Abstract
INTRODUCTION
Voluntary physical activity levels are regulated by sex hormones. The purpose of this study was to determine the effect of the endocrine disruptor benzyl butyl phthalate (BBP) on the regulation of physical activity in mice.
METHODS
Mouse dams were treated with 500 mg·kg−1·day−1 of BBP or vehicle on gestation days 9–16. Pups were weaned and analyzed for voluntary physical activity levels, puberty development, sex hormone levels, and body composition over a 20 week period.
RESULTS
Seventy-three offspring from BBP treated dams were studied (n=43 males, n=30 females). Endocrine disruption was indicated by decreased anogenital distances in BBP-treated male offspring at 10 (p=0.001) and 20 weeks (p=0.038) and delayed vaginal openings in BBP-treated female offspring (p=0.001). Further, there was a significant decrease in serum testosterone concentration in male mice between control and BBP at 10 weeks (p=0.039) and at 20 weeks (p=0.022). In female mice there was a significant increase in serum testosterone concentration in BBP mice at 20 weeks (p=0.002), and a significant increase in estrogen (estradiol) concentrations at 20 weeks in the control female mice (p=0.015). Overall, BBP mice ran significantly less distance (males, p=0.008; females, p=0.042) than controls. Other than a significant increase in BBP-treated males in fat mass at 20 weeks (p=0.040), there was no significant decrease in weight, lean mass, or fat mass in either female or male mice, regardless of treatment.
CONCLUSION
Maternal endocrine disruption altered hormone response, but not body composition in either sex of offspring, with a corresponding decreased activity throughout early adulthood in all offspring. These results suggest that exposure to common environmental endocrine disruptors in utero, can reduce and alter physical activity levels in offspring.
Keywords: endocrine disruption, voluntary wheel running, sex hormones, environmental toxicant
INTRODUCTION
It is well known that physical inactivity is the underlying cause of a large variety of chronic health conditions, is the second leading actual cause of death in the United States (in conjunction with poor diet), and is a major contributor to obesity and diabetes (23, 26). The regulation of physical activity is primarily influenced by genetic/biological mechanisms (25–92% influence; reviewed in 19) and unique environmental exposures (8–52% influence; 14, 36). Sex hormones have been identified as potent biological regulators of daily activity (4, 18) and decreased levels of circulating sex hormones, specifically testosterone, inhibit voluntary physical activity, which can be rescued with hormone replacement (2, 3). Given that there are multiple environmental endocrine disruptors (ED) that directly affect sex hormone level and functioning (24), it is an intriguing possibility that environmental exposure to ED may directly affect physical activity levels (37). Though disruption in hormone levels or activity can cause a variety of physiological problems (5, 7) there is little evidence regarding the impact of ED exposure on voluntary physical activity levels. Given that benzyl butyl phthalate (BBP) is ubiquitous and found in a variety of plastic and personal care products like deodorants, lotions, nail polishes, fragrances, and body soaps (12, 16), it is unknown whether this established endocrine disruptor affects physical activity levels. Thus, we hypothesized that in utero exposure to BBP would alter physical activity in offspring.
METHODS
This protocol conformed to the standards of humane animal care and was approved by the Texas A&M University Institutional Animal Care and Use Committee (AUP 2012–0274). Twelve female breeder mice (C57Bl/6J inbred mice; Jackson Laboratory, Bar Harbor, ME) were housed two to a cage and paired with one male breeder (n=6). Female mice were evaluated every 12 hours for the presence of a vaginal plug that indicated gestation day 0. At gestation day 0, the female mouse was placed in an individual cage for the remainder of pregnancy. Pregnant female mice were administered a gavage treatment of either a control substance (100µl of sesame oil) or BBP (500 mg·kg−1·day−1 in a vehicle of 100µl of sesame oil; 6) on gestation days 9–16 when organ system development and testosterone production occurs (30). While the BBP dosage we used was based on previous studies (6, 30), it is estimated that fetuses are exposed to 1/100–1/1000 of the mother’s dose of BBP (27). Thus, we estimate that the fetuses were exposed to ≈ 0.5–5 mg·kg−1·day−1 which places these exposure rates slightly above the US EPA safe dose for humans of 0.2 mg·kg−1·day−1 (38).
The resulting pups were weaned at three weeks of age and then housed individually for the remainder of the experiment until sacrifice (Table 1). We also used a cohort of sentinel C57Bl/6J mice (four males, four females) developed from our breeding protocol to ensure that the offspring of our animals were as active as past animals in this strain (20) and to eliminate the possibility that our breeding procedures were introducing an unknown variable that was altering physical activity. The sentinels were pups born from male/female breeder pairs in our animal care facility that received no treatment during pregnancy - i.e. no BBP or no control oil - and were randomly assigned to the sentinel group. Mice from the different treatment groups were sacrificed at 4 weeks, 10 weeks or 20 weeks and are referred to as “Control 4 weeks” or “BBP 4 weeks”"Control 10 weeks” or “BBP 10 weeks” and “Control 20 weeks” or “BBP 20 weeks,” respectively.
Table 1.
