Abstract
Objective
The goal of the present study was to examine distributed attentional functions in long-term but currently abstinent methamphetamine (MA) abusers using a task that measures attentional alertness, orienting, and conflict resolution.
Methods
Thirty currently abstinent MA abusers (1 month–5 years) and 22 healthy non-substance using adults were administered a multimodal version of the Attentional Network Task (ANT-I). In this task subjects identified the direction of a centrally presented arrow using a key press. Analyses examined the interaction between alerting tones, location cueing and congruency between the target arrows and flanking distractor stimuli.
Results
All participants were faster when an auditory tone preceded the trial onset (p < 0.001), on trials in which a valid cue preceded the location of the target arrow (p < 0.001), and on congruent trials (i.e., when all display arrows faced in the same direction) (p < 0.001). Of primary interest was the finding that MA abusers were more influenced by the conflict between the peripheral arrows and the central target arrow (p = 0.009). There were also correlations between length of drug sobriety and executive function as well as between drug-induced psychiatric symptoms and alertness.
Conclusions
These results suggest that chronic MA abusers display cognitive deficits that may reflect a specific vulnerability to distraction on a task of executive function. These findings are consistent with other studies that have reported deficits in anterior attentional systems and top-down cognitive control.
Keywords: Methamphetamine, Attention, Conflict, Attentional network, ANT
1. Introduction
Addiction is a brain disease that involves dysfunction and morphological changes in the neural systems that regulate responsivity to reward and pleasure (Jentsch & Taylor, 1999; Kalivas & Volkow, 2005). In addicted individuals, the ability to refrain from activities that will result in negative consequences is often compromised due to changes in the neurobiology of the brain that govern decision-making and self-control (Jentsch & Taylor, 1999). It has been shown through elegant animal work (Cadet & Krasnova, 2009; Yamamoto, Moszczynska, & Gudelsky, 2010) as well as neuroimaging studies of the addicted brain (Volkow, Fowler, & Wang, 2004) that neural changes occurring after long-term drug use are widely distributed, comprising multiple brain regions and diverse neurotransmitters (Kalivas & Volkow, 2005; Koob, 1998; Koob & Moal, 2006; Nestler & Malenka, 2004). Thus any investigations that delve into the behavioral sequelae of drug abuse should consider a network approach to the investigation of cognitive function.
One drug that has taken center stage in the global arena of substance abuse and addiction is methamphetamine (MA). In the past decade the use of the stimulant MA has increased in the general population, with worldwide abuse of amphetamines surpassing that of cocaine and opiates combined (Nations, 2004). It is now estimated that approximately 5% of the adult population in the United States have used MA on at least one occasion with worldwide use estimated to be 33 million users (Roehr, 2005). MA is known to damage the dopaminergic, serotonergic and glutamatergic systems (Davidson, Gow, Lee, & Ellinwood, 2001; Quinton & Yamamoto, 2006; Yamamoto & Bankson, 2005). The highly addictive nature of MA, as well as its neurotoxicity to the human brain that can produce clinical, physical and psychiatric symptoms, makes MA a major public and mental health concern in the 21st century.
Long-term MA abuse causes widespread damage to many brain regions including the striatum, prefrontal cortex, anterior cingulate cortex and amygdala (Davidson, Lee, & Ellinwood, 2005; Davidson et al., 2001). Such distributed damage may contribute to the broad range of cognitive deficits observed in MA dependent human subjects. Cognitive deficits have been observed in MA dependent individuals with increased performance deficits appearing on tasks that require the suppression of task irrelevant information (Kalechstein, Newton, & Green, 2003; Monterosso, Aron, Cordova, Xu, & London, 2005; Salo, Leamon, Natsuaki, Moore, Waters, & Nordahl, 2008; Salo et al., 2007), decision-making (Paulus, Hozack, Frank, Brown, & Schuckit, 2003; Paulus, Tapert, & Schuckit, 2005), and working memory (Chang et al., 2002; McKetin & Mattick, 1997, 1998). In contrast, implicit tasks of cognitive function appear to be less affected (Salo, Leamon, et al., 2008; Salo et al., 2011). Although numerous studies have probed focused higher-level cognitive function in MA abusers, to the best of our knowledge, few have examined the interaction of basic bottom-up driven processes with more top-down behavioral regulation (Hoffman et al., 2006; Kalechstein et al., 2003; Monterosso et al., 2005, 2006, 2007; Nordahl, Salo, & Leamon, 2003; Paulus et al., 2003, 2005; Salo, Ursu, Buonocore, Leamon, & Carter, 2009; Salo et al., 2002, 2005, 2006, 2007; Simon, Domier, Sim, Richardson, Rawson, & Ling, 2002; Simon, Richardson, Dacey, Glynn, Domier, Rawson, 2002; Simon et al., 2000). Given the fact that MA has widespread effects on the human brain it was of interest to study cognitive deficits in MA abusers using a task that measures a distributed network of attentional function, the Attentional Network Task (ANT).
The ANT is a validated test that measures multiple cognitive operations within a single paradigm and thus is a powerful tool for the investigation of how potential cognitive deficits following long-term MA abuse may interact with each other (Fan, McCandliss, Sommer, Raz, & Posner, 2002). Three attentional networks have been defined in both anatomical and functional terms (Posner & Fan, 2008; Posner & Petersen, 1990). These functions involve alerting, orienting, and executive attention. The ANT measures responses to unpredictably occurring non-spatial cues to test the alerting network, spatial cueing (Posner, 1980) to test the orienting network and attentional conflict (i.e., flanker interference) to test the executive network (Eriksen & Eriksen, 1974). Alerting refers to the tonic maintenance of attention over prolonged periods but also to engaging “bursts” of phasic attention under certain situations where extra attention is needed, such as in the ANT (Sturm & Willmes, 2001). Orienting refers to either the voluntary (endogenous or under conscious control) or reflexive (endogenous or captured by bottom-up attention capture) orienting of attention (Posner, 1980; Posner & Petersen, 1990). Executive control of attention refers to more complex operations such as detecting and resolving conflicts between different activated task sets (Botvinick, Braver, Barch, Carter, & Cohen, 2001; Bush, Luu, & Posner, 2000; MacDonald, Cohen, Stenger, & Carter, 2000).
