Abstract
Background
Obese patients with established coronary artery disease have reduced mortality compared to normal or low body mass index (BMI) patients. The reason for the relation is not yet clearly understood. We sought to evaluate the association of BMI and waist circumference (WC) at the time of presentation in patients with myocardial infarction (MI) with one-year adverse cardiac events.
Methods
In this prospective cohort study, we included consecutive patients with acute MI admitted to a tertiary care hospital during a period of one year. Upon admission, BMI and WC were measured. Patients were followed-up for a period of one year and the primary composite outcome of death or non-fatal MI was correlated with BMI and WC categories.
Results
There were 703 patients (males 559 (79.5%)). Combined non-fatal MI and death at one year was 128 (18.2%). Incidence of primary outcome was 25.0% in low BMI group, 19.9% in normal BMI group, 13.1% in overweight group, 13.4% in class I obese, and 11.1% in class II obese groups. In univariate analysis, the inverse correlation was significant (p value = 0.007). In one-year follow-up period, 12.8% in high and 20.8% in normal WC groups had primary outcome (p value = 0.01). Both BMI and WC lost their predictive value in multivariate analysis.
Conclusions
Low BMI and normal WC were associated with a worse short-term outcome in patients with acute MI. Neither BMI nor WC independently predicted cardiac events or death after acute MI.
Keywords: Obesity paradox, Body mass index, Myocardial infarction
1. Introduction
Obesity, which is established as a risk factor for the development of cardiovascular diseases, has assumed epidemic proportions globally in adults as well as in children. Body mass index (BMI) is the most accepted parameter for the definition and categorization of obesity. Based on BMI, an individual is classified as underweight (BMI <18.5 kg/m2), normal (18.5–22.9 kg/m2), overweight (23–24.9 kg/m2), class I obese (25–29.9 kg/m2), or class II obese (>30 kg/m2).1 Excess abdominal fat deposition is established as a cardiovascular risk factor over and above the general obesity. Waist circumference (WC) is an accepted parameter for measuring this abdominal obesity. High WC is defined as WC greater than 90 cm in men and 80 cm in women.1
Because of its maladaptive effects on various cardiovascular risk factors and its adverse effects on cardiovascular structure and function, obesity has a major impact on cardiovascular diseases, such as coronary artery diseases (CAD),2 heart failure (HF),3 sudden cardiac death,4 and atrial fibrillation,5 and is associated with reduced overall survival. Based on these observations, virtually all national and international guidelines recommend weight loss for overweight and obese patients for the primary and secondary prevention of cardiovascular disease.6, 7
Although obesity is clearly a risk factor for developing CAD and HF, in patients in whom these diseases are established, obesity is reported to have an inverse correlation with all-cause mortality,8 cardiovascular mortality,9 and need for repeat revascularization.10 It was found that obese patients with established CAD had reduced mortality compared with normal BMI patients, whether treated medically, by percutaneous coronary intervention (PCI) or by coronary artery bypass surgery (CABG). The highest mortality rates are observed in patients with a very low BMI (<18.5 kg/m2). This observation has been referred to as the ‘obesity paradox’.10, 11 Though there are several studies related to obesity paradox, the reason for the paradoxical U- or J-shaped relation between BMI and adverse outcome is not yet understood. Several explanations have been suggested for this phenomenon.
Most of the previous studies on obesity paradox are retrospective in nature. BMI was the most commonly used epidemiological measure of obesity in these studies. It does not directly distinguish between central from peripheral adiposity.12 Other indices having better predictive power, but less commonly used, include WC, waist-to-hip ratio, and weight-to-height ratio.13 There are scant data published on paradoxical relation of obesity and CAD, particularly in patients with acute coronary syndromes from India.
2. Methods
In this prospective cohort study, patients admitted to cardiology department of a tertiary hospital with a diagnosis of acute ST elevation myocardial infarction (STEMI) or acute non-ST elevation myocardial infarction (NSTEMI) were included. ST-elevation MI and NSTEMI were defined according to American College of Cardiology Foundation/American Heart Association (ACCF/AHA) guidelines.14, 15
Exclusion criteria were history of myocardial infarction (MI) in the last six months, severe valvular heart disease, conditions where anthropometric measurements were not possible and severe non-cardiac illness limiting survival to less than one year.