Summary demographics of mice. Average weight (g), fat mass (g), and lean mass (g) values were taken on final week of sacrifice.
| Males | Females | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Treatment | Wheel Exposure (weeks) |
Sacrificed Age (weeks) |
# of Males (n=43) |
# of Females (n=30) |
Average Weight (g) ± SD |
Average Fat Mass (g) ± SD |
Average Lean Mass (g) ± SD |
Average Weight (g) ± SD |
Average Fat Mass (g) ± SD |
Average Lean Mass (g) ± SD |
|
Sentinel Animals |
4 | 10 | 4 | 4 | 25.20 ± 1.24 | 1.61 ± 0.20 | 21.98 ± 0.66 | 19.82 ± 0.63 | 1.83 ± 0.29 | 16.81 ± 0.54 |
| BBP | -- | 4 | 7 | 3 | 14.15 ± 2.34 | 1.22 ± 0.31 | 12.48 ± 2.02 | 13.14 ± 0.57 | 1.39 ± 0.31 | 11.13 ± 0.19 |
| 8 | 10 | 8 | 6 | 23.70 ± 0.76 | 1.72 ± 0.51 | 20.86 ± 0.44 | 19.93 ± 1.80 | 1.87 ± 0.63 | 14.66 ± 1.30 | |
| 8 | 20 | 6 | 5 | 27.10 ± 0.76 | 2.42 ± 0.33 | 23.10 ± 0.72 | 24.00 ± 1.12 | 2.58 ± 0.37 | 20.16 ± 0.66 | |
| Control | -- | 4 | 6 | 2 | 14.08 ± 1.08 | 2.21 ± 0.17 | 12.49 ± 0.88 | 11.91 ± 0.19 | 1.23 ± 0.07 | 10.36 ± 0.14 |
| 8 | 10 | 6 | 6 | 24.23 ± 0.61 | 1.67 ± 0.11 | 21.63 ± 0.73 | 19.90 ± 0.60 | 1.73 ± 0.31 | 17.14 ± 0.47 | |
| 8 | 20 | 6 | 4 | 26.83 ± 1.19 | 2.00 ± 0.26 | 23.86 ± 0.86 | 23.79 ± 2.16 | 2.18 ± 0.65 | 20.18 ± 1.69 | |
Wheel Running Activity
Beginning at eight weeks of age, physical activity measurements were determined on all animals by measurement of daily distance (km/day), duration (min/day), and speed (m·min−1) of wheel running using our standard lab protocol (21). In brief, running wheels were mounted to the cage tops of standard rat cages and were equipped with a computer (BC500, Sigma Sport, Batavia, IL) to record running distance and duration. Running wheels had a 450mm circumference and a 40mm wide, solid running surface. Running distance and duration data were collected on a daily basis in the morning, sensor alignment and wheel resistance checked and adjusted as needed, and an average daily running speed was calculated from the corresponding distance and duration measures.
Body Composition
Body composition in all mice was measured weekly using magnetic resonance imaging (MRI) using an EchoMRI (EchoMRI, Houston, TX). In brief, mice were first weighed, placed in the appropriate sized MRI tube, and then inserted into the MRI. The body analysis was completed in 30 seconds with the mouse awake and resulted in direct measurements of total body fat (grams) and lean mass (grams).
Puberty Development
As indices of puberty development and whether BBP-treatment disrupted pubertal development, anogenital distances in males and vaginal openings in females were used. In female pups, the beginning of mouse estrous cycling was indicated by the day of vaginal opening (33). This identification was performed daily by holding the mouse face up and examining the vaginal region with a spatula until the vaginal opening was visibly present. Previous studies (28) have suggested that in utero phthalate exposure delays vaginal openings in female offspring. As noted above, in males, anogenital distance [AD - distance from the anus to the base of the genitalia (22)] was measured weekly using a digital caliper (Mitutoyo Digimatic). Several studies (29, 30, 32) have shown that in utero exposure to phthalates significantly decreases the anogenital distance.
Sex Steroid Analysis
On the day of sacrifice, blood samples were extracted via cardiac puncture using a 20 gauge needle, and the blood was spun down with cold centrifugation at 1000 RPM at 4°C for 30 minutes to obtain the serum. Testosterone (ng/ml) was measured in triplicate via ELISA per manufacturer’s instructions (Alpco Serum Testosterone, Salen, NH) for all male and female samples, and estrogen (17–beta estradiol) (pg/ml) was measured in pooled triplicate via ELISA per manufacturer’s instructions (MyBioSource, San Diego, CA) for all female samples.
Statistical Analysis
Physical activity data (distance, duration, and speed) was analyzed by a two-way ANOVA (week×treatment) with 12 levels (8–19 weeks) for time and 2 levels for treatment (BBP vs. Control). In addition, body composition, sex steroid analysis, and puberty development was analyzed by an ANOVA. Alpha levels were set to 0.05 a priori (JMP v.12, SAS, Inc. Cary, NC). A Tukey’s post-hoc test was used to assess significant main effects or interactions.
RESULTS
Seventy-three pups were analyzed for this experiment (Table 1) with an average litter size of six pups in the control mice and four pups in the BBP mice, plus eight sentinel mice (four males and four females).