It has been proposed that these processes are subserved by different anatomical brain networks (Fan, Byrne, Worden, Guise, McCandliss, Fossella, 2007). Alerting is thought to be subserved by the thalamus, frontal and parietal brain regions and is modulated by the midbrain locus coeruleus/norepinephrine system (Coull, Frith, Frackowiak, & Grasby, 1996; Fan et al., 2002). Orienting is thought to be subserved by the frontal eye fields and parietal regions while the executive control network is thought to be subserved by the anterior cingulate cortex and frontal regions (Fan, McCandliss, Fossella, Flombaum, & Posner, 2005).
However recent behavioral evidence suggests that although these processes are distinct, they are also integrated and interact with each other (Fuentes & Campoy, 2008). For instance experimental data suggests that alerting inhibits and orienting enhances executive control (Callejas, Lupianez, Funes, & Tudela, 2005; Fuentes & Campoy, 2008). Functional MRI evidence also suggests that although the brain networks subserving each of these attention constructs are distinct, they also overlap to some degree (Fan et al., 2005). Fan and colleagues found that there was overlap in the thalamus and left fusiform between alerting and conflict but no overlap between conflict and orienting. Furthermore, alerting modulates activity in the executive control network (Fan, Kolster, Ghajar, Suh, Knight, Sarkar, 2007). Studies in participants with schizophrenia (Lopez et al., 2011), PTSD (Leskin & White, 2007) and in those who heavily use cannabis (Abdullaev, Posner, Nunnally, & Dishion, 2010) suggest impairments in the executive control network and conflict resolution.
The ANT is designed to evaluate alerting, orienting and executive control within a single 30-min testing session that can be easily performed (Fan et al., 2002). A published study with 40 normal adult subjects reported that the ANT produced reliable single subject estimates of each of these three functions (Fan et al., 2002). Reaction time (RT) measures obtained from the ANT can be used to quantify the processing efficiency within each of these networks (i.e., alerting, orienting and executive function) as well as potential interactions between the three systems (Callejas, Lupianez, & Tudela, 2004; Callejas et al., 2005). Recent studies have modified the ANT to further assess the interaction of alertness, orienting and executive function; some of them combining auditory and visual stimuli (Callejas et al., 2004, 2005). The goal of the present study was to examine a range of attentional functions in long-term but currently abstinent MA abusers using a modified version of the ANT that contains both visual and auditory stimuli (Callejas et al., 2004).
2. Material and methods
2.1. Subjects
Two groups were studied: 30 MA-abusers and 22 non-substance abusing control subjects. The MA-abusing group was recruited from six substance abuse treatment centers (one inpatient clinic and five outpatient clinics) and met DSM-IV criteria for lifetime MA dependence determined from the Structured Clinical Interview (SCID) (First, Spitzer, Gibbon, & Williams, 1995). Random urine screens were performed at the referring sites as well as on-site oral toxicology screens on the day of testing.1 All of the subjects were found to be drug-free on the day of testing. On average the healthy non-substance abusing adults and the MA abusers differed in age [F(1,50) = 16.23, p < 0.01] and years of education [F(1,49) = 27.80, p < 0.01]. For the MA-abusing subjects, inclusion criteria were (1) lifetime diagnosis of MA dependence according to DSM-IV criteria; (2) age range between 18 and 52 years. Exclusion criteria for the MA group were: (1) past abuse or dependence on: opiates, inhalants, hallucinogens and sedatives; (2) alcohol abuse or dependence within the past 5 years; (3) cocaine abuse or dependence within the past 5 years; (4) cannabis abuse within the past 4 weeks; (5) treatment or hospitalization for non-drug related DSM-IV Axis I psychiatric disorders; (6) medical or neurological illness or trauma which would affect the CNS (e.g., stroke or seizure disorder); (7) severe hepatic, endocrine, renal disease, or history of loss of consciousness of over 30 min and; (8) self-reported HIV positive status; (9) compound skull fracture or clear neurological sequelae of head trauma. The healthy non-substance abusing adults were recruited from the local community. Exclusion criteria for healthy non-substance abusing adults were: (1) past abuse or dependence on any substance according to DSM-IV criteria (except for nicotine) as well as exclusion criteria five through eight. None of the subjects met DSM-IV criteria for non-substance related DSM-IV Axis I psychiatric disorders. After complete description of the study to the subjects, written informed consent was obtained. A modest stipend was paid for participation in the study.
2.2. Apparatus
Stimuli were generated using E-Prime Ver. 1 and were presented on a 14″ VGA color monitor. A personal computer (PC) running Windows XP controlled stimuli presentation and data collection.
Participants viewed the screen from a distance of 65 cm, and all responses were collected using two key presses (C and M) on the computer keyboard.
2.3. Stimuli
Experimental stimuli consisted of a row of five arrows pointing leftward or rightward, against a gray background (see Fig. 1). The target of interest was the central arrow. In addition to the presentation of the target arrows, the following stimulus events occurred to measure the contribution of the three cognitive systems of interest:
Fig. 1.
Sequence of trial events.
