
| Stage I | 239 | 53 | 2.50 (1.14, 5.45) | 303 | 79 | 1.88 (0.90, 3.95) |
| Stage 0** | 90 | 8 | 1.00 | 65 | 9 | 1.00 |
Controls refer to individuals who are alive
In situ, localized
Table 5.
Adjusted Stratum Specific- measures of association for the outcome of death (deceased vs. not deceased at date of last follow-up OR for vital statistics
| African-American | European-American | |||||
|---|---|---|---|---|---|---|
| African- American |
No. of controls |
No. | OR (CI) | No. of controls |
No. | OR (CI) |
| Menopause | ||||||
| Yes | 244 | 110 | 1.78 (1.21, 2.62) | 359 | 148 | 2.98 (1.82, 4.89) |
| No | 248 | 77 | 1.00 | 155 | 30 | 1.00 |
| Diabetes | ||||||
| Yes | 113 | 57 | 1.52 (0.61, 3.77) | 63 | 10 | 1.92 (0.55,6.66) |
| No | 379 | 130 | 1.00 | 451 | 168 | 1.00 |
| Diabetes Medication |
||||||
| Yes | 96 | 48 | 1.05 (0.40, 2.75) | 53 | 6 | 0.16 (0.04, 0.74) |
| No | 396 | 139 | 1.00 | 461 | 172 | 1.00 |
| Breast Cancer | ||||||
| Stage III | 8 | 38 | 69.4 (23.8, 203.) | 8 | 35 | 37.8 (13.0, 110.) |
| Stage II | 156 | 88 | 7.49 (3.43, 16.3) | 138 | 55 | 3.07 (1.41, 6.69) |
| Stage I | 239 | 53 | 2.79 (1.27, 6.14) | 303 | 79 | 1.83 (0.87, 3.88) |
| Stage 0 | 90 | 8 | 1.00 | 65 | 9 | 1.00 |
Discussion
Our findings show that significant racial disparities in late-stage BrCA exist after accounting for diabetes medication and age at menopause. Studying reasons for differences in BrCA outcome has the potential for decreasing health disparities related to late-stage BrCA and poor survival. The point estimates for BrCA risk (Table 5) shows the magnitude of the effect under study: AAs suffer twice the mortality rate compared to EAs at virtually every disease stage (compared to in situ). AA women who have BrCA at younger ages also tend to have larger tumors and higher rates of local/ distant metastasis. These women have twice the incidence of invasive BrCA than do EAs.16 The significantly reduced risk of late-stage BrCA associated with menopause in AAs supports this (Table 3) and is consistent with what we know about the differences in the epidemiology of BrCA by menopausal status. 17,18 More studies need to be conducted to understand this relationship, but based on emerging knowledge policymakers may have to consider race/ethnic- appropriate clinical guidelines to encourage earlier mammograms among AA women who are more likely to have late-stage breast cancer diagnosed at younger ages. 19,20
The DAG 21 helped us identify necessary variables to control for prior to conducting the study. An “unblocked backdoor path” (i.e., a path that has an arrow pointing into the exposure that ends with an arrow pointing to the outcome), such as menopause, is important to adjust for as it may otherwise bias results in our study (Figure 1).12 Our results show that age ≥55 years (which is our proxy for menopause) played a significant role in understanding the relationship between diabetes and BrCA. Since 94% of the US female population is menopausal by age 54, this cut-off point is a reasonable proxy for menopause. 22
Confining the analysis to cases of BrCA allows us to compare women at each stage of BrCA. Findings from our study show that women with more advanced stages of BrCA have an increased chance of death; however, the odds of death for EA were not significantly different between stage 0 and stage I and became significant in the last 2 stages. For AA, when compared to stage 0, the odds of death among those with stage III cancer was nearly 70 times (OR=69.4 CI: 23.8, 203.0) higher; nearly twice the differential observed in EAs (OR=37.8 CI: 13.0, 110.0). The reasons for this dramatic difference are important to understand. Possible explanation include the interaction differences and BrCA, lack of access to health care services, pre-existing or co-morbid medical conditions, and genetic factors that may result in metabolic obesity (i.e., AAs appear to have attributes associated with obesity evident at relatively lower total body weight ). 23–25 This latter factor may help to explain the differential effect of T2DM by race.