Sex Steroids
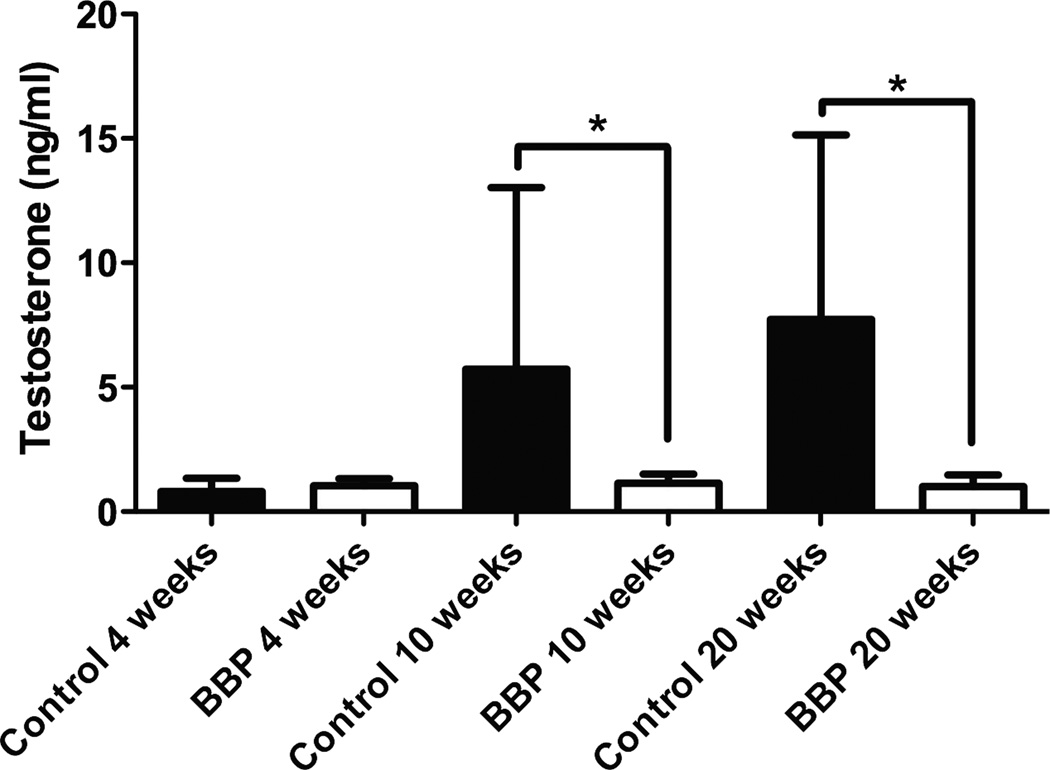
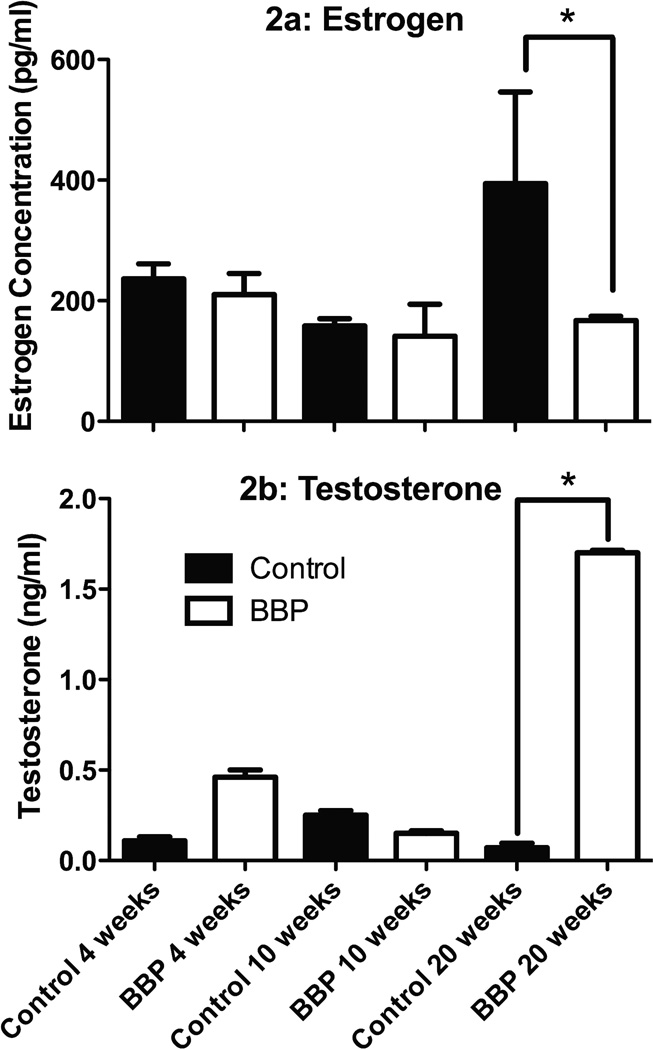
There was a significant decrease in serum testosterone concentration values in BBP male mice compared to control at 10 weeks (p=0.039) and at 20 weeks (p=0.022; Figure 1). There was no significance in serum testosterone concentration values in male mice between control and BBP at 4 weeks (p=0.403; Figure 1). The average coefficient of variance between triplicates from the male samples was 2.23%. When testosterone concentration values were computed, any value outside three standard deviations above or below the mean were eliminated from the analysis; using these standards, we eliminated one data point from the BBP 4 week group, two data points from BBP 20 week group, and two data points from the Control 20 week group. In the female mice, there was no significant difference in serum estradiol concentration levels between control and BBP at 4 weeks (p=0.997), 10 weeks (p=0.999), but the control mice had significantly (p=0.015) higher values at 20 weeks (Figure 2a). The coefficient of variation between triplicates of the estrogen samples averaged 3.12%. In addition, testosterone concentration levels were higher in the female BBP 20 week group versus the control 20-week group (p=0.002; Figure 2b). However, there was no significant difference in serum testosterone concentration levels in the females between the BBP and control groups at 4 weeks (p=0.427) or 10 weeks (p=0.137; Figure 2b). The coefficient of variation values within the testosterone triplicates of the female samples averaged 5.12%.
Fig. 1.
Serum testosterone levels in male control and BBP mice. *Significantly (p<0.05) different between control and BBP-treated animals. Significant difference in serum testosterone concentration values in male mice between control and BBP at 10 weeks (p=0.039) and at 20 weeks (p=0.022). No significance in serum testosterone concentration values in male mice between control and BBP at 4 weeks (p=0.403).
Fig. 2.
Sex hormone levels in female mice. *Significantly (p<0.05) different between control and BBP-treated animals. Panel A: Estradiol concentration values were not significantly different between control and BBP at 4 weeks (p=0.997) or 10 weeks (p=0.999), yet there was significance at 20 weeks (p=0.015). Panel B: Testosterone levels were significantly different between between female BBP 20 weeks and control 20 weeks (p=0.002). No significant difference in serum testosterone concentration values in female mice between control and BBP at 4 weeks (p=0.427) or 10 weeks (p=0.137).