Alerting. The Alerting variable consisted of a short duration high frequency tone that had two levels (presence and absence). Spatial Cueing: The Cueing variable had three levels: (1) Valid trials: an asterisk was presented at the same location as the subsequent target stimulus; (2) Invalid trials: the asterisk was presented on the alternative position to that of the target stimulus; and (3) No-cue trials: no asterisk was presented to the participants. The orienting cue was non-predictive of target location. Congruency: The congruency variable had two levels (congruent and incongruent). Each central target arrow was flanked on either side by two arrows that either pointed: in the same direction (congruent condition) or in the opposite direction (incongruent condition).
Each trial consisted of the following events. (1) A fixation period was presented for a random variable duration (400–1600 millisecond [ms]); (2) In half of the trials a 2000 Hz auditory warning cue was presented for 50 ms; (3) A short fixation period occurred for 400 ms after the warning cue onset; (3) On 2/3 of the trials a 50 ms spatial cue was presented (half of the times in valid and half of the times in invalid location); (4) One hundred msec after the spatial cue onset the target and flankers appeared simultaneously. The target could appear either above or below the fixation point. The target and flankers were presented until the participant responded, but for no longer than 3000 ms. The fixation cross appeared at the center of the screen during the whole trial and following a key press response, the target and flankers disappeared immediately until the next trial began. Each trial lasted for 5100 ms. One practice block and six experimental blocks were administered. Each experimental block consisted of 48 trials for a total of 288 trials.
2.4. Procedure
Participants were instructed to focus on a centrally located fixation cross throughout the task, and to respond as quickly and accurately as possible. The participants’ task was to indicate the direction of the centrally presented arrow by pressing one key (C) for the left direction and another key (M) for the right direction. During the practice trials, but not during the experimental trials, subjects received feedback from the computer on their speed and accuracy. The practice block took approximately 2 min and each experimental block took approximately 5 min. Total task time was approximately 30 min.
2.5. Statistical analysis
Mean RTs for responses for every condition were computed for each subject. Only correct responses were included in the RT analyses. Mean RTs were trimmed to exclude any response that exceeded 2500 ms and any response that was faster than 100 ms. Analysis of variance procedures for repeated measures were used to analyze the data in a 2 × 2 × 3 × 2 mixed design with group as a between-subjects factor (MA vs. healthy non-substance abusing adults), alertness (tone vs. no tone), cue validity (valid vs. invalid × no cue) and congruency (congruent vs. incongruent) as within-subjects factors. Planned comparisons were carried out to measure differences in RT between incongruent and congruent trials (congruency effect) as well as between invalid and valid trials (cueing effect). Further analyses were conducted to examine error responses as well as speed-accuracy patterns. Correlational analyses were also conducted to examine the relationship between drug use patterns (i.e., years use, time drug abstinent) and the experimental variables. Given our previously published findings on cognitive differences between short-term (12 months and less) and long-term abstinent MA abusers (12 months and longer), planned comparisons were conducted looking at these two subgroups of MA abusers (Salo, Nordahl, Galloway, Moore, Waters, & Leamon, 2009).
3. Results
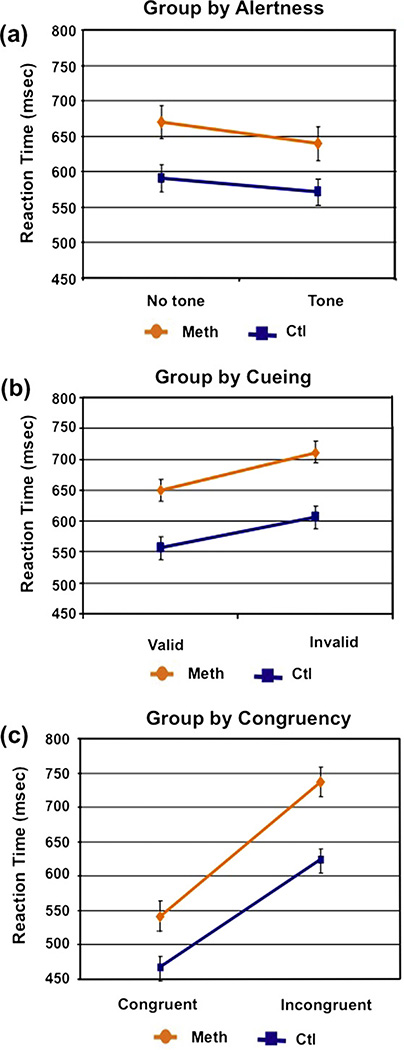
A main effect of group was observed (F(1,50) = 12.64; p < 0.001) in that the MA abusers exhibited slower RTs across conditions (685 ms) compared to healthy non-substance using adults (584 ms). Statistically significant differences were also observed for each of the three main effects. Responses were faster in both groups for alerting trials (F(1,50) = 103.42; p < 0.0001, d = 0.45), cued trials (F(2,100) = 130.44; p < 0.0001, d = 0.48) and congruent trials (F(1,50) = 333.90; p < 0.0001, d = 0.80). See Table 2. Significant interactions were observed between alerting and cueing (F(2,100) = 26.97; p < 0.0001), alerting and congruency (F(1,50) = 5.97; p = 0.02) as well as group and congruency (F(1,50) = 7.41; p = 0.009, d = 0.80). MA abusers exhibited larger congruency effects than healthy non-substance abusing adults, namely they exhibited longer RTs to incongruent than congruent trials and this difference was larger than in the control participants. Gender differences were examined but none emerged across any of the variables. Since RTs were slower in the MA abusers compared to healthy non-substance abusing adults we co varied for RT in our analysis and found that group differences in congruency endured when baseline RT was taken into consideration (p < 0.05). We also employed both age and education as covariates in the analyses and the group differences in congruency remained significant (see Fig. 2).