Baseline data were collected regarding conventional CAD risk factors (diabetes mellitus, hypertension, smoking, and dyslipidemia), ECG manifestations, biochemical values, Thrombolysis in myocardial infarction (TIMI) score, and Killip class at presentation. Overnight fasting blood samples were collected on the morning after admission for blood lipid and blood sugar measurements. Details of reperfusion procedures (thrombolysis or PCI) were noted. Details of pre-discharge coronary angiogram (CAG) and revascularization, if any, were recorded.
Anthropometric parameters were measured during admission to hospital. Height was measured by wall-mounted tape to the nearest centimeter. Subjects were asked to stand upright without shoes, with their back against the wall, heels together, and eyes directed forward. Weight was measured with portable weighing scale kept on a firm horizontal surface. The subjects were asked to wear light clothing and remove footwear. Weight was recorded to the nearest kilogram. WC was measured using a non-stretchable measuring tape. The subjects were asked to stand erect in a relaxed position with both feet together. Waist girth was measured at the midpoint between the iliac crest and the lower margin of the ribs at the end of expiration, to the nearest centimeter. Patients were categorized into BMI and WC groups according to WHO Classification of BMI and WC in Asian adults.16
Follow-up was done at one month, three months, six months, and one year after discharge. Follow-up was performed either in special clinic conducted for study or by telephonic interview. Patients who report events over phone were called to special clinic for verification of records.
Primary outcome was a composite of death due to any cause or non-fatal MI at one year. Secondary outcome was in-hospital mortality. BMI and WC at admission were correlated with both primary and secondary outcomes.
Statistical analysis was performed using Statistical Package for Social Science software (SPSS Inc Chicago, Illinois version 18). Qualitative variables, expressed as numbers and percents, were compared by the Chi-square test. We used univariate analysis to determine the effect of factors affecting one-year outcomes. Factors that were significant predictors of outcomes were used as independent variables in multiple logistic regression analysis to determine independent predictors of one-year outcome. A p-value of less than 0.05 was considered as statistically significant.
3. Results
There were 703 patients (males 559 (79.5%), females 144 (20.4%)). Of the total group, 100 (14.2%) were underweight, 351 (49.9%) were of normal weight, and 122 (17.3%) were overweight. Class I obesity was seen in 112 (15.9%) and class II obesity in 18 (2.5%) patients (Table 1). High WC was measured in 227 (32%) among a total of 703 patients.
Table 1.
Patient categorization according to BMI.
| BMI groups | Number (%) |
|---|---|
| Total | 703 |
| Underweight (BMI1) | 100 (14.2) |
| Normal weight (BMI2) | 351 (49.9) |
| Overweight (BMI3) | 122 (17.3) |
| Obese class I (BMI4) | 112 (15.9) |
| Obese class II (BMI5) | 22 (3.12) |
BMI, body mass index.
The baseline characteristics according to BMI subgroups are summarized in Table 2. There were statistically significant differences in age, TIMI score at admission, ejection fraction, and lipid values at admission. Patients in low BMI groups were older and were having higher TIMI score and lower ejection fraction, while there was a trend of higher lipid values among patients in higher BMI groups. The baseline characteristics were comparable between subgroups of patients with high or normal WC.
Table 2.
Baseline characteristics of BMI subgroups.
| BMI1 | BMI2 | BMI3 | BMI4 | BMI5 | p-Value | |
|---|---|---|---|---|---|---|
| Age, mean (SD) | 58.69 (9.3) | 56.68 (11.2) | 52.98 (10.7) | 54.92 (11.1) | 52.78 (9.3) | 0.001 |
| Diabetes% | 26 | 28.2 | 34.4 | 34.8 | 33.33 | 0.440 |
| Past history CVA% | 4 | 3 | 4.9 | 8.03 | 0 | 0.322 |
| Old CAD% | 10 | 8.5 | 12.2 | 11.6 | 16.66 | 0.606 |
| Dyslipidemia% | 17 | 23.3 | 28.6 | 30.3 | 22.22 | 0.031a |
| Hypertension% | 31 | 35.8 | 37.7 | 30.32 | 16.66 | 0.769 |
| Smoking% | 55 | 59.5 | 59.8 | 50 | 50 | 0.663 |
| TIMI average | 2.68 | 2.82 | 2.40 | 2.49 | 1.83 | 0.037 |
| Primary PCI% | 6.5 | 7.6 | 10.5 | 3.9 | 0 | 0.475 |
| LVEF% | 54 | 58 | 59 | 60 | 58 | <0.001 |
| Serum cholesterol | 176 | 186 | 188 | 189 | 173 | 0.183 |
| Serum triglycerides | 107 | 114 | 118 | 124 | 127 | 0.092 |
| PCI in 1 year | 37 | 38.1 | 32.78 | 30.3 | 27.7 | 0.501 |
SD, standard deviation; LVEF, left ventricular ejection fraction; PCI, percutaneous coronary intervention; CVA, cerebrovascular accident.