T2DM is characterized by modifications in insulin levels, 26 which plays a vital role in the rate of cell proliferation and estrogen levels.14 The relationship between insulin receptors and BrCA suggests that the mechanisms involved in diabetes also may affect BrCA. 14 It is possible that the higher prevalence of T2DM in AA may be responsible for the increased incidence of late-stage BrCA and poorer survival. Previous research about the co-occurrence of DM and BrCA is limited in their ability to analyze by race or ethnicity. The association between diabetes and BrCA stage may be masked by diabetes medication. In Table 4, the univariate model shows that medication significantly protects EA’s from BrCA death (OR: 0.30 CI: 0.13, 0.72). Even though data on diabetes medication was not significant among AA in the crude or adjusted model, it is interesting to note that the point estimates are in inverse directions for the odds of death, which increase for AA women and significantly decrease for their EA counterparts. DM medications may modify BrCA risk differentially; for example, two diabetes medications can have different effects: metformin may reduce the risk while glargine increases BrCA risk. 13 Having more detailed information on the use of medications could reduce measurement bias.
With access to the same quality care, 27 evidence suggests that AAs and EAs respond homogeneously to similar treatments given at the same stage of cancer. Therefore, lack of access to essential preventive services may be an important underlying factor leading to AA experiencing almost twice as many BrCA deaths as EA. Other work indicates that participants with T2DM experienced a 2-fold increased risk of incident BrCA, which would align with our findings. The presence of pre-existing conditions in AA populations also might play an important role in determining BrCA survival, which is an under-studied topic. Compared to those individuals with no co-morbidities, participants with 3 or more co-morbidities had a significantly higher risk of BrCA (Hazard Ratio: 2.1 CI: 1.3–3.3).28 Because of the high prevalence of T2DM we need to understand its role as a potential cause of cancer. It is not clear if diabetes directly increases BrCA mortality in AAs; however, there is evidence that both diabetic women 29 and AA women present with later-stage BrCA. 30 Patients with T2DM often have other co-morbidities and because of the simultaneous management of these chronic conditions they are less likely to be screened for BrCA. Furthermore, diabetes and other co-morbid conditions are significantly associated with chemotherapy toxicity, infection, fever, anemia, neutropenia and all-cause mortality. 31 Controlling for co-morbidities could improve AA cancer prognosis. These findings raise questions for further research.
The genome-wide association studies (GWAS) have narrowed susceptible loci identified for BrCA that could link genetic factors to the risk of developing cancers. Being able to identify and treat genetic components of BrCA would allow researchers to focus on differences in cultural and ecological factors that could influence cancer susceptibility. AAs have the highest cancer burden of all racial minority groups.7 Evidence suggests AAs suffer more toxic side effects from certain therapies than EAs 32 due to differential biological responses. More research needs to be done to see the role of co-morbidities and genetic differences. 28 However, isolating certain factors, such as a specific gene related to the aforementioned co-morbidities and BrCA, is time-consuming, costly and may be difficult in large samples. 33 Arguments can be made for both the genetic- based differences in cancer susceptibility and the importance of modifiable exposures. 7 Further studies should consider the genetic and non-genetic factors that contribute to varying effects of T2DM medication on BrCA survival by race.