Table 2.
Mean reaction time (RT) in milliseconds (% accuracy) across conditions.
| No alerting tone | Alerting tone | |||||
|---|---|---|---|---|---|---|
| No cue | Valid cue | Invalid cue | No cue | Valid cue | Invalid cue | |
| MA abusers | ||||||
| Congruent | 673.33 (.99) | 620.87 (1.0) | 658.20 (1.0) | 603.89 (1.0) | 578.85 (.99) | 635.58 (.99) |
| Incongruenta | 771.58 (.99) | 720.31 (1.0) | 782.37 (.98) | 725.51 (.98) | 678.44 (.99) | 768.92 (.98) |
| Healthy adults | ||||||
| Congruent | 574.86 (.99) | 532.96 (.99) | 567.23 (.99) | 525.91 (.99) | 505.68 (1.0) | 549.34 (.99) |
| Incongruent | 645.75 (.99) | 608.35 (.99) | 655.22 (.98) | 606.53 (.98) | 579.04 (.98) | 656.62 (.97) |
Significantly different from control group.
Fig. 2.
Group interactions across task conditions: (a) group by alerting; (b) group by orienting; and (c) group by executive function. Y-axes for all figures are scaled from 450 ms to 800 ms.
ANOVA procedures were also employed to analyze accuracy across conditions. Analyses revealed that the two groups did not differ in overall accuracy (F(1,50) = .84; p = 0.63) nor in any of the three experimental conditions. Main effects were observed for alerting (F(1,50) = 5.67; p = 0.02), cueing (F(2,100) = 4.95; p = 0.009), and congruency (F(1,50) = 20.13; p = 0.001). Specifically, all subjects made fewer errors when: (1) an alerting tone appeared prior to the appearance of the stimulus; and (2) the flanking errors were congruent with the central target arrow. Surprisingly, all subjects made fewer errors when an invalid spatial cue preceded the appearance of the target stimulus. Error rates were extremely low across all subjects (i.e., less than 2%).
3.1. Correlations with years of use, length of remission
Using regression analyses for all 30 MA abusers we examined the relationship between months of abstinence, years of usage, and age at first MA use. No significant correlations were observed between these drug use patterns and any of the three processes (i.e., alertness, cueing, and congruency). Spearman correlations were also carried out to examine the relationship between task performance and drug use patterns separately in the short-term and long-term abstinent MA abusers. A significant correlation was observed in the short-term abstinent MA group between months of sobriety and the congruency effect (r = 0.68; p = 0.01). Specifically, those MA abusers with the shortest length of MA sobriety are the ones who displayed the greatest flanker interference when the direction of the flanker arrows was in conflict with the central target arrow. No correlations were observed in the long-term abstinent MA abusers when measuring months of sobriety and the three cognitive variables (orienting, cueing and congruency).
3.2. Measures of drug induced clinical symptoms and cognitive variables
Given our previous published findings that reported a correlation between childhood reports of attentional deficits and frequency of MA-induced psychotic symptoms, it was of interest to examine whether any such relationship existed in the present study (Salo, Nordahl, Leamon, Natsuaki, Moore, Waters, 2008). Planned analyses looked at patterns of MA-induced psychotic symptoms and performance on the ANT. No significant correlations emerged. However, post hoc group comparisons revealed that there was a trend for those MA abusers with a history of frequent MA-induced psychosis to exhibit faster RTs on those trials in which an alerting tone was present compared to those MA abusers with no history of MA-induced psychosis (p = 0.07) (Leamon et al., 2010).
4. Discussion
The results of this study suggest that attentional deficits following long-term MA abuse are most pronounced on tasks that tap into the executive system as measured by the flanker conflict component of the ANT. Although the ANT measures the alerting and orienting systems through target tones and spatial cueing, no group differences emerged on those measures. In contrast, in a task of spatial conflict using flanker stimuli, the MA dependent subjects were significantly slower on conditions in which the direction of the central target arrow and the surrounding flankers were in conflict with each other. No group differences were observed in task accuracy in any of the three conditions which suggest that any group differences observed are not a result of task difficulty.
The data in this study are consistent with other studies that have used this multimodal task version (Callejas et al., 2004, 2005) as well as those that have employed the version with visual stimuli alone (Fan et al., 2002, 2005). Specifically, in this study main effects of the three attentional networks were observed in the predicted direction (i.e., alerting, orienting, and executive function). All subjects were: (1) faster when an alerting tone was present; (2) faster when a valid cue predicted the target location; and (3) slower when the flanking arrows pointed in the opposite direction to that of the central arrow. In addition, significant interactions were also observed between alerting and the other two systems, spatial cueing and conflict resolution. Thus the presence of an auditory tone prior to the appearance of the target speeded the key press response in both groups, with an increased RT benefit to those targets which had been validly cued. No interaction was observed between spatial cueing and congruency (i.e., the ability to resolve conflict when stimuli are validly cued).
The behavioral findings in this study are quite consistent with imaging studies of long-term MA abusers as they relate to the brain regions thought to be involved in the three attentional networks (Fan et al., 2002, 2005). Group differences were observed on the task thought to be associated with the ACC and lateral frontal cortex (i.e., the flanker conflict task). Studies have shown that the flanker task activates an area of the anterior cingulate that overlaps brain regions activated by other conflict tasks (Fan, Flombaum, McCandliss, Thomas, & Posner, 2003). Several studies have reported both neurochemical and functional abnormalities within the ACC (Nordahl et al., 2002; Paulus et al., 2003, 2005; Salo et al., 2007; Taylor et al., 2007) as well as the prefrontal cortex (Paulus et al., 2002, 2003, 2005; Salo, Ursu, et al., 2009) of long-term MA abusers. Furthermore the group differences in congruency effects are also consistent with the findings within the MA literature that suggest that the most pronounced cognitive deficits produced by MA are on tasks that tap into measures of top-down executive control (Gonzalez, Bechara, & Martin, 2007; Monterosso et al., 2005; Nordahl et al., 2003; Paulus et al., 2003, 2005; Salo, Ursu, et al., 2009; Salo et al., 2007, 2000).