Puberty Development
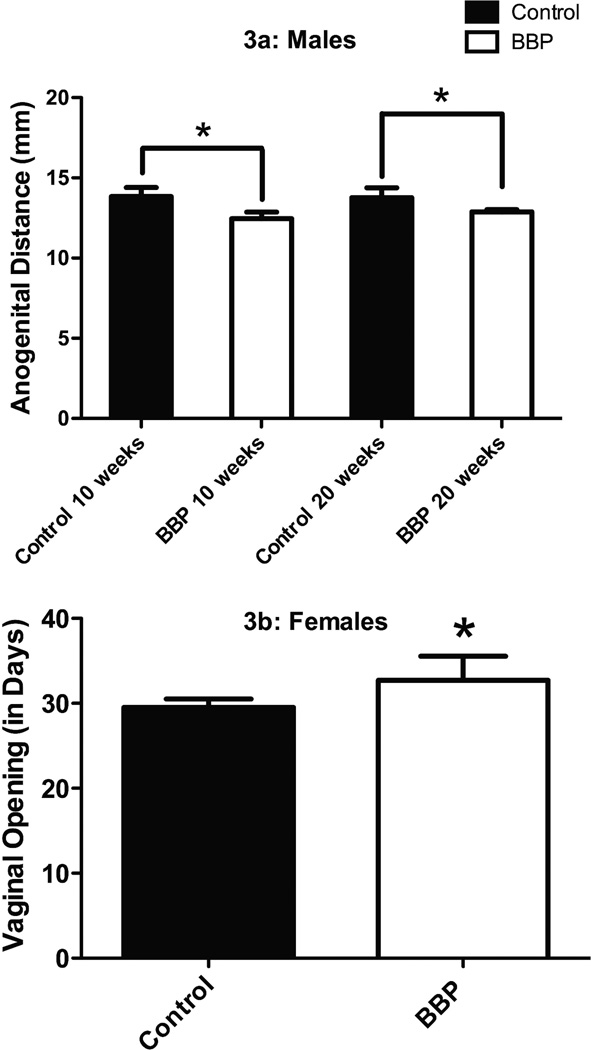
As supported by the literature (29, 30, 32), in the male mice, anogenital distances in BBP mice were significantly smaller compared to control mice at 10 (p=0.001) and 20 weeks (p=0.038; Figure 3a). Additionally, similar to previous phthalate work (28) vaginal openings in female BBP mice were significantly delayed compared to control mice (p=0.001; Figure 3b).
Fig. 3.
Indices of pubetal development in male and female mice. *Significantly (p<0.05) between control and BBP-treated animals. Panel A: Anogenital difference were significantly different between BBP male offspring and control offspring. Anogenital distances were significantly smaller in BBP mice compared to control mice at 10 weeks (p=0.001) and 20 weeks (p=0.038). Panel B: Vaginal openings were significantly delayed between BBP and control females (p=0.001).
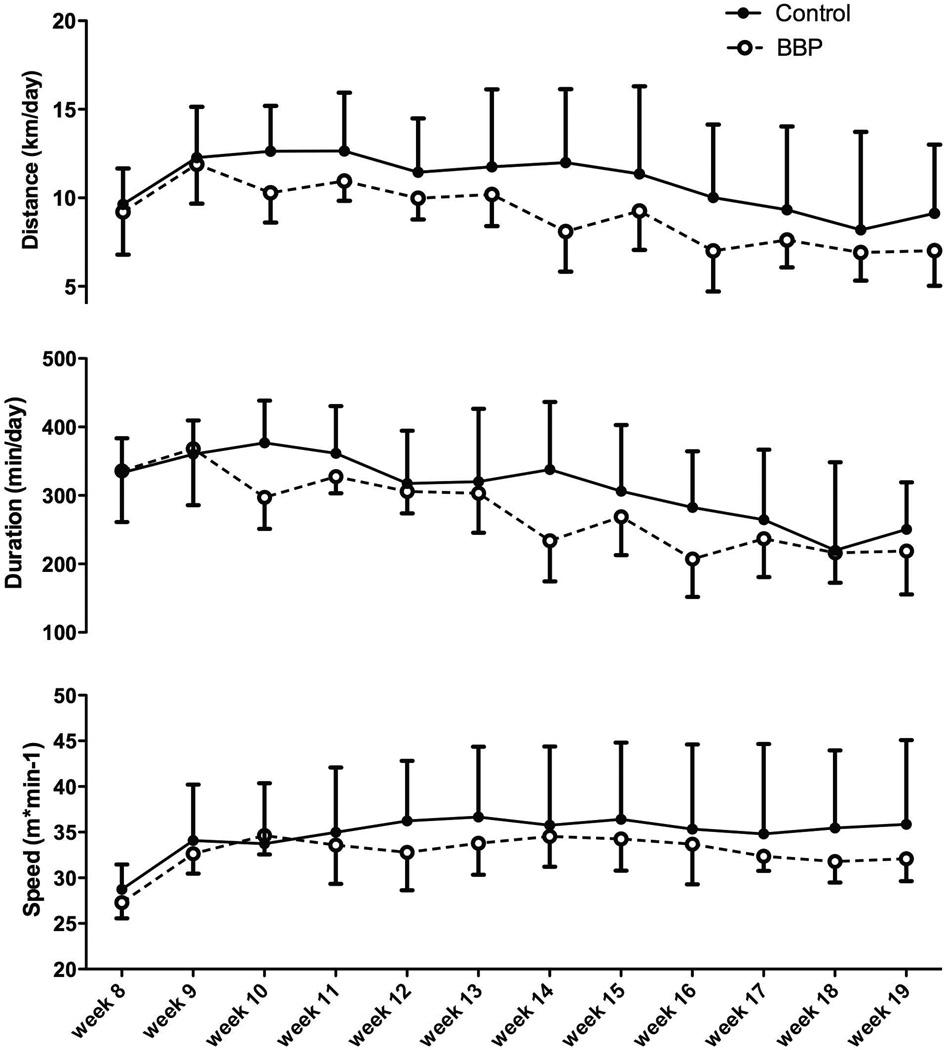
Voluntary Wheel Running Data
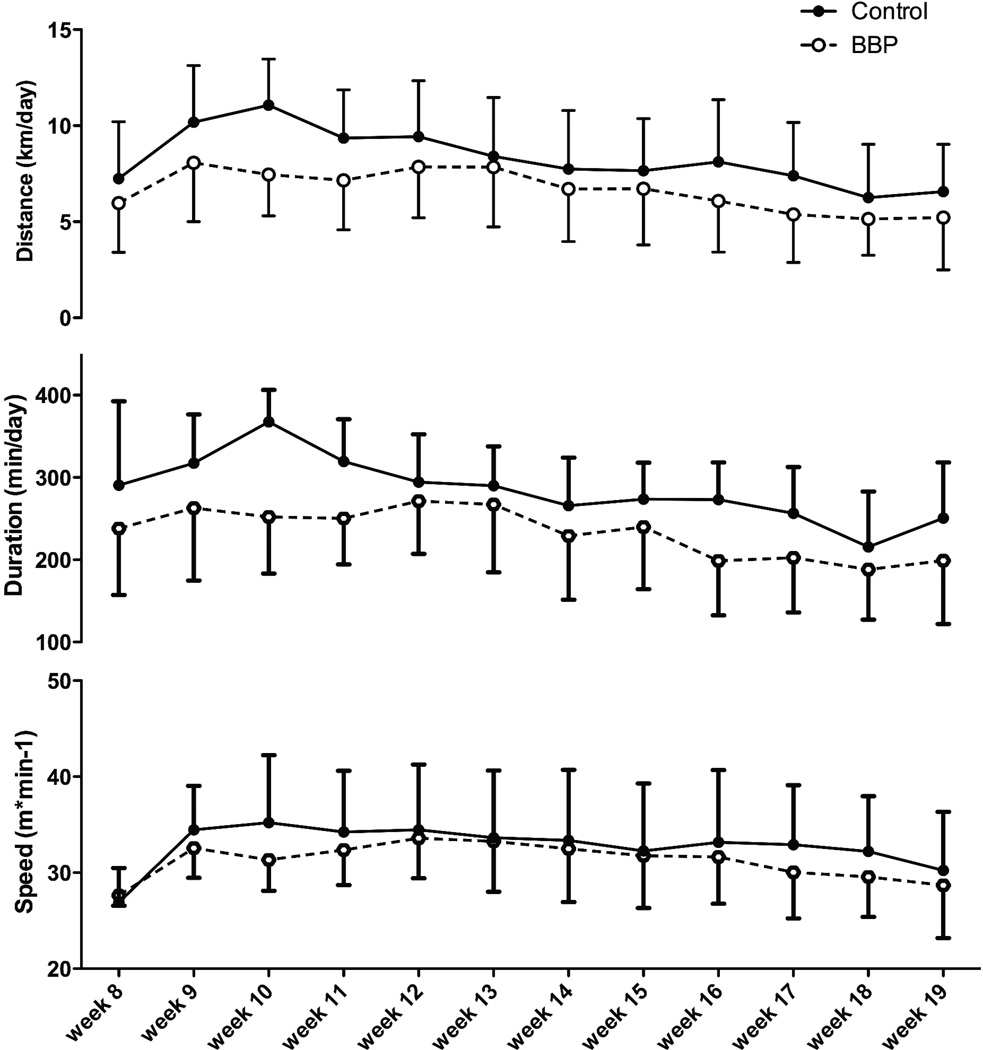
Overall, in male mice, the BBP-offspring showed a significant decrease in distance (p=0.008) and duration of exercise (p=0.005), but not speed (p=0.495) as compared to control-vehicle mice (Figure 4). On average, BBP male mice ran 20% less than controls across the experiment. In the BBP female mice, much like the BBP male mice, there was a significant decrease in distance (p=0.042) and duration (p=0.015) across the 20-week time period, but no significant alteration in speed (p=0.742) between control and treatment mice (Figure 5). On average, BBP female mice ran 15% less than controls across the experiment. The control mice (treated with oil gavage) did not differ in activity levels as compared to the sentinel mice (p=0.355; data not shown).
Fig. 4.
Physical activity indices of male mice. The BBP offspring showed a significant overall decrease in distance (p=0.008) and duration of exercise (p=0.005), but not speed (p=0.495) as compared to control mice.
Fig. 5.
Physical activity indices of female mice. The BBP offspring showed a significant overall decrease in distance (p=0.042) and duration of exercise (p=0.015), but not speed (p=0.742) as compared to control mice.
Body Composition
Overall, at each sacrifice time point, there were no differences in body weight, lean mass, and fat mass between either male or female BBP or control mice, with the exception of BBP-treated male mice that showed a higher fat mass at 20 weeks than control male mice (Table 1). As would be anticipated due to growth, all mice weights and body compositions increased across the 20 weeks of the experiment.