Underweight (BMI1), Normal weight (BMI2), Overweight (BMI3), Obese class I (BMI4) and Obese class II (BMI5).
Linear-by-linear association value.
3.1. BMI and outcomes
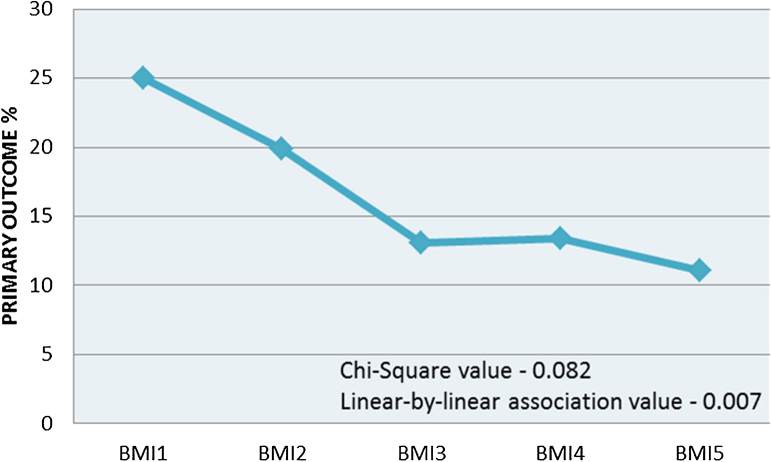
At one year, there were 40 (5.6%) deaths including 18 in-hospital deaths. The incidence of primary outcome was 128 (18.2%). At one-year follow-up, 25.0% of low BMI group, 19.9% of normal BMI group, 13.1% of overweight group, 13.4% of class I obese group, and 11.1% of class II obese group had the composite primary outcome. In univariate analysis, the inverse correlation of obesity and primary outcome was significant by linear-by-linear association (p value = 0.007) (Fig. 1 and Table 3). In-hospital mortality alone showed no significant trend among BMI groups.
Fig. 1.
Correlation of body mass index categories and primary outcome (death or non-fatal myocardial infarction at one year). Underweight (BMI1), Normal weight (BMI2), Overweight (BMI3), Obese class I (BMI4) and Obese class II (BMI5).
Table 3.
BMI and primary outcome (death or non-fatal MI at 1 year).
| BMI category | Number (%) | p-Value |
|---|---|---|
| Total | 128 (18.2%) | |
| Underweight | 25 (25.0%) | Linear-by-linear association value (chi-square value for trend) −0.007 |
| Normal weight | 70 (19.9%) | |
| Overweight | 16 (13.1%) | |
| Obese class I | 15 (13.4%) | |
| Obese class II | 2 (11.1%) |
BMI, body mass index.
Subgroup analysis was also done to find out whether any difference exists regarding the effect of BMI and the primary outcome between the subgroups formed on the basis of sex, diabetes mellitus, and hypertension. When separately analyzed, no correlation could be observed between BMI and the primary outcome in any of the subgroups probably due to smaller sample sizes in each of the subgroup (Table 6).
Table 6.
BMI and primary outcome: subgroup analysis.
| Underweight |
Overweight |
Obese | |
|---|---|---|---|
| Relative risk (95% CI) | |||
| Male | 1.30 (0.82–2.07 | 0.56 (0.30–1.02) | 0.68 (0.39–1.16) |
| Female | 1.12 (0.50–2.48) | 1.09 (0.44–2.68) | 0.57 (0.18–1.83) |
| Diabetic | 1.73 (0.94–3.19) | 0.54 (0.22–1.32) | 0.80 (0.39–1.66) |
| Non-diabetic | 1.06 (0.63–1.79) | 0.72 (0.39–1.32) | 0.56 (0.29–1.08) |
| Hypertensive | 1.40 (0.77–2.56) | 0.19 (0.05–0.76) | 0.41 (0.16–1.11) |
| Non-hypertensive | 1.19 (0.71–2.02) | 0.01 (0.58–1.75) | 0.81 (0.46–1.44) |
All comparisons are against the group with normal BMI.