There were further findings of interest related to drug-induced clinical symptoms and periods of sobriety in the MA abusers. Among those MA abusers with less than 1 year sobriety, the magnitude of flanker interference was related to months since last use of MA. Specifically, those MA abusers with longer periods of sobriety within the initial year showed increased ability to ignore the distracting spatial flanker. These findings are consistent with other studies that have reported gradual improvements in cognitive performance during the first year of drug abstinence (Salo, Nordahl, et al., 2009; Simon, Dean, Cordova, Monterosso, & London, 2010). The lack of correlations in the long-term MA abusers is not as easy to explain. It may be that the range of time abstinent in this group is too narrow to observe any significant correlations or it may be that the improvements within the first year are greater in magnitude than those which occur at more distant periods of sobriety.
The significant trends between frequency of MA-induced psychotic episodes and a subset of the cognitive variables are intriguing. In particular the relationship between the alerting tones and reported incidences of MA paranoia frequency which may suggest a state of hyper-alertness to the presence of the tones in this task. The alerting system has been associated with the frontal and parietal regions of the right hemisphere. This is thought to be due to the cortical distribution of the brain’s norepinephrine system (NE) (Coull et al., 1996; Marrocco, Witte, & Davidson, 1994). We have previously reported links between MA paranoia frequency and cognition on a Stroop Conflict task in which analyses revealed that those subjects who reported experiencing frequent episodes of paranoia exhibited lower Stroop RT interference compared to those who reported infrequent episodes (Salo et al., 2002). Collectively these findings between the frequency of paranoid episodes and the cognitive variables (i.e., alerting and Stroop interference) could suggest greater vigilance and focused attention in those MA subjects with a history of frequent paranoid episodes. More research is needed to examine this issue.
4.1. Limitations
To minimize the possibility that group differences were due to pre-existing abnormalities in the MA-abusers, we excluded those who had non-drug-related Axis I disorders. It is always a concern that previous abuse of other illicit drugs may have a persistent effect on cognition. Thus in order to reduce the potential effects of other drugs of abuse, we studied subjects whose primary drug of choice was MA and whose abuse of other drugs was limited and distant. See Table 1 for statistics on other drug use. It is possible that chronic tobacco use may potentiate the affects of MA by degrading the ability of the brain to metabolize DA (Brody et al., 2004). Post-hoc analyses failed to reveal differences on the flanker conflict task between the 16 MA-abusers who had abused cannabis in the past (p = 0.25) or the 25 tobacco smokers (p = 0.73) vs. those who were non-smokers in this sample.
Table 1.
Characteristics of research participants.
| Control subjects (n = 22) |
MA abusers (n = 30) |
|
|---|---|---|
| Age, y, mean (SD) | 29.96 (8.5) | 39.6 (8.5)a |
| Females | 10 | 12 |
| Subject’s education, y, mean (SD) | 15.86 (1.9) | 12.8 (2.1)a |
| Right-handed | 21 | 24 |
| MA use variables | ||
| Duration, y, mean (SD) | 14.5 (5.8) | |
| Mos abstinent, mean (SD) | 14.4 (15.0) | |
| Age of first use, y, mean (SD) | 20.1 (7.5) | |
| Route of use | ||
| Snort only | 0 | |
| Smoke only | 4 | |
| IV only | 3 | |
| Multiple routes | 23 | |
| Past history of substance abuse | ||
| Tobacco smokers | 4 | 25 |
| Alcohol abusers | – | 16 |
| Mean years since last alcohol use | – | 13.29 (17.9) |
| Cannabis users | – | 16 |
| Mean months since last cannabis use |
– | 10.5 |
| Cocaine abusers | – | 11 |
| Mean years since last cocaine use |
– | 17.89 (6.9) |
| MDMA abusers | – | 0 |
| Opiate abusers | – | 0 |
Significantly different from control group.
4.2. Conclusions
This current study provides an important direction for our cognitive work in MA abusers by examining a broad range of attentional functions. As addiction may represent abnormal stimulation of some normal processes coupled with pathological functioning of others, the dissociation of attentional processes is essential to identify which processes are intact vs. those which are dysfunctional. Increased knowledge about the neural mechanisms underlying behaviors that promote and sustain substance use will help to guide treatment strategies and will constitute an important contribution to the neuroscience of drug addiction.
Acknowledgments
This work was supported by DA021847 and DA0121847 to RS.