DISCUSSION
To our knowledge, this is the first study to examine the effect of a common and ubiquitous environmental toxicant – BBP (12, 16) – on physical activity levels in both male and female mice. In the current study, with maternal exposure to BBP, we observed significant decreases in testosterone levels in males as well as changes in sex hormone driven physiological parameters including decreased anogenital distances. We also observed a decreased estradiol level and a delayed vaginal opening in the treated female offspring. Most importantly, we observed an overall significant inhibition in distance run, primarily as a consequence of decreased duration of daily activity, in both male and female offspring. This study shows that in utero exposure to a physiologically relevant dosage of a common environmental toxicant decreases physical activity levels in mice.
Routes of phthalate exposure can be through ingestion, food, medical devices, or pharmaceuticals (34). Not all sources, pathways, and rates of human exposure are well understood; however, BBP is used in a variety of products that can leach onto a person’s skin or contaminate the food they eat making it hard to quantify just how much a person is exposed to these chemicals on a daily basis (34). One criticism of much ED research has been the lack of physiological relevance of the applied dosages. Gledhill et al. (9) concluded in 1980 that there was insufficient published data to make any determination on safe levels of BBP exposure in the U.S. Since that report, there has been little progress on determining ‘safe’ levels of BBP exposure. The most current guidelines for daily exposure to various phthalates (including BBP) from the European Commission suggest that safe exposure levels for humans for BBP is 0.5 mg·kg−1·1·k−1(8). In the present experiment, the BBP dosage of 500 mg·kg−1·1·k−1 given to the dams significantly exceed the Commission guidelines and would appear to not be physiologically relevant; however, fetuses are estimated to only be exposed to 1/100–1/1000 of the mother’s dose of BBP (27). Thus, in this study, we estimate that the fetuses were exposed to ≈ 0.5–5 mg·kg−1·1·k−1 which places these exposure rates above the US EPA safe dose for humans of 0.2 mg·kg−1·1·k−1(38). However, it should be considered that while in our study we exposed the fetuses to only one endocrine disruptor, adult pregnant females (and consequently the fetus) are routinely exposed on a daily basis to dozens of different endocrine disruptors which will further compound the dosages (25). Thus, the dosage that we employed, even though using only one endocrine disruptor, is likely indicative of the total disruptor load that a pregnant female (and her fetus) encounters on a daily basis.
Wheel Running Activity
Past studies in our lab (3, 4) have shown that testosterone is a primary regulator of physical activity in both male and female mice with estrogen also affecting physical activity, albeit at a lower level. Supporting these earlier results and confirming our initial hypothesis, BBP male mice showed decreased testosterone and AD distance, both indicative of disrupted testosterone production, and demonstrated an overall decrease in daily activity across the 20 weeks of the study. The decrease in daily activity was primarily due to a reduced duration of running without a change in speed of activity (Figure 4). In total, our data suggests that in males, interfering with testosterone production in utero by BBP administration reduces circulating testosterone in male mice leading to decreased activity levels.
Similar to the male mice, in the female mice we observed a decrease in estradiol as well as a delayed vaginal opening, which is an index associated with disrupted endocrine functioning (28). Associated with these alterations was a significant difference in distance and duration of exercise, but not speed in the BBP mice, confirming our hypothesis that exposure to BBP will alter physical activity. While the estradiol levels at 20-weeks were decreased in the BBP mice, the potential mechanism responsible for this decrease in activity in the female mice, is less clear. Specifically, the lack of an alteration in estradiol earlier in the lifespan, coupled with the higher levels of testosterone in the 20-week old BBP-treated females, was surprising. BBP is known to be primarily anti-androgenic and only weakly estrogenic (11) which may be why female BBP-treated offspring did not demonstrate altered estradiol levels until the 20 week period. However, it is unclear from the literature why testosterone was increased in the BBP-treated females at 20 weeks of age. While this difference in testosterone levels may be indicative of a complex dysregulation of the endocrine axis in the female mice similar to that observed when alterations in testosterone occurred before weaning (13), these results require further investigation to confirm this speculation.
In context, alterations in physical activity by common environmental endocrine disruptors, such as BBP, is important because BBP is ubiquitous in cosmetics and personal care products used daily by most people, especially by women (1, 12, 16, 40). To our knowledge, this is the first data to suggest that a common endocrine disruptor can significantly inhibit physical activity in offspring. However, there has been general concern with the role of BBP and other phthalates on human health by scientific bodies such as The American Council on Science and Health (15, 17).
The adipocyte has been hypothesized as a target of endocrine disrupting chemicals (31) and It has been speculated that a dose-response exists between ED exposure, the time frame when exposure occurred (i.e. in utero, puberty), and how much body composition is altered (39). While there have not been studies specifically targeting BBP, other phthalate exposure has resulted in increased body fat accumulation. For example, Hao et al. (10) found that male offspring born to mothers dosed with low doses of mono-2-ethylhexyl phthalate (MEHP) (0.05, 0.25, or 0.5 kg/mg) had an increased body weight and fat storage with similar results available in female mice (35). However, In general, we did not observe a significant difference in weight, fat mass, or lean mass in the BBP groups versus the control groups at most time points, with the exception of the week 20 male BBP animals having a higher body fat percentage (Table 1). Given that all male and female mice had access to a running-wheel at the beginning of week eight until sacrifice, it is probable that the physical activity the mice performed on a daily basis was enough to maintain their body composition within normal levels.
In summary, exposure to a physiological dose of a phthalate in utero in mice caused significant alterations in reproductive indices and a reduction of daily physical activity in both male and female offspring. The decreased activity was primarily the result of a reduction in duration of daily activity. In the male offspring, testosterone levels were reduced in early to mid-life, with reductions in testosterone associated with the reduced activity levels. Intriguingly, the female BBP mice, while exhibiting an overall decreased activity level and delayed pubertal signs, only showed changes in estradiol at 20 weeks of age and showed increased testosterone concentration, suggesting a complex dysregulation of sex hormone production by the weakly anti-estrogenic BBP. However, taken together, our observations show that in utero exposure to physiologically relevant levels of a common environmental toxicant (BBP) can cause significant changes in sex hormones concentrations later in life in the offspring with associated significantly inhibited daily activity levels in offspring.. These results provide a potential environmental factor to investigate as reductions in childhood activity levels are considered.