3.2. WC and outcomes
At one-year follow-up 12.8% in high WC group and 20.8% in normal WC group had the composite primary outcome (p value = 0.01) (Table 4). Secondary outcome of in-hospital mortality was 0.9% in high WC group and 3.4% in normal WC group (p value = 0.052).
Table 4.
Waist circumference and primary outcome (death or non-fatal MI at 1 year).
| Waist circumference category | Number (%) | p-Value |
|---|---|---|
| Total | 128 (18.2%) | |
| High waist circumference | 29 (12.8%) | 0.010 |
| Normal waist circumference | 99 (20.8%) |
MI, myocardial infarction.
Other parameters, which were found to be predictive of one-year outcomes, were age, TIMI risk score, and Killip class at admission. But on multivariate analysis, none of these parameters were found to have significant predictive value for the primary outcome (Table 5).
Table 5.
Multivariate analysis of primary outcome.
| p-Value | Adjusted ORa | 95% CI for OR |
||
|---|---|---|---|---|
| Lower | Upper | |||
| BMI | 0.756 | |||
| BMI1 vs 2 | 0.310 | 0.757 | 0.443 | 1.296 |
| BMI1 vs 3 | 0.207 | 0.618 | 0.293 | 1.304 |
| BMI1 vs 4 | 0.283 | 0.643 | 0.287 | 1.440 |
| BMI1 vs 5 | 0.621 | 0.663 | 0.130 | 3.390 |
| Waist circumference | 0.130 | 1.526 | 0.884 | 2.634 |
OR, odds ratio.
After adjusting age, TIMI class, creatinine at admission, KILLIP class at admission, Reference category BMI 1.
4. Discussion
Metabolic cardiovascular risk factors continuously worsen with increasing BMI, which is described in many previous studies.17, 18, 19, 20 Our study showed an increasing trend for incidence of dyslipidemia toward higher BMI subgroups. Despite that, our study demonstrated a paradoxical relationship between BMI and outcomes after acute MI. But after multivariate analysis, these associations were found non-significant, which is consistent with most of the previous studies from different parts of the world.19, 20, 21 Study of the relation between BMI, WC, and death after acute MI by Zeller et al. included 2229 consecutive patients with acute MI.19 Patients were classified according to BMI and WC. Increased BMI was associated with a reduced death rate, with a 5% risk reduction for each unit increase in BMI. WC as a continuous variable had no impact on all-cause death. A combination of decreased muscle mass and increased abdominal adiposity, which may be termed sarcopenic obesity, which supposedly elevates mortality risk in CAD, was identified in their study population. Such a group was not identified in our sample. The primary outcome in that study was cardiovascular mortality. We could not keep cardiovascular mortality as the primary outcome due to small sample size. After adjustment for baseline predictors of death, neither BMI nor WC was an independent predictor of death after acute MI. Inverse relationship between BMI and outcomes was found due to confounding factors interfering with survival, as seen in our study.
A major impact of younger age in the apparent protection conferred by obesity has been reported in many studies.22, 23 As obese patients with acute coronary syndromes are younger, they are more likely to be referred to experts for secondary prevention, to receive treatment for co-morbidities, a more aggressive lifestyle modification, and optimization of medical treatment. This may be a plausible reason for the enhanced survival in such individuals. In the present study population also, age was significantly lower in the higher BMI group patients, which could have contributed to the better outcome.
A study on “obesity paradox” in Korean patients undergoing primary PCI in STEMI by Kang et al. was recently published.20 Three thousand eight hundred and twenty-four STEMI patients from Korean Acute MI Registry who underwent primary PCI were selected. They were divided into four groups according to BMI. In-hospital mortality, revascularization at one year, mortality at one year and overall mortality were compared. Obese patients had significantly lower in-hospital and overall mortalities. But obesity was not an independent predictor of overall mortality after multivariate analysis. Overweight and obese groups were significantly younger, had better LVEF, and were more likely to be men with a higher incidence of hypertension, diabetes, and hyperlipidemia. Obese STEMI patients treated by primary PCI were associated with lower mortality, which was explained by younger age, hemodynamic stability at admission, and better use of medical treatment. In present study population also, LVEF was significantly low and TIMI score was significantly high in the lower BMI groups. The size of the coronary arteries increases with increasing BMI, and small size of the vessels is a risk factor for worse outcome after PCI and CABG.24 This study did not look into this aspect.