Footnotes
Drugs screened in the random urine toxicology included: alcohol, amphetamine, methamphetamine, MDMA, cocaine, benzodiazepines, barbiturates, THC, morphine, codeine, hydro- and oxycodone
References
- Abdullaev Y, Posner MI, Nunnally R, Dishion TJ. Functional MRI evidence for inefficient attentional control in adolescent chronic cannabis abuse. Behavioural Brain Research. 2010;215(1):45–57. doi: 10.1016/j.bbr.2010.06.023. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Braver TS, Barch DM, Carter CS, Cohen JD. Conflict monitoring and cognitive control. Psychological Review. 2001;108(3):624–652. doi: 10.1037/0033-295x.108.3.624. [DOI] [PubMed] [Google Scholar]
- Brody AL, Olmstead RE, London ED, Farahi J, Meyer JH, Grossman P, et al. Smoking-induced ventral striatum dopamine release. American Journal of Psychiatry. 2004;161(7):1211–1218. doi: 10.1176/appi.ajp.161.7.1211. [DOI] [PubMed] [Google Scholar]
- Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends in Cognitive Sciences. 2000;4(6):215–222. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- Cadet JL, Krasnova IN. Molecular bases of methamphetamine-induced neurodegeneration. International Review of Neurobiology. 2009;88:101–119. doi: 10.1016/S0074-7742(09)88005-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callejas A, Lupianez J, Funes MJ, Tudela P. Modulations among the alerting, orienting and executive control networks. Experimental Brain Research. 2005;167(1):27–37. doi: 10.1007/s00221-005-2365-z. [DOI] [PubMed] [Google Scholar]
- Callejas A, Lupianez J, Tudela P. The three attentional networks: On their independence and interactions. Brain and Cognition. 2004;54(3):225–227. doi: 10.1016/j.bandc.2004.02.012. [DOI] [PubMed] [Google Scholar]
- Chang L, Ernst T, Speck O, Patel H, DeSilva M, Leonido-Yee M, et al. Perfusion MRI and computerized cognitive test abnormalities in abstinent methamphetamine users. Psychiatry Research. 2002;114(2):65–79. doi: 10.1016/s0925-4927(02)00004-5. [DOI] [PubMed] [Google Scholar]
- Coull JT, Frith CD, Frackowiak RS, Grasby PM. A fronto-parietal network for rapid visual information processing: A PET study of sustained attention and working memory. Neuropsychologia. 1996;34(11):1085–1095. doi: 10.1016/0028-3932(96)00029-2. [DOI] [PubMed] [Google Scholar]
- Davidson C, Gow AJ, Lee TH, Ellinwood EH. Methamphetamine neurotoxicity: Necrotic and apoptotic mechanisms and relevance to human abuse and treatment. Brain Research Reviews. 2001;36(1):1–22. doi: 10.1016/s0165-0173(01)00054-6. [DOI] [PubMed] [Google Scholar]
- Davidson C, Lee TH, Ellinwood EH. Acute and chronic continuous methamphetamine have different long-term behavioral and neurochemical consequences. Neurochemistry International. 2005;46(3):189–203. doi: 10.1016/j.neuint.2004.11.004. [DOI] [PubMed] [Google Scholar]
- Eriksen BA, Eriksen CW. Effects of noise letters on the identification of a target letter in a nonsearch task. Perception & Psychophysics. 1974;16:143–149. [Google Scholar]
- Fan J, Byrne J, Worden MS, Guise KG, McCandliss BD, Fossella J, et al. The relation of brain oscillations to attentional networks. Journal of Neuroscience. 2007;27(23):6197–6206. doi: 10.1523/JNEUROSCI.1833-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan J, Flombaum JI, McCandliss BD, Thomas KM, Posner MI. Cognitive and brain consequences of conflict. Neuroimage. 2003;18(1):42–57. doi: 10.1006/nimg.2002.1319. [DOI] [PubMed] [Google Scholar]
- Fan J, Kolster R, Ghajar J, Suh M, Knight RT, Sarkar R, et al. Response anticipation and response conflict: An event-related potential and functional magnetic resonance imaging study. Journal of Neuroscience. 2007;27(9):2272–2282. doi: 10.1523/JNEUROSCI.3470-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan J, McCandliss BD, Fossella J, Flombaum JI, Posner MI. The activation of attentional networks. Neuroimage. 2005;26(2):471–479. doi: 10.1016/j.neuroimage.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Fan J, McCandliss BD, Sommer T, Raz A, Posner MI. Testing the efficiency and independence of attentional networks. Journal of Cognitive Neuroscience. 2002;14(3):340–347. doi: 10.1162/089892902317361886. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer L, Gibbon M, Williams JBW. Structured clinical interview for DSM-IV axis I disorders. New York, NY: Biometrics Research Department; 1995. [Google Scholar]
- Fuentes LJ, Campoy G. The time course of alerting effect over orienting in the attention network test. Experimental Brain Research. 2008;185(4):667–672. doi: 10.1007/s00221-007-1193-8. [DOI] [PubMed] [Google Scholar]
- Gonzalez R, Bechara A, Martin EM. Executive functions among individuals with methamphetamine or alcohol as drugs of choice: Preliminary observations. Journal of Clinical and Experimental Neuropsychology. 2007;29(2):155–159. doi: 10.1080/13803390600582446. [DOI] [PubMed] [Google Scholar]
- Hoffman WF, Moore M, Templin R, McFarland B, Hitzemann RJ, Mitchell SH. Neuropsychological function and delay discounting in methamphetamine-dependent individuals. Psychopharmacology (Berlin) 2006;188(2):162–170. doi: 10.1007/s00213-006-0494-0. [DOI] [PubMed] [Google Scholar]
- Jentsch JD, Taylor JR. Impulsivity resulting from frontostriatal dysfunction in drug abuse: Implications for the control of behavior by reward-related stimuli. Psychopharmacology (Berlin) 1999;146(4):373–390. doi: 10.1007/pl00005483. [DOI] [PubMed] [Google Scholar]