Strengths
This research study has many strengths. The large sample allowed us to have a unique look at the incidence of BrCA among women with and without a diagnosis of diabetes mellitus in both AA and EA populations. It also highlighted the discrepancies in BrCA for these two major racial groups. In addition, this dataset combined the strengths of both high-quality BrCA information (from a gold-rated state cancer registry) with the very detailed information on diabetes diagnosis and medication use from administrative claims data that avoided having to rely on patient self-report data which can often be biased. 34,35
Limitations
One potential limitation to this study is the misclassification of diabetes mellitus depending on disease onset and the period for determining T2DM. Approximately 100,000 people do not know that they have diabetes in South Carolina, 5 which has the potential change our results towards or away from the null. We had no direct information on menopausal status and, therefore, had to rely on age as a proxy. Another limitation to this study could be detection bias; women with T2DM might be less likely to get screened for BrCA. 14 This would lead to misclassification of the outcome variable. Misclassifying our exposure or outcome of interest could have important consequences on study results. However, this effect was reduced by linking the Medicaid data to SCCCR and considering those using diabetes medication and diagnosed with diabetes as ‘diabetic.’ Both of these factors helped to reduce error. Additionally, registry datasets do not provide information on potential confounders such as diet, physical activity, serum lipids, blood pressure, family history, and anthropometric measurements from which we could compute waist to hip ratio or body mass index. These factors could have shed light on some of the underlying mechanisms that play a role in the relationship between T2DM and BrCA stage-at-diagnosis and survival. Because T2DM is strongly associated with sedentary behavior and, concomitantly, fitness, BMI could have served as a rough proxy for physical activity or fitness, both known risk factors for BrCA. 18,36–38
Conclusion
In this study we provide evidence of racial disparities in BrCA survival rate among AAs and EAs. The crude data shows T2DM had an opposite effect on AA and EA: AA had higher odds of developing BrCA and diabetes may even protect EAs from death. This might be due to diabetes management by race; the types of medications used; differential access to health care services and presence of co-morbidities; genetic components; changes in insulin level; and cell proliferation. The presence of these factors might mask BrCA symptoms and lead to late-stage diagnosis in AAs. The results suggest that the negative association between diabetes and BrCA is stronger in AAs, especially among pre-menopausal women.
Future studies should delve into the various factors that may affect BrCA trajectories by race/ ethnicity. Understanding this association may promote healthy preventive measures for pre-diabetics or better diabetes management tools. Educating the public about the correlation between diabetes and BrCA could improve adherence to certain preventive health guidelines. Furthermore, patients suffering from multiple conditions should be encouraged to continue regular screenings. Due to similarly high incidence and mortality rates among AA in the southeastern part of the US, future research should examine the relationship between diabetes and breast cancer by state.
Acknowledgments
Funding: This work was supported in part by the Department of Defense (W81XWH-04-1-0703), the National Cancer Institute (U54 CA153461; JR Hébert, P.I.) and by a NIH research training grant (T32-GM081740).
Abbreviations
- AA
African American
- EA
European American
- SC
South Carolina
- BrCA
Breast Cancer
- T2DM
Type 2 Diabetes Mellitus
Footnotes
Conflict of interest: None of the authors have conflicts of interest to disclose
Compliance with Ethical Standards” on the title page when submitting a paper:
Ethical approval: This article does not contain any studies with human participants or animals performed by any of the authors.
Contributor Information
Marsha Samson, Email: msamson@email.sc.edu.
Swann Arp Adams, Email: adamss@mailbox.sc.edu.
Olubunmi Orekoya, Email: orekoya@email.sc.edu.
James R. Hebert, Email: jhebert@sc.edu.
Reference
- 1.Adams SA, et al. Breast cancer disparities in South Carolina: early detection, special programs, and descriptive epidemiology. J. S. C. Med. Assoc. 1975. 2006;102:231–239. [PMC free article] [PubMed] [Google Scholar]
- 2.Maskarinec G, Sen C, Koga K, Conroy SM. Ethnic Differences in Breast Cancer Survival: Status and Determinants. Womens Health Lond. Engl. 2011;7:677–687. doi: 10.2217/whe.11.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Healthy People Living in Healthy Communities Report - ML-006048.pdf. 2009 at < http://www.scdhec.gov/library/ML-006048.pdf>.
- 4.Hébert JR, et al. Mapping Cancer Mortality-to-Incidence Ratios to Illustrate Racial and Sex Disparities in a High-risk Population. Cancer. 2009;115:2539–2552. doi: 10.1002/cncr.24270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bolick-Aldrich S. DHEC: SC Central Cancer Registry - Overview. South Carolina Department of Health and Environmental Control. at < http://www.scdhec.gov/Health/DiseasesandConditions/Cancer/CancerStatisticsReports/CancerRegistry/>.