Acknowledgments
Research reported in this publication was supported by matching pilot grants from the Texas A&M College of Education and Human Development and the Texas A&M Center for Translational Environmental Health Research (NIH P30ES023512). The authors would also like to thank Clayton Cruthirds of the Biology of Physical Activity Lab, Dr. Jim Fluckey of the Muscle Biology Lab, Adriana Coletta of the Exercise & Sport Nutrition Lab, and finally Jean Kovar of the Comparative Medicine Program for their assistance in this project. Additionally, we would like to thank Dr. David Threadgill and the Texas A&M Institute for Genome Sciences and Society for making available the MRI body composition device. The results of the present study do not constitute endorsement by ACSM
Footnotes
The authors of the current study do not report any conflicts of interest.
REFERENCES
- 1.Al-Saleh I, Elkhatib R. Screening of phthalate esters in 47 branded perfumes. Environ Sci Pollut Res Int. 2015 doi: 10.1007/s11356-015-5267-z. [DOI] [PubMed] [Google Scholar]
- 2.Bowen RS, Ferguson DP, Lightfoot JT. Effects of aromatase inhibition on the physical activity levels of male mice. Journal of Steroids & Hormonal Science. 2011;1(1):1–7. doi: 10.4172/2157-7536.S1-001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bowen RS, Knab AM, Hamilton AT, McCall JR, Moore-Harrison TL, Lightfoot JT. Effects of supraphysiological doses of sex steroids on wheel running activity in mice. Steroids & Hormonal Science. 2012;3(2):110. doi: 10.4172/2157-7536.1000110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bowen RS, Turner MJ, Lightfoot JT. Sex hormone effects on physical activity levels: why doesn't Jane run as much as Dick? Sports Medicine. 2011;41(1):73–86. doi: 10.2165/11536860-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carlsen E, Giwercman A, Keiding N, Skakkebaek NE. Evidence for decreasing quality of semen during past 50 years. Bmj. 1992;305(6854):609–613. doi: 10.1136/bmj.305.6854.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clewell R, Campbell J, Ross S, Gaido K, Clewell HJ, Anderson M. Assessing the relevance of in vitro measures of phthalate inhibition of steroidogenesis for in vivo response. Toxicol In Vitro. 2010;24:327–334. doi: 10.1016/j.tiv.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 7.Colborn T, vom Saal FS, Soto AM. Developmental effects of endocrine-disrupting chemicals in wildlife and humans. Environmental Health Perspectives. 1993;101(5):378–384. doi: 10.1289/ehp.93101378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.European Commission. Directorate-General for Health & Consumers. [Date accessed: February 13, 2015];Scientific Committee on Health and Environmental Risks Opinion on phthalates in school supplies. 2008 Available from: http://ec.europa.eu/health/ph_risk/committees/04_scher/docs/scher_o_106.pdf.
- 9.Gledhill WE, Kaley RG, Adams WJ, et al. An environmental safety assessment of butyl benzyl phthalate. Environmental Science & Technology. 1980;14(3):301–305. doi: 10.1021/es60163a001. [DOI] [PubMed] [Google Scholar]
- 10.Hao C, Cheng X, Xia H, Ma X. The endocrine disruptor mono-(2-ethylhexyl) phthalate promotes adipocyte differentiation and induces obesity in mice. Biosci Rep. 2012;32:619–629. doi: 10.1042/BSR20120042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harris CA, Pirkko H, Parker MG, Sumpter JP. The estrogentic activity of phthalate esters in vitro. Environmental Health Perspectives. 1997;105(8):802–811. doi: 10.1289/ehp.97105802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hubinger JC, Havery DC. Analysis of consumer cosmetic products for phthalate esters. Journal of cosmetic science. 2006;57(2):127–137. [PubMed] [Google Scholar]
- 13.Jang H, Bhasin S, Guameri T, et al. The effects of a single developmentally entrained pulse of testosterone in female neonatal mice on reproductive and metabolic functions in adult life. Endocrinology. 2015;156(10):3737–3746. doi: 10.1210/EN.2015-1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Joosen A, Gielen M, Vlietinck R, Westerterp K. Genetic analysis of physical activity in twins. The American journal of clinical nutrition. 2005;82(6):1253–1259. doi: 10.1093/ajcn/82.6.1253. [DOI] [PubMed] [Google Scholar]
- 15.Kamrin MA. Phthalate risks, phthalate regulation, and public health: a review. Journal of toxicology and environmental health. Part B Critical reviews. 2009;12(2):157–174. doi: 10.1080/10937400902729226. [DOI] [PubMed] [Google Scholar]
- 16.Koo HJ, Lee BM. Estimated exposure to phthalates in cosmetics and risk assessment. Journal of Toxicology and Environmental Health. Part A. 2004;67(23–24):1901–1914. doi: 10.1080/15287390490513300. [DOI] [PubMed] [Google Scholar]
- 17.Koop CE, Juberg DR, Benedek EP, et al. A scientific evaluation of health effects of two plasticizers used in medical devices and toys: a report from the american council on science and health. Medscape General Medicine. 1999:E14. [PubMed] [Google Scholar]