- Kalechstein AD, Newton TF, Green M. Methamphetamine dependence is associated with neurocognitive impairment in the initial phases of abstinence. Journal of Neuropsychiatry and Clinical Neurosciences. 2003;15(2):215–220. doi: 10.1176/jnp.15.2.215. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Volkow ND. The neural basis of addiction: A pathology of motivation and choice. American Journal of Psychiatry. 2005;162(8):1403–1413. doi: 10.1176/appi.ajp.162.8.1403. [DOI] [PubMed] [Google Scholar]
- Koob GF. Circuits, drugs, and drug addiction. Advances in Pharmacology. 1998;42:978–982. doi: 10.1016/s1054-3589(08)60910-2. [DOI] [PubMed] [Google Scholar]
- Koob GF, Moal L. Neurobiology of addiction. London: Academic Press; 2006. [Google Scholar]
- Leamon MH, Flower K, Salo RE, Nordahl TE, Kranzler HR, Galloway GP. Methamphetamine and paranoia: The methamphetamine experience questionnaire. American Journal on Addictions. 2010;19(2):155–168. doi: 10.1111/j.1521-0391.2009.00014.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leskin LP, White PM. Attentional networks reveal executive function deficits in posttraumatic stress disorder. Neuropsychology. 2007;21(3):275–284. doi: 10.1037/0894-4105.21.3.275. [DOI] [PubMed] [Google Scholar]
- Lopez SG, Fuster JI, Reyes MM, Collazo TM, Quinones RM, Berazain AR, et al. Attentional network task in schizophrenic patients and theirs unaffected first degree relatives: A potential endophenotype. Actas Espanolas de Psiquiatria. 2011;39(1):32–44. [PubMed] [Google Scholar]
- MacDonald AW, 3rd, Cohen JD, Stenger VA, Carter CS. Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science. 2000;288(5472):1835–1838. doi: 10.1126/science.288.5472.1835. [DOI] [PubMed] [Google Scholar]
- Marrocco RT, Witte EA, Davidson MC. Arousal systems. Current Opinion in Neurobiology. 1994;4(2):166–170. doi: 10.1016/0959-4388(94)90067-1. [DOI] [PubMed] [Google Scholar]
- McKetin R, Mattick RP. Attention and memory in illicit amphetamine users. Drug and Alcohol Dependence. 1997;48(3):235–242. doi: 10.1016/s0376-8716(97)00132-4. [DOI] [PubMed] [Google Scholar]
- McKetin R, Mattick RP. Attention and memory in illicit amphetamine users: Comparison with non-drug-using controls. Drug and Alcohol Dependence. 1998;50(2):181–184. doi: 10.1016/s0376-8716(98)00022-2. [DOI] [PubMed] [Google Scholar]
- Monterosso JR, Ainslie G, Xu J, Cordova X, Domier CP, London ED. Frontoparietal cortical activity of methamphetamine-dependent and comparison subjects performing a delay discounting task. Human Brain Mapping. 2006 doi: 10.1002/hbm.20281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monterosso JR, Ainslie G, Xu J, Cordova X, Domier CP, London ED. Frontoparietal cortical activity of methamphetamine-dependent and comparison subjects performing a delay discounting task. Human Brain Mapping. 2007;28(5):383–393. doi: 10.1002/hbm.20281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monterosso JR, Aron AR, Cordova X, Xu J, London ED. Deficits in response inhibition associated with chronic methamphetamine abuse. Drug and Alcohol Dependence. 2005;79(2):273–277. doi: 10.1016/j.drugalcdep.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Nations U. The world drug problem: A status report. Vienna: United Nations; 2004. [Google Scholar]
- Nestler EJ, Malenka RC. The addicted brain. Scientific American. 2004;290(3):78–85. doi: 10.1038/scientificamerican0304-78. [DOI] [PubMed] [Google Scholar]
- Nordahl TE, Salo R, Leamon M. Neuropsychological effects of chronic methamphetamine use on neurotransmitters and cognition: A review. Journal of Neuropsychiatry and Clinical Neurosciences. 2003;15(3):317–325. doi: 10.1176/jnp.15.3.317. [DOI] [PubMed] [Google Scholar]
- Nordahl TE, Salo R, Possin K, Gibson DR, Flynn N, Leamon M, et al. Low N-acetyl-aspartate and high choline in the anterior cingulum of recently abstinent methamphetamine-dependent subjects: A preliminary proton MRS study. Magnetic resonance spectroscopy. Psychiatry Research. 2002;116(1–2):43–52. doi: 10.1016/s0925-4927(02)00088-4. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Hozack N, Frank L, Brown GG, Schuckit MA. Decision making by methamphetamine-dependent subjects is associated with error-rate-independent decrease in prefrontal and parietal activation. Biological Psychiatry. 2003;53(1):65–74. doi: 10.1016/s0006-3223(02)01442-7. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Hozack NE, Zauscher BE, Frank L, Brown GG, Braff DL, et al. Behavioral and functional neuroimaging evidence for prefrontal dysfunction in methamphetamine-dependent subjects. Neuropsychopharmacology. 2002;26(1):53–63. doi: 10.1016/S0893-133X(01)00334-7. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Tapert SF, Schuckit MA. Neural activation patterns of methamphetamine-dependent subjects during decision making predict relapse. Archives of General Psychiatry. 2005;62(7):761–768. doi: 10.1001/archpsyc.62.7.761. [DOI] [PubMed] [Google Scholar]
- Posner MI. Orienting of attention. Quarterly Journal of Experimental Psychology. 1980;32(1):3–25. doi: 10.1080/00335558008248231. [DOI] [PubMed] [Google Scholar]
- Posner MI, Fan J, editors. Topics in integrative neuroscience; from cells to cognition. Cambridge University Press; 2008. [Google Scholar]