- 6.Giovannucci E, et al. Diabetes and cancer: a consensus report. Diabetes Care. 2010;33:1674–1685. doi: 10.2337/dc10-0666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wallace TA, Martin DN, Ambs S. Interactions among genes, tumor biology and the environment in cancer health disparities: examining the evidence on a national and global scale. Carcinogenesis. 2011;32:1107–1121. doi: 10.1093/carcin/bgr066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cavicchia PP, et al. Racial disparities in colorectal cancer incidence by type 2 diabetes mellitus status. Cancer Causes Control CCC. 2013;24:277–285. doi: 10.1007/s10552-012-0095-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Devesa SS, et al. Recent cancer trends in the United States. J. Natl. Cancer Inst. 1995;87:175–182. doi: 10.1093/jnci/87.3.175. [DOI] [PubMed] [Google Scholar]
- 10.Greenland S, Pearl J, Robins JM. Causal diagrams for epidemiologic research. Epidemiol. Camb. Mass. 1999;10:37–48. [PubMed] [Google Scholar]
- 11.Robins JM. Data, design, and background knowledge in etiologic inference. Epidemiol. Camb. Mass. 2001;12:313–320. doi: 10.1097/00001648-200105000-00011. [DOI] [PubMed] [Google Scholar]
- 12.Fleischer NL, Diez Roux AV. Using directed acyclic graphs to guide analyses of neighbourhood health effects: an introduction. J. Epidemiol. Community Health. 2008;62:842–846. doi: 10.1136/jech.2007.067371. [DOI] [PubMed] [Google Scholar]
- 13.Timofeeva OA, et al. Enhanced expression of SOS1 is detected in prostate cancer epithelial cells from African-American men. Int. J. Oncol. 2009;35:751–760. [PMC free article] [PubMed] [Google Scholar]
- 14.Pollak M. Insulin and insulin-like growth factor signalling in neoplasia. Nat. Rev. Cancer. 2008;8:915–928. doi: 10.1038/nrc2536. [DOI] [PubMed] [Google Scholar]
- 15.Huang Y, Malone KE, Cushing-Haugen KL, Daling JR, Li CI. Relationship between menopausal symptoms and risk of postmenopausal breast cancer. Cancer Epidemiol. Biomark. Prev. Publ. Am. Assoc. Cancer Res. Cosponsored Am. Soc. Prev. Oncol. 2011;20:379–388. doi: 10.1158/1055-9965.EPI-10-0998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Anders CK, Johnson R, Litton J, Phillips M, Bleyer A. Breast Cancer Before Age 40 Years. Semin. Oncol. 2009;36:237–249. doi: 10.1053/j.seminoncol.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Keum N, et al. Adult weight gain and adiposity-related cancers: a dose-response meta-analysis of prospective observational studies. J. Natl. Cancer Inst. 2015;107 doi: 10.1093/jnci/dju428. [DOI] [PubMed] [Google Scholar]
- 18.La Vecchia C, Giordano SH, Hortobagyi GN, Chabner B. Overweight, obesity, diabetes, and risk of breast cancer: interlocking pieces of the puzzle. The Oncologist. 2011;16:726–729. doi: 10.1634/theoncologist.2011-0050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aizer AA, et al. Lack of reduction in racial disparities in cancer-specific mortality over a 20-year period. Cancer. 2014;120:1532–1539. doi: 10.1002/cncr.28617. [DOI] [PubMed] [Google Scholar]
- 20.Adams SA, et al. Racial disparities in breast cancer mortality in a multiethnic cohort in the Southeast. Cancer. 2012;118:2693–2699. doi: 10.1002/cncr.26570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Textor J, Hardt J, Knüppel S. DAGitty: a graphical tool for analyzing causal diagrams. Epidemiol. Camb. Mass. 2011;22745 doi: 10.1097/EDE.0b013e318225c2be. [DOI] [PubMed] [Google Scholar]
- 22.Age at Menopause. (Vital and Health Statistics - National Center for Health Statistics) at < http://www.cdc.gov/nchs/data/series/sr_11/sr11_019.pdf>.