In the present study, a worse outcome was observed in patients with low WC. But most of the previous studies showed worse outcome in patients with high WC along with normal or low BMI, which presumably reflects the presence of visceral obesity with low muscle mass and a lack of functional subcutaneous adipose tissue.19 Functional adipose tissue produces hormones that may have cardio-protective effects in MI.25
4.1. Limitations of the study
There are significantly large number of males in the study group compared to females. This might have affected the results. Similarly, there were significant differences between the study groups with reference to age, dyslipidemia, hypertension and ejection fraction, which might have acted as confounding factors. We did not have data on cause-specific mortality mainly due to small sample size and relatively short follow-up duration. 15% of the patients had missing data. These patients would have had higher number of events, and therefore, their exclusion from the analysis might have produced biased results. We did not have prior sample size estimation, and hence, the absence of correlation between BMI and outcomes on multivariate analysis cannot be taken as a definitive result. Larger studies are required for definite evidence.
5. Conclusion
Low BMI and normal WC are associated with a worse short-term outcome in patients with acute MI. After adjusting for other variables, neither BMI nor WC independently predicts cardiac events or death after acute MI.
Conflicts of interest
The authors have none to declare.
References
- 1.World Health Organization; Geneva, Switzerland: 2000. Obesity: Preventing and Managing the Global Epidemic. Report on a WHO Consultation. [PubMed] [Google Scholar]
- 2.Calle E., Thun M.J., Petrelli J.M. Body mass index and mortality in a prospective cohort of U.S. adults. N Engl J Med. 1999;341:1097–1105. doi: 10.1056/NEJM199910073411501. [DOI] [PubMed] [Google Scholar]
- 3.Kenchaiah S., Evans J., Levy D. Obesity and the risk of heart failure. N Engl J Med. 2002;347:305–313. doi: 10.1056/NEJMoa020245. [DOI] [PubMed] [Google Scholar]
- 4.Flegal K.M., Graubard B.I., Williamson D.F., Gail M.H. Cause-specific excess deaths associated with underweight, overweight, and obesity. JAMA. 2007;298:2028–2037. doi: 10.1001/jama.298.17.2028. [DOI] [PubMed] [Google Scholar]
- 5.Wanahita N., Messerli F.H., Bangalore S. Atrial fibrillation and obesity-results of a meta-analysis. Am Heart J. 2008;155:310–315. doi: 10.1016/j.ahj.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 6.Willett W.C., Dietz W.H., Colditz G.A. Guidelines for healthy weight. N Engl J Med. 1999;341:427–434. doi: 10.1056/NEJM199908053410607. [DOI] [PubMed] [Google Scholar]
- 7.Klein S., Burke L.E., Bray G.A. Clinical implications of obesity with specific focus on cardiovascular disease: a statement for professionals from the American Heart Association Council on Nutrition, Physical Activity, and Metabolism: endorsed by the American College of Cardiology Foundation. Circulation. 2004;110:2952–2967. doi: 10.1161/01.CIR.0000145546.97738.1E. [DOI] [PubMed] [Google Scholar]
- 8.Oreopoulos A., Padwal R., Norris C. Effect of obesity on short and long-term mortality post coronary revascularization: a meta-analysis. Obesity. 2008;16:442–450. doi: 10.1038/oby.2007.36. [DOI] [PubMed] [Google Scholar]
- 9.Oreopoulos A., Padwal R., Kalantar-Zadeh K. Body mass index and mortality in heart failure: a meta- analysis. Am Heart J. 2008;156:13–22. doi: 10.1016/j.ahj.2008.02.014. [DOI] [PubMed] [Google Scholar]