- Posner MI, Petersen SE. The attention system of the human brain. Annual Review of Neuroscience. 1990;13:25–42. doi: 10.1146/annurev.ne.13.030190.000325. [DOI] [PubMed] [Google Scholar]
- Quinton MS, Yamamoto BK. Causes and consequences of methamphetamine and MDMA toxicity. AAPS Journal. 2006;8(2):E337–E347. doi: 10.1007/BF02854904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roehr B. Half a million Americans use methamphetamine every week. BMJ. 2005:476. doi: 10.1136/bmj.331.7515.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salo R, Nordahl TE, Buonocore MH, Natsuaki YT, Moore CD, Waters C, et al. Spatial inhibition and the visual cortex: A magnetic resonance spectroscopy imaging study. Neuropsychologia. 2011;49(5):830–838. doi: 10.1016/j.neuropsychologia.2011.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salo R, Leamon MH, Natsuaki Y, Moore C, Waters C, Nordahl TE. Findings of preserved implicit attention in methamphetamine dependent subjects. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2008;32(1):217–223. doi: 10.1016/j.pnpbp.2007.08.012. [DOI] [PubMed] [Google Scholar]
- Salo R, Nordahl TE, Galloway GP, Moore CD, Waters C, Leamon MH. Drug abstinence and cognitive control in methamphetamine-dependent individuals. Journal of Substance Abuse Treatment. 2009;1 doi: 10.1016/j.jsat.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salo R, Nordahl TE, Leamon MH, Natsuaki Y, Moore CD, Waters C, et al. Preliminary evidence of behavioral predictors of recurrent drug-induced psychosis in methamphetamine abuse. Psychiatry Research. 2008;157(1–3):273–277. doi: 10.1016/j.psychres.2007.04.018. [DOI] [PubMed] [Google Scholar]
- Salo R, Nordahl TE, Moore C, Waters C, Natsuaki Y, Galloway GP, et al. A dissociation in attentional control: Evidence from methamphetamine dependence. Biological Psychiatry. 2005;57(3):310–313. doi: 10.1016/j.biopsych.2004.10.035. [DOI] [PubMed] [Google Scholar]
- Salo R, Nordahl TE, Natsuaki Y, Leamon MH, Galloway GP, Waters C, et al. Attentional control and brain metabolite levels in methamphetamine abusers. Biological Psychiatry. 2006 doi: 10.1016/j.biopsych.2006.07.031. [DOI] [PubMed] [Google Scholar]
- Salo R, Nordahl TE, Natsuaki Y, Leamon MH, Galloway GP, Waters C, et al. Attentional control and brain metabolite levels in methamphetamine abusers. Biological Psychiatry. 2007;61(11):1272–1280. doi: 10.1016/j.biopsych.2006.07.031. [DOI] [PubMed] [Google Scholar]
- Salo R, Nordahl TE, Possin K, Leamon M, Gibson DR, Galloway GP, et al. Preliminary evidence of reduced cognitive inhibition in methamphetamine-dependent individuals. Psychiatry Research. 2002;111(1):65–74. doi: 10.1016/s0165-1781(02)00111-7. [DOI] [PubMed] [Google Scholar]
- Salo R, Ursu S, Buonocore MH, Leamon MH, Carter C. Impaired prefrontal cortical function and disrupted adaptive cognitive control in methamphetamine abusers: A functional magnetic resonance imaging study. Biological Psychiatry. 2009;65(8):706–709. doi: 10.1016/j.biopsych.2008.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon SL, Domier C, Carnell J, Brethen P, Rawson R, Ling W. Cognitive impairment in individuals currently using methamphetamine. American Journal on Addictions. 2000;9(3):222–231. doi: 10.1080/10550490050148053. [DOI] [PubMed] [Google Scholar]
- Simon SL, Domier CP, Sim T, Richardson K, Rawson RA, Ling W. Cognitive performance of current methamphetamine and cocaine abusers. Journal of Addictive Diseases. 2002a;21(1):61–74. doi: 10.1300/j069v21n01_06. [DOI] [PubMed] [Google Scholar]
- Simon SL, Dean AC, Cordova X, Monterosso JR, London ED. Methamphetamine dependence and neuropsychological functioning: Evaluating change during early abstinence. Journal of Studies on Alcohol Drugs. 2010;71(3):335–344. doi: 10.15288/jsad.2010.71.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon SL, Richardson K, Dacey J, Glynn S, Domier CP, Rawson RA, et al. A comparison of patterns of methamphetamine and cocaine use. Journal of Addictive Diseases. 2002b;21(1):35–44. doi: 10.1300/j069v21n01_04. [DOI] [PubMed] [Google Scholar]
- Sturm W, Willmes K. On the functional neuroanatomy of intrinsic and phasic alertness. Neuroimage. 2001;14(1 Pt 2):S76–S84. doi: 10.1006/nimg.2001.0839. [DOI] [PubMed] [Google Scholar]
- Taylor MJ, Schweinsburg BC, Alhassoon OM, Gongvatana A, Brown GG, Young-Casey C, et al. Effects of human immunodeficiency virus and methamphetamine on cerebral metabolites measured with magnetic resonance spectroscopy. Journal of Neurovirology. 2007;13(2):150–159. doi: 10.1080/13550280701194230. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ. The addicted human brain viewed in the light of imaging studies: Brain circuits and treatment strategies. Neuropharmacology. 2004;47(Suppl. 1):3–13. doi: 10.1016/j.neuropharm.2004.07.019. [DOI] [PubMed] [Google Scholar]
- Yamamoto BK, Bankson MG. Amphetamine neurotoxicity: Cause and consequence of oxidative stress. Critical Reviews in Neurobiology. 2005;17(2):87–117. doi: 10.1615/critrevneurobiol.v17.i2.30. [DOI] [PubMed] [Google Scholar]
- Yamamoto BK, Moszczynska A, Gudelsky GA. Amphetamine toxicities: Classical and emerging mechanisms. Annals of the New York Academy of Sciences. 2010;1187:101–121. doi: 10.1111/j.1749-6632.2009.05141.x. [DOI] [PMC free article] [PubMed] [Google Scholar]