- 23.Hébert JR, et al. C-reactive protein levels in African Americans: a diet and lifestyle randomized community trial. Am. J. Prev. Med. 2013;45:430–440. doi: 10.1016/j.amepre.2013.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hu FB. Globalization of Diabetes The role of diet, lifestyle, and genes. Diabetes Care. 2011;34:1249–1257. doi: 10.2337/dc11-0442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kelishadi R, et al. Effects of a lifestyle modification trial among phenotypically obese metabolically normal and phenotypically obese metabolically abnormal adolescents in comparison with phenotypically normal metabolically obese adolescents. Matern. Child. Nutr. 2010;6:275–286. doi: 10.1111/j.1740-8709.2009.00207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yerrabothala S, Shaaban H, Capo G, Maroules M, Debari VA. The impact of diabetes mellitus on breast cancer outcomes: a single center retrospective study. Pathol. Oncol. Res. POR. 2014;20:209–214. doi: 10.1007/s12253-013-9666-5. [DOI] [PubMed] [Google Scholar]
- 27.Chu QD, et al. Race/Ethnicity has no effect on outcome for breast cancer patients treated at an academic center with a public hospital. Cancer Epidemiol. Biomark. Prev. Publ. Am. Assoc. Cancer Res. Cosponsored Am. Soc. Prev. Oncol. 2009;18:2157–2161. doi: 10.1158/1055-9965.EPI-09-0232. [DOI] [PubMed] [Google Scholar]
- 28.Patterson RE, et al. Medical Comorbidities Predict Mortality in Women with a History of Early Stage Breast Cancer. Breast Cancer Res. Treat. 2010;122:859–865. doi: 10.1007/s10549-010-0732-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Michels KB, et al. Type 2 Diabetes and Subsequent Incidence of Breast Cancer in the Nurses? Health Study. Diabetes Care. 2003;26:1752–1758. doi: 10.2337/diacare.26.6.1752. [DOI] [PubMed] [Google Scholar]
- 30.Kaplan MA, et al. Type 2 diabetes mellitus and prognosis in early stage breast cancer women. Med. Oncol. Northwood Lond. Engl. 2012;29:1576–1580. doi: 10.1007/s12032-011-0109-4. [DOI] [PubMed] [Google Scholar]
- 31.Srokowski TP, Fang S, Hortobagyi GN, Giordano SH. Impact of diabetes mellitus on complications and outcomes of adjuvant chemotherapy in older patients with breast cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2009;27:2170–2176. doi: 10.1200/JCO.2008.17.5935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hasan S, Dinh K, Lombardo F, Kark J. Doxorubicin cardiotoxicity in African Americans. J. Natl. Med. Assoc. 2004;96:196–199. [PMC free article] [PubMed] [Google Scholar]
- 33.Tammemagi CM, Nerenz D, Neslund-Dudas C, Feldkamp C, Nathanson D. Comorbidity and survival disparities among black and white patients with breast cancer. JAMA. 2005;294:1765–1772. doi: 10.1001/jama.294.14.1765. [DOI] [PubMed] [Google Scholar]
- 34.Cook C. Mode of administration bias. J. Man. Manip. Ther. 2010;18:61–63. doi: 10.1179/106698110X12640740712617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Donaldson SI, Grant-Vallone EJ. Understanding Self-Report Bias in Organizational Behavior Research. J. Bus. Psychol. 2002;17:245–260. [Google Scholar]
- 36.Gouveri E, Papanas N, Maltezos E. The female breast and diabetes. The Breast. 2011;20:205–211. doi: 10.1016/j.breast.2011.02.019. [DOI] [PubMed] [Google Scholar]
- 37.Hong T, et al. Preoperative serum C-reactive protein levels and early breast cancer by BMI and menopausal status. Cancer Invest. 2013;31:279–285. doi: 10.3109/07357907.2013.789898. [DOI] [PubMed] [Google Scholar]
- 38.Peel JB, et al. A prospective study of cardiorespiratory fitness and breast cancer mortality. Med. Sci. Sports Exerc. 2009;41:742–748. doi: 10.1249/MSS.0b013e31818edac7. [DOI] [PMC free article] [PubMed] [Google Scholar]