- 10.Gruberg L., Mercado N., Milo S. Arterial Revascularization Therapies Study I. Impact of body mass index on the outcome of patients with multivessel disease randomized to either coronary artery bypass grafting or stenting in the ARTS trial: the obesity paradox II? Am J Cardiol. 2005;95:439–444. doi: 10.1016/j.amjcard.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 11.Romero-Corral A., Montori V.M., Somers V.K. Association of body weight with total mortality and with cardiovascular events in coronary artery disease: a systematic review of cohort studies. Lancet. 2006;368:666–678. doi: 10.1016/S0140-6736(06)69251-9. [DOI] [PubMed] [Google Scholar]
- 12.James W.P. Assessing obesity: are ethnic differences in body mass index and waist classification criteria justified? Obese Rev. 2005;6:179–181. doi: 10.1111/j.1467-789X.2005.00214.x. [DOI] [PubMed] [Google Scholar]
- 13.Litwin S.E. Which measures of obesity best predict cardiovascular risk? J Am Coll Cardiol. 2008;52:616–619. doi: 10.1016/j.jacc.2008.05.017. [DOI] [PubMed] [Google Scholar]
- 14.Antman E.M., Anbe D.T., Armstrong P.W. ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction: a report of the ACC/AHA Task Force on Practice Guidelines (Committee to Revise the 1999 Guidelines for the Management of Patients with Acute Myocardial Infarction) Circulation. 2004;110:e82. [PubMed] [Google Scholar]
- 15.Anderson J.L., Adams C.D., Antman E.M. ACC/AHA 2007 guidelines for the management of patients with unstable angina/non-ST-elevation myocardial infarction: executive summary. A report of the ACC/AHA Task Force on Practice Guidelines. Circulation. 2007;116:803. [Google Scholar]
- 16.Misra A., Chowbey P., Makkar B.M., for Consensus Group Consensus statement for diagnosis of obesity, abdominal obesity and the metabolic syndrome for Asian Indians and recommendations for physical activity, medical and surgical management. JAPI. 2009;57:163–170. [PubMed] [Google Scholar]
- 17.Gupta R., Agrawal A., Misra A. Metabolic cardiovascular risk factors worsen continuously across the spectrum of body mass index in Asian Indians. Indian Heart J. 2012;6403:236–244. doi: 10.1016/S0019-4832(12)60079-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pi-Sunyer F.X. The obesity epidemic: pathophysiology and consequences of obesity. Obese Res. 2002;10:97–104. doi: 10.1038/oby.2002.202. [DOI] [PubMed] [Google Scholar]
- 19.Zeller M., Steg P.G., Ravisy J. Relation between body mass index, waist circumference, and death after acute myocardial infarction. Circulation. 2008;118:482–490. doi: 10.1161/CIRCULATIONAHA.107.753483. [DOI] [PubMed] [Google Scholar]
- 20.Kang W.Y., Jeong M.H., Ahn Y.K. Obesity paradox in Korean patients undergoing primary percutaneous coronary intervention in ST-segment elevation myocardial infarction. J Cardiol. 2010;55:84–91. doi: 10.1016/j.jjcc.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 21.Buchholz E.M., Rathore S.S., Reid K.J. Body mass index and mortality in acute myocardial infarction patients. Am J Med. 2012;125:796–803. doi: 10.1016/j.amjmed.2012.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mehta L., Devlin W., McCullough P.A. Impact of body mass index on outcomes after percutaneous coronary intervention in patients with acute myocardial infarction. Am J Cardiol. 2007;99:906–910. doi: 10.1016/j.amjcard.2006.11.038. [DOI] [PubMed] [Google Scholar]
- 23.Madala M.C., Franklin B.A., Chen A.Y. Obesity and age of first non-ST-segment elevation myocardial infarction. J Am Coll Cardiol. 2008;52:979–985. doi: 10.1016/j.jacc.2008.04.067. [DOI] [PubMed] [Google Scholar]
- 24.O’Connor N.J., Morton J.R., Birkmeyer J.D. Effect of coronary artery diameter in patients undergoing coronary bypass surgery. Northern New England Cardiovascular Disease Study Group. Circulation. 1996;93:652–655. doi: 10.1161/01.cir.93.4.652. [DOI] [PubMed] [Google Scholar]
- 25.Kershaw E.E., Flier J.S. Adipose tissue as an endocrine organ. J Clin Endocrinol Metab. 2004;89:2548–2556. doi: 10.1210/jc.2004-0395. [DOI] [PubMed] [Google Scholar]