- 18.Lightfoot JT. Sex hormones' regulation of rodent physical activity: a review. International Journal of Biological Sciences. 2008;4(3):126–132. doi: 10.7150/ijbs.4.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lightfoot JT. Current understanding of the genetic basis for physical activity. The Journal of Nutrition. 2011;141(3):526–530. doi: 10.3945/jn.110.127290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lightfoot JT, Leamy L, Pomp D, et al. Strain screen and haplotype association mapping of wheel running in inbred mouse strains. Journal of Applied Phyioslogy. 2010;109:623–634. doi: 10.1152/japplphysiol.00525.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lightfoot JT, Turner MJ, Daves M, Vordermark A, Kleeberger SR. Genetic influence on daily wheel running activity level. Physiological Genomics. 2004;19(3):270–276. doi: 10.1152/physiolgenomics.00125.2004. [DOI] [PubMed] [Google Scholar]
- 22.Manno F. Measurement of the digit lengths and the anogential distance in mice. Physiology & Behavior. 2008;93:364–368. doi: 10.1016/j.physbeh.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 23.Manson J, Skerrett P, Greenland P, VanItallie T. The escalating pandemics of obesity and sedentary lifestyle: a call to action for clinicians. Archives of Internal Medicine. 2004;164(3):249–258. doi: 10.1001/archinte.164.3.249. [DOI] [PubMed] [Google Scholar]
- 24.Meeker JD. Exposure to environmental endocrine disrupting compounds and men's health. Maturitas. 2010;66(3):236–241. doi: 10.1016/j.maturitas.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 25.Meeker JD, Sathyanarayana S, Swan SH. Phthalates and other additives in plastics: human exposure and associated health outcomes. Philos Trans R Soc Lond B Biol Sci. 2009;364(1526):2097–2113. doi: 10.1098/rstb.2008.0268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mokdad A, Marks J, Stroup D, Gerberding J. Actual causes of death in the United States, 2000. The Journal of the American Medical Association. 2004;291(10):1238–1245. doi: 10.1001/jama.291.10.1238. [DOI] [PubMed] [Google Scholar]
- 27.Moral R, Sanucci-Pereira J, Wang R, Russo I, Lamartiniere C, Russo J. In utero exposure to butl benzyl phthalate induces modifications in the morphology and the gene expression profile of the mammary gland: an experiment study in rats. Environmental Health : a global access science source. 2011;10(5):1–12. doi: 10.1186/1476-069X-10-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moyer B, Hixon M. Reproductive effects in F1 adult females exposedin uteroto moderate to high doses of mono-2-ethylhexylphthalate (MEHP) Reproductive Toxicology. 2012;34:43–50. doi: 10.1016/j.reprotox.2012.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mylchreest E, Cattley R, Foster P. Male reproductive tract malformations in rats following gestational and lactational exposure to Di(n-butyl) phthalate: an antiandrogenic mechanism? Toxicological Sciences. 1998;43:47–60. doi: 10.1006/toxs.1998.2436. [DOI] [PubMed] [Google Scholar]
- 30.Nagao T, Ohta R, Marumo H, Shindo T, Yoshimura S, Ono H. Effect of butyl benzyl phthalate in Sprague-Dawley rats after gavage administration: a two-generation reproductive study. Reproductive Toxicology. 2000;14(6):513–532. doi: 10.1016/s0890-6238(00)00105-2. [DOI] [PubMed] [Google Scholar]
- 31.Newbold R. Impact of environmental endocrine disrupting chemicals on the development of obesity. Hormones. 2010;9(3):206–217. doi: 10.14310/horm.2002.1271. [DOI] [PubMed] [Google Scholar]
- 32.Parks LG, Ostby JS, Lambright CR, et al. The plasticizer diethylhexyl phthalate induces malformations by decreasing fetal testosterone synthesis during sexual differentiation in the male rat. Toxicological Sciences. 2000;58(2):339–349. doi: 10.1093/toxsci/58.2.339. [DOI] [PubMed] [Google Scholar]
- 33.Ramirez V, Sawyer C. Advancement of puberty in the female rat by estrogen. Endocrinology. 1965;76:1158–1168. doi: 10.1210/endo-76-6-1158. [DOI] [PubMed] [Google Scholar]
- 34.Schettler T. Human exposure to phthalates via consumer products. International Journal of Andrology. 2006;29(1):134–139. doi: 10.1111/j.1365-2605.2005.00567.x. [DOI] [PubMed] [Google Scholar]
- 35.Schmidt J, Schaedlich K, Fiandanese N, Pocar P, Fischer B. Effects of Di(2-ethylhexyl) phthalate (DEHP) on female fertility and adipogenesis in C3H/N mice. Environmental health perspectives. 2012;120(8):1123–1129. doi: 10.1289/ehp.1104016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stubbe J, Boomsma D, Vink J, et al. Genetic influences on exercise participation in 37,051 twin pairs from seven countries. PloS One. 2006;1:e22. doi: 10.1371/journal.pone.0000022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Troiano R, Berrigan D, Dodd K, Masse L, Tilert T, McDowell M. Physical activity in the United States measured by accelerometer. Medicine and Science in Sports and Exercise. 2008;40(1):181–188. doi: 10.1249/mss.0b013e31815a51b3. [DOI] [PubMed] [Google Scholar]
- 38.United States Environmental Protection Agency. Integrated Risk Information System. [Date accessed: March 1, 2015];Butyl Benzyl Phthlate. Available from: http://www.epa.gov/iris/subst/0293.htm.
- 39.vom Saal F, Akingbeml B, Belcher S, et al. Chapel Hill bisphenol A expert panel consensus statement: Integration of mechanisms, effects in animals and potential to impact human health at current levels of exposure. Reproductive toxicology. 2007;24(2):131–138. doi: 10.1016/j.reprotox.2007.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Witorsch RJ, Thomas JA. Personal care products and endocrine disruption: A critical review of the literature. Critical Reviews in Toxicology. 2010;40(Suppl 3):1–30. doi: 10.3109/10408444.2010.515563. [DOI] [PubMed] [Google Scholar]